Abstract
Hyperglycemia increases insulin flux through the endoplasmic reticulum (ER) of pancreatic β-cells, and the unfolded protein response pathway is required to enhance insulin processing. Pancreatic and duodenal homeobox 1 (PDX1), a key pancreatic transcription factor, regulates insulin along with targets involved in insulin processing and secretion. Here we find that PDX1 is a direct transcriptional regulator of ER oxidoreductin-1-like β (Ero1lβ), which maintains the oxidative environment of the ER to facilitate disulfide bond formation. PDX1 deficiency reduced Ero1lβ transcript levels in mouse islets and mouse insulinoma (MIN6) cells; moreover, PDX1 occupied the Ero1lβ promoter in β-cells. ERO1lβ levels were induced by high glucose concentrations and by the reducing agent dithiothreitol, indicating potential roles in adaptation to increased oxidative protein folding load in the β-cell ER. In MIN6 cells, small interfering RNA-mediated silencing of Ero1lβ decreased insulin content and increased susceptibility to ER stress-induced apoptosis. These findings demonstrate roles for the PDX1 target ERO1lβ in maintaining insulin content and regulating cell survival during ER stress.
Glucose stimulates insulin transcription, biosynthesis, and secretion. Preproinsulin is translated and imported into the endoplasmic reticulum (ER) where it is cleaved into proinsulin. Proinsulin forms three disulfide bonds in the oxidative environment of the ER and is subsequently delivered to secretory granules, where prohormone convertases cleave the molecule into insulin and C-peptide. The β-cell is thought to process up to one million insulin molecules per minute (1). Glucose induces the unfolded protein response (UPR) to compensate for the increased protein synthetic load on the ER (2–4). Although the UPR has adaptive functions to enhance protein folding capacity, under conditions of chronic or insurmountable ER stress, the UPR pathway can activate apoptosis. Signs of ER stress have been observed in the β-cells of patients with type 2 diabetes, and ER stress is thought to contribute to β-cell failure and insulin resistance leading to disease progression (5–7). The Akita mouse model, which harbors a cysteine to tyrosine mutation of insulin 2 that prevents proper disulfide bond formation, has reduced insulin content, neonatal diabetes, and activated ER stress response illustrating the association between proper disulfide bond formation in the ER and β-cell function (8–10).
ER oxidoreductin 1 (ERO1) was initially discovered in yeast as a mediator of disulfide bond formation (11, 12). ERO1 transfers electrons from protein disulfide isomerase (PDI) to molecular oxygen, allowing PDI to transfer disulfide bonds to cargo proteins (13, 14). The importance of ERO1 for cell survival during ER stress has been illustrated in several models. Studies in yeast demonstrated that ERO1 mutation increased susceptibility to cell death from the reducing agent dithiothreitol (DTT) whereas overexpression of ERO1 enhanced cell survival at high concentrations of DTT (11, 12). DTT reduced the disulfide bonds of proteins within the ER, and ERO1 mutants were unable to process these reduced proteins, leading to their accumulation and degradation (11). In Caenorhabditis elegans, ero-1 RNA interference decreased susceptibility to tunicamycin-induced cell death by lowering levels of reactive oxygen species (15, 16). Mammalian cells have two ERO1-like genes, the ubiquitously expressed Ero1lα and the pancreas-enriched Ero1lβ (17, 18). During conditions of ER stress, ERO1-like α (ERO1lα), a target of the proapoptotic factor CCAAT enhancer binding protein homologous protein (CHOP), increased inositol triphosphate (IP3)-induced calcium release and apoptosis in macrophages (16, 19). ERO1lα and ERO1lβ are retained in the ER through interactions with PDI and ERp44 (20). ERp44 directly binds and inhibits the IP3 receptor type 1; thus, ERO1lα may potentially enhance IP3-induced calcium release via inhibition of ERp44 (19, 21). In summary, these disparate models suggest roles for ERO1 and ERO1-like genes in adapting to unfolded proteins, determining the level of reactive oxygen species, and regulating calcium mediated apoptosis pathways.
ERO1lβ is abundantly expressed in pancreatic islets (18). A study of differentially regulated genes in β-cell-enriched tissues from type 2 diabetic patients found significant reduction of Ero1lβ levels compared with tissues from healthy donors, suggesting Ero1lβ deficiency may contribute to the pathogenesis of type 2 diabetes (22). We previously demonstrated that ERO1lβ is downstream of the transcription factor pancreatic and duodenal homeobox 1 (PDX1), which is required to maintain ER homeostasis during high-fat-diet feeding, a physiologically relevant model of type 2 diabetes (23). A recent study of Ero1lβ gene trap mice has demonstrated the importance of ERO1lβ in insulin biosynthesis; islets from homozygous Ero1lβ mutant mice showed delayed processing of proinsulin to insulin, which was associated with decreased pancreatic insulin content and impaired glucose tolerance (24). ERO1lβ deficiency promoted colony formation of MIN6 cells harboring the Akita mutation; however, heterozygous Ero1lβ mutation exacerbated the hyperglycemia of Akita mice (24). The mechanisms for these results remain to be elucidated, and the effect of ERO1lβ deficiency on cell death was not directly addressed (24).
High glucose concentrations stimulate β-cells to increase insulin secretion and biosynthesis, which increases the demand on the β-cell ER. PDX1 has been shown to regulate ER homeostasis to meet the increased insulin demand from high-fat-diet feeding. Here we show that islet-enriched ERO1lβ is induced by glucose and directly regulated by PDX1. ERO1lβ deficiency in MIN6 cells delays proinsulin folding to its native disulfide state and reduces insulin content, leading to impaired insulin secretion. Impaired protein folding may lead to ER stress, which when severe or prolonged, can cause cell death. Furthermore, we find that ERO1lβ deficiency increases phosphorylation of c-Jun N-terminal kinase (JNK) and increases susceptibility to ER stress-induced cell death. These data show that ERO1lβ deficiency limits insulin content and impairs cell survival during ER stress in MIN6 cells.
Materials and Methods
Animals
Pdx1+/− mice were maintained on a C57BL/6 background. Male Pdx1+/− and littermate wild-type control mice were killed at 20 wk of age. To harvest islets, the pancreata were removed and digested with collagenase (Worthington Biochemical Corp., Lakewood, NJ) at 37 C for 16 min. The pancreata were washed twice with Hanks' buffered saline with 0.02% BSA and separated using a Ficoll gradient (25). The islets were handpicked three rounds before harvesting for RNA. To measure the effect of glucose on Ero1lβ transcript levels, islets were harvested from male C57BL/6 mice and incubated in 2, 5, 10, or 16 mm glucose in RPMI 1640 media (Invitrogen, Carlsbad, CA) with 0.2% fetal bovine serum and 0.1% BSA. All animal procedures were approved by the Institutional Animal Care and Use Committee at the University of Pennsylvania.
Cell culture
MIN6 cells were grown in DMEM (Invitrogen) with 25 mm glucose supplemented with 10% fetal bovine serum. In experiments involving silencing of Pdx1, MIN6 cells (passages 25–35) were Amaxa nucleofected with 0.3 nmol of ON-TARGETplus nontargeting small interfering RNA (siRNA) pool or 0.3 nmol of Pdx1 ON-TARGETplus SMARTpool (Dharmacon, Lafayette, CO). In experiments involving silencing of Ero1lβ, MIN6 cells were nucleofected with 1 nmol of ON-TARGETplus nontargeting siRNA pool or 1 nmol of Ero1lβ ON-TARGETplus SMARTpool (Dharmacon). For all experiments, cells from each nucleofection were harvested 72 or 96 h after nucleofection to measure silencing efficiency by RT-PCR or Western blot. To observe the effect of reducing conditions on ERO1lβ levels, 1 m dithiothreitol (Fisher Scientific, Pittsburgh, PA) was prepared in H2O and diluted to 1 mm in DMEM immediately before treatment. MIN6 cells were treated for 0, 1, 2, 4, and 6 h before harvesting for RNA and protein. Actinomycin D (Sigma Chemical Co., St. Louis, MO) was dissolved in dimethylsulfoxide (DMSO) for a stock concentration of 10 mg/ml and diluted to 10 μg/ml in DMEM before treatment.
Quantitative RT-PCR
MIN6 cells were harvested in Trizol reagent (Invitrogen), and RNA was isolated using the Trizol protocol. Samples were treated with Turbo DNA-free (Ambion, Austin, TX) before RT using Superscript II (Invitrogen). Quantitative real-time PCR was performed with SYBR green using a Bio-Rad (Hercules, CA) iCycler. Primers for mouse Pdx1, Ero1lβ, Ero1lα, sXbp1, tXbp1, and Chop were previously described (23).
Western blot
MIN6 cells were harvested in lysis buffer [50 mm Tris-HCl (pH 8.0), 150 mm NaCl, 1% Nonidet P-40, 1% protease inhibitor cocktail, 1% phosphatase inhibitor cocktail]. Proteins were separated by SDS-PAGE and transferred to nitrocellulose membrane. The membrane was blocked with 5% milk for 1 h, then incubated overnight at 4 C with PDX1 antibody (R&D Systems, Minneapolis, MN), ERO1lβ antibody (Proteintech Group, Inc., Chicago, IL), phospho-JNK antibody (Cell Signaling, Beverly, MA) or JNK antibody (Cell Signaling) at 1:1000 dilution, or CHOP antibody (Santa Cruz Biotechnology, Santa Cruz, CA) at 1:500 dilution in PBS plus 0.05% Tween. For the loading control, the membrane was incubated for 1 h at room temperature in anti-cyclophilin A (Cell Signaling) diluted 1:50,000 or anti-β-actin (Sigma) diluted 1:5000. The membrane was incubated for 1 h at room temperature in horseradish peroxidase-goat antimouse or horseradish peroxidase-goat antirabbit secondary antibodies (Santa Cruz) and developed using ECL (Amersham Biosciences, Piscataway, NJ). For the insulin immunoblot, samples were run under nonreducing conditions and immunoblotted with anti-insulin (Linco, St. Charles, MO) diluted 1:1000 or anti-α tubulin (Sigma) diluted 1:3000.
Chromatin immunoprecipitation (ChIP) sequencing (Seq)
Primary islets from 6- to 8-wk-old male CD1 mice and MIN6 cells were used for ChIP Seq studies. PDX1 ChIP was performed as previously described (23, 26). DNA and proteins were cross-linked and immunoprecipitated using PDX1-specific antiserum (27). ChIP Seq was performed as previously described (28). Briefly, DNA was modified and ligated to adapters according to the Ilumina protocol before size selection with 2% agarose separation. The DNA was PCR amplified, purified with QIAquick PCR purification kit (Qiagen, Valencia, CA), and analyzed by Agilent 2100 Bioanalyzer (Agilent, Santa Clara, CA). The Illumina protocol was followed for cluster generation and sequence alignment to the mouse genome. Only sequence tags uniquely mapping to the Ero1lβ locus were analyzed for this study. Using isotype-matched normal goat serum and PDX1 antibody (Santa Cruz), ChIP were performed in MIN6 cells to confirm occupancy using the following primer sequences for Ero1lβ: region 1 (forward, GTTCACCCATGCTCAGTTCC; reverse, GACAGGTGGTGAGGCATGAT), region 2 (forward, GTTCCCTAGCCTCATGTTCC; reverse, GTGAGTCCATCCGTCATGTG), region 3 (forward, GGGCGTGATCATAACTGAGG; reverse, CAGCAGCGACTGATGTACCA), and region 4 (forward, ACGAGCTGTCACTGTCATCCT; reverse, GGCAGCTCAGTCAGGAAAAG).
Electrophoretic mobility shift assay
EMSA were preformed as previously described (29). Briefly, 4 μl of in vitro translated product was incubated with 1 ng radioactive labeled oligonucleotide probe containing the potential PDX1 binding sites in the presence of 1 μg polydeoxyinosinic deoxycytidylic acid. To show specificity, PDX1 antiserum was added to reduce electrophoretic mobility of the complex and produce a supershifted band. Samples were separated on a 5% nondenaturing polyacrylamide gel and detected by autoradiography. Probe sequences for Ero1lβ were as follows: 2A (forward, CTACAGATTAGAGCCTGGT; reverse, ACCAGGCTCTAATCTGTAG), 2B (forward, AGTAACAGATCATCTGTACT; reverse, AGTACAGATGATCTGTTACT), 2C (forward, TCAATGGGAAATCATCACTG; reverse, CAGTGATGATTTCCCATTGA), and 2D (forward, GTACTAATTGACAAAAATTGGT; reverse, ACCAATTTTTGTCAATTAGTAC).
Glucose-stimulated insulin secretion
At 72 h after nucleofection, MIN6 cells were washed once with KRBH [15 mm HEPES, 120 mm NaCl, 4.7 mm KCl, 1.2 mm MgSO4, 1.2 mm KH2PO4, 20 mm NaHCO3, 2 mm CaCl2, 0.1% BSA (pH 7.4)]. The cells were incubated in glucose-free KRBH for 1 h and incubated in 2.5 mm glucose KRBH for 30 min, and then 10% of the volume was collected to measure basal insulin secretion. A 250 mm stock glucose solution was added for a final concentration of 27.5 mm glucose. The cells were incubated in 27.5 mm glucose for 30 min to measure glucose-stimulated insulin secretion. The cells were harvested with lysis buffer [20 mm Tris-HCl (pH 7.4), 150 mm NaCl, 1 mm Na2EDTA, 1 mm EGTA, 1% Triton X-100, and 1% protease inhibitor cocktail] to measure insulin content and protein concentration. An ultrasensitive mouse insulin ELISA (Crystal Chem, Downers Grove, IL) was used to measure insulin secretion and content. A micro-BCA protein assay kit (Thermo Scientific, Rockford, IL) was used to measure protein concentration.
Pulse chase
At 72 h after nucleofection, MIN6 cells were labeled with 100 ucCi [35S]cysteine/methionine (MP Biomedical, Solon, OH) in DMEM lacking cysteine and methionine. Cells were washed and incubated on ice with PBS containing N-ethylmaleimide (Sigma) and then lysed with SDS lysis buffer [150 mm NaCl, 1% Nonidet P-40, 0.1% SDS, 2 mm EDTA, 10 mm Tris-HCl (pH 7.4), 1% protease inhibitor cocktail, 10 mm N-ethylmaleimide]. Lysates were immunoprecipitated overnight with guinea pig antiinsulin (Linco) and then denatured with SDS sample buffer. For the reduced samples, DTT was added, and all samples were analyzed by Tris-tricine-urea-SDS-PAGE.
Calcium imaging
MIN6 cells were nucleofected and plated on coverslips. After 72 h, the cells were washed with KRBH (114 mm NaCl, 5 mm KCl, 24 mm NaHCO3, 1 mm MgCl2, 2.2 mm CaCl2, 1 mm NaHPO4, 10 mm HEPES, 0.25% BSA, 1.25 mm probenecid) and loaded with 2.5 μm fura-2 in KRBH with 1.67 mm glucose for 45 min at 37 C. The coverslip was mounted on the stage of an inverted microscope (Eclipse TE2000; Nikon, Melville, NY) and perfused with 1.67 mm glucose, 16.7 mm glucose, and 1.67 mm glucose in KRBH for 10 min each and then perfused with 30 mm KCl in KRBH for 5 min. Intracellular calcium levels were measured and analyzed as previously reported (30). Peak high-glucose- and KCl-stimulated intracellular calcium levels were analyzed for 20 cells per nucleofection and five nucleofections for nontargeting and Ero1lβ siRNA pools.
Annexin V staining
MIN6 cells were nucleofected and 72 h later treated with vehicle (DMSO) or 10 μg/ml tunicamycin for 24 h. Floating cells were harvested and combined with adherent cells, which were trypsinized into single-cell suspensions. The cells were stained with annexin V and propidium iodide according to the protocol from the fluorescein isothiocyanate-annexin V apoptosis detection kit I (BD PharMingen, San Diego, CA) and analyzed with a BD FACS Calibur.
Statistical analysis
Data are presented as mean ± sem. Two-tailed Student's t test was used to determine statistical significance. Differences of P < 0.05 were considered to be significant.
Results
ERO1lβ is directly regulated by PDX1
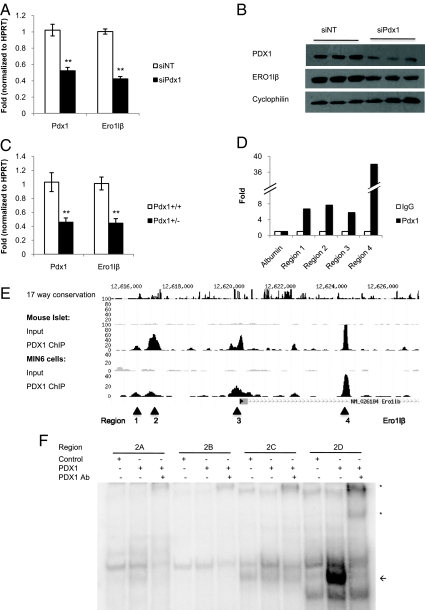
Because PDX1 is a critical pancreatic transcription factor upstream of a number of genes involved in regulating homeostasis of the endoplasmic reticulum, PDX1 regulation of ERO1lβ was further investigated (23). Mouse insulinoma MIN6 cells were nucleofected with pooled siRNA duplexes targeting Pdx1 (siPdx1) or control nontargeting siRNA duplexes (siNT). Pdx1 and Ero1lβ transcript levels were measured 72 h after nucleofection by quantitative RT-PCR. Silencing of Pdx1 caused a significant 57% reduction in Ero1lβ transcript levels (Fig. 1A). Pdx1 silencing also caused a notable reduction in ERO1lβ protein levels by Western blot analysis (Fig. 1B). To confirm the regulation of Ero1lβ by PDX1 in vivo, islets were harvested from Pdx1+/+ and Pdx1+/− mice. RT-PCR showed a significant 57% reduction in Ero1lβ transcript levels in Pdx1+/− islets, indicating that PDX1 regulates ERO1lβ in vivo (Fig. 1C). Previous studies have shown that PDX1 is upstream of Ero1lα as well, likely through an indirect mechanism involving PDX1 regulation of Atf4 (23). Using primer sets with similar amplification efficiencies, the transcript levels of Ero1lα and Ero1lβ were compared by RT-PCR. Ero1lβ is significantly more abundantly expressed than Ero1lα in MIN6 cells (Supplemental Fig. 1A, published on The Endocrine Society's Journals Online web site at http://jcem.endojournals.org) and in primary mouse islets (Supplemental Fig. 1B); thus, subsequent studies focused on ERO1lβ. To confirm whether PDX1 regulation of ERO1lβ involved direct occupancy of PDX1 on the Ero1lβ promoter, we examined global PDX1 occupancy data from ChIP with massively parallel sequencing (ChIP Seq) in mouse islets and MIN6 cells and identified three regions of PDX1 occupancy near the promoter and strong occupancy at an intronic region (Fig. 1E). To confirm PDX1 occupancy, PDX1 ChIP was performed in MIN6 cells demonstrating 6.7-fold enrichment at region 1, 7.7-fold enrichment at region 2, 5.8-fold enrichment at region 3, and 38-fold enrichment at region 4 compared with isotype-matched IgG control (Fig. 1D). The albumin promoter was used as a negative control. To further analyze binding by EMSA, we examined the regions for conservation across species and the presence of PDX1 consensus binding motifs and identified four potential PDX1 binding sites within region 2. Regions 1 and 4 were not well conserved, and region 3 did not contain a PDX1 binding motif within the peak region. Radiolabeled probes for the putative binding sites were incubated with control (in vitro translation from blank vector pCMX) or in vitro-translated PDX1. Incubating the site 2D probe with PDX1 produced a distinct band, which was supershifted using PDX1 antiserum, whereas PDX1 did not form complexes with the probes for sites 2A, 2B, and 2C, further suggesting binding specificity (Fig. 1F).
Fig. 1.
Ero1lβ is directly regulated by the pancreatic transcription factor PDX1. A, Ero1lβ transcript was significantly reduced in MIN6 cells nucleofected with pooled siRNA duplexes targeting Pdx1. Results were normalized to hypoxanthine-guanine phosphoribosyltransferase (Hprt) (n = 9). **, P < 0.0001. B, Western blot showing decreased ERO1lβ protein levels in MIN6 cells nucleofected with siPdx1, representative of six experiments each performed in triplicate. C, Ero1lβ transcript levels were significantly reduced in islets from Pdx1+/− mice compared with Pdx1+/+ mice (n = 4–6 mice). **, P < 0.005. D, PDX1 ChIP in MIN6 cells showing enrichment of regions 1, 2, 3, and 4, which correspond to locations shown in E, representative of three independent ChIPs. E, Mouse islet and MIN6 cell PDX1 ChIP Seq profiles for Ero1lβ. F, EMSA showing radiolabeled probes incubated with control in vitro-translated product or PDX1 in vitro-translated product. Arrow indicates bound probe, and asterisks indicate supershift resulting from addition of PDX1 antiserum (Ab).
ERO1lβ levels are affected by glucose concentrations and redox environment
Because glucose increases demand for insulin biosynthesis, the effect of glucose concentration on ERO1lβ levels was assessed. Mouse islets were harvested and incubated in 2, 5, 10, or 16 mm glucose containing medium for 24 h before harvesting for RNA. Quantitative RT-PCR showed statistically significant inductions of Ero1lβ transcript in higher (10 and 16 mm) glucose conditions compared with lower (2 and 5 mm) glucose conditions, implying that Ero1lβ levels are dynamic in a glucose range that is physiologically relevant (Fig. 2). Although β-cells are not exposed to DTT physiologically, DTT is a pharmacological tool to simulate the reducing effect that occurs during cotranslational translocation of cysteine-rich proteins into the ER lumen. DTT was found to induce ERO1lβ protein level in MIN6 cells over a short time course (Fig. 3A). Although DTT induced positive controls spliced Xbp1 and total Xbp1, Ero1lβ transcript was not induced; in fact, it decreased after DTT treatment, suggesting the effect of DTT on ERO1lβ was not transcriptionally mediated (Fig. 3B). To confirm that DTT regulation of ERO1lβ is not transcriptional, we treated with the transcriptional inhibitor actinomycin D. Treatment with actinomycin D attenuated the induction of a positive control, CHOP, by DTT (Fig. 3, C and D); however, actinomycin D did not affect the induction of ERO1lβ protein, supporting a posttranscriptional mechanism (Fig. 3D). Interestingly, Pdx1 transcript was unchanged by DTT (Fig. 3B), but PDX1 protein decreased, suggesting that DTT may have posttranslational effects on both ERO1lβ and PDX1 (Fig. 3A). The reduction in Ero1lβ transcript may be due to the decrease in PDX1, but clearly other factors are involved in posttranscriptional regulation of ERO1lβ protein levels in the setting of DTT treatment.
Fig. 2.
Ero1lβ levels are affected by glucose concentrations. Mouse islets were incubated in 2, 5, 10, or 16 mm glucose for 24 h. RT-PCR showed significant increases in Ero1lβ transcript in islets cultured in 10 and 16 mm glucose compared with 2 mm glucose or compared with 5 mm glucose (n = 3). *, P < 0.05 compared with 2 mm glucose; ^, P < 0.01 compared with 5 mm glucose.
Fig. 3.
ERO1lβ levels are affected by the reducing agent DTT. A, MIN6 cells were treated with 1 mm DTT for 0, 1, 2, 4, and 6 h before harvest; Western blot showing ERO1lβ and PDX1 levels over time, representative of three independent experiments. B, Ero1lβ, Pdx1, sXbp1, and tXbp1 transcripts were measured after 0, 1, 2, and 4 h treatment with 1 mm DTT (n = 3). *, P < 0.05. C, MIN6 cells were treated with 1 mm DTT, 10 μg/ml actinomycin D, or 1 mm DTT and 10 μg/ml actinomycin D. Chop transcript was measured by RT-PCR after 4 h treatment as a positive control for actinomycin D (n = 3). *, P < 0.05 compared with vehicle; ^, P < 0.05 compared with 1 mm DTT. D, ERO1lβ protein levels were measured by Western blot after 4 h treatment (n = 2), representative of three independent experiments.
Silencing of Ero1lβ in MIN6 cells reduces insulin content
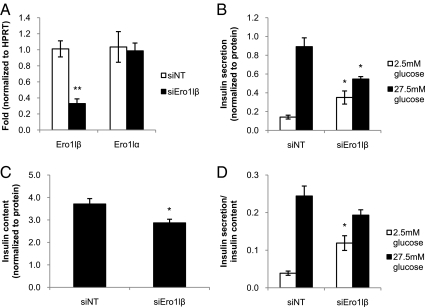
Given the abundance of ERO1lβ in pancreatic islets and the role of ERO1 in disulfide bond formation and potentially in ER calcium regulation, ERO1lβ may have a role in determining insulin content by regulation of insulin biosynthesis or in regulating insulin secretion by regulation of ER calcium flux. Ero1lβ mutant mice were reported to have reduced pancreatic insulin content and reduced insulin secretion (24). To clarify the effects of ERO1lβ deficiency on insulin secretion relative to insulin content, glucose-stimulated insulin secretion assays were performed. MIN6 cells were nucleofected with Ero1lβ-targeted siRNA duplexes (siEro1lβ) or nontargeting siRNA control. RT-PCR showed a 68% reduction in Ero1lβ transcript levels in MIN6 cells nucleofected with siEro1lβ compared with the nontargeting control, and no compensatory change in Ero1lα transcript levels was observed (Fig. 4A). At 72 h after nucleofection, the cells were incubated in glucose-free KRBH buffer for 1 h, 2.5 mm glucose for 30 min, and 27.5 mm glucose for 30 min. The media were collected to measure insulin secretion, and the cells were lysed to measure insulin content and protein concentration. All results were normalized by protein concentration. In the nontargeting siRNA nucleofected MIN6 cells, insulin secretion was 6-fold greater in high glucose than in low glucose. In MIN6 cells nucleofected with siEro1lβ, insulin secretion was only 1.6-fold greater in high glucose compared with low glucose (Fig. 4B). Ero1lβ silencing resulted in an elevation of basal insulin secretion under low-glucose conditions and a significant 39% reduction in glucose-stimulated insulin secretion. Insulin content was measured in ERO1lβ-deficient cells to assess whether reduced content contributes to the impaired insulin secretion. Ero1lβ silencing resulted in a significant 23% decrease in insulin content (Fig. 4C). When insulin secretion was normalized to insulin content, the difference between glucose-stimulated insulin secretion from siNT and siEro1lβ nucleofected cells was not statistically significant (Fig. 4D). The data from glucose-stimulated insulin secretion assays showed that Ero1lβ silencing reduced insulin content, leading to impaired insulin secretion. To confirm the role of ERO1lβ in maintaining insulin content, proinsulin and insulin levels were measured by Western blot (Supplemental Fig. 2A). ERO1lβ deficiency reduced insulin protein levels, whereas proinsulin levels were unaffected at steady state. To further examine insulin processing, pulse-chase analysis was performed in MIN6 cells nucleofected with siEro1lβ or siNT, and lysates were immunoprecipitated for proinsulin after 0-, 2-, and 5-min chase periods. Nonreducing gel electrophoresis showed that less proinsulin achieved the native confirmation in ERO1lβ-deficient cells (Supplemental Fig. 2B). Thus, ERO1lβ deficiency impaired proinsulin folding and decreased insulin content.
Fig. 4.
Silencing of Ero1lβ in MIN6 cells reduces insulin content. A, siRNA targeting of Ero1lβ reduced Ero1lβ transcript levels by 68% and did not affect Ero1lα transcript levels (n = 3). **, P < 0.005. B, At 72 h after nucleofection, glucose-stimulated insulin secretion assays were performed. Silencing Ero1lβ significantly increased basal insulin secretion and reduced glucose-stimulated insulin secretion (n = 6). *, P < 0.05. C, Silencing Ero1lβ in MIN6 cells significantly reduced insulin content (n = 6). *, P < 0.05. D, The difference between glucose-stimulated insulin secretion from ERO1lβ-deficient cells and from nontargeting control cells was not statistically significant after normalizing for insulin content.
siRNA targeting of Ero1lβ does not significantly affect glucose- or KCl-stimulated intracellular calcium
During glucose-stimulated insulin secretion in β-cells, glucose enters the cell via the glucose transporter 2, is phosphorylated by glucokinase, and undergoes glycolysis and the tricarboxylic acid cycle to produce ATP. The increase in the ATP to ADP ratio leads to closure of the K/ATP channel, which depolarizes the membrane to open the voltage-gated calcium channel, allowing an influx of calcium that in turn leads to calcium-induced calcium release from the ER. The resulting elevation in intracellular calcium levels causes exocytosis of insulin granules (31). In a previous study of macrophages, ERO1lα was found to affect IP3-induced calcium release (19). In β-cells, IP3-induced calcium release contributes to insulin exocytosis (31). Calcium imaging was performed to determine whether proximal defects in the insulin secretion pathway or changes in intracellular calcium contributed to the decreased glucose-stimulated insulin secretion after Ero1lβ silencing. MIN6 cells nucleofected with siEro1lβ or siNT were stained with the fluorescent calcium dye fura-2. Cells were perfused with 1.67 mm glucose in KRBH for 10 min, 16.7 mm glucose for 10 min, 1.67 mm glucose for 10 min, and then 30 mm KCl for 5 min. Representative calcium traces show that high glucose concentrations and KCl increased intracellular calcium levels in MIN6 cells (Fig. 5A). Peak calcium levels in high glucose and KCl were quantified from five nucleofections per siRNA (20 cells per nucleofection). ERO1lβ deficiency did not cause significant differences in peak intracellular calcium levels after glucose or KCl stimulation in MIN6 cells, nor were basal intracellular calcium levels affected (Fig. 5B). Because glucose- and KCl-stimulated intracellular calcium levels were not altered by ERO1lβ deficiency, the defect is likely limited to the distal steps in the glucose-stimulated insulin secretion pathway. Although membrane channels and other proteins involved in insulin secretion transit through the ER and require disulfide bond formation, the impairment in ERO1lβ deficiency seems most pronounced in the processing of insulin, the predominant protein product of β-cells.
Fig. 5.
siRNA targeting of Ero1lβ does not significantly affect high-glucose- or KCl-stimulated intracellular calcium. A, Representative calcium traces. MIN6 cells were perfused with 1.67 mm glucose for 10 min, 16.7 mm glucose for 10 min, 1.67 mm glucose for 10 min, and 30 mm KCl for 5 min. B, Quantification of peak calcium concentrations during high glucose and KCl perfusion; n = 5 nucleofections, 20 cells per nucleofection.
Silencing of Ero1lβ increases susceptibility to tunicamycin-induced cell death in MIN6 cells
ERO1 has prosurvival roles in yeast through adaptation to ER stress and proapoptotic roles in C. elegans by increasing oxidative stress (11, 12, 15, 19). Furthermore, ERO1lα has proapoptotic roles in macrophages by mediating calcium-induced apoptosis (19). ERO1lβ deficiency in MIN6 cells expressing the Akita mutant insulin was shown to improve cell survival as measured by colony formation, but ERO1lβ heterozygous mutation exacerbated the hyperglycemia of the Akita mutant mice (24). To clarify the role of ERO1lβ in ER stress-induced cell death, MIN6 cells were nucleofected with siEro1lβ, causing a 78% reduction in protein levels (Fig. 6A). The JNK signaling pathway is thought to be activated during ER stress by inositol-requring enzyme 1 and TNF receptor-associated factor 2 (32). ER stress activates inositol-requring enzyme 1 causing recruitment of TNF receptor-associated factor 2, which interacts with apoptosis signaling regulated kinase 1 leading to phosphorylation and activation of JNK. Western blot analysis of MIN6 cells nucleofected with siEro1lβ showed increased phosphorylated JNK, suggesting increased stress (Fig. 6B). Annexin V staining, which detects exposed phosphatidylserine on the outer leaflet of cell membranes, was used to measure apoptosis. To examine the potential baseline cell death and membrane damage from Amaxa nucleofection, nonnucleofected and nucleofected cells were cultured for 96 h and stained with annexin V, showing a modest though significant effect of nucleofection (Supplemental Fig. 3). Compared with nucleofection with nontargeting siRNAs, Ero1lβ silencing did not significantly alter the percentage of annexin V-positive cells in the vehicle-treated groups, indicating that ERO1lβ deficiency alone did not lead to apparent changes in apoptosis rates in MIN6 cells. Addition of 10 μg/ml tunicamycin, an inhibitor of N-glycosylation that leads to protein misfolding and ER stress, significantly increased cell death compared with vehicle treatment in the siNT nucleofected cells, and the effect was exacerbated in the siEro1lβ nucleofected cells (Fig. 6C). Our findings indicate that ERO1lβ deficiency increased susceptibility to tunicamycin-induced cell death in MIN6 cells, suggesting a potential explanation for the Ero1lβ mutation worsening the hyperglycemic phenotype of the Akita mutant mice, which model ER stress (24).
Fig. 6.
Silencing of Ero1lβ increases susceptibility to tunicamycin-induced cell death in MIN6 cells. A, Western blot showing nucleofection with siEro1lβ significantly reduced ERO1lβ protein levels. Quantification of ERO1lβ Western blot showed 78% reduction in protein levels (n = 3). **, P < 0.0005. B, Western blot showing siEro1lβ nucleofection increased phosphorylation of JNK, representative of three independent experiments each performed in triplicate. C, At 72 h after nucleofection, the cells were treated with vehicle (DMSO) or 10 μg/ml tunicamycin for 24 h. Floating and adherent cells were harvested and stained with annexin V and analyzed by flow cytometry; n = 3 nucleofections, 20,000 cells counted per nucleofection. *, P < 0.05 compared with vehicle; ^, P < 0.005 compared with siNT with tunicamycin treatment.
Discussion
We previously demonstrated that PDX1 influences the susceptibility of pancreatic β-cells to ER stress and regulates a broad repertoire of genes involved in maintaining ER homeostasis, which is consistent with its role to mediate β-cell compensation for genetic and diet-induced forms of insulin resistance (23, 33–35). Although multiple targets likely mediate the functions of PDX1 in β-cell compensation, here we show that PDX1 directly binds to and regulates the gene encoding Ero1lβ, a β-cell-enriched oxidoreductase that mediates disulfide bond formation. Although we observe PDX1 occupancy at a number of regions near the Ero1lβ transcriptional start site, the EMSA data suggest direct interaction likely occurs at a site 3.4 kb upstream of the transcriptional start site, which contains a well-conserved PDX1 binding motif. Possible explanations for the ChIP Seq and ChIP results illustrating multiple regions of occupancy include PDX1 interaction via cofactors or DNA looping mechanisms that may bring PDX1 in proximity to regions involved in transcriptional regulation of Ero1lβ. Similar to PDX1 deficiency in cells and in vivo, siRNA-mediated reduction of Ero1lβ levels in MIN6 cells decreased insulin content and enhanced susceptibility to ER stress-induced cell death, suggesting that ERO1lβ could mediate some of the critical effects of PDX1 on β-cell function and survival.
Proinsulin is estimated to constitute up to 50% of the protein synthesized by β-cells after high glucose stimulation (1). High glucose concentrations cause induction of ER targets as an adaptive response to increase insulin biosynthesis (2–4). Here we identify glucose as a regulator of Ero1lβ; the specific mechanism may involve targeting of Ero1lβ by a glucose-responsive transcription factor or regulation by conditions in the ER as part of a general adaptive response to increased proinsulin folding load. Using DTT, we also identify redox environment as a determinant of ERO1lβ levels. The use of DTT mimics the reducing conditions that occur during increased synthesis of cysteine-rich proteins that need to form disulfide bonds within the ER lumen. ERO1lβ induction by DTT may be an adaptive response to maintain the oxidizing environment within the ER. Interestingly, DTT did not increase Ero1lβ transcript levels, indicating posttranslational regulation may lead to the induction of ERO1lβ protein levels in reducing conditions. A transcription-independent mechanism was also supported by the persistence of the effect after treatment with actinomycin D, which is in contrast to earlier studies showing DTT treatment-induced Ero1lβ mRNA in non-β-cell lines (18). The precise mechanisms of ERO1lβ regulation during ER stress in β-cells appear to be complex and remain to be elucidated. We observed that DTT treatment decreased PDX1 levels, leading us to speculate that although PDX1 may have a role in regulating ER targets to couple insulin biosynthesis to insulin transcription, it may not be induced to promote recovery during severe pathological ER stress. Further studies are needed to clarify the role of PDX1 during ER stress and its regulation by conditions of ER stress.
ERO1lβ deficiency impaired proinsulin folding and reduced insulin content, leading to impaired glucose-stimulated insulin secretion. Changes in intracellular calcium do not appear to contribute to the impairment in insulin secretion during ERO1lβ deficiency. Our findings are consistent with those from Ero1lβ mutant mice demonstrating the importance of ERO1lβ for proinsulin maturation and maintenance of insulin content (24). Silencing of Ero1lβ was associated with increased basal insulin secretion, which might reflect insulin released during apoptosis in the ERO1lβ-deficient MIN6 cells. Although annexin V staining did not demonstrate a statistically significant increase in the level of cell death in vehicle-treated cells, a mild increase in cell death could potentially affect the insulin measurement during incubation in low glucose. Other potential explanations for increased basal insulin secretion that remain to be investigated include secretion of misfolded forms of insulin or indirect effects of ERO1lβ on other ER proteins involved in basal insulin secretion. Pdx1+/− mice also have decreased insulin content and impaired insulin secretion (23). Although PDX1 regulates several targets involved in glucose-stimulated insulin secretion including insulin, the present results suggest that ERO1lβ might contribute to the diabetic phenotype of these animals and possibly to β-cell impairment in Maturity Onset Diabetes of the Young (MODY4) patients with mutations in PDX1.
Studies in various systems have suggested roles for ERO1 and ERO1-like genes in cell survival and apoptosis (11, 12, 15, 19). Although β-cell mass and apoptosis were not directly measured in the Ero1lβ mutant mice, they were reported to have increased glucagon-positive cells in the core of their islets, which is reminiscent of other models in which a similar phenotype is secondary to a decrease in the number of insulin-producing β-cells (36, 37). Alternatively, the UPR pathway components may have significant roles during development leading to changes in islet architecture (38). Lentiviral silencing of Ero1lβ in MIN6 cells harboring the Akita mutation increased cell survival. In contrast, heterozygous Ero1lβ mutation worsened the hyperglycemic phenotype of the Akita heterozygous mice, and differences in oxygen conditions, cell death pathways, and insulin signaling were proposed as potential explanations for the different effects of ERO1lβ deficiency in MIN6 cells vs. in vivo (24). Here, Ero1lβ silencing increased susceptibility to tunicamycin-induced cell death, which is in contrast to the finding that Ero1lβ silencing promoted colony formation of MIN6 cells expressing the Akita mutant insulin (24). Possible explanations include differences in the gene silencing, in the measurement of cell survival, or in the culture conditions. We delivered siRNA using a transient transfection approach and measured apoptosis directly with annexin V staining, whereas in the previous report, MIN6 cells were infected with lentivirus containing Ero1lβ-targeted short hairpin RNA to generate a stable cell line, and colony formation was used as a measure of cell survival (24). Our results are consistent with the previous finding that heterozygous Ero1lβ mutation worsened the phenotype of the Akita heterozygous mice, which have been shown to have increased ER stress (24), leading us to hypothesize that the in vivo phenotype may be due either to exacerbation of the decrease in insulin production or to an increase in β-cell apoptosis as shown in this report. We also find increased JNK phosphorylation in ERO1lβ-deficient MIN6 cells, which suggests that ERO1lβ deficiency may cause a basal level of stress leading to a predisposition to apoptosis in the setting of severe ER stress. This is consistent with the observation in the β-cells of Ero1lβ mutant mice of increased oxidized proinsulin aggregates, which may instigate ER stress (24). Our findings indicate that ERO1lβ deficiency reduces insulin content and increases susceptibility to ER stress-induced cell death, and Ero1lβ was previously shown to be significantly reduced in β-cell-enriched tissues from human type 2 diabetic patients (22), suggesting that ERO1lβ deficiency may be one of several perturbations that contribute to β-cell failure during type 2 diabetes.
Insulin is a small peptide hormone with enormous significance for patients with diabetes. The specific mechanisms of initiating insulin biosynthesis in the ER involving ER chaperones and oxidoreductases are still being elucidated. Investigating these mediators of insulin production may lead to therapeutic targets that increase the efficiency of the β-cell ER to sustain insulin biosynthesis and prevent the β-cell failure leading to the progression of type 2 diabetes.
Acknowledgments
We acknowledge J. K. Foskett and R. J. Lee for assistance with the calcium imaging experiments and F. R. Papa for helpful suggestions. We also thank A. Suen and K. H. Cheung for advice on calcium imaging in MIN6 cells.
Disclosure Summary: The authors have nothing to disclose.
This work was supported by the National Institutes of Health Grants P01 DK49210 (to D.A.S.), F30 DK085931 (to C.K.), and R01 DK48280 (to P.A.).
Footnotes
- ChIP
- Chromatin immunoprecipitation
- CHOP
- CCAAT enhancer binding protein homologous protein
- DMSO
- dimethylsulfoxide
- DTT
- dithiothreitol
- ER
- endoplasmic reticulum
- ERO1
- ER oxidoreductin 1
- ERO1lα
- ERO1-like α
- IP3
- inositol triphosphate
- JNK
- c-Jun N-terminal kinase
- PDI
- protein disulfide isomerase
- PDX1
- pancreatic and duodenal homeobox 1
- Seq
- sequencing
- siNT
- nontargeting siRNA duplex
- siRNA
- small interfering RNA
- UPR
- unfolded protein response.
References
- 1. Scheuner D, Kaufman RJ. 2008. The unfolded protein response: a pathway that links insulin demand with β-cell failure and diabetes. Endocr Rev 29:317–333 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Bensellam M, Van Lommel L, Overbergh L, Schuit FC, Jonas JC. 2009. Cluster analysis of rat pancreatic islet gene mRNA levels after culture in low-, intermediate- and high-glucose concentrations. Diabetologia 52:463–476 [DOI] [PubMed] [Google Scholar]
- 3. Elouil H, Bensellam M, Guiot Y, Vander Mierde D, Pascal SM, Schuit FC, Jonas JC. 2007. Acute nutrient regulation of the unfolded protein response and integrated stress response in cultured rat pancreatic islets. Diabetologia 50:1442–1452 [DOI] [PubMed] [Google Scholar]
- 4. Lipson KL, Fonseca SG, Ishigaki S, Nguyen LX, Foss E, Bortell R, Rossini AA, Urano F. 2006. Regulation of insulin biosynthesis in pancreatic β-cells by an endoplasmic reticulum-resident protein kinase IRE1. Cell Metab 4:245–254 [DOI] [PubMed] [Google Scholar]
- 5. Laybutt DR, Preston AM, Akerfeldt MC, Kench JG, Busch AK, Biankin AV, Biden TJ. 2007. Endoplasmic reticulum stress contributes to β-cell apoptosis in type 2 diabetes. Diabetologia 50:752–763 [DOI] [PubMed] [Google Scholar]
- 6. Ozcan U, Cao Q, Yilmaz E, Lee AH, Iwakoshi NN, Ozdelen E, Tuncman G, Görgün C, Glimcher LH, Hotamisligil GS. 2004. Endoplasmic reticulum stress links obesity, insulin action, and type 2 diabetes. Science 306:457–461 [DOI] [PubMed] [Google Scholar]
- 7. Huang CJ, Lin CY, Haataja L, Gurlo T, Butler AE, Rizza RA, Butler PC. 2007. High expression rates of human islet amyloid polypeptide induce endoplasmic reticulum stress mediated β-cell apoptosis, a characteristic of humans with type 2 but not type 1 diabetes. Diabetes 56:2016–2027 [DOI] [PubMed] [Google Scholar]
- 8. Wang J, Takeuchi T, Tanaka S, Kubo SK, Kayo T, Lu D, Takata K, Koizumi A, Izumi T. 1999. A mutation in the insulin 2 gene induces diabetes with severe pancreatic β-cell dysfunction in the Mody mouse. J Clin Invest 103:27–37 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Yoshioka M, Kayo T, Ikeda T, Koizumi A. 1997. A novel locus, Mody4, distal to D7Mit189 on chromosome 7 determines early-onset NIDDM in nonobese C57BL/6 (Akita) mutant mice. Diabetes 46:887–894 [DOI] [PubMed] [Google Scholar]
- 10. Oyadomari S, Koizumi A, Takeda K, Gotoh T, Akira S, Araki E, Mori M. 2002. Targeted disruption of the Chop gene delays endoplasmic reticulum stress-mediated diabetes. J Clin Invest 109:525–532 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Pollard MG, Travers KJ, Weissman JS. 1998. Ero1p: a novel and ubiquitous protein with an essential role in oxidative protein folding in the endoplasmic reticulum. Mol Cell 1:171–182 [DOI] [PubMed] [Google Scholar]
- 12. Frand AR, Kaiser CA. 1998. The ERO1 gene of yeast is required for oxidation of protein dithiols in the endoplasmic reticulum. Mol Cell 1:161–170 [DOI] [PubMed] [Google Scholar]
- 13. Frand AR, Kaiser CA. 1999. Ero1p oxidizes protein disulfide isomerase in a pathway for disulfide bond formation in the endoplasmic reticulum. Mol Cell 4:469–477 [DOI] [PubMed] [Google Scholar]
- 14. Tu BP, Weissman JS. 2002. The FAD- and O(2)-dependent reaction cycle of Ero1-mediated oxidative protein folding in the endoplasmic reticulum. Mol Cell 10:983–994 [DOI] [PubMed] [Google Scholar]
- 15. Harding HP, Zhang Y, Zeng H, Novoa I, Lu PD, Calfon M, Sadri N, Yun C, Popko B, Paules R, Stojdl DF, Bell JC, Hettmann T, Leiden JM, Ron D. 2003. An integrated stress response regulates amino acid metabolism and resistance to oxidative stress. Mol Cell 11:619–633 [DOI] [PubMed] [Google Scholar]
- 16. Marciniak SJ, Yun CY, Oyadomari S, Novoa I, Zhang Y, Jungreis R, Nagata K, Harding HP, Ron D. 2004. CHOP induces death by promoting protein synthesis and oxidation in the stressed endoplasmic reticulum. Genes Dev 18:3066–3077 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Cabibbo A, Pagani M, Fabbri M, Rocchi M, Farmery MR, Bulleid NJ, Sitia R. 2000. ERO1-L, a human protein that favors disulfide bond formation in the endoplasmic reticulum. J Biol Chem 275:4827–4833 [DOI] [PubMed] [Google Scholar]
- 18. Pagani M, Fabbri M, Benedetti C, Fassio A, Pilati S, Bulleid NJ, Cabibbo A, Sitia R. 2000. Endoplasmic reticulum oxidoreductin 1-lβ (ERO1-Lβ), a human gene induced in the course of the unfolded protein response. J Biol Chem 275:23685–23692 [DOI] [PubMed] [Google Scholar]
- 19. Li G, Mongillo M, Chin KT, Harding H, Ron D, Marks AR, Tabas I. 2009. Role of ERO1-α-mediated stimulation of inositol 1,4,5-triphosphate receptor activity in endoplasmic reticulum stress-induced apoptosis. J Cell Biol 186:783–792 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Otsu M, Bertoli G, Fagioli C, Guerini-Rocco E, Nerini-Molteni S, Ruffato E, Sitia R. 2006. Dynamic retention of Ero1α and Ero1β in the endoplasmic reticulum by interactions with PDI and ERp44. Antioxid Redox Signal 8:274–282 [DOI] [PubMed] [Google Scholar]
- 21. Higo T, Hattori M, Nakamura T, Natsume T, Michikawa T, Mikoshiba K. 2005. Subtype-specific and ER lumenal environment-dependent regulation of inositol 1,4,5-trisphosphate receptor type 1 by ERp44. Cell 120:85–98 [DOI] [PubMed] [Google Scholar]
- 22. Marselli L, Thorne J, Dahiya S, Sgroi DC, Sharma A, Bonner-Weir S, Marchetti P, Weir GC. 2010. Gene expression profiles of β-cell enriched tissue obtained by laser capture microdissection from subjects with type 2 diabetes. PLoS One 5:e11499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Sachdeva MM, Claiborn KC, Khoo C, Yang J, Groff DN, Mirmira RG, Stoffers DA. 2009. Pdx1 (MODY4) regulates pancreatic β-cell susceptibility to ER stress. Proc Natl Acad Sci USA 106:19090–19095 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Zito E, Chin KT, Blais J, Harding HP, Ron D. 2010. ERO1-β, a pancreas-specific disulfide oxidase, promotes insulin biogenesis and glucose homeostasis. J Cell Biol 188:821–832 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Scharp DW, Kemp CB, Knight MJ, Ballinger WF, Lacy PE. 1973. The use of ficoll in the preparation of viable islets of Langerhans from the rat pancreas. Transplantation 16:686–689 [DOI] [PubMed] [Google Scholar]
- 26. Deramaudt TB, Sachdeva MM, Wescott MP, Chen Y, Stoffers DA, Rustgi AK. 2006. The PDX1 homeodomain transcription factor negatively regulates the pancreatic ductal cell-specific keratin 19 promoter. J Biol Chem 281:38385–38395 [DOI] [PubMed] [Google Scholar]
- 27. Stoffers DA, Zinkin NT, Stanojevic V, Clarke WL, Habener JF. 1997. Pancreatic agenesis attributable to a single nucleotide deletion in the human IPF1 gene coding sequence. Nat Genet 15:106–110 [DOI] [PubMed] [Google Scholar]
- 28. Gao N, LeLay J, Vatamaniuk MZ, Rieck S, Friedman JR, Kaestner KH. 2008. Dynamic regulation of Pdx1 enhancers by Foxa1 and Foxa2 is essential for pancreas development. Genes Dev 22:3435–3448 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Goudet G, Delhalle S, Biemar F, Martial JA, Peers B. 1999. Functional and cooperative interactions between the homeodomain PDX1, Pbx, and Prep1 factors on the somatostatin promoter. J Biol Chem 274:4067–4073 [DOI] [PubMed] [Google Scholar]
- 30. Cheung KH, Mei L, Mak DO, Hayashi I, Iwatsubo T, Kang DE, Foskett JK. 2010. Gain-of-function enhancement of IP3 receptor modal gating by familial Alzheimer's disease-linked presenilin mutants in human cells and mouse neurons. Sci Signal 3:ra22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Dyachok O, Tufveson G, Gylfe E. 2004. Ca2+-induced Ca2+ release by activation of inositol 1,4,5-trisphosphate receptors in primary pancreatic β-cells. Cell Calcium 36:1–9 [DOI] [PubMed] [Google Scholar]
- 32. Urano F, Wang X, Bertolotti A, Zhang Y, Chung P, Harding HP, Ron D. 2000. Coupling of stress in the ER to activation of JNK protein kinases by transmembrane protein kinase IRE1. Science 287:664–666 [DOI] [PubMed] [Google Scholar]
- 33. Kulkarni RN, Jhala US, Winnay JN, Krajewski S, Montminy M, Kahn CR. 2004. PDX-1 haploinsufficiency limits the compensatory islet hyperplasia that occurs in response to insulin resistance. J Clin Invest 114:828–836 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Brissova M, Shiota M, Nicholson WE, Gannon M, Knobel SM, Piston DW, Wright CV, Powers AC. 2002. Reduction in pancreatic transcription factor PDX-1 impairs glucose-stimulated insulin secretion. J Biol Chem 277:11225–11232 [DOI] [PubMed] [Google Scholar]
- 35. Kushner JA, Ye J, Schubert M, Burks DJ, Dow MA, Flint CL, Dutta S, Wright CV, Montminy MR, White MF. 2002. Pdx1 restores β-cell function in Irs2 knockout mice. J Clin Invest 109:1193–1201 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Harding HP, Zeng H, Zhang Y, Jungries R, Chung P, Plesken H, Sabatini DD, Ron D. 2001. Diabetes mellitus and exocrine pancreatic dysfunction in perk−/− mice reveals a role for translational control in secretory cell survival. Mol Cell 7:1153–1163 [DOI] [PubMed] [Google Scholar]
- 37. Zhang P, McGrath B, Li S, Frank A, Zambito F, Reinert J, Gannon M, Ma K, McNaughton K, Cavener DR. 2002. The PERK eukaryotic initiation factor 2 α kinase is required for the development of the skeletal system, postnatal growth, and the function and viability of the pancreas. Mol Cell Biol 22:3864–3874 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Zhang W, Feng D, Li Y, Iida K, McGrath B, Cavener DR. 2006. PERK EIF2AK3 control of pancreatic β-cell differentiation and proliferation is required for postnatal glucose homeostasis. Cell Metab 4:491–497 [DOI] [PubMed] [Google Scholar]