Abstract
HeLa cell nuclei with DNA labeled with [3H] thymidine have been preincubated under varying conditions and then incubated with micrococcal nuclease. Aliquots, removed at increasing times, were analyzed for mononucleosomal size DNA and for acid-soluble DNA, the ratios were plotted and a slope was determined. Preincubation with ATP and a regenerating system increased the slope 2 fold. Optimum ATP concentrations were above 0.25 mM. The ATP effect was reversed by novobiocin. No inhibition of the ATP effect was observed with nalidixic acid, coumermycin, oxolinic acid, VM-26, aphidicolin, or 3 amino-benzamide. NAD or cAMP or cGMP had no effect with or without ATP. Other nucleoside triphosphates could substitute to varying degrees for ATP as could ATP analogues. Nuclei from log phase cells showed no ATP effect, but log phase cells, partially depleted of ATP by incubation with deoxyglucose, showed the effect. The effect was lost in nuclei on long-term storage. No evidence was found for differential degradation of core histones, histone H-1 or DNA, and there was no evidence of nucleosome sliding.
Full text
PDF



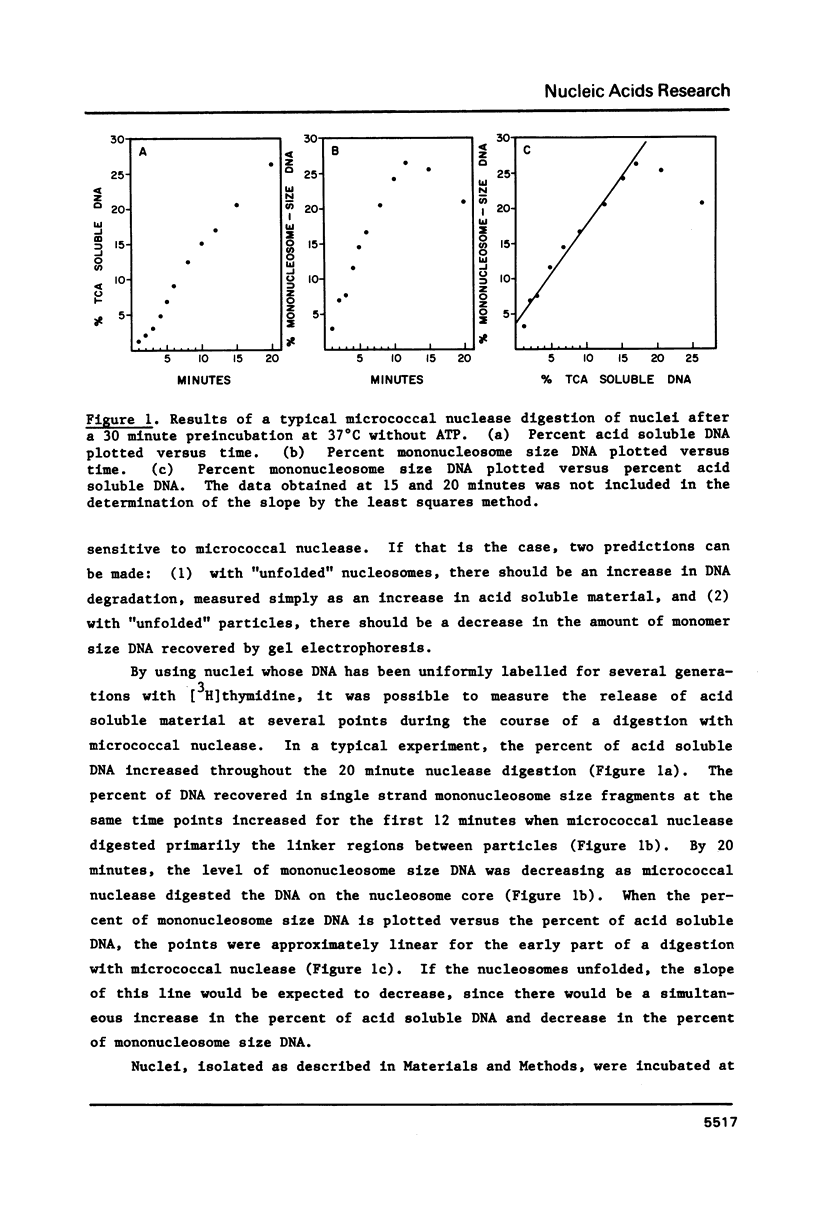
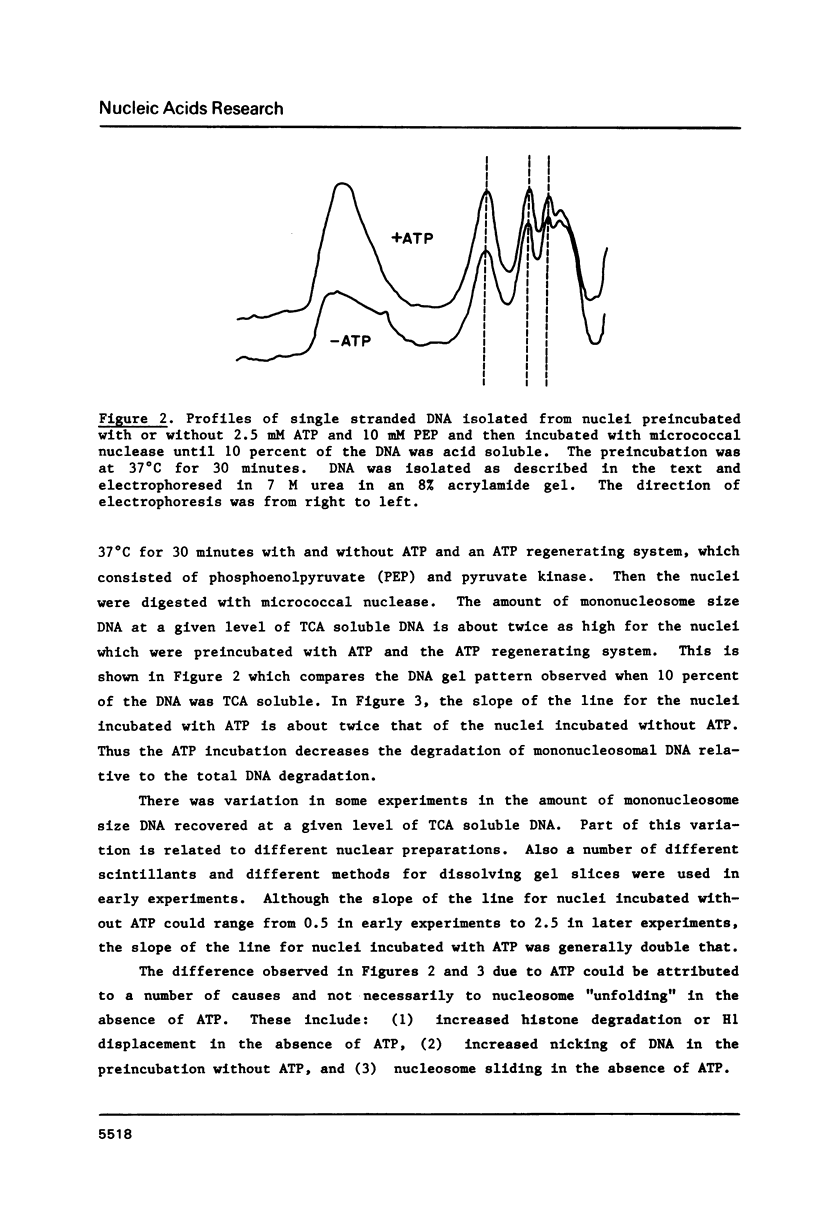
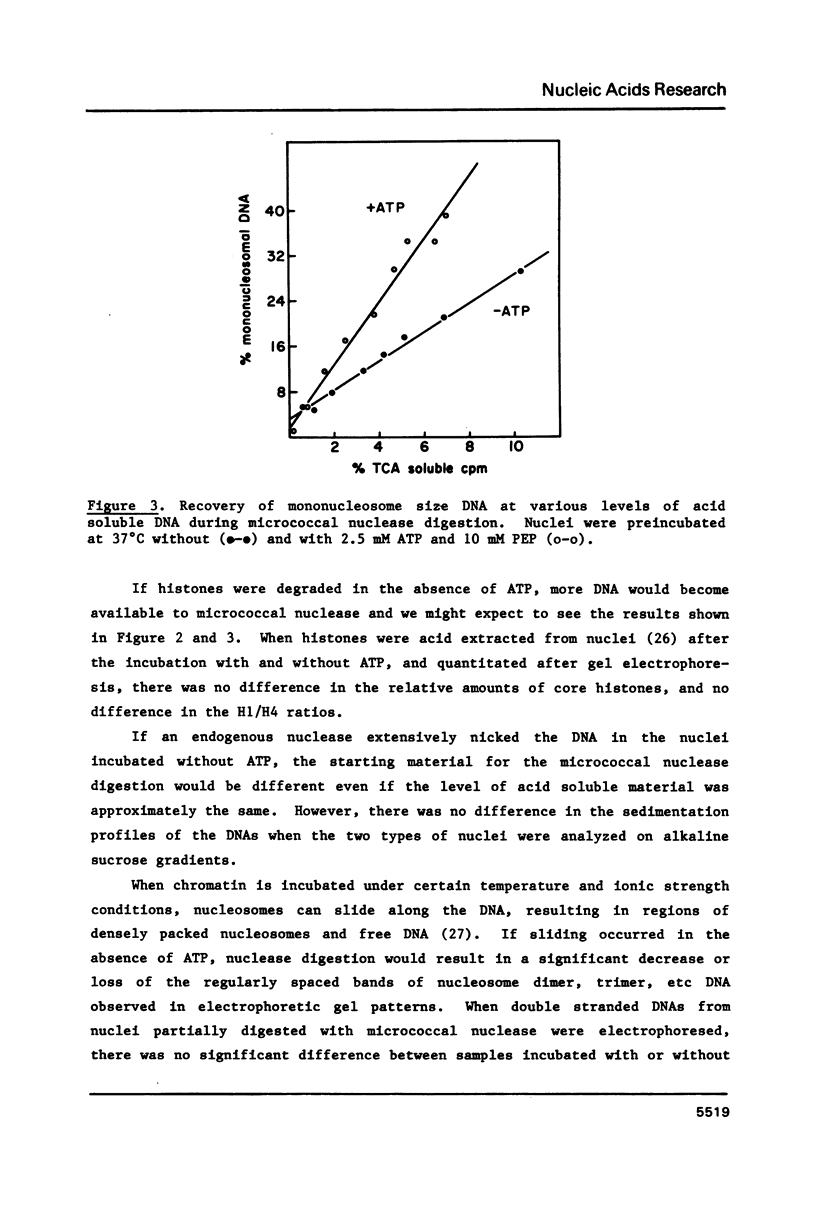
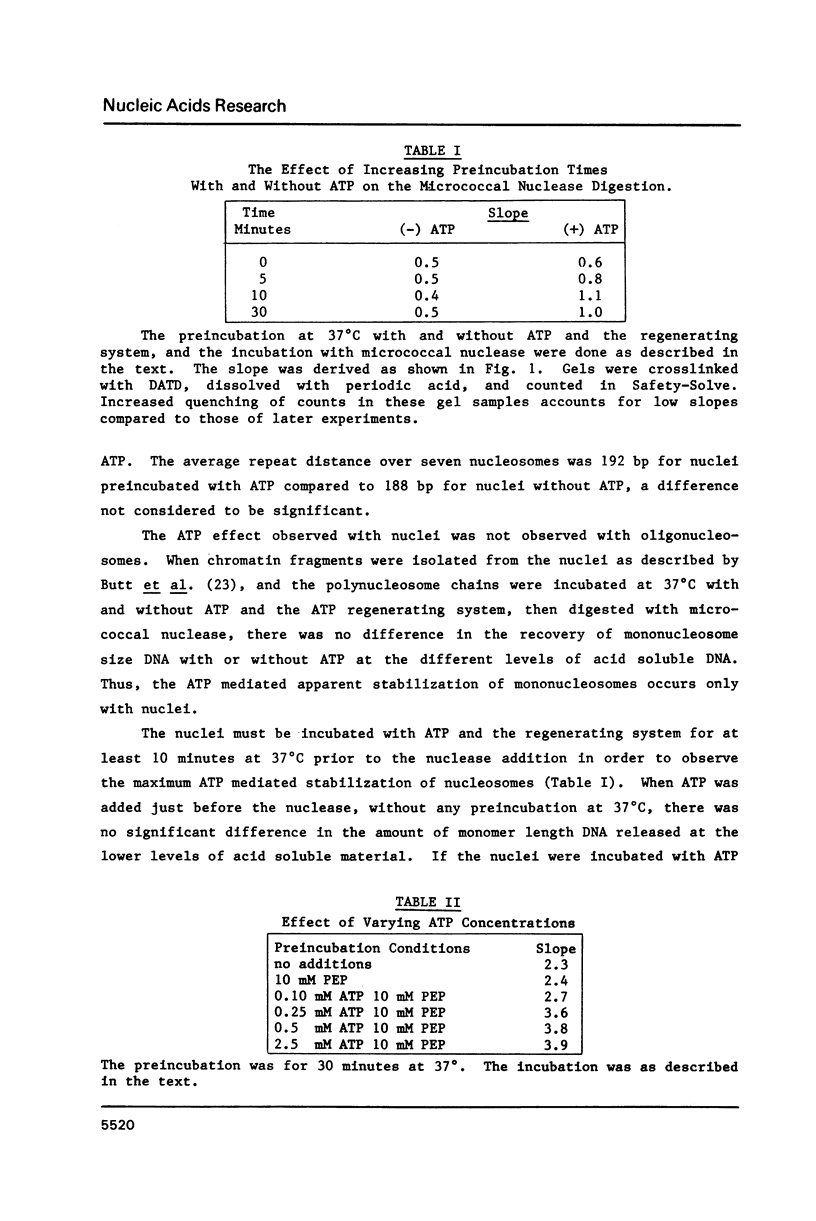

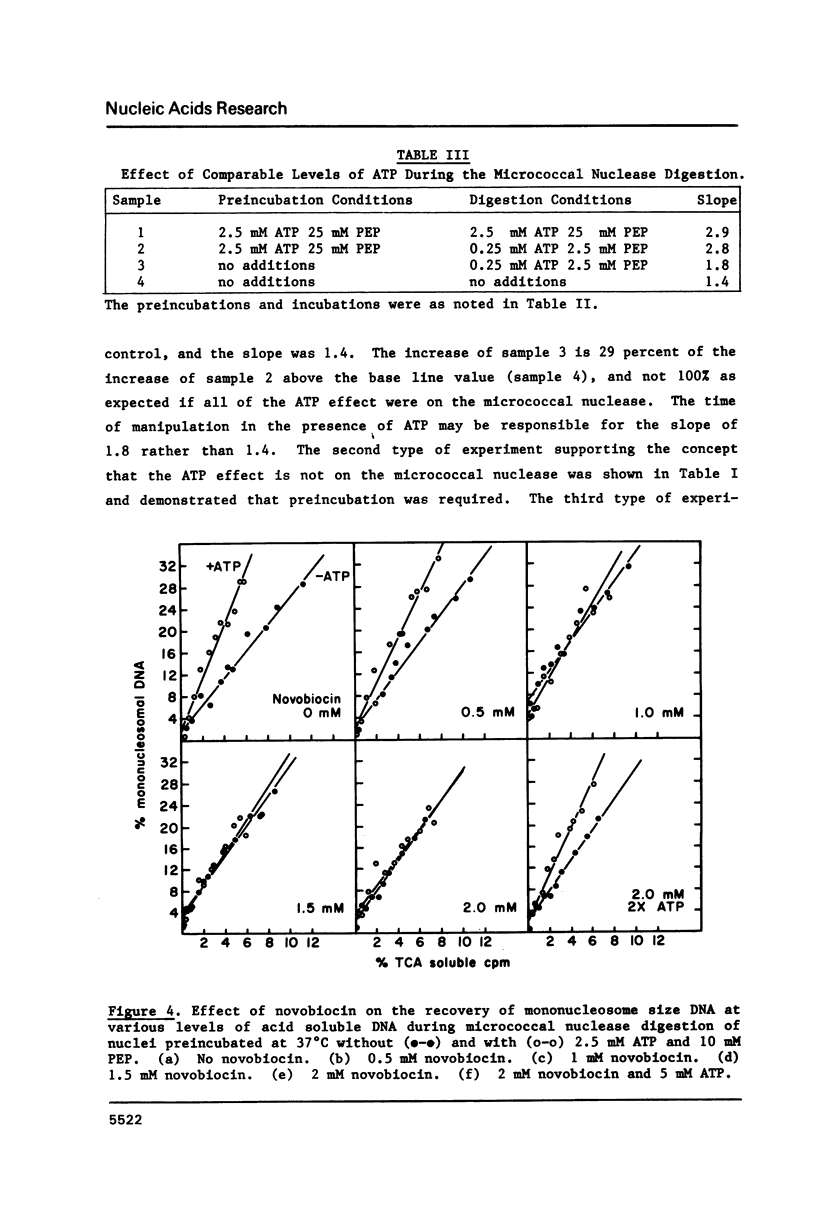






Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bodell W. J., Kaufmann W. K., Cleaver J. E. Enzyme digestion of intermediates of excision repair in human cells irradiated with ultraviolet light. Biochemistry. 1982 Dec 21;21(26):6767–6772. doi: 10.1021/bi00269a023. [DOI] [PubMed] [Google Scholar]
- Burlingame R. W., Love W. E., Wang B. C., Hamlin R., Nguyen H. X., Moudrianakis E. N. Crystallographic structure of the octameric histone core of the nucleosome at a resolution of 3.3 A. Science. 1985 May 3;228(4699):546–553. doi: 10.1126/science.3983639. [DOI] [PubMed] [Google Scholar]
- Butt T. R., Jump D. B., Smulson M. E. Nucleosome periodicity in HeLa cell chromatin as probed by micrococcal nuclease. Proc Natl Acad Sci U S A. 1979 Apr;76(4):1628–1632. doi: 10.1073/pnas.76.4.1628. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen G. L., Yang L., Rowe T. C., Halligan B. D., Tewey K. M., Liu L. F. Nonintercalative antitumor drugs interfere with the breakage-reunion reaction of mammalian DNA topoisomerase II. J Biol Chem. 1984 Nov 10;259(21):13560–13566. [PubMed] [Google Scholar]
- Ciarrocchi G., Linn S. A cell-free assay measuring repair DNA synthesis in human fibroblasts. Proc Natl Acad Sci U S A. 1978 Apr;75(4):1887–1891. doi: 10.1073/pnas.75.4.1887. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clarkson J. M., Mitchell D. L. The effect of various inhibitors of DNA synthesis on the repair of DNA photoproducts. Biochim Biophys Acta. 1983 Sep 9;740(4):355–361. doi: 10.1016/0167-4781(83)90082-9. [DOI] [PubMed] [Google Scholar]
- Collins A., Johnson R. Novobiocin; an inhibitor of the repair of UV-induced but not X-ray-induced damage in mammalian cells. Nucleic Acids Res. 1979 Nov 10;7(5):1311–1320. doi: 10.1093/nar/7.5.1311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dresler S. L., Lieberman M. W. Requirement of ATP for specific incision of ultraviolet-damaged DNA during excision repair in permeable human fibroblasts. J Biol Chem. 1983 Oct 25;258(20):12269–12273. [PubMed] [Google Scholar]
- Edenberg H. J. Novobiocin inhibition of simian virus 40 DNA replication. Nature. 1980 Jul 31;286(5772):529–531. doi: 10.1038/286529a0. [DOI] [PubMed] [Google Scholar]
- Glikin G. C., Ruberti I., Worcel A. Chromatin assembly in Xenopus oocytes: in vitro studies. Cell. 1984 May;37(1):33–41. doi: 10.1016/0092-8674(84)90298-8. [DOI] [PubMed] [Google Scholar]
- Gordon V. C., Knobler C. M., Olins D. E., Schumaker V. N. Conformational changes of the chromatin subunit. Proc Natl Acad Sci U S A. 1978 Feb;75(2):660–663. doi: 10.1073/pnas.75.2.660. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Halligan B. D., Edwards K. A., Liu L. F. Purification and characterization of a type II DNA topoisomerase from bovine calf thymus. J Biol Chem. 1985 Feb 25;260(4):2475–2482. [PubMed] [Google Scholar]
- Harrington R. E. Optical model studies of the salt-induced 10-30-nm fiber transition in chromatin. Biochemistry. 1985 Apr 9;24(8):2011–2021. doi: 10.1021/bi00329a032. [DOI] [PubMed] [Google Scholar]
- Heller E. P., Goldthwait D. A. Release of 3-methyladenine from linker and core DNA of chromatin by a purified DNA glycosylase. Cancer Res. 1983 Dec;43(12 Pt 1):5747–5753. [PubMed] [Google Scholar]
- Igo-Kemenes T., Hörz W., Zachau H. G. Chromatin. Annu Rev Biochem. 1982;51:89–121. doi: 10.1146/annurev.bi.51.070182.000513. [DOI] [PubMed] [Google Scholar]
- Louters L., Chalkley R. In vitro exchange of nucleosomal histones H2a and H2b. Biochemistry. 1984 Jan 31;23(3):547–552. doi: 10.1021/bi00298a023. [DOI] [PubMed] [Google Scholar]
- Lowry O. H., Berger S. J., Chi M. M., Carter J. G., Blackshaw A., Outlaw W. Diversity of metabolic patterns in human brain tumors--I. High energy phosphate compounds and basic composition. J Neurochem. 1977 Dec;29(6):959–977. doi: 10.1111/j.1471-4159.1977.tb06500.x. [DOI] [PubMed] [Google Scholar]
- Mattern M. R., Paone R. F., Day R. S., 3rd Eukaryotic DNA repair is blocked at different steps by inhibitors of DNA topoisomerases and of DNA polymerases alpha and beta. Biochim Biophys Acta. 1982 Apr 26;697(1):6–13. doi: 10.1016/0167-4781(82)90038-0. [DOI] [PubMed] [Google Scholar]
- McGhee J. D., Felsenfeld G. Nucleosome structure. Annu Rev Biochem. 1980;49:1115–1156. doi: 10.1146/annurev.bi.49.070180.005343. [DOI] [PubMed] [Google Scholar]
- McGhee J. D., Nickol J. M., Felsenfeld G., Rau D. C. Higher order structure of chromatin: orientation of nucleosomes within the 30 nm chromatin solenoid is independent of species and spacer length. Cell. 1983 Jul;33(3):831–841. doi: 10.1016/0092-8674(83)90025-9. [DOI] [PubMed] [Google Scholar]
- Miller K. G., Liu L. F., Englund P. T. A homogeneous type II DNA topoisomerase from HeLa cell nuclei. J Biol Chem. 1981 Sep 10;256(17):9334–9339. [PubMed] [Google Scholar]
- Mitra S., Sen D., Crothers D. M. Orientation of nucleosomes and linker DNA in calf thymus chromatin determined by photochemical dichroism. Nature. 1984 Mar 15;308(5956):247–250. doi: 10.1038/308247a0. [DOI] [PubMed] [Google Scholar]
- Noll M., Kornberg R. D. Action of micrococcal nuclease on chromatin and the location of histone H1. J Mol Biol. 1977 Jan 25;109(3):393–404. doi: 10.1016/s0022-2836(77)80019-3. [DOI] [PubMed] [Google Scholar]
- Perez-Grau L., Bordas J., Koch M. H. Chromatin superstructure: synchrotron radiation X-ray scattering study on solutions and gels. Nucleic Acids Res. 1984 Mar 26;12(6):2987–2996. doi: 10.1093/nar/12.6.2987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Price J. A., Heller E., Goldthwait D. A. The release of 3-methyladenine from nucleosomal DNA by a 3-methyladenine DNA glycosylase. Carcinogenesis. 1983;4(2):145–152. doi: 10.1093/carcin/4.2.145. [DOI] [PubMed] [Google Scholar]
- Prior C. P., Cantor C. R., Johnson E. M., Littau V. C., Allfrey V. G. Reversible changes in nucleosome structure and histone H3 accessibility in transcriptionally active and inactive states of rDNA chromatin. Cell. 1983 Oct;34(3):1033–1042. doi: 10.1016/0092-8674(83)90561-5. [DOI] [PubMed] [Google Scholar]
- Richmond T. J., Finch J. T., Rushton B., Rhodes D., Klug A. Structure of the nucleosome core particle at 7 A resolution. Nature. 1984 Oct 11;311(5986):532–537. doi: 10.1038/311532a0. [DOI] [PubMed] [Google Scholar]
- Ryoji M., Worcel A. Structure of the two distinct types of minichromosomes that are assembled on DNA injected in Xenopus oocytes. Cell. 1985 Apr;40(4):923–932. doi: 10.1016/0092-8674(85)90352-6. [DOI] [PubMed] [Google Scholar]
- Simpson R. T. Structure of the chromatosome, a chromatin particle containing 160 base pairs of DNA and all the histones. Biochemistry. 1978 Dec 12;17(25):5524–5531. doi: 10.1021/bi00618a030. [DOI] [PubMed] [Google Scholar]
- Smerdon M. J., Lieberman M. W. Distribution within chromatin of deoxyribonucleic acid repair synthesis occurring at different times after ultraviolet radiation. Biochemistry. 1980 Jun 24;19(13):2992–3000. doi: 10.1021/bi00554a025. [DOI] [PubMed] [Google Scholar]
- Smith C. A., Hanawalt P. C. Phage T4 endonuclease V stimulates DNA repair replication in isolated nuclei from ultraviolet-irradiated human cells, including xeroderma pigmentosum fibroblasts. Proc Natl Acad Sci U S A. 1978 Jun;75(6):2598–2602. doi: 10.1073/pnas.75.6.2598. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Snyder R. D., Van Houten B., Regan J. D. Studies on the inhibition of repair of ultraviolet- and methyl methanesulfonate-induced damage in the DNA of human fibroblasts by novobiocin. Nucleic Acids Res. 1982 Oct 11;10(19):6207–6219. doi: 10.1093/nar/10.19.6207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spadafora C., Oudet P., Chambon P. Rearrangement of chromatin structure induced by increasing ionic strength and temperature. Eur J Biochem. 1979 Oct;100(1):225–235. doi: 10.1111/j.1432-1033.1979.tb02053.x. [DOI] [PubMed] [Google Scholar]
- Sugino A., Higgins N. P., Brown P. O., Peebles C. L., Cozzarelli N. R. Energy coupling in DNA gyrase and the mechanism of action of novobiocin. Proc Natl Acad Sci U S A. 1978 Oct;75(10):4838–4842. doi: 10.1073/pnas.75.10.4838. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Villeponteau B., Lundell M., Martinson H. Torsional stress promotes the DNAase I sensitivity of active genes. Cell. 1984 Dec;39(3 Pt 2):469–478. doi: 10.1016/0092-8674(84)90454-9. [DOI] [PubMed] [Google Scholar]
- Weintraub H. Histone-H1-dependent chromatin superstructures and the suppression of gene activity. Cell. 1984 Aug;38(1):17–27. doi: 10.1016/0092-8674(84)90522-1. [DOI] [PubMed] [Google Scholar]
- Weintraub H., Worcel A., Alberts B. A model for chromatin based upon two symmetrically paired half-nucleosomes. Cell. 1976 Nov;9(3):409–417. doi: 10.1016/0092-8674(76)90085-4. [DOI] [PubMed] [Google Scholar]
- Wilhelm M. L., Wilhelm F. X. Conformation of nucleosome core particles and chromatin in high salt concentration. Biochemistry. 1980 Sep 2;19(18):4327–4331. doi: 10.1021/bi00559a028. [DOI] [PubMed] [Google Scholar]
- Worcel A., Strogatz S., Riley D. Structure of chromatin and the linking number of DNA. Proc Natl Acad Sci U S A. 1981 Mar;78(3):1461–1465. doi: 10.1073/pnas.78.3.1461. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu H. M., Dattagupta N., Hogan M., Crothers D. M. Unfolding of nucleosomes by ethidium binding. Biochemistry. 1980 Feb 19;19(4):626–634. doi: 10.1021/bi00545a004. [DOI] [PubMed] [Google Scholar]
- Yasuda H., Matsumoto Y., Mita S., Marunouchi T., Yamada M. A mouse temperature-sensitive mutant defective in H1 histone phosphorylation is defective in deoxyribonucleic acid synthesis and chromosome condensation. Biochemistry. 1981 Jul 21;20(15):4414–4419. doi: 10.1021/bi00518a028. [DOI] [PubMed] [Google Scholar]


