Abstract
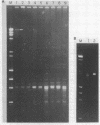
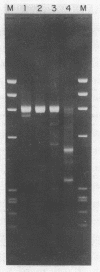
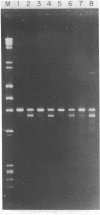
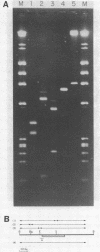
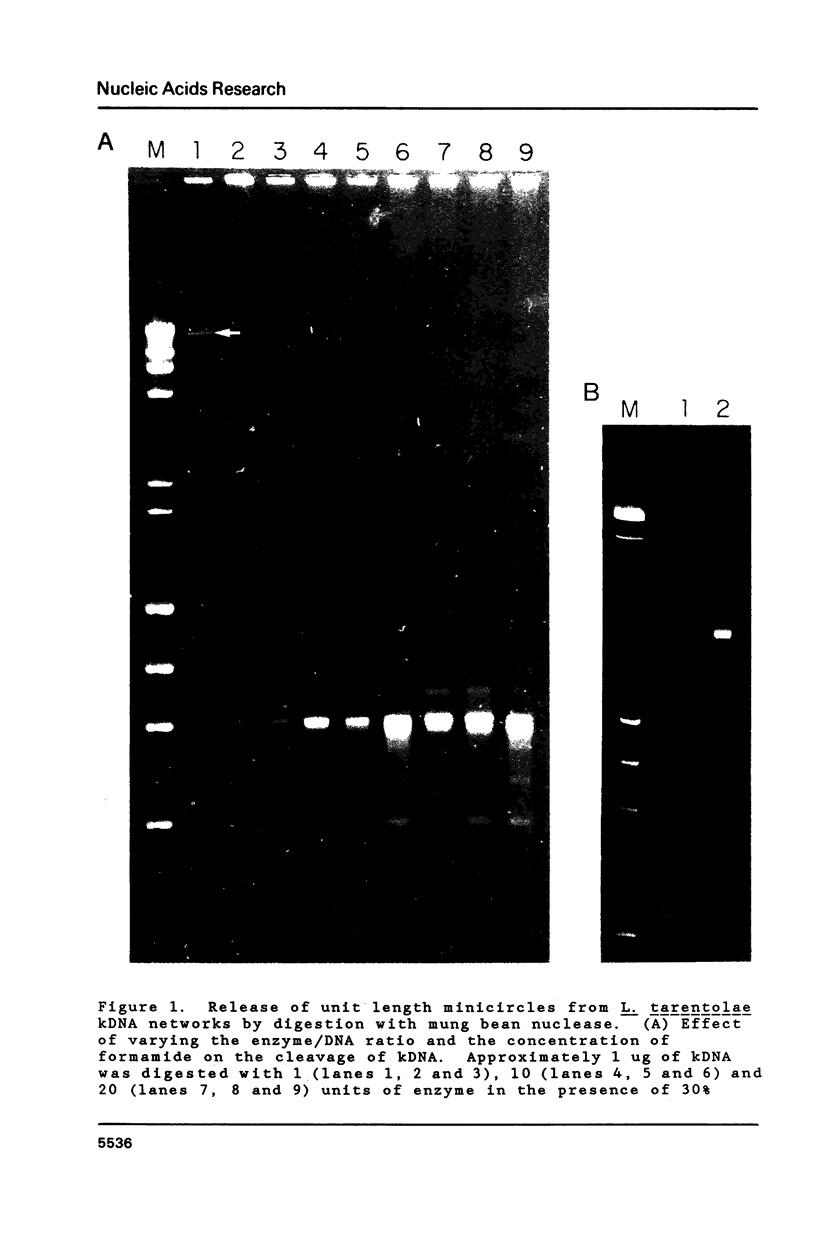
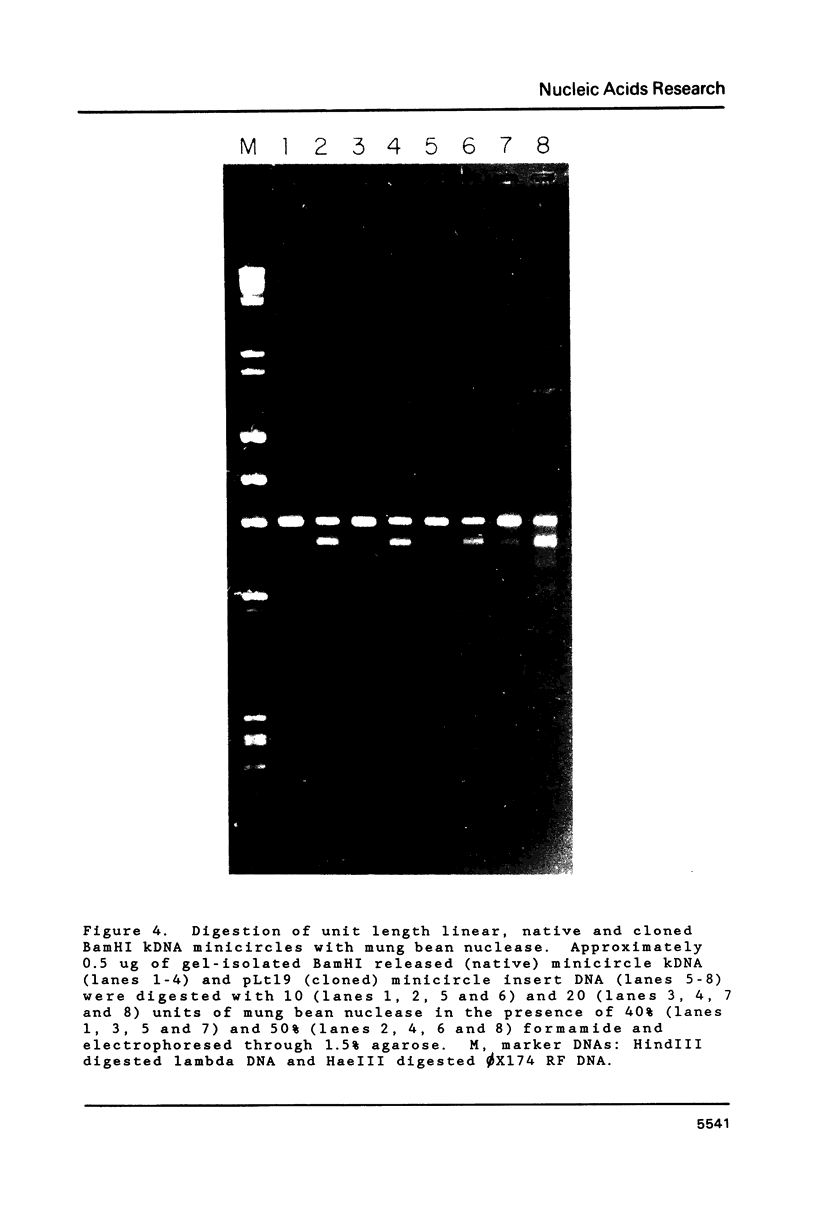
Multiple sequence classes of kinetoplast minicircle DNA from Leishmania tarentolae were cleaved by mung bean nuclease in the presence of formamide, yielding unit length linear molecules which retained the anomalous electrophoretic mobility in acrylamide characteristic of minicircle DNA. No specific cleavage site sequence common to all minicircle sequence classes was apparent, although the main region of nuclease cleavage was localized approximately 350 bp from the unique SmaI restriction site of the conserved region found in all minicircle sequence classes. Covalent closure of the minicircle substrate was not a requirement for cleavage, as linearized network-derived or cloned minicircles were also cleaved by mung bean nuclease at similar locations. The partial sequences of several new minicircle sequence classes released from the network by mung bean nuclease are also reported.
Full text
PDF




















Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Arnot D. E., Barker D. C. Biochemical identification of cutaneous leishmanias by analysis of kinetoplast DNA. II. Sequence homologies in Leishmania kDNA. Mol Biochem Parasitol. 1981 May;3(1):47–56. doi: 10.1016/0166-6851(81)90076-1. [DOI] [PubMed] [Google Scholar]
- Benne R., De Vries B. F., Van den Burg J., Klaver B. The nucleotide sequence of a segment of Trypanosoma brucei mitochondrial maxi-circle DNA that contains the gene for apocytochrome b and some unusual unassigned reading frames. Nucleic Acids Res. 1983 Oct 25;11(20):6925–6941. doi: 10.1093/nar/11.20.6925. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Benton W. D., Davis R. W. Screening lambdagt recombinant clones by hybridization to single plaques in situ. Science. 1977 Apr 8;196(4286):180–182. doi: 10.1126/science.322279. [DOI] [PubMed] [Google Scholar]
- Borst P., Fase-Fowler F., Hoeijmakers J. H., Frasch A. C. Variations in maxi-circle and mini-circle sequences in kinetoplast DNAs from different Trypanosoma brucei strains. Biochim Biophys Acta. 1980 Dec 11;610(2):197–210. doi: 10.1016/0005-2787(80)90001-5. [DOI] [PubMed] [Google Scholar]
- Brunk C. F., Simpson L. Comparison of various ultraviolet sources for fluorescent detection of ethidium bromide-DNA complexes in polyacrylamide gels. Anal Biochem. 1977 Oct;82(2):455–462. doi: 10.1016/0003-2697(77)90183-x. [DOI] [PubMed] [Google Scholar]
- Chen K. K., Donelson J. E. Sequences of two kinetoplast DNA minicircles of Tryptanosoma brucei. Proc Natl Acad Sci U S A. 1980 May;77(5):2445–2449. doi: 10.1073/pnas.77.5.2445. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Donelson J. E., Majiwa P. A., Williams R. O. Kinetoplast DNA minicircles of Trypanosoma brucei share regions of sequence homology. Plasmid. 1979 Oct;2(4):572–588. doi: 10.1016/0147-619x(79)90055-6. [DOI] [PubMed] [Google Scholar]
- Hagerman P. J. Evidence for the existence of stable curvature of DNA in solution. Proc Natl Acad Sci U S A. 1984 Aug;81(15):4632–4636. doi: 10.1073/pnas.81.15.4632. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hensgens L. A., Brakenhoff J., De Vries B. F., Sloof P., Tromp M. C., Van Boom J. H., Benne R. The sequence of the gene for cytochrome c oxidase subunit I, a frameshift containing gene for cytochrome c oxidase subunit II and seven unassigned reading frames in Trypanosoma brucei mitochondrial maxi-circle DNA. Nucleic Acids Res. 1984 Oct 11;12(19):7327–7344. doi: 10.1093/nar/12.19.7327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hoeijmakers J. H., Borst P. Kinetoplast DNA in the insect trypanosomes Crithidia luciliae and Crithidia fasciculata. II. Sequence evolution of the minicircles. Plasmid. 1982 May;7(3):210–220. doi: 10.1016/0147-619x(82)90002-6. [DOI] [PubMed] [Google Scholar]
- Hoeijmakers J. H., Weijers P. J., Brakenhoff G. J., Borst P. Kinetoplast DNA in the insect trypanosomes Crithidia luciliae and Crithidia fasciculata. III. Heteroduplex analysis of the C. luciliae minicircles. Plasmid. 1982 May;7(3):221–229. doi: 10.1016/0147-619x(82)90003-8. [DOI] [PubMed] [Google Scholar]
- Hughes D., Simpson L., Kayne P. S., Neckelmann N. Autonomous replication sequences in the maxicircle kinetoplast DNA of Leishmania tarentolae. Mol Biochem Parasitol. 1984 Nov;13(3):263–275. doi: 10.1016/0166-6851(84)90118-x. [DOI] [PubMed] [Google Scholar]
- Kanehisa M. I. Los Alamos sequence analysis package for nucleic acids and proteins. Nucleic Acids Res. 1982 Jan 11;10(1):183–196. doi: 10.1093/nar/10.1.183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kidane G. Z., Hughes D., Simpson L. Sequence heterogeneity and anomalous electrophoretic mobility of kinetoplast minicircle DNA from Leishmania tarentolae. Gene. 1984 Mar;27(3):265–277. doi: 10.1016/0378-1119(84)90071-4. [DOI] [PubMed] [Google Scholar]
- Kowalski D. Changes in site specificity of single-strand-specific endonucleases on supercoiled PM2 DNA with temperature and ionic environment. Nucleic Acids Res. 1984 Sep 25;12(18):7071–7086. doi: 10.1093/nar/12.18.7071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kroeker W. D., Kowalski D. Gene-sized pieces produced by digestion of linear duplex DNA with mung bean nuclease. Biochemistry. 1978 Aug 8;17(16):3236–3243. doi: 10.1021/bi00609a010. [DOI] [PubMed] [Google Scholar]
- Leon W., Frasch A. C., Hoeijmakers J. H., Fase-Fowler F., Borst P., Brunel F., Davison J. Maxi-circles and mini-circles in kinetoplast DNA from trypanosoma cruzi. Biochim Biophys Acta. 1980 Apr 30;607(2):221–231. doi: 10.1016/0005-2787(80)90075-1. [DOI] [PubMed] [Google Scholar]
- Macina R. A., Sanchez D. O., Affranchino J. L., Engel J. C., Frasch A. C. Polymorphisms within minicircle sequence classes in the kinetoplast DNA of Trypanosoma cruzi clones. Mol Biochem Parasitol. 1985 Jun;16(1):61–74. doi: 10.1016/0166-6851(85)90049-0. [DOI] [PubMed] [Google Scholar]
- Marini J. C., Effron P. N., Goodman T. C., Singleton C. K., Wells R. D., Wartell R. M., Englund P. T. Physical characterization of a kinetoplast DNA fragment with unusual properties. J Biol Chem. 1984 Jul 25;259(14):8974–8979. [PubMed] [Google Scholar]
- Maslov D. A., Kolesnikov A. A., Zaitseva G. N. Conservative and divergent base sequence regions in the maxicircle kinetoplast DNA of several trypanosomatid flagellates. Mol Biochem Parasitol. 1984 Jul;12(3):351–364. doi: 10.1016/0166-6851(84)90091-4. [DOI] [PubMed] [Google Scholar]
- McCutchan T. F., Hansen J. L., Dame J. B., Mullins J. A. Mung bean nuclease cleaves Plasmodium genomic DNA at sites before and after genes. Science. 1984 Aug 10;225(4662):625–628. doi: 10.1126/science.6330899. [DOI] [PubMed] [Google Scholar]
- Messing J., Vieira J. A new pair of M13 vectors for selecting either DNA strand of double-digest restriction fragments. Gene. 1982 Oct;19(3):269–276. doi: 10.1016/0378-1119(82)90016-6. [DOI] [PubMed] [Google Scholar]
- Morel C., Chiari E., Camargo E. P., Mattei D. M., Romanha A. J., Simpson L. Strains and clones of Trypanosoma cruzi can be characterized by pattern of restriction endonuclease products of kinetoplast DNA minicircles. Proc Natl Acad Sci U S A. 1980 Nov;77(11):6810–6814. doi: 10.1073/pnas.77.11.6810. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Muhich M. L., Neckelmann N., Simpson L. The divergent region of the Leishmania tarentolae kinetoplast maxicircle DNA contains a diverse set of repetitive sequences. Nucleic Acids Res. 1985 May 10;13(9):3241–3260. doi: 10.1093/nar/13.9.3241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Muhich M. L., Simpson L., Simpson A. M. Comparison of maxicircle DNAs of Leishmania tarentolae and Trypanosoma brucei. Proc Natl Acad Sci U S A. 1983 Jul;80(13):4060–4064. doi: 10.1073/pnas.80.13.4060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Payne M., Rothwell V., Jasmer D. P., Feagin J. E., Stuart K. Identification of mitochondrial genes in Trypanosoma brucei and homology to cytochrome c oxidase II in two different reading frames. Mol Biochem Parasitol. 1985 May;15(2):159–170. doi: 10.1016/0166-6851(85)90117-3. [DOI] [PubMed] [Google Scholar]
- Ponzi M., Birago C., Battaglia P. A. Two identical symmetrical regions in the minicircle structure of Trypanosoma lewisi kinetoplast DNA. Mol Biochem Parasitol. 1984 Sep;13(1):111–119. doi: 10.1016/0166-6851(84)90105-1. [DOI] [PubMed] [Google Scholar]
- Pustell J., Kafatos F. C. A convenient and adaptable package of computer programs for DNA and protein sequence management, analysis and homology determination. Nucleic Acids Res. 1984 Jan 11;12(1 Pt 2):643–655. doi: 10.1093/nar/12.1part2.643. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sanger F., Nicklen S., Coulson A. R. DNA sequencing with chain-terminating inhibitors. Proc Natl Acad Sci U S A. 1977 Dec;74(12):5463–5467. doi: 10.1073/pnas.74.12.5463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sheflin L. G., Kowalski D. Altered DNA conformations detected by mung bean nuclease occur in promoter and terminator regions of supercoiled pBR322 DNA. Nucleic Acids Res. 1985 Sep 11;13(17):6137–6154. doi: 10.1093/nar/13.17.6137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sheflin L. G., Kowalski D. Mung bean nuclease cleavage of a dA + dT-rich sequence or an inverted repeat sequence in supercoiled PM2 DNA depends on ionic environment. Nucleic Acids Res. 1984 Sep 25;12(18):7087–7104. doi: 10.1093/nar/12.18.7087. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shlomai J., Zadok A. Kinetoplast DNA minicircles of trypanosomatids encode for a protein product. Nucleic Acids Res. 1984 Nov 12;12(21):8017–8028. doi: 10.1093/nar/12.21.8017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Simpson A. M., Simpson L. Kinetoplast DNA and RNA of Trypanosoma brucei. Mol Biochem Parasitol. 1980 Dec;2(2):93–108. doi: 10.1016/0166-6851(80)90034-1. [DOI] [PubMed] [Google Scholar]
- Simpson A. M., Simpson L., Livingston L. Transcription of the maxicircle kinetoplast DNA of Leishmania tarentolae. Mol Biochem Parasitol. 1982 Oct;6(4):237–252. doi: 10.1016/0166-6851(82)90057-3. [DOI] [PubMed] [Google Scholar]
- Simpson L., Braly P. Synchronization of Leishmania tarentolae by hydroxyurea. J Protozool. 1970 Nov;17(4):511–517. doi: 10.1111/j.1550-7408.1970.tb04719.x. [DOI] [PubMed] [Google Scholar]
- Simpson L., Simpson A. M., Kidane G., Livingston L., Spithill T. W. The kinetoplast DNA of the hemoflagellate protozoa. Am J Trop Med Hyg. 1980 Sep;29(5 Suppl):1053–1063. doi: 10.4269/ajtmh.1980.29.1053. [DOI] [PubMed] [Google Scholar]
- Sloof P., Van den Burg J., Voogd A., Benne R., Agostinelli M., Borst P., Gutell R., Noller H. Further characterization of the extremely small mitochondrial ribosomal RNAs from trypanosomes: a detailed comparison of the 9S and 12S RNAs from Crithidia fasciculata and Trypanosoma brucei with rRNAs from other organisms. Nucleic Acids Res. 1985 Jun 11;13(11):4171–4190. doi: 10.1093/nar/13.11.4171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Staden R. An interactive graphics program for comparing and aligning nucleic acid and amino acid sequences. Nucleic Acids Res. 1982 May 11;10(9):2951–2961. doi: 10.1093/nar/10.9.2951. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wirth D. F., Pratt D. M. Rapid identification of Leishmania species by specific hybridization of kinetoplast DNA in cutaneous lesions. Proc Natl Acad Sci U S A. 1982 Nov;79(22):6999–7003. doi: 10.1073/pnas.79.22.6999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu H. M., Crothers D. M. The locus of sequence-directed and protein-induced DNA bending. Nature. 1984 Apr 5;308(5959):509–513. doi: 10.1038/308509a0. [DOI] [PubMed] [Google Scholar]
- de la Cruz V. F., Lake J. A., Simpson A. M., Simpson L. A minimal ribosomal RNA: sequence and secondary structure of the 9S kinetoplast ribosomal RNA from Leishmania tarentolae. Proc Natl Acad Sci U S A. 1985 Mar;82(5):1401–1405. doi: 10.1073/pnas.82.5.1401. [DOI] [PMC free article] [PubMed] [Google Scholar]
- de la Cruz V. F., Neckelmann N., Simpson L. Sequences of six genes and several open reading frames in the kinetoplast maxicircle DNA of Leishmania tarentolae. J Biol Chem. 1984 Dec 25;259(24):15136–15147. [PubMed] [Google Scholar]
- de la Cruz V. F., Simpson A. M., Lake J. A., Simpson L. Primary sequence and partial secondary structure of the 12S kinetoplast (mitochondrial) ribosomal RNA from Leishmania tarentolae: conservation of peptidyl-transferase structural elements. Nucleic Acids Res. 1985 Apr 11;13(7):2337–2356. doi: 10.1093/nar/13.7.2337. [DOI] [PMC free article] [PubMed] [Google Scholar]