Abstract
Polychlorinated biphenyls (PCB) are industrial chemicals linked to developmental deficits that may be caused in part by disrupting thyroid hormone (TH) action by either reducing serum TH or interacting directly with the TH receptor (TR). Individual PCB congeners can activate the TR in vitro when the metabolic enzyme cytochrome P4501A1 (CYP1A1) is induced, suggesting that specific PCB metabolites act as TR agonists. To test this hypothesis in vivo, we compared two combinations of PCB congeners that either activate the TR (PCB 105 and 118) or not (PCB 138 and 153) in the presence or absence of a PCB congener (PCB 126) that induces CYP1A1 in vitro. Aroclor 1254 was used as a positive control, and a group treated with propylthiouracil was included to characterize the effects of low serum TH. We monitored the effects on TH signaling in several peripheral tissues by measuring the mRNA expression of well-known TH-response genes in these tissues. Aroclor 1254 and its component PCB 105/118/126 reduced total T4 to the same extent as that of propylthiouracil but increased the expression of some TH target genes in liver. This effect was strongly correlated with CYP1A1 expression supporting the hypothesis that metabolism is necessary. Effects were gene and tissue specific, indicating that tissue-specific metabolism is an important component of PCB disruption of TH action and that PCB metabolites interact in complex ways with the TR. These are essential mechanisms to consider when evaluating the health risks of contaminant exposures, for both PCB and other polycyclic compounds known to interact with nuclear hormone receptors.
Polychlorinated biphenyls (PCB) are a family of industrial compounds consisting of two linked phenyl rings and varying degrees of chlorination, resulting in 209 different congeners (1, 2). Their production was banned in the 1970s after more than a billion kilograms were produced (3), but they remain persistent and ubiquitous environmental contaminants that are routinely found in samples of human and animal tissues (reviewed in Ref. 4). PCB exposure is associated with measures of neurotoxicity (5) including reduced intelligence quotient and reduced response inhibition (6–9). However, PCB exposures, along with exposures to other organochlorine compounds, are also associated with nonneural diseases/disorders such as diabetes (10).
An important mechanism by which PCB produce adverse health effects appears to be by acting on the thyroid hormone (TH) system. PCB are known to cause a reduction in serum TH concentrations in animals (11) and have been associated with reduced TH levels in some (12) but not all (13) human studies. Mechanistic studies in animals reveal that PCB exposure causes a reduction in serum TH but that the outcomes are not fully consistent with reduced serum TH produced by other means, such as by propylthiouracil (PTU) exposure. For example, PCB exposure significantly reduces serum total and free T4 but does not cause an increase in serum TSH (14, 15). In addition, PCB exposure does not produce changes in central neurotransmitter levels (16), eye opening, tooth eruption, or body weight (17) in a manner that is consistent with reduced serum TH produced by PTU treatment.
PCB may also interact directly with TH receptor(s) (TR). The technical mixture Aroclor 1254 (A1254) significantly reduces serum TH levels but also produces thyroid hormone-like actions in the developing brain, including an increase in expression of the TH-response genes NSP-A, Oct-1, and RC3 in the fetal cortex (18), RC3 and myelin basic protein (19) in the postnatal forebrain, and Purkinje cell protein-2 (20) in the cerebellum, and it can oppose the effect of hypothyroidism on elements of cerebellar histogenesis (20). Thus, PCB may disrupt TH signaling by both causing a reduction in serum total or free T4 or T3 and/or by interacting directly with the TR to act as an agonist. To test this hypothesis, we found that a limited mixture of six PCB congeners significantly reduced serum TH but simultaneously activated the expression of the TH-response gene malic enzyme (ME) mRNA in the liver (21). We then identified specific PCB congeners among this mixture of six PCBs that could activate a TH response element (direct repeat four; DR4) in a luciferase assay (21). However, this activation was observed only in the presence of the entire mixture of six PCB congeners, or in the presence of PCB 126, PCB 105, and PCB 118. Furthermore, using a pharmacological approach, we demonstrated that the agonistic effect of a limited PCB mixture required activation of the aryl hydrocarbon receptor (AhR) and induction of the metabolic enzyme cytochrome P4501A1 (CYP1A1) (21). Thus, we proposed that some PCB congeners (i.e. PCB 126) can activate the AhR causing the induction of CYP1A1, which hydroxylates other PCB (i.e. PCB 105 and/or PCB 118) to form a metabolite(s) that acts as a TR agonist.
Considering these findings, we hypothesize that some PCB congeners (e.g. PCB 126) can activate the AhR, which then induces the expression of the phase I enzyme CYP1A1. CYP1A1 is then required for the hydroxylation of other PCB (e.g. PCB 105 and PCB 118), which then act as TR agonists. If true, the consequences of PCB exposure on TH signaling may differ across tissues, depending on its capacity to metabolize PCB or to take up (transport) PCB metabolites. In addition, considering our previous in vivo work, we predicted that the effects of specific PCB metabolites on TH signaling would be gene specific. To test this hypothesis, we compared the in vivo effects of two combinations of PCB congeners that we previously demonstrated either to activate the TR (PCB 105 and PCB 118) or not (PCB 138 and PCB 153), in the presence or absence of the coplanar, dioxin-like PCB congener (PCB 126) that induces CYP1A1 (21). For a positive control, we used the commercial mixture A1254, for which we have previously characterized several effects on TH signaling in vivo (18–22) using an environmentally relevant dose. In addition, we included a group treated with a dose of PTU calibrated to produce the same reduction in serum TH as that produced by A1254 (23) both to differentiate between PCB effects on serum TH levels and tissue TH signaling and to determine whether serum TH levels predict the consequences of PCB exposure on TH signaling in vivo. We characterized TH signaling in several tissues by measuring the mRNA expression of well-known TH-response genes in these different tissues. Because TH regulates different genes in different tissues, the TH response genes we used in this study are different in the various tissues.
Materials and Methods
Animals and experimental treatments
All animal procedures were performed in accordance with the National Institutes of Health Guidelines for the Care and Use of Laboratory Animals and were approved by the Institutional Animal Care and Use Committee at the University of Massachusetts Amherst (Amherst, MA). Time-pregnant Sprague Dawley rats (n = 42), purchased from Charles River Laboratories (Raleigh, NC), arrived in our facility 2 d after insemination on gestational day (G) 2. The animals were housed in individual polycarbonate plastic cages with cellulose fiber bedding, with a 12-h light, 12-h dark cycle and free access to food (Rodent diet 5001; LabDiet, PMI Nutrition International, St. Louis, MO) and water. Dams were assigned to different treatment groups (Table 1) and trained to eat half of an untreated wafer (vanilla wafers; Keebler Co., Battle Creek, MI) from G2 to G6. The assembled PCB mixtures were purchased from Accustandard (Accustandard Inc., New Haven, CT) and arrived in our laboratory dissolved in methanol. These mixtures were applied to half a wafer as described below, and the methanol was allowed to evaporate in a fume hood for 4–5 h before feeding to the dams. Treatments began on G6 and continued daily until postnatal day (P) 16. The dose was calibrated to dam body weight measured every second day. On P4, litters with more than seven pups were culled to equalize litter size. One male pup from each litter was weighed and killed on each of P14, P15, and P16. Trunk blood was collected for analysis of total serum T4 concentration at the time the animals were killed. Livers and pituitaries were collected on P15, heart on P14, and muscle on P16. The number of different tissues we could collect was restricted to ensure that all tissues were collected within a 2-h window in the morning (from 900 to 1100 h). Our previous work indicated that there were no large differences between the measures taken from pups on P14 and P15 (24), and we show that serum T4 levels were not different among the pups killed on these different days (Supplemental Fig. 1, published on The Endocrine Society's Journals Online web site at http://endo.endojournals.org). Pups in different treatment groups were killed in a fashion that was independent of timing. All tissues were immediately frozen on dry ice and stored at −80 C until further analysis.
Table 1.
Treatment groups
| Treatments | Number of animals | Dose (mg/kg · d)a | Dose of single congener (mg/kg · d) |
|---|---|---|---|
| Control | 5 | 0 | 0 |
| A1254 | 5 | 5 | |
| PCB 105 | 6 | 1.05 | 0.37 |
| PCB 118 | 0.68 | ||
| PCB 105 | 6 | 1.051 | 0.37 |
| PCB 118 | 0.68 | ||
| PCB 126 | 0.001 | ||
| PCB 138 | 6 | 0.475 | 0.3 |
| PCB 153 | 0.175 | ||
| PCB 138 | 6 | 0.476 | 0.3 |
| PCB 153 | 0.175 | ||
| PCB 126 | 0.001 | ||
| PTU | 6 | 3 ppm |
Dose was based on the concentrations of these congeners in A1254 at 5 mg/kg · d (51).
RIA
Serum total T4 was measured by a RIA as described earlier (20) with the following modifications. First, after a 1-h incubation at 37 C with primary antibody, 100 μl of secondary antibody was added (secondary donkey antirabbit antibody magnetic beads; Amersham, GE Healthcare Biosciences, Piscataway, NJ) and incubated at 4 C for 4 h. Tubes were centrifuged to pellet beads and bound 125I-T4 was counted. Nonspecific binding was less than 8% and the intraassay variation was less than 10%. Total T3 was measured in 100 μl of serum added to T3 antibody-coated tubes according to the manufacturer's instructions (MP Biomedicals, Irvine, CA).
RNA isolation
Total RNA was extracted using TRIzol reagent (Invitrogen Corp., Carlsbad, CA) and a Bullet blender homogenizer (Next Advance Inc., Averill Park, NY) according to the manufacturer's instructions. Total RNA was quantified using a NanoDrop spectrometer (Thermo Scientific, Wilmington, DE), and RNA integrity was determined by using an Agilent 2100 bioanalyzer (Agilent Technologies, Inc., Santa Clara, CA). Only RNA with a mRNA integrity number greater than 8.5 was used for these studies (25).
Quantitative RT-PCR
Total RNA (1 μg) was reverse transcribed into cDNA using the high-capacity cDNA reverse transcription kit (Applied Biosystems, Inc., Foster City, CA) following the manufacturer's instructions. For quantitative PCR, a 10-μl reaction contained 1 μl cDNA template, 5 μl FastStart universal SYBR Green master (Rox) kit (Roche Diagnostics Corp., Indianapolis, IN), 400 or 300 nm forward and reverse primers (Supplemental Table 1) each, and nuclease-free water. Quantitative PCR was performed on a Mx3000P real-time PCR machine (Agilent Technologies, Inc., La Jolla, CA). A melting curve analysis provided single-peak verification, and mRNA levels were determined with the change in cycle threshold value method with β-actin as reference gene (26).
Preparation of microsomal fraction
Microsomal fractions were prepared from the same tissues that were used in isolating RNA to be able to compare CYP1A1 mRNA levels with CYP1A1 enzyme activity within the same pup. Microsomal fractions were prepared by homogenizing frozen tissue in a buffer containing 250 mm sucrose, 1 mm dithiothreitol, 0.5 mm EDTA, 10% glycerol, 0.25 mm KCl, and 10 mm HEPES (pH 7.4). The homogenates were centrifuged at 9000 × g for 22 min at 4 C. The resulting supernatant was centrifuged at 105,000 × g for 1 h at 4 C. The pellets were resuspended in the homogenization buffer and microsomal protein concentration was determined using fluorescamine as described by Kennedy and Jones in 1994 (27).
Ethoxyresorufin-O-deethylation (EROD) measurement
Microsomal EROD activities were assayed using a fluorometric microplate reader (Polarstar Optima; BMG Labtech, Inc., Cary, NC) according to the protocol described by Paul et al. in 2010 (28). Each well of the 96-well plate contained 175 μl, including the substrate (50 μl of 1.5 nm ethoxyresorufin), 50 μl of 1:100 diluted samples, and 50 μl of 0.05 mm Tris buffer (pH 8.0). Reduced nicotinamide adenine dinucleotide phosphate (NADPH) (25 μl) was added to each sample-containing well to start the reaction, and the fluorescence signal was measured every 55 sec for 11 min at 37 C. A resorufin standard concentration curve was used and normalized against the concentration of proteins in the sample.
Statistical analysis
GraphPad Prism 4.0 software (GraphPad Software, Inc., San Diego, CA) was used to determine statistical significance between treatment groups using one-way ANOVA followed by Newman-Keuls multiple comparison tests. One pup per litter was used for each end point (liver and pituitary on P15, heart on P14, and skeletal muscle on P16); comparisons across littermates were never made. Linear and nonlinear regressions and Bartlett's test for unequal variance were performed using the same software. For the linear and nonlinear regressions each data point represents the mean of one treatment group ± sem. Statistical outliers in the data sets were identified using the Grubbs test (http://www.graphpad.com/quickcalcs/). Data were then analyzed using a one-way ANOVA followed by Newman-Keuls post hoc tests where appropriate.
Results
TH and body weight in pups on P15
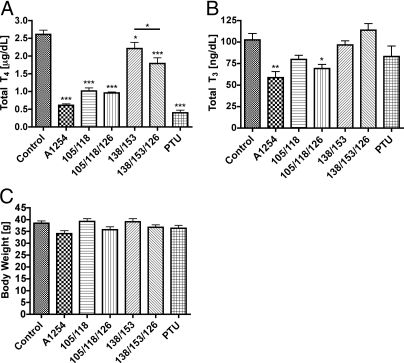
Serum total T4 levels were significantly lower in pups whose dam was exposed to one of the mixtures of PCB compared with pups born to control dams (Fig. 1A). Serum total T4 was lowest in pups exposed to PTU or with the combination of PCB 105/118/126 or A1254. Total T4 levels in pups exposed to PCB 138 and 153, with or without PCB 126, were suppressed to a lesser extent than were those of pups in other exposure groups. Total serum T3 levels were significantly lower only in the pups exposed to A1254 and PCB mixture 105/118/126 compared with the control pups (Fig. 1B). Pup body weight was not significantly different among the treatment groups (Fig. 1C).
Fig. 1.
Exposure effects on serum T4, T3, and body weight of P15 pups. A, Total serum T4 levels in pups on P15. One-way ANOVA revealed significant differences among the treatments for pups (F6,30 = 51.96; P < 0.0001). B, Total serum T3 levels in pups on P15. One-way ANOVA revealed significant differences among the treatments for pups (F6,33 = 6.229; P = 0.0002). C, Body weights of P15 pups were not different among the treatment groups (F6,33 = 2.472; P = 0.0438). Serum total T4 and body weights exhibited identical results in pups killed on P14 and on P16.
Exposure effects on TH signaling in liver
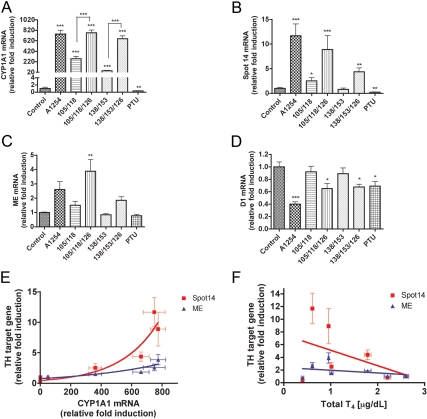
CYP1A1 mRNA
CYP1A1 mRNA levels in A1254-exposed pups were about 750-fold greater than those of control animals (Fig. 2A), and this was similar to that of the combination of PCB 105/118/126 or with PCB 138/153/126. CYP1A1 mRNA levels in pups exposed to the two PCB congeners 105 and 118 were about 300-fold higher than observed in control animals. The pups exposed to PCB 138/153 exhibited a 50-fold higher level of CYP1A1 mRNA expression compared with control pups. CYP1A1 mRNA levels were significantly reduced in animals exposed to PTU compared with control animals.
Fig. 2.
Exposure effects on mRNA expression in liver of P15 pups. A, CYP1A1 mRNA expression in liver was significantly induced by all treatments but PTU (F6,33 = 75.74; P < 0.0001). However, pups treated with PCB mixtures containing PCB126 exhibited significantly greater CYP1A1 mRNA induction than pups in the other groups. B, S14 mRNA levels were significantly different among the treatment groups (F6,31 = 8.727; P < 0.0001). S14 mRNA levels were greatest in animals treated with A1254 and with PCB 105/118/126. C, ME mRNA also was significantly different among the treatment groups (F6,31 = 7.263; P < 0.0001). D, D1 mRNA levels were significantly affected by treatment (F6,33 = 7.023; P < 0.0001). D1 mRNA levels were significantly reduced by A1254, PTU, and those PCB mixtures containing PCB126. Bartlett's test showed unequal variance of data sets for CYP1A1 and S14, so the data were log transformed for the purpose of statistical analysis only. E, Regression analysis revealed a strong nonlinear correlation between CYP1A1 mRNA levels and the mRNA encoding S14 (r2 = 0.5616) and ME (r2 = 0.4691). F, A weaker positive linear correlation was observed between serum total T4 levels and S14 (r2 = 0.1312), but this was not observed with ME mRNA levels (r2 = 0.05417).
Spot 14 (S14) mRNA
S14 is directly regulated by the TR (29) and is involved in lipogenesis (30). Treatment with PTU decreases its expression as we predicted (Fig. 2B) (31). S14 mRNA levels were significantly higher in pups exposed to A1254 (by ∼12-fold) compared with pups in the control group (Fig. 2B), and this was similar to that observed in pups exposed to PCB 105/118/126. Pups exposed to PCB 138/153/126 displayed 4-fold higher levels of S14 mRNA than pups in the control group. The expression levels of S14 mRNA in PCB 105/118 exposed pups were also elevated about 2.5-fold compared with the S14 mRNA levels of controls. Linear regression analysis revealed a tight positive exponential relationship between CYP1A1 and S14 mRNA expression (r2 = 0.5616; Fig. 2E). Because S14 is known to be regulated by TH, we also regressed S14 mRNA against serum total T4, revealing a weaker but still positive relationship (r2 = 0.1312; Fig. 2F).
ME mRNA
ME mRNA expression tended to be increased relative to controls in pups exposed to A1254, but this was not statistically significant (Fig. 2C). Pups exposed to PCB 105/118/126 expressed significantly higher ME mRNA levels than controls. There was no significant reduction of ME expression in the pups in the PTU exposure group compared with the control group (Fig. 2C). Linear regression revealed an exponential positive relationship between mRNA coding for ME and for CYP1A1 (r2 = 0.4691; Fig. 2E). ME expression in the liver was not correlated with serum T4 levels (r2 = 0.05417; Fig. 2F).
Deiodinase type I (D1) mRNA
In pups exposed to A1254, D1 mRNA levels were reduced by 60% compared with those in the control groups (Fig. 2D). The pups exposed to PCB 105/118/126 and PCB 138/153/126 exhibited a significant 30% reduction of D1 expression compared with control pups. Animals exposed to PTU also exhibited 30% lower D1 mRNA levels than unexposed animals. D1 mRNA and CYP1A1 mRNA expression in the PTU group exhibited no correlation (r2 = 0.3665; Table 2), whereas T4 serum levels in the PTU-exposed pups were linearly correlated with D1 expression (r2 = 0.4448; Table 2). In PCB-exposed pups, D1 mRNA expression was significantly correlated with CYP1A1 mRNA (r2 = 0.7428; Table 2), whereas D1 mRNA levels and serum T4 levels were not significantly correlated (r2 = 0.5225; Table 2).
Table 2.
Correlation between D1 mRNA expression and total T4 serum levels or CYP1A1 mRNA expression
| Treatmentsa | Total T4 (μg/dl) | CYP1A1 mRNA (relative fold induction) |
|---|---|---|
| D1 mRNA (relative fold induction) | ||
| PTU | ||
| r2 | 0.4448 | 0.3665 |
| P value | 0.025 | 0.0636 |
| PCB | ||
| r2 | 0.5225 | 0.7428 |
| P value | 0.1046 | 0.0273 |
Analysis for PCB treatment includes all PCB mixtures and control, whereas PTU contains PTU treatment group and control.
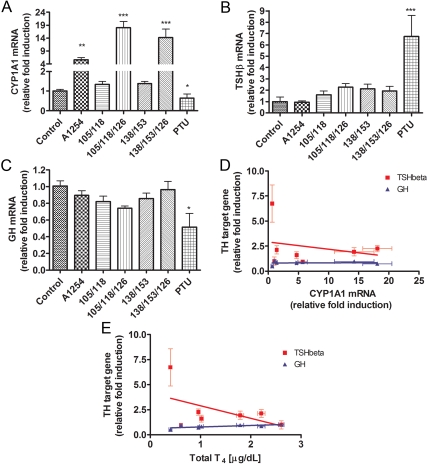
Exposure effects on TH signaling in pituitary
CYP1A1 mRNA
In the pituitary on P15, A1254-exposed pups exhibited about 5.5-fold higher CYP1A1 mRNA levels compared with controls (Fig. 3A). Pups exposed to PCB mixtures with three PCB congeners expressed significantly greater levels of CYP1A1 than controls; 18-fold in pups exposed to PCB 105/118/126 and 14-fold in pups exposed to PCB 138/153/126. Pups exposed to PTU exhibited a significant reduction in CYP1A1 mRNA expression compared with controls. CYP1A1 mRNA levels in pups exposed to PCB 138/153 or PCB 105/118 did not differ from those detected in controls (Fig. 3A).
Fig. 3.
Exposure effects on mRNA expression in pituitary of P15 pups. A, CYP1A1 mRNA was significantly affected by treatments (F6,33 = 28.85; P < 0.0001). Interestingly, mixtures of three PCB congeners containing PCB 126 produced the greatest induction of CYP1A1 mRNA. B, TSHβ mRNA was significantly affected by treatment (F6,33 = 6.172; P = 0.0002); it was increased in PTU-treated animals. C, GH mRNA levels were significantly affected by treatment (F6,33 = 3.345; P = 0.0011); it was significantly reduced in PTU-treated animals. D, CYP1A1 mRNA levels were weakly associated with mRNA expression of TSHβ (r2 = 0.03361) but not GH (r2 = 0.0071). E, Serum total T4 levels were negatively associated with TSHβ mRNA (r2 = 0.1365) and positively associated with GH mRNA levels (r2 = 0.2001).
Thyroid-stimulating hormone (TSH)-β mRNA
The expression of the β-subunit of TSH is increased in hypothyroidism (32). PTU-exposed pups exhibited about 7-fold higher levels of the mRNA coding for the β-subunit of TSH (Fig. 3B) compared with control pups. Animals exposed to the various combinations of PCB displayed no differences in TSHβ mRNA levels compared with controls (Fig. 3B). Regression analysis revealed no correlation between CYP1A1 mRNA expression and TSHβ mRNA (r2 = 0.03361; Fig. 3E). In contrast, the expression of TSHβ mRNA was weakly correlated with serum T4 levels when all groups were included in the analysis (r2 = 0.1365; Fig. 3F).
GH mRNA
Pups exposed to PTU displayed significantly lower GH mRNA levels compared with controls (Fig. 3C) as expected (32, 33). PCB-exposed animals exhibited GH mRNA levels similar to the unexposed controls (Fig. 3C). Regression analysis revealed no correlation between CYP1A1 expression and GH mRNA (r2 = 0.0071; Fig. 3E). GH expression was weakly related to serum T4 levels (r2 = 0.2001; Fig. 3F).
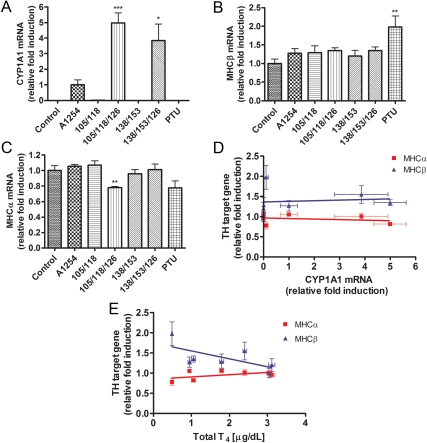
Exposure effects on TH signaling in heart
CYP1A1 mRNA
In the heart on P14, CYP1A1 mRNA expression was not detected in control pups. CYP1A1 mRNA in pups exposed to A1254 was therefore used to assess the relative induction of CYP1A1 mRNA in the other groups. In pups exposed to PCB 105/118/126 or PCB 138/153/126, CYP1A1 mRNA in heart was 5-fold or 4-fold higher than in the A1254-exposed pups, respectively. In pups exposed to the other treatments, CYP1A1 mRNA was not detected (Fig. 4A).
Fig. 4.
Exposure effects on mRNA expression in heart of P14 pups. Heart tissue was collected on P14. A, CYP1A1 mRNA was significantly affected by treatments (F6,33 = 17.41; P < 0.0001). CYP1A1 mRNA levels were not detected in controls, PTU-treated animals, and animals treated with PCB that did not include PCB 126. Therefore, CYP1A1 mRNA levels were normalized with respect to the A1254-treated animals (see text). B, MHCβ mRNA levels were significantly affected by treatment (F6,32 = 3.155; P = 0.0152); they were significantly elevated in PTU-treated animals. C, MHCα mRNA levels were significantly affected by treatment (F6,32 = 4.045; P = 0.004); they were significantly reduced in animals treated with PCB 105/118/126 and tended to be reduced in PTU-treated animals. Bartlett's test showed unequal variance in the data sets for MHCα, so the data were log transformed for the purpose of statistical analysis only. D, There was no significant correlation between CYP1A1 mRNA levels and MHCα (r2 = 0.027) or MHCβ mRNAs (r2 = 0.0035). E, Serum total T4 levels were only weakly correlated with MHCα (r2 = 0.1004) or MHCβ mRNAs (r2 = 0.1421).
Myosin heavy-chain (MHC)-β mRNA
MHCβ is also a direct target for TH action in the heart, and its levels are increased perinatally in hypothyroid animals (34). PTU-exposed pups exhibited significantly greater MHCβ mRNA levels compared with control pups. In contrast, PCB-exposed pups did not exhibit a difference in expression of MHCβ mRNA compared with controls (Fig. 4B). Regression analysis revealed no association between CYP1A1 and MHCβ mRNAs in pup hearts (r2 = 0.0035; Fig. 4F) and an inverse linear correlation between MHCβ mRNA and total T4 levels (r2 = 0.1421; Fig. 4G).
MHCα mRNA
MHCα is also a direct target for TH action in the heart, and its levels are decreased perinatally in hypothyroid animals (35). The expression of MHCα mRNA in pups exposed to PCB 105/118/126 was significantly reduced by nearly 25% compared with MHCα mRNA levels observed in control pups. Pups exposed to other PCB mixtures or PTU did not exhibit a difference in MHCα mRNA expression compared with control animals (Fig. 4C). CYP1A1 mRNA expression in the heart was not correlated with the expression of MHCα (r2 = 0.027; Fig. 4F). Serum T4 levels were positively associated with MHCα mRNA expression (r2 = 0.1004; Fig. 4G).
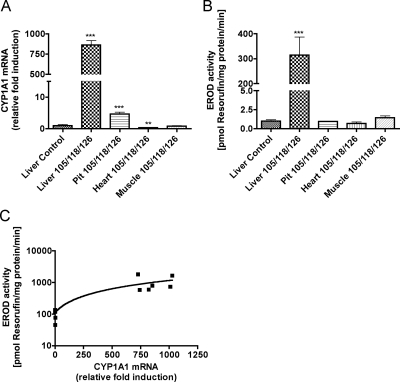
CYP1A1 induction and activity in different tissues
Because we measured CYP1A1 mRNA levels in different tissues in separate assays, it was not possible to directly compare differences in CYP1A1 mRNA expression in different tissues. Therefore, we performed a separate experiment to determine the relative levels of CYP1A1 mRNA levels and enzyme activity in the different tissues. CYP1A1 mRNA levels in the different tissues of PCB 105/118/1260-exposed pups were compared with the basal expression of the livers obtained from the control pups (Fig. 5). CYP1A1 mRNA levels were 860-fold higher in PCB-exposed liver but only 5-fold higher in pituitary compared with the control livers. In heart, CYP1A1 mRNA levels were 70% lower in PCB-exposed pups compared with control livers. In PCB-exposed skeletal muscle, CYP1A1 mRNA levels reached only the basal line observed in control livers (Fig. 5A). EROD was used to measure the activity of CYP1A1 enzyme in the different tissues. EROD activity was elevated relative to control livers (by 320-fold) only in the liver of pups exposed to the PCB mixture 105/118/126 (Fig. 5B). EROD was not significantly elevated above that of the control liver in any other tissue. The CYP1A1 mRNA levels correlated in a linear fashion with the CYP1A1 enzyme activity (r2 = 0.8493; Fig. 5C).
Fig. 5.
Relative induction of CYP1A1 mRNA and activity across tissues. TH signaling appeared to be differentially affected by PCB exposure, perhaps because CYP1A1 mRNA and activity were induced to a different extent. To test this, we measured expression levels of CYP1A1 mRNA (A) and enzyme activity (B) in various tissues of pups, using liver from control animals as our point of reference. CYP1A1 mRNA levels were significantly induced by PCB 105/118/126 in liver and pituitary but not in heart or skeletal muscle (F5,29 = 178; P < 0.0001). Likewise, CYP1A1 enzyme activity (EROD) was induced in liver only (F5,29 = 62.15; P < 0.0001). Bartlett's test showed unequal variance in the data sets for CYP1A1 mRNA and EROD, so the data were log transformed for the purpose of statistical analysis only. C, Linear regression of CYP1A1 mRNA and enzyme activity revealed a strong correlation between the abundance of mRNA and enzyme activity (r2 = 0.8493; y-axis was log scaled).
Discussion
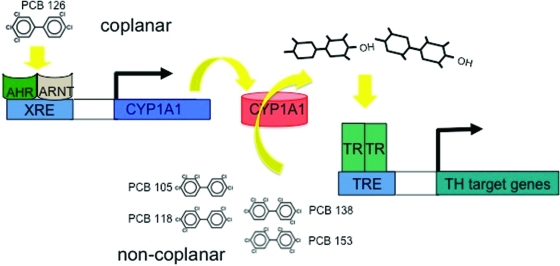
Our current findings strongly support the working hypothesis that selective PCB congeners can act as TH agonists on the TR only if they are metabolized by CYP1A1 (Fig. 6). CYP1A1 mRNA and enzyme activity was highly induced by A1254 in liver, and this was simultaneously associated with a 10-fold increase in S14 mRNA expression, a direct target of TH action, despite a significant reduction in serum total T4. This effect was also observed in animals treated only with the combination of PCB 105, PCB 118, and PCB 126. As predicted, the combination of PCB 105 and PCB 118 was not as effective at enhancing S14 mRNA levels in the absence of PCB 126; this lesser impact on S14 mRNA appears to be related to the lesser induction of CYP1A1 inasmuch as the correlation between CYP1A1 and S14 mRNA expression across groups is high. The combination of PCB 138 and PCB 153 induced a small but significant increase in S14 mRNA expression, and this was fully dependent on the presence of PCB 126. These effects were generally consistent with effects observed on ME mRNA expression in liver but not with effects on the expression of D1 mRNA. We did not observe these effects in the pituitary or heart (Table 3).
Fig. 6.
Congener-specific metabolism by CYP1A1. Noncoplanar PCB, like PCB 105, PCB 118, PCB 138, and PCB 153 can act on TR in vivo, after being metabolized by CYP1A1, which is induced by a dioxin-like compound like PCB 126. TRE, Thyroid hormone-responsive element; ARNT, aryl hydrocarbon receptor nuclear translocator; XRE, xenobiotic-responsive element.
Table 3.
Overview of exposure effects in different tissues
| Exposure effects compared to control | A1254 | PCB 105/118 | PCB 105/118/126 | PCB 138/153 | PCB 138/153/126 | PTU |
|---|---|---|---|---|---|---|
| Total T4 | −−− | −−− | −−− | − | −−− | −−− |
| Total T3 | −− | − | ||||
| Body weight | ||||||
| Liver | ||||||
| CYP1A1 | +++ | ++ | +++ | ++ | +++ | −− |
| S14 | +++ | + | +++ | ++ | −− | |
| ME | ++ | |||||
| D1 | −−− | − | − | − | ||
| Pituitary | ||||||
| CYP1A1 | + | ++ | ++ | − | ||
| TSHβ | ++ | |||||
| GH | − | |||||
| Hearta | ||||||
| CYP1A1 | +++ | + | ||||
| MHCβ | ++ | |||||
| MHCα | −− |
CYP1A1 mRNA expression normalized against A1254. +, Significant increase compared to control; −, significant decrease compared to control.
We were surprised that the combination of PCB 138/153/126 slightly but significantly induced S14 mRNA because these congeners were not effective at inducing luciferase activity from a direct repeat four thyroid hormone-responsive element in GH3 cells (21). It is possible that CYP1A1 activity is higher in the liver than in GH3 cells, resulting in a more effective production of hydroxylated metabolites. Moreover, in GH3 cells, these PCB were incubated with cells for only 24 h, which may not have been enough time for the production of these metabolites. The hydroxylated metabolites of these PCB congeners have been described (36). PCB 105 and PCB 118 form 4-OH-PCB 120 and 4-OH-PCB 107, respectively, and PCB 138 and PCB 153 form 3-OH-PCB 153 and 4-OH-PCB 146, respectively. All of these PCB metabolites have been measured in maternal and cord blood and in breast milk (37). Moreover, 4-OH-PCB107 is uniquely, and negatively, associated with measures of cognitive function in children (38). Thus, PCB metabolism in general may be an important mechanism by which PCB exposure interferes with TH signaling.
PCB exposure increased S14 mRNA levels but suppressed D1 mRNA levels. This is paradoxical if PCBs are acting uniformly as TH agonists because TH positively regulates both S14 and D1 mRNA expression in the liver, as evidenced by previous studies (e.g. Ref. 31) and by the current observation that PTU treatment significantly reduced both D1 and S14 mRNA levels. It is theoretically possible that D1 mRNA expression was reduced because serum T4 levels were reduced in PCB-treated animals. However, this seems unlikely because D1 mRNA levels were not significantly correlated with circulating levels of T4 (r2 = 0.5225; Table 2) when only PCB exposed and control animals were included in the regression but were highly correlated when only the PTU and control groups were included (r2 = 0.4448; Table 2). Considering this, it is possible that PCB metabolites can exert agonistic or antagonistic effects on TR function, depending on the structure of the thyroid hormone responsive element that drives the sensitivity of the gene to TH or perhaps on the structure of the metabolite itself.
Other laboratories have found antagonistic actions of PCB and PCB metabolites. The Koibuchi laboratory has proposed that these environmental chemicals can bind to an allosteric site on the TR, causing it to dissociate from DNA (39). Considering this, it is curious that we have nearly uniformly found agonist effects of PCB on the TR in vitro (40). Although it is unclear how to reconcile these two kinds of in vitro observations, it is important to note that in vivo, we have found what appear to be both agonistic and antagonistic actions of PCB on TH-response genes (20, 21, 41). Thus, different PCB congeners may exert different actions on the TR in a manner that is perhaps dependent on cell context or DNA sequence.
It is unlikely that our current findings are attributable to the estrogenic actions of these PCB. Some PCB congeners have estrogenic properties but estrogen-regulated genes in the liver were not affected by these treatments (Supplemental Fig. 2). In the liver, only A1254 treatment produced a reduction in Lipin1 mRNA levels, indicating that the A1254 contains congeners acting on the estrogen receptor but that these are not PCB 105, PCB 118, PCB 126, PCB 138 or PCB 153. Thus, although these data indicate that A1254 may possess weak estrogenic properties, these properties are not attributable to those PCB congeners or metabolites that selectively exert actions on the TR.
PTU treatment significantly elevated pituitary TSHβ mRNA levels and reduced GH mRNA levels as expected, but this was not observed in animals treated with PCB. Therefore, PCB treatment apparently failed to affect TH signaling in the pituitary gland by either reducing serum T4 or exerting direct actions on the TR. PCB treatment reduces both free and total T4 and T3; therefore, it is enigmatic that serum TSH does not respond (42). Our observations are consistent with the interpretation that the PCB-induced reduction in serum TH is not opposed by a compensatory activation of the hypothalamic-pituitary-thyroid axis. We also measured TRH mRNA levels in the paraventricular nucleus of the animals reported here and observed no effect of PCB treatment (data not shown). These findings in the pituitary are parallel to our findings in heart and skeletal muscle (Supplemental Fig. 3). It is theoretically possible that TH-like PCB metabolites in these other tissues compensates for low TH in the blood. This seems unlikely in that it might require some kind of interaction with the cellular machinery required to control intracellular free T3 levels such that there is a perfect balance with the reduction in serum free T4.
The U.S. Environmental Protection Agency recognizes PCB as unavoidable toxins and recently established a temporary maximum tolerance for PCBs in various food sources such as dairy and meat (43). For example, the maximum tolerance of PCB for dairy milk and cheese is 1.5 mg/liter (or kilograms). It is 3 mg/kg for poultry. In our current studies, we used concentrations in the microgram per kilogram range of individual PCB, and a recent study reported human fetal liver concentrations of total PCBs in the microgram per kilogram range (44). These observations suggest that the human population remains exposed to PCB and that the concentrations we have used in the current experiment are within the range observed in people.
These findings hold potentially important implications for understanding the impacts of persistent organic pollutants on the human population. Several of these chemicals, including flame retardants such as polybrominated diphenyl ethers (45), bisphenol-A and its halogenated derivatives (46–49), and the antibacterial agent triclosan (50), are also known to interact directly with the TR in a fashion similar to what we have found with PCB. Our study suggests that people with elevated liver (or placental) CYP1A1 (e.g. in smokers) may be more sensitive to the these bioaccumulating chemicals than others because of an enhanced ability to produce hydroxylated persistent organic pollutants. Moreover, these data clearly indicate that hormone levels may not reflect the mechanism by which these chemicals exert toxic effects on thyroid hormone signaling. These implications strongly suggest that current practices for evaluating the risk to population health of exposures to these chemicals may not be based on the proper end points.
Acknowledgments
This work was supported by Grant ES010026 from the National Institutes of Environmental Health Sciences.
Disclosure Summary: The authors have nothing to disclose.
Footnotes
- A1254
- Aroclor 1254
- AhR
- aryl hydrocarbon receptor
- CYP1A1
- cytochrome P4501A1
- D1
- deiodinase type I
- EROD
- ethoxyresorufin-O-deethylation
- G
- gestational day
- ME
- malic enzyme
- MHC
- myosin heavy chain
- P
- postnatal day
- PCB
- polychlorinated biphenyl
- PTU
- propylthiouracil
- S14
- spot 14
- TH
- thyroid hormone
- TR
- TH receptor.
References
- 1. Tilson HA, Kodavanti PRS. 1997. Neurochemical effects of polychlorinated biphenyls: an overview and identification of research needs. Neurotoxicology 13:727–744 [PubMed] [Google Scholar]
- 2. Erickson MD. 2001. PCB properties, uses, occurrence, and regulatory history. In: Robertson LW, Hansen LG. eds. PCBs: recent advances in environmental toxicology and health effects. Lexington, KY: The University Press of Kentucky; xii-xxx [Google Scholar]
- 3. Erickson MD. 1986. Analytical Chemistry of PCBs. Boston: Butterworth [Google Scholar]
- 4. Yang JM, Salmon AG, Marty MA. 2010. Development of TEFs for PCB congeners by using an alternative biomarker—thyroid hormone levels. Regul Toxicol Pharmacol 56:225–236 [DOI] [PubMed] [Google Scholar]
- 5. Schantz SL, Widholm JJ, Rice DC. 2003. Effects of PCB exposure on neuropsychological function in children. Environ Health Perspect 111:357–576 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Stewart PW, Lonky E, Reihman J, Pagano J, Gump BB, Darvill T. 2008. The relationship between prenatal PCB exposure and intelligence (IQ) in 9-year-old children. Environ Health Perspect 116:1416–1422 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Stewart PW, Sargent DM, Reihman J, Gump BB, Lonky E, Darvill T, Hicks H, Pagano J. 2006. Response inhibition during differential reinforcement of low rates (DRL) schedules may be sensitive to low-level polychlorinated biphenyl, methylmercury, and lead exposure in children. Environ Health Perspect 114:1923–1929 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Stewart P, Reihman J, Gump B, Lonky E, Darvill T, Pagano J. 2005. Response inhibition at 8 and 9 1/2 years of age in children prenatally exposed to PCBs. Neurotoxicol Teratol 27:771–780 [DOI] [PubMed] [Google Scholar]
- 9. Stewart PW, Reihman J, Lonky EI, Darvill TJ, Pagano J. 2003. Cognitive development in preschool children prenatally exposed to PCBs and MeHg. Neurotoxicol Teratol 25:11–22 [DOI] [PubMed] [Google Scholar]
- 10. Everett CJ, Frithsen IL, Diaz VA, Koopman RJ, Simpson WM, Jr, Mainous AG., 3rd 2007. Association of a polychlorinated dibenzo-p-dioxin, a polychlorinated biphenyl, and DDT with diabetes in the 1999–2002 National Health and Nutrition Examination Survey. Environ Res 103:413–418 [DOI] [PubMed] [Google Scholar]
- 11. Zoeller TR. 2010. Environmental chemicals targeting thyroid. Hormones (Athens) 9:28–40 [DOI] [PubMed] [Google Scholar]
- 12. Herbstman JB, Sjödin A, Apelberg BJ, Witter FR, Halden RU, Patterson DG, Panny SR, Needham LL, Goldman LR. 2008. Birth delivery mode modifies the associations between prenatal polychlorinated biphenyl (PCB) and polybrominated diphenyl ether (PBDE) and neonatal thyroid hormone levels. Environ Health Perspect 116:1376–1382 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Salay E, Garabrant D. 2009. Polychlorinated biphenyls and thyroid hormones in adults: a systematic review appraisal of epidemiological studies. Chemosphere 74:1413–1419 [DOI] [PubMed] [Google Scholar]
- 14. Kato Y, Ikushiro SI, Takiguchi R, Haraguchi K, Koga N, Uchida S, Sakaki T, Yamada S, Kanno J, Degawa M. 2007. A novel mechanism for polychlorinated biphenyls-induced decrease in serum thyroxine level in rats. Drug Metab Dispos 35:1949–1955 [DOI] [PubMed] [Google Scholar]
- 15. Goldey ES, Kehn LS, Lau C, Rehnberg GL, Crofton KM. 1995. Developmental exposure to polychlorinated biphenyls (Aroclor 1254) reduces circulating thyroid hormone concentrations and causes hearing deficits in rats. Toxicol Appl Pharmacol 135:77–88 [DOI] [PubMed] [Google Scholar]
- 16. Zahalka EA, Ellis DH, Goldey ES, Stanton ME, Lau C. 2001. Perinatal exposure to polychlorinated biphenyls Aroclor 1016 or 1254 did not alter brain catecholamines nor delayed alternation performance in Long-Evans rats. Brain Res Bull 55:487–500 [DOI] [PubMed] [Google Scholar]
- 17. Goldey ES, Kehn LS, Rehnberg GL, Crofton KM. 1995. Effects of developmental hypothyroidism on auditory and motor function in the rat. Toxicol Appl Pharmacol 135:67–76 [DOI] [PubMed] [Google Scholar]
- 18. Gauger KJ, Kato Y, Haraguchi K, Lehmler HJ, Robertson LW, Bansal R, Zoeller RT. 2004. Polychlorinated biphenyls (PCBs) exert thyroid hormone-like effects in the fetal rat brain but do not bind to thyroid hormone receptors. Environ Health Perspect 112:516–523 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Zoeller RT, Dowling AL, Vas AA. 2000. Developmental exposure to polychlorinated biphenyls exerts thyroid hormone-like effects on the expression of RC3/neurogranin and myelin basic protein messenger ribonucleic acids in the developing rat brain. Endocrinology 141:181–189 [DOI] [PubMed] [Google Scholar]
- 20. Bansal R, Zoeller RT. 2008. Polychlorinated biphenyls (Aroclor 1254) do not uniformly produce agonist actions on thyroid hormone responses in the developing rat brain. Endocrinology 149:4001–4008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Gauger KJ, Giera S, Sharlin DS, Bansal R, Iannacone E, Zoeller RT. 2007. Polychlorinated biphenyls 105 and 118 form thyroid hormone receptor agonists after cytochrome P4501A1 activation in rat pituitary GH3 cells. Environ Health Perspect 115:1623–1630 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Bansal R, You SH, Herzig CT, Zoeller RT. 2005. Maternal thyroid hormone increases HES expression in the fetal rat brain: an effect mimicked by exposure to a mixture of polychlorinated biphenyls (PCBs). Brain Res 156:13–22 [DOI] [PubMed] [Google Scholar]
- 23. Sharlin DS, Tighe D, Gilbert ME, Zoeller RT. 2008. The balance between oligodendrocyte and astrocyte production in major white matter tracts is linearly related to serum total thyroxine. Endocrinology 149:2527–2536 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Sharlin DS, Gilbert ME, Taylor MA, Ferguson DC, Zoeller RT. 2010. The nature of the compensatory response to low thyroid hormone in the developing brain. J Neuroendocrinol 22:153–165 [DOI] [PubMed] [Google Scholar]
- 25. Fleige S, Pfaffl MW. 2006. RNA integrity and the effect on the real-time qRT-PCR performance. Mol Aspects Med 27:126–139 [DOI] [PubMed] [Google Scholar]
- 26. Pfaffl MW. 2001. A new mathematical model for relative quantification in real-time RT-PCR. Nucleic Acids Res 29:e45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Kennedy SW, Jones SP. 1994. Simultaneous measurement of cytochrome P4501A catalytic activity and total protein concentration with a fluorescence plate reader. Anal Biochem 222:217–223 [DOI] [PubMed] [Google Scholar]
- 28. Paul KB, Hedge JM, DeVito MJ, Crofton KM. 2010. Short-term exposure to triclosan decreases thyroxine in vivo via upregulation of hepatic catabolism in young Long-Evans rats. Toxicol Sci 113:367–379 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Jump DB, Tao TY, Towle HC, Oppenheimer JH. 1986. Dissociation of hepatic messenger ribonucleic acidS14 levels and nuclear transcriptional rates in suckling rats. Endocrinology 118:1892–1896 [DOI] [PubMed] [Google Scholar]
- 30. Zhu Q, Mariash A, Margosian MR, Gopinath S, Fareed MT, Anderson GW, Mariash CN. 2001. Spot 14 gene deletion increases hepatic de novo lipogenesis. Endocrinology 142:4363–4370 [DOI] [PubMed] [Google Scholar]
- 31. Takeuchi Y, Murata Y, Sadow P, Hayashi Y, Seo H, Xu J, O'Malley BW, Weiss RE, Refetoff S. 2002. Steroid receptor coactivator-1 deficiency causes variable alterations in the modulation of t(3)-regulated transcription of genes in vivo. Endocrinology 143:1346–1352 [DOI] [PubMed] [Google Scholar]
- 32. Rodríguez-García M, Jolín T, Santos A, Pérez-Castillo A. 1995. Effect of perinatal hypothyroidism on the developmental regulation of rat pituitary growth hormone and thyrotropin genes. Endocrinology 136:4339–4350 [DOI] [PubMed] [Google Scholar]
- 33. Walker P, Dussault JH. 1980. Hypothalamic somatostatin and pituitary and serum growth hormone concentrations during postnatal development in rats exposed chronically to propylthiouracil or a low iodine diet. J Dev Physiol 2:111–117 [PubMed] [Google Scholar]
- 34. Sweadner KJ, McGrail KM, Khaw BA. 1992. Discoordinate regulation of isoforms of Na, K-ATPase and myosin heavy chain in the hypothyroid postnatal rat heart and skeletal muscle. J Biol Chem 267:769–773 [PubMed] [Google Scholar]
- 35. Chizzonite RA, Zak R. 1984. Regulation of myosin isoenzyme composition in fetal and neonatal rat ventricle by endogenous thyroid hormones. J Biol Chem 259:12628–12632 [PubMed] [Google Scholar]
- 36. Sjodin A, Tullsten AK, Klasson Wehler E. 1998. Identification of the parent compounds to selectively retained hydroxylated PCB metabolites in rat blood plasma. Organohalogen Compounds 37:365–368 [Google Scholar]
- 37. Guvenius DM, Aronsson A, Ekman-Ordeberg G, Bergman A, Norén K. 2003. Human prenatal and postnatal exposure to polybrominated diphenyl ethers, polychlorinated biphenyls, polychlorobiphenylols, and pentachlorophenol. Environ Health Perspect 111:1235–1241 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Park HY, Park JS, Sovcikova E, Kocan A, Linderholm L, Bergman A, Trnovec T, Hertz-Picciotto I. 2009. Exposure to hydroxylated polychlorinated biphenyls (OH-PCBs) in the prenatal period and subsequent neurodevelopment in eastern Slovakia. Environ Health Perspect 117:1600–1606 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Miyazaki W, Iwasaki T, Takeshita A, Tohyama C, Koibuchi N. 2008. Identification of the functional domain of thyroid hormone receptor responsible for polychlorinated biphenyl-mediated suppression of its action in vitro. Environ Health Perspect 116:1231–1236 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. You SH, Gauger KJ, Bansal R, Zoeller RT. 2006. 4-Hydroxy-PCB106 acts as a direct thyroid hormone receptor agonist in rat GH3 cells. Mol Cell Endocrinol 257–258:26–34 [DOI] [PubMed] [Google Scholar]
- 41. Sharlin DS, Bansal R, Zoeller RT. 2006. Polychlorinated biphenyls exert selective effects on cellular composition of white matter in a manner inconsistent with thyroid hormone insufficiency. Endocrinology 147:846–858 [DOI] [PubMed] [Google Scholar]
- 42. Martin L, Klaassen CD. 2010. Differential effects of polychlorinated biphenyl congeners on serum thyroid hormone levels in rats. Toxicol Sci 117:36–44 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. 2010. 21CFR109.30. Code of federal regulations. In: U.S. Environmental Protection Agency, ed. Washington, D.C.: U.S. Government [Google Scholar]
- 44. Doucet J, Tague B, Arnold DL, Cooke GM, Hayward S, Goodyer CG. 2009. Persistent organic pollutant residues in human fetal liver and placenta from Greater Montréal, Québec: a longitudinal study from 1998 through 2006. Environ Health Perspect 117:605–610 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Li F, Xie Q, Li X, Li N, Chi P, Chen J, Wang Z, Hao C. 2010. Hormone activity of hydroxylated polybrominated diphenyl ethers on human thyroid receptor-β: in vitro and in silico investigations. Environ Health Perspect 118:602–606 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Heimeier RA, Shi YB. 2010. Amphibian metamorphosis as a model for studying endocrine disruption on vertebrate development: effect of bisphenol A on thyroid hormone action. Gen Comp Endocrinol 168:181–189 [DOI] [PubMed] [Google Scholar]
- 47. Sun H, Shen OX, Wang XR, Zhou L, Zhen SQ, Chen XD. 2009. Anti-thyroid hormone activity of bisphenol A, tetrabromobisphenol A and tetrachlorobisphenol A in an improved reporter gene assay. Toxicol In Vitro 23:950–954 [DOI] [PubMed] [Google Scholar]
- 48. Moriyama K, Tagami T, Akamizu T, Usui T, Saijo M, Kanamoto N, Hataya Y, Shimatsu A, Kuzuya H, Nakao K. 2002. Thyroid hormone action is disrupted by bisphenol A as an antagonist. J Clin Endocrinol Metab 87:5185–5190 [DOI] [PubMed] [Google Scholar]
- 49. Kitamura S, Jinno N, Ohta S, Kuroki H, Fujimoto N. 2002. Thyroid hormonal activity of the flame retardants tetrabromobisphenol A and tetrachlorobisphenol A. Biochem Biophys Res Commun 293:554–559 [DOI] [PubMed] [Google Scholar]
- 50. Veldhoen N, Skirrow RC, Osachoff H, Wigmore H, Clapson DJ, Gunderson MP, Van Aggelen G, Helbing CC. 2006. The bactericidal agent triclosan modulates thyroid hormone-associated gene expression and disrupts postembryonic anuran development. Aquat Toxicol 80:217–227 [DOI] [PubMed] [Google Scholar]
- 51. Frame GM, Cochran JW, Bowadt SS. 1996. Complete PCB congener distributions for 17 Aroclor mixtures determined by 3 HRGC systems optimized for comprehensive, quantitative, congener-specific analysis. J High Resol Chromatogr 19:657–668 [Google Scholar]