Abstract
The ubiquitously expressed and highly promiscuous Protein Phosphatase 1 (PP1) regulates many cellular processes. Targeting PP1 to specific locations within the cell allows for the regulation of PP1 by conferring substrate specificity. In the present study, we identified AKAP79 as a novel PP1 regulatory subunit. Immunoprecipitaiton of the AKAP from rat brain extract found that the PP1 catalytic subunit co-purified with the anchoring protein. This is a direct interaction, demonstrated by pulldown experiments using purified proteins. Interestingly, the addition of AKAP79 to purified PP1 catalytic subunit decreased phosphatase activity with an IC50 of 811±0.56 nM of the anchoring protein. Analysis of AKAP79 identified a PP1 binding site that conformed to a consensus PP1 binding motif (FxxR/KxR/K) in the first 44 amino acids of the anchoring protein. This was confirmed when a peptide mimicking this region of AKAP79 was able to bind PP1 by both pulldown assay and Surface Plasmon Resonance. However, PP1 was still able to bind to AKAP79 upon deletion of this region, suggestion additional sites of contact between the anchoring protein and the phosphatase. Importantly, this consensus PP1 binding motif was found not to be responsible for PP1 inhibition, but rather enhanced phosphatase activity, as deletion of this domain resulted in an increased inhibition of PP1 activity. Instead, a second interaction domain localized to residues 150–250 of AKAP79 was required for the inhibition of PP1. However, the inhibitory actions of AKAP79 on PP1 are substrate dependent, as the anchoring protein did not inhibit PP1 dephosphorylation of phospho-PSD-95, a substrate found in AKAP79 complexes in the brain. These combined observations suggest that AKAP79 acts as a PP1 regulatory subunit that can direct PP1 activity towards specific targets in the AKAP79 complex.
Protein Phosphatase 1 is a ubiquitously expressed serine/threonine phosphatase that controls many cell processes such as cell cycle progress, protein synthesis, muscle contraction, carbohydrate metabolism and neuronal signaling(1–3). In vitro studies have demonstrated that the isolated catalytic subunit of PP1 is nondiscriminatory. Therefore, mechanisms that promote specificity of PP1 action despite its promiscuous nature are of considerable interest(4, 5). Increasing evidence suggest that precision of action is achieved by phosphatase targeting or regulatory subunits. These proteins function to direct the enzyme to specific subcellular compartments and, in many cases, modulate phosphatase activity(5, 6).
Multiple PP1 targeting proteins have been shown to dictate PP1 location within the cell, and PP1 targeting has been found at multiple sites including glycogen particles, F-actin, and the nucleus(6). While this diverse family shares little similarity in overall homology, numerous studies have shown these proteins contain at least one of several common docking motifs that are responsible for PP1binding(4, 5). The first and most well characterized consensus PP1 binding sequence is the RVxF motif, found in inhibitor-1, NIPP-1, spinophillin, and GM(6–10). Other consensus sites include the MyPhone and SILK motifs, found in the myosin phosphtase targeting subunit Mypt1 and inhibitor-2, respectively(11). Furthermore, an additional motif consisting of FxxR/KxR/K was identified in several Bcl-2 proteins to direct PP1 targeting to this class of proteins(12, 13).
As PP1 targeting proteins function not only to localize the phosphatase, but to focus PP1 activity toward a particular substrate, additional PP1 binding sites are commonly found in each regulatory protein that generate specificity of PP1 actions(5, 6). For example, spinophilin targets the phosphatase to ion channels in the brain, allowing for dephosphorylation of specific substrates while inhibiting phosphatase action towards others(14, 15). Similarly, PP1 binding to GADD34 inhibits catalytic activity towards phosphorylase a, but does not block the actions of the phosphatase towards eIF-2a(16). These second sites of interaction create precision in target selection while modulating phosphatase activity.
Several multivalent anchoring proteins have been found to co-localize protein kinases and protein phosphatases simultaneously(17). A prototypical example of such anchoring proteins are the A-kinase-anchoring proteins (AKAPs), which coordinate cAMP signaling modules, and a subset of these also bind to PP1 and consequently regulate its activity(17). A prototypical example is the NMDA receptor-associated AKAP yotiao, which synchronizes receptor phosphorylation by maintaining a pool of anchored PKA and PP1 with the ion channel(18). By localizing the enzymes responsible for both signal activation and signal termination, these proteins orchestrate bi-directional phosphorylation events in a spatial-temporal manner.
Here, we present evidence that the neuronally expressed AKAP79 acts as a PP1 regulatory subunit. This scaffold protein has previously been shown to localize protein kinase A, protein kinase C and protein phosphatase 2B to the post-synaptic density in the hippocampus(19–21). Our work demonstrates a direct interaction between AKAP79 and PP1 that can be isolated from both rat brain extract and transfected HEK293 cells. Surface Plasmon Resonance assays demonstrated a high affinity association between the anchoring protein and PP1, with two sites of contact between AKAP79 and the PP1 catalytic subunit. Consistent with other PP1 binding proteins, full length AKAP79 attenuated PP1 catalytic active, displaying an IC50 of 811±0.56 nM(13, 22, 23). Mapping studies identified a consensus, high affinity PP1 targeting motif in the first 44 amino acids of the anchoring protein that surprisingly stimulated phosphatase activity. However, a second site of interaction between AKAP79 and PP1 was found to be responsible for inhibition of phosphatase activity towards histone 2A. However, this inhibitory action is substrate dependent, as AKAP79 did not inhibit the dephosphorylation of PSD-95 by PP1. Thus, AKAP79 acts as a PP1 regulatory subunit and our data suggests that interactions between the anchoring protein and the phosphatase can direct PP1 activity towards specific targets.
Experimental Procedures
Preparation of rat brain extract
Frozen adult rat brains (Pel-Freez Biologicals) were homogenized in ice-cold lysis buffer (20 mM HEPES, pH 7.4, 100 mM NaCl, 5 mM EDTA, 0.5% Triton X-100 (w/v), 2 μg/ml leupeptin and pepstatin, 1 mM benzamidine, 1 mM 4-(2-aminoethyl)benzenesulfonyl fluoride). Centrifugation at 16,000 × g for 10 min clarified each lysate. Supernatant was used for immunoprecipitation assays.
HEK-293 cell transfection
For analysis of AKAP79 binding to PP1, HEK-293 cells at 50% confluency were transfected by calcium phosphate precipitation with pEGFP-AKAP79 or control pEGFP plasmid cDNA expression constructs (10 μg) overnight under 5% CO2 at 37° C. Cells were harvested 18 hours later for cell extract and immunoprecipitation.
Expression Constructs
For the AKAP79 deletion constructs, human AKAP79 was PCR amplified using pEGFP-AKAP79 and sub-cloned into the EcoRI-Xho sites of pet32a. In order to make a bacterial recombinant PSD-95, we obtained Addgene plasmid 15463, PSD-95 in pcDNA3(24). PSD-95 was amplified by PCR, and then sub-cloned into the EcoRI/Xho sites of pet32a. PP2B-GST was obtained from Dr. Michael Kapiloff, University of Miami, Miller School of Medicine.
Bacterial expression and purification recombiant proteins
Recombinant AKAP79 fragments and full-length PSD-95 were expressed as His6-tagged/S-Tag fusions using pET vectors (Novagen) in bacteria (T7 Express; New England Biolabs). Bacteria were grown to OD595 0.8 then induced with IPTG (1 mM final concentration) for 3 hours. After centrifugation, the bacteria were lysed in binding buffer (20 mM HEPES, pH 7.4, 5 mM imidizole, 0.5M NaCl) for 2 hours. Supernatant was collected by centrifugation and protein purified by Ni-agarose pulldown overnight (0.5 mL of 50% slurry; Ni-NTA solution, Qiagen). Ni-agarose was washed 3× in binding buffer and protein was eluted with binding buffer + 800 mM imidizole. Protein was concentrated with Amicon Ultra centrifugal filters (Millipore) and concentration determined by Bradford assay.
Immunoprecipitation
For analysis of AKAP150 (the rodent homolog of AKAP79) binding to PP1 from rat brain extract, AKAP150 immune complexes were isolated by incubation of 0.5 ml brain extract with 2 μl diluted rabbit serum anti-AKAP150 (Upstate Biotechnology) with protein G-Sepharose (20 μl, 50% slurry) overnight at 4° C. Control experiments were performed by incubating rat brain extracts with a non-related IgG at the same concentration overnight at 4° C. After microcentrifugation, the protein G-Sepharose was washed 3× with lysis buffer and immune complexes were eluted with SDS-PAGE sample buffer (20 μl). Samples were run on 10% SDS-PAGE gels. For analysis of AKAP79 binding to PP1 from transfected HEK-293 cells, endogenous PP1 complexes were isolated by incubation of 0.5 ml transfected HEK-293 cell lysate with 2 μg mouse monoclonal anti-PP1 (Santa Cruz Biotechnology) or 2 μg goat polyclonal anti-PP1 with protein G-Sepharose (20 μL, 50% slurry) overnight at 4° C. After microcentrifugation, the protein G-Sepharose was washed 3× with lysis buffer and immune complexes were eluted with SDS-PAGE sample buffer (20 μL). Samples were run on 10% SDS-PAGE gels.
Recombinant S-Tag, AKAP79 Protein and AKAP79 Peptide Binding Assays
Recombinant S-Tag AKAP79 full length proteins, and truncation mutants encompassing amino acids 1-150, 1-250, 1-350 and 44-300 (1 μg), were incubated with S-protein agarose (20 μL; Novagen) in 0.5 mL HSE buffer (20 mM HEPES, pH 7.4, 100 mM NaCl, 0.5% Triton X-100 (w/v)) with 1 μg PP1 catalytic subunit (New England BioLabs) for 2 hours. The mixture was centrifuged (3000 × g) and washed 3× with HSE buffer. Protein complexes were eluted from S-protein agarose with 20 μL SDS-PAGE sample buffer and run on 10% SDS-PAGE gels.
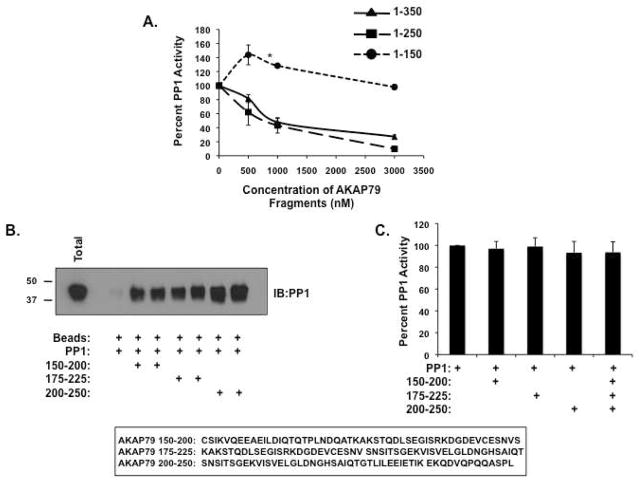
Biotinylated AKAP79 synthetic peptides amino acids 25–49 wild-type (AERQKEKASMLCFKRRKKAAKALKP) and mutant (AERQKEKASMLCAKRAKAAAKALKP) were purchased from Chi Scientific. For peptide binding assays, 1 μg of peptide was incubated with streptavidin beads (20 μL; Invitrogen) for 2 hours and then washed 3× with HSE buffer to remove unbound peptide. For PP1 binding to the AKAP79 peptide, 1 μg of PP1 catalytic subunit was added to the protein complex with 0.5 mL HSE buffer and incubated for 2 hours at 4° C. Similar experiments using peptides encompassing amino acids 150-200 (CSIKVQEEAEILDIQTQTPLNDQATKAKSTQDLSEGISRKDGDEVCESNVS), 175–225 (KAKSTQDLSEGISRKDGDEVCESNV SNSITSGEKVISVELGLDNGHSAIQT), and 200–250 (SNSITSGEKVISVELGLDNGHSAIQTGTLILEEIETIKEKQDVQPQQASPL) of AKAP79 were performed.
For assays demonstrating the AKAP79 peptide binds to PP1, calmodulin (Calbiochem), PKCα (Calbiochem), PP2B (Calbiochem) or PSD-95, 1 μg of peptide was incubated with strepavidin beads (20 μL; Invitrogen) for 2 hours and then washed 3× with HSE buffer to remove unbound peptide. Next, 50 nM PP1α catalytic subunit, 0.3 μg of PKCα protein, 1 μg of calmodulin, 1 μg PP2B, 1 μg PSD-95. After a 2 hour incubation, the complexes were isolated and visualization of proteins was accomplished by western blot analysis.
For competition experiments using PKC, PP1 and AKAP79, S-tagged beads were charged with (0.5 μg) AKAP79 before the addition of PKC (0.3 μg) and increasing concentrations of PP1 (10–1.2 μg). After a 2 hour incubation, the complexes were isolated and visualization of bound proteins was accomplished by western blot analysis.
To measure if AKAP79 can bind both PP1 and calmodulin concurrently, AKAP79 (30 nM) and PP1 (200 nM) were incubated with Calmodulin sepharose (GE Healthcare, 20 μl). After a 2 hour incubation, the isolated complex was washed and run on 10% SDS-PAGE gels to analyze PP1 binding.
For assays using the PP1 binding peptide to compete for PP1 binding to AKAP79, S-tagged beads were charged with (0.5 μg) AKAP79 before the addition of PP1 (0.5 μg) and increasing concentrations of peptide (0–3.2 μM). After a 2 hour incubation, the complexes were isolated and visualization of bound proteins was accomplished by western blot analysis.
Phosphatase Assay
Phosphatase activity was measured by using 32P-labeled histone as substrate. Histone 2A was radiolabeled in reactions containing 250 mM MOPS, pH 7.4, 2.5 mM magnesium acetate, 100 mM ß-mercaptoethanol, purified PKA catalytic subunit, 1 μm ATP, 20 μm histone, and 1 mCi of [γ-32P]-ATP (6000 Ci/mmol). The reaction was terminated by the addition of 50% trichloroacetic acid, and [32P]-histone was purified from free radionucleotide by centrifugation. The [32P]-histone pellet was washed with 1 ml of ether/ethanol/HCl (4:1:0.1) once and 1 ml of ether/ethanol (4:1) three times. The substrate was then suspended in 200 μl of PP1 assay buffer (25mM Tris, pH 7.4, 1mM dithiothreitol, and 10mM MgCl2). For assays where PSD-95 was the substrate for PP1, purified PSD-95 attached to S-beads (25 μg), was phosphorylated by PKA for 8 hours. The complex was washed extensively, and PSD-95 was then eluted from the beads and concentrated. For phosphatase assays, 1 μl of phospho-PSD-95 was incubated with PP1 in the presence or absence of AKAP79 (800 nM).
To measure phosphatase activity from rat brain extract, immunoprecipitated AKAP150 (the rodent ortholog of AKAP79) protein complexes were washed twice in HSE buffer and once in PP1 reaction buffer. The immunoprecipitates were incubated at 30° C in 20 μl of PP1 assay buffer containing 100,000 cpm of [32P]-histone. Reactions were terminated by the addition of 100 μl of 20% trichloroacetic acid followed by a 10-minute centrifugation. Trichloracetic acid supernatants (100 μl) containing released 32PO4 were measured by scintillation counting.
To measure phosphatase activity from purified AKAP79 constructs, purified protein (50–5000 nM) and/or AKAP79 synthetic peptide (50–5000 nM) was incubated with 50 nM of purified PP1α (New England Biolabs) and 50 μl of PP1 assay buffer containing 100,000 cpm of [32P]-histone and phosphatase activity was measured as detailed above. For experiments designed to measure the effect of PKC on AKAP79-mediated inhibition of PP1 activity, 300 ng of purified PKCα was added to the reaction and phosphatase activity was determined.
For phosphatase assays utilizing trypsin, 1 μM trypsin was used to digest 800 nM AKAP79 and 50 nM PP1. Assays were then performed as described.
Surface Plasmon Resonance
SPR analysis was performed using a BIAcore T100. PP1 catalytic subunits were covalently immobilized using NHS (N-hydroxysuccinamide) and EDC [1-ethyl-3-(3-dimethylaminopropyl)-carbodiimide] (Biacore amine coupling kit) to the surface of a sensor chip (BIAcore type CM5). The amount of ligand bound (in resonance units )RUs)) was 150 RU. Protein analytes (AKAP79 peptides or full length AKAP79) were diluted at increasing concentrations (6.25–200 nM) in HBS buffer (10 mM Hepes (pH 7.4), 150 mM NaCl and 0.005% Surfactant P20) and were injected over the sensor surface at a flow rate of 30 μl/min for 300 s. Post injection phase, dissociation was monitored in HBS buffer for 300 s at the same flow rate. The surface was regenerated between injections using 10 mM NaCl at a flow rate of 50 μl/min for 30 s. Obtained sensorgrams were all processed by BIAcore T100 evaluation software.
Results
Identification of AKAP79 as a PP1 binding protein
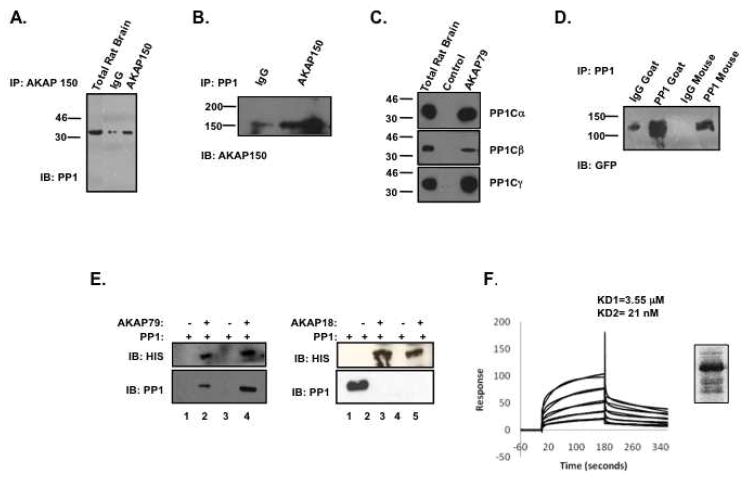
Initial studies on the AKAP79 signaling complex in the brain identified the association of multiple protein kinases, including protein kinase A and protein kinase C(25). However, only one phosphatase, protein phosphatase 2B, had been found to bind AKAP79(20). As protein phosphatases are key signal terminators, we hypothesized that AKAP79 could associate with other phosphatases as well. Therefore, AKAP79 was immunoprecipitated from rat brain extract and association with additional protein phosphatases were investigated. As shown in Figure 1A, protein phosphatase 1 (PP1) was found in AKAP150 (the rodent ortholog of human AKAP79) immunoprecipitates isolated from rat brain extract. Importantly, this association was increased over IgG control, demonstrating the specificity of the interaction. Reciprocal experiments were performed demonstrating that PP1 also immunoprecipitates AKAP150 (Figure 1B). In contrast, protein phosphatase 2A was not present in AKAP150 immunoprecipitates, suggesting that AKAP150 does not indiscriminately interact with phosphatases (data not shown). Next, we investigated which PP1 isoform could associate with AKAP79 complexes isolated from rat brain extract by pulldown assay. Figure 1C shows PP1α, PP1β, and PP1γ catalytic subunit can all associate with the AKAP. To confirm this interaction in a heterologous system, GFP-tagged AKAP79 was transiently transfected into HEK293 cells and association with PP1 was determined by immunoprecipitation of the phosphatase with two distinct antibodies, a goat polyclonal and a mouse monoclonal antibody (Figure 1D). Under both conditions, AKAP79 co-precipitated with endogenous PP1, suggesting this association is not cell-specific.
Figure 1. Identification of AKAP79 as a PP1 binding protein.
(A) Whole rat brain extracts were prepared as described in the Materials and Methods section. Clarified lysates were subjected to immunoprecipitation using either IgG control or AKAP150/79. After an overnight incubation, the complex was subjected to immunoblot analysis for PP1 catalytic subunit. Rat brain extract total (5%) was run on same gel as IgG control and AKAP150/79 samples, but molecular weight marker, which separated the lanes, has been removed from picture, n=3. (B) Rat brain extracts were used to immunoprecipitate IgG control or PP1 catalytic subunit. After purification of the complexes, western blot analysis was performed to demonstrate AKAP150 association. (C) S-tagged beads charged with AKAP79 (3 μg) were incubated with rat brain extract for 7 hours. The complexes were isolated and association of the different isoforms of PP1 catalytic subunit was determined by western blot analysis; n=3. (D) HEK293 cells were transiently transfected with GFP-tagged AKAP79. Cells were lysed 18 hours later in lysis buffer and the lysate was clarified by centrifugation. PP1 catalytic subunit was immunoprecipitated with either a goat polyclonal PP1 catalytic subunit antibody, or a mouse monoclonal antibody to PP1 catalytic subunit, and the appropriate IgG controls. After an overnight incubation, the PP1 complex was isolated and then subjected to western blot analysis using an antibody specific for GFP; n=3. (E) Pulldown assays were performed using S-protein beads charged with or without bacterially expressed S-tagged AKAP79 or AKAP18 in the presence of PP1α catalytic subunit. After a 2 hour incubation, the complex was isolated and association of AKAP79 and PP1 were determined by western blot, n=3. (F) SPR was performed by immobilizing PP1α catalytic subunit (150RUs) on a CM-5 chip and measuring the response when passing over a range of AKAP79 protein concentrations (6.25-200 nM). SPR affinity graph fitted to a heterogeneous, two-ligand model suggestive of two PP1 binding sites on AKAP79. Affinity of the two binding sites are 3.55 μM and 21 nM, as calculated by BIAcore T100 evaluation software. Protein stain of 5 μg of AKAP79 protein used for BIAcore measurements.
To investigate if this interaction is direct or due to an intermediary protein, pulldown assays were performed using bacterially expressed, S-tagged AKAP79 and commercially available PP1α catalytic subunit. This subunit is both catalytically active and has been shown to bind to a multitude of anchoring proteins(26, 27). As shown in Figure 1E, PP1 was only found with S-beads charged with AKAP79. However, the phosphatase did not bind to beads charged with another anchoring protein, AKAP18, demonstrating the specificity of association.
Next we investigated the binding characteristics of AKAP79 and PP1α catalytic subunit by Surface Plasma Resonance (SPR). The binding properties of immobilized PP1 were measured over a range of AKAP79 concentrations (6.25–200 nM). As shown in Figure 1F, SPR data between the two proteins was best fitted to a heterogeneous two-ligand model, suggesting the AKAP contains two PP1 binding domains. This is consistent with other PP1 binding proteins, such as DARPP-32, inhibitor-1, spinophillin and neurabin, which contain two PP1 binding sites, one that directs the association and another involved in regulation of PP1 activity. SPR analysis of the first PP1 binding domain gave an on rate of 6.8×105 1/Ms (ka), an off rate of 2.44 1/sec (kd) and an affinity constant of 3.55 μM (KD). The second PP1 binding site on AKAP79 gave an on rate of 1.9×105 1/Ms (ka), an off rate of 0.00234 1/sec (kd) and an affinity constant of 21 nM (KD). Thus, AKAP79 exhibits a high affinity to PP1. Taken together, the data presented in Figure 1 suggest that AKAP79 and PP1 catalytic subunit interact directly.
Binding of PP1 to AKAP79 inhibits phosphatase activity
Previous work from several investigators has shown PP1 binding proteins function not only by directing the activity of the phosphatase to specific substrates by localizing PP1 to discrete subcellular compartments, but also by regulating the activity of the phosphatase(6). In fact, binding of the phosphatase to several binding proteins such as NIPP-1, AKAP220, and spinophilin inhibit PP1 activity in vitro(8, 22, 27). Therefore, we investigated the effect of AKAP79 on PP1 activity. To begin, we measured phosphatase activity in AKAP79 complexes isolated from rat brain extract using a general PP1 substrate, PKA-phosphorylated histone 2A. As shown in Figure 2A, no significant amount of PP1 activity over IgG was seen in AKAP79 immunoprecipitates, although PP1 immunoreactivity in AKAP79 complexes can be seen under similar conditions (as seen in Figure 1A). This observation was confirmed using pulldowns of Stagged AKAP79 and purified PP1. As shown in Figure 2B, no PP1 activity was seen in AKAP79 pulldowns using purified AKAP79 and PP1α catalytic subunit, suggesting that binding of PP1 to AKAP79 inhibits phosphatase activity.
Figure 2. Regulation of PP1 activity by AKAP79.
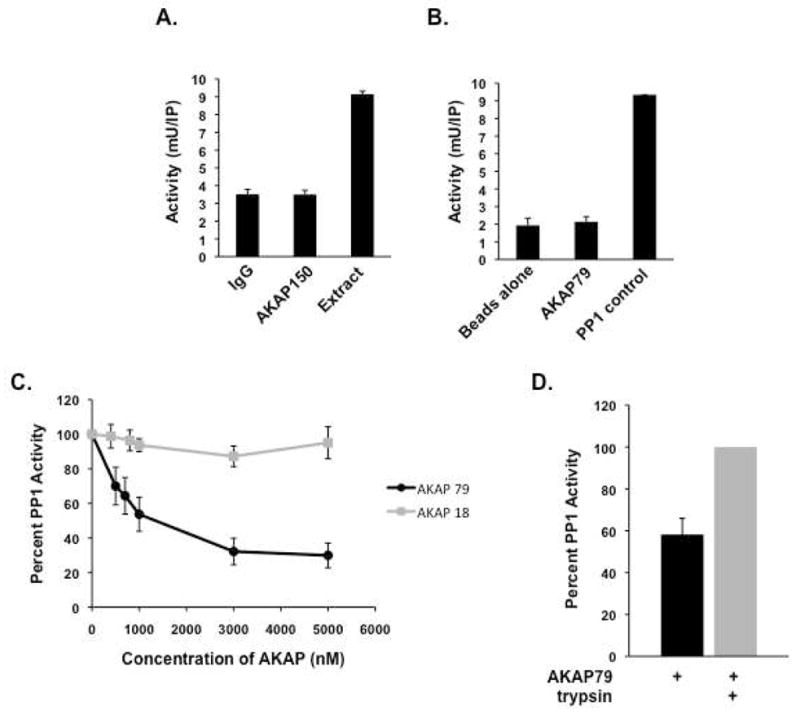
(A) AKAP150/PP1 complexes were immunoprecipitated from rat brain extract and assayed for phosphatase activity using 32-P-labeled histone as a substrate as described in the Materials and Methods; n=3. (B) Bacterially expressed AKAP79 and PP1α catalytic subunit were incubated together for two hours and isolated complexes were assayed for phosphatase activity; n=3. (C) Dose-dependent inhibition of PP1 by AKAP79 or AKAP18 was assayed by incubating increasing amounts of purified AKAP79 or AKAP18 (0-5000 nM) with PP1α catalytic subunit (50 nM). PP1 activity was then measured using 32-P-labeled histone as a substrate; n=3. (D) AKAP79 inhibition of PP1 was performed in the presence and absence of trypsin (1 μM) using AKAP79 (800 nM) and PP1 (50 nM); n=3.
To further investigate this observation, we determined the dose-dependent effect of AKAP79 on PP1 activity (Figure 2C). Increasing concentrations of AKAP79 were added to purified PP1α catalytic subunit, and the resulting changes in PP1 activity were measured. Inhibition of PP1α was seen starting with 300 nM of AKAP79, and the IC50 for inhibition was determined to be 811±0.56 nM. As a control, similar experiments performed with identical concentrations of another AKAP (AKAP18) did not affect PP1 activity (Figure 2C). Furthermore, in order to demonstrate the specificity of the interaction, AKAP79 was treated with trypsin to digest the AKAP, and phosphatase activity was measured. Importantly, PP1 is not affected by trypsin and is still able to dephosphorylate phospho-proteins(28, 29). Figure 2D demonstrates that trypsin digestion of AKAP79 relieved the inhibitory affect of the AKAP on PP1 activity. Taken together, the ability of AKAP79 to inhibit PP1 activity suggests that when PP1 binds to AKAP79, the anchoring protein either occludes the active site on the phosphatase, or induces a conformational change in the PP1 active site, thereby inhibiting phosphatase activity.
A conserved PP1 binding motif on AKAP79 binds to the phosphatase
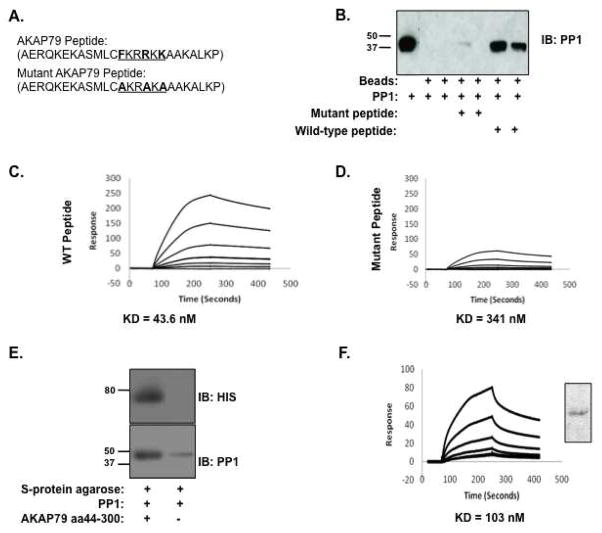
Next, we set out to define the amino acids required for PP1 binding. Several S-tagged, bacterially expressed AKAP79 deletion constructs (shown in Figure 3A) were incubated with PP1α catalytic subunit, and association with the phosphatase was demonstrated by western blot analysis. PP1 was found in all the pulldowns, suggesting at least one binding domain for the phosphatase is contained in the first 150 amino acids of AKAP79 (Figure 3B, top box).
Figure 3. Mapping the binding site of PP1 on AKAP79.
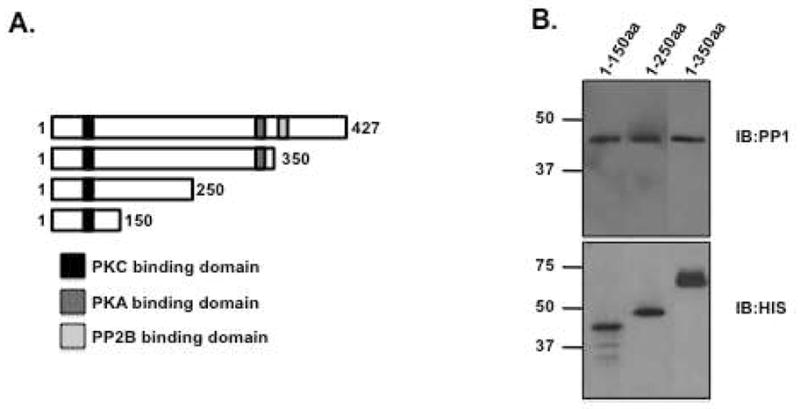
(A) Schematic of AKAP79 deletion constructs. Known protein binding sequences are labeled, PKC-black box, amino acids 31-52; PKA-dark grey box, amino acids 388–409; PP2B-light grey box, amino acids 315-360. (B) Bacterially expressed S-tagged AKAP79 deletion constructs (amino acids 1-150, 1-250 and 1-350) were immobilized on S-protein agarose and incubated with PP1α catalytic subunit for two hours. Association of the phosphatase was demonstrated by western blot. Western blots were spliced together from experiments on each AKAP79 fragment and to exclude duplicate sample lanes; n=3.
Several conserved PP1 binding motifs have previously been identified and are considered to be important for the recognition and binding of PP1 to its anchoring proteins. Examination of the first 150 amino acids revealed that residues 37–42 of AKAP79 harbored a potential PP1 binding motif that corresponded well with an FxxR/KxR/K template (Schematic shown in Figure 4A), but not with other known targeting sequences including the RVxF, MyPhone, or SILK motifs. To test if this region was sufficient for PP1 association, a biotinylated peptide mimicking amino acids 25–49 of the anchoring protein was synthesized and subsequently examined by a PP1 pulldown assay. As shown in Figure 4B, the AKAP79-derived peptide bound to PP1, but a control peptide, where the key residues were mutated to alanines, could not.
Figure 4. Identification of a conserved PP1 binding motif on AKAP79.
(A) Diagram depicting the sequences of the synthesized peptides, corresponding to amino acids 25-49 on AKAP79, that contain the proposed PP1 binding motif FxxR/KxR/K. In the control peptide, key residues are mutated to alanines (AxxAxA). (B) Pulldown assay utilizing synthetic wild-type or mutated AKAP79 peptides. Peptides were immobilized on streptavidin agarose for two hours, and incubated with PP1α catalytic subunit for two hours. Association of the phosphatase was demonstrated by western blot. Western blot was spliced to exclude molecular weight marker; n=3. PP1 total (lane 1) represents 1% of input. (C, D) Affinities of PP1α binding to the AKAP79 peptides were determined by SPR. PP1α catalytic was immobilized on a CM-5 chip and peptides passed over at increasing concentrations (6.25-200 nM). Affinity of wild-type AKAP79 peptide for PP1 is 43.6 nM while mutant AKAP79 peptide has an affinity of 341 nM. Affinities were calculated by BIAcore T100 evaluation software. (E) AKAP79 mutant consisting of amino acids 44-300 (PP1 binding site deletion) was assayed for PP1 binding. Purified mutant AKAP79 protein was immobilized on S-Protein agarose for two hours, before incubation with PP1α catalytic subunit for two hours. Association of the phosphatase was demonstrated by western blot; n=3. (F) The affinity of PP1α binding to AKAP79 44-300 was determined by SPR. PP1α catalytic was immobilized on a CM-5 chip and AKAP79 protein was passed over at increasing concentrations (6.25-200 nM). Affinity of the mutant AKAP79 protein for PP1 is 103 nM. Affinities were calculated by BIAcore T100 evaluation software. Protein stain of 1 μg of protein used for BIAcore measurements.
In agreement, SPR analysis revealed that the wild-type peptide has an affinity constant of 43.6 nM (KD) for PP1α catalytic subunit (Figure 4C), whereas the mutated peptide exhibited ~10 fold decrease in affinity (341 nM) (Figure 4D). Taken together, these data suggest this region of AKAP79 is sufficient for high-affinity association with PP1.
Next, we determined the effect on PP1 binding to AKAP79 when this region was deleted from the anchoring protein. An S-tagged mutant AKAP79 protein consisting of amino acids 44-300 was bacterially purified and incubated with purified PP1α catalytic subunit. After isolation of the complex using S-protein agarose, PP1 association was determined by western blot. As shown in Figure 4E (lower panel), PP1 continued to bind to AKAP79 when this region was deleted, suggesting that other sites on the anchoring protein also contribute to PP1 binding, consistent with our previous SPR analysis of full-length AKAP79. This is also consistent with other PP1 binding proteins, where mutation of one domain was not sufficient to significantly decrease PP1 binding(27). To further analyze the effect of deleting this PP1 domain from AKAP79, SPR analysis was performed (Figure 4F). The binding properties of immobilized PP1 were measured over a range of AKAP79 concentrations (6.25–200 nM). SPR analysis of gave an on rate of 2.88×105 1/Ms (ka), an off rate of 0.003 1/sec (kd) and an affinity constant of 103 nM (KD). These data demonstrate that while this domain contributes to the binding of PP1 to AKAP79, the phosphatase can still associate with the anchoring protein when this domain is eliminated, albeit at a lower affinity.
Overlapping binding of PP1 to the PKC/calmodulin binding domain on AKAP79
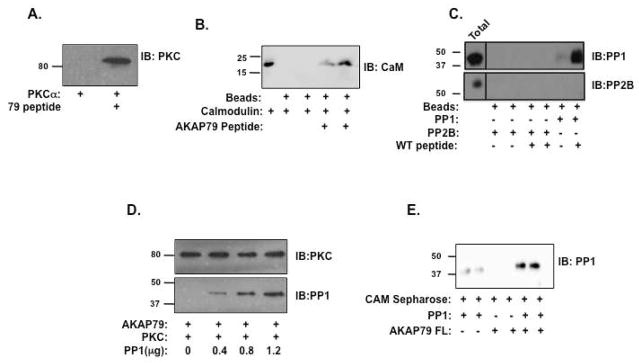
Previous studies have demonstrated that amino acids 31–52 of AKAP79 confer the direct association of PKC and calmodulin to the AKAP79 signaling complex(30, 31). This domain overlaps with at least one of the newly defined PP1 binding domain on AKAP79, suggesting that binding of PP1 and PKC/calmodulin might be mutually exclusive, as is the case for PKC and Ca2+/Calmodulin(32). To test this hypothesis, we began by demonstrating that the PP1 binding peptide can also bind PKC. As shown in Figure 5A, this peptide does bind to bacterially purified PKCα, suggesting PP1 and PKC have a common targeting domain on the AKAP. This was confirmed with calmodulin as well (Figure 5B). However, the peptide did not bind to another AKAP79 associated protein PP2B (Figure 5C), as the binding sites for this phosphatase is contained in amino acids 315-360(33). However, AKAP79 is required to bring PP1 to PP2B complexes (Supplemental Data 1). Next, we performed competition assays using purified AKAP79, PKCα and PP1α. Recombinant, S-tagged AKAP79 (0.5 μg) was incubated with recombinant PKCα (0.3 μg) and increasing concentrations of PP1α catalytic subunit (0–1.2 μg), before isolation of the complex using S-protein agarose. As shown in Figure 5D, the addition of PP1 (bottom blot) could not prevent the binding of PKC to AKAP79 (top blot), suggesting that the second site of PP1 interaction was sufficient to allow for PP1 binding to AKAP79 in the presence of PKC. To determine if AKAP79 signaling complexes can contain both PP1 and calmodulin, we utilized calmodulin-sepharose to pulldown PP1 in the presence and absence of AKAP79. As shown in Figure 5F, AKAP79 significantly increases PP1 binding to calmodulin-sepharose, suggesting that AKAP79 signaling complex can contain both PP1 and calmodulin/PKC.
Figure 5. PP1 Binds AKAP79 in the presence of PKC and calmodulin.
(A) Streptavidin agarose was incubated for two hours in the presence and absence of AKAP79 wild-type peptide, and then charged beads were incubated with recombinant PKCα for two hours. PKC association was demonstrated by western blot, n=3. (B) Streptavidin agarose was incubated for two hours in the presence and absence of AKAP79 wild-type peptide (1 μg), and then charged beads were incubated with recombinant calmodulin (1 μg) for 2 hours. Calmodulin association was demonstrated by western blot, n=3. (C) Streptavidin agarose was incubated for two hours in the presence and absence of AKAP79 WT peptide (1 μg), and then charged beads were incubated with recombinant PP1 or PP2B (1 μg) for two hours. Associated PP1 and PP2B were demonstrate by western blot, n=3. (D) S-tagged beads were charged with recombinant AKAP 79 (0.5 μg) before the addition of PKC (0.3 μg) and increasing amounts of PP1 (0-1.2 μg). Binding of proteins were determined by western blot, n=3. (E) PP1 (200 nM) was incubated with calmodulin sepharose (20 μL) for 2 hours before the addition of AKAP79 (30 nM). The isolated complex was washed and association of PP1 was determined by immunoblot; n=3.
Mapping the region on AKAP79 responsible for inhibition of PP1
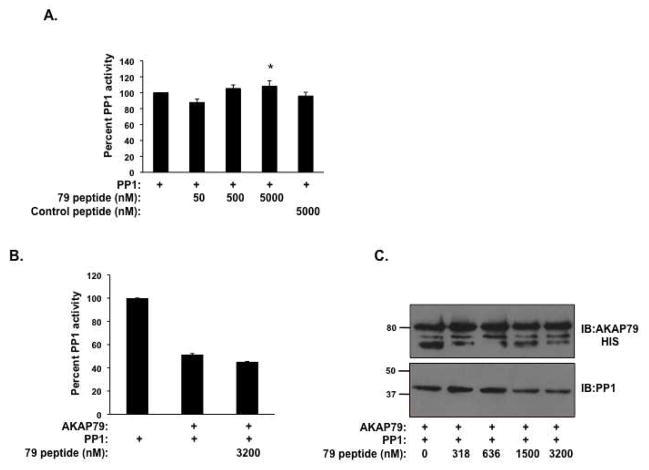
As our work shown in Figure 2 found that AKAP79 binding to PP1 inhibits phosphatase activity, we sought to describe the regions on AKAP79 that are involved in this process. Our initial SPR data suggests two sites on the anchoring protein interact with PP1. Therefore, we began this investigation by determining if the region we previously defined for anchoring of PP1 (Figure 3) was involved in regulation of PP1 activity. Increasing concentrations of the AKAP79 peptide was incubated with purified PP1α catalytic subunit and the effect on phosphatase activity was determined. As shown in Figure 6A, this peptide at high concentrations (5 μM) did not significantly reduce phosphatase activity. In fact, we witnessed a small, but significant increase in PP1 activity (P<0.05). Therefore, we suggest this domain on AKAP79 is involved in anchoring PP1 to AKAP79, but other regions on the anchoring protein are responsible for inhibiting the phosphatase. This has been found for other PP1 anchoring proteins that inhibit phosphatase activity.
Figure 6. Peptide competition of PP1 binding to AKAP79.
(A) Increasing amounts of the AKAP79 PP1 binding peptide (50-5000 nM) and mutant AKAP79 PP1 binding peptide (5000 nM) (depicted in Figure 3) were incubated with PP1α catalytic subunit (50 nM) and assayed for phosphatase activity using 32-P-labeled histone as a substrate as described in the Materials and Methods, n=3 (*=P<0.05). (B) Phosphatase activity was determined using 50 nM PP1α catalytic subunit in the presence and absence of 800 nM AKAP79 protein, with or without 3.2 μM consensus motif peptide; n=3. (C) Competition of PP1 binding to AKAP79 by adding increasing amounts of consensus motif peptide. S-protein agarose was charged with 1000 nM AKAP79 in an overnight incubation before the addition of 50 nM PP1α catalytic subunit and increasing concentrations of consensus motif peptide (0-3200 nM). After a 2 hour incubation, the complex was isolated and bound proteins were identified by western blot, n=3.
While this region of AKAP79 is not responsible for inhibition of PP1 activity, it may be a valuable tool to disrupt phosphatase binding to the AKAP. Multiple studies have demonstrated that while the consensus PP1 binding domain found in proteins like Inhibitor-1, DARPP-32 and spinophillin does not inhibit PP1 activity, it can prevent the inhibitory affect of the full-length protein on the PP1 catalytic subunit by competing for binding of the holoenzyme to the targeting subunit(13, 22). Therefore, we determined if this region of the anchoring protein could attenuate the AKAP79-mediated inhibition of PP1. In Figure 6B, we incubated 3.2 μM of the consensus motif peptide with recombinant (800 nM) AKAP79 and (50 nM) purified PP1α catalytic subunit, and measured the affect on phosphatase activity. While this region of AKAP79 can clearly bind to PP1 in vitro and did not affect phosphatase activity by itself, it was unable to prevent the AKAP79-induced inhibition of PP1 activity. Furthermore, the peptide was unable to fully compete PP1α catalytic subunit binding to the complex (Figure 6C, lower band). However, at high concentrations (3200 nM), PP1 binding was decreased by the peptide by 55%. This data suggests that the additional PP1-binding domains contained in other regions of AKAP79 display high affinity binding to the phosphatase, and disrupting one PP1-binding domain is not sufficient to compete off PP1. This has been shown for other high affinity PP1 binding proteins, where mutation of one consensus PP1 binding domain did not disrupt phosphatase association(23, 29).
Next, we attempted to map the region on AKAP79 that was responsible for inhibition of PP1 activity. PP1 activity assays were performed using increasing concentrations of recombinant AKAP79 deletion constructs and purified PP1α catalytic subunit. Deleting amino acids 350-427 or 250-427 did not prevent inhibition of PP1 by the anchoring protein (Figure 7A, solid line with triangles or dotted line with squares). However, when PP1 was incubated with amino acids 1-150 of AKAP79, phosphatase activity was no longer inhibited (dotted line with circles), suggesting amino acids 150-250 contained the inhibitory domain. Furthermore, phosphatase activity was enhanced 60% when PP1 was incubated with up to 1000 nM of the anchoring protein, suggesting the previously defined PP1 binding domain can function to enhance phosphatase activity. To further define this domain on AKAP79, we made peptide fragments encompassing amino acids 150-200, 175-225, and 200-250 of AKAP79. While all the fragments were able to bind to PP1 in pulldown assays (Figure 7B), none were able to inhibit PP1 activity both independently and in combination (Figure 7C), suggesting a three dimensional structure of the AKAP that cannot be recapitulated in a peptide is needed for inhibition of PP1 activity.
Figure 7. Mapping the PP1 inhibition domain on AKAP79.
(A) Increasing concentrations (0-3000 nM) of AKAP79 deletion constructs (amino acids 1-150, 1-250 and 1-350) were incubated with PP1α catalytic subunit (50 nM), and the effect on phosphatase activity was determined using 32-P-labeled histone as a substrate as described in the Materials and Methods; n=3 (*=P>0.02). (B) Streptavidin agarose was incubated with AKAP79 peptides (amino acids 150-200, 175-225, or 200-250) for two hours. Charged beads were then incubated with recombinant PP1α catalytic subunit for 2 hours. Associated PP1 was demonstrated by western blot, n=3. (C) AKAP79 peptides (1 μg) were added to PP1 activity assays and phosphatase activity was measured 32-P-labeled histone as a substrate as described in the Materials and Methods; n=3.
Amino Acids 1-44 of AKAP79 function to enhance PP1 activity
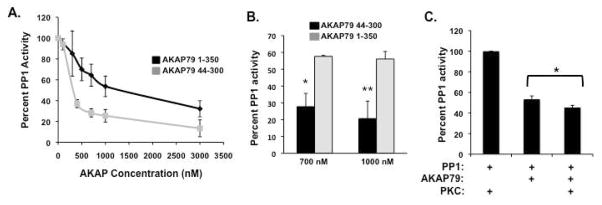
If amino acids 1-44 act to enhance PP1 activity, then deletion of this domain should result in greater inhibition of PP1 by AKAP79. To investigate the role of these amino acids, we first determined the dose-dependent effect of AKAP79 lacking amino acids 1-44 on PP1 activity (Figure 8A, grey squares). Inhibition of PP1α catalytic subunit was seen starting with 100 nM of the AKAP79 mutant, and the IC50 for inhibition was determined to be 343.8 nM +/− .84. To confirm this observation, we compared the effect of either 700 nM AKAP79 1-350 or 700 nM AKAP79 44-300 on phosphatase activity. As shown in Figure 8B, incubation with AKAP79 44-300 had a stronger effect on PP1 activity than AKAP79 1-350, resulting in 72% inhibition as compared to 45% with AKAP79 1-350. This effect was even stronger with a higher concentration of the anchoring protein, where 80% of PP1 activity was inhibited by 1000 nM AKAP79 44-300.
Figure 8. Deletion of the conserved PP1 binding domain on AKAP79 increased the inhibition of PP1 activity.
(A) Dose-dependent inhibition of PP1 by AKAP79 1-350 or AKAP79 44-300 was determined by incubating increasing amounts of purified AKAP79 (0-5000 nM) with PP1α catalytic subunit (50 nM). PP1 activity was then measured using 32-P-labeled histone as a substrate; n=3 (B). Direct comparison of the PP1 inhibitory affect on PP1. Either AKAP79 1-350 or 44-300 (700 mM or 1000 mM) was incubated with PP1α catalytic subunit (50 nM) and PP1 activity was then measured using 32-P-labeled histone as a substrate; n=3 (*=P<0.03, **P<0.05). (C) PP1 activity was determined as described in the presence or absence of AKAP79 (800 nM) or 200 nM PKC; n=3 (*=P<0.03)
Our data shown in Figure 5 demonstrates that amino acids 1-44 also contain the PKC and calmodulin binding domains. We hypothesized that AKAP79 complexes containing both PKC and PP1 should exhibit an increased inhibition of PP1 due to PKC blockage of the “PP1 enhancer” domain. To test this hypothesis, purified PKC (200 nM) was added to PP1 phosphatase activity assays in the presence and absence of AKAP79. Similar to experiments performed above, the addition of 800 nM AKAP79 blocked PP1 activity by 53±3.21%. Importantly, when PKC was added along with the anchoring protein, PP1 activity was decreased to 44±1.48%. However, control experiments found PKC alone did not significantly decrease PP1 activity. Therefore, AKAP79 complex composition greatly affects the activity of the bound phosphatase.
PP1 Target specificity is defined by AKAP79
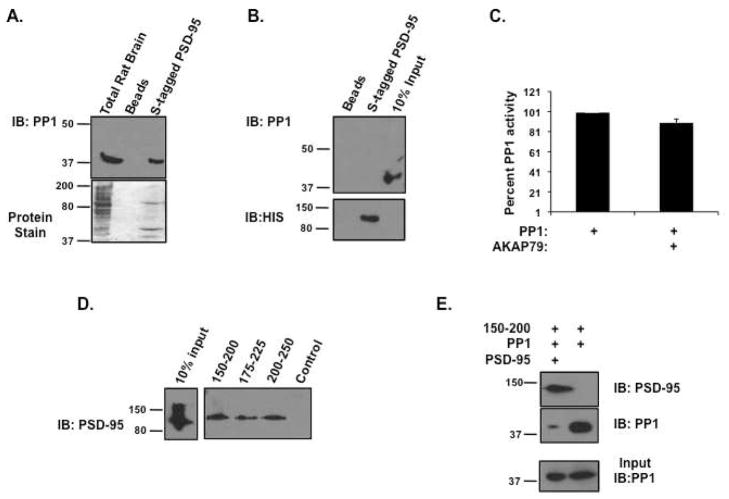
Many PP-1 binding proteins function to define the substrate recognition of PP1 complexes, directing phosphatase activity towards specific targets in the complex(16, 34). As the experiments detailed above were performed using a general PP1 substrate that is not found in endogenous AKAP79 complexes, we were interested if the same inhibitory affect would be present on a more likely target for AKAP79-bound PP1. Several studies have demonstrated that AKAP79 binds directly to PSD-95 in the brain, and this association is responsible for directing the actions of the AKAP towards other, indirectly associated substrates(19, 35). Interestingly, PSD-95 is also a phospho-protein, containing many sites of phosphorylation that are regulated by PP1 activity(36–38). In order to address the effect of AKAP79 on PP1 dephosphorylation of this substrate, we began by investigating if PSD-95 can associate with PP1. Pulldown experiments from rat brain extract using S-beads charged with purified PSD-95 found PP1 could associate with the protein (Figure 9A). However, a direct interaction could not be detected (Figure 9B). Next, phosphatase activity assays using phosphorylated PSD-95, PP1α, and 800 nM AKAP79 were performed (Figure 9C). While this concentration of AKAP79 is sufficient for 50% inhibition of PP1 activity towards phospho-histone, it did not significantly block the ability of PP1 to dephosphorylate PSD-95, suggesting AKAP79 may act to direct PP1 activity towards this specific target.
Figure 9. AKAP79 directs PP1 activity towards phospho-PSD-95.
(A) S-tagged beads charged with PSD-95 (3 μg) were incubated with rat brain extract for 7 hours. The complexes were isolated and association of PP1 catalytic subunit was determined by western blot analysis; n=3. (B) Pulldown assays were performed using S-protein beads charged with or without bacterially expressed S-tagged PSD-95 in the presence of PP1α catalytic subunit. After a 2 hour incubation, the complex was isolated and association of PP1 was determined by western blot, n=3. (C) PP1 activity was determined as described using 32-P-labeled PSD-95 as a substrate in the presence or absence of AKAP79 (800 nM); n=3. (D) Streptavidin agarose was incubated with AKAP79 peptides (amino acids 150-200, 175-225, or 200-250) for two hours. Charged beads were then incubated with recombinant PSD-95 (1 μg) for 2 hours. Associated PSD-95 was demonstrated by western blot, n=3. (E) Streptavidin agarose was incubated with AKAP79 peptide encompassing amino acids 150-200 for two hours. Charged beads were then incubated with recombinant PP1 (1 μg) in the presence and absence of PSD-95 (5 μg) for 2 hours. Associated proteins were demonstrated by western blot, n=3.
In order to begin to understand why AKAP79 did not inhibit PP1 activity towards PSD-95, we hypothesized that PSD-95 may compete with PP1 for binding to the inhibitory region on AKAP79 found in amino acids 150-250. In support, Figure 9D shows PSD-95 associated with the peptides mimicking the inhibitory domain on AKAP79 (shown in Figure 7). Furthermore, the addition of PSD-95 (5 μg) to pulldown assays containing 1 μg of the AKAP79 peptide encompassing amino acids 150-250 and PP1 (1 μg) significantly reduced the ability of the peptide to associate with PP1. Taken together, these data suggest that in AKAP79 complexes containing PSD-95, PP1 can only associate with the first PP1 binding site on the AKAP that is not responsible for PP1 inhibition. Therefore, PP1 is active and can phosphorylate targets in the complex.
Discussion
PP1 is a ubiquitously expressed protein phosphatase with a large number of potential substrates, raising the question of how specificity in phosphatase action occurs. Accumulating evidence suggests that the interaction of the catalytic subunit of PP1 with targeting and regulatory subunits accounts for the explicit action of the phosphatase(5, 6). Our work shown here demonstrates that the neuronally expressed A-Kinase anchoring protein AKAP79 is a PP1 targeting subunit, as a direct interaction between PP1 and AKAP79 can be isolated from both rat brain extract and transfected 293 cells. Furthermore, this association displays a relatively high binding affinity, well within the range of the estimated concentrations of PP1 and AKAP79 in the brain. This data suggests that AKAP79 will function to focus the action of PP1 towards specific substrates in the brain.
Both experimental and structural data suggest that most PP1 binding proteins contain multiple sites of interaction with the phosphatase(6, 13, 15, 34). A primary site consisting of a conserved PP1 binding motif targets the phosphatase while additional sites on the regulatory subunit are involved in regulating PP1 activity and defining the substrate specificity of the phosphatase. In agreement with this idea, SPR analysis and biochemical characterization of the AKAP79 and PP1 interaction suggest the phosphatase interacts with two sites on the anchoring protein. The first site, contained in amino acids 1-44, binds PP1 with high affinity and enhances phosphatase activity. An additional site of interaction found in amino acids 150-250 also associates with PP1, but suppresses phosphatase activity towards a histone substrate.
Importantly, full length AKAP79 acts as an inhibitor of PP1 activity, displaying an IC50 of 811±0.56 nM. This data suggest that the AKAP sequesters the inactive phosphatase to discrete regions in the cell in order to localize the phosphates close to its targets. This scaffolding model is similar to that of PP2B/AKAP79 interactions, where the association of PP2B to the anchoring protein inhibits phosphatase activity, but the subcellular localization of PP2B by AKAP79 is critical for regulation of ion channel activity(33, 39). In support of this, our SPR analysis demonstrated a fast on and off rate of PP1 binding to AKAP79, allowing for PP1 to function at targeted regions in the cell.
Previous work demonstrated that AKAP79 provides a scaffold for at least three distinct enzymes - PKA, PKC, and PP2B, as well as the signaling molecule calmodulin(25). Interestingly, one of the PP1 interactions on AKAP79 found in amino acids 1-44 described here overlaps with the previously defined binding domain for PKC and calmodulin, suggesting binding may be mutually exclusive between these molecules. However, isolated AKAP79 signaling complexes were found to contain both calmodulin and PP1, suggesting that the second site of PP1 interaction is sufficient to direct anchoring of the phosphatase. Importantly, in AKAP79 complexes containing both PKC and PP1, greater inhibition of PP1 activity is seen, suggesting that the stimulatory site is occupied leaving only the inhibitor site. Therefore, AKAP79 complex composition and which enzymes are associated at similar times would greatly determine the activity of PP1.
An emerging theme in the field of PP1 signaling is that each regulatory subunit binds to PP1 in such a way that it restricts phosphatase activity, only allowing the dephosphorylation of a particular substrate(29). For example, structural analysis of the PP1/spinophilin interaction demonstrated that the binding of spinophilin to PP1 blocks one of the three putative substrate binding sites on PP1(15). The functional implication of this is that spinophilin inhibits PP1 activity towards phosphorylatase a, but not the tail sequence of the Glutamate receptor 1 (GluR1). We therefore looked at how changing the substrate would affect PP1 activity in the AKAP79 complex. Importantly, the inhibitory actions of AKAP79 were lost when we used a more endogenous substrate, PSD-95. While the structural and molecular underpinnings for this lack of inhibition are unknown at present, it may be due to a competition between PSD-95 and PP1 for binding to amino acids 150-250 of AKAP79, the region on the anchoring protein containing the PP1 inhibitory domain. This work is currently ongoing.
PP1 regulatory and targeting subunits function to sequester both the phosphatase and phospho-substrate into a discrete signaling compartment, thereby defining the spatial-temporal window for phosphatase activity. By binding to AKAPs, PP1 will be contained in the same signaling unit as various protein kinases, providing a mechanism to coordinate the phosphorylation and de-phosphorylation of target proteins. A prime example of this is the AKAP yotiao, which nucleates a complex consisting of PP1, PKA, and the NMDA-type glutamate-receptor(18). Under basal conditions, the active phosphtase limits channel activity until stimulation of the kinase overcomes PP1 activity, allowing for phosphorylation of the channel and increased channel opening. While the direct substrate for AKAP79-regulated PP1 in the brain is currently unknown, we are investigating the possibility of PSD-95 as one such substrate. NMDA treatment of neurons isolated from the rat hippocampus resulted in dephosphorylation of PSD-95 by a PP1-mediated mechanism(36). Our work demonstrated here found AKAP79-bound PP1 can dephosphorylate PSD-95 in vitro. By anchoring both PSD-95 and PP1, AKAP79 would help in the speed of action of the phosphatase towards this substrate. However, other targets for AKAP79-bound PP1 can also be explored, such as the GluR1 and NMDA receptor, both of which are PP1 substrates and indirectly associate with AKAP79(19).
Supplementary Material
Acknowledgments
We would like to acknowledge John Redden, Arpita Singh, and Maximilian Vargas for helpful discussion of this work.
This work was funded, in whole or in part, by National Institutes of Health Grants HL82705 (K.L.D.-K.) and NS046661 (S.J.T), as well as a University of Connecticut Institutional Grant (A.L.).
Abbreviations
- AKAP
A-kinase anchoring protein
- PP1
protein phosphatase 1
- SPR
Surface Plasmon Resonance
Footnotes
To demonstrate that AKAP79 can link PP1 to PP2B, purified PP1 catalytic subunit was subjected to pulldown assay using GST-tagged PP2B A subunit in the presence and absence of AKAP79. As shown in Supplemental Figure 1, PP1 was only seen in the PP2B complex in the presence of AKAP79, suggesting the anchoring protein organizes a trimeric complex consisting of AKAP79, PP1 and PP2B. Supplemental material may be accessed free of charge online at http://pubs.acs.org.
Contributor Information
Andrew. V. Le, Pat and Jim Calhoun Center for Cardiology, University of Connecticut Health Center, Farmington, CT 06030
Steven. J. Tavalin, Department of Pharmacology, University of Tennessee Health Science Center, Memphis, Tennessee 38163
Kimberly L. Dodge-Kafka, Pat and Jim Calhoun Center for Cardiology, University of Connecticut Health Center, Farmington, CT 06030, 860-679-2452, Fax: 860-679-1426, dodge@uchc.edu
References
- 1.Shenolikar S, Nairn AC. Protein phosphatases: recent progress. Adv Second Messenger Phosphoprotein Res. 1991;23:1–121. [PubMed] [Google Scholar]
- 2.Shenolikar S. Protein serine/threonine phosphatases--new avenues for cell regulation. Annu Rev Cell Biol. 1994;10:55–86. doi: 10.1146/annurev.cb.10.110194.000415. [DOI] [PubMed] [Google Scholar]
- 3.Aggen JB, Nairn AC, Chamberlin R. Regulation of protein phosphatase-1. Chem Biol. 2000;7:R13–23. doi: 10.1016/s1074-5521(00)00069-7. [DOI] [PubMed] [Google Scholar]
- 4.Virshup DM, Shenolikar S. From promiscuity to precision: protein phosphatases get a makeover. Mol Cell. 2009;33:537–545. doi: 10.1016/j.molcel.2009.02.015. [DOI] [PubMed] [Google Scholar]
- 5.Bollen M. Combinatorial control of protein phosphatase-1. Trends Biochem Sci. 2001;26:426–431. doi: 10.1016/s0968-0004(01)01836-9. [DOI] [PubMed] [Google Scholar]
- 6.Cohen PT. Protein phosphatase 1--targeted in many directions. J Cell Sci. 2002;115:241–256. doi: 10.1242/jcs.115.2.241. [DOI] [PubMed] [Google Scholar]
- 7.Doherty MJ, Moorhead G, Morrice N, Cohen P, Cohen PT. Amino acid sequence and expression of the hepatic glycogen-binding (GL)-subunit of protein phosphatase-1. FEBS Lett. 1995;375:294–298. doi: 10.1016/0014-5793(95)01184-g. [DOI] [PubMed] [Google Scholar]
- 8.Hsieh-Wilson LC, Allen PB, Watanabe T, Nairn AC, Greengard P. Characterization of the neuronal targeting protein spinophilin and its interactions with protein phosphatase-1. Biochemistry. 1999;38:4365–4373. doi: 10.1021/bi982900m. [DOI] [PubMed] [Google Scholar]
- 9.McAvoy T, Allen PB, Obaishi H, Nakanishi H, Takai Y, Greengard P, Nairn AC, Hemmings HC., Jr Regulation of neurabin I interaction with protein phosphatase 1 by phosphorylation. Biochemistry. 1999;38:12943–12949. doi: 10.1021/bi991227d. [DOI] [PubMed] [Google Scholar]
- 10.Kreivi JP, Trinkle-Mulcahy L, Lyon CE, Morrice NA, Cohen P, Lamond AI. Purification and characterisation of p99, a nuclear modulator of protein phosphatase 1 activity. FEBS Lett. 1997;420:57–62. doi: 10.1016/s0014-5793(97)01485-3. [DOI] [PubMed] [Google Scholar]
- 11.Hendrickx A, Beullens M, Ceulemans H, Den Abt T, Van Eynde A, Nicolaescu E, Lesage B, Bollen M. Docking motif-guided mapping of the interactome of protein phosphatase-1. Chem Biol. 2009;16:365–371. doi: 10.1016/j.chembiol.2009.02.012. [DOI] [PubMed] [Google Scholar]
- 12.Ayllón V, Cayla X, Garcia A, Fleischer A, Rebollo A. Protein phosphatase 1 interacts wtih protein required for meiosis and other cellular processes in Saccharomyces cerevisiae. Mol Cell Biol. 2002;16:4199–4206. doi: 10.1128/mcb.16.8.4199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Huang H-B, Horiuchi A, Watanabe T, Shih SR, Tsay HJ, Li HC, Greengard P, Nairn AC. Characterization of the inhibition of protein phosphatase-1 by DARPP-32 and inhibitor-2. J Biol Chem. 1999;274:7870–7878. doi: 10.1074/jbc.274.12.7870. [DOI] [PubMed] [Google Scholar]
- 14.Yan Z, Hsieh-Wilson L, Feng J, Tomizawa K, Allen PB, Fienberg AA, Nairn AC, Greengard P. Protein phosphatase 1 modulation of neostriatal AMPA channels: regulation by DARPP-32 and spinophilin. Nat Neurosci. 1999;2:13–17. doi: 10.1038/4516. [DOI] [PubMed] [Google Scholar]
- 15.Ragusa MJ, Dancheck B, Critton DA, Nairn AC, Page R, Peti W. Spinophilin directs protein phosphatase 1 specificity by blocking substrate binding sites. Nat Struct Mol Biol. 17:459–464. doi: 10.1038/nsmb.1786. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Connor JH, Weiser DC, Li S, Hallenbeck JM, Shenolikar S. Growth arrest and DNA damage-inducible protein GADD34 assembles a novel signaling complex containing protein phosphatase 1 and inhibitor 1. Mol Cell Biol. 2001;21:6841–6850. doi: 10.1128/MCB.21.20.6841-6850.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Bauman AL, Scott JD. Kinase- and phosphatase-anchoring proteins: harnessing the dynamic duo. Nat Cell Biol. 2002;4:E203–206. doi: 10.1038/ncb0802-e203. [DOI] [PubMed] [Google Scholar]
- 18.Westphal RS, Tavalin SJ, Lin JW, Alto NM, Fraser ID, Langeberg LK, Sheng M, Scott JD. Regulation of NMDA receptors by an associated phosphatase-kinase signaling complex. Science. 1999;285:93–96. doi: 10.1126/science.285.5424.93. [DOI] [PubMed] [Google Scholar]
- 19.Colledge M, Dean RA, Scott GK, Langeberg LK, Huganir RL, Scott JD. Targeting of PKA to glutamate receptors through a MAGUK-AKAP complex. Neuron. 2000;27:107–119. doi: 10.1016/s0896-6273(00)00013-1. [DOI] [PubMed] [Google Scholar]
- 20.Coghlan VM, Perrino BA, Howard M, Langeberg LK, Hicks JB, Gallatin WM, Scott JD. Association of protein kinase A and protein phosphatase 2B with a common anchoring protein. Science. 1995;267:108–111. doi: 10.1126/science.7528941. [DOI] [PubMed] [Google Scholar]
- 21.Klauck TM, Faux MC, Labudda K, Langeberg LK, Jaken S, Scott JD. Coordination of three signaling enzymes by AKAP79, a mammalian scaffold protein. Science. 1996;271:1589–1592. doi: 10.1126/science.271.5255.1589. [DOI] [PubMed] [Google Scholar]
- 22.Beullens M, Van Eynde A, Vulsteke V, Connor J, Shenolikar S, Stalmans W, Bollen M. Molecular determinants of nuclear protein phosphatase-1 regulation by NIPP-1. J Biol Chem. 1999;274:14053–14061. doi: 10.1074/jbc.274.20.14053. [DOI] [PubMed] [Google Scholar]
- 23.Schillace RV, Scott JD. Association of the type 1 protein phosphatase PP1 with the A-kinase anchoring protein AKAP220. Curr Biol. 1999;9:321–324. doi: 10.1016/s0960-9822(99)80141-9. [DOI] [PubMed] [Google Scholar]
- 24.Zhang J, Vinuela A, Neely MH, Hallett PJ, Grant SG, Miller GM, Isacson O, Caron MG, Yao WD. Inhibition of the dopamine D1 receptor signaling by PSD-95. J Biol Chem. 2007;282:15778–15789. doi: 10.1074/jbc.M611485200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Dodge K, Scott JD. AKAP79 and the evolution of the AKAP model. FEBS Lett. 2000;476:58–61. doi: 10.1016/s0014-5793(00)01671-9. [DOI] [PubMed] [Google Scholar]
- 26.Connor JH, Kleeman T, Barik S, Honkanen RE, Shenolikar S. Importance of the beta12-beta13 loop in protein phosphatase-1 catalytic subunit for inhibition by toxins and mammalian protein inhibitors. J Biol Chem. 1999;274:22366–22372. doi: 10.1074/jbc.274.32.22366. [DOI] [PubMed] [Google Scholar]
- 27.Schillace RV, Voltz JW, Sim AT, Shenolikar S, Scott JD. Multiple interactions within the AKAP220 signaling complex contribute to protein phosphatase 1 regulation. J Biol Chem. 2001;276:12128–12134. doi: 10.1074/jbc.M010398200. [DOI] [PubMed] [Google Scholar]
- 28.Steen R, Beullens M, Landsverk HB, Bollen M, Collas P. AKAP149 is a novel PP1 specificier required to maintain nuclear envelope integrity in G1 phase. J Cell Science. 2003;116:2237–2246. doi: 10.1242/jcs.00432. [DOI] [PubMed] [Google Scholar]
- 29.Bollen M, Peti W, Ragusa MJ, Beullens M. The extended PP1 toolkit: designed to create specificity. Trends Biochem Sci. 2010;35:450–458. doi: 10.1016/j.tibs.2010.03.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Faux MC, Scott JD. Regulation of the AKAP79-protein kinase C interaction by Ca2+/Calmodulin. J Biol Chem. 1997;272:17038–17044. doi: 10.1074/jbc.272.27.17038. [DOI] [PubMed] [Google Scholar]
- 31.Brooks IM, Tavalin SJ. CaMKII inhibitors disrupt AKAP79-dependent PKC signaling to GluA1 AMPA receptors. J Biol Chem. 2010 doi: 10.1074/jbc.M110.183558. Epub ahead of print. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Nikandrova YA, Jiao Y, Baucum AJ, Tavalin SJ, Colbran RJ. Ca2+/calmodulin-dependent protein kinase II binds to and phosphorylates a specific SAP97 splice variant to disrupt association with AKAP79/150 and modulate alpha-amino-3-hydroxy-5-methyl-4-isoxazolepropionic acid-type glutamate receptor (AMPAR) activity. J Biol Chem. 2010;285:923–934. doi: 10.1074/jbc.M109.033985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Dell'Acqua ML, Dodge KL, Tavalin SJ, Scott JD. Mapping the protein phosphatase-2B anchoring site on AKAP79. Binding and inhibition of phosphatase activity are mediated by residues 315–360. J Biol Chem. 2002;277:48796–48802. doi: 10.1074/jbc.M207833200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Ragusa MJ, Dancheck B, Critton DA, Nairn AC, Page R, Peti W. Spinophilin directs protein phosphatase 1 specificity by blocking substrate binding sites. Nat Struct Mol Biol. 2010;17:459–464. doi: 10.1038/nsmb.1786. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Dell'Acqua ML, Smith KE, Gorski JA, Horne EA, Gibson ES, Gomez LL. Regulation of neuronal PKA signaling through AKAP targeting dynamics. Eur J Cell Biol. 2006;85:627–633. doi: 10.1016/j.ejcb.2006.01.010. [DOI] [PubMed] [Google Scholar]
- 36.Kim MJ, Futai K, Jo J, Hayashi Y, Cho K, Sheng M. Synaptic accumulation of PSD-95 and synaptic function regulated by phosphorylation of serine-295 of PSD-95. Neuron. 2007;56:488–502. doi: 10.1016/j.neuron.2007.09.007. [DOI] [PubMed] [Google Scholar]
- 37.Sabio G, Reuver S, Feijoo C, Hasegawa M, Thomas GM, Centeno F, Kuhlendahl S, Leal-Ortiz S, Goedert M, Garner C, Cuenda A. Stress- and mitogen-induced phosphorylation of the synapse-associated protein SAP90/PSD-95 by activation of SAPK3/p38gamma and ERK1/ERK2. Biochem J. 2004;380:19–30. doi: 10.1042/BJ20031628. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Gardoni F, Polli F, Cattabeni F, Di Luca M. Calcium-calmodulin-dependent protein kinase II phosphorylation modulates PSD-95. Eur J Neurosci. 2006;24:2694–2704. doi: 10.1111/j.1460-9568.2006.05140.x. [DOI] [PubMed] [Google Scholar]
- 39.Tavalin SJ, Colledge M, Hell JW, Langeberg LK, Huganir RL, Scott JD. Regulation of GluR1 by the A-kinase anchoring protein 79 (AKAP79) signaling complex shares properties with long-term depression. J Neurosci. 2002;22:3044–3051. doi: 10.1523/JNEUROSCI.22-08-03044.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.