Abstract
The transcriptional regulator GATA1 is crucially involved in megakaryocytopoiesis and erythropoiesis. Mutations of the gene which is located on the X chromosome have been associated with platelet and red blood cell abnormalities. We identified a family with a GATA1G208R mutation in whom a low male birth rate and frequent miscarriages among heterozygous females suggested increased fetal death in male hemizygotes. Female mutation carriers had normal or near normal hemoglobin levels and platelet counts ranging from normal to severely reduced, probably reflecting skewed X chromosome inactivation. Platelets were dimorphous, and thrombocytopenia was associated with erythroblastosis. The only living male mutation carrier had severe macrothrombocytopenia with life-threatening bleeding episodes, moderate to severe anemia, eosinopenia, skeletal abnormalities, and abundant extra-medullary hematopoiesis. Long-term sequelae in the 50-year-old patient included unilateral nephrectomy following misinterpretation of paraspinal hematopoiesis as renal cancer, spinal stenosis which was possibly favored by progressive bone marrow expansion, and severe secondary gout.
Keywords: Anemia, Erythroblastosis, Extramedullary hematopoiesis, GATA1, Thrombocytopenia
Introduction
The transcriptional regulator GATA1 coordinates the development of platelets, erythrocytes, eosinophils, and mast cells. Germline mutations of its gene which is located on the X chromosome have been associated with abnormal thrombocytopoiesis and erythropoiesis [1]. One of the mutations, GATA1G208R, which has been described in two kindreds [2, 3], causes severe thrombocytopenia and dyserythropoietic anemia in affected males. The morphological abnormalities associated with this mutation showed striking similarities with the blood and bone marrow findings in one of our patients who had been diagnosed with atypical congenital dyserythropoietic anemia in 1972 [4, 5]. Gene sequencing confirmed hemizygosity for the GATA1G208R mutation which was also detected in a maternal great aunt and her daughter. We here describe the clinical course of the affected family members.
Patients, materials, and methods
Index patient
The index patient (IV-2; Fig. 1) manifested with extensive mucocutaneous bleeding in his first weeks of life. Severe thrombocytopenia (12/nl) and mild macrocytic anemia with occasional erythroblasts on the blood smear were first documented when he was 4 months old. During infancy and childhood, epistaxis and gastrointestinal hemorrhage necessitated repeated blood transfusions. Treatment with prednisone, vitamins, or iron remained ineffective.
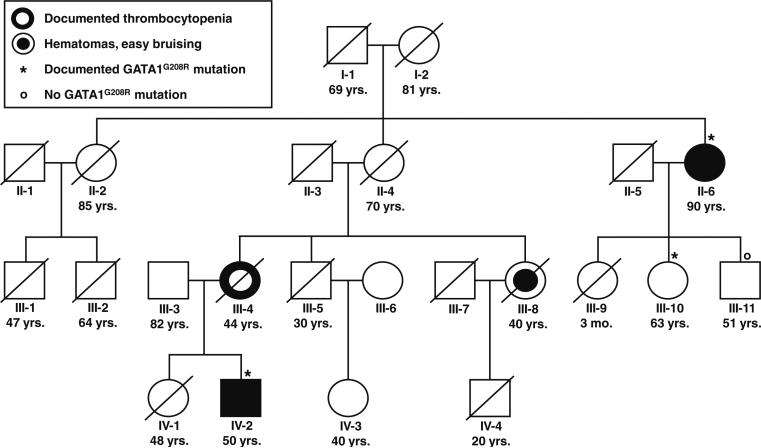
Fig. 1.
Pedigree of a family affected by a GATA1G208R mutation. The age (mo. months, yrs. years) indicated below each subject relates to the age at writing the manuscript or the age at death of living or deceased family members, respectively
At age 12, a diagnosis of atypical congenital dyserythropoietic anemia was made [4]. The patient had hepatosplenomegaly, turricephalus with frontal bossing and widened diploe, strabismus, and unilateral cryptorchidism. Hematological abnormalities included moderate anemia (hemoglobin 10.0 g/dl, normal range [NR] 13.7–17.2) with signs of increased red cell destruction (bilirubin 1.4 mg/dl, NR<1.2; lactate dehydrogenase [LDH] 403 U/l, NR 100–240; haptoglobin 0.01 g/dl, NR 0.3–2.0) and inadequate erythropoietic regeneration (reticulocytes 4.0%, NR 0.4–1.6), severe thrombocytopenia (13/nl, NR 140–320), eosinopenia, and a left shift of neutrophilic granulocytes (Table 1). Radiolabeling studies demonstrated increased, but inefficient erythropoiesis with reduced erythrocyte survival and predominant red cell sequestration in the spleen (Table 2). Hemoglobin electrophoresis was normal (HbA 97.4%; HbA2 2.3%, NR≤3.0; HbF 0.3%, NR≤0.4; no abnormal hemoglobin).
Table 1.
Blood cell parameters in a hemizygous male patient and three heterozygous female relatives affected by a GATA1G208R mutation
| Subject (no. on pedigreea) | Ageb (years) | Hemoglobin (g/dl) | MCV (fl) | Reticulocytes (%) | Erythroblasts (/nl) | TNC (/nl) | WBC (/nl) | Differential WBC count (%) |
Platelets (/nl) | ||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Neutrophilsc | E | B | M | L | |||||||||
| Index patient before splenectomy | |||||||||||||
| Index patient (IV-2) | 0.9 | 12.3 | n.d. | 5.9 | ++d | 26.5 | n.d. | Slight left shiftd | 16 | ||||
| Index patient (IV-2) | 12 | 10.0 | 106 | 4.0 | 0.4 | 9.2 | 8.8 | 3-1-1-1-6-47 | 0 | 2 | 8 | 31 | 13 |
| Index patient after splenectomy | |||||||||||||
| Index patient (IV-2) | 13 | 11.4 | 105 | 4.0 | 30.4 | 41.1 | 10.7 | 2-1-2-2-1-72 | 0 | 2 | 8 | 10 | 35 |
| Index patient (IV-2) | 48 | 7.4 | 110 | 2.4 | 96.0 | 126.3 | 30.3 | 0-2-1-0-1-89 | 0 | 0 | 5 | 2 | 31 |
| Other family members affected by the mutation | |||||||||||||
| Mother (III-4) | 34 | 13.6 | 90 | 1.0 | 0.5 | 10.0 | 9.5 | 0-0-0-0-1-65 | 1 | 1 | 6 | 26 | 76 |
| Aunt (III-10) | 63 | 13.7 | 89 | 1.1 | 0 | 7.5 | 7.5 | 0-0-0-0-0-68 | 4 | 0 | 4 | 24 | 230e |
| Great aunt (II-6) | 90 | 11.2 | 102 | 2.7 | 0.5 | 9.4 | 8.9 | 0-0-0-0-2-55 | 1 | 6 | 19 | 17 | 17 |
MCV mean corpuscular volume, TNC total nucleated cells, WBC white blood cells, E eosinophils, B basophils, M monocytes, L lymphocytes, n.d. not done
cf. Fig. 1
Age at analysis
Left to right: myeloblasts–promyelocytes–myelocytes–metamyelocytes–band forms–polymorphonuclear granulocytes
Present, but not enumerated
Dimorphous platelets (mainly small, some very large; cf. Fig. 4b)
Table 2.
Radiolabeling studies in a hemizygous male carrier of a GATA1G208R mutation at age 12 (subject IV-2; cf. Fig. 1)
| Parameter | Unit | Normal rangea | IV-2 |
|---|---|---|---|
| Ferrokinetic study (injection of 8 μCi 59Fe) | |||
| Plasma iron clearance (t1/2) | min | 70–140 | 40 |
| Plasma iron transport rate | mg/day | 21–42 | 82 |
| Plasma iron turnover | mg/100 ml blood/day | 0.45–0.90 | 1.78 |
| Iron utilization | % | 70–90 | 22b |
| Erythrocyte survival time (erythrocyte labeling with 60 μCi 51Cr) | |||
| 51Cr erythrocyte survival (t1/2) | days | 25–30 | 16 |
| Spleen–liver ratio | – | 0.7–2.0 | 3.2 |
Normal range for male adults
Determined after 17 days
At age 13, splenectomy was performed (weight, 500 g) resulting in a moderate increase in platelet count (30–40/nl) and a decrease in bleeding episodes. After splenectomy the ratio between erythroblasts and leukocytes rose from about 5:100 to over 200:100, causing a significant increase in total nucleated blood cells (Table 1; Fig. 2). The erythroblasts were highly abnormal, with condensed nuclei, irregular, frequently serrated nuclear outline, and gross granules reacting positive for non-heme iron on Prussian blue (Perls’) stain. The erythrocytes were characterized by anisocytosis, poikilocytosis, marked basophilic stippling, and frequent inclusion of Pappenheimer bodies. The platelets were large, with variably developed, often unequally distributed, granulation. The bone marrow revealed erythropoietic hyperplasia (myeloid-to-erythroid ratio 1:4.4, NR 1.5–3.3) with atypical mature erythroblasts resembling those in the peripheral blood, increased iron deposition in red cell precursors with about 5% ringed sideroblasts, an increase in megakaryocytes with hypolobulated nuclei, and normal granulopoiesis (Fig. 2).
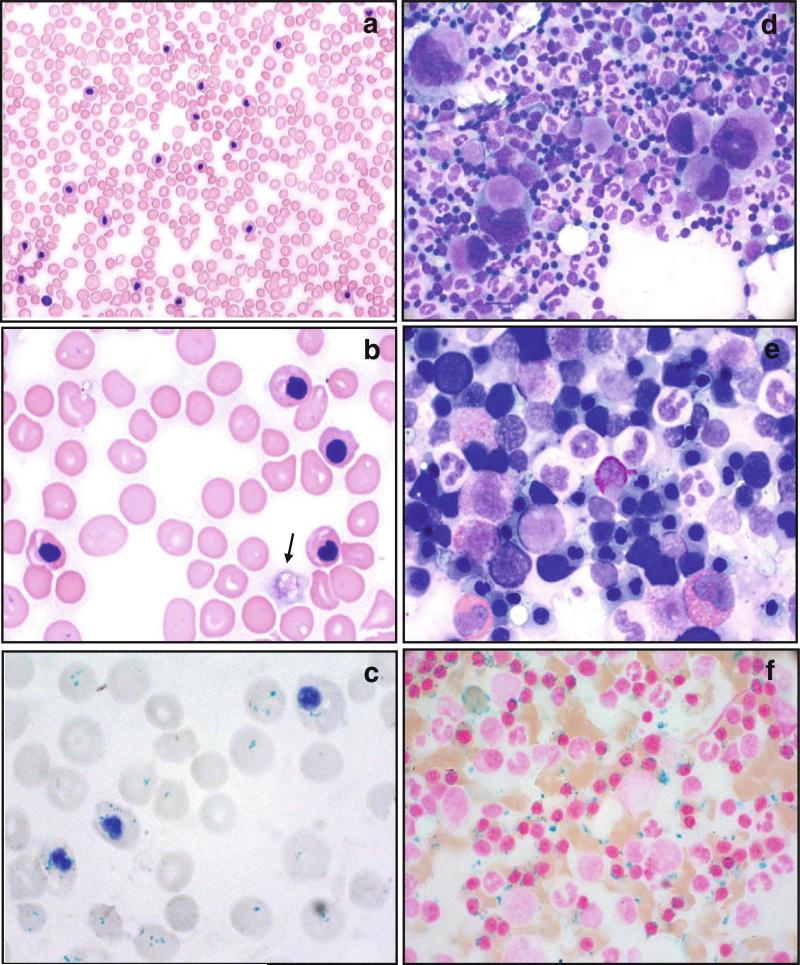
Fig. 2.
Blood and bone marrow cell morphology in a hemizygous male carrier of a GATA1G208R mutation (subject IV-2; cf. Fig. 1). a Blood smear at age 39 (26 years after splenectomy; May–Grünwald–Giemsa stain): markedly increased nucleated cell (“leukocyte”) count due to numerous mature-appearing nucleated red blood cells. Most nuclei have an irregular shape. Note the anisocytosis, poikilocytosis, and thrombocytopenia. b Same blood smear, higher magnification: four erythroblasts with condensed nuclei of irregular shape, marked basophilic stippling of several erythrocytes, one very large hypogranulated platelet (macrothrombocyte; arrow). c Blood smear at age 48 (Prussian blue reaction, hematoxylin): gross granules reacting positive for non-heme iron in erythrocytes (Pappenheimer bodies) and erythroblasts. d Bone marrow cytology at age 45: high cellularity, multitude of megakaryocytes of variable size, often hypolobulated or with small marginal nuclei. e Bone marrow cytology at age 39: increased number of mature erythroblasts with irregularly shaped nuclei and karyorrhexis. f Bone marrow cytology at age 39 (Prussian blue reaction, nuclear fast red): markedly increased iron deposition with 5% ringed sideroblasts
At age 25, an asymptomatic paraspinal tumor encasing the right kidney was removed (weight, 700 g). Histology revealed extensive extramedullary hematopoiesis without evidence of malignancy [5]. During the subsequent two decades, hemoglobin leveled off at 10–12 g/dl, allowing the patient to work as a nurse. At age 45, he experienced life-threatening gastric hemorrhage, leaving him with impaired renal function, deterioration of anemia (Table 1), and renewed transfusion dependency. He subsequently developed tophaceous gout (uric acid 13.8 g/dl, NR 3.5–7.2; Fig. 3a), bilateral cataracts related to glucocorticosteroid treatment of gouty arthritis, and, at age 49, paraparesis of the lower limbs due to stenosis of the lumbar spine. Whether this was related to bone marrow expansion resulting from inefficient hematopoiesis could not be resolved. Whole-body magnetic resonance imaging revealed intrathoracic paraspinal masses (Fig. 3b), likely representing extramedullary hematopoiesis [6].
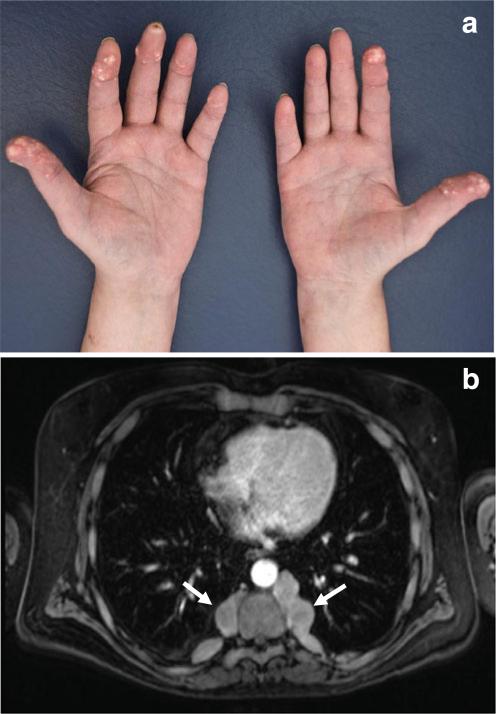
Fig. 3.
Long-term sequelae in a hemizygous male carrier of a GATA1G208R mutation at age 48 (subject IV-2; cf. Fig. 1). a Cutaneous deposits of firm yellow-colored material in the fingers from which microscopically identifiable urate crystals were recovered (tophaceous gout). b Intrathoracic paraspinal masses, most likely representing extramedullary hematopoiesis (arrows)
Family
The patient's mother (III-4; Fig. 1) had three miscarriages of unknown gender, all between months 3 and 5 of gestation, and gave birth to the index patient and his elder sister (IV-1) of whom no hematological abnormalities were reported. The mother suffered from easy bruising, and, at age 38, had a moderately decreased platelet count, a normal hemoglobin level, and occasional erythroblasts on the blood smear (Table 1). The father (III-3) has no hematological abnormalities.
The patient's maternal grandmother (II-4) had no apparent bleeding disposition. She gave birth to two daughters and a son. Her younger sister (II-6) suffered from easy bruising and menorrhage. She had a male miscarriage and gave birth to two daughters and a son, none of whom ever experienced increased bleeding. At age 64, asymptomatic thrombocytopenia was first documented. Starting at about age 80, she developed increasing mucocutaneous hemorrhage. Idiopathic thrombocytopenia was suspected, but glucocorticosteroids remained ineffective. At age 90, there was severe thrombocytopenia (17/nl), mild macrocytic anemia (hemoglobin 11.2 g/dl) with some degree of hemolysis (bilirubin 2.4 mg/dl; LDH 397 U/l; haptoglobin 0.15 g/dl) and erythroblastosis (Table 1; Fig. 4a), and slight splenomegaly. Her second daughter (III-10) suffered two miscarriages of unknown gender in months 3 and 4 of gestation, respectively.
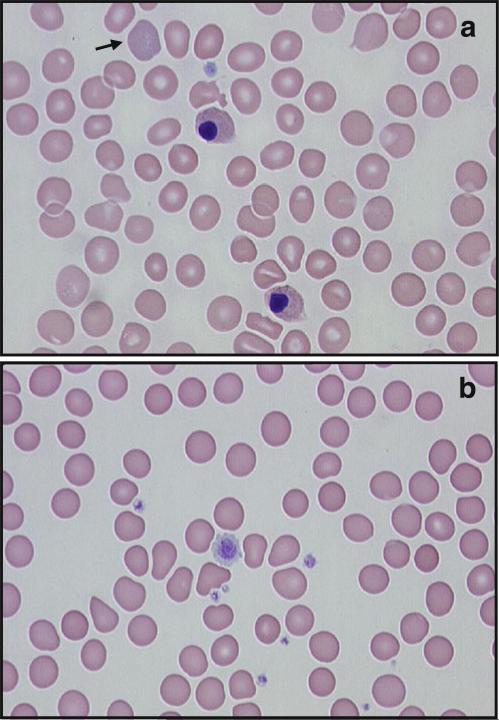
Fig. 4.
Blood cell abnormalities in two heterozygous carriers of a GATA1G208R mutation (May–Grünwald–Giemsa stain). a Blood smear of a heterozygous female (subject II-6; cf. Fig. 1) with severe thrombocytopenia at age 90: two erythroblasts, one with irregular nuclear outline, basophilic stippling in an erythrocyte (arrow) and in the erythroblasts, a single platelet with poor granulation. b Blood smear of a heterozygous female (subject III-10) without thrombocytopenia at age 63: platelet dimorphism
Investigations
Subjects provided informed consent according to institutional guidelines. Mutation analysis was performed on blood cells employing published methods [3, 7, 8].
Results and discussion
Four members of the family (II-6, III-10, III-11, and IV-2) participated in the mutation analysis. The index patient (IV-2) harbored a hemizygous G to A transition at nucleotide 622 in exon 4 of GATA1 predicting a G208R amino acid change in the N-terminal zinc finger domain of GATA1 which is crucial for interaction with its coregulator FOG1 [1]. The patient inherited the mutant allele from his mother (III-4) who also had a reduced platelet count. While his maternal grandmother (II-4) never suffered from abnormal bleeding, one of her sisters (II-6) had severe thrombocytopenia. She also carried the GATA1 mutation and passed it on to her second daughter (III-10), but not to her son (III-11; Fig. 1). Her daughter's platelet count was normal, but the platelets were dimorphous with a majority of small and a minority of unusually large elements (Fig. 4b).
Several aspects of this family deserve special attention. First, in contrast to previous cases with GATA1G208R mutations [2, 3], the disease course of our index patient spans five decades. The leading symptom was hemorrhage. Splenectomy resulted in a moderate increase in platelets and pronounced erythroblastosis suggesting a red cell denucleation defect with sequestration of nucleated cells in the spleen. Anemia due to inefficient erythropoiesis, hemolysis, and bleeding was compensated by erythropoietic hyperplasia which led to skeletal abnormalities (turricephalus and possibly spinal stenosis) and abundant extramedullary blood cell formation. Increased cell turnover eventually caused severe gout.
The index patient was the only male with increased bleeding in a four-generation pedigree. The five obligate female carriers of the mutation (I-2, II-4, II-6, III-4, and III-10) gave birth to eight daughters and three sons. A total of six miscarriages, all between months 3 and 5 of gestation, were observed in the three women of whom detailed information was available (II-6, III-4, and III-10). It is tempting to speculate that the GATA1G208R mutation predisposes male carriers to fetal death.
In heterozygous females platelet dimorphism is likely to be explained by differential X chromosome inactivation. Different degrees of skewing of X chromosome inactivation may lead to widely varying platelet counts [9]. In the index patient's great aunt (II-6), bleeding increased with increasing age. This may be explained by age-dependent skewing of X chromosome inactivation which is observed in about half of females above age 60 and may be caused by oligoclonal hematopoiesis or hemizygous stem cell selection [10, 11]. It is of interest that thrombocytopenia was not associated with severe anemia, left shift of neutrophils, or absence of eosinophils which were consistent findings in the index patient (Table 1). Explanations include varying sensitivities of blood cell lineages to mutated GATA1 [12], preferential derivation of different lineages from different stem cells, and compensatory mechanisms unrelated to GATA1.
In summary, this is the first report describing the natural history of GATA1G208R-related cytopenia. Male patients should be carefully monitored for potentially disabling extramedullary hematopoiesis. Whether this may be prevented by hypertransfusion or early hematopoietic stem cell transplantation as practiced in other ineffective erythropoiesis syndromes [13] remains to be shown. Female patients may present as steroid-refractory “idiopathic” thrombocytopenia which may be related to GATA1 mutations more frequently than hitherto known. In such cases, concomitant erythroblastosis may lead the way to the right diagnosis.
Acknowledgment
We thank the index patient and his relatives for providing the family history and Ms. Rosi Leichtle for managing the data in the German Registry of Congenital Dyserythropoietic Anemia.
Footnotes
Present Address: C. P. Kratz Clinical Genetics Branch, Division of Cancer Epidemiology and Genetics, National Cancer Institute, Bethesda, MD 20852, USA
Contributor Information
Ulrich Dührsen, Klinik für Hämatologie, Universitätsklinikum Essen, Hufelandstraße 55, 45122 Essen, Germany.
Christian P. Kratz, Klinik IV: Hämatologie und Onkologie, Zentrum für Kinderheilkunde und Jugendmedizin der Universität Freiburg, Mathildenstraße 1, 70106 Freiburg, Germany
Christian Flotho, Klinik IV: Hämatologie und Onkologie, Zentrum für Kinderheilkunde und Jugendmedizin der Universität Freiburg, Mathildenstraße 1, 70106 Freiburg, Germany.
Thomas Lauenstein, Institut für Diagnostische und Interventionelle Radiologie und Neuroradiologie, Universitätsklinikum Essen, Hufelandstraße 55, 45122 Essen, Germany.
Martin Bommer, Zentrum für Innere Medizin, Universitätsklinikum Ulm, Albert-Einstein-Allee 23, 89081 Ulm, Germany.
Erika König, Klinik für Hämatologie, Universitätsklinikum Essen, Hufelandstraße 55, 45122 Essen, Germany.
Günter Brittinger, Klinik für Hämatologie, Universitätsklinikum Essen, Hufelandstraße 55, 45122 Essen, Germany.
Hermann Heimpel, Zentrum für Innere Medizin, Universitätsklinikum Ulm, Albert-Einstein-Allee 23, 89081 Ulm, Germany.
References
- 1.Ciovacco WA, Raskind WH, Kacena MA. Human phenotypes associated with GATA-1 mutations. Gene. 2008;427:1–6. doi: 10.1016/j.gene.2008.09.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Del Vecchio GC, Giordani L, De Santis A, De Mattia D. Dyserythropoietic anemia and thrombocytopenia due to a novel mutation in GATA-1. Acta Haematol. 2005;114:113–116. doi: 10.1159/000086586. [DOI] [PubMed] [Google Scholar]
- 3.Kratz CP, Niemeyer CM, Karow A, Volz-Fleckenstein M, Schmitt-Gräff A, Strahm B. Congenital transfusion-dependent anemia and thrombocytopenia with myelodysplasia due to a recurrent GATA1G208R germline mutation. Leukemia. 2008;22:432–434. doi: 10.1038/sj.leu.2404904. [DOI] [PubMed] [Google Scholar]
- 4.König E, Osieka R, Brittinger G. Atypische kongenitale dyserythropoietische Anämie mit Thrombocytopenie. Verh Dtsch Ges Inn Med. 1973;79:490–492. [PubMed] [Google Scholar]
- 5.Schmidt U, Richter HJ, Samandari S. Atypische kongenitale dyserythropoietische Anämie. Schweiz med Wschr. 1987;117:1776–1780. [PubMed] [Google Scholar]
- 6.Heimpel H, Dührsen U, Hofbauer P, Rigamonti-Wermlinger V, Kreuser ED, Schwarz K, Solenthaler M, Pauls S. Bulky extramedullary hematopoiesis is not a rare complication of congenital dyserythropoietic anemia. Ann Hematol. 2009;88:937–941. doi: 10.1007/s00277-009-0735-5. [DOI] [PubMed] [Google Scholar]
- 7.Nichols KE, Crispino JD, Poncz M, White JG, Orkin SH, Maris JM, Weiss MJ. Familial dyserythropoietic anaemia and thrombocytopenia due to an inherited mutation in GATA1. Nat Genet. 2000;24:266–270. doi: 10.1038/73480. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Hollanda LM, Lima CS, Cunha AF, Albuquerque DM, Vassallo J, Ozelo MC, Joazeiro PP, Saad ST, Costa FF. An inherited mutation leading to production of only the short isoform of GATA-1 is associated with impaired erythropoiesis. Nat Genet. 2006;38:807–812. doi: 10.1038/ng1825. [DOI] [PubMed] [Google Scholar]
- 9.Balduini CL, Pecci A, Loffredo G, Izzo P, Noris P, Grosso M, Bergamaschi G, Rosti V, Magrini U, Ceresa IF, Conti V, Poggi V, Savoia A. Effects of the R216Q mutation of GATA-1 on erythropoiesis and megakaryocytopoiesis. Thromb Haemost. 2004;91:129–140. doi: 10.1160/TH03-05-0290. [DOI] [PubMed] [Google Scholar]
- 10.Abkowitz JL, Taboada M, Shelton GH, Catlin SN, Guttorp P, Kiklevich JV. An X chromosome gene regulates hematopoietic stem cell kinetics. Proc Natl Acad Sci U S A. 1998;95:3862–3866. doi: 10.1073/pnas.95.7.3862. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Busque L, Paquette Y, Provost S, Roy DC, Levine RL, Mollica L, Gilliland DG. Skewing of X-inactivation ratios in blood cells of aging women is confirmed by independent methodologies. Blood. 2009;113:3472–3474. doi: 10.1182/blood-2008-12-195677. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Mehaffey MG, Newton AL, Gandhi MJ, Crossley M, Drachman JG. X-linked thrombocytopenia caused by a novel mutation of GATA-1. Blood. 2001;98:2681–2688. doi: 10.1182/blood.v98.9.2681. [DOI] [PubMed] [Google Scholar]
- 13.Rund D, Rachmilewitz E. Beta-thalassemia. N Engl J Med. 2005;353:1135–1146. doi: 10.1056/NEJMra050436. [DOI] [PubMed] [Google Scholar]