Abstract
Estrogen has pronounced effects on thermoregulation, but the anatomic sites of integration between the reproductive and thermoregulatory axes are unknown. In this study, we tested whether estradiol-17β (E2) treatment would alter the activity of thermoregulatory brain regions responding to mild changes in ambient temperature (TAMBIENT). Core and tail skin temperatures were recorded at the ambient temperatures of 20, 24, or 31 C in ovariectomized (OVX) rats with and without E2. Neuronal activity was evaluated by counting the number of Fos-immunoreactive cells in the brains of rats killed 90 min after exposure to one of the three ambient temperatures. Of 14 brain areas examined, the median preoptic nucleus (MnPO) was the only site that exhibited increased Fos immunoreactivity at the high TAMBIENT of 31 C. At 24 C, OVX rats exhibited increased numbers of MnPO Fos-immunoreactive cells, compared with OVX + E2 rats. Interestingly, tail skin vasomotion and MnPO Fos expression were affected in a similar manner by TAMBIENT and E2 treatment. In the arcuate nucleus and anteroventral periventricular nucleus (AVPV), Fos immunoreactivity was highest at the low TAMBIENT of 20 C, with inhibitory (arcuate nucleus) and stimulatory (AVPV) effects of E2. No other areas responded to both TAMBIENT and E2 treatment. These results implicate the MnPO, the arcuate nucleus, and the AVPV as sites of integration between the reproductive and thermoregulatory axes. Combined with studies showing the importance of MnPO neurons in heat-defense pathways, the MnPO emerges as a likely site for E2 modulation of thermoregulatory vasomotion.
Thermoregulation is altered by the levels of ovarian hormones throughout a woman's life span. Changes occur across the menstrual cycle, with oral contraceptive use, pregnancy, and most dramatically as a result of the menopause. During the menopausal transition and early postmenopausal phase, most women experience hot flushes, a syndrome consisting of the episodic activation of heat dissipation mechanisms (1). Hot flushes are secondary to estrogen withdrawal because they are triggered by conditions that lower serum estrogens and are effectively treated by estrogen replacement (2, 3). Hot flushes are temporally linked to pulses of LH in peripheral plasma (4), although clinical studies indicate that neither LH nor GnRH is required for hot flush symptoms to occur (5, 6). These data provide evidence of a central neural trigger for hot flushes related to the hypothalamic control of pulsatile GnRH secretion (7). Despite the high prevalence of this syndrome, little is understood about the etiology of hot flushes or the biological effects of estrogens on thermoregulation in general.
Tail skin vasodilatation is an important heat dissipation effector in rodents (8). Fluctuations in tail skin temperature are characteristic of normal thermoregulation within the thermoneutral zone, in which body temperature is regulated only by dry heat loss mechanisms such as active changes in skin vasomotion (9). In intact cycling rats, these large fluctuations in tail skin temperature are reduced on the night of proestrous (10), consistent with a shift in the thermoneutral zone with increasing serum estrogen (11). Of note, the average tail skin temperature is increased by ovariectomy, and this effect is reversed by treatment with estrogens (10–17). Estrogen treatment of ovariectomized (OVX) rats also alters the ambient temperature (TAMBIENT) threshold for the initiation of skin vasodilatation, resulting in a shift in the thermoneutral zone (11). Moreover, increased heat escape behavior occurs in OVX rats, compared with estrogen-replaced controls (13). Thus, OVX rats demonstrate increased activation of heat dissipation effectors, compared with OVX rats with estrogen replacement. Although the mean increase in tail skin temperature induced by ovariectomy of rats does not mimic the episodic syndrome of flushing in women (12), studies of these phenomena are useful in understanding how estrogen modifies thermoregulatory heat-defense mechanisms from a basic science perspective.
Skin vasodilatation and other thermoregulatory effectors are hierarchically controlled by the hypothalamus, with the preoptic area playing a critical role (18, 19). Independent autonomic effectors involved in heat defense are regulated by preoptic neurons that integrate information from core body and skin temperature as well as other physiological conditions (19, 20). Previous studies have shown that estradiol-17β (E2) modulates the electrical activity of warm-sensitive preoptic neurons in tissue slice preparations (21). Moreover, estrogen receptor-α mRNA is expressed in many nuclei in the rostral hypothalamus, including the median preoptic nucleus (MnPO), anteroventral periventricular nucleus (AVPV), and medial preoptic area (22). Based on these data, we hypothesized that neurons in the rostral hypothalamus/preoptic area could mediate the effects of estrogens on tail skin vasodilatation. However, it is not known whether E2 modifies the activity of warm-sensitive thermoregulatory centers in vivo, and little information is available on the sites of integration between the reproductive and thermoregulatory axes.
In the present study, we determined whether E2 treatment of OVX rats modified the activation of hypothalamic regions that respond to mild changes in ambient temperature. Fos immunohistochemistry was chosen as a marker of activity based on numerous studies showing increased hypothalamic Fos expression in response to a variety of reproductive (23) or thermoregulatory stimuli (24–27). Ambient temperatures were selected near the upper and lower boundaries of the thermoneutral zone, based on our previous studies (11). Additional core and tail skin recordings were performed to confirm the thermoregulatory state of the animals at these selected ambient temperatures. Because tail skin vasodilatation is elevated at warm ambient temperatures and increased in OVX rats without estrogen replacement (11, 13–16, 28), an important goal was to determine the effects of estrogen on Fos immunoreactivity in hypothalamic thermoregulatory nuclei that responded to high ambient temperatures.
Materials and Methods
Animal care procedures
Animal protocols were approved by the University of Arizona Animal Care and Use Committee and conformed to National Institutes of Health guidelines. Female Sprague Dawley rats (150–200 g, Harlan Sprague Dawley; Harlan Laboratories, Indianapolis, IN) were housed with free access to food and water on a 12-h light, 12-h dark schedule (lights on at 0700 h) in the Animal Care Facility at the University of Arizona. Because high levels of phytoestrogens in standard rat chow can affect tail skin temperatures (15, 29), rats were fed low-phytoestrogen rat chow (Harlan Teklad 2014; Harlan Laboratories, Houston, TX).
Rats were ovariectomized under general anesthesia and implanted ip with a Subcue temperature data logger (Canadian Analytical Technologies, Inc., Calgary, Alberta, Canada). Ten days later, rats were anesthetized with isoflurane gas and implanted with two SILASTIC capsules (sc; Dow Corning, Midland, MI) (30 mm, 1.57 mm inner diameter, 3.18 mm outer diameter; Dow Corning, Midland, MI) containing either E2 in sesame oil (180 μg/ml, OVX + E2 group) or saline (OVX group). The SILASTIC capsules were plugged on each end with 5-mm wood sticks and silicone glue, resulting in an effective capsule length of 20 mm. Similar SILASTIC capsules have been shown to deliver physiological concentrations of E2 for 5 wk after implantation (30). In our hands, this treatment results in serum E2 levels of 24.8 ± 0.6 pg/ml and suppression of serum LH when measured 3–4 wk after implantation (11). Moreover, we have previously shown that this E2 treatment alters the thermoregulatory system in OVX rats (11). Saline was used as a vehicle control instead of sesame oil because of initial concerns that phytoestrogens in the sesame oil could exert an independent effect on thermoregulation. However, we have subsequently detected no difference in tail skin temperature or core temperatures of OVX rats with sesame oil capsules vs. saline capsules (10).
Experiment 1: measurements of core and tail skin temperatures of OVX + E2 and OVX rats exposed to ambient temperatures of 20, 24, and 31 C
These experiments were performed between 0800 and 1500 h, 9–25 d after E2 or saline capsule implantation. To minimize stress, rats were habituated to the recording procedures for at least three sessions before data acquisition. An IT-18 thermocouple (Physitemp Inc., Clifton, NJ) was taped 5–6 cm from the base of the tail. This thermocouple was protected with a Nalgene T-tube fitted over 1 in. of the rat's tail and polyethylene tubing over the wire. Rats were placed in a grid plastic cage (6 in. × 6 in. × 4 in.) with food and an attached water bottle. The thermocouple was threaded through the cage roof, taped to a swivel, and inserted into a TC4000 or QuadTemp data logger (Madgetech, Inc., Contoocook, NH). This experimental set-up allowed the rats freedom of movement, unlimited access to food and water, and the ability to regulate temperature by changes in posture and grooming. The plastic cages were placed in an environmental chamber (Forma model 3940; Thermo Scientific, San Jose, CA) with ambient temperatures (TAMBIENT) set at 20, 24, or 31 C and humidity set at 50%. Core temperature (TCORE) was recorded every 10–15 min and tail skin temperature (TSKIN) was recorded every 5 sec. A separate thermocouple was placed in the environmental chamber to record TAMBIENT. Data were recorded after 1 h of acclimation to the environmental chamber for each TAMBIENT. In a first protocol, temperatures of 12 OVX + E2 and 11 OVX rats were recorded for a 1-h session at each TAMBIENT in a random order, with two recordings per day (11). In a second protocol, 11 OVX + E2 and 11 OVX rats were recorded twice at each TAMBIENT for 2–3 h, with one recording per day and 1 wk separating the two sessions at each TAMBIENT. The results of these two protocols were not significantly different, and these data were pooled. In addition, the length of time after estrogen replacement did not affect the TCORE or TSKIN results, consistent with our recent demonstration that the effects of E2 on the tail skin temperature in OVX rats during the light-phase are stable from 8 to 21 d after treatment (10).
Data analysis
Skin vasomotion was indirectly evaluated by the heat loss index (HLI) (9): HLI = (TSKIN − TAMBIENT)/ (TCORE − TAMBIENT).
During tail skin vasoconstriction, HLI decreases to a theoretical minimum of 0 due to TSKIN approaching TAMBIENT. During tail skin vasodilatation, HLI increases to a theoretical maximum of 1 due to TSKIN approaching TCORE (9). Average TCORE and HLI were calculated for each rat at each TAMBIENT, and these values were used to generate group averages. Differences between groups were tested by two-way ANOVA (TAMBIENT × E2) using SigmaPlot Software (Systat Software, Inc., San Jose, CA). If there were significant overall effects of TAMBIENT or E2, post hoc comparisons were made using Tukey's multiple comparisons tests (α = 0.05).
Experiment 2: analysis of Fos-immunoreactive (ir) cells in the brain nuclei of OVX + E2 and OVX rats exposed to ambient temperatures of 20, 24, and 31 C
Fifty rats were ovariectomized and implanted with sc capsules as described above (OVX + E2, n = 25; OVX, n = 25). To minimize stress, rats were habituated to the experimental procedures in at least five sessions over 10 d. Between 10 and 21 d after capsule implantation, each rat was placed in a plastic grid cage, with food and water, and this cage was placed inside an environmental chamber set at 20 C. After 1–3 h of acclimation at 20 C, the chamber temperature was either changed to 24 or 31 C or kept at 20 C. After 90 min, each rat was removed, administered an overdose of sodium pentobarbital (100 mg/kg, ip), and perfused via the ascending aorta with heparinized saline followed by 4% paraformaldehyde in 0.1 m PBS (pH 7.4). The rats were killed between 1000 and 1400 h. The brain was removed, postfixed for 1 h in 4% paraformaldehyde, and cryoprotected in ascending solutions of sucrose in PBS (pH 7.4) (10, 20, and 30%). Each brain was blocked using a rat brain matrix (Braintree Scientific, Braintree, MA), and frozen sections in the coronal plane (40 μm thickness) were then taken with a sliding microtome. Sections were stored at −20 C in cryoprotectant solution (31).
The Fos antiserum (AB-5, lot D00007099; EMD Biosciences Inc., La Jolla, CA) was generated in the rabbit against amino acids 4–17 of human c-Fos. This specificity of this antibody has been verified by preabsorption experiments (32, 33) and negative staining of sections from Fos knockout mice (32). Every 10th section was Nissl stained to aid with matching sections to plates of a rat brain atlas (34). Sections from the same levels of the brain were processed simultaneously for Fos immunohistochemistry. Free-floating brain sections were rinsed in 0.1 m PBS (pH 7.4) (3 × 10 min), treated with 0.3% H2O2 in PBS for 30 min, and rinsed again. Slices were then immersed for 60 min in blocking solution (3% normal goat serum in PBS with 0.3% Triton X-100) and incubated for 40 h at 4 C with a Fos antiserum in blocking solution (1:20,000, optimal concentration determined by prior titration). Slices were rinsed, incubated for 2 h with biotinylated goat antirabbit IgG (1:250; Vector Laboratories, Burlingame, CA) in blocking solution, and rinsed again. Sections were treated with a Vectastain Elite ABC kit for 90 min and rinsed, and then Fos immunoreactivity was visualized with a standard diaminobenzidine reaction (0.5 mg/ml diaminobenzidine, 0.01% H2O2 in PBS, 5–6 min). Control sections with omission of the primary antibody did not exhibit staining.
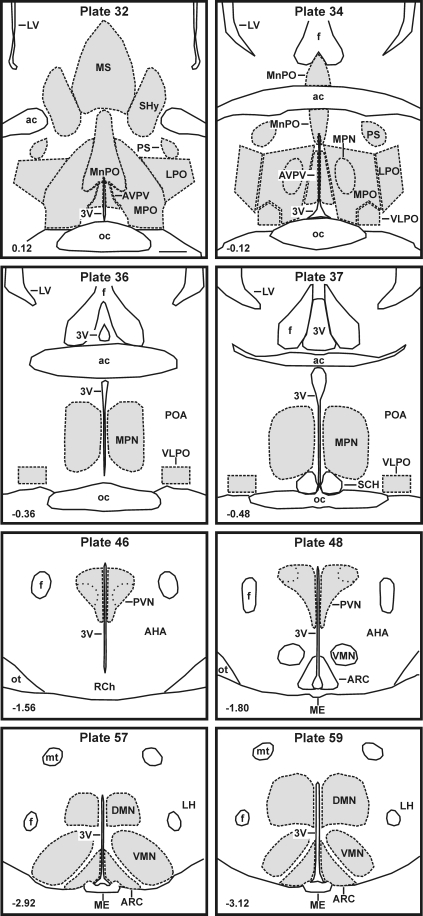
Fos-ir neurons were identified by dark brown nuclear staining and were counted in selected brain areas using an image-combining computer microscope consisting of a motorized stage (LUDL Electronics, Hawthorne, NY), Lucivid miniature monitor (MicroBrightfield, Colchester, VT), and Neurolucida software (MicroBrightfield). The borders of specific brain areas were manually digitized with the assistance of Nissl-stained sections and a rat brain atlas (34). We focused on brain areas of the rostral hypothalamus and adjacent septal regions based on their role in thermoregulation: AVPV, lateral preoptic area, medial preoptic area, medial preoptic nucleus, medial septal nucleus, MnPO, parastrial nucleus, septohypothalamic nucleus, and the ventrolateral preoptic area. Additional nuclei that have been previously implicated in thermoregulatory (dorsomedial nucleus, paraventricular nucleus) or reproductive processes (arcuate nucleus, ventromedial nucleus) were studied. The AVPV, lateral preoptic area, MnPO, medial septum, medial preoptic area, parastrial nucleus, and septohypothalamic nucleus were outlined in brain slices matched to plates 32 and 34 of the rat brain atlas (34); the medial preoptic nucleus in sections matched to plates 36 and 37; the paraventricular nucleus in sections matched to plates 46 and 48; and the arcuate nucleus, dorsomedial nucleus, and ventromedial nucleus to plates 57 and 59 (Fig. 1). The ventrolateral preoptic area was delineated by a 300- × 200-mm box combined with a 300- × 100-mm triangle (plate 34, Fig. 1) or a 500- × 300-mm box (plates 36 and 37, Fig. 1) based on previous studies (34–36).
Fig. 1.
Line drawings adapted from the Paxinos and Watson rat brain atlas (34) showing the location of areas analyzed for Fos immunoreactivity. The corresponding atlas plate is listed in the top center, and the distance from bregma is at the bottom left of each drawing. Analyzed regions (shaded areas) were outlined with the aid of Nissl-stained sections. Scale bar (upper left drawing), 500 μm (applies to all). 3V, Third ventricle; ac, anterior commissure; AHA, anterior hypothalamic area; ARC, arcuate nucleus; DMN, dorsomedial nucleus; f, fornix; LH, lateral hypothalamus; LPO, lateral preoptic area; LV, lateral ventricle; ME, median eminence; MPN, medial preoptic nucleus; MPO, medial preoptic area; MS, medial septal nucleus; mt, mammillothalamic tract; oc, optic chiasm; ot, optic tract; POA, preoptic area; PS, parastrial nucleus; PVN, paraventricular nucleus; RCh, retrochiasmatic area; SCH, suprachiasmatic nucleus; SHy, septohypothalamic nucleus; VLPO, ventrolateral preoptic area; VMN, ventromedial nucleus.
During data acquisition and analysis, experimenters were blinded to the experimental groups. Fos-ir cells were manually mapped and counted within the digitized border of each brain region. All brain regions were counted bilaterally (one to three sections per brain area) except for the MnPO, which is a midline nucleus. These data were averaged to give the number of cells per unilateral section, or in case of the MnPO, the number of cells per section. For simplicity, these data are reported as the number of cells per section. The number of cells per section was calculated for each rat, and these numbers were used to generate group means and se. Data were analyzed using SigmaPlot software with two-way ANOVA (TAMBIENT × E2). If there were significant overall effects for TAMBIENT or E2, post hoc comparisons were made using Tukey's multiple comparison test (α = 0.05).
Results
Thermoregulatory status of OVX + E2 and OVX rats exposed to ambient temperatures of 20, 24, and 31 C
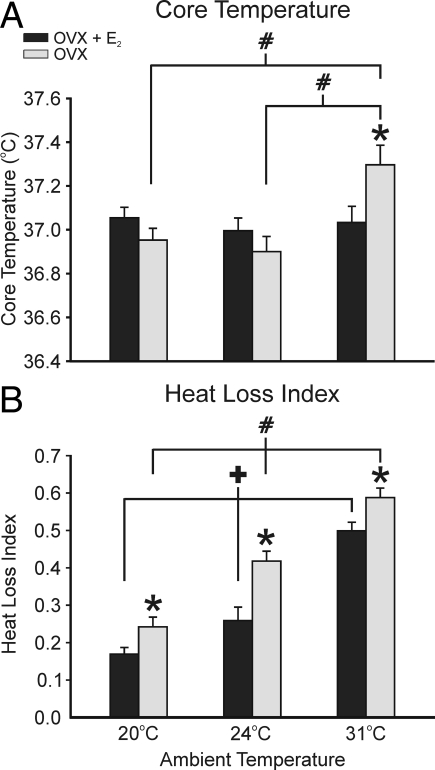
Core temperatures of OVX + E2 rats were normothermic at the environmental temperatures of 20, 24, and 31 C (37.06 ± 0.05, 37.00 ± 0.06, and 37.03 ± 0.07, respectively, mean ± sem). The TCORE of OVX rats was not significantly different from the OVX + E2 groups at the ambient temperatures of 20 and 24 C (36.95 ± 0.05 and 36.90 ± 0.07 C). However, at the highest TAMBIENT of 31 C, the average TCORE of OVX animals was significantly increased (with respect to all other OVX and OVX + E2 groups) to 37.30 ± 0.09 C (Fig. 2A).
Fig. 2.
Average TCORE (A) and HLI (B) in OVX + E2 and OVX rats exposed to ambient temperatures of 20, 24, or 31 C. A, At 20 and 24 C, the average core temperatures were not significantly different between the OVX + E2 and OVX animals. At the TAMBIENT of 31 C, the OVX rats (but not the OVX + E2 rats) exhibited a significant increase in TCORE compared with OVX rats at 20 and 24 C and OVX + E2 rats. B, HLI was significantly increased from 20 to 24 C and from 24 to 31 C in both the OVX + E2 and the OVX rats, reflecting increased skin vasodilatation at higher ambient temperatures. At all ambient temperatures, the OVX rats exhibited a significantly higher HLI than the OVX + E2 rats (n = 22–23 rats/group). Values represent means ± sem. *, Significant difference between OVX + E2 and OVX rats; #, significant effect of TAMBIENT in OVX rats; +, significant effect of TAMBIENT in OVX + E2 rats.
In both OVX + E2 and OVX rats, the HLI, a measure of tail skin vasomotion, was significantly increased from 20 to 24 C and from 24 to 31 C. At all three ambient temperatures, the HLI was significantly elevated in the OVX vs. OVX + E2 rats (Fig. 2B).
The number of Fos-ir cells was significantly increased in the MnPO at the warm TAMBIENT of 31 C and increased in OVX, compared with the OVX + E2-treated rats at 24 C
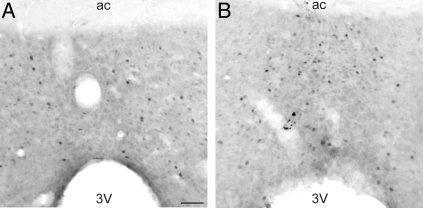
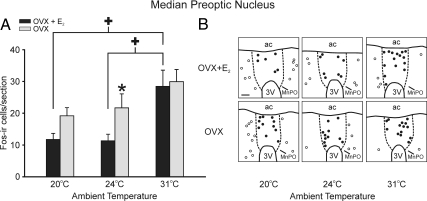
Of 14 examined brain areas, the MnPO was the only area that exhibited significantly more Fos-ir cells in rats exposed to the TAMBIENT of 31 C (Figs. 3 and 4). The number of MnPO Fos-ir cells was the most elevated at the highest TAMBIENT in both OVX + E2 and OVX rats. At the ambient temperature of 24 C, OVX rats had significantly greater numbers of Fos-ir cells in the MnPO, compared with OVX + E2 rats. A similar elevation of Fos immunoreactivity in OVX animals was also observed at the TAMBIENT of 20 C, but this effect did not reach significance (Fig. 4). Comparison of Figs. 4 and 2B shows that the number of MnPO Fos-ir cells and the HLI, reflecting skin vasomotion, were affected in a similar manner by both TAMBIENT and E2 treatment.
Fig. 3.
Photomicrographs of Fos immunoreactivity in the MnPO of OVX + E2 rats exposed to the ambient temperatures of 24 C (A) and 31 C. (B). The MnPO was the only area (of 14 areas examined) that demonstrated significantly increased numbers of Fos-ir cells in response to the higher ambient temperature. 3V, Third ventricle; ac, anterior commissure. Scale bar in A, 50 μm and applies to both A and B.
Fig. 4.
A, Average number of Fos-ir cells per section in the MnPO in OVX + E 2 and OVX rats exposed to ambient temperatures of 20, 24, and 31 C. Fos immunoreactivity was significantly increased in the MnPO of OVX + E 2 rats at the TAMBIENT of 31 C, compared with rats exposed to ambient temperatures of 20 and 24 C. At 24 C, Fos immunoreactivity was significantly elevated in the OVX rats, compared with the OVX + E2 rats (n = 8 rats/group). Values represent means ± sem. B, Representative computer-assisted maps of Fos-ir cells in the MnPO (closed circles) and adjacent preoptic area (open circles) of OVX + E2 (top panels) and OVX (bottom panels) rats at three ambient temperatures. This map is at the level of plate 34 from the Paxinos and Watson rat brain atlas (34). The boundaries of the MnPO (dotted lines) were drawn with the aid of adjacent Nissl-stained sections. 3V, Third ventricle; ac, anterior commissure. Scale bar in upper left, 100 μm for all maps. *, Significant difference between OVX + E2 and OVX rats; +, significant effect of TAMBIENT in OVX + E2 rats.
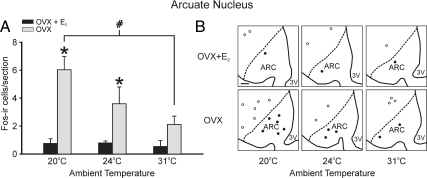
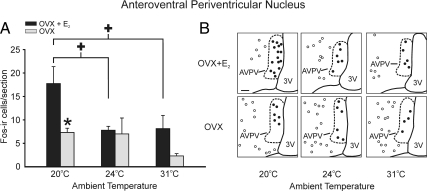
The numbers of Fos-ir cells were significantly increased in the arcuate nucleus and AVPV at the low TAMBIENT of 20 C, with inhibitory (arcuate nucleus) and stimulatory (AVPV) effects of E2
In the arcuate nucleus, the number of Fos-ir cells was highest at the low TAMBIENT of 20 C but only in the OVX group (Fig. 5). In E2-treated rats, the number of Fos-ir cells in the arcuate nucleus was consistently low, regardless of the TAMBIENT. At both 20 and 24 C, OVX rats exhibited significantly more Fos-ir cells in the arcuate nucleus than OVX + E2 rats. The number of Fos-ir cells in the AVPV was also highest in rats exposed to the low TAMBIENT of 20 C (Fig. 6). In contrast to both the MnPO and arcuate nucleus, the number of Fos-ir cells in the AVPV was highest in the OVX + E2 treated rats. This effect was significant only in rats exposed to the low TAMBIENT of 20 C.
Fig. 5.
A, Average numbers of Fos-ir cells per section in the arcuate nucleus in OVX + E2 and OVX rats exposed to ambient temperatures of 20, 24, and 31 C. Increased numbers of Fos-ir cells were detected at the TAMBIENT of 20 C in the arcuate nucleus of OVX rats, compared with OVX rats at 31 C. E2 significantly reduced Fos-ir in the arcuate nucleus at the ambient temperatures of 20 and 24 C. Values represent means ± sem (n = 6–8 rats/group). B, Representative computer-assisted maps of Fos-ir cells in the arcuate nucleus (closed circles) and adjacent ventromedial nucleus (open circles) of OVX + E2 (top panels) and OVX (bottom panels) rats at three ambient temperatures. This map is at the level of plate 57 from the Paxinos and Watson rat brain atlas (34). The boundary of the arcuate nucleus (dotted line) was drawn with the aid of adjacent Nissl-stained sections. 3V, Third ventricle; ARC, arcuate nucleus. Scale bar in upper left, 100 μm for all maps. *, Significant difference between OVX and OVX + E2 rats; #, significant effect of TAMBIENT in OVX rats.
Fig. 6.
A, Average numbers of Fos-ir cells per section in the AVPV in OVX + E2 and OVX rats exposed to ambient temperatures of 20, 24, and 31 C. E2 treatment increased the number of Fos-ir cells in rats exposed to the TAMBIENT of 20 C in the AVPV (n = 6–8 rats/group). Values represent means ± sem. B, Representative computer-assisted maps of Fos-ir cells in the AVPV (closed circles) and adjacent medial preoptic area (open circles) of OVX + E2 (top panels) and OVX (bottom panels) rats at three ambient temperatures. This map is at the level of plate 32 from the Paxinos and Watson rat brain atlas (34). The boundary of the AVPV (dotted line) was drawn with the aid of adjacent Nissl-stained sections. 3V, Third ventricle. Scale bar in upper left, 100 μm for all maps. *, Significant difference between OVX and OVX + E2 rats; +, significant effect of TAMBIENT in OVX + E2 rats.
Multiple areas exhibited increased numbers of Fos-ir cells at the TAMBIENT of 20 C, without significant overall effects of E2, and other areas exhibited no effect of either TAMBIENT or E2
The dorsomedial nucleus, lateral preoptic area, medial preoptic nucleus, medial septal nucleus, and parastrial nucleus exhibited significantly increased numbers of Fos-ir cells at the lowest TAMBIENT of 20 C, compared with the highest TAMBIENT of 31 C (Table 1). However, because two-way ANOVA revealed no overall effect of E2 in these areas, post hoc tests for estrogen effects were not performed.
Table 1.
Number of Fos-ir cells in select brain areas in OVX and OVX + E2 rats exposed to mild changes in TAMBIENT
| Brain area | 20 C |
24 C |
31 C |
||||
|---|---|---|---|---|---|---|---|
| OVX + E2 | OVX | OVX + E2 | OVX | OVX + E2 | OVX | ||
| Significant increase in Fos-ir at 20 C (compared with 31 C), no effect of E2 | Dorsomedial nucleus | 5.0 ± 0.9 | 9.8 ± 1.8 | 4.1 ± 0.8 | 3.2 ± 0.7 | 3.2 ± 1.2 | 4.4 ± 1.4 |
| Lateral preoptic area | 8.3 ± 1.9 | 11.7 ± 1.5 | 8.1 ± 1.9 | 6.5 ± 1.2 | 5.0 ± 0.6 | 5.3 ± 0.9 | |
| Medial preoptic nucleus | 7.4 ± 0.9 | 7.3 ± 1.0 | 6.7 ± 1.6 | 7.3 ± 2.0 | 4.4 ± 0.8 | 3.9 ± 0.6 | |
| Medial septal nucleus | 28.6 ± 3.8 | 30.2 ± 7.0 | 17.8 ± 1.8 | 23.8 ± 8.1 | 15.2 ± 3.5 | 14.2 ± 3.3 | |
| Parastrial nucleus | 7.1 ± 1.1 | 13.8 ± 2.1 | 9.9 ± 2.3 | 9.2 ± 2.7 | 6.6 ± 0.7 | 5.9 ± 1.1 | |
| No significant effect of TAMBIENT or E2 | Medial preoptic area | 26.7 ± 3.6 | 29.7 ± 4.5 | 19.1 ± 3.7 | 25.1 ± 6.0 | 21.4 ± 2.8 | 20.5 ± 2.8 |
| Septohypothalamic nucleus | 15.6 ± 2.7 | 16.4 ± 4.2 | 11.7 ± 3.1 | 13.4 ± 4.2 | 12.3 ± 2.6 | 8.6 ± 1.4 | |
| Ventrolateral preoptic area | 3.0 ± 0.7 | 2.9 ± 0.3 | 1.7 ± 0.2 | 2.3 ± 0.6 | 2.5 ± 0.8 | 3.3 ± 0.6 | |
| Ventromedial nucleus | 3.0 ± 1.7 | 4.7 ± 0.7 | 3.8 ± 1.2 | 2.1 ± 0.5 | 1.9 ± 0.7 | 3.2 ± 1.0 | |
Numbers represent mean ± sem (n = 5–8 rats/group).
There was no effect of either TAMBIENT or E2 on Fos immunoreactivity in the medial preoptic area, septohypothalamic nucleus, ventrolateral preoptic nucleus, and ventromedial nucleus (Table 1). Because of the low numbers of For-ir cells detected in the magnocellular and parvocellular regions of the paraventricular nucleus (one or less Fos-ir cell per section), statistical analysis was not performed on these areas.
Discussion
The reproductive axis modifies mammalian thermoregulation, as shown by the changes in body temperature during the menstrual cycle and pregnancy (37, 38), and by the occurrence of hot flushes in humans in response to gonadal hormone withdrawal (2, 3). These phylogenetically old, homeostatic systems are regulated at the level of the hypothalamus, but little is known about the anatomic sites of integration. To address this issue, we used Fos immunohistochemistry to examine the response of brain areas to mild changes in environmental temperature and to determine whether these responses are modified by E2 treatment. Because the OVX rats without estrogen replacement exhibit increased activation of skin vasodilatation and an increased sensitivity to warm ambient temperatures (11, 39), we were particularly interested in the effect of E2 on warm-responsive hypothalamic nuclei.
Mild ambient temperature stimuli were selected to avoid the confounding effects of stress and to minimize changes in core body temperature that could directly stimulate thermosensitive hypothalamic neurons. The selection of these ambient temperatures was based on our previous study documenting a shift in the thermoneutral zone from 26.1 to 31.9 C in OVX + E2 rats to 21.9 to 29.7 C in OVX rats (11). Thus, in both groups, the TAMBIENT of 20 C was below the thermoneutral zone, and 31 C was near the upper limit of thermoneutrality. The intermediate TAMBIENT (24 C) was neutral for OVX rats but subneutral for the OVX + E2 rats (11). In the present study, additional measurements of tail skin and core temperature were taken to confirm the effects of E2 at these ambient temperatures. As expected, the heat loss mechanism of skin vasodilatation was increased at the higher ambient temperatures and decreased by E2 treatment of OVX animals (11, 13). Moreover, at the high TAMBIENT of 31 C, the average TCORE was increased by approximately 0.3 C in the OVX but not in the OVX + E2 rats. These data agree with the previous demonstration that E2 replacement allows OVX rats to maintain their core temperatures closer to normothermic levels when exposed to a warm environment (11, 39).
Remarkably, the MnPO was the only site demonstrating increased numbers of Fos-ir cells at 31 C, compared with 20 and 24 C. The selectivity of this response diverges from previous studies in which male rats, when exposed to high ambient temperatures, exhibited increased Fos immunoreactivity in numerous areas in addition to the MnPO (25–27). This discrepancy could be a gender effect, but it is more likely due to the higher ambient temperatures used in the previous studies, which ranged from 33 to 47 C. Similarly, the lowest ambient temperature used in this study, although subneutral, was very mild compared with the cold environments (4–10 C) used to elicit Fos immunoreactivity in OVX female rats (40) and male rats (24, 26, 27, 41). Changes in core body temperature, increased activation of peripheral thermosensors, and/or recruitment of additional thermoeffectors or other physiological systems might contribute to the higher levels of Fos activation observed in the previous studies.
The MnPO contains warm-sensitive neurons (42), but the increased Fos immunoreactivity in the MnPO at 31 C in E2-treated rats cannot be explained by higher body temperatures because the TCORE of these animals was not significantly elevated. We also considered the possibility that activity in the MnPO could be modulated indirectly by other physiological circuits. MnPO neurons have been implicated in the control of osmotic balance (43), but the low Fos immunoreactivity in the magnocellular paraventricular nucleus (one or less Fos-ir cell per section) indicates that changes in this factor are unlikely the cause of increased Fos immunoreactivity in the MnPO at the highest ambient temperature. Changes in osmotic balance are also unlikely to explain the Fos activation at 31 C because the rats had free access to water and food. Alternatively, Fos immunoreactivity in the MnPO can be altered by sleep (44, 45), and sleep patterns are affected by both ambient temperature and estrogen status (46–50). However, neither ambient temperature nor estradiol treatment altered Fos immunoreactivity in the ventrolateral preoptic nucleus, an area that exhibits robust sleep-dependent Fos immunoreactivity (45). More importantly, previous studies have reported that baseline sleep patterns during the light phase in rats are not significantly altered by estrogen treatment (50) or by mild increases in ambient temperature (23–30 C) (51). These considerations suggest that sleep patterns in this study did not differ substantially across treatment groups and thus were unlikely to cause the observed effects in the MnPO. In contrast, the changes in ambient temperature would have been detected by warm-sensitive skin thermoreceptors that project via brain stem relays to the MnPO (52). Thus, it seems likely that the increase in MnPO Fos-ir at 31 C was due to changes in ambient temperature and not secondary to alterations in other homeostatic systems.
Our findings are consistent with recent studies implicating the MnPO as an important area for the control of body temperature through the dilatation and constriction of skin blood vessels (27, 41, 52–55). The MnPO is part of the neuronal circuitry regulating heat-defense responses (52), and it is the first site in the preoptic area to receive peripheral temperature information (52, 54). Modulation of MnPO neurons by local injections of pharmacological agents changes the activity of sympathetic fibers controlling tail vasomotion (55). The MnPO may influence skin vasomotion through projections to other regions of the hypothalamus (19, 52, 54) or to the rostral medullary raphe in which sympathetic vasoconstrictor neurons control tail vasomotion (41, 56, 57). In a separate proposed pathway, MnPO neurons activated by high ambient temperatures were shown to project to the rostral periaqueductal gray (27), a midbrain site that may convey signals for thermoregulatory vasodilatation (58, 59). In addition to its role in thermoregulatory vasomotion, the MnPO is implicated in the facilitation of heat-escape behavior during osmotic stimulation (60) and brown adipose tissue thermogenesis (19, 54, 61).
Given the important role of MnPO neurons in thermoregulatory vasomotion, our results suggest that this nucleus could mediate the effects of E2 on skin vasomotion in OVX rats (10, 11, 13–16, 28). Of the 14 brain regions examined, the MnPO was the only area that increased Fos immunoreactivity in response to the high TAMBIENT of 31 C. Importantly, the effects of TAMBIENT and E2 on MnPO Fos immunoreactivity occurred in parallel to their effects on tail skin vasomotion. Moreover, at the TAMBIENT of 24 C, Fos immunoreactivity was significantly elevated in the MnPO of OVX rats, compared with OVX + E2 rats. Using similar experimental conditions, we previously observed that 24 C was above the ambient temperature threshold for skin vasodilatation in OVX rats but below the threshold (subneutral) in OVX + E2 rats (11). Thus, differential activation of MnPO neurons could underlie the lower ambient threshold for skin vasodilatation in the OVX rats.
In addition to the MnPO, the arcuate nucleus and AVPV were significantly affected by TAMBIENT and E2. Both areas exhibited increased Fos immunoreactivity at the lowest TAMBIENT, with E2 treatment having opposite effects. In rats exposed to 20 C, E2 reduced Fos immunoreactivity in the arcuate nucleus and increased Fos immunoreactivity in the AVPV. These effects on Fos immunoreactivity might reflect a role of these areas in thermoregulation. Electrical stimulation of the medial basal hypothalamus (primarily the arcuate and ventromedial nuclei) influences the activity of thermosensitive neurons in the preoptic area (62). Pharmacological inhibition of the AVPV influences the activity of sympathetic nerve fibers supplying tail skin blood vessels (55). The arcuate nucleus and AVPV project to thermoregulatory areas in the preoptic/septal regions (63–65) including the MnPO (66). However, it is also possible that the Fos-ir changes in the arcuate nucleus and AVPV reflect the effects of ambient temperature on other physiological circuits. For example, the arcuate nucleus and the AVPV are key areas in the sex steroid regulation of reproduction (67–69) and cold environments interact with photoperiod to affect the reproductive axis (70). Alternatively, the changes in the number of Fos-ir cells in the arcuate nucleus could reflect activity in energy balance circuits. Food intake is increased during cold acclimation (71), decreased during warm acclimation (72), and decreased by systemic or hypothalamic E2 treatment (73). Further studies are necessary to understand the physiological significance of the TAMBIENT and E2 modulation of Fos in the arcuate nucleus and AVPV.
In summary, Fos immunoreactivity was modified by both mild changes in TAMBIENT and E2 in three areas: the MnPO, arcuate nucleus, and AVPV. The arcuate nucleus has a well-established role in energy balance, and both the arcuate nucleus and the AVPV are key components of the reproductive neuroendocrine axis. In contrast, the MnPO has a demonstrated role in the thermoregulatory axis, receiving thermal information as a part of a heat-defense pathway (52). There was a close correspondence between MnPO activation and tail skin vasodilatation and an inhibitory effect of E2 on both parameters. Based on these data, we hypothesize that the MnPO plays an important role in the E2, modulation of thermoregulatory vasomotion.
Acknowledgments
We thank Dr. Nathaniel McMullen, Dr. Andrej Romanovsky, and Dr. Alex Steiner for useful advice on the experimental design. Elizabeth Shih and Jessica Brown provided technical assistance. We would also like to thank the following people for critical reading of the manuscript: Dr. Nathaniel McMullen, Dr. Andrej Romanovsky, Dr. Andrew Dacks, Melinda Smith, Marina Cholanian, and Hemalini Williams.
This work was supported by the National Institutes of Health (NIH), National Institute on Aging Grant R01 AG032315, and the Arizona Biomedical Research Commission. P.A.D. was supported by NIH Predoctoral Training Program in Neuroscience Grant 5T32-AG007434, the Achievement Rewards for College Scientists Foundation, NIH National Institute on Aging Predoctoral Training Fellowship 1F31-AG030881, and the Evelyn McKnight Brain Institute.
Disclosure Summary: The authors have nothing to disclose.
Footnotes
- AVPV
- Anteroventral periventricular nucleus
- E2
- estradiol-17β
- HLI
- heat loss index
- ir
- immunoreactive
- MnPO
- median preoptic nucleus
- OVX
- ovariectomized
- TAMBIENT
- ambient temperature
- TCORE
- core temperature
- TSKIN
- skin temperature.
References
- 1. Freedman RR. 2001. Physiology of hot flashes. Am J Hum Biol 13:453–464 [DOI] [PubMed] [Google Scholar]
- 2. Stearns V, Ullmer L, López JF, Smith Y, Isaacs C, Hayes DF. 2002. Hot flushes. Lancet 360:1851–1861 [DOI] [PubMed] [Google Scholar]
- 3. Santoro N. 2008. Symptoms of menopause: hot flushes. Clin Obstet Gynecol 51:539–548 [DOI] [PubMed] [Google Scholar]
- 4. Casper RF, Yen SS, Wilkes MM. 1979. Menopausal flushes: a neuroendocrine link with pulsatile luteinizing hormone secretion. Science 205:823–825 [DOI] [PubMed] [Google Scholar]
- 5. Casper RF, Yen SSC. 1981. Menopausal flushes: effect of pituitary gonadotropin desensitization by a potent luteinizing hormone-releasing factor agonist. J Clin Endocrinol Metab 53:1056–1058 [DOI] [PubMed] [Google Scholar]
- 6. Gambone J, Meldrum DR, Laufer L, Chang RJ, Lu JK, Judd HL. 1984. Further delineation of hypothalamic dysfunction responsible for menopausal hot flashes. J Clin Endocrinol Metab 59:1097–1102 [DOI] [PubMed] [Google Scholar]
- 7. Casper RF, Yen SSC. 1985. Neuroendocrinology of menopausal flushes: an hypothesis of flush mechanism. Clin Endocrinol (Oxf) 22:293–312 [DOI] [PubMed] [Google Scholar]
- 8. Gordon CJ. 1990. Thermal biology of the laboratory rat. Physiol Behav 47:963–991 [DOI] [PubMed] [Google Scholar]
- 9. Romanovsky AA, Ivanov AI, Shimansky YP. 2002. Selected contribution: ambient temperature for experiments in rats: a new method for determining the zone of thermal neutrality. J Appl Physiol 92:2667–2679 [DOI] [PubMed] [Google Scholar]
- 10. Williams H, Dacks PA, Rance NE. 2010. An improved method for recording tail skin temperature in the rat reveals changes during the estrous cycle and effects of ovarian steroids. Endocrinology 151:5389–5394 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Dacks PA, Rance NE. 2010. Effects of estradiol on the thermoneutral zone and core temperature in ovariectomized rats. Endocrinology 151:1187–1193 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Kobayashi T, Tamura M, Hayashi M, Katsuura Y, Tanabe H, Ohta T, Komoriya K. 2000. Elevation of tail skin temperature in ovariectomized rats in relation to menopausal hot flushes. Am J Physiol Regul Integr Comp Physiol 278:R863–R869 [DOI] [PubMed] [Google Scholar]
- 13. Hosono T, Chen XM, Miyatsuji A, Yoda T, Yoshida K, Yanase-Fujiwara M, Kanosue K. 2001. Effects of estrogen on thermoregulatory tail vasomotion and heat-escape behavior in freely moving female rats. Am J Physiol Regul Integr Comp Physiol 280:R1341–R1347 [DOI] [PubMed] [Google Scholar]
- 14. Berendsen HH, Weekers AH, Kloosterboer HJ. 2001. Effect of tibolone and raloxifene on the tail temperature of oestrogen-deficient rats. Eur J Pharmacol 419:47–54 [DOI] [PubMed] [Google Scholar]
- 15. Opas EE, Rutledge SJ, Vogel RL, Rodan GA, Schmidt A. 2004. Rat tail skin temperature regulation by estrogen, phytoestrogens and tamoxifen. Maturitas 48:463–471 [DOI] [PubMed] [Google Scholar]
- 16. Sipe K, Leventhal L, Burroughs K, Cosmi S, Johnston GH, Deecher DC. 2004. Serotonin 2A receptors modulate tail-skin temperature in two rodent models of estrogen deficiency-related thermoregulatory dysfunction. Brain Res 1028:191–202 [DOI] [PubMed] [Google Scholar]
- 17. Bowe J, Li XF, Kinsey-Jones J, Heyerick A, Brain S, Milligan S, O'Byrne K. 2006. The hop phytoestrogen, 8-prenylnaringenin, reverses the ovariectomy-induced rise in skin temperature in an animal model of menopausal hot flushes. J Endocrinol 191:399–405 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Boulant JA. 2000. Role of the preoptic-anterior hypothalamus in thermoregulation and fever. Clin Infect Dis 31:S157–S161 [DOI] [PubMed] [Google Scholar]
- 19. Romanovsky AA, Almeida MC, Garami A, Steiner AA, Norman MH, Morrison SF, Nakamura K, Burmeister JJ, Nucci TB. 2009. The transient receptor potential vanilloid-1 channel in thermoregulation: a thermosensor it is not. Pharmacol Rev 61:228–261 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Romanovsky AA. 2007. Thermoregulation: some concepts have changed. Functional architecture of the thermoregulatory system. Am J Physiol Regul Integr Comp Physiol 292:R37–R46 [DOI] [PubMed] [Google Scholar]
- 21. Silva NL, Boulant JA. 1986. Effects of testosterone, estradiol, and temperature on neurons in preoptic tissue slices. Am J Physiol Regul Intergr Comp Physiol 250:R625–R632 [DOI] [PubMed] [Google Scholar]
- 22. Simerly RB, Chang C, Muramatsu M, Swanson LW. 1990. Distribution of androgen and estrogen receptor mRNA-containing cells in the rat brain: an in situ hybridization study. J Comp Neurol 294:76–95 [DOI] [PubMed] [Google Scholar]
- 23. Hoffman GE, Lyo D. 2002. Anatomical markers of activity in neuroendocrine systems: are we all ‘fos-ed out?’ J Neuroendocrinol 14:259–268 [DOI] [PubMed] [Google Scholar]
- 24. Kiyohara T, Miyata S, Nakamura T, Shido O, Nakashima T, Shibata M. 1995. Differences in Fos expression in the rat brains between cold and warm ambient exposures. Brain Res Bull 38:193–201 [DOI] [PubMed] [Google Scholar]
- 25. McKitrick DJ. 2000. Expression of Fos in the hypothalamus of rats exposed to warm and cold temperatures. Brain Res Bull 53:307–315 [DOI] [PubMed] [Google Scholar]
- 26. Bratincsák A, Palkovits M. 2004. Activation of brain areas in rat following warm and cold ambient exposure. Neuroscience 127:385–397 [DOI] [PubMed] [Google Scholar]
- 27. Yoshida K, Konishi M, Nagashima K, Saper CB, Kanosue K. 2005. Fos activation in hypothalamic neurons during cold or warm exposure: projections to periaqueductal gray matter. Neuroscience 133:1039–1046 [DOI] [PubMed] [Google Scholar]
- 28. Kobayashi T, Ushijima O, Chen JT, Shiraki M, Ohta T, Kiyoki M. 1995. Basal tail skin temperature elevation and augmented response to calcitonin gene-related peptide in ovariectomized rats. J Endocrinol 146:431–437 [DOI] [PubMed] [Google Scholar]
- 29. Pan Y, Anthony MS, Binns M, Clarkson TB. 2001. A comparison of oral micronized estradiol with soy phytoestrogen effects on tail skin temperatures of ovariectomized rats. Menopause 8:171–174 [DOI] [PubMed] [Google Scholar]
- 30. Ström JO, Theodorsson E, Theodorsson A. 2008. Order of magnitude differences between methods for maintaining physiological 17β-oestradiol concentrations in ovariectomized rats. Scand J Clin Lab Invest 68:814–822 [DOI] [PubMed] [Google Scholar]
- 31. Watson RE, Jr, Wiegand SJ, Clough RW, Hoffman GE. 1986. Use of cryoprotectant to maintain long-term peptide immunoreactivity and tissue morphology. Peptides 7:155–159 [DOI] [PubMed] [Google Scholar]
- 32. Elmquist JK, Ahima RS, Elias CF, Flier JS, Saper CB. 1998. Leptin activates distinct projections from the dorsomedial and ventromedial hypothalamic nuclei. Proc Natl Acad Sci USA 95:741–746 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Patronas P, Horowitz M, Simon E, Gerstberger R. 1998. Differential stimulation of c-fos expression in hypothalamic nuclei of the rat brain during short-term heat acclimation and mild dehydration. Brain Res 798:127–139 [DOI] [PubMed] [Google Scholar]
- 34. Paxinos G, Watson C. 2007. The rat brain in stereotaxic coordinates. Burlington, MA: Elsevier Inc; [DOI] [PubMed] [Google Scholar]
- 35. Sherin JE, Elmquist JK, Torrealba F, Saper CB. 1998. Innervation of histaminergic tuberomammillary neurons by GABAergic and galaninergic neurons in the ventrolateral preoptic nucleus of the rat. J Neurosci 18:4705–4721 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Gvilia I, Xu F, McGinty D, Szymusiak R. 2006. Homeostatic regulation of sleep: a role for preoptic area neurons. J Neurosci 26:9426–9433 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Stephenson LA, Kolka MA. 1993. Thermoregulation in women. Exerc Sport Sci Rev 21:231–262 [PubMed] [Google Scholar]
- 38. Eliason HL, Fewell JE. 1997. Thermoregulatory control during pregnancy and lactation in rats. J Appl Physiol 83:837–844 [DOI] [PubMed] [Google Scholar]
- 39. Baker MA, Dawson DD, Peters CE, Walker AM. 1994. Effects of estrogen on thermoregulatory evaporation in rats exposed to heat. Am J Physiol Regul Integr Comp Physiol 267:R673–R677 [DOI] [PubMed] [Google Scholar]
- 40. Uchida Y, Tokizawa K, Nakamura M, Mori H, Nagashima K. 2010. Estrogen in the medial preoptic nucleus of the hypothalamus modulates cold responses in female rats. Brain Res 1339:49–59 [DOI] [PubMed] [Google Scholar]
- 41. Yoshida K, Li X, Cano G, Lazarus M, Saper CB. 2009. Parallel preoptic pathways for thermoregulation. J Neurosci 29:11954–11964 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Travis KA, Johnson AK. 1993. In vitro sensitivity of median preoptic neurons to angiotensin II, osmotic pressure, and temperature. Am J Physiol Regul Integr Comp Physiol 264:R1200–R1205 [DOI] [PubMed] [Google Scholar]
- 43. Whyte DG, Johnson AK. 2005. Thermoregulatory role of periventricular tissue surrounding the anteroventral third ventricle (AV3V) during acute heat stress in the rat. Clin Exp Pharmacol Physiol 32:457–461 [DOI] [PubMed] [Google Scholar]
- 44. Gong H, Szymusiak R, King J, Steininger T, McGinty D. 2000. Sleep-related c-Fos protein expression in the preoptic hypothalamus: effects of ambient warming. Am J Physiol Regul Integr Comp Physiol 279:R2079–R2088 [DOI] [PubMed] [Google Scholar]
- 45. Szymusiak R, Gvilia I, McGinty D. 2007. Hypothalamic control of sleep. Sleep Med 8:291–301 [DOI] [PubMed] [Google Scholar]
- 46. Schmidek WR, Hoshino K, Schmidek M, Timo-Iaria C. 1972. Influence of environmental-temperature on sleep-wakefulness cycle in rat. Physiol Behav 8:363–371 [DOI] [PubMed] [Google Scholar]
- 47. Mahapatra AP, Mallick HN, Kumar VM. 2005. Changes in sleep on chronic exposure to warm and cold ambient temperatures. Physiol Behav 84:287–294 [DOI] [PubMed] [Google Scholar]
- 48. Kumar D, Mallick HN, Kumar VM. 2009. Ambient temperature that induces maximum sleep in rats. Physiol Behav 98:186–191 [DOI] [PubMed] [Google Scholar]
- 49. Nowakowski S, Meliska CJ, Martinez LF, Parry BL. 2009. Sleep and menopause. Curr Neurol Neurosci Rep 9:165–172 [DOI] [PubMed] [Google Scholar]
- 50. Deurveilher S, Rusak B, Semba K. 2009. Estradiol and progesterone modulate spontaneous sleep patterns and recovery from sleep deprivation in ovariectomized rats. Sleep 32:865–877 [PMC free article] [PubMed] [Google Scholar]
- 51. Gao BO, Franken P, Tobler I, Borbély AA. 1995. Effect of elevated ambient temperature on sleep, EEG spectra, and brain temperature in the rat. Am J Physiol 268:R1365–R1373 [DOI] [PubMed] [Google Scholar]
- 52. Nakamura K, Morrison SF. 2010. A thermosensory pathway mediating heat-defense responses. Proc Natl Acad Sci USA 107:8848–8853 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. Lazarus M, Yoshida K, Coppari R, Bass CE, Mochizuki T, Lowell BB, Saper CB. 2007. EP3 prostaglandin receptors in the median preoptic nucleus are critical for fever responses. Nat Neurosci 10:1131–1133 [DOI] [PubMed] [Google Scholar]
- 54. Nakamura K, Morrison SF. 2008. A thermosensory pathway that controls body temperature. Nat Neurosci 11:62–71 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55. Tanaka M, McKinley MJ, McAllen RM. 2009. Roles of two preoptic cell groups in tonic and febrile control of rat tail sympathetic fibers. Am J Physiol Regul Integr Comp Physiol 296:R1248–R1257 [DOI] [PubMed] [Google Scholar]
- 56. Nakamura K, Matsumura K, Kaneko T, Kobayashi S, Katoh H, Negishi M. 2002. The rostral raphe pallidus nucleus mediates pyrogenic transmission from the preoptic area. J Neurosci 22:4600–4610 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57. Nakamura Y, Nakamura K, Morrison SF. 2009. Different populations of prostaglandin EP3 receptor-expressing preoptic neurons project to two fever-mediating sympathoexcitatory brain regions. Neuroscience 161:614–620 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58. Zhang YH, Hosono T, Yanase-Fujiwara M, Chen XM, Kanosue K. 1997. Effect of midbrain stimulations on thermoregulatory vasomotor responses in rats. J Physiol 503:177–186 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59. Nagashima K, Nakai S, Tanaka M, Kanosue K. 2000. Neuronal circuitries involved in thermoregulation. Auton Neurosci 85:18–25 [DOI] [PubMed] [Google Scholar]
- 60. Konishi M, Kanosue K, Kano M, Kobayashi A, Nagashima K. 2007. The median preoptic nucleus is involved in the facilitation of heat-escape/cold-seeking behavior during systemic salt loading in rats. Am J Physiol Regul Integr Comp Physiol 292:R150–R159 [DOI] [PubMed] [Google Scholar]
- 61. Romanovsky AA, Sugimoto N, Simons CT, Hunter WS. 2003. The organum vasculosum laminae terminalis in immune-to-brain febrigenic signaling: a reappraisal of lesion experiments. Am J Physiol Regul Integr Comp Physiol 285:R420–R428 [DOI] [PubMed] [Google Scholar]
- 62. Hori T, Kiyohara T, Osaka T, Shibata M, Nakashima T. 1982. Responses of preoptic thermosensitive neurons to mediobasal hypothalamic stimulation. Brain Res Bull 8:677–683 [DOI] [PubMed] [Google Scholar]
- 63. Chronwall BM. 1985. Anatomy and physiology of the neuroendocrine arcuate nucleus. Peptides 6(Suppl 2):1–11 [DOI] [PubMed] [Google Scholar]
- 64. Gu GB, Simerly RB. 1997. Projections of the sexually dimorphic anteroventral periventricular nucleus in the female rat. J Comp Neurol 384:142–164 [PubMed] [Google Scholar]
- 65. Simonian SX, Spratt DP, Herbison AE. 1999. Identification and characterization of estrogen receptor-α containing neurons projecting to the vicinity of the gonadotropin-releasing hormone perikarya in the rostral preoptic area of the rat. J Comp Neurol 411:346–358 [DOI] [PubMed] [Google Scholar]
- 66. Krajewski SJ, Burke MC, Anderson MJ, McMullen NT, Rance NE. 2010. Forebrain projections of arcuate neurokinin B neurons demonstrated by anterograde tract-tracing and monosodium glutamate lesions in the rat. Neuroscience 166:680–697 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67. Barraclough CA. 1973. Sex steroid regulation of reproductive neuroendocrine processes. In: Greep RO, Astwood EB. eds. Handbook of physiology, endocrinology II, part 1. Baltimore: Waverly Press; 29–56 [Google Scholar]
- 68. Herbison AE. 1998. Multimodal influence of estrogen upon gonadotropin-releasing hormone neurons. Endocr Rev 19:302–330 [DOI] [PubMed] [Google Scholar]
- 69. Simerly RB. 2002. Wired for reproduction: organization and development of sexually dimorphic circuits in the mammalian forebrain. Annu Rev Neurosci 25:507–536 [DOI] [PubMed] [Google Scholar]
- 70. Kriegsfeld LJ, Ranalli NJ, Bober MA, Nelson RJ. 2000. Photoperiod and temperature interact to affect the GnRH neuronal system of male prairie voles (Microtus ochrogaster). J Biol Rhythms 15:306–316 [DOI] [PubMed] [Google Scholar]
- 71. Leung PM, Horwitz BA. 1976. Free-feeding patterns of rats in response to changes in environmental temperature. Am J Physiol 231:1220–1224 [DOI] [PubMed] [Google Scholar]
- 72. Rousset B, Cure M, Jordan D, Kervran A, Bornet H, Mornex R. 1984. Metabolic alterations induced by chronic heat exposure in the rat: the involvement of thyroid function. Pflugers Arch 401:64–70 [DOI] [PubMed] [Google Scholar]
- 73. Geary N. 2000. Estradiol and appetite. Appetite 35:273–274 [DOI] [PubMed] [Google Scholar]