Abstract
During embryogenesis, sexually dimorphic organogenesis is achieved by hormones produced in the gonad. The external genitalia develop from a single primordium, the genital tubercle, and their masculinization processes depend on the androgen signaling. In addition to such hormonal signaling, the involvement of nongonadal and locally produced masculinization factors has been unclear. To elucidate the mechanisms of the sexually dimorphic development of the external genitalia, series of conditional mutant mouse analyses were performed using several mutant alleles, particularly focusing on the role of hedgehog signaling pathway in this manuscript. We demonstrate that hedgehog pathway is indispensable for the establishment of male external genitalia characteristics. Sonic hedgehog is expressed in the urethral plate epithelium, and its signal is mediated through glioblastoma 2 (Gli2) in the mesenchyme. The expression level of the sexually dimorphic genes is decreased in the glioblastoma 2 mutant embryos, suggesting that hedgehog signal is likely to facilitate the masculinization processes by affecting the androgen responsiveness. In addition, a conditional mutation of Sonic hedgehog at the sexual differentiation stage leads to abnormal male external genitalia development. The current study identified hedgehog signaling pathway as a key factor not only for initial development but also for sexually dimorphic development of the external genitalia in coordination with androgen signaling.
Differentiated male and female external genitalia enable highly efficient internal fertilization. External genitalia exhibit substantial sexual dimorphisms in many animal species. In mice, anatomical sexual dimorphisms of the genital tubercle (GT) (embryonic external genitalia) are first visible at embryonic day (E)16.5 (1). The urethral fold and the prepuce develop more prominently in male than that of female GT. As a result, male urethra forms a tubular structure within the glans. In addition, the prospective corporal body is bilaterally segmented in the male GT mesenchyme. The male-specific GT differentiation is established by androgen secreted from the testis. In contrast, female external genitalia display few of such characteristic processes due to a lack of androgen stimulation. Among reproductive organ formation, male and female external genitalia arise from the common anlage and differentiate by the respective hormone environment from midembryogenesis in mice. Although significant progress of our understanding for its developmental processes has been achieved by genetic analyses on mice, molecular mechanisms of sexually dimorphic development of the GT are not yet known.
During the past few decades, disorders of sexual development are among the most common human birth defects. Hypospadias, in which the urethral meatus is located at the ventral side of the penis, has been described as a malformation with a high prevalence as human birth defects (2, 3). In most cases, the etiology of such disorders is still obscure. Thus, a better understanding of the developmental mechanisms of GT will shed light on the causative mechanisms of genital malformations.
Initially, both male and female GT are morphologically identical. The GT as a common anlage develops from the cloacal region starting at E10.5 (1). The after outgrowth is the consequence of mesenchymal swelling around the cloaca. The outgrowing GT is accompanied with the formation of urethral plate epithelium (UPE), the future urethral epithelium, in the ventral (lower) side. These developmental events are completely androgen independent. During these processes, Sonic hedgehog (Shh) expressed in the endodermal epithelium plays an essential role in the regulation of epithelial-mesenchymal interactions (4–6). Shh induces the expression of various genes in the bilateral mesenchyme to the urethra and triggers signal cascade for initial GT outgrowth. Accordingly, Shh knockout (KO) mice display a complete GT agenesis (4, 6).
The hedgehog signal is mediated through its membrane receptor complex consisting of Patched (Ptc) and Smoothened (Smo). In the absence of hedgehog ligands, Ptc blocks Smo activity, and glioblastoma (Gli) transcriptional factor is inactivated by proteolytic processing. Hedgehog binding to Ptc unleashes Smo activity, which promotes transcriptional activation of the Gli. In vertebrates, three distinct Gli family proteins, Gli1, Gli2 and Gli3, are involved in the transcription response to the hedgehog signaling (7, 8). Mutant mice analyses have revealed that Gli1 and Gli2 function primarily as activators, whereas Gli3 acts mainly as a repressor, although some overlapping roles among Gli proteins are also reported (9–12). Loss of Gli2 or Gli3 is embryonic lethal, whereas Gli1 is dispensable for normal development. Gli1 itself is a target of hedgehog signal; hence, it appears to participate in a positive-feedback loop in the hedgehog pathway. It is widely accepted the hedgehog pathway controls multiple developmental processes. However, the contribution of Shh and each Gli family protein for the GT sexual development remains unclear.
Reciprocal interactions between epithelium and mesenchyme in the urogenital organs have been shown by tissue graft experiments. For instance, mesenchymal androgen receptor is necessary for the androgen-dependent epithelial cell differentiation of the prostate (13, 14). In contrast, the epithelium is required for differentiation and spatial organization of the smooth muscle (15, 16). In addition, the inductive effect of the epithelium on mesenchymal growth and differentiation has been demonstrated for GT development (17, 18), although such epithelium-derived factor is still not identified. Despite the previous description of hormonal (androgen) control of the sexual development (19), the involvement of nongonadal and locally produced masculine factors is not yet known. In this article, we demonstrated that hedgehog pathway is indispensable for the masculinization processes of male external genitalia. We showed that Shh expressed in the UPE regulates GT mesenchymal differentiation through Gli2. Genetic disruption of hedgehog signal led to a hypospadias-like phenotype. Intriguingly, conditional gene inactivation of Shh at the sexual differentiation stage induced a female-like structure of the male GT. These results reveal newly identified functions of Shh-Gli signaling for sexually dimorphic development during embryogenesis.
Materials and Methods
Mouse
The mutant alleles used herein were Gli1 (12), Gli2 (10), Gli3 (20), Shh (21), Shhlox (22), CAGGS-CreERTM (23), Del5-LacZ (24), and R26-SmoM2 (25). All experimental procedures and protocols were approved by the Committee on the Animal Research at Kumamoto University. Embryos for each experiment were collected from more than three independent pregnant females. Noon on the day when a vaginal plug was detected was designated as E0.5.
The tamoxifen (TM)-inducible Cre recombinase system removes the floxed sequence of the target genome (26). TM (Sigma, St. Louis, MO) was dissolved in sesame oil (Kanto Chemical, Tokyo, Japan) at a final concentration of 10 mg/ml. Two milligrams of TM per 40 g body weight was administrated (ip) to the pregnant mice. Under these conditions, no overt teratologic effects nor sexual disorders of the reproductive organs were observed (24).
For androgen treatment, pregnant mice were administrated (ip) a daily single injection of 100 mg/kg body weight testosterone propionate (TP) (Wako, Osaka, Japan) for 5 d starting from E13.5 and killed 24 h later after the last injection (E18.5).
The concentration of testosterone in the testes of the mice at E18.5 was measured by Clinical Pathology Laboratory, Inc. (Kagoshima, Japan) using a chemiluminescent immunoassay (27). More than seven embryos per group were collected, and the average relative testosterone contents were evaluated for further analysis. Error bars represent the se. A statistical analysis was performed using Student's t test or Welch's t test followed by F-test; differences with P < 0.05 were considered to be significant.
Histology, LacZ staining, immunohistochemistry, and in situ hybridization for gene expression analysis
Hematoxylin and eosin staining and LacZ staining were performed by standard procedures as previously described (24). For immunohistochemistry, paraformaldehyde-fixed, paraffin-embedded sections were treated for antigen retrieval (microwave treatment 10 min in citrate buffer; pH 6.0). The sections were incubated at a 1:100 dilution of anti-p450Scc antibody (American Research Products, Belmont, MA). Immunofluorescence analysis was performed with Alexa Fluor 546 antirabbit IgG (Invitrogen, Carlsbad, CA) and counterstained with Hoechst 33342 (Sigma).
For in situ hybridization, the sections were deparaffinized, rehydrated, incubated in 1 μg/ml proteinase K for 7 min at 37 C, and refixed with 4% paraformaldehyde for 10 min at room temperature. After washing in PBS containing 0.1% Tween 20, overnight hybridization was performed in a buffer (50% formamide, 5× saline sodium citrate, 50 μg/ml yeast tRNA, 1% sodium dodecyl sulfate, and 50 μg/ml heparin) with 1 μg/ml probe at 68 C. After washing with 5× saline sodium citrate and 50% formamide for 1 h at 68 C, the slides were incubated in blocking solution [10% blocking reagent (Roche, Mannheim, Germany) in 100 mm maleate buffer and Tris-buffered saline with Tween 20 (140 mm NaCl, 2.7 mm KCl, 0.1% Tween 20, 25 mm Tris-HCl; pH 7.5)] for 2 h. Antidigoxigenin antibody (Roche) in a blocking solution was added to the slides and incubated for 1 h. After washing with Tris-buffered saline with Tween 20, the sections were equilibrated in NTMT buffer [100 mm NaCl, 50 mm MgCl2, 0.1% Tween 20 and 100 mm Tris-HCl (pH 9.5)], including 2 mm levamisole (Sigma) and incubated in color solution [3.5 μg nitroblue tetrazolium chloride (Sigma) and 1.75 μg 5-bromo-4-chloro-3-indolyl-phosphate (Sigma) per milliliter of NTMT buffer]. The templates used in this study were kindly provided from J. Motoyama (Gli1 and Ptc1), C. Shukunami (Shh), C. Niehrs (Dkk2), and B. Capel (Scc). The template of Sfrp1 was obtained by standard RT-PCR procedures. The primer sequences were: secreted frizzled-related protein (Sfrp)1, TTC TAC ACC AAG CCC CCG CAG, GAT GGG CCC CAG CTT CAA GG. The preparation of the digoxigenin-labeled probes was performed according to the manufacturer's instructions (Roche).
Quantitative RT-PCR analysis
The changes in gene expression were confirmed and quantified using the 7500 real-time PCR system (Applied Biosystems, Foster City, CA) according to the manufacturer's instructions. Total RNA (1 μg), isolated with ISOGEN (Nippongene, Tokyo, Japan) from the mesenchyme adjacent to the UPE of GT (blue area shown by figure 3A), was used in RT-PCR reactions carried out with SuperScript III (Invitrogen) and SYBR Green master mix (Applied Biosystems). The relative RNA equivalents for each sample were determined by standardization with the ribosomal protein L8 levels. More than three pools of samples per group were tested in triplicate, and the average relative RNA equivalents per sample were analyzed further. Error bars represent se. The statistical comparisons among the experimental groups were assessed by ANOVA. When F ratios were significant (P < 0.05), Scheffé post hoc tests between two groups were done, and P < 0.05 was considered as statistically significant. The primer sequences were: Dickkopf (Dkk)2, TGT CTG AAG CAC AGG CTG GAT, CTT CTG GAG CCT CTG ATG GC; Sfrp1, AAG GAG AGG CAG AAT CCT TTC A, TTT CCA AAC CGG CCA ACA; and ribosomal protein L8, ACA GAG CCG TTG TTG GTG TTG, CAG CAG TTC CTC TTT GCC TTG T.
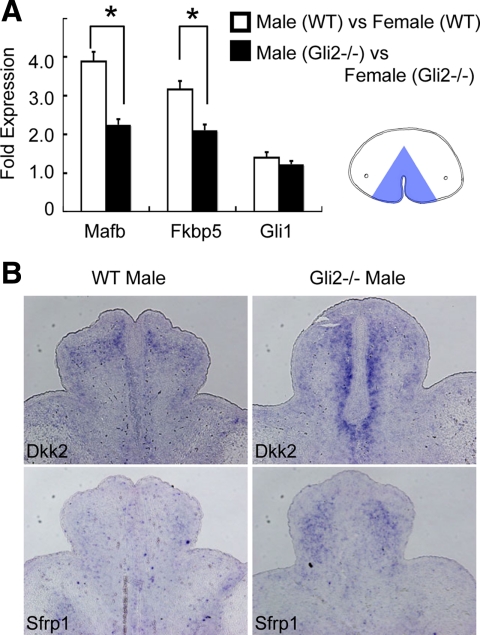
Fig. 3.
Impaired masculine characteristics in the male Gli2 mutant GT. A, The fold expression of MafB and Fkbp5 between male and female GT is decreased in the Gli2 mutant GT. Gli1 expression does not show sexually dimorphic expression pattern. RNA was isolated from the mesenchyme adjacent to the UPE (blue area in A). The relative RNA equivalents for each sample were determined by standardization with ribosomal protein L8 levels. Statistical significance is indicated by asterisks, with P < 0.05. B, The expression of Dkk2 and Sfrp1 is up-regulated in the Gli2 mutant GT in comparison with those of the wild-type (WT) male GT at E15.5. Their expression pattern is shown by the representative transverse sections.
Results
Expression pattern of hedgehog pathway genes
The expression pattern of Shh, Gli1, and Ptc1 was examined in the GT at E15.5, the critical time points to the onset of androgen-induced sexually dimorphic development of the GT (28). Shh mRNA was specifically expressed in the UPE (Fig. 1A, arrow). In contrast, Gli1 and Ptc1, representative hedgehog-responsive genes, were expressed in the mesenchyme adjacent to the UPE (Fig. 1, B and C, arrowheads), indicating that Shh mediates epithelial-mesenchymal interactions during GT sexual differentiation. Sexually dimorphic expression was not observed among these genes (data not shown). The mesenchymal differentiation has been shown to be tightly associated with GT masculinization (28);, the function of hedgehog signaling pathway was, therefore, investigated.
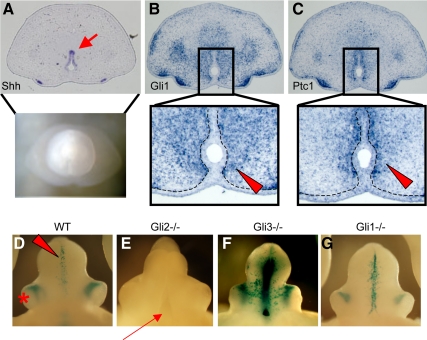
Fig. 1.
Expression pattern of hedgehog signaling genes in the male GT at E15.5. A, Shh is expressed in the UPE (arrow in the cross-section). B and C, Gli1 (B) and Ptc1 (C), representative hedgehog-responsive genes, are expressed in the mesenchyme adjacent to the UPE (arrowheads in the highly magnified pictures). Dotted lines indicate the boundary between the epithelium and mesenchyme of the developing GT. D–G, The LacZ staining pattern of the del5-LacZ line with several Gli mutant allelic backgrounds at E15.5. In the wild-type mouse embryos (WT), the LacZ signals are detected in the mesenchymal region adjacent to the UPE (D, arrowhead). An asterisk indicates the preputial gland. The Gli2 KO embryos exhibit a hypoplasic GT and groove-like structure in the ventral GT (arrow) without LacZ signals derived from the del5-LacZ allele (E). The ablation of Gli3 leads to enhanced LacZ signals, although lacking overt morphological GT defects (F). The Gli1 mutant GT does not show apparent GT phenotypes, and the LacZ signal is detected almost unaltered (G). No sexually dimorphic expression pattern is observed, and the representative images from male embryos are shown.
Gli2 as a main regulatory gene mediating hedgehog signaling during GT development
In vertebrates, three distinct Gli family proteins mediate Shh signal transduction. To determine the role of each Gli transcription factor in the GT development, several Gli mutants were analyzed by crossing with alleles of the hedgehog-responsive indicator mouse line, del5-LacZ, at E15.5 (24). In the wild-type embryos, the hedgehog responsiveness was detected in the mesenchyme adjacent to the UPE (Fig. 1D; the red arrowhead indicates the del5-LacZ-derived mesenchymal signals). The expression pattern derived from the del5-LacZ allele was basically similar to the endogenous Gli1 and Ptc1 expressing region (data not shown). This LacZ signal was completely lost associated with the GT hypoplasia and cleft at the proximal ventral midline in the Gli2 mutants (Fig. 1E). The LacZ signal in the Gli3 mutants driven by the del5-LacZ allele was augmented, indicating that Gli3 appears to function as a hedgehog signal repressor (Fig. 1F). However, the ablation of Gli3 does not affect GT development per se (Fig. 1F). In the case of Gli1 mutants, no apparent phenotypes were observed with almost the same level and pattern of the LacZ signal as wild-type GT (Fig. 1G). Hence, these data suggest that Gli2 is a major regulator mediating hedgehog signal during sexually dimorphic development.
Differential GT abnormalities in Gli2 KO male and female embryos
To investigate the Gli2 function for the GT masculinization, Gli2 mutants were analyzed at E18.5, when sexual dimorphisms is apparent. In the wild-type embryos, morphological sexual dimorphisms are apparent with a well-developed prepuce and tubular urethra in the male GT (Fig. 2A). The more drastic genital defect in the Gli2 mutant male could be due to an open urethral groove-like structure and preputial fusion defects (Fig. 2A; serial sections covering the proximal to the distal GT region all showed such abnormalities). Notably, this male mutant GT phenotype was much more prominent than the degree of the female mutant GT (Fig. 2A). Desert hedgehog (Dhh), the other hedgehog ligand, could affect fetal Leydig cell differentiation, which plays a crucial role in androgen production (29, 30). The more drastic genital defect in the Gli2 male mutant GT can be due to the abnormal testis development and/or androgen production. To exclude such a possibility, the testis of the Gli2 KO embryos was examined. p450Scc, a marker gene for Leydig cell differentiation, was expressed normally (Fig. 2B). In fact, the testosterone content in the testis was not altered among Gli2-wild-type, heterozygote and homozygote mouse embryos (Fig. 2C). Furthermore, the expression of 5α-reductase in the GT and the length of the anogenital distance, a reliable marker for masculinization and fetal androgen exposure, were not altered (data not shown). Gli2 male mutants treated with TP at E13.5-E17.5 failed to recover the GT defects (Fig. 2D). These results indicate that the more severe phenotype of the Gli2 male mutant GT is not caused by androgen deficiency.
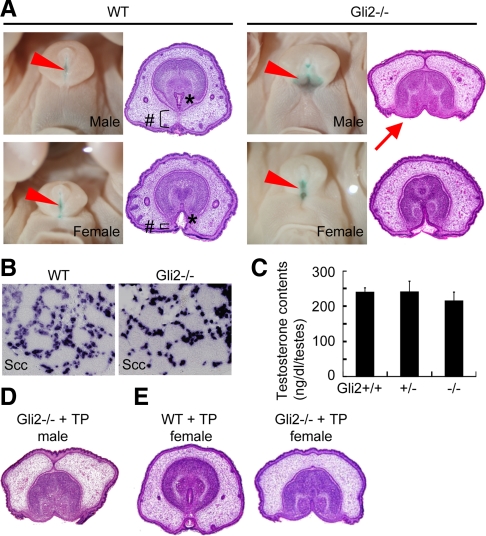
Fig. 2.
Requirement of Shh-Gli2 signal for masculine processes. A, Wild-type (WT) male GT at E18.5 shows a well-developed prepuce (#) and a tubular urethra (*). In the Gli2 KO mutant embryonic GT, the urethra uncovered by epidermal epithelium is shown by methyl green staining (shown by arrowheads). The male mutant GT exhibits more severe phenotypes than those of the female mutants. The histological section shows that the urethral epithelium is exposed to the outer surface of the Gli2 KO male embryos (arrow). B and C, Gli2 male mutant GT does not show defects in androgen signaling pathway. The expression of p450Scc is not changed in the Gli2 mutant testis at E16.5 (B). No significant differences of the testosterone content are observed irrespective of Gli2 genotypes. D and E, Intrauterine androgen treatment experiments for the Gli2 mutants. TP treatment does not recover the GT phenotype of the Gli2 male mutants (D). The wild-type female embryonic GT treated with TP is masculinized displaying a similar morphology to the male GT (E, compare with wild-type male and female GT in A), but mutant female GT does not show masculine phenotypes (E).
Wild-type female GT was masculinized when embryos were treated with TP (Fig. 2E, compare with the wild-type GT in Fig. 2A) (31, 32). However, Gli2 mutant female GT was not masculinized with the TP treatment (Fig. 2E), also suggesting a requirement of Gli2 for GT masculinization.
Decreased responsiveness for androgen signaling in the Gli2 mutant GT
To examine how hedgehog signal affects GT masculinization, the responsiveness to androgen was analyzed in the Gli2 mutants. Mafb and Fkbp5, representative androgen-responsive genes that are expressed in the GT mesenchyme, showed a sexually dimorphic expression (Fig. 3A) (33). Notably, the degree of the differential gene expression between male and female GT was decreased in the Gli2 mutant embryos, indicating that the responsiveness of androgen was indeed decreased. Dkk2 and Sfrp1 have been demonstrated as feminized marker genes in the GT (28). Their expression level was increased in the Gli2 mutant male than that of wild-type male GT (Fig. 3B), indicating that gene expression status of the Gli2 male mutant GT shifts toward female-like pattern. On the other hand, androgen did not seem to affect the hedgehog signal responsiveness in the GT, because the Gli1 expression was not altered in such embryos (Fig. 3A).
The roles of Gli3 in the GT development
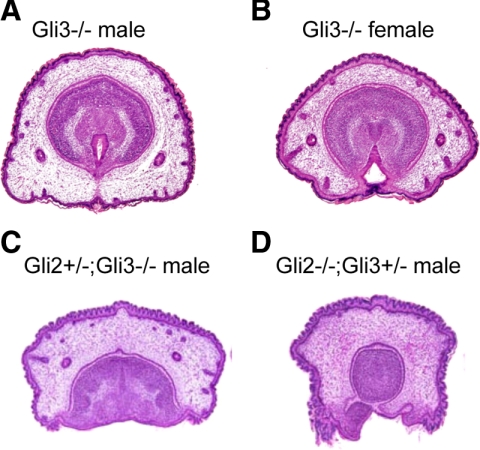
In contrast to the drastic phenotype of the Gli2 mutants, the Gli3 mutant GT developed morphologically normal and showed a proper sexual differentiation (Fig. 4, A and B), whereas a few mutant embryos exhibited a developmental defect with a lack of dorsal part of the GT (two out of 16 embryos, one male and one female) (data not shown). A genetic compensation among several Gli genes has been reported during organogenesis in developmental context-dependent manners (10, 11). Thus, Gli2 and Gli3 compound mutant embryos were analyzed in the current study. Gli2 heterozygotes (Gli2+/−) and double heterozygotes (Gli2+/−;Gli3+/−) were phenotypically normal during development and adult life (data not shown). Loss of Gli3 alleles (Gli2+/−;Gli3−/−) led to severe defects in the prepuce and urethral formation similar to the phenotype of the Gli2 homozygous background (Gli2−/−) (Fig. 4C, compare with Fig. 2A). Furthermore, additional loss of a Gli3 allele in the Gli2 homozygotes (Gli2−/−;Gli3+/−) induced drastic defects in the GT development with underdeveloped glans without urethral epithelium (Fig. 4D). Although Gli3 basically functions as a hedgehog signal repressor in the normal GT development (see Fig. 1F), it appeared to also possess an activator function and partially compensate for the hedgehog signal transduction when Gli2 allele was mutated.
Fig. 4.
Morphological analysis of compound mutants for Gli2 and Gli3 alleles. A and B, Gli3 mutant male (A) and female GT (B) exhibit normal sexually dimorphic development at E18.5. C, The GT of the Gli2+/−;Gli3−/− embryos shows a groove-like structure similar to the phenotype of the Gli2 homozygotes. D, The Gli2−/−;Gli3+/− embryos display drastic GT defects with underdeveloped glans, which does not contain the urethra.
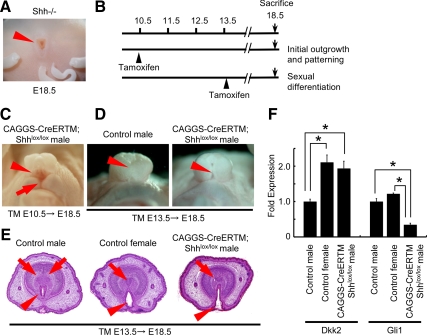
Shh signaling is required for masculinization of the GT
The Shh mutants display a complete GT agenesis as described previously (Fig. 5A) (4, 6, 34). To examine whether Shh signal relayed by Gli2 plays an important role in the process of male sexual differentiation, Shh-conditional mutant analyses were employed. Shh-floxed mice were mated with the CAGGS-CreERTM line (23) to conditionally inactivate the hedgehog signal. The TM was treated at E10.5 and E13.5, just before the GT protrusion and the sexual differentiation, respectively, and the corresponding GT phenotype was analyzed (Fig. 5B). The conditional mutant mice treated with TM at E10.5 displayed a severe lower (ventral) defect with a groove-like structure in the GT (Fig. 5C), possibly due to impaired initial outgrowth and early patterning. On the other hand, the conditional male mutant embryos treated with TM at E13.5 exhibited demasculinized GT phenotypes. Their prepuce remained open showing a slit in the ventral midline of the GT (Fig. 5D, arrowhead), resulting in a failure to form a tubular structure (Fig. 5E, arrowhead). In addition, although control male GT showed the condensation of a bilaterally segmented prospective corporal body, such a structure was undifferentiated in the mutant male GT resembling the female GT (Fig. 5E, arrows). In addition, Dkk2 expression as a feminized marker was increased in the male mutants (Fig. 5F). As expected, Gli1 expression was decreased in the mutant GT (Fig. 5F). The androgen production was normal judged by their anogenital distance, indicating that androgen signal was not affected in the mutants.
Fig. 5.
Requirement of Shh in both initial development and sexual differentiation of the GT. A, The Shh null embryos show a GT agenesis and a persistent cloaca. B, TM treatment timeline; TM is treated at E10.5 or E13.5 to investigate the effect of Shh for the initial development and sexual differentiation of the GT, respectively. C, Conditional Shh KO embryos treated with TM at E10.5 display a severe lower (ventral) GT abnormality with a groove-like structure (arrowheads). An arrow indicates a cleft in the scrotal region. D and E, Male mutant embryos treated with TM at E13.5 display the abnormal masculinization phenotypes of the GT. Note the slit-like defect in the ventral midline of the mutant GT (D, arrowhead). The urethral fold is unfused, resulting in an impaired tubular urethra (E, arrowhead), and the prospective corporal body is underdeveloped (E, arrows). F, Dkk2 expression is increased, whereas Gli1 expression is decreased in the mutant male GT. The relative RNA equivalents for each sample were determined by standardization with ribosomal protein L8 levels. Statistical significance is indicated by asterisks, with P < 0.05.
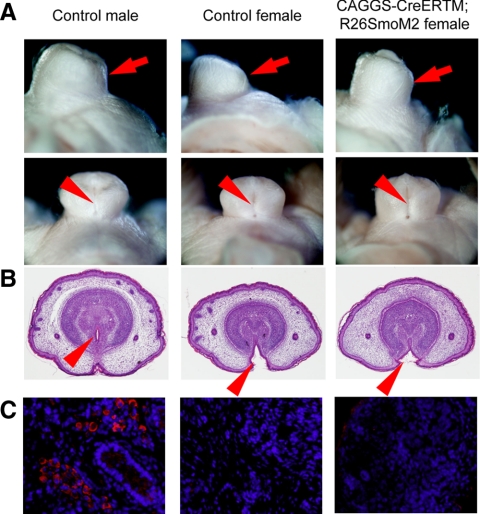
Accelerated prepuce development in the female GT of gain-of-function mutants for hedgehog signaling
To test whether hedgehog signal activation could induce the masculine phenotype of the female GT, a constitutively activated Smo allele (SmoM2) was conditionally expressed using the CAGGS-CreERTM allele (25, 35). After TM treatment to mice at E13.5, the phenotype of CAGGS-CreER;R26-SmoM2 female embryos was examined at E18.5. The mutant female GT showed a well-developed prepuce, particularly in the ventral side, in comparison with that of the control female embryos (Fig. 6A, arrow). However, the urethral fold was not fused in the GT ventral midline similar to the normal female GT (Fig. 6, A and B). In addition, the expression level of the sexually dimorphic genes, MafB and Dkk2, was not altered between control and mutant female GT (data not shown). These results suggest that the activation of hedgehog signal alone could partially masculinize the female GT but not sufficient for complete sexual reversal of the GT in the current experimental condition.
Fig. 6.
Prepuce hyperplasia by constitutively activated hedgehog signal in the female GT. A and B, The female GT of R26SmoM2 mutant embryos shows a prepuce hyperplasia, particularly in its ventral side (arrow). In the mutant embryos, a slit-like structure is observed in the ventral midline of the GT (A, arrowhead) as a consequence of improper incorporation of the urethra into the glans region (B, arrowhead). C, No significant differences of p450Scc expression are observed between mutant and control ovaries, whereas high level of its expression is detected in the control male testis.
It has been reported that conditionally activated hedgehog pathway in the embryonic ovary can ectopically induce the expression of steroid synthesis enzyme and produce androgen (36). However, p450Scc expression was barely detected in both mutant and control ovary, in contrast to the high level expression in the testis (Fig. 6C). The internal reproductive organ development and the length of anogenital distance were not affected in the current conditional mutant mouse embryos (data not shown). These data indicate that the well-developed prepuce is not attributed to the ectopic androgen production in the female mutant embryos.
Discussion
External genitalia formation consists of a series of complex developmental processes. Among such processes, the establishment of sexual dimorphisms is a unique developmental program during embryogenesis. It has been shown that androgen signaling hormonally establishes the male sexual characteristics (19). However, its mediating genes, often termed as effector genes, involving for the masculine process have been poorly understood. The presence of effectors of androgen signaling has been assumed, although the elucidation of such genes has been barely performed. The secreted growth factor, Shh, is one of the major developing regulators during embryogenesis and coordinately interacts with various signaling molecules to promote cell proliferation, survival, and differentiation. Hedgehog signaling is essential for the protrusion and initial outgrowth of the GT; hence, mice with a target deletion of Shh allele show a GT agenesis (4, 6, 34, 37). In addition, it was reported that the extent of GT outgrowth is correlated with the duration of Shh signaling, with longer Shh exposures leading to more extensive outgrowth (38). We here demonstrate that hedgehog signaling affects not only organ growth but also mesenchymal cell differentiation cooperatively with androgen signaling. This would be a novel function of hedgehog signaling mediating sexually dimorphic development in the male GT.
Epithelial-mesenchymal interactions mediated by growth factors are required for the genital organ development. Previous tissue graft experiments have demonstrated the inductive effect of the epithelium on the mesenchymal growth and differentiation in the GT (17, 18). Various growth factors have been shown to function during initial GT outgrowth and patterning (1). However, epithelium-derived developmental regulators and its functions, particularly during late organogenesis for sexually dimorphic development, are still not determined. Shh expressed in the endodermal epithelium elicited mesenchymal gene expression during GT sexual differentiation. Mutant mice for Gli2 and Shh showed abnormal male external genitalia formation. Based on the current results, it is suggested that Shh signaling has a key role in not only initial GT development but also during sexual differentiation of the external genitalia. Shh is therefore a possible candidate for the epithelium-derived factor that regulates mesenchymal growth and differentiation for male external genitalia. Although hedgehog signaling is necessary for male GT development, the activation of hedgehog signaling is not sufficient for induction of complete sex-reversal GT in the female embryos. A constitutively activated Smo (SmoM2) expression induced a hyperplasic prepuce in the ventral side, but failed to achieve urethral fusion in the female GT. Thus, additional factors may be necessary for induction of the GT masculinization, which involve various growth factors, including bone morphogenetic protein, fibroblast growth factor, and Int and Wg (wingless) in Drosophila (Wnt) signalings like the case for prostate development (39–42).
It has been recently demonstrated that Shh possesses multiple functions for anogenital and urogenital organ formation (28, 43–45). Although there are some conflicting data (46, 47), androgen can induce Shh expression in the embryonic prostate (48, 49). However, this induction of Shh expression by androgen may not be elicited in the developing GT. The Gli1 and Ptc1 expression levels were not altered between male and female GT. Thus, hedgehog signaling does not appear to be a downstream target of androgen of this developmental context. On the other hand, androgen responsiveness was decreased in Gli2 mutants when compared for sexually dimorphic gene expression between male and female GT. FK506 binding protein (Fkbp5) is one of the molecular chaperones that maintain a stimulatory effect on the androgen receptor-mediated transcriptional activity (50, 51). The mutants for Fkbp4, another gene belonging to the same family with Fkbp5, display the decreased level of androgen signal sensitivity, which results in a GT hypoplasia (52). These results are in agreement with the current observation that the androgen responsiveness was down-regulated in the Gli2 mutants. Notably, female marker genes, Dkk2 and Sfrp1, were abundantly expressed in the Gli2 male mutants in comparison with those of the wild-type male GT. Increased level of Wnt-inhibitory genes, such as Dkk2 and Sfrp1, can lead to down-regulation of Wnt/β-catenin signaling, which activity is necessary for masculine process for GT development (28). These observations suggest that hedgehog signal may facilitate masculine processes upon modulating the androgen responsiveness in the mesenchyme. Androgen treatment to the Gli2 female mutant embryos did not induce GT masculinization. This also supports the notion that androgen is not sufficient for induction of male GT development in the absence of hedgehog signaling.
In contrast to severe phenotypes of Gli2 mutants, GT in the Gli3 mutants were morphologically normal and showed proper sexual differentiation, even though the del5-derived LacZ signal was augmented. Thus, the role of Gli3 as a hedgehog repressor during GT development remains unknown, so far. However, double mutants analysis revealed that Gli3 can compensate for Gli2 function as a hedgehog activator when Gli2 alleles are mutated. The compensatory role of Gli3 has been known particularly in the developing foregut and hindgut, such as trachea, esophagus, lung, and anorectal organ (11, 44). To our knowledge, the current result would be the first case showing a redundant role of Gli2 and Gli3 function in the reproductive organ. Most double homozygotes (Gli2−/−;Gli3−/−) die around E10.5 (10, 11), and a few survivor did not exhibit GT protrusion like the case of Shh KO mutants (Miyagawa, S., and G. Yamada, unpublished data). This also suggests a redundant role of Gli2 and Gli3 function throughout GT development.
Hedgehog pathway-mediated embryonic masculinization can also be attributed to androgen production through Dhh, the other member of the mammalian hedgehog family ligand. Inactivation of the Dhh gene leads to defects of fetal Leydig cell differentiation, resulting in the insufficient production of androgen and male-to-female sex-reversal in both the internal and external reproductive organs in such mice (30, 53). Although Gli2 has been suggested to be a primary activator of Shh-mediated transcription, androgen production and testicular development is normal in the Gli2 mutant embryos. Dhh-mediated hedgehog signaling in the testis is possibly compensated by the Gli3 protein. Recently, conditionally expressed SmoM2 driven by SF1-Cre transgenic mice ectopically express steroid synthesis-related gene expression, such as Cyp17, which is not expressed in the normal embryonic ovary (36). In contrast, the current constitutively activated SmoM2 driven by CAGGS-CreERTM allele did not elicit excessive androgen production judged by p450Scc expression (54, 55). These observations may suggest the existence of the critical time point of hedgehog-mediated Leydig cell differentiation. SF1-Cre allele induces the target gene recombination in the undifferentiated stage of steroid synthesizing cells, whereas the current mutant embryos were conditionally expressing SmoM2 at later stages.
Hypospadias is one of the most frequent birth defects often considered as disorders of masculinization. There is a controversy about the causative mechanisms of hypospadias. Both experimentally and clinically, any defects in androgen pathway cause urogenital defects, including hypospadias (1). Hypospadias is generally considered as caused by multifactorial factors; thus, familial cases for the hypospadias are rather rare among total patients. Moreover, only a small portion of heritable hypospadias can be attributed to such defects (56). The mutations of several growth factors, including hedgehog signaling, potentially induce early embryonic defects or lethality in severe cases. Hence, the contribution of growth factor signaling to the sexual differentiation has been generally unclear. Taking an advantage of conditionally mutant analyses, the current results shed light on hedgehog signaling as a newly identified crucial regulator for external genitalia masculinization and its possible relation with urogenital disorders.
Acknowledgments
We thank Dr. A. Joyner, Dr. A. McMahon, Dr. P. Chambon, Dr. L. Ma, Dr. M. Renfree, Dr. H. Yao, Dr. T.P. Yamaguchi, Dr. S. Hayashi, Dr. H. Sasaki, Dr. F. Umehara, and Dr. H. Nishida for their support. We also thank T. Tanaka, C. Inoue, K. Tanaka, C. Nakahara, and S. Miyaji for their assistance.
This work was supported by Grant-in-Aid for Young Scientists B, and for Scientific Research on Innovative Areas “Molecular Mechanisms for Establishment of Sex Differences (22132006)” and by the Global Center of Excellence program “Cell Fate Regulation Research and Education Unit”; by a grant for Child Health and Development and a Health Sciences Research Grant from the Ministry of Health, Labor, and Welfare; by the National Institute of Environmental Health Sciences Grant R01ES016597-01A1.
Disclosure Summary: The authors have nothing to disclose.
Footnotes
- Dhh
- Desert hedgehog
- Dkk
- Dickkopf
- E
- embryonic day
- Fkbp5
- Fk506 binding protein
- Gli
- glioblastoma
- GT
- genital tubercle
- KO
- knockout
- Ptc
- Patched
- Sfrp
- secreted frizzled-related protein
- Shh
- Sonic hedgehog
- Smo
- Smoothened
- TM
- tamoxifen
- TP
- testosterone propionate
- UPE
- urethral plate epithelium
- Wg
- wingless
- Wnt
- Int and Wg in Drosophila.
References
- 1. Yamada G, Suzuki K, Haraguchi R, Miyagawa S, Satoh Y, Kamimura M, Nakagata N, Kataoka H, Kuroiwa A, Chen Y. 2006. Molecular genetic cascades for external genitalia formation: an emerging organogenesis program. Dev Dyn 235:1738–1752 [DOI] [PubMed] [Google Scholar]
- 2. Baskin LS, Himes K, Colborn T. 2001. Hypospadias and endocrine disruption: is there a connection? Environ Health Perspect 109:1175–1183 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Skakkebaek NE, Rajpert-De Meyts E, Main KM. 2001. Testicular dysgenesis syndrome: an increasingly common developmental disorder with environmental aspects. Hum Reprod 16:972–978 [DOI] [PubMed] [Google Scholar]
- 4. Haraguchi R, Mo R, Hui C, Motoyama J, Makino S, Shiroishi T, Gaffield W, Yamada G. 2001. Unique functions of sonic hedgehog signaling during external genitalia development. Development 128:4241–4250 [DOI] [PubMed] [Google Scholar]
- 5. Haraguchi R, Suzuki K, Murakami R, Sakai M, Kamikawa M, Kengaku M, Sekine K, Kawano H, Kato S, Ueno N, Yamada G. 2000. Molecular analysis of external genitalia formation: the role of fibroblast growth factor (Fgf) genes during genital tubercle formation. Development 127:2471–2479 [DOI] [PubMed] [Google Scholar]
- 6. Perriton CL, Powles N, Chiang C, Maconochie MK, Cohn MJ. 2002. Sonic hedgehog signaling from the urethral epithelium controls external genital development. Dev Biol 247:26–46 [DOI] [PubMed] [Google Scholar]
- 7. Ingham PW, McMahon AP. 2001. Hedgehog signaling in animal development: paradigms and principles. Genes Dev 15:3059–3087 [DOI] [PubMed] [Google Scholar]
- 8. Nieuwenhuis E, Hui CC. 2005. Hedgehog signaling and congenital malformations. Clin Genet 67:193–208 [DOI] [PubMed] [Google Scholar]
- 9. Bai CB, Auerbach W, Lee JS, Stephen D, Joyner AL. 2002. Gli2, but not Gli1, is required for initial Shh signaling and ectopic activation of the Shh pathway. Development 129:4753–4761 [DOI] [PubMed] [Google Scholar]
- 10. Mo R, Freer AM, Zinyk DL, Crackower MA, Michaud J, Heng HH, Chik KW, Shi XM, Tsui LC, Cheng SH, Joyner AL, Hui C. 1997. Specific and redundant functions of Gli2 and Gli3 zinc finger genes in skeletal patterning and development. Development 124:113–123 [DOI] [PubMed] [Google Scholar]
- 11. Motoyama J, Liu J, Mo R, Ding Q, Post M, Hui CC. 1998. Essential function of Gli2 and Gli3 in the formation of lung, trachea and oesophagus. Nat Genet 20:54–57 [DOI] [PubMed] [Google Scholar]
- 12. Park HL, Bai C, Platt KA, Matise MP, Beeghly A, Hui CC, Nakashima M, Joyner AL. 2000. Mouse Gli1 mutants are viable but have defects in SHH signaling in combination with a Gli2 mutation. Development 127:1593–1605 [DOI] [PubMed] [Google Scholar]
- 13. Cunha GR, Chung LW, Shannon JM, Reese BA. 1980. Stromal-epithelial interactions in sex differentiation. Biol Reprod 22:19–42 [DOI] [PubMed] [Google Scholar]
- 14. Prins GS, Putz O. 2008. Molecular signaling pathways that regulate prostate gland development. Differentiation 76:641–659 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Cunha GR, Battle E, Young P, Brody J, Donjacour A, Hayashi N, Kinbara H. 1992. Role of epithelial-mesenchymal interactions in the differentiation and spatial organization of visceral smooth muscle. Epithelial Cell Biol 1:76–83 [PubMed] [Google Scholar]
- 16. Hayward SW, Haughney PC, Rosen MA, Greulich KM, Weier HU, Dahiya R, Cunha GR. 1998. Interactions between adult human prostatic epithelium and rat urogenital sinus mesenchyme in a tissue recombination model. Differentiation 63:131–140 [DOI] [PubMed] [Google Scholar]
- 17. Kurzrock EA, Baskin LS, Li Y, Cunha GR. 1999. Epithelial-mesenchymal interactions in development of the mouse fetal genital tubercle. Cells Tissues Organs 164:125–130 [DOI] [PubMed] [Google Scholar]
- 18. Murakami R, Mizuno T. 1986. Proximal-distal sequence of development of the skeletal tissues in the penis of rat and the inductive effect of epithelium. J Embryol Exp Morphol 92:133–143 [PubMed] [Google Scholar]
- 19. Jost A. 1953. Problems of fetal endocrinology: the gonadal and hypophyseal hormones. Recent Prog Horm Res 8:379–418 [DOI] [PubMed] [Google Scholar]
- 20. Hui CC, Joyner AL. 1993. A mouse model of greig cephalopolysyndactyly syndrome: the extra-toesJ mutation contains an intragenic deletion of the Gli3 gene. Nat Genet 3:241–246 [DOI] [PubMed] [Google Scholar]
- 21. Chiang C, Litingtung Y, Lee E, Young KE, Corden JL, Westphal H, Beachy PA. 1996. Cyclopia and defective axial patterning in mice lacking sonic hedgehog gene function. Nature 383:407–413 [DOI] [PubMed] [Google Scholar]
- 22. Dassule HR, Lewis P, Bei M, Maas R, McMahon AP. 2000. Sonic hedgehog regulates growth and morphogenesis of the tooth. Development 127:4775–4785 [DOI] [PubMed] [Google Scholar]
- 23. Hayashi S, McMahon AP. 2002. Efficient recombination in diverse tissues by a tamoxifen-inducible form of Cre: a tool for temporally regulated gene activation/inactivation in the mouse. Dev Biol 244:305–318 [DOI] [PubMed] [Google Scholar]
- 24. Haraguchi R, Motoyama J, Sasaki H, Satoh Y, Miyagawa S, Nakagata N, Moon A, Yamada G. 2007. Molecular analysis of coordinated bladder and urogenital organ formation by hedgehog signaling. Development 134:525–533 [DOI] [PubMed] [Google Scholar]
- 25. Jeong J, Mao J, Tenzen T, Kottmann AH, McMahon AP. 2004. Hedgehog signaling in the neural crest cells regulates the patterning and growth of facial primordia. Genes Dev 18:937–951 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Feil R, Wagner J, Metzger D, Chambon P. 1997. Regulation of Cre recombinase activity by mutated estrogen receptor ligand-binding domains. Biochem Biophys Res Commun 237:752–757 [DOI] [PubMed] [Google Scholar]
- 27. Nishida H, Miyagawa S, Vieux-Rochas M, Morini M, Ogino Y, Suzuki K, Nakagata N, Choi HS, Levi G, Yamada G. 2008. Positive regulation of steroidogenic acute regulatory protein gene expression through the interaction between Dlx and GATA-4 for testicular steroidogenesis. Endocrinology 149:2090–2097 [DOI] [PubMed] [Google Scholar]
- 28. Miyagawa S, Satoh Y, Haraguchi R, Suzuki K, Iguchi T, Taketo MM, Nakagata N, Matsumoto T, Takeyama K, Kato S, Yamada G. 2009. Genetic interactions of the androgen and Wnt/β-catenin pathways for the masculinization of external genitalia. Mol Endocrinol 23:871–880 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Huang CC, Yao HH. 2010. Diverse functions of hedgehog signaling in formation and physiology of steroidogenic organs. Mol Reprod Dev 77:489–496 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Yao HH, Whoriskey W, Capel B. 2002. Desert hedgehog/patched 1 signaling specifies fetal Leydig cell fate in testis organogenesis. Genes Dev 16:1433–1440 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Welsh M, Saunders PT, Fisken M, Scott HM, Hutchison GR, Smith LB, Sharpe RM. 2008. Identification in rats of a programming window for reproductive tract masculinization, disruption of which leads to hypospadias and cryptorchidism. J Clin Invest 118:1479–1490 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Wilson MJ. 1983. Inhibition of development of both androgen-dependent and androgen-independent pigment cells in scrotal skin dermis of the rat by antiandrogen treatment during fetal growth. Endocrinology 112:321–325 [DOI] [PubMed] [Google Scholar]
- 33. Nishida H, Miyagawa S, Matsumaru D, Wada Y, Satoh Y, Ogino Y, Fukuda S, Iguchi T, Yamada G. 2008. Gene expression analyses on embryonic external genitalia: identification of regulatory genes possibly involved in masculinization processes. Congenit Anom 48:63–67 [DOI] [PubMed] [Google Scholar]
- 34. Yucel S, Liu W, Cordero D, Donjacour A, Cunha G, Baskin LS. 2004. Anatomical studies of the fibroblast growth factor-10 mutant, sonic hedge hog mutant and androgen receptor mutant mouse genital tubercle. Adv Exp Med Biol 545:123–148 [DOI] [PubMed] [Google Scholar]
- 35. Xie J, Murone M, Luoh SM, Ryan A, Gu Q, Zhang C, Bonifas JM, Lam CW, Hynes M, Goddard A, Rosenthal A, Epstein EH, Jr, de Sauvage FJ. 1998. Activating smoothened mutations in sporadic basal-cell carcinoma. Nature 391:90–92 [DOI] [PubMed] [Google Scholar]
- 36. Barsoum IB, Bingham NC, Parker KL, Jorgensen JS, Yao HH. 2009. Activation of the Hedgehog pathway in the mouse fetal ovary leads to ectopic appearance of fetal Leydig cells and female pseudohermaphroditism. Dev Biol 329:96–103 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Miyagawa S, Moon A, Haraguchi R, Inoue C, Harada M, Nakahara C, Suzuki K, Matsumaru D, Kaneko T, Matsuo I, Yang L, Taketo MM, Iguchi T, Evans SM, Yamada G. 2009. Dosage-dependent hedgehog signals integrated with Wnt/β-catenin signaling regulate external genitalia formation as an appendicular program. Development 136:3969–3978 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Seifert AW, Zheng Z, Ormerod BK, Cohn MJ. 2010. Sonic hedgehog controls growth of external genitalia by regulating cell cycle kinetics. Nat Commun 1:23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Huang L, Pu Y, Hu WY, Birch L, Luccio-Camelo D, Yamaguchi T, Prins GS. 2009. The role of Wnt5a in prostate gland development. Dev Biol 328:188–199 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Huang L, Pu Y, Alam S, Birch L, Prins GS. 2005. The role of Fgf10 signaling in branching morphogenesis and gene expression of the rat prostate gland: lobe-specific suppression by neonatal estrogens. Dev Biol 278:396–414 [DOI] [PubMed] [Google Scholar]
- 41. Grishina IB, Kim SY, Ferrara C, Makarenkova HP, Walden PD. 2005. BMP7 inhibits branching morphogenesis in the prostate gland and interferes with notch signaling. Dev Biol 288:334–347 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Cook C, Vezina CM, Allgeier SH, Shaw A, Yu M, Peterson RE, Bushman W. 2007. Noggin is required for normal lobe patterning and ductal budding in the mouse prostate. Dev Biol 312:217–230 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Lin C, Yin Y, Veith GM, Fisher AV, Long F, Ma L. 2009. Temporal and spatial dissection of Shh signaling in genital tubercle development. Development 136:3959–3967 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Mo R, Kim JH, Zhang J, Chiang C, Hui CC, Kim PC. 2001. Anorectal malformations caused by defects in sonic hedgehog signaling. Am J Pathol 159:765–774 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Seifert AW, Bouldin CM, Choi KS, Harfe BD, Cohn MJ. 2009. Multiphasic and tissue-specific roles of sonic hedgehog in cloacal septation and external genitalia development. Development 136:3949–3957 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Pu Y, Huang L, Birch L, Prins GS. 2007. Androgen regulation of prostate morphoregulatory gene expression: Fgf10-dependent and -independent pathways. Endocrinology 148:1697–1706 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Pu Y, Huang L, Prins GS. 2004. Sonic hedgehog-patched Gli signaling in the developing rat prostate gland: lobe-specific suppression by neonatal estrogens reduces ductal growth and branching. Dev Biol 273:257–275 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Lamm ML, Catbagan WS, Laciak RJ, Barnett DH, Hebner CM, Gaffield W, Walterhouse D, Iannaccone P, Bushman W. 2002. Sonic hedgehog activates mesenchymal Gli1 expression during prostate ductal bud formation. Dev Biol 249:349–366 [DOI] [PubMed] [Google Scholar]
- 49. Podlasek CA, Barnett DH, Clemens JQ, Bak PM, Bushman W. 1999. Prostate development requires sonic hedgehog expressed by the urogenital sinus epithelium. Dev Biol 209:28–39 [DOI] [PubMed] [Google Scholar]
- 50. Barent RL, Nair SC, Carr DC, Ruan Y, Rimerman RA, Fulton J, Zhang Y, Smith DF. 1998. Analysis of FKBP51/FKBP52 chimeras and mutants for Hsp90 binding and association with progesterone receptor complexes. Mol Endocrinol 12:342–354 [DOI] [PubMed] [Google Scholar]
- 51. Febbo PG, Lowenberg M, Thorner AR, Brown M, Loda M, Golub TR. 2005. Androgen mediated regulation and functional implications of fkbp51 expression in prostate cancer. J Urol 173:1772–1777 [DOI] [PubMed] [Google Scholar]
- 52. Cheung-Flynn J, Prapapanich V, Cox MB, Riggs DL, Suarez-Quian C, Smith DF. 2005. Physiological role for the cochaperone FKBP52 in androgen receptor signaling. Mol Endocrinol 19:1654–1666 [DOI] [PubMed] [Google Scholar]
- 53. Clark AM, Garland KK, Russell LD. 2000. Desert hedgehog (Dhh) gene is required in the mouse testis for formation of adult-type Leydig cells and normal development of peritubular cells and seminiferous tubules. Biol Reprod 63:1825–1838 [DOI] [PubMed] [Google Scholar]
- 54. Hu MC, Hsu NC, El Hadj NB, Pai CI, Chu HP, Wang CK, Chung BC. 2002. Steroid deficiency syndromes in mice with targeted disruption of Cyp11a1. Mol Endocrinol 16:1943–1950 [DOI] [PubMed] [Google Scholar]
- 55. Shih MC, Hsu NC, Huang CC, Wu TS, Lai PY, Chung BC. 2008. Mutation of mouse Cyp11a1 promoter caused tissue-specific reduction of gene expression and blunted stress response without affecting reproduction. Mol Endocrinol 22:915–923 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56. Holmes NM, Miller WL, Baskin LS. 2004. Lack of defects in androgen production in children with hypospadias. J Clin Endocrinol Metab 89:2811–2816 [DOI] [PubMed] [Google Scholar]