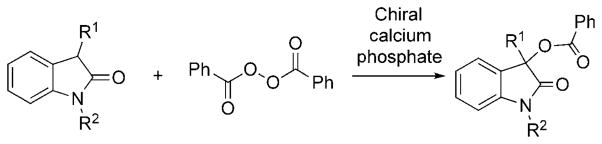
3-Hydroxy-2-oxindoles are structural motifs present in a number of natural products and biologically active compounds.[1, 2] Among these molecules, 3-aryl-3-hydroxyoxindoles represent an important class of molecules that have found broad applications in medicinal chemistry. One such example is SM-130686 (Scheme 1), a compound exhibiting potent activity with respect to growth hormone release.[2a] The absolute configuration of the hydroxy group at the C3 position was shown to further modulate the biological activity.[2c] It is therefore of high importance to introduce asymmetry at the C3 position with high enantiocontrol. To date, only a limited number of approaches have been reported, which outline the preparation of chiral 3-hydroxy-2-oxindoles. One type of approach calls for the asymmetric nucleophilic addition of organometallic reagents[3] or electron-rich reagents[4–6] to isatins. The second approach entails asymmetric hydroxylation of 3-substituted 2-oxindoles.[7] Despite these developments, the available methodologies are often limited and a new methodology is highly desirable, considering the importance of chiral 3-substituted oxindoles.
Scheme 1.
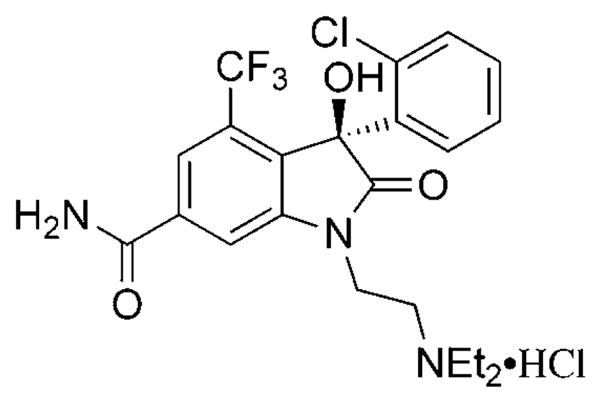
Structure of SM-130686.
Since the independent reports by Akiyama and Terada in 2004,[8] chiral phosphoric acids have proven to be versatile catalysts and have subsequently been applied to a variety of transformations with high stereocontrol.[9] Moreover, the alkali or alkaline earth derived salts of chiral phosphoric acids have proven to be highly effective catalysts in several recent reports.[10]
Benzoyl peroxide (BPO) is a readily available oxylation reagent, which has been known for decades.[11] Nonetheless, asymmetric oxylation using BPO are very rare.[12] Herein, we describe, to the best of our knowledge, the first example of a highly enantioselective benzoyloxylation of an oxindole with BPO catalyzed by a chiral calcium phosphate (Scheme 2).[13] By comparison to published reports, this work provides access to 3-hydroxyoxindole derivatives with the highest stereoselectivity to date.
Scheme 2.
Enantioselective benzoyloxylation of oxindoles.
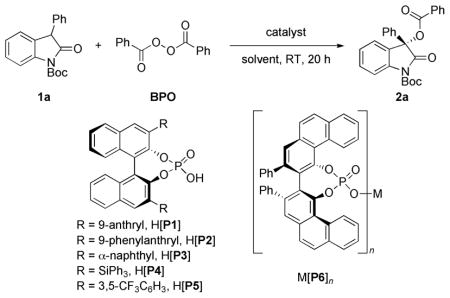
We began our investigation with 3-phenyloxindole 1a and BPO as substrates, and toluene as the solvent, as a starting point for optimization studies. Chiral phosphoric acids purified by silica gel column chromatography, were then screened. Catalysts H[P1], H[P4], and H[P6] (Table 1, entries 1, 4, and 6) imparted meagre stereoselectivity. H[P6], a VAPOL-derived phosphoric acid, proved to be the best catalyst when TBME was the solvent (Table 1, entries 7– 9). The reverse selectivity was observed in DCM (Table 1, entry 10).[14] To our delight, an upgrade to 99% ee was obtained using diethyl ether (Table 1, entry 11). Interestingly, H[P6] washed with 6N HCl exhibited poor catalytic efficiency and enantioselectivity under the same conditions (Table 1, entry 12). Correlation of this result to that of a recent report by Ishihara and co-workers,[10a] showing a high abundance of chiral phosphate salts in the absence of a final HCl wash of the chiral phosphoric acid/salt mixture obtained by silica gel purification, directed us to propose the active catalytic species to be that of a chiral phosphate salt.[15] To identify the metal counterion, several variants of P6 were prepared and evaluated. Na[P6] and K[P6] afforded the product with no selectivity (Table 1, entries 13 and 14). Ca[P6]2 and Sr[P6]2 both induced remarkably high selectivity (>99%) (Table 1, entries 15 and 16). Ba[P6]2 allowed for a significantly lower enantioselectivity (7 %) (Table 1, entry 17). Mg[P6]2 furnished the product with 60% ee, but with the opposite configuration (Table 1, entry 18), presumably due to a difference on coordination spheres compared to calcium.[16] To our delight, excellent enantioselectivity (95%) is still observed with Ca[P6]2, even when the catalyst loading is reduced to 0.10 mol% (Table 1, entry 22).
Table 1.
Screening of catalysts and solvents.[a]
 | ||||
|---|---|---|---|---|
| Solvent | Catalyst (mol %) | Yield [%][b] | ee [%][c] | |
| 1 | toluene | H[P1] purified on silica gel (5) | 77 | 50 |
| 2 | toluene | H[P2] purified on silica gel (5) | 65 | 0 |
| 3 | toluene | H[P3] purified on silica gel (5) | 79 | 30 |
| 4 | toluene | H[P4] purified on silica gel (5) | 80 | 40 |
| 5 | toluene | H[P5] purified on silica gel (5) | 84 | 0 |
| 6 | toluene | H[P6] purified on silica gel (5) | 81 | 45 |
| 7 | TBME | H[P1] purified on silica gel (5) | 80 | 72 |
| 8 | TBME | H[P4] purified on silica gel (5) | 78 | 36 |
| 9 | TBME | H[P6] purified on silica gel (5) | 80 | 96 |
| 10 | DCM | H[P6] purified on silica gel (5) | 56 | −36 |
| 11 | ether[d] | H[P6] purified on silica gel (5) | 81 | 99 |
| 12 | ether | H[P6] washed with HCl (5) | 11 | 15 |
| 13 | ether | Na[P6] (5) | 62 | 2 |
| 14 | ether | K[P6] (5) | 18 | 2 |
| 15 | ether | Ca[P6]2 (2.5) | 83 | > 99 |
| 16 | ether | Sr[P6]2 (2.5) | 82 | > 99 |
| 17 | ether | Ba[P6]2 (2.5) | 51 | 7 |
| 18 | ether | Mg[P6]2 (2.5) | 80 | −60 |
| 19 | ether | Ca[P6]2 (1.0) | 82 | 99 |
| 20 | ether | Ca[P6]2 (0.5) | 81 | 98 |
| 21 | ether | Ca[P6]2 (0.25) | 81 | 97 |
| 22 | ether | Ca[P6]2 (0.10) | 80 | 95 |
Reaction conditions: 1a (1.0 equiv), BPO (1.1 equiv), catalyst (x mol %), solvent (0.1 M) under argon.
Yield of isolated products.
Determined by HPLC analysis on a chrial stationary phase. TBME: tert-butyl methyl ether.
Ether in entries 11–22 means diethyl ether.
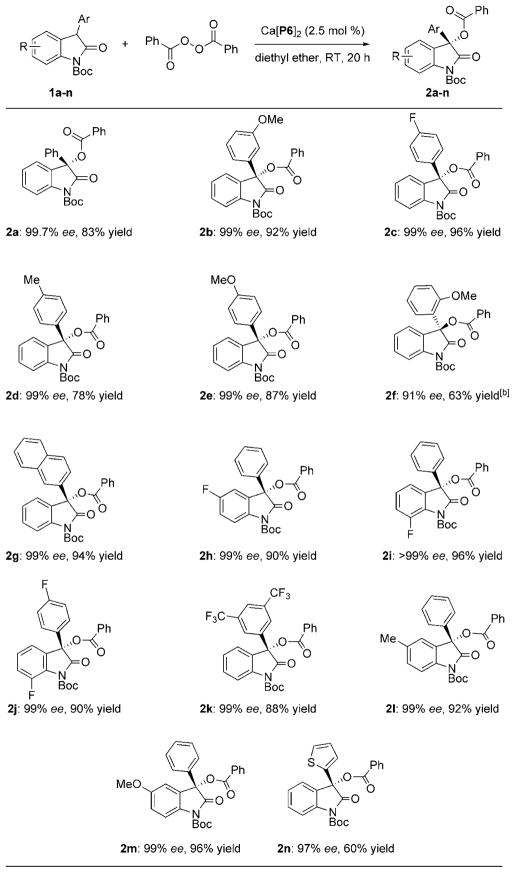
With the optimized reaction conditions in hand, we turned our attention to the scope of the asymmetric benzoyloxylation of 3-aryloxindoles with Ca[P6]2. As shown in Table 2, introduction of either electron-donating or electron-withdrawing groups on the 3-aryl ring or the arene ring of the oxindole have little effect on the enantioselectivity (2a–2m). The majority of products were obtained with 99% ee and good yield. It is worthy of note that 3-aryloxindoles bearing a heteroatom can provide the desired product with excellent enantioselectivity (2n). Unfortunately, no product was detected using 3-benzyloxindole due to lower reactivity.
Table 2.
Substrate scope for the asymmetric benzoyloxylation of oxindoles.[a]
|
Reaction conditions: 1 a–n (1.0 equiv), BPO (1.1 equiv), Ca[P6]2 (2.5 mol %), diethyl ether (0.1 M) at room temperature under argon. Yields refer to isolated product. Enantiomeric excess was determined by HPLC analysis using either a chiral AD-H or OD-H column.
(R)-Ca[P6]2 was used as the catalyst.
Determination of the absolute configuration of the products, as well as potential synthetic utility of this methodology is shown in Scheme 3. Boc-deprotection followed by reduction of the benzoyl group of 2a yielded known compound 4a in two steps with good overall yield and excellent retention of chirality.[17]
Scheme 3.
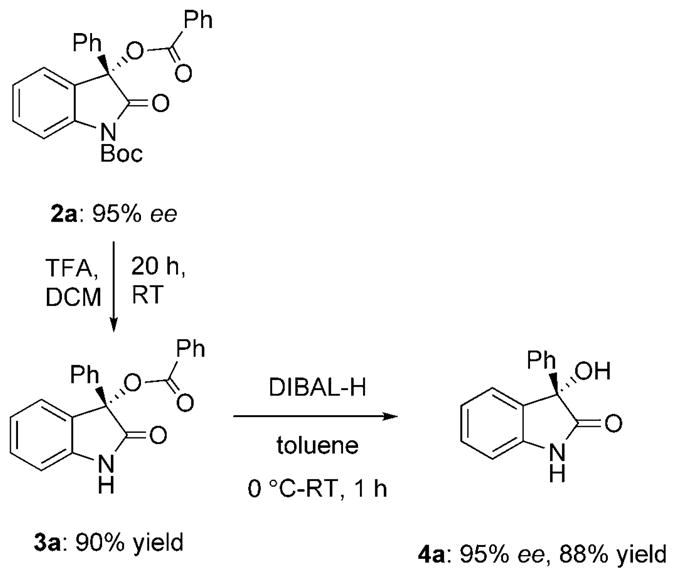
Transformation of 2 a to known hydroxyoxindole 4 a. TFA = trifluoroacetic acid, DIBAL-H = diisobutylaluminum hydride.
While a detailed mechanism for this novel transformation is unknown, we propose that the bifunctional nature of the chiral calcium phosphate salt allows for activation of both the nucleophile and the electrophile, as shown in Scheme 4. Two characteristics of calcium were considered in developing this plausible transition state. First, the low electronegativity of calcium should lead to a significant increase in the Brønsted basicity of the chiral phosphate counteranion. Second, calcium’s various coordination sites presumably allow for a greater number of favorable electrostatic interactions.[18] The coordination between calcium and the carbonyl oxygens of both BPO and the Boc-group of the oxindole serve not only to activate the electrophile but also force the two substrates to be in closer proximity to one another, in the chiral environment. These interactions coupled with the hydrogen-bonding interactions between the hydroxy group of the oxindole tautomer and the P=O moiety of the catalyst can be used to rationalize the unprecedented enantioselectivity observed.
Scheme 4.
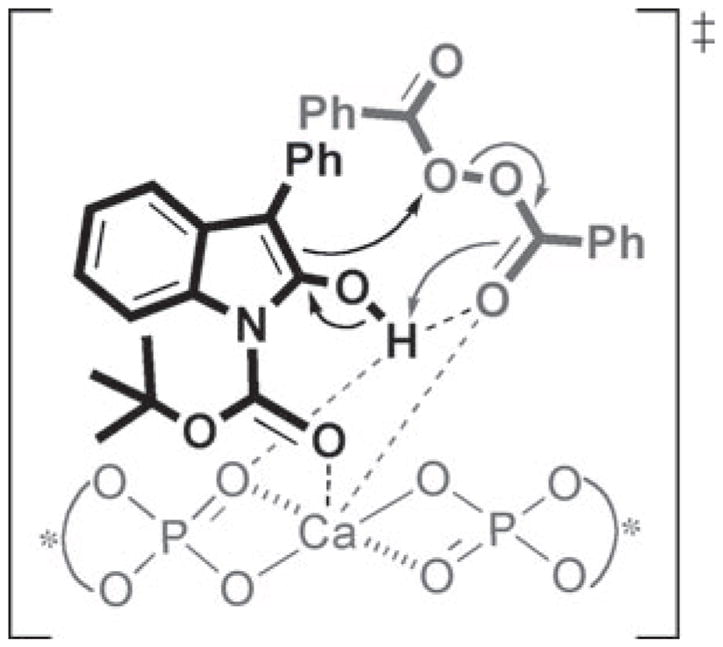
Proposed transition state for the Ca[P6]2-catalyzed benzoyloxylation of oxindole 1 a.
In conclusion, we report a novel asymmetric benzoyloxylation of 3-aryl-2-oxindoles catalyzed by a chiral VAPOL calcium phosphate salt. This transformation utilizes readily available benzoyl peroxide as a benzoyloxylation reagent. A series of 3-aryl-3-benzoyloxindoles are obtained with good yields and excellent enantioselectivities. Further studies of the benzoyloxylation of additional nucleophiles are currently under investigation in our laboratory and will be reported in due course.
Experimental Section
General procedure: Oxindole 1 (0.10 mmol, 1.0 equiv), benzoyl peroxide (0.11 mmol, 26.6 mg, 1.1 equiv), and Ca[P6]2 (2.5 mol%, 3.2 mg) were added to a flame-dried test tube. The vessel was placed under vacuum and the atmosphere exchanged with argon three times before the addition of ether (1.0 mL). The reaction was stirred at room temperature for 20 h and the reaction mixture then purified directly by silica gel column chromatography (eluent: hexanes/ethyl acetate 15:1 to 2:1) to afford pure product 2.
Supplementary Material
Footnotes
We thank the National Institutes of Health (NIH GM-082935) and the National Science Foundation CAREER program (NSF-0847108) for financial support. We also thank Matthew J. Kaplan for preparation of the catalyst and helpful suggestions.
Supporting information for this article is available on the WWW under http://dx.doi.org/10.1002/anie.201006595.
References
- 1.a) Tang YQ, Sattler I, Thiericke R, Grabley S, Feng XZ. Eur J Org Chem. 2001:261–267. [Google Scholar]; b) Gan CY, Kam TS. Tetrahedron Lett. 2009;50:1059–1061. [Google Scholar]; c) Suzuki H, Morita H, Shiro M, Kobayashi J. Tetrahedron. 2004;60:2489–2495. [Google Scholar]; d) Kohno J, Koguchi Y, Nishio M, Nakao K, Kuroda M, Shimizu R, Ohnuki T, Komatsubara S. J Org Chem. 2000;65:990–995. doi: 10.1021/jo991375+. [DOI] [PubMed] [Google Scholar]
- 2.a) Tokunaga T, Hume WE, Umezome T, Okazaki K, Ueki Y, Kumagai K, Hourai S, Nagamine J, Seki H, Taiji M, Noguchi H, Nagata R. J Med Chem. 2001;44:4641–4649. doi: 10.1021/jm0103763. [DOI] [PubMed] [Google Scholar]; b) Tokunaga T, Hume WE, Nagamine J, Kawamura T, Taiji M, Nagata R. Bioorg Med Chem Lett. 2005;15:1789–1792. doi: 10.1016/j.bmcl.2005.02.042. [DOI] [PubMed] [Google Scholar]; c) Hewawasam P, Erway M, Moon SL, Knipe J, Weiner H, Boissard CG, Post-Munson DJ, Gao Q, Huang S, Gribkoff VK, Meanwell NA. J Med Chem. 2002;45:1487–1499. doi: 10.1021/jm0101850. [DOI] [PubMed] [Google Scholar]
- 3.a) Tomita D, Yamatsugu K, Kanai M, Shibasaki M. J Am Chem Soc. 2009;131:6946–6948. doi: 10.1021/ja901995a. [DOI] [PubMed] [Google Scholar]; b) Shintani R, Inoue M, Hayashi T. Angew Chem. 2006;118:3431–3434. doi: 10.1002/anie.200600392. [DOI] [PubMed] [Google Scholar]; Angew Chem Int Ed. 2006;45:3353–3356. doi: 10.1002/anie.200600392. [DOI] [PubMed] [Google Scholar]; c) Itoh J, Han SB, Krische MJ. Angew Chem. 2009;121:6431–6434. [Google Scholar]; Angew Chem Int Ed. 2009;48:6313–6316. doi: 10.1002/anie.200902328. [DOI] [PMC free article] [PubMed] [Google Scholar]; d) Grant CD, Krische MJ. Org Lett. 2009;11:4485–4487. doi: 10.1021/ol9018562. [DOI] [PMC free article] [PubMed] [Google Scholar]; e) Funabashi K, Jachmann M, Kanai M, Shibasaki M. Angew Chem. 2003;115:5647–5650. doi: 10.1002/anie.200351650. [DOI] [PubMed] [Google Scholar]; Angew Chem Int Ed. 2003;42:5489–5492. doi: 10.1002/anie.200351650. [DOI] [PubMed] [Google Scholar]; f) Toullec PY, Jagt RBC, de Vries JG, Feringa BL, Minnaard AL. Org Lett. 2006;8:2715–2718. doi: 10.1021/ol0608101. [DOI] [PubMed] [Google Scholar]; g) Lai H, Huang Z, Wu Q, Qin Y. J Org Chem. 2009;74:283–288. doi: 10.1021/jo802036m. [DOI] [PubMed] [Google Scholar]
- 4.For examples using electron-rich nucleophiles, see: Hanhan NV, Sahin AH, Chang TW, Fettinger JC, Franz AK. Angew Chem. 2010;122:756–759. doi: 10.1002/anie.200904393.Angew Chem Int Ed. 2010;49:744–747. doi: 10.1002/anie.200904393.Chauhan P, Chimni SS. Chem Eur J. 2010;16:7709–7713. doi: 10.1002/chem.201000846.Nakamura T, Shirokawa S, Hosokawa S, Nakazaki A, Kobayashi S. Org Lett. 2006;8:677–679. doi: 10.1021/ol052871p.
- 5.For aldol-type reactions catalyzed by secondary amines, see: Luppi G, Cozzi PG, Monari M, Kaptein B, Broxterman QB, Tomasini C. J Org Chem. 2005;70:7418–7421. doi: 10.1021/jo050257l.Itoh T, Ishikawa H, Hayashi Y. Org Lett. 2009;11:3854–3857. doi: 10.1021/ol901432a.Malkov AV, Kabeshov MA, Bella M, Kysilka O, Malyshev DA, Pluháčková K, Kočovský P. Org Lett. 2007;9:5473–5476. doi: 10.1021/ol7023983.Nakamura S, Hara N, Nakashima H, Kubo K, Shibata N, Toru T. Chem Eur J. 2008;14:8079–8081. doi: 10.1002/chem.200800981.Hara N, Nakamura S, Shibata N, Toru T. Chem Eur J. 2009;15:6790–6993. doi: 10.1002/chem.200900944.Luppi G, Monari M, Correa RJ, Violante FA, Pinto AC, Kaptein B, Broxterman QB, Garden SJ, Tomasini C. Tetrahedron. 2006;62:12017–12024.Chen JR, Liu XP, Zhu XY, Li L, Qiao YF, Zhang JM, Xiao WJ. Tetrahedron. 2007;63:10437–10444.
- 6.For an MBH reaction catalyzed by an alkaloid, see: Liu Y-L, Wang B-L, Cao J-J, Chen L, Zhang Y-X, Wang C, Zhou J. J Am Chem Soc. 2010;132:15176–15178. doi: 10.1021/ja107858z.
- 7.a) Ishimaru T, Shibata N, Nagai J, Nakamura S, Toru T, Kanemasa S. J Am Chem Soc. 2006;128:16488–16489. doi: 10.1021/ja0668825. [DOI] [PubMed] [Google Scholar]; b) Sano D, Nagata K, Itoh T. Org Lett. 2008;10:1593–1595. doi: 10.1021/ol800260r. [DOI] [PubMed] [Google Scholar]; c) Bui T, Candeias NR, Barbas CF., III J Am Chem Soc. 2010;132:5574–5575. doi: 10.1021/ja101032j. [DOI] [PubMed] [Google Scholar]
- 8.a) Akiyama T, Itoh J, Yokota K, Fuchibe K. Angew Chem. 2004;116:1592–1594. doi: 10.1002/anie.200353240. [DOI] [PubMed] [Google Scholar]; Angew Chem Int Ed. 2004;43:1566–1568. doi: 10.1002/anie.200353240. [DOI] [PubMed] [Google Scholar]; b) Uraguchi D, Terada M. J Am Chem Soc. 2004;126:5356–5357. doi: 10.1021/ja0491533. [DOI] [PubMed] [Google Scholar]
- 9.For reviews, see: Akiyama T. Chem Rev. 2007;107:5744–5758. doi: 10.1021/cr068374j.Terada M. Synthesis. 2010:1929–1982.Terada M. Chem Commun. 2008:4097–4112. doi: 10.1039/b807577h.Doyle AD, Jacobsen EN. Chem Rev. 2007;107:5713–5743. doi: 10.1021/cr068373r.
- 10.a) Hatano M, Moriyama K, Maki T, Ishihara K. Angew Chem. 2010;122:3911–3914. doi: 10.1002/anie.201000824. [DOI] [PubMed] [Google Scholar]; Angew Chem Int Ed. 2010;49:3823–3826. doi: 10.1002/anie.201000824. [DOI] [PubMed] [Google Scholar]; b) Klussmann M, Ratjen L, Hoffmann S, Wakchaure V, Goddard R, List B. Synlett. 2010:2189–2192. [Google Scholar]; c) Hatano M, Ikeno T, Matsumura T, Torii S, Ishihara K. Adv Synth Catal. 2008;350:1776–1780. [Google Scholar]; d) Shen K, Liu X, Cai Y, Lin L, Feng X. Chem Eur J. 2009;15:6008–6014. doi: 10.1002/chem.200900210. [DOI] [PubMed] [Google Scholar]
- 11.For representative examples, see: Augustine RL. J Org Chem. 1963;28:581–582.Hecker SJ, Werner KM. J Org Chem. 1993;58:1762–1765.
- 12.a) Kano T, Mii H, Maruoka K. J Am Chem Soc. 2009;131:3450–3451. doi: 10.1021/ja809963s. [DOI] [PubMed] [Google Scholar]; b) Vaismaa MJP, Yau SCY, Tomkinson NCO. Tetrahedron Lett. 2009;50:3625–3627. [Google Scholar]; c) Gotoh H, Hayashi Y. Chem Commun. 2009:3083–3085. doi: 10.1039/b902287b. [DOI] [PubMed] [Google Scholar]
- 13.Selected examples of enantioselective reactions with chiral Ca catalysts: Suzuki T, Yamagiwa N, Matsuo Y, Sakamoto S, Yamaguchi K, Shibasaki M, Noyori R. Tetrahedron Lett. 2001;42:4669–4671.Kumaraswamy G, Sastry MNV, Jena N. Tetrahedron Lett. 2001;42:8515–8517.Kumaraswamy G, Jena N, Sastry MNV, Padmaja M, Markondaiah B. Adv Synth Catal. 2005;347:867–871.Saito S, Tsubogo T, Kobayashi S. J Am Chem Soc. 2007;129:5364–5365. doi: 10.1021/ja0709730.Tsubogo T, Saito S, Seki K, Yamashita Y, Kobayashi S. J Am Chem Soc. 2008;130:13321–13332. doi: 10.1021/ja8032058.Kobayashi S, Tsubogo T, Saito S, Yamashita Y. Org Lett. 2008;10:807–809. doi: 10.1021/ol702958w.Poisson T, Yamashita Y, Kobayashi S. J Am Chem Soc. 2010;132:7890–7892. doi: 10.1021/ja102555a.Poisson T, Tsubogo T, Yamashita Y, Kobayashi S. J Org Chem. 2010;75:963–965. doi: 10.1021/jo902383b.Sr catalysts: Agostinho M, Kobayashi S. J Am Chem Soc. 2008;130:2430–2431. doi: 10.1021/ja710332h.Kobayashi S, Yamaguchi M, Agostinho M, Schneider U. Chem Lett. 2009;38:296–297.
- 14.For a similar solvent effect, see: Zhou J, Ye MC, Huang ZZ, Tang Y. J Org Chem. 2004;69:1309–1320. doi: 10.1021/jo035552p.
- 15.For a recent report demonstrating the importance of reacidification, see: Rueping M, Theissmann T, Kuenkel A, Koenigs RM. Angew Chem. 2008;120:6903–6906. doi: 10.1002/anie.200802139.Angew Chem Int Ed. 2008;47:6798–6801. doi: 10.1002/anie.200802139.
- 16.For a review, see: Bartók M. Chem Rev. 2010;110:1663–1705. doi: 10.1021/cr9002352.
- 17.For details, see the Supporting Information.
- 18.Cotton FA, Wilkinson G, Gaus PL, editors. Basic Inorganic Chemistry. 3. Wiley; New York: 1995. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.