Abstract
P450 oxidoreductase (POR) is a two-flavin protein that reduces microsomal P450 enzymes and some other proteins. Preparation of active bacterially expressed human POR for biochemical studies has been difficult because membrane-bound proteins tend to interact with column matrices. To reduce column-protein interactions and permit more vigorous washing, human POR lacking 27 N-terminal residues (N-27 POR) was modified to carry a C-terminal Gly3His6-tag (N-27 POR-G3H6). When expressed in Escherichia coli, N-27 POR-G3H6 could be purified to apparent homogeneity by a modified, single-step nickel-nitrilotriacetic acid affinity chromatography, yielding 31 mg POR per liter of culture, whereas standard purification of native N-27 POR required multiple steps, yielding 5 mg POR per liter. Both POR proteins had absorption maxima at 375 and 453 nm and both reduced cytochrome c with indistinguishable specific activities. Using progesterone as substrate for bacterially expressed purified human P450c17, the Michaelis constant for 17α-hydroxylase activity supported by N-27 POR or N-27 POR-G3H6 were 1.73 or 1.49 μm, and the maximal velocity was 0.029 or 0.026 pmol steroids per picomole P450 per minute, respectively. Using 17-hydroxypregnenolone as the P450c17 substrate, the Michaelis constant for 17,20 lyase activity using N-27 POR or N-27 POR-G3H6 was 1.92 or 1.89 μm and the maximal velocity was 0.041 or 0.042 pmol steroid per picomole P450 per minute, respectively. Thus, N-27 POR-G3H6 is equally active as native N-27 POR. This expression and purification system permits the rapid preparation of large amounts of highly pure, biologically active POR and may be generally applicable for the preparation of membrane-bound proteins.
Preparation of biochemically useful quantities of pure, catalytically active, membrane-bound proteins is difficult, partly because membrane-bound proteins are usually hydrophobic and hence may adhere to column matrices; the consequent multistep washing and elution procedures reduce yields. Using human P450 oxidoreductase (POR) as an example, we describe a modification of standard His-tag chromatography that may be generally applicable. POR is a microsomal redox protein containing FMN and FAD moieties; the FAD receives electrons from reduced nicotinamide adenine dinucleotide phosphate (NADPH) and then transfers them to the FMN, which then transfers them to a recipient protein (1). POR donates electrons to all microsomal forms of P450 and to several non-P450 enzymes. Microsomal P450 enzymes participate in the synthesis of steroids, fatty acids, and prostaglandins and in xenobiotic and drug disposal. POR gene knockouts in mice cause embryonic lethality (2, 3) and human missense mutations cause disordered steroidogenesis, ambiguous genitalia, and Antley-Bixler syndrome (4, 5). The human POR gene is highly polymorphic (6). POR variants affect xenobiotic metabolism in vitro (7–9) and in vivo (10). The activity of a specific POR variant to support the activity of one P450 does not predict its ability to support the activity of other P450s (5–7); therefore, the ability of each POR sequence variant to support the activity of each P450 must be studied separately. For example, the disease-causing mutant A287P has 20–40% of wild-type (WT) activity to support the 17α-hydroxylation and 17,20 lyase activity of P450c17 (4, 5) and 14–27% of WT activity to support reactions catalyzed by CYP2D6 and CYP3A4 (8, 9) but no detectable activity to support activation of 7-ethoxymethoxy-3-cyanocoumarin by CYP1A2 or CYP2C19 (7), and normal activity to support CYP19A1 (11).
Biochemical studies of POR require large amounts of pure, catalytically active protein. We describe a method for preparing bacterially expressed, catalytically active, soluble human POR lacking 27 amino-terminal residues (N-27 POR) by virtue of modifying the conventional approach of adding four C-terminal histidine residues for nickel-affinity chromatography by substituting a C-terminal extension consisting of three glycines followed by six histidines (G3H6). The glycines provide rotational freedom and distance between the POR and the matrix material of the column, and the larger number of histidines permits more vigorous washing. These innovations permit one-step purification scheme, dramatically increasing yields of apparently pure, catalytically active protein.
Materials and Methods
Reagents and supplies
Ni2+-nitrilotriacetic acid (NTA) agarose was from QIAGEN (Valencia, CA), and rabbit anti-His-tag antibody was from Genscript (Piscataway, NJ). Glucose-6-phosphate, nicotinamide adenine dinucleotide phosphate, and glucose-6-phosphate dehydrogenase were from EMD Bioscience (La Jolla, CA); histidine was from Sigma-Aldrich (St. Louis, MO); and prestained SDS-PAGE markers were from Bio-Rad (Hercules, CA).
Construction of human POR expression plasmid pET22b-POR-G3H6
Plasmid pET22-N-27POR, expressing human POR lacking 27 N-terminal residues (5), was used as the template to amplify human N-27 POR cDNA using the forward primer, 5′-ATTCGGATCCGAATTCTATGACGGACATG-3′ containing an EcoRI site (underlined) and the reverse primer 5′-CTTATACTCGAGCTAGTGGTGGTGGTGGTGGTGGCCGCCGCCGCTCCACACGTCCAG-3′ containing an XhoI site (underlined); the nucleotides shown in boldface encode the three glycine and six histidine residues (G3H6). The 50-μl PCR reaction contained 100 ng pET22b-N-27POR DNA, 2 mm deoxynucleotide triphosphate mix, 10 pmol of each primer, 5 μl of 10× Taq buffer, and 1 μl (5 U) of Taq DNA polymerase (Fermentas, Glen Burnie, MD). Initial denaturation was at 94 C for 3 min, followed by 35 cycles of PCR (94 C for 30 sec, 60 C for 30 sec, 72 C for 1 min), concluding at 72 C for 10 min. The PCR products were separated by electrophoresis in 1% agarose gel containing 1 μg/ml ethidium bromide. The single 2-kb band was excised, purified (QIAquick PCR purification kit; QIAGEN), cut with EcoRI and XhoI and cloned into pET22b cut with the same enzymes. The resulting plasmid, pET22b-N-27POR-G3H6, was sequenced to confirm the correct structure.
Expression of native N-27 POR and N-27 POR-G3H6
Human N-27 POR and N-27 POR-G3H6 were expressed in Escherichia coli CD41(DE3) (Lucigen, Middleton, WI) as described (5). E. coli CD41(DE3) containing pET22b-N-27POR or pET22b-N-27POR-G3H6 was grown in terrific broth at room temperature to an OD of 0.4, and then POR expression was induced with 0.4 mm isopropyl-1-thio-β-d-galactopyranoside and incubated at 28 C for 48 h. Bacterial spheroplasts and membrane proteins were prepared as described (5) and stored at −80 C until the purification step. N-27 POR was purified by 2′,5′-ADP-Sepharose affinity chromatography as described (5). N-27 POR-G3H6 was purified using nickel-NTA affinity column chromatography. All purification steps were done at 4 C. The N-27POR-G3H6 pellet from 500 ml culture was suspended in 3 ml of 20 mm potassium phosphate buffer (pH 7.4), 20% glycerol, 0.1 mm EDTA, 0.1 mm phenylmethylsulfonyl fluoride, 0.2% cholate, and 0.2% Triton X-100 and shaken gently for 2 h, and the insoluble material was pelleted at 100,000 × g for 90 min. The supernatant was diluted 5-fold with equilibration buffer [50 mm potassium phosphate buffer (pH 7.4), 20% glycerol, 0.1 mm EDTA, 0.1 mm phenylmethylsulfonyl fluoride, 0.1% cholate, and 0.1% Triton X-100] and mixed with 1.5 ml nickel-NTA agarose previously equilibrated with equilibration buffer. The mixture was shaken gently for 2 h, poured into a 10 × 1 cm, 6-ml column, and washed with 15 volumes of washing buffer [50 mm potassium phosphate buffer (pH 7.4), 20% glycerol, 0.15 m NaCl, 0.1% cholate and 0.1% Triton X-100, 20 mm histidine]. The N-27 POR-G3H6 was eluted dropwise with 2 column volumes (3 ml) of elution buffer [50 mm potassium phosphate buffer (pH 7.4), 20% glycerol, 0.15 m NaCl, 0.1% cholate, 0.1% Triton X-100, and 200 mm histidine]. Eluted protein was dialyzed three times against 20 mm potassium phosphate buffer (pH 7.4) and 20% glycerol.
Purified N-27 POR-G3H6 was analyzed by 10% SDS-PAGE and transferred to Immobilon-FL transfer membrane (Millipore, Bedford MA). The membrane was probed with 1:2000 rabbit polyclonal anti-His-tag (Genscript), washed, and incubated with 1:10,000 goat antirabbit infrared-labeled antibody (Li-COR Bioscience, Lincoln, NE) at room temperature for 1 h. The membrane was washed three times and the POR protein bands were detected in the green fluorescent channel (700 nm) on an Odyssey Infrared imaging system (LI-COR Bioscience).
Spectral analyses
The absorption spectra of 0.1 μm N-27 POR and N-27 POR-G3H6 were determined between 300 and 700 nm in 1 m Tris-HCl (pH 8.1) using a Spectramax M2 (Molecular Devices, Sunnyvale, CA).
POR assays based on cytochrome c
The capacities of native N-27 POR and N-27 POR-G3H6 to reduce cytochrome c were measured by quantitating the change in absorbance at 550 nm when oxidized cytochrome c is reduced (12); reduction rates were calculated using the extinction coefficient ε550 = 21.0 mm−1/cm−1 for reduced cytochrome c (13). Spectrophotometric assays were performed in a reaction volume of 1 ml in disposable 1-ml cuvettes. The assay system contained an NADPH regeneration system consisting of 0.1 m Tris-HCl (pH 7.8), 2 mm glucose-6-phosphate, 3 U of glucose-6-phosphate dehydrogenase, 40 μm cytochrome c, and 5 μm NADPH. Assays were performed with 6 pmol purified N-27 POR or N-27 POR-G3H6. The change in absorbance at 550 nm was monitored against time, and specific activities were expressed as (micromoles reduced cytochrome c)(min)−1(milligrams of POR)−1.
Assay of P450c17 activities
Plasmid pCWH17mod(G3H6) (14) was expressed in E. coli JM109 and human P450c17 (containing a C terminal G3H6 tag) associated with bacterial membranes was extracted and purified as described (14). The abilities of N-27 POR and N-27 POR-G3H6 to support the 17α-hydroxylase and 17,20 lyase activities of P450c17 were assayed as described (5). Each 200-μl reaction contained 30 pmol of purified human P450c17 and 60 pmol purified human N-27 POR or N-27 POR-G3H6 for 17α-hydroxylase assays and 40 pmol of human P450c17 and 80 pmol human N-27 POR or N-27 POR-G3H6 for 17,20 lyase assays, 10 μg of 1,2-didodecanoyl-sn-glycero-3-phosphocholine, 50 mm potassium phosphate buffer (pH 7.4) and sonicated on ice for 10 min. The reaction mixtures then received 6 mm potassium acetate, 10 mm MgCl2, 1 mm reduced glutathione, 20% glycerol, and radiolabeled steroid substrate: [14C]progesterone (25 nCi; 55 mCi/mmol; PerkinElmer, Norwalk, CT) for 17α-hydroxylase activity or [3H]17α-hydroxypregnenolone (25 nCi; 60 mCi/mmol; American Radiolabeled Chemicals, St. Louis, MO) for 17,20 lyase activity. Cytochrome b5 (20 pmol), which acts allostericly to facilitate the 17,20 lyase activity of P450c17 (15, 16) was added for 17,20 lyase assays. Reactions were started by adding 4 μl of 100 mm NADPH, incubated at 37 C for 90 min, stopped by adding 450 μl of 1:1 ethyl acetate/isooctane, and mixed vigorously, and then the supernatants containing the steroids were dried with nitrogen and dissolved in 20 μl of dichloromethane. Extracted steroids were analyzed by thin-layer chromatography on silica gel plates (PE SIL G/UV; Whatman, Middlesex, UK) developed with 3:1 chloroform/ethyl acetate. The signals were quantitated by phosphoimaging and analyzed using GraphPad Prism 3 (GraphPad Software, San Diego, CA). Michaelis constant (Km) and maximal velocity (Vmax) data are given as mean ± sem of three independent experiments, each performed in duplicate.
Results
Construction, expression, and purification of N-27 POR and N-27 POR-G3H6
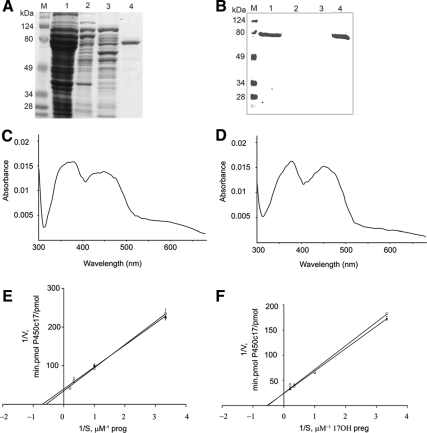
To build the expression vector for N-27 POR-G3H6, cDNA encoding human N-27 POR was PCR amplified from pWT22-N-27POR (5) using a 3′ primer containing nucleotides encoding the C-terminal G3H6 extension. A single PCR product of 2018 bp was obtained and confirmed by sequencing. This plasmid was transfected into bacteria, induced with isopropyl-1-thio-β-d-galactopyranoside and the resulting protein prepared by Ni-NTA chromatography and analyzed by SDS-PAGE. A Coomassie-stained gel displaying the membranes, flowthrough, wash, and eluted fractions showed a single homogenous protein band of N-27 POR-G3H6 at approximately 75 kDa (Fig. 1A). Immunoblotting using anti-His tag antiserum identified N-27 POR-G3H6 in the membrane fraction and in the purified eluate but not in the flowthrough or wash (Fig. 1B). The yield of purified N-27 POR-G3H6 was about 31 mg/liter (by Bradford assay); by contrast, purification of native N-27 POR or N-27 POR-G3H6 by conventional ADP-Sepharose affinity chromatography yielded 4–5 mg of native N-27 POR or POR-G3H6 per liter of culture. Based on the amount of total membrane protein in the cultures, the amount of protein loaded in each lane of the gels and the apparently equivalent amounts of POR in lanes 1 and 4, we estimated our yield to be 50–55%. The purified N-27 POR-G3H6 and native N-27 POR were both visibly yellow, indicating the presence of flavin. The absorption spectra of oxidized forms of both proteins showed maxima at 375 and 453 nm, as expected, for POR (17, 18); the spectra of the two preparations did not differ substantially (Fig. 1, C and D).
Fig. 1.
Characterization of N-27 POR-G3H6. A, SDS-PAGE of purified N-27 POR-G3H6. B, Immunoblot of purified N-27 POR-G3H6. In A and B, lane M, molecular weight markers; lane 1, membrane protein; lane 2, flowthrough; lane 3, wash; lane 4, eluted fraction. C and D, Absorbance spectra. C, N-27 POR-G3H6. D, N-27 POR. E and F, Lineweaver-Burk analyses; circles represent the activity of N-27 POR and triangles represent the activity of N-27 POR-G3H6. E, 17α-Hydroxylase activity. F, 17,20-Lyase activity. Error bars represent the mean ± sem of at least three independent experiments.
Characterization of enzymatic activity
The conventional assay for POR activity is the reduction of cytochrome c (12, 19). In this assay, the specific activities of the purified N-27POR and N-27POR-G3H6 were indistinguishable at 10.0 ± 0.5 and 10.2 ± 0.9 μmol/min·mg protein, respectively. However, this assay is nonphysiological because cytochrome c is a mitochondrial protein, whereas POR is in the endoplasmic reticulum. Therefore, we assayed the ability of the two POR preparations to support the 17α-hydroxylase and 17,20 lyase activities of bacterially expressed human P450c17 because 17,20 lyase activity is very sensitive to minor perturbations in electron transfer (20, 21) and POR structure (4, 5, 8). The 17α-hydroxylase activity of P450c17 is measured by conversion of radiolabeled progesterone to 17-hydroxyprogesterone because human P450c17 has minimal 17,20 lyase activity with 17-hydroxyprogesterone as substrate (15), and 17,20 lyase activity is measured by conversion of radiolabeled 17-hydroxypregnenolone to dehydroepiandrosterone. Autoradiography of chromatograms of incubations with several substrate concentrations permitted calculation of Km and Vmax from Lineweaver-Burk plots (Fig. 1, E and F). There were no differences in the hydroxylase and lyase activities of P450c17 using the two forms of POR (Table 1).
Table 1.
Catalytic activities of human P450c17 supported by N-27 POR and N-27 POR-G3H6
| 17α-Hydroxylase |
17,20 Lyase |
|||
|---|---|---|---|---|
| N-27 POR | N-27 POR-G3H6 | N-27 POR | N-27 POR-G3H6 | |
| Km (μm) | 1.73 ± 0.154 | 1.49 ± 0.344 | 1.92 ± 0.095 | 1.89 ± 0.341 |
| Vmax (pmol steroid/pmol P450/min) | 0.029 ± 0.0007 | 0.026 ± 0.004 | 0.041 ± 0.002 | 0.042 ± 0.005 |
Discussion
Because POR is required for catalysis by all microsomal P450 enzymes, its structure has been of considerable interest. Trypsin-cleaved, 72-kDa rat POR lacking 56 N-terminal residues has been crystallized (22); however, although this truncated POR can transfer electrons to cytochrome c, it is inactive with cytochrome P450. The crystal structure of catalytically active yeast POR has been reported with some significant differences in flavin binding compared with rat POR (23), but preparation of biochemically useful quantities of highly purified, catalytically active mammalian POR has been daunting.
We report a protocol for expression and purification of catalytically active human POR as N-27 POR-G3H6. The protocol depends on a modified C-terminal histidine tag that permits more robust washing of the protein affixed to the Ni-NTA column. Expression of proteins fused to polyhistidine tags has been used for more than 35 yr (24); however, both the length and position of His tags can affect the level of protein expression and its suitability for functional and structural studies (25). Our procedure gave a higher yield of purified N-27 POR-G3H6 (31 mg/liter) compared with WT POR (5 mg/liter) or N-27 POR-G3H6 (4 mg/liter) purified by 2′,5′-ADP-Sepharose affinity chromatography. The yield of purified N-27 POR-G3H6 is much higher than the yield of POR (∼2 mg/liter) by coexpression of cytochrome P450 and POR in E. coli (26).
The purified N-27 POR-G3H6 and the conventionally purified POR had the same specific activity for cytochrome c reduction and indistinguishable activities to support the 17α-hydroxylase and 17,20 lyase activities of human P450c17. The 17,20 lyase activity of human P450c17 is exquisitely sensitive to mild perturbations in electron transfer (1, 20, 21); hence, the indistinguishable values for the Km and Vmax of this reaction strongly indicate that the two forms of POR are catalytically identical. Thus, the analyses show that N-27 POR-G3H6 behaves identically to its parental POR, indicating that the deletion of 27 N-terminal residues and the addition of the C-terminal G3H6 extension have no effect on POR function.
Deleting the N-terminal membrane-binding domain may facilitate the high expression of POR, but deleting more than 27 residues impairs or ablates interactions with microsomal P450 enzymes (27, 28). Addition of histidine tags is widely used and typically have little or no effect on protein function because histidine has a neutral isoelectric point and rarely alters to protein immunogenicity, protein structure, function, or secretion (29). The insertion of three glycines between POR and six histidines was designed to improve the flexibility between the POR moiety and the histidine tag, which was lengthened from four to six residues to permit more stringent washing conditions (14). Using this strategy permits the single-step purification of N-27 POR-G3H6 on the nickel-NTA affinity column rather than three steps of column chromatography (diethylaminoethyl-cellulose, ADP-Sepharose, and hydroxyapatite) in the conventional protocols (28, 30). Moreover, nickel-NTA column chromatography is less expensive than using 2′,5′-ADP-Sepharose affinity chromatography per milligram of purified POR. Because this approach has been successful with P450c17 (14) and POR, it may be also applicable for large-scale production of other membrane-bound proteins.
Acknowledgments
This work was supported by National Institutes of Health Grant R01 GM073020 (to W.L.M.).
Current address for D.S.: Laboratory of Immunology, Chulabhorn Research Institute, Bangkok 10210, Thailand.
Disclosure Summary: D.S. and W.L.M. have nothing to declare.
Footnotes
- G3H6
- Three glycines followed by six histidines
- Km
- Michaelis constant
- NADPH
- reduced nicotinamide adenine dinucleotide phosphate
- N-27 POR
- POR lacking 27 amino-terminal residues
- NTA
- Ni2+-nitrilotriacetic acid
- POR
- P450 oxidoreductase
- Vmax
- maximal velocity
- WT
- wild type.
References
- 1. Miller WL. 2005. Regulation of steroidogenesis by electron transfer. Endocrinology 146:2544–2550 [DOI] [PubMed] [Google Scholar]
- 2. Shen AL, O'Leary KA, Kasper CB. 2002. Association of multiple developmental defects and embryonic lethality with loss of microsomal NADPH-cytochrome P450 oxidoreductase. J Biol Chem 277:6536–6541 [DOI] [PubMed] [Google Scholar]
- 3. Otto DM, Henderson CJ, Carrie D, Davey M, Gundersen TE, Blomhoff R, Adams RH, Tickle C, Wolf CR. 2003. Identification of novel roles of the cytochrome P450 system in early embryogenesis: effects on vasculogenesis and retinoic acid homeostasis. Mol Cell Biol 23:6103–6116 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Flück CE, Tajima T, Pandey AV, Arlt W, Okuhara K, Verge CF, Jabs EW, Mendonça BB, Fujieda K, Miller WL. 2004. Mutant P450 oxidoreductase causes disordered steroidogenesis with and without Antley-Bixler syndrome. Nat Genet 36:228–230 [DOI] [PubMed] [Google Scholar]
- 5. Huang N, Pandey AV, Agrawal V, Reardon W, Lapunzina PD, Mowat D, Jabs EW, Van Vliet G, Sack J, Flück CE, Miller WL. 2005. Diversity and function of mutations in P450 oxidoreductase in patients with Antley-Bixler syndrome and disordered steroidogenesis. Am J Hum Genet 76:729–749 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Huang N, Agrawal V, Giacomini KM, Miller WL. 2008. Genetics of P450 oxidoreductase: sequence variation in 842 individuals of four ethnicities and activities of 15 missense mutations. Proc Natl Acad Sci USA 105:1733–1738 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Agrawal V, Huang N, Miller WL. 2008. Pharmacogenetics of P450 oxidoreductase: effect of sequence variants on activities of CYP1A2 and CYP2C19. Pharmacogenet Genomics 18:569–576 [DOI] [PubMed] [Google Scholar]
- 8. Agrawal V, Choi JH, Giacomini KM, Miller WL. 2010. Substrate-specific modulation of CYP3A4 activity by genetic variants of cytochrome P450 oxidoreductase. Pharmacogenet Genomics 20:611–618 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Sandee D, Morrissey K, Agrawal V, Tam HK, Kramer MA, Tracy TS, Giacomini KM, Miller WL. 2010. Effects of genetic variants of human P450 oxidoreductase on catalysis by CYP2D6 in vitro. Pharmacogenet Genomics 20:677–686 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Tomalik-Scharte D, Maiter D, Kirchheiner J, Ivison HE, Fuhr U, Arlt W. 2010. Impaired hepatic drug and steroid metabolism in congenital adrenal hyperplasia due to P450 oxidoreductase deficiency. Eur J Endocrinol 163:919–924 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Pandey AV, Kempná P, Hofer G, Mullis PE, Flück CE. 2007. Modulation of human CYP19A1 activity by mutant NADPH P450 oxidoreductase. Mol Endocrinol 21:2579–2595 [DOI] [PubMed] [Google Scholar]
- 12. Lu AY, Junk KW, Coon MJ. 1969. Resolution of the cytochrome P-450-containing ω-hydroxylation system of liver microsomes into three components. J Biol Chem 244:3714–3721 [PubMed] [Google Scholar]
- 13. Vermilion JL, Ballou DP, Massey V, Coon MJ. 1981. Separate roles for FMN and FAD in catalysis by liver microsomal NADPH-cytochrome P-450 reductase. J Biol Chem 256:266–277 [PubMed] [Google Scholar]
- 14. Wang YH, Tee MK, Miller WL. 2010. Human cytochrome P450c17: single step purification and phosphorylation of serine 258 by protein kinase A. Endocrinology 151:1677–1684 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Auchus RJ, Lee TC, Miller WL. 1998. Cytochrome b5 augments the 17,20-lyase activity of human P450c17 without direct electron transfer. J Biol Chem 273:3158–3165 [DOI] [PubMed] [Google Scholar]
- 16. Pandey AV, Miller WL. 2005. Regulation of 17,20 lyase activity by cytochrome b5 and by serine phosphorylation of P450c17. J Biol Chem 280:13265–13271 [DOI] [PubMed] [Google Scholar]
- 17. Oprian DD, Coon MJ. 1982. Oxidation-reduction states of FMN and FAD in NADPH-cytochrome P-450 reductase during reduction by NADPH. J Biol Chem 257:8935–8944 [PubMed] [Google Scholar]
- 18. Kurzban GP, Howarth J, Palmer G, Strobel HW. 1990. NADPH-cytochrome P-450 reductase. Physical properties and redox behavior in the absence of the FAD moiety. J Biol Chem 265:12272–12279 [PubMed] [Google Scholar]
- 19. Lamb DC, Warrilow AG, Venkateswarlu K, Kelly DE, Kelly SL. 2001. Activities and kinetic mechanisms of native and soluble NADPH-cytochrome P450 reductase. Biochem Biophys Res Commun 286:48–54 [DOI] [PubMed] [Google Scholar]
- 20. Geller DH, Auchus RJ, Mendonça BB, Miller WL. 1997. The genetic and functional basis of isolated 17,20-lyase deficiency. Nat Genet 17:201–205 [DOI] [PubMed] [Google Scholar]
- 21. Geller DH, Auchus RJ, Miller WL. 1999. P450c17 mutations R347H and R358Q selectively disrupt 17,20-lyase activity by disrupting interactions with P450 oxidoreductase and cytochrome b5. Mol Endocrinol 13:167–175 [DOI] [PubMed] [Google Scholar]
- 22. Wang M, Roberts DL, Paschke R, Shea TM, Masters BS, Kim JJ. 1997. Three-dimensional structure of NADPH-cytochrome P450 reductase: prototype for FMN- and FAD-containing enzymes. Proc Natl Acad Sci USA 94:8411–8416 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Lamb DC, Kim Y, Yermalitskaya LV, Yermalitsky VN, Lepesheva GI, Kelly SL, Waterman MR, Podust LM. 2006. A second FMN binding site in yeast NADPH-cytochrome P450 reductase suggests a mechanism of electron transfer by diflavin reductases. Structure 14:51–61 [DOI] [PubMed] [Google Scholar]
- 24. Porath J, Carlsson J, Olsson I, Belfrage G. 1975. Metal chelate affinity chromatography, a new approach to protein fractionation. Nature 258:598–599 [DOI] [PubMed] [Google Scholar]
- 25. Mohanty AK, Wiener MC. 2004. Membrane protein expression and production: effects of polyhistidine tag length and position. Protein Expr Purif 33:311–325 [DOI] [PubMed] [Google Scholar]
- 26. Dong J, Porter TD. 1996. Coexpression of mammalian cytochrome P450 and reductase in Escherichia coli. Arch Biochem Biophys 327:254–259 [DOI] [PubMed] [Google Scholar]
- 27. Andersen JF, Utermohlen JG, Feyereisen R. 1994. Expression of house fly CYP6A1 and NADPH-cytochrome P450 reductase in Escherichia coli and reconstitution of an insecticide-metabolizing P450 system. Biochemistry 33:2171–2177 [DOI] [PubMed] [Google Scholar]
- 28. Hayashi S, Omata Y, Sakamoto H, Hara T, Noguchi M. 2003. Purification and characterization of a soluble form of rat liver NADPH-cytochrome P-450 reductase highly expressed in Escherichia coli. Protein Expr Purif 29:1–7 [DOI] [PubMed] [Google Scholar]
- 29. Stiborova H, Kostal J, Mulchandani A, Chen W. 2003. One-step metal-affinity purification of histidine-tagged proteins by temperature-triggered precipitation. Biotechnol Bioeng 82:605–611 [DOI] [PubMed] [Google Scholar]
- 30. Kimura S, Iyanagi T. 2003. High-level expression of porcine liver cytochrome P-450 reductase catalytic domain in Escherichia coli by modulating the predicted local secondary structure of mRNA. J Biochem 134:403–413 [DOI] [PubMed] [Google Scholar]