Abstract
Two recent papers published in Immunity and Cell Host & Microbe underline the great importance of B cells and of antibodies (Abs) in orchestrating crucial T helper cell type 2 (Th2) protective immune responses to gastrointestinal nematodes. The findings in animal models now raise major questions as to how B cells and Abs carry out these functions in humans. Here we discuss recent technological advances in humanizing animal models at the level of both Abs and their Fc-receptors, that might provide some answers.
From polyclonal to monoclonal
We have read with great interest the important work by Wojciechowski et al. [1] and the highly complimentary article by McCoy et al. [2], in which the authors demonstrate that B cells and antibodies (Abs) play an essential role in protection to the gastrointestinal nematode, Heligmosomoides polygyrus, now renamed Heligmosomoides bakeri [3]. The two papers highlight that B cells are crucial to generation of T helper cell type 2 (Th2) responses that lead to control of parasites. Although this expansion and maturation of protective Th2 cells by B cells was independent of Ab synthesis, the ability to limit parasites was clearly attributable to the presence of specific Ab. The important contribution made by Abs was demonstrated by passive transfer of immune serum (or the IgG fraction) into naïve recipient C57BL/6 mice [2]. Surprisingly, passive transfer of immune serum into μ–MT mice was without protective effect [1], presumably as a consequence of a genetic defect in the mice (or some other uncharacterized deficiency) on which the functionality of Abs relies, and that indirectly prevents Abs from clearing parasites?
In contrast to the work of Wojciechowski and McCoy using passive transfer of immune serum containing polyclonal Abs (principally IgG1), many groups have shown passive transfer of protective immunity using monoclonal Abs (mAbs). For example, mAbs of the IgA class (significantly not IgG or IgM) are capable of transferring protection against gastrointestinal nematodes Trichuris muris [4] and Trichinella britovi [5]. Such experiments have not been possible with H. bakeri (and many other helminth diseases), because of the lack of mAbs. In the most critical passive transfer experiments, both studies used polyclonal anti-serum that might contain Abs with a predilection for triggering inhibitory receptors (e.g. FcγRIIB; see Figure 1), contain irrelevant specificities (blocking Abs for example), cytokines, or other serum factors that cloud the picture that might otherwise be seen with purified mAbs.
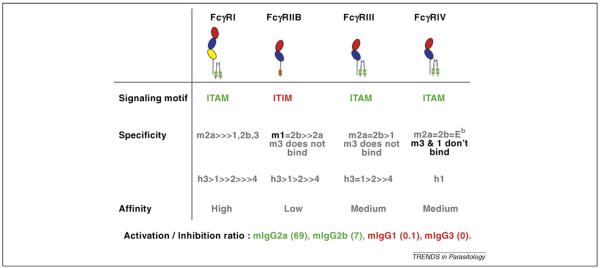
Figure 1.
The activation/inhibitory (A:I) ratios of the four mouse (m) IgG subclasses for binding murine Fcγ-receptors. Note that, in contrast with mIgG2a or mIgG2b, mIgG1 prefers to bind inhibitory FcγRIIB (CD32), offering a possible explanation as to why this subclass is ineffective in many passive transfer experiments of both infection and tumour mouse models (see Ref. [6] for calculation of A:I ratios, and Ref. [7] for a discussion of their applicability to parasite systems). Abbreviations: ITAM, immunoreceptor tyrosine-based activation motif; ITIM, immunoreceptor tyrosine-based inhibition motif.
And the war of the subclasses
With this in mind, it becomes crucial to identify the mouse IgG subclass responsible for the protective effects observed by Wojciechowski and McCoy. Among mouse or murine IgG subclasses, IgG2a and IgG2b are considered to be the most potent activators and dominate in successful passive-transfer experiments in both murine infection and tumor models [6–8]. Such functional distinctions have been attributed to differences in their capacity to fix complement, and/or recruit relevant Fcγ-receptors. This is important because much published research overemphasizes the importance of IgG1 to protection by H. bakeri. Mouse IgG1 is not a subclass associated with protective properties, as it possesses an extremely low activation to inhibition (A:I) ratio (0.1 compared to 69 and 7 for IgG2a and IgG2b, respectively), as a consequence of its preference for binding the inhibitory FcγRIIB receptor (Figure 1) [6–8]. It will therefore be fascinating to determine if these passive-transfer experiments can be emulated with mAbs, and, if so, with which IgG subclass and to which antigens.
It is very difficult to ensure the absolute purity of any individual subclass isolated from mouse sera, and minor contamination with a highly biologically active subclass (or potent cytokine) can give misleading results. Purifying parasite-specific Abs of any given IgG subclass, from small volumes of mouse blood, also results in ng to μg quantities of protein that are difficult to standardize. Furthermore, antigen-directed isotype restriction, means that different subclasses do not recognize identical epitope populations. Because epitope density has a major influence on the efficiency of effector mechanisms, such as Ab-dependent cell mediated cytotoxicity (ADCC), it must follow that the importance of polyclonal IgG might not be representative of the fundamental role played by any individual subclass or antigen in vivo. Recombinant epitope-matched mouse mAbs for all the subclasses are now required to overcome these important limitations seen with polyclonal reagents. These can be generated from combinatorial Ab libraries by cloning variable (V) genes into plasmids containing mouse γ1, γ2a, γ2b or γ3 constant regions for expression by cell lines [7].
Fc-receptors all knocked out?
The implication that affinity-matured IgG1 is therefore a protective subclass might imply that mouse FcγRs are involved (Figure 1). However, contradictory findings have been reported for helminth parasites using mice deficient in the common γ-chain, essential for the expression of many but not all FcRs [6–8]. Indeed, Wojciechowski et al. suggest that γ-chain-deficient animals are susceptible to challenge infections, whereas McCoy et al. conclude that FcγRs play only a very small, but reproducible, role in mediating parasite rejection. How can these apparent differences be reconciled? It is important to appreciate that work in the γ-chain-deficient mouse cannot provide definitive evidence for rejecting the role of FcγRs in their observed findings [1,2]. There might be as yet unidentified FcγRs involved in the observed response, and the α-chain of many FcRs might associate with signaling proteins other than the common γ-chain. For example, mouse IgG3-opsonized Cryptococcus neoformans can still be phagocytosed by macrophages from these same FcRγ−/− mice [9]. This effect is probably mediated via an undefined FcR that does not require γ-chain for function because, of the known FcRs, only murine FcγRI binds mouse IgG3 [10] (see Figure 1). More problematic is that FcRγ−/− mice have been found to express partially functional FcγRI in more recent mouse knockouts [11,12]. It is now known that the α-chain of FcγRI can mediate major histocompatibility complex (MHC) class II Ag presentation without active γ-chain signaling [13,14], and that the α-chain can interact with Periplakin to control receptor endocytosis and IgG binding capacity [13,14]. These issues might therefore be more clearly resolved in mice deficient in α-chains for these FcRs, for example, in quintuplet Fc-deficient mice used recently to highlight the importance of FcγRIV to the function of mouse IgE [15]. If the assertion made by the authors as to the importance of IgG1 is correct, might one expect greater levels of protection in mice deficient in FcγRIIB, that are no longer able to bind IgG1 and are therefore incapable of mounting inhibitory responses (Figure 1)?
Neutralizing Fabs or regulatory Fcs
Another possibility is that the protection offered by passively transferred IgG is mediated not only by FcγRs, but also by other mechanisms. For example, in malaria, Abs can inhibit processing of key parasite antigens and the subsequent invasion of red blood cells by mechanisms that are independent of Fc-receptors [16]. The passive transfer of Fabs or F(ab’)2, derived from their specific IgG (free of Fc), would have provided evidence for the role of the Fc over the Fab in their findings. However, that passively transferred Abs do not protect in μ-MT animals would argue against Fc-independent mechanisms of Ab action.
One unifying hypothesis that fits the data obtained is that Abs simply neutralize the immunomodulatory factors secreted by the highly immune suppressive adult worms, thereby allowing Th2-driven responses to gain dominance, together with ensuing inflammatory responses, leading to protection [17,18]. Indeed, earlier work from our group [19] demonstrated that IgG1 (almost undetectable IgG2a) was the predominant subclass found at mucosal surfaces, and much of it was directed against excretory/secretory (ES) molecules from adult worms. That IgG1 is the major subclass found at mucosal surfaces in mice makes sense in light of the low A:I ratio ascribed to this subclass [6,7]. It has also recently been shown that the Fc portion of IgG contains T-cell epitopes that are capable of specifically activating CD4+CD25HiFoxP3+ natural T regulatory cells (Tregs) [20]. In vivo administration of Fc ‘tregitopes’ resulted in suppression of immune responses to known immunogens, and suggests a potential immunosuppressive activity of polyclonal IgG1, as seen during H. bakeri primary infections with adult worms [17,18,21]. The immunosuppressive effects of adult worms and the polyclonal IgG1 they induce might therefore be related to the activity of Tregs. Such an abundance of polyclonal IgG1 might tip the resulting immune response towards tolerance of worms rather than their elimination [20]. The identification of the molecules seen by these IgG1 s and how they interfere with adult worms must be priorities for future research. Understanding the role (if any), of the other subclasses, e.g. by chimerization of V genes from these IgG1s, might yield novel findings concerning the role of the Fc in this process. However, a mechanism of survival based purely on the induction of Tregs is unlikely to explain why adult worms can be transplanted directly into the intestines of immune mice where they quickly establish and survive for many weeks [22].
Human, mouse or human–mouse chimeras?
Immunity in rodents might also bear little resemblance to immunity in humans. Now that the importance of Abs in controlling nematode infections in rodents has been firmly established, making the system more relevant to humans becomes critical. This could be addressed by engineering human mAbs for testing in mouse models transgenic for human Fc-receptors, as has recently been done with malaria to highlight the importance of human FcγRI[7,23]. That this approach might hold promise for investigating Ab-mediated immunity to nematodes has been demonstrated by the observation that human IgG can passively protect against larval Strongyloides stercoralis in recipient mice [24]. Similar approaches have been used to examine human immune responses to whipworms by reconstitution of SCID mice with human lymphocytes [25]. The relevance of mouse models to infection in humans is particularly important to address, because knockout mice are increasingly being used in models of parasite infection. It is therefore vital to recognize the differences between FcR systems in mice and in humans. For example, there are no mouse equivalents of human FcγRIIA, FcγRIIC, FcγRIIIB and FcαRI [7,8]. Looking at FcγRIIA in particular, this receptor has its own unique immunoreceptor tyrosine-based activation (ITAM) motif and is therefore capable of signal transduction and phagocytosis in the absence of the common γ-chain or other associated subunits. Such careful considerations will prove important in the design of Ab-based therapies and vaccination protocols for helminth diseases of humans and animals.
It is clear from these two important publications that Ab is an important component of immune responses leading to the control of parasites. The priority now is to identify the antigens involved and to elucidate the Fc-receptor/IgG subclasses that contribute optimally to this protection. Only detailed and precise answers to these questions will lead to accurate in vitro assays that better reflect our expectations for future vaccines.
Acknowledgements
We thank the Wellcome Trust, the Medical Research Council UK and the Sir Halley Stewart trust for funding work in our laboratories.
Footnotes
The authors declare no conflicts of interest
References
- 1.Wojciechowski W, et al. Cytokine-producing effector B cells regulate type 2 immunity to H. polygyrus. Immunity. 2009;30:421–433. doi: 10.1016/j.immuni.2009.01.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.McCoy KD, et al. Polyclonal and specific antibodies mediate protective immunity against enteric helminth infection. Cell Host Microbe. 2008;16:362–373. doi: 10.1016/j.chom.2008.08.014. [DOI] [PubMed] [Google Scholar]
- 3.Behnke J, Harris P. Heligmosomoides bakeri or Heligmosomoides polygyrus? Am. J. Trop. Med. Hyg. 2009;80:684–685. [PubMed] [Google Scholar]
- 4.Roach TI, et al. Trichuris muris: antigen recognition and transfer of immunity in mice by IgA monoclonal antibodies. Parasit. Immunol. 1991;13:1–12. doi: 10.1111/j.1365-3024.1991.tb00258.x. [DOI] [PubMed] [Google Scholar]
- 5.Inaba T, et al. Monoclonal IgA antibody-mediated expulsion of Trichinella from the intestine of mice. Parasitology. 2003;126:591–598. doi: 10.1017/s003118200300310x. [DOI] [PubMed] [Google Scholar]
- 6.Nimmerjahn F, Ravetch JV. Divergent immunoglobulin γ subclass activity through selective Fc receptor binding. Science. 2005;310:1510–1512. doi: 10.1126/science.1118948. [DOI] [PubMed] [Google Scholar]
- 7.Pleass RJ. Fc-receptors and immunity to malaria: from models to vaccines. Parasit. Immunol. 2009;31:529–538. doi: 10.1111/j.1365-3024.2009.01101.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Pleass RJ, Woof JM. Fc receptors and immunity to parasites. Trends Parasitol. 2001;17:545–551. doi: 10.1016/s1471-4922(01)02086-4. [DOI] [PubMed] [Google Scholar]
- 9.Yuan R, et al. Ab-mediated modulation of Cryptococcus neoformans infection is dependent on distinct Fc receptor functions and IgG subclasses. J. Exp. Med. 1998;187:641–648. doi: 10.1084/jem.187.4.641. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Gavin AL, et al. Identification of the mouse IgG3 receptor: implications for antibody effector function at the interface between innate and adaptive immunity. J. Immunol. 1998;160:20–23. [PubMed] [Google Scholar]
- 11.Ioan-Facsinay A, et al. FcgammaRI (CD64) contributes substantially to severity of arthritis, hypersensitivity responses, and protection from bacterial infection. Immunity. 2002;16:391–402. doi: 10.1016/s1074-7613(02)00294-7. [DOI] [PubMed] [Google Scholar]
- 12.Barnes N, et al. FcgammaRI-deficient mice show multiple alterations to inflammatory and immune responses. Immunity. 2002;16:379–389. doi: 10.1016/s1074-7613(02)00287-x. [DOI] [PubMed] [Google Scholar]
- 13.van Vugt MJ, et al. The FcgammaRIa (CD64) ligand binding chain triggers major histocompatibility complex class II antigen presentation independently of its associated FcR gamma-chain. Blood. 1999;94:808–817. [PubMed] [Google Scholar]
- 14.Beekman JM, et al. Direct interaction between FcgammaRI (CD64) and periplakin controls receptor endocytosis and ligand binding capacity. Proc. Natl. Acad. Sci. USA. 2004;101:10392–10397. doi: 10.1073/pnas.0401217101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Mancardi DA, et al. FcgammaRIV is a mouse IgE receptor that resembles macrophage FcepsilonRI in humans and promotes IgE-induced lung inflammation. J. Clin. Invest. 2008;118:3738–3750. doi: 10.1172/JCI36452. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Collins CR, et al. An inhibitory antibody blocks interactions between components of the malarial invasion machinery. PLoS Pathog. 2009;5(1):e1000273. doi: 10.1371/journal.ppat.1000273. Epub 2009 Jan 23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Pleass RJ, Bianco AE. The role of adult worms in suppressing functional protective immunity to Heligmosomoides polygyrus bakeri challenge infections. Parasit. Immunol. 1994;16:619–628. doi: 10.1111/j.1365-3024.1994.tb00318.x. [DOI] [PubMed] [Google Scholar]
- 18.Behnke JM. Evasion of immunity by nematode parasites causing chronic infections. Adv. Parsitol. 1987;26:1–71. doi: 10.1016/s0065-308x(08)60294-8. [DOI] [PubMed] [Google Scholar]
- 19.Pleass RJ, Bianco AE. Irradiated larval vaccination and antibody responses evaluated in relation to the expression of immunity to Heligmosomoides polygyrus. Parasitol. Res. 1996;82:445–453. doi: 10.1007/s004360050143. [DOI] [PubMed] [Google Scholar]
- 20.De Groot AS, et al. Activation of natural regulatory T cells by IgG Fc-derived peptide ‘Tregitopes’. Blood. 2008;112:3303–3311. doi: 10.1182/blood-2008-02-138073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Chapman CB, et al. IgG1 hypergammaglobulinaemia in chronic parasitic infections in mice: evidence that the response reflects chronicity of antigen exposure. Aus. J. Exp. Biol. Med. Sci. 1979;57:389–400. doi: 10.1038/icb.1979.39. [DOI] [PubMed] [Google Scholar]
- 22.Robinson M, et al. Immunity to adult Heligmosomoides polygyrus (Nematospiroides dubius): survival or rejection of adult worms following transplantation to mice refractory to larval challenge. J. Helminthol. 1988;62:221–231. doi: 10.1017/s0022149x00011561. [DOI] [PubMed] [Google Scholar]
- 23.McIntosh RS, et al. The importance of human FcgammaRI in mediating protection to malaria. PLoS Pathog. 2007 May 18;3(5):e72. doi: 10.1371/journal.ppat.0030072. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Kerepesi LA, et al. Human immunoglobulin G mediates protective immunity and identifies protective antigens against larval Strongyloides stercoralis in mice. J. Infect. Dis. 2004;189:1282–1290. doi: 10.1086/382484. [DOI] [PubMed] [Google Scholar]
- 25.Taylor MD, Else KT. Human Trichuris-specific antibody responses in vaccinated hu-PBL-SCID mice. Parasit. Immunol. 2002;24:1–13. doi: 10.1046/j.0141-9838.2001.00435.x. [DOI] [PubMed] [Google Scholar]