Abstract
The efficacy of exercise as primary prevention of obesity is the subject of intense investigation. Here, we show that voluntary exercise in a mouse strain susceptible to diet-induced obesity (C57B6J) decreases fat mass and increases energy expenditure. In addition, exercise attenuates obesity in mice fed a high-fat diet (HFD). Using FosB immunoreactivity as a marker of chronic neuronal activation, we found that exercise activates leptin receptor-positive neurons in the ventromedial hypothalamic nucleus, involved in homeostatic control of energy balance. FosB immunoreactivity in the ventromedial hypothalamic nucleus is decreased in sedentary mice exposed to HFD but is increased in exercised mice independent of adiposity. To determine whether the antiobesity effects of voluntary exercise improve central nervous system (CNS) leptin action, we measured the anorectic and weight reducing effects of intracerebroventricular (ICV) leptin in sedentary and exercised mice exposed to HFD (EH), as well as in sedentary mice that have been calorie restricted (SR) to match the fat mass of EH mice. ICV leptin was ineffective in lowering food intake and body weight (BW) in sedentary mice exposed to HFD mice. The anorectic potency of leptin was partially restored in EH and SR groups. However, ICV leptin significantly lowered BW in EH but not SR mice. Thus, exercise leads to the maintenance of a lower BW and leaner composition, as well as to improved CNS leptin action, independent of fat mass. These results support the notion that physical exercise directly influences the responsiveness of the CNS circuits involved in energy homeostasis by allowing the defense of a lowered BW.
An increased sedentary lifestyle is recognized as a major risk factor for obesity and associated disorders, such as cardiovascular disease, type 2 diabetes, and depression (1). Increased physical activity reduces the risk of obesity and associated metabolic disorders (2, 3). This might be ascribed to the reduction in body weight (BW) associated with increased physical activity, although some health benefits of exercise are often independent of weight loss (4).
The role of physical exercise as a primary intervention for obesity and weight reduction is the subject of intense scrutiny. Some human studies report large individual variability in the weight loss response to exercise (5, 6). In other studies, exercise alone resulted in less weight loss than that predicted by the increased energy expenditure of physical activity (7). This compensation is believed to occur because, in keeping with the adipostatic hypothesis, the increased energy expenditure during exercise triggers central nervous system (CNS) responses that increase appetite and food intake that consequently prevent the depletion of fat stores (8, 9). On the other hand, some studies indicate that exercise does not always increase appetite and food intake but rather increases postprandial satiation (10). Similarly, animal studies support the notion that exercise can lower BW without increasing food intake (11, 12). The lack of compensatory hyperphagia, in the face of weight loss, suggests that exercise modifies the responses of the CNS to adiposity signals. Indeed, exercise is known to increase sensitivity to the adiposity hormone leptin, whose plasma levels are proportional to fat stores (13, 14).
In this report, we test the hypothesis that voluntary exercise alters central control of energy balance regardless of diet composition. We show that voluntary exercise promotes increased energy expenditure in mice exposed to low-fat diet or high-fat diet (HFD), resulting in a lower fat mass. To explore the CNS mechanisms underlying these effects of exercise, we mapped the areas of the brain that are activated by chronic voluntary exercise and determined whether exercise prevents diet-induced leptin resistance independent of fat mass.
Materials and Methods
Animals
Ten-week-old male C57B6J mice were purchased from The Jackson Laboratory (Bar Harbor, ME). Long form of the leptin receptor (LRb)-green fluorescent protein (GFP) mice were a kind gift of Martin Myers (15). In all experiments, mice were individually housed in large rodent cages, acclimated for 1 wk in study housing room before collection of baseline parameters. Throughout the studies, mice were maintained on a 12-h light, 12-h dark cycle (energy balance experiment: 0600 h on and 1800 h off; leptin action experiment: 0200 h on and 1400 h off). All animal protocols were approved by the Institutional Animal Care and Use Committee of the University of Cincinnati.
Experimental design
Energy balance experiment
To assess the effect of exercise and diet on energy balance and body composition, the home cages were equipped with either blocked (sedentary group) or mobile (exercised group) running wheels, which were available at all times. Simultaneously, mice received ad libitum access to either chow diet (13% kcal fat, chow catalog no. 7012; Harlan Laboratories, Indianapolis, IL) or HFD (60%kcal fat, catalog no. D12492, Research Diets, Inc., New Brunswick, NJ). Four experimental groups (n = 7 per group) included: sedentary chow (SC)-, sedentary HFD (SH)-, exercised chow (EC)-, and exercised HFD (EH)-fed mice. Food intake was measured three times a week for 6 wk, and body composition was measured every 2 wk. At the end of the experiment, mice were removed from exercise cages at 2 h after light onset and anesthetized with euthasol at 4–7 h after light onset. Blood was collected via cardiac puncture, the descending aorta was clamped, and the upper body of the mice was perfused with 0.1 m PBS followed by 4% paraformaldehyde. After perfusion, brains were harvested and placed in 4% paraformaldehyde for 24 h followed by transition to 30% sucrose solution. A separate cohort of mice was placed in calorimetry cages after a 6-wk exposure to running wheels and ad libitum access to chow.
Leptin action experiment
To assess the effect of exercise on CNS leptin sensitivity, 10-wk-old mice were implanted with an indwelling cannula into the third ventricle [intracerebroventricularly (ICV)] as described below. After recovery from surgery (∼1 wk), mice were randomized into four groups: 1) SC (n = 8); 2) SH (n = 8); 3) EH (n = 11); 4) and SH restricted (SR) (n = 8) to 80–85% of daily calories ingested by the SH group. This calorie restriction was effective in matching SR fat mass to the EH group. After 4 wk, mice were tested for ICV leptin sensitivity. One week after the behavioral tests, mice were euthanized with carbon dioxide, and their fat mass was measured by fat dissection, because in vivo MRI measures were not possible due to ICV catheters.
Running activity
Exercised mice were single housed in cages containing running wheels (Lafayette Instrument Co., Lafayette, IN). Running activity was monitored continuously via a computerized system (Lafayette Instruments Computerized Animal Wheel Monitoring System; Lafayette Instrument Co.). Distance (meters) and velocity (meters per minute) were recorded in 10-min time intervals.
Body composition and food intake
In the energy balance experiment, BW was measured weekly at 2 h after light onset. Body composition was assessed biweekly via nuclear magnetic resonance spectroscopy (EchoMRI; Echo Medical Systems, Houston, TX). Change in BW and fat mass was calculated as the difference of basal weight from final weight. Food intake was measured every 2–3 d via food hopper weight, and cumulative caloric intake was calculated by the total gram weight of food eaten multiplied by the caloric content of the diet (3.41 and 5.24 kcal, chow and HFD, respectively). Change in energy intake was calculated by subtracting the total kcal of food eaten during the baseline week from the total kcal eaten during the final week of treatment. In the ICV leptin experiments, to ensure matching of BW of EH and SR mice, BW and food hopper weight were recorded daily throughout the study at 6 h after the onset of light. The SR group was calorie restricted by providing 85% of the average intake of ad libitum-fed mice the day before and allocated in two daily allotments: one consisting of 25–30% of their daily allowance at 6 h into the light cycle and a second allotment with the remaining 70–75% at dark onset.
Energy expenditure
Indirect calorimetry was performed as described previously (16). After 6 wk of voluntary exercise, a separate cohort of mice and their controls were moved to metabolic chambers (TSE/SciPro, Midland, MI), in which fluid, food intake, activity, and gas exchanges can be monitored. After 24 h of acclimation, oxygen consumption, CO2 production, and locomotor activity were measured continually for 2 d.
FosB-LRb colocalization experiment (see figure 5)
Fig. 5.
Exercise-activated FosB+ neurons in VMH coexpress the LRb. GFP and FosB IR, respectively, in VMH of LRb-GFP mice after 4 wk of voluntary running wheel exercise; magnification, ×10 (A and B); ×20 magnification for GFP and FosB IR, respectively (C and D). Merged images depicting colocalization (arrowheads) of LRb (GFP) and FosB IR (E).
To determine whether ventromedial hypothalamus (VMH) neurons activated by exercise were also expressing the LRb, we used LRb-GFP mice (n = 5), which express GFP in all cells that contain the LRb. After a 4-wk exposure to the running wheels, mice were removed from exercise cages 6 h into light period and injected with euthasol at 8–10 h after light onset and prepared for immunofluorescence immunohistochemistry (IHC).
Immunohistochemistry
Perfused and fixed brains were sectioned using a microtome. Free floating 40-μm sections were washed in 0.1 m Krebs phosphate buffer and 0.1 m Krebs phosphate buffered saline solution (KPBS) solutions where appropriate. Sections were then incubated in 1% hydrogen peroxide/KPBS and rinsed thoroughly with KPBS. Sections were then blocked by incubation in 4% horse serum with 0.4% Triton X-100. Rabbit anti-FosB (sc-7203; Santa Cruz Biotechnology, Inc., Santa Cruz, CA) was diluted to 1:500 in 4% horse serum with 0.4% Triton X-100 and incubated overnight. The secondary antibody, biotinylated goat antirabbit IgG (1:250) (BA-1000; Vector Laboratories, Burlingame, CA), was applied followed by incubation in avidin-biotin complex (1:500) (Vectastain ABC PK6100; Vector Laboratories) and development with diaminobenzidine for 8 min. After washing, sections were mounted and images were taken using a Zeiss light microscope (Carl Zeiss Imaging Solutions, Thornwood, NY) and analyzed using Axio-vision software (Carl Zeiss Imaging Solutions). Images of coronal sections taken at a distance from bregma of +1.18, −1.70, −0.82, −1.46, and −1.46 mm were used to analyze the core of the nucleus accumbens (NAc), dentate gyrus of the hippocampus (DG), periventricular nucleus (PVN), arcuate nucleus (ARC), and VMH, respectively, with the use a mouse brain atlas (17). The number of cells per unit area was calculated using a standard voxel within the region of interest, as described (18).
Single label immunofluorescence
The sections were rinsed in 0.1 phosphate buffer, followed by additional washes in 0.1 m PBS; sections were then incubated in 1% hydrogen peroxide/PBS and rinsed thoroughly with PBS. Sections were then blocked by incubation in 4% horse serum with 0.4% Triton X-100. Rabbit anti-FosB (sc-48; Santa Cruz Biotechnology, Inc.) antibody diluted 1:250 in 4% horse serum with 0.4% Triton X-100 (Fisher Scientific, Pittsburgh, PA) was applied to the sections for overnight incubation. Sections were then incubation in Alexa Fluor 488 goat antimouse IgG diluted (1:200) (A11001; Invitrogen, Carlsbad, CA). Slides were cover slipped with Gelvatol mounting media containing 1,4-diazabicyclo[2.2.2]octane antifade agent (Electron Microscopy Sciences, Hatfield, PA).
Double label immunofluorescence
The sections were rinsed in 0.1 phosphate buffer, followed by additional washes in 0.1 m PBS; sections were then incubated in 1% hydrogen peroxide/PBS and rinsed thoroughly with PBS. Sections were then blocked by incubation in 4% horse serum with 0.4% Triton X-100. Rabbit anti-FosB (sc-48; Santa Cruz Biotechnology, Inc.) antibody diluted 1:100 in 4% horse serum with 0.4% Triton X-100 (Fisher Scientific) was applied to the sections for overnight incubation. The secondary antibody, Biotinylated goat antirabbit IgG (1:250) (BA-1000; Vector Laboratories), was applied followed by incubation in avidin-biotin complex (1:500) (Vectastain ABC PK6100; Vector Laboratories) and an incubation in biotinyl tyramide signal amplification solution (1:250) (NEL700A; PerkinElmer, Boston, MA). Cyanine three streptavidin (1:200) (Cy3; Jackson ImmunoResearch, West Grove, PA) was then applied to the sections. The sections were then washed with PBS and incubated in 4% goat serum + 0.4% Triton X-100 blocking solution followed by rabbit anti-GFP (A11122; Invitrogen), formulated in the same blocking solution, overnight at room temperature followed by incubation in Alexa Fluor 488 goat antimouse IgG diluted (1:200) (A11001; Invitrogen). Slides were cover slipped with Gelvatol mounting media containing 1,4-diazabicyclo[2.2.2]octane antifade agent (Electron Microscopy Sciences).
Third ventricular cannulation and ICV leptin treatment
Briefly, mice were anesthetized with ketamine/xylazine (100 mg/kg and 10 mg/kg, respectively) and implanted stereotaxically with a 26 G stainless steel cannula (Plastics One, Inc.) into the third ventricle (coordinates in millimeters from bregma −1.8 anterior-posterior, −4.8 dorsal-ventral, 0.0 medial-lateral), which were cemented and secured to the skull by one to two placement screws. Three days after surgery, cannula placement was verified behaviorally by testing the hyperphagic response to an ICV injection of neuropeptide Y in saline (1 ug/ul). ICV placement was deemed correct if food intake post-neuropeptide Y was more than or equal to 6 g in 4 h. After recovery and exercise and diet treatments, each mouse received ICV injections of leptin (2 μg/2 μl; PeproTech, Inc., Rocky Hill, NJ) or vehicle (sterile saline) 4 h before the onset of dark and placed in a clean sedentary or exercise treatment cage without food. The experimental diet food was returned at onset of dark, and food intake was measured at 2, 4, and 24 h. Animals were provided with their treatment diet to avoid possible changes in food intake due to novelty or palatability. BW was measured at time of injection and after 24 h. The leptin dose (2 μg) was selected from a pilot study, because it elicited a 50% reduction in food intake at 4 h in comparable mice. Leptin and vehicle treatments were given 1 wk apart to allow wash-out of the drug and achieve within subjects design.
Hormone analysis
Plasma insulin and leptin were measured with a multiplex kit (Lincoplex Millipore, St. Charles, MO).
Statistical analysis
Data are shown as mean value with sem. Data expressed as changes from baseline and within subjects comparisons were compared by Student's t test; groups were compared with two-way ANOVA or two-way repeated measures ANOVA followed by Tukey post hoc test for multiple comparisons, as described in figure legends and text. Statistical analysis was performed using GraphPad Prism software (GraphPad Prism, La Jolla, CA).
Results
Voluntary exercise increases leanness and energy expenditure
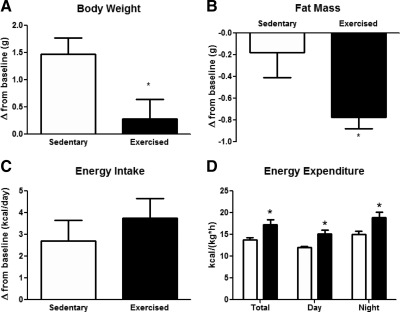
To determine the effect of voluntary exercise on energy homeostasis and body composition, mice were exposed to blocked (sedentary), or mobile (exercised) running wheels for 6 wk. After 1 wk of adaptation, exercised mice ran a stable daily distance (8.73 ± 0.19 km) at a consistent average speed (15.21 ± 0.45 m/min). BW was not significantly different between groups (Supplemental Fig. 1A, published on The Endocrine Society's Journals Online web site at http://endo.endojournals.org). However, change in BW from baseline showed that chow-fed sedentary mice gained weight, whereas exercised mice did not [1.47 ± 0.30 g and 0.29 ± 0.35 g, respectively; t(12) = 2.55, P < 0.05] (Fig. 1A). Exercise mice lost significant fat mass compared with sedentary [−0.77 ± 0.11 g and −0.18 ± 0.23 g, respectively; t(12) = 2.33, P < 0.05] (Fig. 1B), resulting in a lower fat mass at the end of the study (Supplemental Fig. 1B and Table 1). Lean body mass was not affected by exercise (Table 1). Food intake, measured as change in average daily caloric intake from baseline or cumulative calorie intake (Fig. 1C and Supplemental Fig. 1C, respectively), was not significantly different in the two groups. Energy expenditure measured by indirect calorimetry, immediately after a 6-wk exercise treatment, and normalized by either BW (Fig. 1D) or lean body mass (Supplemental Fig. 2A), was significantly increased in exercised mice [exercise, F(1,1) = 12.64; P < 0.05] (Fig. 1D). Simultaneous recording of spontaneous locomotor activity in the calorimeter cages, measured by beam breaks, did not differ among groups (Supplemental Fig. 2C). Respiratory quotient was significantly increased in the exercised group, suggesting that exercise increased glucose oxidation [F(1,11) = 12.64, P < 0.05] (Supplemental Fig. 2B).
Fig. 1.
Voluntary running wheel exercise decreases fat mass and increases energy expenditure without increasing food intake. Changes in body weight (BW) (A), fat mass (B), and daily energy intake (C) in mice fed chow ad libitum with or without access to voluntary running wheel exercise for 6 wk. Energy expenditure (D) in mice fed chow ad libitum after a 6-wk exercise treatment. Each value is the mean ± sem for n = 6–7 mice per group. A and B: *, P < 0.05 Student's t test; D: *, P < 0.05, significantly different from sedentary control by ANOVA followed by Tukey post hoc test.
Table 1.
Body composition and plasma values
| Chow |
High fat |
|||
|---|---|---|---|---|
| Sedentary | Exercised | Sedentary | Exercised | |
| Insulin (ng/ml) | ||||
| Final | 0.67 ± 0.20 | 0.42 ± 0.13 | 2.20 ± 0.21a | 1.175 ± 0.29b |
| Leptin (ng/ml) | ||||
| Final | 2.08 ± 1.09 | 0.43 ± 0.15a | 41.80 ± 5.87a | 26.42 ± 5.57b |
| BW (g) | ||||
| Baseline | 26.86 ± 0.63 | 26.99 ± 0.45 | 27.00 ± 0.46 | 26.90 ± 0.61 |
| Final | 28.33 ± 0.70 | 27.27 ± 0.44 | 37.03 ± 1.05a | 29.73 ± 1.13 |
| Fat mass (g) | ||||
| Baseline | 1.12 ± 0.20 | 1.11 ± 0.09 | 1.08 ± 0.12 | 1.11 ± 0.11 |
| Final | 0.94 ± 0.17 | 0.33 ± 0.08a | 10.47 ± 0.84a | 3.42 ± 0.77a,b |
| Lean mass (g) | ||||
| Baseline | 23.56 ± 0.66 | 23.70 ± 0.46 | 23.87 ± 0.48 | 23.97 ± 0.35 |
| Final | 24.56 ± 0.49 | 24.02 ± 0.41 | 23.73 ± 0.42 | 23.54 ± 0.38 |
| Averaged daily caloric intake (kcal) | ||||
| Baseline | 15.71 ± 0.42 | 16.75 ± 0.91 | 16.13 ± 0.62 | 17.37 ± 0.97 |
| Final | 16.25 ± 0.64 | 17.31 ± 0.45 | 17.30 ± 0.36 | 17.76 ± 0.84 |
All values are mean ± sem for n = 6–7 mice.
P < 0.05, different from sedentary chow control via two-way ANOVA followed by Tukey post hoc test.
P < 0.05, different from sedentary HFD-fed control by two-way ANOVA followed by Tukey post hoc test.
Exercise prevents diet-induced obesity
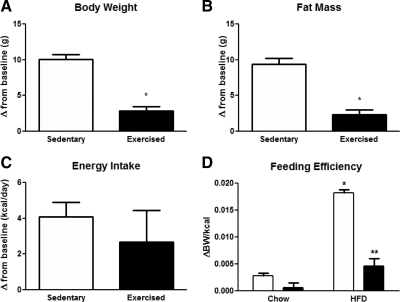
To determine whether voluntary exercise can prevent diet-induced obesity, we exposed mice to HFD and running wheels for 6 wk, and we compared energy balance and body composition to sedentary mice exposed to the same diet. Exercise markedly attenuated HFD-induced weight gain [t(12) = 7.59, P < 0.001; Fig. 2A and Supplemental Fig. 1D]. Fat mass at the end of the study was significantly increased in the sedentary HF group compared with baseline [10.47 ± 0.84 g vs. 1.08 ± 0.12 g, respectively; t(6) = 11.22, P < 0.05] (Supplemental Fig. 1E and Table 1). Although both sedentary and exercised groups gained fat mass on HFD, the change in fat mass was markedly and significantly lower in exercised mice compared with sedentary [2.31 ± 0.67 g vs. 9.39 ± 0.84 g, t(12) = 6.605, P < 0.0001] (Fig. 2B). Cumulative and daily food intake was not changed in sedentary vs. exercised mice regardless of diet (Fig. 2C, Table 1, and Supplemental Fig. 1, C and F). Because both HFD and exercise affected BW without changing daily calorie intake, we calculated the feeding efficiency in each group (Fig. 2D). Interestingly, HFD markedly increased feeding efficiency [diet, F(1,1) = 101.80; P < 0.0001] (Fig. 2D), whereas exercise greatly attenuated this increase [interaction: F(1,1) = 35.17, P < 0.05; exercise: F(1,1) = 66.82, P < 0.0001] (Fig. 2D). HFD did not change running wheel activity. Average night-time distance and velocity were not significantly different between chow and HF groups (Supplemental Fig. 3). Additionally, exercised mice had a similar running profile regardless of diet (data not shown), resulting in similar cumulative running distance (Supplemental Fig. 3).
Fig. 2.
Exercise prevents the obesigenic effects of HFD. Changes in BW (A), fat mass (B), and daily energy intake (C) in mice fed HFD ad libitum with or without access to running wheels for 6 wk. Feeding efficiency (D) of chow and HFD-fed sedentary and exercised mice. Each value is the mean ± sem for n = 6–7 mice per group. A and B: *, P < 0.05 Student's t test; D: *, P < 0.05, significantly different from sedentary control; **, P < 0.05, significantly different from SH by two-way ANOVA followed by Tukey post hoc test.
After 6 wk, hormonal analysis revealed that exercise decreased both insulin and leptin levels (nonsignificant) in the chow-fed group (Table 1). Exposure to HFD resulted in hyperinsulinemia and hyperleptinemia in sedentary animals [insulin: F(1,1) = 28.21, P < 0.0001; leptin: F(1,1) = 64.64, P < 0.0001] (Table 1). Exercise reduced hyperleptinemia to levels consistent with the reduced fat mass of exercised mice on HFD [F(1,1) = 4.34, P < 0.05]. Exercise also reduced the hyperinsulinemia induced by HFD, suggesting improved insulin sensitivity [F(1,1) = 8.81, P < 0.01] (Table 1).
Effect of diet and exercise on chronic neuronal activation
To determine the effect of diet and exercise on the chronic activation of CNS neurons, we measured the expression of ΔFosB (Figs. 3A and 4A, respectively), a transcription factor that is induced and accumulates in specific regions of the brain after chronic perturbations (19, 20). FosB antibodies do not discriminate between FosB and ΔFosB. However, unlike FosB (0.5–2 h half-life), ΔFosB has a very long half-life (1–2 wk) (20). Thus, to eliminate exercise- or diet-dependent activation of FosB in the short term, we harvested brains at least 4 h after the last bout of exercise or food availability. FosB immunoreactivity (IR) was not changed in areas of the hypothalamus known to control energy homeostasis, such as the ARC and PVN (Table 2). However, exercise significantly increased FosB IR in the dorsomedial portion of the VMH (dmVMH) of both chow and HFD animals compared with their sedentary controls [exercise, F(1,1) = 8.82; P < 0.05] (Fig. 3, B and C). HFD exposure decreased FosB IR in both sedentary and exercised groups [diet, F(1,1) = 13.45; P < 0.05] (Fig. 3, B and C), suggesting that exercise and HFD have opposite effects on the activity of neurons that control energy balance and/or metabolic homeostasis (Fig. 3, B and D). To determine whether the effects of exercise and HFD on neuronal activation were dependent on fat mass, we measured FosB IR in sedentary mice receiving chow or HFD (SC and SH groups), exercised mice receiving HFD (EH group), and sedentary mice receiving HFD, but calorie restricted to match their BW and fat mass to that of exercise mice (SR group). Sedentary mice gained BW and fat mass, whereas exercised mice on HFD did not (Supplemental Fig. 4). Again, FosB IR was decreased in SH and increased in EH mice, but it was not significantly increased in SR mice in comparison with SH, indicating that the effect of exercise on VMH neuronal activation is independent of its weight-reducing effects [F(3,12) = 3.85; P < 0.05] (Fig. 3, D and E).
Fig. 3.
Exercise activates a distinct subset of neurons in VMH. Diagram of the mouse brain depicting the hypothalamus (A). Quantitative analysis of FosB IR of the dmVMH from SC, EC, SH, and EH groups (B) and representative corresponding IHC images (C). Quantitative analysis of FosB IR of the dmVMH of SC, SH, EH, and SR groups (D) and representative corresponding IHC images (E). Each value is the mean ± sem for n = 5–7 mice per group. B: *, P < 0.05, significantly different from SC control; **, P < 0.05, significantly different from SH mice by two-way ANOVA followed by Tukey post hoc test; D: *, P < 0.05, by one-way ANOVA followed by Tukey post hoc test. v, Ventricle (third).
Fig. 4.
Exercise activates a distinct subset of neurons in NAc. Diagram of the mouse brain depicting the core of the NAc (A). Quantitative analysis of FosB IR of the NAc from SC, EC, SH, and EH groups (B) and representative corresponding IHC images (C). Quantitative analysis of FosB IR of the NAc of SC, SH, EH, and SR groups (D) and representative corresponding IHC images (E). Each value is the mean ± sem for n = 5–7 mice per group. B: *, P < 0.05, significantly different from SC control by two-way ANOVA followed by Tukey post hoc test; D: *, P < 0.05, by one-way ANOVA followed by Tukey post hoc test. ac, Anterior commissure.
Table 2.
Hypothalamic and hippocampal FosB IR (cells/μm2 × 103)
| Chow |
High fat |
|||
|---|---|---|---|---|
| Sedentary | Exercised | Sedentary | Exercised | |
| ARC | 4.90 ± 1.20 | 4.2 ± 1.80 | 5.10 ± 2.90 | 5.30 ± 2.00 |
| PVN | 0.79 ± 0.52 | 0.80 ± 0.53 | 0.82 ± 0.62 | 0.63 ± 0.35 |
| DG | 7.80 ± 1.30 | 8.10 ± 0.80 | 7.50 ± 0.70 | 7.0 ± 1.2 |
All values are mean ± sem for six to seven mice. All comparisons P > 0.05 in two-way ANOVA.
Consistent with previous reports (19), exercise increased FosB IR in the NAc of chow-fed animals (Fig. 4, B and C), confirming that exercise stimulates neuronal pathways involved in reward circuits. In sedentary mice, HFD significantly increased FosB IR in the NAc compared with chow-fed mice but did not potentiate the effect of exercise [interaction, F(1,1) = 9.83; P < 0.05] (Fig. 4, B and C). Unlike the VMH, the NAc of sedentary mice on HFD that were calorically restricted to match the BW of exercised mice (SR) displayed significantly increased FosB IR, compared with SC mice, similar to the effect in SH and EH mice [F(3,13) = 6.11; P < 0.01] (Fig. 4, D and E). FosB expression did not differ in the DG (Table 2).
Neurons activated by exercise are leptin targets
We next determined whether the VMH neurons activated by exercise contain the leptin receptor. To this end, we used a reporter mouse that expresses GFP in all LRb+ cells (LRb-GFP) (15). After 4 wk of voluntary running wheel exercise, GFP colocalized with all FosB+ neurons in dmVMH (Fig. 5, A–E).
Exercise improves leptin resistance in mice fed HFD
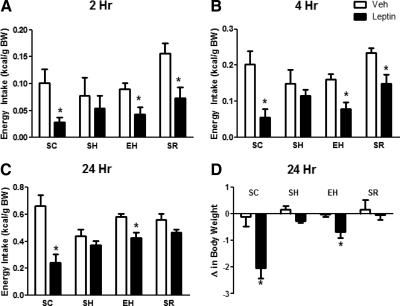
Because voluntary exercise is known to increase CNS sensitivity to leptin (13), we asked whether exercise could prevent the onset of leptin resistance secondary to exposure to HFD and weight gain. ICV leptin at 2, 4, and 24 h resulted in a significant reduction in the caloric intake of chow-fed sedentary mice [interaction, F(3,3) = 5.37; P < 0.01]. By contrast, leptin failed to significantly reduce caloric intake in SH, indicating that they are leptin resistant (Fig. 6C). Exercise partly restored the ability of ICV leptin to significantly reduce food intake at 2, 4, and 24 h (Fig. 6C). To determine whether the effect of exercise on leptin sensitivity was simply secondary to the decreased fat mass of the EH group, we restricted the calorie intake of a group of sedentary mice to prevent weight gain of HFD and match fat mass to that of exercised mice (SR) (Supplemental Fig. 4). ICV leptin significantly decreased food intake in SR mice at 2 and 4 h, but this effect was no longer significant at 24 h (Fig. 6C). ICV leptin did not lower BW of SH or SR mice. By contrast, leptin significantly decreased BW of EH mice (Fig. 6D). Notably, although ICV leptin in the EH group was able to significantly decrease food intake and BW compared with vehicle, this effect did not completely fully restore the anorectic and weight-reducing potency of leptin observed in lean mice on chow diet (SC group). Immediately after the leptin injections, we returned exercised mice to their cages with running wheels, and we measured the effect of ICV leptin on their running activity. We found no differences in the 24-h distance run between vehicle and leptin treatments (8.19 ± 1.72 vs.7.12 ± 1.92 km, respectively).
Fig. 6.
Exercise prevents leptin resistance induced by HFD, independent of body fat. Food intake at 2, 4, and 24 h, respectively, after ICV injection of vehicle (Veh) or leptin (A–C). Change in BW at 24 h after ICV injection of vehicle and leptin (2 μg) (D). Each value is the mean ± sem for n = 8–11 mice per group. *, P < 0.05, different from vehicle treatment by two-way RM ANOVA with Tukey post hoc test.
Discussion
These experiments investigate the effects of voluntary exercise on energy balance during exposure to low-fat diet or HFD. One major finding of these studies is that in this paradigm, voluntary exercise increases energy expenditure and lowers fat mass, underscoring the importance of exercise as primary intervention for weight loss and reduction of body adiposity. Interestingly, our data show that exercised mice display increased energy expenditure for at least 3 d after the cessation of wheel running, because running wheels were not present in the calorimeter cages. This finding suggests that exercise, in addition to expend more energy through enhanced locomotor activity, stimulates adaptive thermogenesis. However, the mechanisms for this effect of exercise are still unknown (21). We cannot ascribe this increase to spontaneous physical activity or to increase lean body mass, because these parameters were not changed in our experiments. It is important to note, however, that this increase in energy expenditure after exercise might be short lived, because we found that exercised mice deprived of running wheels regain fat mass within 2 wk (data not shown).
These results support the notion that exercise reduces adiposity and diet-induced obesity and represents an effective primary intervention for the prevention and treatment of obesity (12). Our data have two major implications: 1) voluntary exercise can promote increased energy expenditure resulting in a lower fat mass and, more importantly, can protect against dietary obesity; and 2) these effects must be mediated by changes in the CNS centers that control energy homeostasis.
Thus, we sought to identify CNS neurons activated by voluntary exercise during exposure to low-fat diet or HFD. We observed that exercise significantly activates a subset of neurons in the dmVMH of the hypothalamus. Interestingly, exercise and HFD were found to have opposite effects on both energy homeostasis and neuronal activation in the VMH, i.e. although exercise promotes leanness and activates VMH neurons, maintenance on a HFD induces weight gain and decreases neuronal activation in VMH. This result suggests that these VMH neurons are implicated in the regulation of BW and respond in opposite directions to environmental conditions, such as diet and exercise.
We found no changes in FosB IR in other hypothalamic nuclei, including the ARC and PVN, which are also known to regulate energy and glucose homeostasis. The lack of FosB IR in these nuclei does not completely rule out their possible involvement in mediating some effects of exercise on energy balance and body composition. The VMH has long been implicated in the control of energy balance and glucose homeostasis (22). In addition, leptin action in the VMH modulates energy homeostasis. The infusion of leptin into the VMH improves glucose metabolism and decreases BW (23), whereas genetic ablation of LRb in the VMH causes hyperphagia and obesity (20). Moreover, the leptin-induced excitability of neurons in the VMH, but not ARC, is diminished in offspring of DIO rats (24). Because we found that exercise-induced FosB+ neurons coexpress LRb, it is possible that these VMH neurons might be implicated in the beneficial effects of exercise via modulation of their leptin sensitivity. Indeed, we found that exercise improves resistance to CNS leptin's anorectic and BW effects in HFD-fed mice, as previously described in DIO rats (13). On the other hand, prevention of weight gain by calorie restriction improved the anorectic action of leptin in the short term (2 and 4 h) but did not improve the ability of leptin to decrease BW (Fig. 6). The better weight-reducing effect of exercise might be explained by a more lasting anorectic effect of leptin at 24 h in exercised compared with calorie-restricted mice (Fig. 6C). It is also possible that ICV leptin is more effective in increasing energy expenditure in exercised than in calorie-restricted mice by activating basal metabolic rate or physical activity. In this regard, there is evidence that ICV leptin can increase spontaneous physical activity in rats (25) and in ob/ob mice but not in wild-type mice (26). Also, leptin can acutely increase wheel running in ob/ob but not in wild-type mice (26). In our experiment, we did not find any change in wheel running in the 24 h after leptin administration. However, because we did not measure spontaneous physical activity or metabolic rate, we cannot exclude or confirm an acute effect of leptin on these parameters. These novel findings suggest that weight loss through exercise improves CNS leptin action via mechanisms distinct from those of weight loss from calorie restriction. Further studies are needed to establish whether CNS leptin action is required for the beneficial effects of exercise and in what areas of the brain exercise improves leptin action.
Interestingly, both exercise and HFD independently activated neurons in the NAc. This is consistent with earlier observations that link the activation of ΔFosB in NAc with increased physical activity (19, 27). The NAc is a major component of the brain's reward circuitry (28). Because fatty foods and leptin have opposite effects on food reward behavior (29), it is possible that exercise might restrain food intake through the activation of these reward circuitries and consequently counteract or compete for the reward and/or motivation associated with the consumption of fatty food. However, our data cannot establish whether there is any interaction between HFD and exercise in NAc. Because calorie restriction in HFD mice does not abolish the effect of diet in NAc activation, the simplest explanation is that exercise has no effect on NAc activation in mice on HFD. Nonetheless, the pattern of neuronal activation of exercise in lean mice points to a potential role for exercise to restrain food intake by modulating homeostatic (hypothalamic VMH) and nonhomeostatic (reward circuits, NAc) centers that control food intake.
In conclusion, we report that voluntary exercise promotes leanness and protects against diet-induced obesity. These effects are associated with improved leptin sensitivity and with exercise-specific neuronal activation in VMH, independent of changes in fat mass. These novel findings underscore the powerful role of increased physical activity in protecting against obesity, and point to the involvement of specific brain areas as key determinants for the beneficial effects of exercise.
Acknowledgments
We thank Stephen C. Woods and James Herman for helpful discussions and Maureen Fitzgerald, Michael Haas, Sonia Lipp, and Jack Magrisso for outstanding technical support.
This work was supported by an American Heart Association predoctoral fellowship grant (K.A.K.C.), the National Institutes of Health Grant DK078283, The American Diabetes Association Research Grant 707RA116, and the Cincinnati Mouse Metabolic Phenotyping Center Grant DK59630 (to S.O.).
Disclosure Summary: The authors have nothing to disclose.
Footnotes
- ARC
- Arcuate nucleus
- BW
- body weight
- CNS
- central nervous system
- DG
- dentate gyrus of the hippocampus
- dmVMH
- dorsomedial portion of the VMH
- EC
- exercised chow
- EH
- exercised HFD
- GFP
- green fluorescent protein
- HFD
- high-fat diet
- ICV
- intracerebroventricular
- IHC
- immunohistochemistry
- IR
- immunoreactivity
- KPBS
- Krebs phosphate buffered saline solution
- LRb
- long form of the leptin receptor
- NAc
- nucleus accumbens
- PVN
- periventricular nucleus
- SC
- sedentary chow
- SH
- sedentary HFD
- SR
- SH restricted
- VMH
- ventromedial hypothalamus.
References
- 1. Sullivan PW, Morrato EH, Ghushchyan V, Wyatt HR, Hill JO. 2005. Obesity, inactivity, and the prevalence of diabetes and diabetes-related cardiovascular comorbidities in the US, 2000–2002. Diabetes Care 28:1599–1603 [DOI] [PubMed] [Google Scholar]
- 2. Hayes C, Kriska A. 2008. Role of physical activity in diabetes management and prevention. J Am Diet Assoc 108:S19–S23 [DOI] [PubMed] [Google Scholar]
- 3. Warburton DER, Nicol CW, Bredin SSD. 2006. Health benefits of physical activity: the evidence. Can Med Assoc J 174:801–809 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Fogelholm M. 2010. Physical activity, fitness and fatness: relations to mortality, morbidity and disease risk factors. A systematic review. Obes Rev 11:202–221 [DOI] [PubMed] [Google Scholar]
- 5. Hopkins M, King NA, Blundell JE. 2010. Acute and long-term effects of exercise on appetite control: is there any benefit for weight control? Curr Opin Clin Nutr Metab Care 13:635–640 [DOI] [PubMed] [Google Scholar]
- 6. Melzer K, Kayser B, Saris WH, Pichard C. 2005. Effects of physical activity on food intake. Clin Nutr 24:885–895 [DOI] [PubMed] [Google Scholar]
- 7. Church TS, Martin CK, Thompson AM, Earnest CP, Mikus CR, Blair SN. 2009. Changes in weight, waist circumference and compensatory responses with different doses of exercise among sedentary, overweight postmenopausal women. PLoS ONE 4:e4515. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Schwartz MW, Woods SC, Porte D, Jr, Seeley RJ, Baskin DG. 2000. Central nervous system control of food intake. Nature 404:661–671 [DOI] [PubMed] [Google Scholar]
- 9. Finlayson G, Caudwell P, Gibbons C, Hopkins M, King N, Blundell J. 20 September 2010. Low fat loss response after medium-term supervised exercise in obese is associated with exercise-induced increase in food reward. J Obes 10.1155/2011/615624 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Martins C, Kulseng B, King NA, Holst JJ, Blundell JE. 2010. The effects of exercise-induced weight loss on appetite-related peptides and motivation to eat. J Clin Endocrinol Metab 95:1609–1616 [DOI] [PubMed] [Google Scholar]
- 11. Mayer J, Marshall NB, Vitale JJ, Christensen JH, Mashayekhi MB, Stare FJ. 1954. Exercise, food intake and body weight in normal rats and genetically obese adult mice. Am J Physiol 177:544–548 [DOI] [PubMed] [Google Scholar]
- 12. Patterson CM, Levin BE. 2008. Role of exercise in the central regulation of energy homeostasis and in the prevention of obesity. Neuroendocrinology 87:65–70 [DOI] [PubMed] [Google Scholar]
- 13. Patterson CM, Bouret SG, Dunn-Meynell AA, Levin BE. 2009. Three weeks of postweaning exercise in DIO rats produces prolonged increases in central leptin sensitivity and signaling. Am J Physiol Regul Integr Comp Physiol 296:R537–R548 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Shapiro A, Matheny M, Zhang Y, Tümer N, Cheng KY, Rogrigues E, Zolotukhin S, Scarpace PJ. 2008. Synergy between leptin therapy and a seemingly negligible amount of voluntary wheel running prevents progression of dietary obesity in leptin-resistant rats. Diabetes 57:614–622 [DOI] [PubMed] [Google Scholar]
- 15. Chee MJ, Myers MG, Jr, Price CJ, Colmers WF. 2010. Neuropeptide Y suppresses anorexigenic output from the ventromedial nucleus of the hypothalamus. J Neurosci 30:3380–3390 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Pfluger PT, Herranz D, Velasco-Miguel S, Serrano M, Tschöp MH. 2008. Sirt1 protects against high-fat diet-induced metabolic damage. Proc Natl Acad Sci USA 105:9793–9798 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Paxinos G, Franklin KBJ. 2001. The mouse brain in stereotaxic coordinates. San Diego, CA: Academic Press [Google Scholar]
- 18. Flak JN, Ostrander MM, Tasker JG, Herman JP. 2009. Chronic stress-induced neurotransmitter plasticity in the PVN. J Comp Neurol 517:156–165 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Werme M, Messer C, Olson L, Gilden L, Thorén P, Nestler EJ, Brené S. 2002. ΔFosB regulates wheel running. J Neurosci 22:8133–8138 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Dhillon H, Zigman JM, Ye C, Lee CE, McGovern RA, Tang V, Kenny CD, Christiansen LM, White RD, Edelstein EA, Coppari R, Balthasar N, Cowley MA, Chua S, Jr, Elmquist JK, Lowell BB. 2006. Leptin directly activates SF1 neurons in the VMH, and this action by leptin is required for normal body-weight homeostasis. Neuron 49:191–203 [DOI] [PubMed] [Google Scholar]
- 21. Lowell BB, Spiegelman BM. 2000. Towards a molecular understanding of adaptive thermogenesis. Nature 404:652–660 [DOI] [PubMed] [Google Scholar]
- 22. Miller NE, Bailey CJ, Stevenson JA. 1950. Decreased “hunger” but increased food intake resulting from hypothalamic lesions. Science 112:256–259 [DOI] [PubMed] [Google Scholar]
- 23. Minokoshi Y, Haque MS, Shimazu T. 1999. Microinjection of leptin into the ventromedial hypothalamus increases glucose uptake in peripheral tissues in rats. Diabetes 48:287–291 [DOI] [PubMed] [Google Scholar]
- 24. Irani BG, Le Foll C, Dunn-Meynell AA, Levin BE. 2009. Ventromedial nucleus neurons are less sensitive to leptin excitation in rats bres to develop diet-induced obesity. Am J Physiol Regul Integr Comp Physiol 296:R521–R527 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Choi YH, Li C, Hartzell DL, Little DE, Della-Ferra MA, Baile CA. 2008. ICV leptin effects on spontaneous physical activity and feeding behavior in rats. Behav Brain Res 188:100–108 [DOI] [PubMed] [Google Scholar]
- 26. Morton GJ, Kaiyala KJ, Fisher JD, Ogimoto K, Schwartz MW, Wisse BE. 2011. Identificantion of a physiological role for leptin in the regulation of ambulatory activity and wheel running in mice. Am J Physiol Endocrinol Metab 300:E392–E401 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Chen J, Kelz MB, Hope BT, Nakabeppu Y, Nestler EJ. 1997. Chronic Fos-related antigens: stable variants of ΔFosB induced in brain by chronic treatments. J Neurosci 17:4933–4941 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Figlewicz DP, Benoit SC. 2009. Insulin, leptin, and food reward: update 2008. Am J Physiol Regul Integr Comp Physiol 296:R9–R19 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Davis JF, Choi DL, Schurdak JD, Fitzgerald MF, Clegg DJ, Lipton JW, Figlewicz DP, Benoit SC. 2011. Leptin regulates energy balance and motivation through action at distinct neural circuits. Biol Psychiatry 69:668–674 [DOI] [PMC free article] [PubMed] [Google Scholar]