Abstract
Dopamine, acting through the dopamine type 2 receptor (Drd2), is the main inhibitor of pituitary prolactin (PRL) secretion and lactotroph proliferation. TGF-β1 is involved, at least in part, in mediating these actions. It was described that TGF-β1 synthesis in rat pituitary lactotrophs is up-regulated by dopamine and down-regulated by estradiol. TGF-β1 is secreted as a large latent complex. The local regulation of cytokine activation in the pituitary has not yet been explored. In this work, we studied pituitary active and total TGF-β1 content, as well as TGF-β1 mRNA, and the in vivo role of dopamine and estradiol on pituitary TGF-β1 levels. Adult female mice (wild type), and female mice with a null mutation in the Drd2 (Drd2−/−), were used. The loss of dopaminergic tone induced a decrease in TGF-β1 mRNA expression, in active and total cytokine content, and in TGF-β type II receptor expression. Dopamine regulation of pituitary TGF-β1 activation process was inferred by the inhibition of active cytokine by in vivo sulpiride treatment. Interestingly, in the absence of dopaminergic tone, estradiol induced a strong increase in active TGF-β1. PRL secretion correlated with active, but not total cytokine. TGF-β1 inhibitory action on lactotroph proliferation and PRL secretion was decreased in Drd2−/− pituitary cells, in correlation with decreased TGF-β type II receptor. The study of the TGF-β1 activation process and its regulation is essential to understand the cytokine activity. As an intermediary of dopamine inhibition of lactotroph function, TGF-β1 and local activators may be important targets in the treatment of dopamine agonist-resistant prolactinomas.
Dopamine plays a key role in maintaining the normal function of lactotrophs by inhibiting cell proliferation and prolactin (PRL) secretion (1, 2). The dopamine type 2 receptor (Drd2) is the predominant subtype in the pituitary gland and mediates these effects (3). Accordingly, our group and others have previously reported that transgenic mice lacking functional Drd2 (Drd2−/−) have chronic hyperprolactinemia and lactotroph hyperplasia and that these traits are more evident in females (4–6).
Even though dopamine is the principal inhibitor of PRL synthesis and secretion, a cohort of peptides and growth factors participate in pituitary hyperplasia generation, and many of these are regulated by dopamine. For example, TGF-β1, which is a multifunctional cytokine with powerful effects on cellular proliferation and differentiation, angiogenesis, and extracellular matrix modification (7), affects pituitary function by contributing, at least in part, to the inhibitory action of dopamine on lactotrophs. TGF-β1 and TGFβ type II receptor (TβRII) are both expressed in rat lactotrophs, and the cytokine is described to be a potent autocrine/paracrine inhibitor of cell proliferation and PRL secretion (8, 9). It has been described that dopamine, acting through the Drd2, reduces the rate of lactotroph proliferation (10) and concomitantly up-regulates TGF-β1 expression and secretion, in vivo and in vitro, and it has been proposed that the cytokine might contribute to the effect of dopamine on lactotrophs. On the other hand, it is well known that estradiol inhibits dopamine release from the hypothalamus and reduces the inhibitory action of dopamine on lactotrophs (11). In female rats, it has been described that in vitro and in vivo treatments with 17β-estradiol decrease the levels of pituitary TGF-β1 and TβRII mRNA and proteins, concomitantly with the increase in PRL levels (8, 12, 13). Therefore, decreased TGF-β1 and TβRII might cooperate in the PRL releasing effect of estradiol.
The folliculostellate (FS) pituitary cells have also been identified as a source of TGF-β1 (14). It is unknown to which extent the two cell types contribute to the total intrapituitary TGF-β1 production, but because Drd2 receptors are found only in lactotrophs, there is no evidence that TGF-β1 production by FS cells is under dopaminergic control.
The biology of the TGF-β is complex. These cytokines are synthesized as homodimeric proproteins (pro-TGF-β). The TGF-β propeptide, also known as the latency-associated protein (LAP), is cleaved from the mature TGF-β 24-kDa dimer in the trans-Golgi by furin-type enzymes. However, both LAP and the mature C-terminal TGF-β remain noncovalently associated. Before secretion, this small latent complex is assembled in covalent association with a molecule of latent TGF-β binding protein, an extracellular matrix component. This large latent TGF-β complex is secreted and incorporated into the extracellular matrix, where it can undergo a highly regulated process of activation, whereby TGF-β is released. Little is known about the tissue-specific regulation of the final process that enables the cytokine to be biologically active (7, 15, 16).
Alterations in dopamine or estradiol function lead to PRL-secreting adenomas in different animal models, and both, dopamine and estradiol, regulate TGF-β1 availability at the pituitary level. In this context, Drd2−/− female mice (5, 17) and the estrogen-treated rat (reviewed in Ref. 18) share the characteristics of increased pituitary weight, hyperprolactinemia, lactotroph hyperplasia, and reduced or absent dopaminergic action at the pituitary level.
In humans, prolactinomas are the most frequent among pituitary tumors. They are usually benign and can be treated with dopaminergic agents. Nevertheless, 15% of these tumors may be resistant to classical pharmacological therapy, become invasive and aggressive, and require extirpation. Alternative therapies would be desired for these tumors. In this context, mice lacking pituitary dopaminergic control represent an interesting model to study dopamine agonist-resistant prolactinomas and could help to unravel the impact of dopamine and estrogens on pituitary TGF-β1 synthesis and activation. The study of pituitary TGF-β1 regulation could provide novel tools as alternative therapies in dopamine agonist-resistant prolactinomas.
In the present study, we sought to investigate the in vivo role of dopamine and estradiol on pituitary TGF-β1. To this end, we studied active and total TGF-β1 content, as well as TGF-β1 secretory and proliferative actions in vitro, in pituitaries of female mice lacking Drd2 in comparison with their wild-type siblings (Drd2+/+). Furthermore, selective pharmacological tools were used in vivo to evaluate effects of Drd2 stimulation and blockade or estradiol administration on active and total pituitary TGF-β1, as well as its mRNA expression in both Drd2+/+ and Drd2−/− female mice.
Materials and Methods
Animals
Female Drd2−/− mice (official strain designation B6; 129S2-Drd2tm1Low/J by the Induced Mutant Resource at The Jackson Laboratory, Bar Harbor, ME), generated by targeted mutagenesis of the Drd2 gene in embryonic stem cells, were used (5, 17). The original F2 hybrid strain (129S2/Sv X C57BL/6J), containing the mutated Drd2 allele, was backcrossed for eight generations to wild-type C57BL/6J mice. Mutant and wild-type mice were generally the product of heterozygote crossings, and in all cases, sibling controls were used. Mice were housed in groups of four or five with mixed genotypes in an air-conditioned room with lights on at 0700 h and off at 1900 h. Animals had free access to laboratory chow and tap water. Drd2+/+, heterozygous, and Drd2−/− mice were identified by PCR of genomic DNA, as described (4). Animals were used at 8 months of age, at which time the pituitaries from Drd2−/− females were hyperplastic. All experimental procedures were reviewed and approved by the institutional animal care and use committee of the Instituto de Biología y Medicina Experimental (Division of Animal Welfare, Office for Protection of Research Risks, National Institutes of Health, A#5072-01).
In vivo experiments
Wild-type female mice were injected ip with saline solution (control group), the Drd2 antagonist sulpiride (10 mg/kg; IVAX Laboratories, Buenos Aires, Argentina), or the Drd2 agonist cabergoline (2 mg/kg; Beta Laboratories, Buenos Aires, Argentina). Animals were killed by decapitation after 30 min (short term) or 24 h (long term) of treatment.
Another set of Drd2−/− and Drd2+/+ mice were injected with estradiol-valerate (0.2 mg/kg sc, Progynon Depot; Schering, Buenos Aires, Argentina) or castor oil (control group) and killed 24 h later. After every treatment, trunk blood was collected, and anterior pituitaries were removed. Sera were kept at −20 C until RIA were performed. Pituitaries were excised as described below for Western blotting, quantitative real-time RT-PCR (Q-RT-PCR), or ELISA assays. At 8 months, pituitary weights were significantly higher in Drd2−/− mice (2.43 ± 0.08 vs. 4.56 ± 0.22 g, wild type vs. Drd2−/−, respectively; P < 0.001).
Western blotting
Anterior pituitaries were homogenized in 80 μl ice-cold buffer containing 50 mm Tris, 10 mm CaCl2, 1 mm MgCl2, 1% Triton X-100 (pH 7.6), and a mix of proteases inhibitors (phenylmethylsulfonylfluoride, tosylphenylalanine chloromethylketone, tert-Amyl methyl ether, N-carbobenzoxy-l-phenylalanine chloromethyl ketone, and tosylamidelysyl chloromethylketone) in a hand-held microtissue homogenizer. The homogenate was centrifuged at 1500 × g for 5 min at 4 C. The supernatant was collected, and protein concentration was determined by the Quant-iT Protein Assay kit and Qubit fluorometer (Invitrogen, Buenos Aires, Argentina); 50 μg protein from each sample were mixed with 5× sample buffer [150 mm Tris-HCl, 10% sodium dodecyl sulfate, 50% glycerol, 0.05% bromophenol blue, and 50 mm dithiotreitol (pH 6.8)] and heated 5 min at 95 C. Samples were loaded on 12% SDS-PAGE and transferred to Hybond-P polyvinylidene difluoride transfer membranes (GE Healthcare, Princeton, NJ). Membranes were incubated over night at 4 C with mouse anti-TβRII antibody (1:500, sc 17791; Santa Cruz Biotechnology, Inc., Santa Cruz, CA) or mouse anti-β-actin antibody (1:5000, ab 6276; Abcam, Cambridge, MA). After washing in PBS 0.05% Tween 20, membranes were incubated 1 h at room temperature with secondary donkey antimouse horseradish peroxidase (1:5000, sc 2314; Santa Cruz Biotechnology, Inc.). Chemiluminescence was detected in a G:box chemi HR16 (Syngene, Frederick, MD). Band intensities were quantified using ImageJ software (National Institutes of Health, Bethesda, MD).
Quantitative real-time RT-PCR
Pituitaries from different experimental groups were collected in RNA-later (Ambion, Austin, TX). Total RNA was extracted from the tissue using the RNeasy Protect mini kit (QIAGEN, Valencia, CA). RT was performed using 750 ng of total RNA, and the resulting cDNA was used for Q-RT-PCR analysis. Q-RT-PCR were performed using specific primers and the QuantiFast SYBR green PCR kit (QIAGEN) on an iCycler Thermal Cycler (Bio-Rad, Hercules, CA). TGF-β1 transcript expression was quantified by comparing the threshold cycle with that of β-actin by using the comparative threshold cycle method. Primers used: TGF-β1 forward, 5′-CAACAATTCCTGGCGTTACC-3′ and reverse, 5′-AGCCCTGTATTCCGTCTCCT-3′; β-actin forward, 5′-AGCCTTCCTTCTTGGGTATGG-3′ and reverse, 5′-GCCACCGATCCACACAGAGTA-3′.
Detection of total and active TGF-β1
ELISA were performed to quantify active or total TGF-β1 content in pituitary homogenates of wild-type and Drd2−/− female mice with a TGF-β1 Emax ImmunoAssay-System (Promega, Madison, WI).
Before pituitaries were excised and homogenized, as described for Western blotting, the homogenates were centrifuged at 5000 × g for 5 min at 4 C. The supernatant protein contents were measured with the QUBIT Fluorometer and the Quant-iT Protein Assay kit (Invitrogen).
TGF-β1 was expressed as pg/mg protein. The minimum detectable dose of biologically active TGF-β1 was 32 pg/ml with less than 3% cross-reactivity with TGF-β2 and TGF-β3 at 10 ng/ml.
To assay total TGF-β1, samples were acidified to pH 2.6 by adding 1 n HCl for 20 min at room temperature, followed by neutralization with 1 n NaOH to pH 7.6.
Cell dispersion and culture
Anterior pituitary cells from 8-month-old wild-type and Drd2−/− female mice were placed in chambers containing freshly prepared Krebs-Ringer bicarbonate buffer without Ca2+ or Mg2+. Buffer contained 14 mm glucose, 1% BSA, 2% MEM amino acids, 1% MEM vitamins (Life Technologies, Inc., Buenos Aires, Argentina), and 2 mm glutamine and was previously gassed during 15 min with 95% O2-5% CO2 and adjusted to pH 7.35–7.40. Buffer was filtered through a 0.45-μm pore diameter membrane (Nalgene, Rochester, NY). Pituitaries were washed three times with Krebs-Ringer bicarbonate buffer and cut into 1-mm pieces. Fragments were washed and incubated in the same buffer containing 0.5% trypsin for 30 min at 37 C in 95% O2-5% CO2, followed by two additional minutes with 50 μl deoxyribonuclease I (1 mg/ml; Worthington Biochemical Corp., Lakewood, NJ). Digestion was ended by adding 1 mg/ml lima bean trypsin inhibitor. Fragments were disassociated to single cells by gentle trituration through Pasteur pipettes. The resulting suspension was filtered through a nylon gauze (160-μm pore size) and centrifuged 10 min at 1000 × g. Before centrifugation, an aliquot of the cellular suspension was taken to quantify pituitary cell yield using a Neubauer chamber. Cell viability, determined by Trypan Blue exclusion, was always greater than 90%. Cells were cultured for 5 d in DMEM, 10% horse serum, and 2.5% fetal bovine serum. Cells were washed and stimulated with 1 ng/ml TGF-β1 for 24 h in DMEM 0.5% BSA medium, without serum. Cell culture was performed as described (19). Media samples were collected after 24 h, and PRL content was analyzed by RIA.
As we previously described in cell culture experiments, we used three wild-type pituitaries and two Drd2−/− pituitaries in each experiment, and 35,000 cells/well were cultured in 96-well plates. After the trypsinization process, we usually recover 1,800,000 cells per pituitary in Drd2−/− mice and 785,000 cells per pituitary in wild types (4). On the other hand, we observed, by immunohistochemistry, that in 8-month-old females, the number of lactotrophs increases 3-fold in Drd2−/− pituitaries compared with those of wild-type pituitaries.
DNA synthesis in pituitary cells in culture
Culture procedure was the same as described above. [3H]thymidine (0.2 μCi/well, 87.7 Ci/mmol; NEN Life Science Products, Waltham, MA) was added to cultures. After 24 h of incubation, medium was discarded, and the cells removed and lysed by treatment with 0.05% trypsin and 0.02% EDTA in deionized water. The reaction was stopped 20 min later by filtering under vacuum through GF/C filters (Whatman, Middlesex, UK) using the Cell Harvester 8 (Nunc, Glastrup, Denmark). After five washes with deionized water, the filters were placed in plastic vials with 3 ml scintillation solution and radioactivity counted in a Beckman counter. Each experiment was repeated five times.
Radioimmunoassay
PRL was measured by RIA using mouse-specific reagents provided by the National Institute of Diabetes and Digestive and Kidney Diseases National Hormone and Pituitary Program (A. F. Parlow; National Hormone and Pituitary Program, Torrance, CA). Assays were performed using 10 μl serum in duplicate or the adequate quantity of diluted medium from cultured cells. Results are expressed in terms of mouse PRL RP3. Intra- and interassay coefficients of variation were 7.2 and 12.8%, respectively.
Statistical analyses
Results are expressed as means ± sem. Student's t test was used to compare data between genotypes in Fig. 1. Dopaminergic drug treatments (Fig. 2) were compared by one-way ANOVA followed by Fisher's protected least significant difference post hoc tests. Estradiol effect on different genotypes was compared by two-way ANOVA followed by Fisher's protected least significant difference post hoc tests. Correlation between TGF-β1 and serum PRL was analyzed with the Spearman's rank correlation test. P < 0.05 was considered significant.
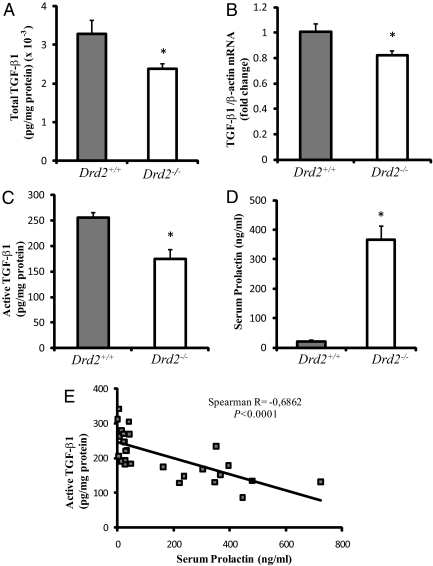
Fig. 1.
Pituitary TGF-β1 alterations in Drd2−/− female mice. A, Total TGF-β1 content measured by ELISA after sample acidification of pituitary homogenates from female Drd2+/+ and Drd2−/− mice. *, P = 0.023, Student's t test, n = 15 and 9, respectively. B, TGF-β1 mRNA levels measured by Q-RT-PCR and normalized to β-actin mRNA levels were decreased in Drd2−/− pituitaries. *, P = 0.027, Student's t test, n = 8 and 6. C, Active TGF-β1, measured by ELISA, was also decreased in pituitaries from Drd2−/− mice. *, P = 0.0002, Student's t test, n = 15 and 12. D, Serum PRL levels were significantly increased in Drd2−/− mice. *, P < 0.0001, Student's t test, n = 15 and 12. E, A significant inverse correlation was found between active TGF-β1 and serum PRL values. Spearman R = −0.686; P < 0.0001. No correlation was found between total TGF-β1 and PRL (data not shown).
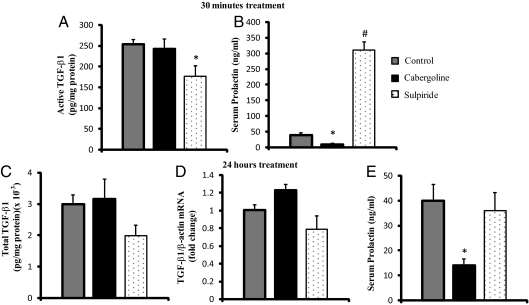
Fig. 2.
Dopaminergic regulation of pituitary TGF-β1. A, The effect of dopaminergic drugs at short time (30 min) on pituitary active TGF-β1 content was measured in Drd2+/+ mice. Cabergoline (2 mg/kg BW, ip) or sulpiride (5 mg/kg BW, ip) was injected, and 30 min later, animals were killed. Cabergoline did not modify active TGF-β1 content, whereas sulpiride induced a decrease in pituitary active TGF-β1. *, P = 0.0031 control vs. sulpiride, (n = 12, 7, and 5). B, Serum PRL levels were assayed by RIA as a control of dopaminergic drug effects. Cabergoline inhibition and sulpiride stimulation of PRL levels were observed. *, P = 0.0007; #, P = 0.0001 vs. control. (n = 13, 15, and 18). C, A 24-h treatment with dopaminergic drugs did not affect total pituitary TGF-β1 (n = 10, 6, and 6). D, TGF-β1 mRNA levels were not modified by dopaminergic drugs after a 24-h treatment (n = 8, 5, and 5). E, Dopaminergic drug effects on serum PRL levels after a 24-h treatment. Cabergoline inhibitory effect on serum PRL was observed. *, P = 0.017 cabergoline vs. control. No effect of sulpiride was found at this time (n = 20, 16, and 22). All data were analyzed by one-way ANOVA and Fisher's protected least significant difference post hoc test.
Results
In vivo experiments
Active and total TGF-β1 content in pituitaries from Drd2+/+ and Drd2−/− mice
As discussed, TGF-β1 is thought to contribute to the inhibitory effect of dopamine on rat lactotrophs, and TGF-β1 mRNA expression and secretion are up-regulated by dopamine (8, 9). However, little is known about active TGF-β1 content and its regulation in the pituitary. We therefore evaluated active and total TGF-β1 in Drd2−/− and Drd2+/+ female mice. We used a sensitive ELISA kit designed to measure biologically active TGF-β1, as well as total TGF-β1, content after acidification of the samples to dissociate the cytokine from its latent complexes. Disruption of the Drd2 caused a decrease in total TGF-β1 content (Fig. 1A) and mRNA levels (Fig. 1B) in pituitaries from female mice. Active TGF-β1 was also decreased in pituitary homogenates from Drd2−/− female mice (Fig. 1C). Less than 8% of total pituitary TGF-β1 was found in the active form (Fig. 1, A and C). As expected, serum PRL was elevated in Drd2−/− mice (Fig. 1D), and serum PRL levels correlated inversely with active, but not total, TGF-β1 values (Fig. 1E).
Dopaminergic regulation of active but not total pituitary TGF-β1
To investigate whether the Drd2 was involved in the TGF-β1 activation process, we assayed in vivo the short-term effect of dopaminergic drugs on active TGF-β1 content in pituitaries from wild-type female mice. After 30 min of in vivo cabergoline stimulation [dopamine agonist, 2 mg/kg body weight (BW), ip], no change in active TGF-β1 levels was found in pituitaries from Drd2+/+ mice, but sulpiride treatment (dopamine antagonist, 5 mg/kg BW, ip) decreased active TGF-β1 (Fig. 2A). Despite the lack of effect of cabergoline on active TGF-β1 content, serum PRL levels were inhibited. Likewise, the stimulatory effect of sulpiride on serum PRL levels was significant (Fig. 2B). An inverse correlation was found between serum PRL and active TGF-β1 values (P < 0.0001).
We next studied the effect of 24 h of treatment with dopaminergic drugs, an exposure that might affect TGF-β1 synthesis. After 24 h of treatment, neither cabergoline nor sulpiride altered total TGF-β1 content or mRNA levels in Drd2+/+ pituitaries (Fig. 2, C and D). Serum PRL levels remained inhibited after 24 h of cabergoline treatment, whereas the PRL releasing effect of sulpiride was not significant at this time (Fig. 2E).
Effect of estradiol administration on active and total pituitary TGF-β1
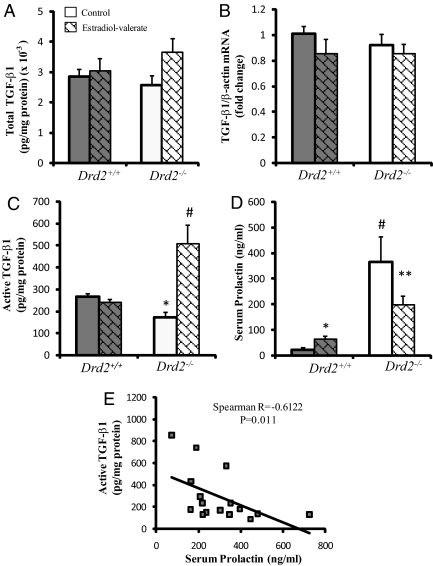
We studied whether estradiol regulates pituitary TGF-β1 in our experimental model. After 24 h of estradiol-valerate treatment (0.2 mg/kg BW, sc), we found no significant effect on total TGF-β1 protein or mRNA levels in either genotype (Fig. 3, A and B). Interestingly, despite no differences in total TGF-β1 content, estradiol induced a marked increase of active TGF-β1 in pituitaries from Drd2−/− mice (Fig. 3C) and no effect in Drd2+/+ mice. Estradiol-valerate increased serum PRL levels in female Drd2+/+ mice, whereas it caused a decrease in PRL levels in Drd2−/− mice (Fig. 3D). We found an inverse correlation between active TGF-β1 and serum PRL values in Drd2−/− mice (Fig. 3E) but not in Drd2+/+ groups where active TGF-β1 was not altered by estradiol treatment.
Fig. 3.
Pituitary TGF-β1 regulation by estradiol. A, No effect of estradiol on total TGF-β1 content in pituitary homogenates of Drd2+/+ and Drd2−/− mice, treated with estradiol valerate (0.2 mg/kg BW, sc, and 24 h), was observed (n = 14, 10, 8, and 9). B, TGF-β1 mRNA levels, assayed by Q-RT-PCR, were not affected by the estradiol treatment. (n = 8, 4, 7, and 6). C, Estradiol treatment induced a strong increase in active TGF-β1 only in pituitaries from Drd2−/− females. Data were analyzed by two-way ANOVA test. Interaction between genotype and drug effects was significant with P < 0.0001. Individual means were compared by Fisher's protected least significant difference test. #, P < 0.0001 Drd2−/− control vs. Drd2−/− estradiol; *, P = 0.001 Drd2+/+ control vs. Drd2−/− control (n = 7, 9, 10, and 12). D, A 24-h estradiol treatment caused opposite effects on serum PRL depending on the genotype. Serum PRL was increased by estradiol in Drd2+/+ females, whereas it was decreased in pituitaries from Drd2−/− mice (compared with genotype-matched control). Drd2−/− control mice showed higher PRL than Drd2+/+ mice as expected. In a two-way ANOVA, interaction between genotype and drug effects was significant with P < 0.0001. Individual means were compared by Fisher's protected least significant difference test. *, P = 0.0036 Drd2+/+ estradiol vs. Drd2+/+ control; **, P = 0.001 Drd2−/− estradiol vs. Drd2−/− control; and P < 0.0001 Drd2+/+ control vs. Drd2−/− control (n = 7, 9, 10, and 12). E, Inverse correlation between active TGF-β1 and serum PRL in Drd2−/− mice (Spearman R = −0.612, P = 0.011).
In vitro experiments
TGF-β1 effects on pituitary cells in culture
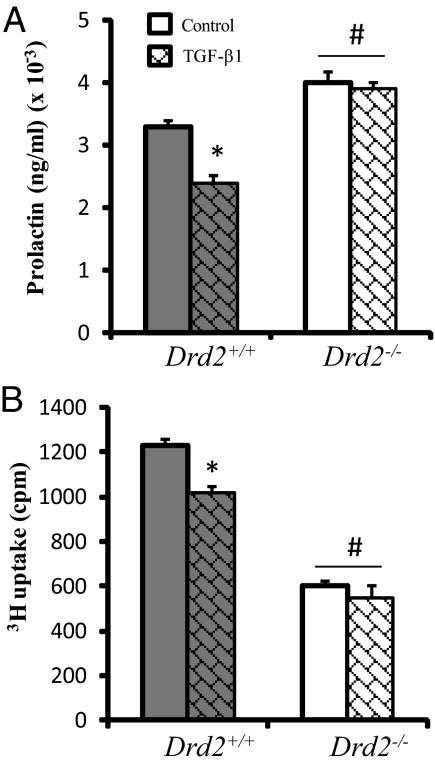
Because TGF-β1 inhibits PRL secretion and cell proliferation, we next explored whether the effect of TGF-β1 on pituitary cells in vitro was altered by disruption of Drd2. TGF-β1 decreased PRL secretion in cells from Drd2+/+ pituitaries (Fig. 4A), whereas TGF-β1 had no effect on pituitary cells from Drd2−/− mice. Furthermore, TGF-β1 decreased 3[H]thymidine uptake in pituitary cells from Drd2+/+ mice (Fig. 4B), whereas no effect was observed in cells from Drd2−/− mice. We observed higher PRL secretion and lower 3[H]thymidine uptake in cells from Drd2−/− mice, as previously described (20).
Fig. 4.
TGF-β1 effect on pituitary cells in culture. A, PRL release was measured by RIA in 24-h serum-free culture medium samples from Drd2+/+ and Drd2−/− pituitary cells. TGF-β1 treatment inhibited PRL release only in cells from Drd2+/+ mice. Drd2−/− cells released higher PRL levels than wild-type ones, two-way ANOVA, interaction genotype vs. drug was significant (P = 0.047). *, P = 0.008 Drd2+/+ TGF-β1 vs. Drd2+/+ control; #, P < 0.03 Drd2−/− vs. drug-matched Drd2+/+, n = 4 independent cultures, with quadruplicate samples. B, 3[H]thymidine uptake in 24-h serum-free culture from Drd2+/+ and Drd2−/− pituitary cells. TGF-β1 treatment caused a decrease in cell proliferation in Drd2+/+ cells without any effect on cells lacking Drd2. Wild-type cells presented higher proliferation than Drd2−/− cells. Two-way ANOVA, interaction genotype vs. drug was found (P = 0.033). *, P = 0.004 Drd2+/+ TGF-β1 vs. Drd2+/+ control; #, P = 0.0002 Drd2−/− vs. drug-matched Drd2+/+, n = 4 independent cultures, with quadruplicate samples.
TβRII expression in pituitary homogenates
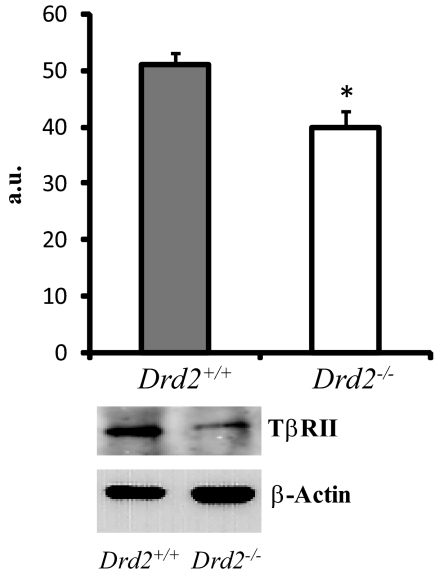
Finally, to evaluate whether the lack of TGF-β1 effect on pituitary cells from Drd2−/− mice could be due to alterations in TβRII concentration, we analyzed TβRII expression by Western blotting. We found decreased TβRII protein expression in Drd2−/− pituitaries compared with those of Drd2+/+ mice (Fig. 5).
Fig. 5.
TβRII expression was measured by Western blotting in pituitary homogenates. Drd2−/− females showed significantly lower TβRII expression. *, P = 0.0075, Student's t test, n = 6 and 6. Representative bands are shown. TβRII band was found at 75 kDa and actin band at 42 kDa. a.u., Arbitrary units.
Discussion
In this study, we examined the balance of active and total pituitary TGF-β1 in wild-type and Drd2−/− female mice and demonstrated that dopamine and estradiol regulate the levels not only of total but also of active TGF-β1. Moreover, the effect of estradiol depended on the presence of a dopaminergic tone. We found that pituitaries from Drd2−/− mice had lower active and total TGF-β1 compared with controls, highlighting the stimulatory role of dopamine on pituitary TGF-β1. This could be suggesting a lower contribution of TGF-β1 by individual Drd2−/− lactotrophs if we consider that the source of pituitary TGF-β1 is limited to lactotrophs and FS cells, the amount of FS cells is not different between genotypes, and lactotroph population is three times higher in pituitaries from Drd2−/− mice (4).
There was a concomitant reduction in pituitary TβRII expression in Drd2−/− mice, which is consistent with data that indicate that dopamine up-regulates TβRII (8, 9). Accordingly, the inhibitory action of TGF-β1 on lactotrophs in vitro was abolished in Drd2−/− pituitary cells. Our results, illustrated in Fig. 6, provide new mechanisms involved in the regulation of pituitary TGF-β1.
Fig. 6.
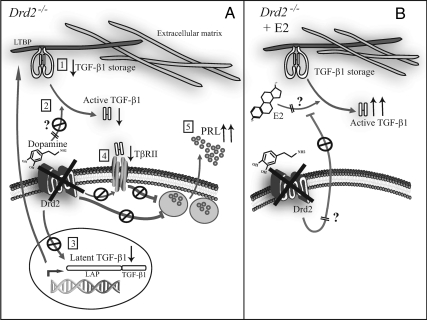
Diagram summarizing dopamine regulation of TGF-β1 inferred from results in mice lactotrophs from Drd2−/− mice. Dopamine, acting through Drd2, regulates normal function of lactotrophs by inhibiting PRL secretion and cell proliferation. TGF-β1 was suggested to partly mediate the inhibitory actions of dopamine. The cytokine is secreted in a latent form (LAP + TGF-β1) in covalent association with the latent TGF-β binding protein (LTBP) and remains sequestered in the extracellular matrix (TGF-β1 storage) until activation occurs. In this work, we found that the loss of dopaminergic regulation (Drd2−/− mice) caused a decrease in total (1) and active (2) TGF-β1 protein levels and mRNA synthesis (3), as well as TβRII expression (4). PRL secretion was highly increased in this model (5) and correlated with the down-regulation of active TGF-β1 (A). A 24-h in vivo estradiol (E2) treatment caused a strong increase in active TGF-β1 only in Drd2−/− mice. We therefore postulate that dopamine might inhibit a positive effect of E2 on TGF-β1 activation (B). Mechanisms underlying regulation of TGF-β1 activation by estradiol and dopamine are not yet elucidated (?).
TGF-β1 has been postulated to mediate, in part, the inhibitory actions of dopamine on lactotroph cell proliferation. To analyze the dopaminergic regulation of pituitary TGF-β1, we assayed in vivo treatments with the Drd2-specific agonist, cabergoline, and the antagonist, sulpiride, in Drd2+/+ mice. We found that a short-term treatment with sulpiride decreased active TGF-β1, suggesting that dopamine modulates TGF-β1 activation, because de novo protein synthesis was probably not altered in a 30-min treatment. The lack of effect of cabergoline on active TGF-β1 may be explained by the fact that, in wild-type mice, the Drd2 receives a constant dopaminergic input from the hypothalamus, and additional Drd2 stimulation may not modify TGF-β1 levels. Neither sulpiride nor cabergoline modified TGF-β1 mRNA expression after 24 h. This may be related to the different time course of action described for dopamine on secretion, synthesis, and cell proliferation in the pituitary (21). Dopamine may modify TGF-β1 activation within minutes to hours, but the effect on synthesis might need more time or involve different intracellular signals. However, the chronic loss of dopaminergic tone caused a decrease in active and total pituitary cytokine content, as well as TGF-β1 mRNA, suggesting that the Drd2 is involved in the regulation of TGF-β1 synthesis and storage, as well as activation. On the other hand, chronic estrogen treatment may counteract the effect of dopamine in rats and may inhibit pituitary TGF-β1 and TβRII expression and increase PRL levels (13), but the estrogenic effect on active cytokine had not been studied. Our results showed that, after 24 h of estradiol-valerate injection, active and total cytokine content, as well as TGF-β1 mRNA levels, remained unaltered in pituitaries from wild-type mice, even though serum PRL levels were increased. This finding suggests that a long-term treatment is necessary to modify TGF-β1 synthesis and storage, but the action of estradiol on serum PRL levels requires a shorter time probably affecting the secretory process. However, the most important finding was that the effect of estradiol on active TGF-β1 levels depended on the dopaminergic tone, because only in the absence of dopamine regulation, in Drd2−/− pituitaries, did estradiol induce a strong increase of the active cytokine (Fig. 6). This unexpected response could reveal a dopaminergic inhibition of a permissive effect of estradiol in the activation of the cytokine. It is known that several mechanisms participate in TGF-β1 activation in different tissues (15, 16, 22), but they have not been explored in the pituitary. As estradiol does not directly activate TGF-β1, the stimulatory effect of estradiol in pituitaries from Drd2−/− female mice may be mediated by the modulation of latent TGF-β activators, for example matrix metalloproteinases (MMP) or thrombospondin-1 among others.
Interestingly the effect of estradiol-valerate in increasing TGF-β1 in Drd2−/− pituitaries correlated with a decrease in serum PRL levels. This result is reminiscent of the estradiol effect sometimes applied to terminate lactation and decrease PRL level in lactating women, in which dopaminergic tone is low and PRL levels are high (23, 24). On the other hand, it is important to note the differences in estradiol sensitivity that exist among experimental models of lactotroph hyperplasia. In the estrogen-treated rat, the sensitivity to estrogen varies in different rat strains and may be caused by differences in the number of FS cells (25). Likewise, in mice, estradiol exerts strain-specific effects (and opposing responses) on PRL release in 129S6 and C57BL/6 mice, whereas comparable increase in pituitary weight is observed (26). We previously reported estradiol inhibition on PRL secretion in C57BL/6 mice (20). On the other hand, differences in doses and experimental procedure (acute or chronic administration) could be also involved in determining estrogen effects. Mechanisms underlying strain-specific responses to estradiol remain largely unknown, but it is important to highlight that the strong increase in active TGF-β1 induced by estradiol that we found could be involved in the concomitant decrease found in serum PRL levels.
It is important to note that less than 8% of total TGF-β was found in the active form. This is similar to what has been described in other tissues (7) and underlines the importance of evaluating biologically active TGF-β1 rather cytokine expression, synthesis, or secretion, when studying TGF-β1. After synthesis, TGF-β are secreted as large latent complexes, and they must undergo a highly regulated activation process to release the mature TGF-β and allow the cytokine to bind to its receptor. Therefore, cytokine activation is a crucial event regulating its biological activity. Several latent TGF-β1 activators have been described, including proteases, thrombospondin-1, the integrin αvβ6, and reactive oxygen species among others. However, their biological importance in releasing TGF-β1 from its latent complex and their local regulation in different tissues are still not fully understood (15, 16). Some of the known activators are present in the pituitary and are candidates for regulation by estradiol: thrombospondin-1 and MMP9 and MMP2 among others (27–29). On the other hand, the effect of dopamine action on different modulators of TGF-β1 activation at the pituitary level has not been described, and less is known about the control mechanism governing TGF-β1 production by FS cells.
These results highlight the importance of TGF-β1 activation and the regulation of this process, particularly in the pituitary, where TGF-β1 may contribute in modulating PRL secretion and lactotroph growth. Increasing knowledge of TGF-β1 control on lactotroph function may be an important tool for future clinical studies. Prolactinomas are the most prevalent type of pituitary tumors in humans and generally respond well to a medical therapy with dopamine agonists. However, for patients exhibiting resistance to dopaminergic drugs, alternative therapies are desired, and TGF-β1 and its local activators may provide important targets in the treatment of dopamine agonist resistant prolactinomas.
Acknowledgments
We thank National Institute of Diabetes and Digestive and Kidney Diseases's National Hormone and Pituitary Program and Dr. A. F. Parlow for prolactin RIA kits.
This work was supported by Consejo de Investigaciones Científicas y Técnicas Grants PIP 2431, 2009 (to G.D.-T.) and PIP 640 (to D.B.-V.), the Agencia Nacional de Promoción Científica y Técnica, Buenos Aires, Argentina Grant PICT N206, 2006 (to D.B.-V.), and by the National Institutes of Health Grant R01 CA034282-25 (to D.B.R.).
Disclosure Summary: The authors have nothing to disclose.
Footnotes
- Drd2
- Dopamine type 2 receptor
- FS
- folliculostellate
- LAP
- latency-associated protein
- MMP
- matrix metalloproteinase
- PRL
- prolactin
- Q-RT-PCR
- quantitative real-time RT-PCR
- TβRII
- TGFβ type II receptor.
References
- 1. Ben-Jonathan N. 1985. Dopamine: a prolactin inhibiting hormone. Endocr Rev 6:564–589 [DOI] [PubMed] [Google Scholar]
- 2. Ben-Jonathan N, Hnasko R. 2001. Dopamine as a prolactin (PRL) inhibitor. Endocr Rev 22:724–763 [DOI] [PubMed] [Google Scholar]
- 3. Missale C, Nash SR, Robinson SW, Jaber M, Caron MG. 1998. Dopamine receptors: from structure to function. Physiol Rev 78:189–225 [DOI] [PubMed] [Google Scholar]
- 4. Díaz-Torga G, Feierstein C, Libertun C, Gelman D, Kelly MA, Low MJ, Rubinstein M, Becú-Villalobos D. 2002. Disruption of the D2 dopamine receptor alters GH and IGF-I secretion and causes dwarfism in male mice. Endocrinology 143:1270–1279 [DOI] [PubMed] [Google Scholar]
- 5. Kelly MA, Rubinstein M, Asa SL, Zhang G, Saez C, Bunzow JR, Allen RG, Hnasko R, Ben-Jonathan N, Grandy DK, Low MJ. 1997. Pituitary lactotroph hyperplasia and chronic hyperprolactinemia in dopamine D2 receptor-deficient mice. Neuron 19:103–113 [DOI] [PubMed] [Google Scholar]
- 6. Cristina C, García-Tornadú I, Díaz-Torga G, Rubinstein M, Low MJ, Becú-Villalobos D. 2006. The dopaminergic D2 receptor knockout mouse: an animal model of prolactinoma. Front Horm Res 35:50–63 [DOI] [PubMed] [Google Scholar]
- 7. Yoshinaga K, Obata H, Jurukovski V, Mazzieri R, Chen Y, Zilberberg L, Huso D, Melamed J, Prijatelj P, Todorovic V, Dabovic B, Rifkin DB. 2008. Perturbation of transforming growth factor (TGF)-β1 association with latent TGF-β binding protein yields inflammation and tumors. Proc Natil Acad Sci USA 105:18758–18763 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Sarkar DK, Kim KH, Minami S. 1992. Transforming growth factor-β1 messenger RNA and protein expression in the pituitary gland: its action on prolactin secretion and lactotropic growth. Mol Endocrinol 6:1825–1833 [DOI] [PubMed] [Google Scholar]
- 9. Sarkar DK, Pastorcic M, De A, Engel M, Moses H, Ghasemzadeh MB. 1998. Role of transforming growth factor-b type I and TGF-b type II receptores in the TGF-b1 regulated gene expression in pituitary prolactin-secreting lactotropes. Endocrinology 139:3620–3628 [DOI] [PubMed] [Google Scholar]
- 10. Sarkar DK, Chaturvedi K, Oomizu S, Boyadjieva NI, Chen CP. 2005. Dopamine, dopamine D2 receptor short isoform, transforming growth factor (TGF)-β1, and TGF-β type II receptor interact to inhibit the growth of pituitary lactotropes. Endocrinology 146:4179–4188 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Sarkar DK, Boyadjieva NI. 2007. Ethanol alters production and secretion of estrogen-regulated growth factors that control prolactin-secreting tumors in the pituitary. Alcohol Clin Exp Res 31:2101–2105 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. De A, Morgan TE, Speth RC, Boyadjieva N, Sarkar DK. 1996. Pituitary lactotrope expresses transforming growth factor b (TGF-b) type II receptor mRNA and protein and contains I-TGF-b1 binding sites. J Endocrinol 149:19–27 [DOI] [PubMed] [Google Scholar]
- 13. Pastorcic M, De A, Boyadjieva N, Vale W, Sarkar DK. 1995. Reduction in the expression and action of transforming growth factor-b1 on lactotropes during estrogen-induced tumorigenesis. Cancer Res 55:4892–4898 [PubMed] [Google Scholar]
- 14. Jin L, Tsumanuma I, Ruebel KH, Bayliss JM, Lloyd RV. 2001. Analysis of homogeneous populations of anterior pituitary folliculostellate cells by laser capture microdissection and reverse transcription-polymerase chain reaction. Endocrinology 142:1703–1709 [DOI] [PubMed] [Google Scholar]
- 15. Annes JP, Munger JS, Rifkin DB. 2003. Making sense of latent TGFβ activation. J Cell Sci 116:217–224 [DOI] [PubMed] [Google Scholar]
- 16. Annes JP, Chen Y, Munger JS, Rifkin DB. 2004. Integrin αVβ6-mediated activation of latent TGF-β requires the latent TGF-β binding protein-1. J Cell Biol 165:723–734 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Asa SL, Kelly MA, Grandy DK, Low MJ. 1999. Pituitary lactotroph adenomas develop after prolonged lactotroph hyperplasia in dopamine D2 receptor-deficient mice. Endocrinology 140:5348–5355 [DOI] [PubMed] [Google Scholar]
- 18. Sarkar DK. 2006. Genesis of prolactinomas: studies using estrogen-treated animals. Front Horm Res 35:32–49 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. González Iglesias A, Díaz-Torga G, Piroli G, Achával-Zaia R, De Nicola AF, Libertun C, Becu-Villalobos D. 2000. Bromocriptine restores Angiotensin II response in pituitary hyperplasia. Mol Cell Endocrinol 165:67–74 [DOI] [PubMed] [Google Scholar]
- 20. Cristina C, Díaz-Torga G, Baldi A, Góngora A, Rubinstein M, Low MJ, Becú-Villalobos D. 2005. Increased pituitary vascular endothelial growth factor-A in dopaminergic D2 receptor knockout female mice. Endocrinology 146:2952–2962 [DOI] [PubMed] [Google Scholar]
- 21. Ben-Jonathan N. 2005. Dopamine and transforming growth factor-β1: an odd couple in growth inhibition of the lactotrophs. Endocrinology 146:4177–4178 [DOI] [PubMed] [Google Scholar]
- 22. Munger JS, Harpel JG, Gleizes PE, Mazzieri R, Nunes I, Rifkin DB. 1997. Latent transforming growth factor-β: structural features and mechanisms of activation. Kidney Int 51:1376–1382 [DOI] [PubMed] [Google Scholar]
- 23. Kochenour NK. 1980. Lactation suppression. Clin Obstet Gynecol 23:1045–1059 [DOI] [PubMed] [Google Scholar]
- 24. Llewellyn-Jones D. 1968. Inhibition of lactation by oestrogens. Br Med J 4:387. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Hentges S, Boyadjieva N, Sarkar DK. 2000. Transforming growth factor-b3 stimulates lactotrope cell growth by increasing basic fibroblast growth factor from folliculo-stellate cells. Endocrinology 141:859–867 [DOI] [PubMed] [Google Scholar]
- 26. Spearow JL, Doemeny P, Sera R, Leffler R, Barkley M. 1999. Genetic variation in susceptibility to endocrine disruption by estrogen in mice. Science 285:1259–1261 [DOI] [PubMed] [Google Scholar]
- 27. Paez-Pereda M, Kuchenbauer F, Arzt E, Stalla GK. 2005. Regulation of pituitary hormones and cell proliferation by components of the extracellular matrix. Braz J Med Biol Res 38:1487–1494 [DOI] [PubMed] [Google Scholar]
- 28. Schultz-Cherry S, Ribeiro S, Gentry L, Murphy-Ullrich JE. 1994. Thrombospondin binds and activates the small and large forms of latent transforming growth factor-β in a chemically defined system. J Biol Chem 269:26775–26782 [PubMed] [Google Scholar]
- 29. Sarkar AJ, Chaturvedi K, Chen CP, Sarkar DK. 2007. Changes in thrombospondin-1 levels in the endothelial cells of the anterior pituitary during estrogen-induced prolactin-secreting pituitary tumors. J Endocrinol 192:395–403 [DOI] [PMC free article] [PubMed] [Google Scholar]