Abstract
The peptide hormone relaxin is a potent vasodilator with therapeutic potential in diseases complicated by vasoconstriction, including heart failure. However, the molecular mediators and magnitude of vasodilation may vary according to duration of exposure and artery type. The objective of these studies was to determine mechanisms of rapid (within minutes) relaxin-induced vasodilation and to examine whether relaxin dilates arteries from different animal species and vascular beds. Rat and mouse small renal, rat mesenteric, and human sc arteries were isolated, mounted in a pressure arteriograph, and treated with recombinant human relaxin (rhRLX; 1–100 ng/ml) after preconstriction with phenylephrine. Rat and mouse small renal as well as human sc arteries dilated in response to rhRLX, whereas rat mesenteric arteries did not. Endothelial removal or pretreatment with l-NG-monomethyl arginine (L-NMMA) abolished rapid relaxin-induced vasodilation; phosphatidylinositol-3-kinase (PI3K) inhibitors also prevented it. In cultured human endothelial cells, rhRLX stimulated nitric oxide (assessed using 4-amino-5-methylamino-2′7′-difluorofluorescein) as well as Akt and endothelial NO synthase (eNOS) phosphorylation by Western blotting but not increases in intracellular calcium (evaluated by fura-2). NO production was attenuated by inhibition of Gαi/o and Akt (using pertussis toxin and the allosteric inhibitor MK-2206, respectively), PI3K, and NOS. Finally, the dilatory effect of rhRLX in rat small renal arteries was unexpectedly potentiated, rather than inhibited, by pretreatment with the vascular endothelial growth factor receptor inhibitor SU5416. We conclude that relaxin rapidly dilates select arteries across a range of species. The mechanism appears to involve endothelial Gαi/o protein coupling to PI3K, Akt, and eNOS but not vascular endothelial growth factor receptor transactivation or increased calcium.
There is growing interest in the clinical potential of the potent vasodilator peptide relaxin in acute heart failure (AHF), partly due to the favorable therapeutic profile of the hormone in the cardiovascular system (1). These effects were initially described using conscious rats, in which chronic relaxin administration decreases systemic vascular resistance and increases cardiac output (mainly through enhanced stroke volume) and global arterial compliance. Relaxin also decreases renal vascular resistance, increases renal plasma flow and/or glomerular filtration rate, and inhibits myogenic constriction of isolated arteries (reviewed in Refs. 2 and 3). In a subsequent phase I study of recombinant human (H2) relaxin (rhRLX) in stable congestive heart failure, similar beneficial alterations in renal and cardiovascular parameters consistent with vasodilation were noted (4). Taken together, these preclinical findings helped motivate a phase I/II trial in AHF, in which rhRLX decreased death or readmission due to renal or heart failure in association with favorable relief of dyspnea and other clinical outcomes in these patients (5), in turn justifying an ongoing phase III trial (1, 5, 6). In view of this potential clinical application, it is important to fully elucidate the mechanisms of relaxin-induced vasodilation.
Endogenous relaxin is a 6-kDa peptide that emanates from the corpus luteum of the ovaries during pregnancy in many species including rats, mice, humans, and nonhuman primates (7). Circulating relaxin concentrations also increase during the luteal phase of the menstrual cycle in humans and nonhuman primates (7). The hormone mediates its effects primarily through the G protein-coupled receptor (GPCR) relaxin family peptide receptor (RXFP)1 (also known as leucine-rich repeat-containing GPCR7), although the related receptor RXFP2 (leucine-rich repeat-containing GPCR8) can also be activated by relaxin in vitro, albeit with much lower efficacy (8, 9). On balance, studies suggest that relaxin is an important vasoregulatory hormone in human pregnancy and that it could be administered to promote vasodilation and increase blood flow outside gestation (2, 3). In addition to renal and cardiovascular adaptations, relaxin has been invoked in several other aspects of maternal physiology during gestation, including implantation and reproductive tissue remodeling (7). However, the relative importance of relaxin in these functions may vary substantially among species.
Over the past decade, the vasodilatory mechanism of relaxin in pregnancy has been partly elucidated (reviewed in Ref. 3). Relaxin increases matrix metalloproteinase-9 or matrix metalloproteinase-2 activity (after hours or days of exposure, respectively) in the vascular wall, which are thought to serve as endothelin (ET) converting enzymes, processing big ET to ET1–32 via cleavage at a Gly-Leu bond (10, 11). ET1–32, in turn, activates the endothelial ETB receptor-nitric oxide synthase (NOS) pathway. Furthermore, emerging evidence suggests vascular endothelial growth factor (VEGF) and placental growth factor are critical (presumably upstream) players in this sustained relaxin vasodilatory pathway (3).
Fisher and colleagues (12) have also described a rapid (i.e. within minutes) dilatory response to rhRLX in isolated human arteries. Interestingly, this effect was observed in vessels obtained from gluteal biopsies, but not pulmonary tissue. Furthermore, relaxin was equipotent with prostaglandin I2 and more efficacious than atrial natriuretic peptide. Both rapid and sustained vasodilatory mechanisms might contribute to the salutary effects of rhRLX in AHF. However, little else is known about rapid relaxin-induced vasodilation. Considering that relaxin's sustained vasodilatory effects are conserved among humans, rats, and mice (3), the first aim of this study was to determine whether relaxin can induce rapid dilation in arteries from both rodents and humans. In view of the finding that the rapid vasodilatory effect of relaxin in humans is artery specific (12), we investigated different arteries in humans (small sc arteries) and in rats (small renal and mesenteric arteries).
Furthermore, the mechanisms involved in rapid relaxin-induced dilation are unknown (with the exception of a requirement for the endothelium, because the response was markedly attenuated after its removal (12). Therefore, our second aim was to determine the mechanism of rapid relaxin-induced dilation in arteries and to further interrogate these pathways using NO production by cultured human vascular cells (which we validate herein as a convenient yet robust model of human arteries). Specifically, we hypothesized that relaxin uses the phosphatidylinositol-3-kinase (PI3K)-Akt-endothelial NOS (eNOS) vasodilatory pathway (13) to effect arterial smooth muscle relaxation. Indeed, PI3K was previously identified as one mediator of the biphasic stimulation of intracellular cAMP that occurs in response to RXFP1 activation in certain cells (via Gαi3-linked βγ-subunits) (14–16). Therefore, we also examined the role of Gαi proteins in this pathway. Finally, we also investigated whether relaxin induces increases in intracellular calcium in cultured human endothelial cells because eNOS is a calcium-sensitive enzyme (13).
Last, we tested the potential role of VEGF receptors in rapid relaxin-induced vasodilation using the VEGF receptor tyrosine kinase inhibitor SU5416. The rationale for this hypothesis was based on several observations: 1) other vasoactive GPCR agonists such as bradykinin can transactivate VEGF receptors (17), 2) relaxin has been shown to increase VEGF in several nonvascular cell types (18), 3) VEGF causes vascular NO production and dilation through PI3K, Akt, and eNOS (19), and 4) VEGF is likely to be involved in relaxin's sustained vasodilatory pathway (3).
Materials and Methods
For details, see Supplemental Materials and Methods (published on The Endocrine Society's Journals Online web site at http://endo.endojournals.org).
Animals and tissue collection
The use of animals was approved by the University of Pittsburgh Institutional Animal Care and Use Committee. Female Long-Evans rats and C57B6/J mice were purchased from Harlan Laboratories (Indianapolis, IN) and housed under standard conditions (12-h light, 12-h dark cycle) with access to a PROLAB RMH 2000 feed containing 0.48% sodium (PME Feeds Inc., St. Louis, MO) and water ad libitum. At approximately 12 wk of age, rats and mice were euthanized by ip pentobarbital injection (60 mg/kg), and small renal and mesenteric arteries were harvested (20). We previously showed that both rat and mouse small renal and mesenteric arteries express the relaxin receptor RXFP1 (21).
Human sc arteries
Subcutaneous fat was obtained from abdominal reduction surgeries under the approval of the University of Pittsburgh Institutional Review Board. Samples from female patients (and one male patient) between 30 and 50 yr of age at the time of surgery were used. Given the local demographics, it is likely that some patients were insulin resistant and that as many as 25–50% were smokers. Despite these comorbidities that can result in endothelial dysfunction, the majority of human arteries studied showed robust responses to rhRLX and methacholine (vide infra). Small arteries were dissected from the fat and prepared for functional studies as described below. These sc arteries express RXFP1 mRNA by RT-PCR (Supplemental Fig. 1).
Arteriography
Arterial segments (internal diameter 100–300 μm at 60 mm Hg) were transferred to a dual-chamber isobaric arteriograph (Living Systems Instruments, Burlington, VT) containing 3 ml HEPES-buffered physiological saline solution (pH 7.4) maintained at 37 C and studied as previously reported (20). In some experiments, 0.1 mmol/liter l-arginine (NOS substrate), 0.1 mmol/liter NG-monomethyl-l-arginine acetate (l-NMMA; competitive inhibitor of NOS), 3 μmol/liter LY294002 or 10 nmol/liter wortmannin (PI3K inhibitors), 3 μmol/liter SU5416 (VEGF receptor tyrosine kinase inhibitor) or dilute dimethylsulfoxide (DMSO) vehicle (all from Sigma-Aldrich, St. Louis, MO) was added to the bath at this point. In another, the endothelium of rat small renal arteries was removed by passing an air bubble of 0.5 ml or smaller through the vessel lumen (20). The VEGF receptor tyrosine kinase and PI3K inhibitor doses selected were calculated to be approximately two to three times the known IC50 for these compounds [SU5416, ∼1 μmol/liter (22); LY294002, 1.4 μmol/liter (23); and wortmannin, ∼4 nmol/liter (24)]. Recombinant human (H2) relaxin (rhRLX; Corthera Inc., San Mateo, CA) was added to the bath in cumulative fashion at doses of 1–100 ng/ml. Vessel internal diameter was assessed using a model 1602-E filar with SM-2 processor (Lasico, Los Angeles, CA) mounted on a TS100 inverted microscope (Nikon, Melville, NY). Measurements were taken only after diameter stabilized, approximately 4 min after the addition of rhRLX or methacholine to the bath. Data are expressed as percent change in diameter from baseline (i.e. a negative change in diameter indicates vessel constriction, whereas a positive change in diameter reflects vasodilation).
Human vascular cell culture and nitric oxide measurement
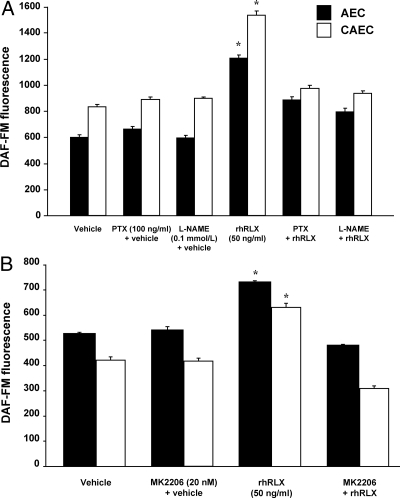
Human coronary artery endothelial cells (CAEC), aortic endothelial cells (AEC), and coronary artery smooth muscle cells (CASMC) (passage 5–7; Lonza, Walkersville, MD) were grown to approximately 80% confluency, and NO production in individual cells was measured using the fluorescent dye 4-amino-5-methylamino-2′7′-difluorofluorescein diacetate (DAF-FM DA; Invitrogen, Carlsbad, CA) (25). Some wells contained 5 μmol/liter LY294002, 10 nmol/liter wortmannin, 20 nmol/liter MK-2206 (an allosteric Akt inhibitor; Selleck Chemicals, Houston, TX) or dilute DMSO vehicle, or 0.1 mmol/liter NG-nitro-l-arginine methyl ester (l-NAME; Sigma-Aldrich). Pertussis toxin (PTX; Sigma-Aldrich) was used to inhibit Gαi/o proteins and was added to the media (100 ng/ml) the day before the experiment was conducted (∼18 h) (26).
Immunoblot detection of total Akt and eNOS and phosphorylated Akt (Ser473) and eNOS (Ser1177)
AEC were grown and subcultured into T-25 flasks or 60-mm dishes. After attaining approximately 80% confluency, medium was replaced with basal medium and cells were cultured in these serum-free conditions for 6 h. Some cells were pretreated with LY294002 (5 μmol/liter) for 30 min at 37 C. Cells were then treated with rhRLX (100 ng/ml) or dilute sodium acetate vehicle and incubated for 1 or 10 min (at 37 C when possible) before removal of the medium and rinsing with 3 ml ice-cold PBS. Cell extracts were then prepared. Protein concentration was measured using the Pierce BCA assay (Thermo Fisher Scientific, Rockford, IL). The 20-μg protein samples were resolved by SDS-PAGE. Rabbit or mouse antihuman antibodies from Cell Signaling Technologies (Akt 4685, p-Akt 9271, eNOS 9586, p-eNOS 9570; Danvers, MA) or BD Transduction Laboratories (p-eNOS 612392; San Jose, CA), respectively, were used.
Measurement of intracellular Ca2+ concentration ([Ca2+]) in endothelial cells
Global cytoplasmic [Ca2+] was measured in cultured human CAEC and AEC using the Ca2+-sensitive fluorescent dye fura-2 as previously reported (27). Cells were stimulated with vehicle (dilute sodium acetate) or rhRLX (1, 10, and 100 ng/ml) in cumulative fashion or with 100 μm ATP (Sigma-Aldrich) by direct addition to the buffer bathing the cells. The resulting Ca2+ images were quantified and expressed as fura-2 ratio.
Statistical analysis
Values are presented as mean ± se. Data were analyzed by one- or two-way ANOVA as appropriate. With few exceptions, arterial dilation data did not deviate significantly from normal distribution, as assessed by skewness and kurtosis and by the Shapiro-Wilk test of normality (PASW Statistics version 18.0; SPSS Inc., Chicago, IL). Therefore, arterial dilation was analyzed using a split-plot design whereby dose was treated as a repeated measure (SAS version 9.2; Cary, NC). If significant main effects or interactions were observed, then individual group means were compared by post hoc t tests. The DAF fluorescence in each cell is an independent observation, and therefore, the number of cells served as the n number. P < 0.05 was considered significant.
Results
Rapid relaxin-induced vasodilation is vessel but not species specific
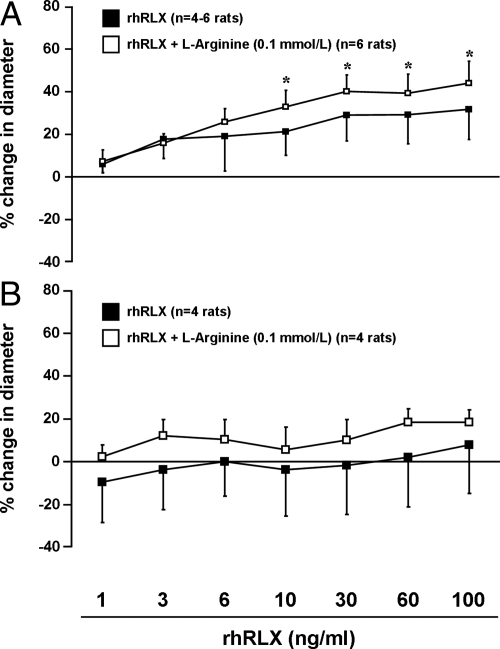
Small renal arteries isolated from virgin female Long-Evans rats (preconstricted to 50% of initial diameter with phenylephrine) dilated in response to rhRLX (1–100 ng/ml) (P < 0.05 by ANOVA; Fig. 1A). However, there was no consistent effect on rat mesenteric arteries (P > 0.05; Fig. 1B). Moreover, small renal arteries treated with the vehicle for rhRLX alone [20 mmol/liter sodium acetate (pH 5.2) diluted identically to rhRLX at each dose] did not dilate (P > 0.05; data not shown).
Fig. 1.
rhRLX induces rapid dilation of rat small renal (A) (P < 0.01 by ANOVA) but not mesenteric (B) (P > 0.05) arteries. The effect on rat small renal arteries was potentiated by pretreatment with l-arginine (0.1 mmol/liter; P < 0.01 by ANOVA). However, post hoc tests did not reach significance between individual rhRLX concentrations in the presence and absence of l-arginine; nor did the dilation beyond 6 and 10 ng/ml reach statistical significance vs. lower doses in arteries with and without l-arginine, respectively. Not all arteries were treated with the full range of rhRLX concentrations, hence the variable sample size. *, P < 0.05 vs. 1 ng/ml rhRLX.
Addition of 0.1 mmol/liter l-arginine (the substrate for NOS) to the bath modestly potentiated the dilatory response of small renal arteries to rhRLX (P < 0.01 by ANOVA; Fig. 1A), but there were no significant differences between the groups for individual rhRLX doses. In mesenteric arteries (Fig. 1B), l-arginine tended to enhance the dilatory effect of rhRLX, but this was not significant (P > 0.05).
rhRLX also dilated mouse small renal arteries (P < 0.05 by ANOVA; Supplemental Fig. 2) and sc arteries from female patients (P < 0.0001; Fig. 2A) as well as from a single male patient (30–35 yr of age; data not shown). The responses of isolated vessels to phenylephrine and methacholine (a positive control for endothelium-dependent dilation) are presented in Supplemental Table 1.
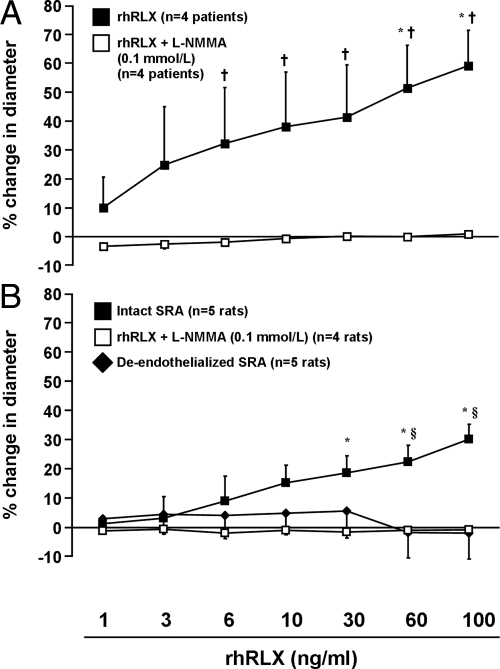
Fig. 2.
A, rhRLX induces rapid dilation of human sc arteries (P < 0.0001 by ANOVA) in a NOS-dependent manner, as shown by pretreatment with l-NMMA (0.1 mmol/liter) for 30 min (P < 0.0001 vs. rhRLX alone). B, l-NMMA pretreatment or endothelial removal also abolishes rapid relaxin-induced dilation in rat small renal arteries (SRA; P < 0.0001 by ANOVA). *, P < 0.05 vs. 1 ng/ml rhRLX; †, P < 0.05 rhRLX vs. rhRLX plus l-NMMA; §, P < 0.01 intact vs. both de-endothelialized and rhRLX plus l-NMMA.
Rapid relaxin-induced vasodilation is blocked by endothelial removal and NOS inhibition
Pretreatment with the NOS inhibitor l-NMMA (0.1 mmol/liter) abolished the effect of rhRLX in human sc arteries (Fig. 2A) and rat small renal arteries (Fig. 2B) (both P < 0.0001 by ANOVA) and attenuated mean dilatory responses to methacholine by approximately 85 and 55%, respectively (Supplemental Table 1). [The magnitude of this reduction produced by l-NMMA in rat small renal arteries is consistent with the recent report that the NO contribution to the acetylcholine response is approximately 50% (49).] Endothelial removal also prevented rapid rhRLX-induced dilation of rat small renal arteries (P < 0.0001; Fig. 2B), and methacholine-induced vasodilation was markedly attenuated by this treatment (Supplemental Table 1), confirming functional disruption of the endothelium.
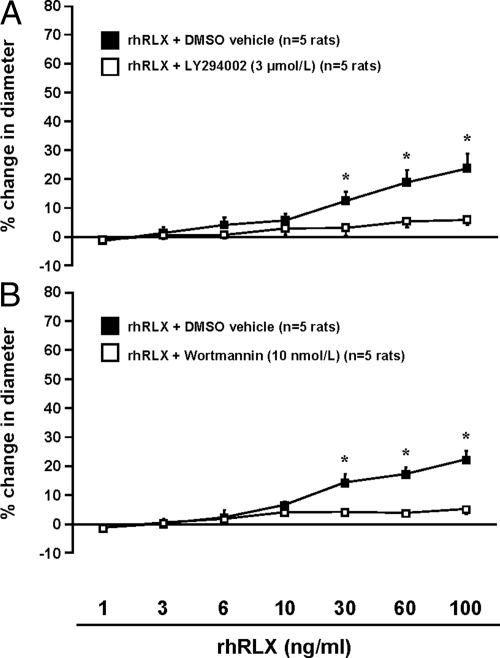
Rapid relaxin-induced vasodilation is blocked by PI3K inhibition
In rat small renal arteries, pretreatment with the PI3K inhibitors LY294002 (3 μmol/liter; Fig. 3A) or wortmannin (10 nmol/liter; Fig. 3B) blocked the response to rhRLX (both P < 0.0001 by ANOVA), although methacholine-induced vasodilation was not affected (Supplemental Table 1). Similarly, rhRLX-induced dilation of a single human sc artery was abolished by pretreatment with LY294002 (3 μmol/liter) (1.2 vs. 41.45% in the presence of DMSO vehicle with 100 ng/ml rhRLX; data not shown).
Fig. 3.
Rapid relaxin-induced vasodilation is attenuated by inhibition of PI3K (P < 0.0001 by ANOVA). Rat small renal arteries were pretreated for 30 min with LY294002 (3 μmol/liter) (A) or wortmannin (10 nmol/liter) (B), and increasing concentrations of rhRLX were added as indicated. Note that two different experiments in A (rhRLX plus LY294002 and rhRLX plus vehicle) and B (rhRLX plus wortmannin and rhRLX plus vehicle) were conducted on different arteries, but from the same rats. *, P < 0.02, DMSO vehicle vs. LY294002 or wortmannin.
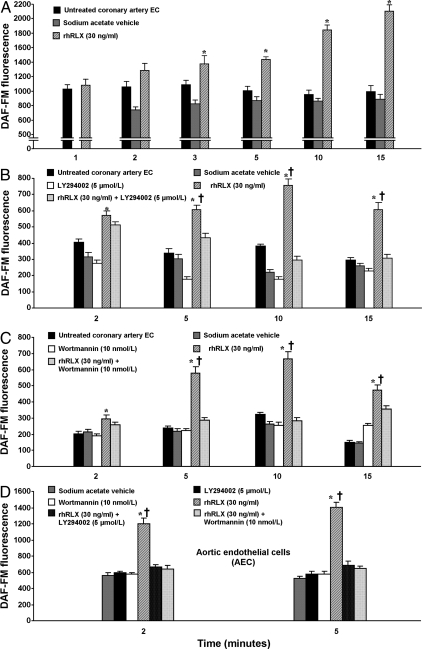
Relaxin induces rapid NO production by cultured human endothelial cells
To determine whether rhRLX directly stimulates NO production in vascular cells, cultured human CAEC and CASMC were treated with rhRLX (30 ng/ml), and NO was assessed via DAF-FM fluorescence. rhRLX stimulated time-dependent accumulation of intracellular NO in CAEC (P < 0.0001 by ANOVA; Fig. 4A), an effect that was replicated in two additional experiments at doses of 30 and 50 ng/ml (data not shown). Compared with the Ca2+ ionophore A23187 (1 μmol/liter), which activates eNOS in ligand-independent fashion, NO production in response to 50 and 100 ng/ml rhRLX was 67 and 86%, respectively, after 10 min of treatment (Supplemental Fig. 3). In control wells treated with vehicle for rhRLX alone or left untreated, intracellular NO levels did not increase (P > 0.05; Fig. 4A). Similarly, there was no increase in NO in CASMC treated with rhRLX compared with untreated cells (P value not significant by ANOVA; Supplemental Fig. 4).
Fig. 4.
Relaxin induces NO in cultured human endothelial cells (EC). A, Time course of cumulative NO production in CAEC treated with rhRLX (30 ng/ml; P < 0.0001 by ANOVA) or dilute sodium acetate vehicle and in untreated cells (representative of three separate experiments). Data were not collected for 1 min vehicle treatment. B and C, This effect is attenuated at 2, 5, 10, and 15 min by pretreatment with the PI3K inhibitors LY294002 (5 μmol/liter) (B) or wortmannin (10 nmol/liter) (C) (P < 0.0001). D, Similar studies were conducted in AEC and are presented here (representative of two separate experiments, each conducted in AEC from different donors; P < 0.0001). Note that the y-axis scales differ because individual cell lots differ in basal NO production. DAF-FM fluorescence is reported in arbitrary densitometric units. *, P < 0.05 for rhRLX vs. untreated cells; †, P < 0.0001 for rhRLX alone vs. rhRLX plus PI3K inhibitor.
Relaxin-induced NO production is mediated by PI3K and Gαi/o, and is associated with Akt and eNOS phosphorylation but not increased intracellular [Ca2+]
CAEC were then pretreated with 5 μmol/liter LY294002 (Fig. 4B) or 10 nmol/liter wortmannin (Fig. 4C) to determine whether rhRLX-induced NO is mediated by PI3K. Both inhibitors blocked production of NO by rhRLX, at least by the 5-min time point and thereafter (P < 0.0001 by ANOVA). In AEC, the increase in intracellular NO induced by rhRLX (30 ng/ml) at 2 and 5 min was completely abrogated by pretreatment with either LY294002 or wortmannin (P < 0.0001; Fig. 4D).
AEC and CAEC were next pretreated overnight with PTX (100 ng/ml) or for 30 min with l-NAME (0.1 mmol/liter) to inhibit Gαi/o and NOS, respectively. NO production in response to rhRLX (50 ng/ml) was greatly attenuated by both inhibitors (both P < 0.0001 by ANOVA; Fig. 5A). Pretreatment of AEC and CAEC with the Akt inhibitor MK-2206 (20 nmol/liter) also prevented NO production in response to rhRLX (Fig. 5B). Notably, neither this nor any of the other inhibitors used resulted in increased rates of endothelial cell apoptosis (data not shown).
Fig. 5.
Relaxin-induced NO is attenuated by inhibition of Gαi/o, NOS, and Akt. AEC and CAEC were pretreated with PTX (100 ng/ml) or l-NAME (0.1 mmol/liter) for 18 h or 30 min, respectively (A) or MK-2206 (20 nmol/liter) for 30 min (B) before the addition of rhRLX (50 ng/ml). DAF-FM fluorescence was then assessed after 5–10 min (P < 0.0001 by ANOVA for both cell types in A and B). Control dishes were treated with the vehicle for rhRLX (dilute sodium acetate) or the relevant inhibitor in the presence of vehicle. AEC and CAEC were from different donors. *, P < 0.0001, rhRLX vs. all other treatments.
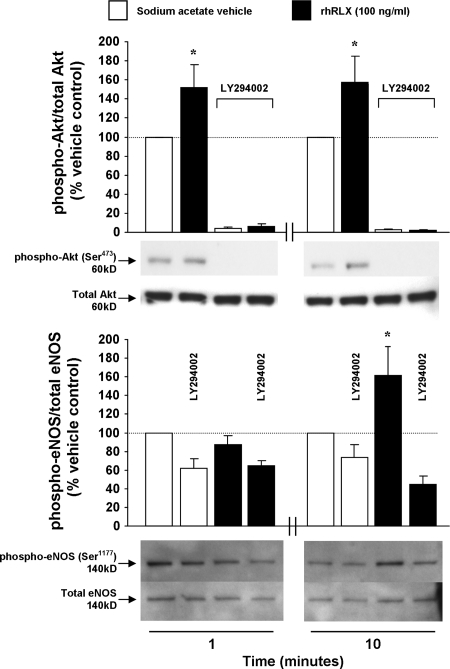
Phosphorylation and/or dephosphorylation of specific serine/threonine residues in Akt and eNOS can mediate their activation (28). Immunoblot analysis of rhRLX-treated (100 ng/ml) AEC demonstrated phosphorylation of Akt (Ser473) and eNOS (Ser1177) compared with vehicle at 1 and 10 min and compared with vehicle at 10 min, respectively; phosphorylation of both was inhibited by LY294002 (Fig. 6).
Fig. 6.
Relaxin induces phosphorylation of Akt and eNOS in aortic endothelial cells (P ≤ 0.005 by ANOVA). Quiescent cells were treated with dilute sodium acetate vehicle or rhRLX (100 ng/ml) for 1 or 10 min, and lysates were immunoblotted. Some dishes were pretreated with LY294002 (5 μmol/liter) for 30 min as indicated. Band densities are expressed as the ratio of phospho-Akt or eNOS to total Akt or eNOS, with vehicle treatment at each time point set at 100% (mean ± sem). Representative blots of n = 4 and n = 3 experiments for total/phospho-Akt and total/phospho -NOS, respectively, are shown. The antibodies used are detailed in the Materials and Methods. *, P < 0.05 vs. vehicle and LY294002 plus rhRLX treatment groups.
An alternative mechanism of agonist-induced eNOS activation (particularly at earlier time points within a minute or two) may be through mobilization of intracellular Ca2+ and subsequent Ca2+/calmodulin binding. However, studies in CAEC and AEC using the fluorescent Ca2+-binding dye fura-2 revealed no change in global cytoplasmic intracellular [Ca2+] upon stimulation with rhRLX (Supplemental Fig. 5).
Lack of a role for VEGF receptor in rapid relaxin-induced vasodilation
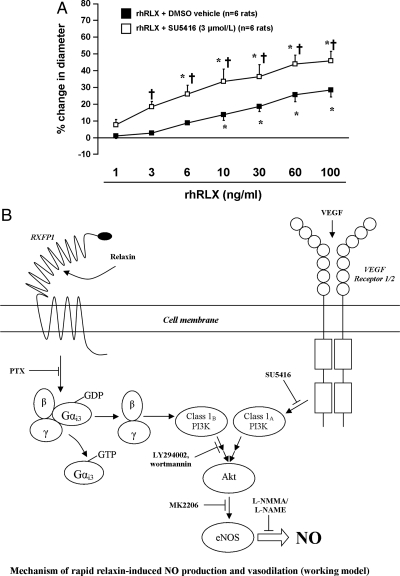
Finally, rat small renal arteries were pretreated with the small-molecule VEGF receptor tyrosine kinase inhibitor SU5416 (3 μmol/liter) to test the hypothesis that VEGF receptor transactivation may play a role in rapid relaxin-induced dilation. Interestingly, the normal dilatory response to rhRLX was significantly (P < 0.0001 by ANOVA) potentiated when arteries were pretreated with SU5416 (Fig. 7A), although the effect of methacholine was not changed (73.4 ± 8.9 vs. 77.7 ± 8.5%, DMSO vehicle vs. SU5416).
Fig. 7.
VEGF receptor tyrosine kinase inhibition potentiates rapid relaxin-induced vasodilation (P < 0.0001 by ANOVA). A, Rat small renal arteries were pretreated with SU5416 (3 μmol/liter) for 30 min, and increasing concentrations of rhRLX (1 - 100 ng/ml) were added as indicated. *, P ≤ 0.02 vs. 1 ng/ml rhRLX; †, P < 0.01 for DMSO vs. SU5416. B, Working model of the mechanism of rapid relaxin-induced vasodilation in endothelial cells. Relaxin activates RXFP1, leading to Gαi3 activation and dissociation of the corresponding βγ-subunits (blocked by PTX), which in turn activates the class IB PI3Kγ. PI3Kγ phosphorylates phosphatidylinositol lipids in the inner leaflet of the cell membrane (not shown; blocked by LY294002 and wortmannin), which promotes phosphorylation of Akt and eNOS, leading to NO generation (blocked by l-NAME/l-NMMA). This pathway shares common downstream effector components with the VEGF pathway, which could explain the potentiation of rapid relaxin-induced vasodilation in the presence of SU5416.
Discussion
The major findings of this study were 1) rhRLX rapidly dilates rat and mouse small renal as well as human sc arteries, 2) this effect is endothelium and NO dependent, and 3) PI3K mediates rapid rhRLX-induced dilation in isolated rat small renal arteries, whereas VEGF receptor transactivation is not involved. We further showed that rhRLX directly induces NO in human coronary artery and AEC (but not CASMC) in a Gαi/o-, Akt-, and PI3K-dependent manner. This was accompanied by increases in the phosphorylation of specific Akt and eNOS residues known to be associated with vasodilation but not by increases in intracellular calcium.
rhRLX-induced dilation of resistance arteries from human gluteal biopsies was first reported by Fisher et al. (12). Before these findings, it had been shown that porcine relaxin does not affect the contractility of isolated human umbilical artery strips (29) and that human relaxin does not abrogate agonist-induced tension in human myometrial or placental villous arteries (30). Fisher et al. (12) also showed that rhRLX has no vasodilatory activity in human pulmonary vessels. Therefore, relaxin-induced vasodilation in the human is specific for certain vascular beds.
Our studies in the rat are in general agreement with this concept (12), insofar as rhRLX dilated small renal but not mesenteric vessels. However, the reasons underlying this vascular bed specificity remain unclear. We previously demonstrated expression of RXFP1 mRNA and protein in rat and mouse mesenteric and small renal arteries as well as the aorta, and that female small renal arteries expressed more RXFP1 mRNA than male arteries (21), suggesting that an absence of the relaxin receptor per se cannot account for this observation. Rather, regional vascular differences in the cellular and subcellular organization of the relaxin signaling pathway and its coupling to vasodilatory effectors may be responsible. Alternatively, endothelial RXFP1 expression may be lower in mesenteric compared with small renal arteries.
Interestingly, rhRLX-induced vasodilation of rat small renal arteries was potentiated overall by l-arginine, although there was insufficient power to detect differences at individual rhRLX doses. Nonetheless, this observation supports the so-called arginine paradox, which states that eNOS activity is enhanced by extracellular l-arginine despite intracellular concentrations being greatly in excess of the Km for eNOS (28). Indeed, we previously reported that l-arginine caused inhibition of myogenic reactivity of small renal arteries from wild-type but not relaxin knockout mice, suggesting a requirement for the NOS substrate in the extracellular milieu to unmask this dilatory effect of local, vascular-derived relaxin (21).
We further confirmed that rhRLX-induced vasodilation is endothelium dependent (12) and extended these findings (by pretreating arteries and endothelial cells with l-arginine analogs) to show that NO mediates the effect. Somewhat unexpected was the relatively high fluorescent signal that persisted in endothelial cells after l-NAME treatment. One possible explanation for the background noise is auto-oxidation of DAF-FM, which elicits the same fluorescent signal as NO (31, 32). Despite this, the data clearly suggest that relaxin activates eNOS to effect rapid arterial dilation.
Using two structurally different inhibitors, we also demonstrated that rapid relaxin-induced vasodilation and NO production are mediated by PI3K. Thus, relaxin joins a long list of vasodilatory stimuli also known to be PI3K dependent, including shear stress (33), estrogen (34), and peptide agonists such as VEGF (19), insulin (35), and ghrelin (36). The class IB PI3Kγ, a heterodimer composed of a p101 regulatory subunit and a p110 catalytic subunit, is known to be regulated by GPCR (37, 38). Although some studies suggest that the p110γ catalytic subunit can be activated directly by Gβγ (39, 40), other work has shown that under physiological conditions, Gβγ likely binds p101 and thereby stimulates recruitment of p110γ to the plasma membrane (39, 41).
One caveat to PI3K inhibition in rat small renal arteries is that this treatment modestly attenuated phenylephrine preconstriction, necessitating the use of higher concentrations of the vasoconstrictor to reduce arterial diameter by 50% (EC50 in rat small renal arteries for LY294002 = 3.3×10−6 m vs. DMSO vehicle alone = 4.5×10−7 m; wortmannin = 1.5×10−6 m vs. DMSO vehicle alone = 6.4×10−7 m; see also Supplemental Table 1). Although unlikely, it is possible that the vasodilatory action of rhRLX, even at the high concentrations employed (up to 100 ng/ml), was insufficient to overcome the additional phenylephrine, independent of any effect of PI3K inhibition. Thus, it was important to show that rhRLX-induced NO production is mediated via PI3K in human CAEC and AEC. In total, we observed rhRLX-induced NO in five lots of endothelial cells (two coronary artery and three aortic) each from different donors, underscoring the reproducibility of this finding. Moreover, rhRLX-induced NO in human endothelial cells was inhibited by both LY294002 and wortmannin, analogous to the inhibition of rapid relaxin-induced dilation of isolated arteries. Interestingly, PI3K inhibitors failed to block rhRLX-induced NO production at early time points in CAEC (Fig. 4, B and C), suggesting a PI3K-independent component, perhaps a Ca2+-mediated increase in eNOS activity. However, we tested this possibility in both CAEC and AEC, and rhRLX failed to increase intracellular calcium in either cell type (Supplemental Fig. 5). In contrast, the PI3K inhibitors did block the early (2 min) induction of NO by rhRLX in AEC. It may be noteworthy, however, that the increase in NO was more robust at this early time point in the AEC than CAEC.
Gβγ also has a crucial upstream role in rhRLX-induced PI3K activation and NO production, because pretreatment with PTX (which inhibits Gαi3 activation and Gβγ subunit dissociation) inhibited NO formation in cultured human endothelial cells. To our knowledge, this is the first demonstration of relaxin using this signaling pathway in a physiological setting. Although the precise mechanism of RXFP1-Gαi3 coupling is not known, the final 10 amino acids of the RXFP1 C-terminal tail, especially Arg752, appear to be critical, as is the presence of functional membrane raft domains (26). This is particularly interesting, because these domains include caveolae, to which eNOS is predominantly localized (42). It is tempting to speculate that the spatial proximity of RXFP1, Gαi3βγ, PI3K, and eNOS within this compartment in endothelial cells may be important in rapid relaxin-induced NO production and vasodilation.
Downstream of PI3K, we showed that rhRLX promotes Akt and eNOS phosphorylation in AEC, reinforcing the functional studies using pharmacological antagonists of PI3K, which together implicate the PI3K-Akt-eNOS pathway in relaxin-induced rapid vasodilation and NO production. The fact that these phosphorylation events were inhibited by LY294002 provides further support for an intermediary role of PI3K between RXFP1 and Akt/eNOS activation. Phosphorylation of Akt was demonstrable at both the 1- and 10-min time points investigated, whereas eNOS phosphorylation was noted at 10 min only, consistent with activation of Akt first followed by that of eNOS. It is likely that if we had investigated additional time points (e.g. 2 or 5 min), eNOS phosphorylation would have been observed earlier than 10 min; however, this will need testing in future studies. That Akt is phosphorylated in response to relaxin and is involved in mediating rapid vasodilation is corroborated by additional results showing that MK-2206, a specific Akt inhibitor (43), completely prevented relaxin-induced NO production in AEC and CAEC.
A surprising finding was that inhibition of VEGF receptor tyrosine kinase potentiated rhRLX-induced vasodilation in rat small renal arteries. This refuted our hypothesis that rhRLX signaling may be mediated by transactivation of the VEGF receptor. On the one hand, a potential explanation for this finding is that, in the absence of endogenous VEGF receptor signaling (i.e. when SU5416 is present), the bioavailability of downstream effectors common to both pathways such as Akt or eNOS is increased, leading to an enhancement of rhRLX-induced signaling and a greater vasodilatory response. On the other hand, Akt and eNOS are likely to be recruited in many cell signaling pathways, and as such, by inhibiting only VEGF receptor signaling, availability of downstream constituents like Akt and eNOS may not be limiting. Another possibility is that SU5416 may also inhibit other growth factor receptor tyrosine kinases, thereby confounding interpretation.
A working model of the mechanism of rapid relaxin-induced vasodilation based on the current findings is shown in Fig. 7B.
Although the physiological significance of rapid relaxin-induced vasodilation is currently uncertain, it is present in at least three mammalian species (mouse, rat, and human) in distinct arterial beds, and is mediated by the same molecular mechanisms. These findings suggest that it is evolutionarily conserved and therefore may be important. Interestingly, serum rhRLX concentrations as low as 1.5 ng/ml were sufficient to induce significant increases in renal plasma flow during acute infusion in healthy human volunteers, in one case within 30 min of initiation of treatment (44). Although the mechanism by which rhRLX induced vasodilation in that study is unknown, the pathway described herein may have been involved.
Another possibility is that local vascular sources of relaxin drive rapid vasodilation. In support of this idea, we recently showed that several different rat artery types express relaxin mRNA and protein, as do small renal arteries from mice and the Tammar wallaby (an Australian marsupial) (21). Dschietzig et al. (45) have also shown, at least at the mRNA level, that human saphenous vein and mammary artery express H1 and H2 relaxins (both of which are thought to activate RXFP1) (46). A key question then is what stimulates relaxin production or release in vascular tissues. This is currently one focus of study in our laboratory.
With respect to AHF, the current data provide strong support for a potential therapeutic role for relaxin. We showed that endothelial cells from human aorta and coronary arteries produced NO in response to rhRLX over a time course consistent with rapid vasodilation. Other investigators have demonstrated relaxin-induced increases in myocardial perfusion of isolated rat and guinea pig hearts (47). Moreover, the clear-cut induction of rapid renal dilation by rhRLX (44) (this study) may in fact have even greater relevance to AHF, because decreased renal function is recognized as one of the most important indicators of poor prognosis in AHF patients (48).
In summary, these data suggest that rapid relaxin-induced dilation could play a physiologically relevant role in the moment-to-moment regulation of arterial tone in several species, vis-à-vis local vascular production of relaxin. Administration of exogenous rhRLX to AHF patients is likely to activate both rapid and sustained vasodilatory mechanisms (3) and thus be salutary by decreasing cardiac afterload and increasing blood flow in specific vascular beds, including the renal circulation.
Study limitations
We provide evidence but not absolute proof that relaxin-induced rapid vasodilation of small arteries is mediated ultimately by increases in NO. We did not actually measure NO in small arteries but showed that relaxin-induced rapid vasodilation was inhibited by NOS inhibitors and potentiated by the NOS substrate, l-arginine. Moreover, we showed that relaxin increases NO production in cultured human endothelial cells.
Many of relaxin's effects are dependent upon or enhanced by estrogen activity in the reproductive tract (reviewed in Ref. 7). Unfortunately, we did not assess the stage of the estrous cycle before rats were euthanized, and we do not have and cannot acquire the patient information that would allow us to report the menstrual cycle status of the women from whom arteries were studied. However, we previously demonstrated that the sustained vasodilatory response to relaxin, which transpires after hours to days of relaxin exposure is observed in ovariectomized female and male rats (reviewed in Refs. 2 and 3). Moreover, the rapid vasodilatory response was also noted in human sc arteries from one man (see Results). In future studies, it will be interesting to test whether the relaxin-induced rapid vasodilation response in arteries or NO production in cultured human endothelial cells is attenuated by estrogen receptor antagonists.
The long-term effect of relaxin, which we have dubbed the sustained vasodilatory pathway, is dependent on arterial VEGF/placental growth factor, gelatinases, the endothelial ETB receptor, and NO. Relaxin uses these molecular mechanisms to evoke long-term renal vasodilation in conscious rats (hours to days) (reviewed in Refs. 2 and 3). Relaxin also uses these molecular mechanisms to inhibit myogenic reconstriction of small renal arteries from rats and mice and small sc arteries from humans (3). The extent to which there may be interdependence or synergy between the rapid and sustained pathways of relaxin vasodilation in arteries remains to be clarified (3). This, too, will be a topic of future studies.
Acknowledgments
We thank Wei Hou (University of Florida) for assistance with statistical analysis and Laura J. Parry (University of Melbourne, Australia) for performing RT-PCR for RXFP1 on human arteries.
This work was supported by NIH RO1 DK63321, NIH RO1 HL67937, and a Grant-in-Aid (Project 00072026) and a Postdoctoral Fellowship (to J.T.M.) from the American Heart Association.
Portions of this work have appeared in abstract form: Matthews JE, Rubin JP, Novak J, Conrad KP. Relaxin (Rlx) induces fast relaxation in some rat and human arteries mediated by PI3 kinase and nitric oxide. Reprod Sci 14(Suppl 1): 114A, 2007; Novak J, Rubin JP, Matthews J, Conrad KP. Relaxin (Rlx) mediated fast relaxation of arteries through PI3 kinase and nitric oxide. FASEB J 21:A1371, 2007; McGuane JT, Sautina L, Debrah JE, Segal MS, Conrad KP. Mechanisms of relaxin-induced rapid arterial relaxation. Reprod Sci 16(Suppl 3):130A, 2009.
Disclosure Summary: K.P.C. reports several use patents for relaxin. The other authors have nothing to disclose.
Footnotes
- AEC
- Aortic endothelial cell
- AHF
- acute heart failure
- [Ca2+]
- Ca2+ concentration
- CAEC
- coronary artery endothelial cell
- CASMC
- coronary artery smooth muscle cell
- DAF-FM
- 4-amino-5-methylamino-2′7′-difluorofluorescein diacetate
- DMSO
- dimethylsulfoxide
- eNOS
- endothelial NOS
- ET
- endothelin
- GPCR
- G protein-coupled receptor
- l-NAME
- NG-nitro-l-arginine methyl ester
- l-NMMA
- NG-monomethyl-l-arginine acetate
- NOS
- nitric oxide synthase
- PI3K
- phosphatidylinositol-3-kinase
- PTX
- pertussis toxin
- rhRLX
- recombinant human relaxin
- RXFP
- relaxin family peptide receptor
- VEGF
- vascular endothelial growth factor.
References
- 1. Teichman SL, Unemori E, Dschietzig T, Conrad K, Voors AA, Teerlink JR, Felker GM, Metra M, Cotter G. 2009. Relaxin, a pleiotropic vasodilator for the treatment of heart failure. Heart Fail Rev 14:321–329 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. McGuane JT, Debrah JE, Debrah DO, Rubin JP, Segal M, Shroff SG, Conrad KP. 2009. Role of relaxin in maternal systemic and renal vascular adaptations during gestation. Ann NY Acad Sci 1160:304–312 [DOI] [PubMed] [Google Scholar]
- 3. Conrad KP. 2010. Unveiling the vasodilatory actions and mechanisms of relaxin. Hypertension 56:2–9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Dschietzig T, Teichman S, Unemori E, Wood S, Boehmer J, Richter C, Baumann G, Stangl K. 2009. Intravenous recombinant human relaxin in compensated heart failure: a safety, tolerability, and pharmacodynamic trial. J Card Fail 15:182–190 [DOI] [PubMed] [Google Scholar]
- 5. Teerlink JR, Metra M, Felker GM, Ponikowski P, Voors AA, Weatherley BD, Marmor A, Katz A, Grzybowski J, Unemori E, Teichman SL, Cotter G. 2009. Relaxin for the treatment of patients with acute heart failure (Pre-RELAX-AHF): a multicentre, randomised, placebo-controlled, parallel-group, dose-finding phase IIb study. Lancet 373:1429–1439 [DOI] [PubMed] [Google Scholar]
- 6. Augoustides JG, Riha H. 2009. Recent progress in heart failure treatment and heart transplantation. J Cardiothorac Vasc Anesth 23:738–748 [DOI] [PubMed] [Google Scholar]
- 7. Sherwood OD. 1994. Relaxin. In: Knobil E, Neill JD, Greenwald GS, Markert CL, Pfaff DW. eds. Physiology of reproduction. New York: Raven Press; 861–1009 [Google Scholar]
- 8. Hsu SY, Nakabayashi K, Nishi S, Kumagai J, Kudo M, Sherwood OD, Hsueh AJ. 2002. Activation of orphan receptors by the hormone relaxin. Science 295:671–674 [DOI] [PubMed] [Google Scholar]
- 9. Debrah JE, Agoulnik AI, Conrad KP. 2008. Changes in arterial function by chronic relaxin infusion are mediated by the leucine rich repeat G coupled Lgr7 receptor. Reprod Sci 15:217 (Abstract) [Google Scholar]
- 10. Fernandez-Patron C, Radomski MW, Davidge ST. 1999. Vascular matrix metalloproteinase-2 cleaves big endothelin-1 yielding a novel vasoconstrictor. Circ Res 85:906–911 [DOI] [PubMed] [Google Scholar]
- 11. Jeyabalan A, Novak J, Danielson LA, Kerchner LJ, Opett SL, Conrad KP. 2003. Essential role for vascular gelatinase activity in relaxin-induced renal vasodilation, hyperfiltration, and reduced myogenic reactivity of small arteries. Circ Res 93:1249–1257 [DOI] [PubMed] [Google Scholar]
- 12. Fisher C, MacLean M, Morecroft I, Seed A, Johnston F, Hillier C, McMurray J. 2002. Is the pregnancy hormone relaxin also a vasodilator peptide secreted by the heart? Circulation 106:292–295 [DOI] [PubMed] [Google Scholar]
- 13. Dimmeler S, Fleming I, Fisslthaler B, Hermann C, Busse R, Zeiher AM. 1999. Activation of nitric oxide synthase in endothelial cells by Akt-dependent phosphorylation. Nature 399:601–605 [DOI] [PubMed] [Google Scholar]
- 14. Halls ML, Bathgate RA, Summers RJ. 2006. Relaxin family peptide receptors RXFP1 and RXFP2 modulate cAMP signaling by distinct mechanisms. Mol Pharmacol 70:214–226 [DOI] [PubMed] [Google Scholar]
- 15. Nguyen BT, Yang L, Sanborn BM, Dessauer CW. 2003. Phosphoinositide 3-kinase activity is required for biphasic stimulation of cyclic AMP by relaxin. Mol Endocrinol 17:1075–1084 [DOI] [PubMed] [Google Scholar]
- 16. Shpakov AO, Kuznetsova LA, Plesneva SA, Pertseva MN. 2004. Role of phosphatidylinositol-3-kinase and protein kinase C zeta in the adenylate cyclase signal mechanism of action of relaxin in muscle tissues of rats and mollusks. Bull Exp Biol Med 138:372–375 [DOI] [PubMed] [Google Scholar]
- 17. Thuringer D, Maulon L, Frelin C. 2002. Rapid transactivation of the vascular endothelial growth factor receptor KDR/Flk-1 by the bradykinin B2 receptor contributes to endothelial nitric-oxide synthase activation in cardiac capillary endothelial cells. J Biol Chem 277:2028–2032 [DOI] [PubMed] [Google Scholar]
- 18. Unemori EN, Erikson ME, Rocco SE, Sutherland KM, Parsell DA, Mak J, Grove BH. 1999. Relaxin stimulates expression of vascular endothelial growth factor in normal human endometrial cells in vitro and is associated with menometrorrhagia in women. Hum Reprod 14:800–806 [DOI] [PubMed] [Google Scholar]
- 19. LeBlanc AJ, Shipley RD, Kang LS, Muller-Delp JM. 2008. Age impairs Flk-1 signaling and NO-mediated vasodilation in coronary arterioles. Am J Physiol 295:H2280–H2288 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Novak J, Ramirez RJJ, Gandley RE, Sherwood OD, Conrad KP. 2002. Myogenic reactivity is reduced in small renal arteries isolated from relaxin-treated rats. Am J Physiol 283:R349–R355 [DOI] [PubMed] [Google Scholar]
- 21. Novak J, Parry LJ, Matthews JE, Kerchner LJ, Indovina K, Hanley-Yanez K, Doty KD, Debrah DO, Shroff SG, Conrad KP. 2006. Evidence for local relaxin ligand-receptor expression and function in arteries. FASEB J 20:2352–2362 [DOI] [PubMed] [Google Scholar]
- 22. Fong TA, Shawver LK, Sun L, Tang C, App H, Powell TJ, Kim YH, Schreck R, Wang X, Risau W, Ullrich A, Hirth KP, McMahon G. 1999. SU5416 is a potent and selective inhibitor of the vascular endothelial growth factor receptor (Flk-1/KDR) that inhibits tyrosine kinase catalysis, tumor vascularization, and growth of multiple tumor types. Cancer Res 59:99–106 [PubMed] [Google Scholar]
- 23. Vlahos CJ, Matter WF, Hui KY, Brown RF. 1994. A specific inhibitor of phosphatidylinositol 3-kinase, 2-(4-morpholinyl)-8-phenyl-4H-1-benzopyran-4-one (LY294002). J Biol Chem 269:5241–5248 [PubMed] [Google Scholar]
- 24. Wipf P, Halter RJ. 2005. Chemistry and biology of wortmannin. Org Biomol Chem 3:2053–2061 [DOI] [PubMed] [Google Scholar]
- 25. Kojima H, Urano Y, Kikuchi K, Higuchi T, Hirata Y, Nagano T. 1999. Fluorescent indicators for imaging nitric oxide production. Angew Chem Int Ed Engl 38:3209–3212 [DOI] [PubMed] [Google Scholar]
- 26. Halls ML, van der Westhuizen ET, Wade JD, Evans BA, Bathgate RA, Summers RJ. 2009. Relaxin family peptide receptor (RXFP1) coupling to G(α)i3 involves the C-terminal Arg752 and localization within membrane raft microdomains. Mol Pharmacol 75:415–428 [DOI] [PubMed] [Google Scholar]
- 27. Jarajapu YP, Knot HJ. 2002. Role of phospholipase C in development of myogenic tone in rat posterior cerebral arteries. Am J Physiol Heart Circ Physiol 283:H2234–H2238 [DOI] [PubMed] [Google Scholar]
- 28. Wyatt AW, Steinert JR, Mann GE. 2004. Modulation of the l-arginine/nitric oxide signalling pathway in vascular endothelial cells. Biochem Soc Symp 71:143–156 [DOI] [PubMed] [Google Scholar]
- 29. Dombrowski MP, Savoy-Moore RT, Swartz K, Churchill PC, Mariona FG, Greenwood FC, Bryant-Greenwood GD, Evans MI. 1986. Effect of porcine relaxin on the human umbilical artery. J Reprod Med 31:467–472 [PubMed] [Google Scholar]
- 30. Petersen LK, Svane D, Uldbjerg N, Forman A. 1991. Effects of human relaxin on isolated rat and human myometrium and uteroplacental arteries. Obstet Gynecol 78:757–762 [PubMed] [Google Scholar]
- 31. Itoh Y, Ma FH, Hoshi H, Oka M, Noda K, Ukai Y, Kojima H, Nagano T, Toda N. 2000. Determination and bioimaging method for nitric oxide in biological specimens by diaminofluorescein fluorometry. Anal Biochem 287:203–209 [DOI] [PubMed] [Google Scholar]
- 32. Balcerczyk A, Soszynski M, Bartosz G. 2005. On the specificity of 4-amino-5-methylamino-2′,7′-difluorofluorescein as a probe for nitric oxide. Free Radic Biol Med 39:327–335 [DOI] [PubMed] [Google Scholar]
- 33. Loufrani L, Retailleau K, Bocquet A, Dumont O, Danker K, Louis H, Lacolley P, Henrion D. 2008. Key role of α1β1-integrin in the activation of PI3-kinase-Akt by flow (shear stress) in resistance arteries. Am J Physiol 294:H1906–H1913 [DOI] [PubMed] [Google Scholar]
- 34. Guo X, Razandi M, Pedram A, Kassab G, Levin ER. 2005. Estrogen induces vascular wall dilation: Mediation through kinase signaling to nitric oxide and estrogen receptors α and β. J Biol Chem 280:19704–19710 [DOI] [PubMed] [Google Scholar]
- 35. Zeng G, Quon MJ. 1996. Insulin-stimulated production of nitric oxide is inhibited by wortmannin. Direct measurement in vascular endothelial cells. J Clin Invest 98:894–898 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Iantorno M, Chen H, Kim J-a, Tesauro M, Lauro D, Cardillo C, Quon MJ. 2007. Ghrelin has novel vascular actions that mimic PI 3-kinase-dependent actions of insulin to stimulate production of NO from endothelial cells. Am J Physiol 292:E756–E764 [DOI] [PubMed] [Google Scholar]
- 37. Stephens L, Smrcka A, Cooke FT, Jackson TR, Sternweis PC, Hawkins PT. 1994. A novel phosphoinositide 3 kinase activity in myeloid-derived cells is activated by G protein βγ-subunits. Cell 77:83–93 [DOI] [PubMed] [Google Scholar]
- 38. Stoyanov B, Volinia S, Hanck T, Rubio I, Loubtchenkov M, Malek D, Stoyanova S, Vanhaesebroeck B, Dhand R, Nürnberg B. 1995. Cloning and characterization of a G protein-activated human phosphoinositide-3 kinase. Science 269:690–693 [DOI] [PubMed] [Google Scholar]
- 39. Krugmann S, Hawkins PT, Pryer N, Braselmann S. 1999. Characterizing the interactions between the two subunits of the p101/p110γ phosphoinositide 3-kinase and their role in the activation of this enzyme by Gβγ subunits. J Biol Chem 274:17152–17158 [DOI] [PubMed] [Google Scholar]
- 40. Leopoldt D, Hanck T, Exner T, Maier U, Wetzker R, Nürnberg B. 1998. Gβγ stimulates phosphoinositide 3-kinase-γ by direct interaction with two domains of the catalytic p110 subunit. J Biol Chem 273:7024–7029 [DOI] [PubMed] [Google Scholar]
- 41. Stephens LR, Eguinoa A, Erdjument-Bromage H, Lui M, Cooke F, Coadwell J, Smrcka AS, Thelen M, Cadwallader K, Tempst P, Hawkins PT. 1997. The Gβγ sensitivity of a PI3K is dependent upon a tightly associated adaptor, p101. Cell 89:105–114 [DOI] [PubMed] [Google Scholar]
- 42. Shaul PW, Smart EJ, Robinson LJ, German Z, Yuhanna IS, Ying Y, Anderson RG, Michel T. 1996. Acylation targets endothelial nitric-oxide synthase to plasmalemmal caveolae. J Biol Chem 271:6518–6522 [DOI] [PubMed] [Google Scholar]
- 43. Hirai H, Sootome H, Nakatsuru Y, Miyama K, Taguchi S, Tsujioka K, Ueno Y, Hatch H, Majumder PK, Pan BS, Kotani H. 2010. MK-2206, an allosteric Akt inhibitor, enhances antitumor efficacy by standard chemotherapeutic agents or molecular targeted drugs in vitro and in vivo. Mol Cancer Ther 9:1956–1967 [DOI] [PubMed] [Google Scholar]
- 44. Smith MC, Danielson LA, Conrad KP, Davison JM. 2006. Influence of recombinant human relaxin on renal hemodynamics in healthy volunteers. J Am Soc Nephrol 17:3192–3197 [DOI] [PubMed] [Google Scholar]
- 45. Dschietzig T, Richter C, Bartsch C, Laule M, Armbruster FP, Baumann G, Stangl K. 2001. The pregnancy hormone relaxin is a player in human heart failure. FASEB J 15:2187–2195 [DOI] [PubMed] [Google Scholar]
- 46. Kern A, Agoulnik AI, Bryant-Greenwood GD. 2007. The low-density lipoprotein class A module of the relaxin receptor (leucine-rich repeat containing G-protein coupled receptor 7): its role in signaling and trafficking to the cell membrane. Endocrinology 148:1181–1194 [DOI] [PubMed] [Google Scholar]
- 47. Bani-Sacchi T, Bigazzi M, Bani D, Mannaioni PF, Masini E. 1995. Relaxin-induced increased coronary flow through stimulation of nitric oxide production. Br J Pharmacol 116:1589–1594 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Schrier RW. 2006. Role of diminished renal function in cardiovascular mortality: marker or pathogenetic factor? J Am Coll Cardiol 47:1–8 [DOI] [PubMed] [Google Scholar]
- 49. Xu Y, Henning RH, van der Want JJ, van Buiten A, van Gilst WH, Buikema H. 2007. Disruption of endothelial caveolae is associated with impairment of both NO- as well as EDHF in acetylcholine-induced relaxation depending on their relative contribution in different vascular beds. Life Sci 80:1678–1685 [DOI] [PubMed] [Google Scholar]