Abstract
Estrogens have been shown to have protective effects on a wide range of cell types and animal models for many neurodegenerative diseases. The present study demonstrates the cytoprotective effects of 17β-estradiol (E2) and estrogen-like compounds in an in vitro model of Friedreich's ataxia (FRDA) using human donor FRDA skin fibroblasts. FRDA fibroblasts are extremely sensitive to free radical damage and oxidative stress, produced here using l-buthionine (S,R)-sulfoximine to inhibit de novo glutathione synthesis. We have shown that the protective effect of E2 in the face of l-buthionine (S,R)-sulfoximine -induced oxidative stress is independent of estrogen receptor-α, estrogen receptor-β or G protein-coupled receptor 30 as shown by the inability of either ICI 182,780 or G15 to inhibit the E2-mediated protection. These cytoprotective effects appear to be dependent on antioxidant properties and the phenolic structure of estradiol as demonstrated by the observation that all phenolic compounds tested were protective, whereas all nonphenolic compounds were inactive, and the observation that the phenolic compounds reduced the levels of reactive oxygen species, whereas the nonphenolic compounds did not. These data show for the first time that phenolic E2-like compounds are potent protectors against oxidative stress-induced cell death in FRDA fibroblasts and are possible candidate drugs for the treatment and prevention of FRDA symptoms.
Friedreich's ataxia (FRDA) is the most common form of inherited ataxia in the world, affecting 1:20,000 to 1:50,000 people in the United States (1–5). It is inherited in an autosomal recessive manner due to a GAA trinucleotide repeat expansion in the first intron of the FXN gene on chromosome 9q13–21, causing gene silencing and a functional absence of the mitochondrial-localizing protein frataxin (1, 5–9). The frataxin protein is responsible for removing iron from around the mitochondria, preventing the formation of reactive oxygen species (ROS) (1, 10–12). The loss of FXN function causes an accumulation of mitochondrial iron, ROS, and impaired function of Fe-S centers in mitochondrial proteins, leading to mitochondrial damage and a decrease in activity of mitochondrial complexes I-III (1, 8, 12–14). The damaged mitochondria is unable to match ATP production to the cell's energy requirements, a common mechanism of cell death in a wide range of neurological disorders including Alzheimer's disease, Parkinson's disease, and Huntington's disease (15–20). FRDA classically presents with a degeneration of the spinocerebellar tracts and posterior columns of the spinal cord, resulting in gait ataxia and tremor (8, 10, 21, 22). Also present are pes cavitus, speech problems, lateral and kyphoscoliosis, tremor, weakness, diabetes, and a 91% incidence of heart disorders, such as hypertrophic cardiomyopathy with interstitial fibrosis, which is the most common cause of early death in FRDA patients (5, 10, 21–24).
The neuroprotective effects of 17β-estradiol (E2) have been clearly documented for more than a decade (25–28) in a variety of disease states involving mitochondrial disruption, but the exact mechanism of action is currently poorly understood (20, 29, 30). Estrogen has been demonstrated to have antioxidant properties (31–35); modulate Ca2+ flux (36); stabilize mitochondrial membrane potential (20, 34, 35); maintain the activity of electron transport chain complexes I, III, and IV; help to maintain aerobic ATP production (34, 37); and promote a favorable balance of antiapoptotic-proapoptotic proteins in the cell (20, 30). Although the neuroprotective effects of estrogens have never been tested in an FRDA model and FRDA shows no gender bias in incidence (21, 22), epidemiological studies of FRDA have shown a better prognosis in female patients (2), and the proposed mitochondrial-support mechanisms of E2 suggests a possible role for E2 in the prevention and treatment of FRDA symptoms (38).
In the present study, we show that estrogens increase cell viability in FRDA fibroblasts subjected to oxidative stressors in a manner that is independent of any known estrogen receptor (ER). In the FRDA fibroblast model, the ability of E2 to provide protection from the pro-oxidant stressors is dependent on the presence of a phenol ring, and the potency of each compound is in part related to the number of phenol groups present in the compounds. This property makes estrogens good candidates to join the list of cellular antioxidants proposed to alleviate Friedreich's ataxia symptoms and modify the disease process (12).
Materials and Methods
Cell culture
Fibroblasts from a 30-yr-old FRDA patient (Coriell Institute, Camden NJ) were maintained in DMEM (ThermoScientific, Waltham, MA) with 10% charcoal-stripped fetal bovine serum (ThermoScientific), 1% GlutaMAX (ThermoScientific), and 1% penicillin-streptomycin (Invitrogen, Carlsbad, CA) at 37 C in 90% humidity and 5% CO2. At the time of treatment, the FRDA fibroblast media were changed to phenol red- and sodium pyruvate-free DMEM (ThermoScientific) and 1% penicillin-streptomycin. All experiments were conducted using cell passages 15–22.
Chemicals and reagents
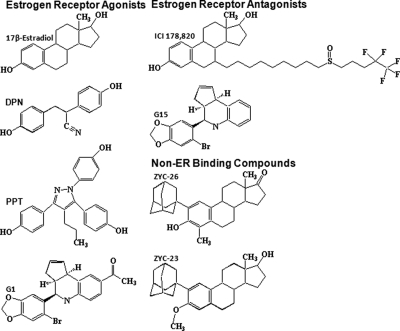
E2 was acquired from Steraloids, Inc. (Newport, RI). l-Buthionine (S,R)-sulfoximine (BSO) was obtained from Sigma-Aldrich (St Louis, MO). G1, an agonist at the membrane ER G protein-coupled receptor (GPR30), and G15, an antagonist at GPR30, were obtained from Calbiochem (San Diego, CA). ICI 182,780, 4,4′,4′-(4-propyl-[1H]-pyrazole-1,3,5-triyl)trisphenol (PPT), and diarylpropiolnitrile (DPN) were purchased from Tocris Bioscience (Ellisville, MO). 2-Adamantyl, 4 methyl estrone (ZYC-26) and 2-adamantyl, 3–0-methyl estradiol (ZYC-23) were synthesized in the laboratories of Covey and colleagues (31). Structures for all steroids are provided in Fig. 1 and were drawn using ChemDraw software (CambridgeSoft, Cambridge, MA).
Fig. 1.
Structures of compounds assessed for protection against BSO toxicity in FRDA fibroblasts.
Treatment paradigm
FRDA fibroblasts were removed from culture with 0.25% trypsin-EDTA (Invitrogen) and plated on 96-well plates at a density of 3000 cells/well in DMEM with 10% charcoal-stripped fetal bovine serum, 1% GlutaMAX, and 1% penicillin-streptomycin. After 24 h the media were removed and replaced with phenol red- and sodium pyruvate-free DMEM with 1% penicillin-streptomycin. The cells were then treated for 48 h with either dimethyl sulfoxide vehicle control (DMSO; Sigma-Aldrich) or 1 mm BSO in the presence of 1 nm to 10 μm E2, DPN, PPT, ZYC-26, ZYC-23, or G1, concentrations of estrogens known to be neuroprotective in various cell lines (39), with and without the ERα and ERβ receptor antagonist ICI 182,780 and the membrane ER receptor (GPR30) antagonist G15.
Lactate dehydrogenase (LDH) cell viability assay
After 48 h of treatment, 50 μl of media was removed from each of the wells of the 96-well treatment plate and placed in a separate 96-well plate. One hundred microliters of a solution consisting of 12 ml of 200 mm (pH 8.2) Tris(hydroxymethyl)aminomethane hydrochloride (Sigma-Aldrich) with 50 μl lactic acid and 4.2 mg iodonitrotetrazolium chloride (Sigma-Aldrich), 1.1 mg phenazine methosulfate (Sigma-Aldrich), and 10.8 mg β-nicotinamide adenine dinucleotide hydrate (Sigma-Aldrich) were added to each well. The absorbance of the resulting reaction was read with a Tecan Infinite M200 plate reader (Tecan Systems, Inc., San Jose, CA) at 490 nm and recorded once the reaction is linear for greater than 2 min. Cell viability for each well (WellX) was determined by the following equation: 100 − [100 (WellX media)/(0.1% Triton X-100 media)]. Measurements were then confirmed by visual inspection of the FRDA fibroblasts.
ROS assay
After 12 h of treatment, the media were removed from each well of the 96-well plate, and 100 μl of a 1 μm 2′,7′-dichlorodihydrofluorescein diacetate (AnaSpec Inc., Fremont, CA) in PBS was added to each well. The plates were returned to a 37 C incubator for 20 min, and then each well was washed three times with PBS and the resulting reaction was read on a Tecan Infinite M200 plate reader with an absorbance of 495 nm and an emission of 529 nm.
Calcien AM cell imaging
Cells were plated on a 96-well plate at a density of 5000 cells/well and then treated identically to those in the LDH cell viability assay. After 48 h of BSO and steroid treatment, the media were removed from each of the wells of a 96-well treatment plate. One hundred microliters of a 1 μg/ml calcien AM (Calbiochem) in phosphate buffer (pH 7.2) (PBS; Fisher Scientific, Pittsburgh, PA) were added to each of the wells, and the plate was incubated for 10 min at 37 C. The cells were then photographed using a Zeiss Axio Observer Z1 inverted microscope (Carl Zeiss, MicroImaging, Thornwood, NY) and Zeiss AxioVision 4.6 image processing software.
Data and statistics
All data are displayed as mean ± 1 sd. These data were analyzed using the ANOVA on the SYSTAT program (Systat Software Inc., Chicago, IL) with Tukey's post hoc test for statistical evaluation against an α-level of 0.05. All bar graphs were made using GraphPad Prism 5 (Graph Pad Inc., La Jolla, CA) and EC50 calculations were made with GraphPad Prism 5. For all groups, n = 8 wells and experiments were repeated three times to ensure consistency.
Results
Effects of BSO on cell viability FRDA fibroblasts
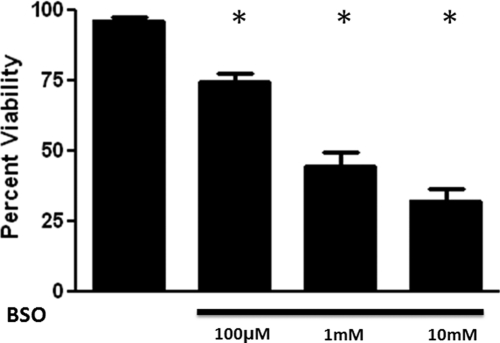
We determined the relative vulnerability of human donor FRDA fibroblasts to the effects of glutathione depletion. We used 100 μm to 10 mm BSO for 48 h to inhibit the rate limiting enzyme in the de novo synthesis of glutathione, γ-glutamyl cysteine synthase. There was a significant reduction in cell viability of FRDA fibroblasts in the presence of BSO. Viability was reduced from 96.3 ± 1.2% in the vehicle control to 74.7 ± 2.8, 44.7 ± 5.0, and 32.3 ± 4.1% in the presence of 100 μm, 1 mm, and 10 mm BSO, respectively (Fig. 2). Inasmuch as 1 mm BSO reduced cell viability by about 50–60%, we used this concentration to assess the cytoprotective activity of each of the compounds.
Fig. 2.
Effects of BSO on cell viability of FRDA fibroblasts. Depicted are mean ± sd (n = 8 per group). *, P < 0.05 vs. vehicle control.
Effects of E2 on cell viability in BSO-treated FRDA fibroblasts
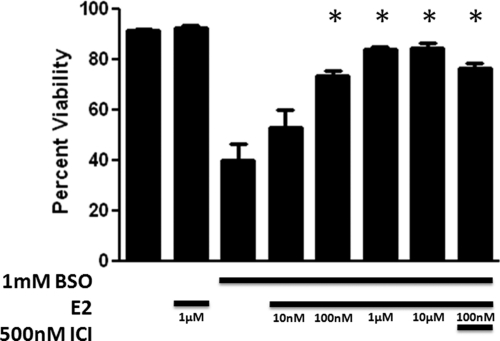
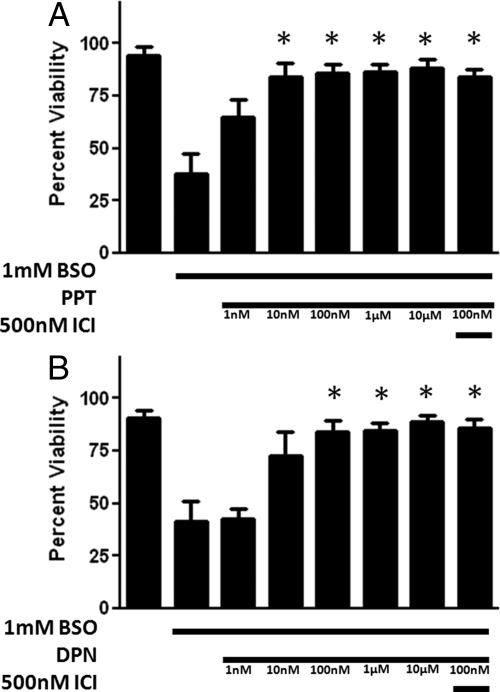
To determine whether E2 exhibited a protective effect against the oxidative damage allowed by BSO-induced glutathione depletion, we tested 10 nm to 10 μm E2 in cells treated with 1 mm BSO (Fig. 3). E2 produced a significant increase in cell viability (P < 0.05) in concentrations ranging from 100 nm to 10 μm (n = 8), with an EC50 of 15.5 nm, and near maximum effects at 1 μm E2. To determine whether these cytoprotective effects were due to either ERα or ERβ, we used 500 nm ICI 182,780, a concentration 1500-fold greater than the 0.29 nm IC50 of ICI 182,780 at both ERα and ERβ (40), in conjunction with 1 mm BSO and 100 nm E2. ICI 182,780 did not significantly reduce the viability of the FRDA fibroblasts compared with 1 mm BSO and 100 nm E2 treatment. In addition, the lack of effect of ICI 182,780 was observed when E2 and ICI 182,780 were given as a 24-h pretreatment (Richardson, T.E.R., unpublished observations). These data indicate that E2 acts by a mechanism that is independent of either ERα or ERβ.
Fig. 3.
Effects of E2 on cell viability in BSO-treated FRDA fibroblasts. Depicted are mean ± sd (n = 8 per group). *, P < 0.05 vs. BSO alone-treated cells. ICI, ICI 182,780.
Effects of ICI 182,780 on cell viability in BSO-treated FRDA fibroblasts
At 500 nm, ICI 182,780 alone provided no significant increase in cell viability against 1 mm BSO treatment. However at 2 μm, a concentration about 7000 times the IC50 of ICI 182,780 at ER, there was a statistically significant increase in cell viability (data not shown). These data are consistent with observations that the protective effects of E2 are due to the phenol ring, included in the structure of both E2 and ICI 182,780 (Fig. 1), and unrelated to either ERα or ERβ.
Effects of nonfeminizing estrogens on cell viability in BSO-treated FRDA fibroblasts
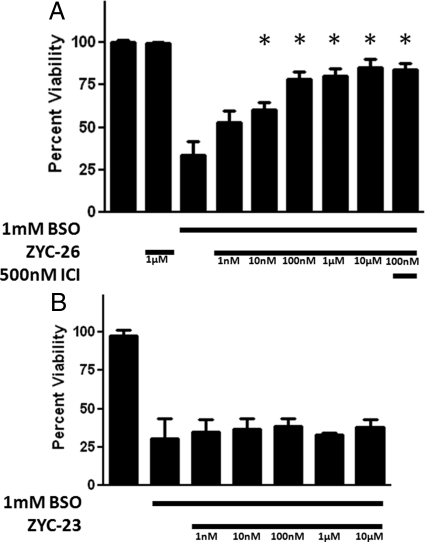
ZYC-26, a nonfeminizing estrogen, has cytoprotective properties in this FRDA fibroblast cell model with statistically significant effects at concentrations ranging from 10 nm to 10 μm (Fig. 4A). Although unable to bind to either ERα or ERβ, ZYC-26 has a phenolic ring as part of its structure, giving it antioxidant properties similar to E2 (31) (Fig. 1). At 100 nm, ZYC-26 increases the cell viability from 33.8 ± 7.59 to 80.37 ± 3.8%, with an EC50 of 23.1 nm. Conversely, ZYC-23, which does not bind to ER (31), had no significant effects on cell viability across the 10 nm to 10 μm concentration range tested (Fig. 4B). These data support the hypothesis that the phenol ring is essential in the protective effects of estrogens and estrogen-like compounds because ZYC-23 has an O-methyl at the 3 position of the phenol ring, eliminating the antioxidant properties of the compound (31) (see Figs. 1 and 8). As is evident in Fig. 4, the phenol ring is essential for maintenance of cell viability in the fibroblast FRDA disease model.
Fig. 4.
Effects of nonfeminizing estrogens on cell viability in BSO-treated FRDA fibroblasts. Depicted are mean ± sd (n = 8 per group). *, P < 0.05 vs. BSO alone-treated cells. ICI, ICI 182,780. A, ZYC-26. B, ZYC-23.
Fig. 8.
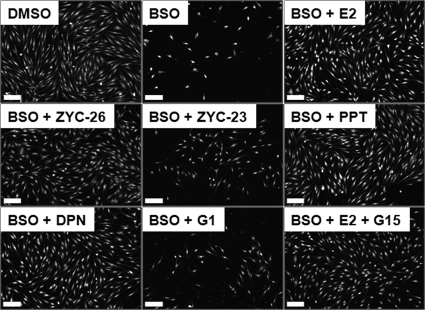
Calcien AM-stained cells visually showing the effects of E2, ZYC-26, ZYC-23, PPT, DPN, G1, and G15 on cell viability in BSO-treated FRDA fibroblasts. BSO concentration was 1 mm and all steroid concentrations were 100 nm. Scale bar, 200 μm.
The effects of ERα- and ERβ-preferring agonists on cell viability in BSO-treated FRDA fibroblasts
PPT is a relatively ERα-specific agonist that has cytoprotective effects in this cell model in the concentration range of 10 nm to 10 μm (Fig. 5A). This compound appears to have greater protective potency than E2. DPN is a relatively selective ERβ agonist with protective effects against BSO-induced cytotoxicity at concentrations ranging from 100 nm to 10 μm (Fig. 5B). Although these compounds are specific for individual ER, the effect of neither compound was inhibited by ICI 182,780, indicating that these compounds act through a mechanism other than their respective ER. Structures of the two compounds (Fig. 1) shows that the ERα agonist PPT has three phenol rings and the ERβ agonist DPN has two phenol rings, suggesting a possible ER-independent mechanism by which these two compounds provide cytoprotection to FRDA fibroblasts in the presence of 1 mm BSO. The extra phenol rings in the PPT molecule also provide a potential explanation for the observation that PPT has significant protective effects at 10 nm with an EC50 of 4.6 nm, whereas E2 does not protect against the BSO insult until it reaches 100 nm concentrations and has an EC50 of 15.5 nm. The extra phenol rings on the DPN molecule also seem to provide additional protective potency, giving it an EC50 value that falls between E2 and PPT at 9.3 nm.
Fig. 5.
Effects of an ERα-preferring agonist, PPT (A) and an ERβ-preferring agonist DPN (B) on cell viability in BSO-treated FRDA fibroblasts. Depicted are mean ± sd (n = 8 per group). *, P < 0.05 vs. BSO alone-treated cells.
Membrane ER (mER)/GPR30 effects on cell viability
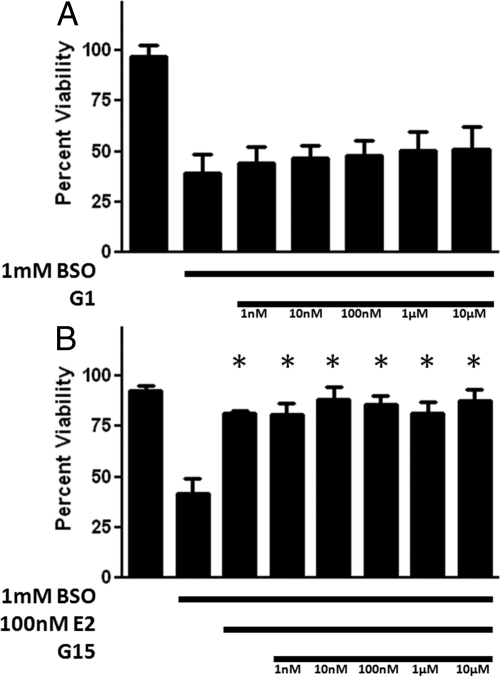
GPR30 is a proposed membrane-associated Gq-protein coupled ER. To assess estrogen actions through this receptor, we tested a range of concentrations of G1, a GPR30-preferring ER agonist. As seen in Fig. 6A, there were no significant protective effects at any tested concentration of G1. In addition, the GPR30 antagonist G15, at concentrations ranging from 1 nm to 10 μm, did not antagonize the cytoprotective effects of 100 nm E2 (Fig. 6B). These data indicate that the membrane-associated ER GPR30 is not involved in the increase in cell viability seen with E2 in FRDA fibroblasts exposed to 1 mm BSO (Fig. 3).
Fig. 6.
Effects of the mER-preferring agonist, G1 (A), on cell viability in BSO-treated FRDA fibroblasts, and effects of the mER-preferring antagonist, G15 (B), on E2-induced enhancement of cell viability in BSO-treated FRDA fibroblasts. Depicted are mean ± sd (n = 8 per group). *, P < 0.05 vs. BSO alone-treated cells.
The effects of estrogen-like compounds on BSO-induced ROS formation
We first determined the time course of ROS formation after BSO administration to FRDA fibroblast. At 1, 2, 3, 6, 12, 18, and 24 h after BSO treatment of FRDA fibroblasts, ROS was determined using a 2′,7′-dichlorodihydrofluorescein diacetate assay. Peak levels of ROS were seen at 12 h of treatment (data not shown). We therefore assessed the effects of compounds on ROS at 12 h after BSO and estrogen treatment.
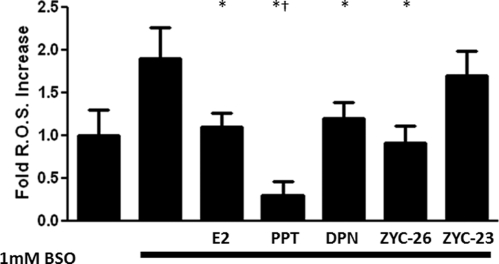
To determine whether the phenol groups present in these estrogen compounds were indeed exerting an antioxidant activity, we measured ROS after BSO in the presence or absence of E2, PPT, DPN, ZYC-26, and ZYC-23 at 100 nm, a concentration that produced near-maximal effects in the phenolic compounds. As can be seen in Fig. 7, BSO doubled ROS concentrations. Those compounds with phenol rings were effective in preventing this BSO-induced rise in ROS, whereas ZYC-23 provided no significant effect. In addition, the group treated with 1 mm BSO and 100 nm PPT, an estrogen-like compound with three phenol rings (Fig. 1), had significantly lower ROS concentrations than either the group treated with 1 mm BSO and 100 nm E2 or the DMSO control group (Fig. 7), an observation consistent with the enhanced potency of PPT.
Fig. 7.
Effects of E2, PPT, DPN, ZYC-26, and ZYC-23 on the formation of ROS in BSO-treated FRDA fibroblasts. All steroid concentrations were 100 nm. Depicted are mean ± sd (n = 8 per group). *, P < 0.05 vs. BSO alone-treated cells, †, P < 0.05 vs. BSO + E2/DPN/ZYC-26-treated cell groups and P < 0.05 vs. DMSO vehicle control-treated cells.
Effects of estrogens on BSO-induced cell death as measured by the calcien AM assay
To achieve a visual confirmation of the damaging effects of BSO and the protective effects of phenolic estrogens, FRDA fibroblasts were treated with BSO (1 mm) with or without simultaneous treatment with each estrogen (100 nm) for 48 h. Cells were then stained with calcien AM (Calbiochem), which stains live cells (31) and photomicrographs were taken. As shown in Fig. 8, the results obtained with the calcien AM assay were consistent with those obtained with the LDH viability assay.
Discussion
The potential role of estrogens in protection against various neurodegenerative diseases, including Alzheimer's disease, Parkinson's disease, Huntington's disease, and ischemic stroke has been known for more than a decade (25–28, 41). The proposed mechanisms by which estrogens provide neuroprotection are varied (for review see Ref. 20), but a clear case has been made for their therapeutic efficacy in neurodegenerative disorders. Previously it was unknown whether estrogens and estrogen-like compounds had any effect on the pathogenesis of Friedreich's ataxia, although the observation that females tend to have less severe symptoms and a later onset of the disease process (2) suggests that there is a beneficial effect of estrogens in this disease process. For the first time, this study presents data showing that estrogens and estrogen-like compounds containing a phenol ring are protective against oxidative stress in FRDA cells and that this process is not mediated by any known ER.
FRDA is the most common form of inherited ataxia in the world, caused by an autosomal recessive trinucleotide repeat expansion in the first intron of the FXN gene on chromosome 9q13–21 (1, 3, 5, 8). FRDA fibroblasts used in this study were taken from a skin-punch biopsy of a 30-yr-old male Caucasian patient with clinical FRDA symptoms. The cells are homozygous for the GAA expansion in the first intron of the Frataxin gene with 541 trinucleotide repeats on one allele and 420 on the second. Human fibroblasts contain both ERα and ERβ (42) as well as the mER GPR30 (43). Human FRDA fibroblasts have been used as a cell model in FRDA studies by several groups for the past 10 yr and have become a standard cell type to use in studying this disease process (12, 13, 44–46). FRDA fibroblasts are extremely vulnerable to oxidative damage caused by glutathione depletion with BSO treatment compared with genetically normal fibroblasts, which require a much greater dose of BSO to obtain a comparable toxicity (13), and it has been shown that antioxidants, specifically those targeted at the mitochondria, are effective at protecting the FRDA fibroblasts from oxidative stress (12). FRDA fibroblasts, although not primary neurons or cardiocytes, are an accepted model for FRDA as a disease process (12, 13). It is thought that the oxidative damage induced by the depletion of glutathione by BSO in this cell type may be analogous to the pathogenic process that is occurring in both the central nervous system and heart, leading to the clinical symptoms of FRDA (1, 8).
In this study, we have shown that estrogen-related compounds are able to protect FRDA fibroblasts from BSO-induced oxidative damage. These effects are independent of the action on ERα, ERβ, or GPR30, as indicated by the lack of inhibition of the cytoprotection induced by E2, ZYC-26, PPT, or DPN by ICI 182,780 or G15 and the lack of efficacy of G1 in promoting cell viability. It appears that the potential protective effects of estrogen-like compounds are dependent on its intrinsic antioxidant properties as determined by the inclusion of a phenol ring in the molecule's structure (Figs. 1 and 7). The protective effect of antioxidants has been shown previously (12) and is reinforced here by the fact that ZYC-26, which has a phenolic ring, is cytoprotective against a BSO-induced insult, whereas ZYC-23, which contains no such structure, is not (Fig. 4). Furthermore, PPT, which contains three phenol rings and has a significant protective effect at 10 nm and an EC50 of 4.6 nm, is a more potent cytoprotectant when compared with E2 or ZYC-26, which contains only one phenol ring and is less protective with EC50 values between 15 and 23 nm (Figs. 3 and 5A). DPN, a compound with two phenol rings, was observed to have intermediate cytoprotective potency, with a EC50 of 9.3 nm (Fig. 5B). In addition, although it is an ERα and ERβ antagonist, ICI 182,780 has a protective effect at concentrations of 2 μm (data not shown), likely due to the phenol ring in its structure (Fig. 1).
All of the phenolic compounds provided a statistically significant reduction in ROS in FRDA fibroblasts when given with 1 mm BSO, whereas compounds without phenol rings did not (Fig. 7). Moreover, the three phenol ring containing PPT (Fig. 1) is far more effective at reducing the levels of ROS than E2 or ZYC-26, which contains one phenol ring, or DPN, which contains two. This is consistent with the finding that PPT is a more potent cytoprotectant against BSO-induced oxidative stress. These observations provide a potential mechanism for the attenuation of the BSO effects seen with all phenolic steroids tested (Figs. 3–5).
These data support the hypothesis that estrogens protect against oxidative damage to the mitochondria in FRDA fibroblasts by direct antioxidant properties, rather than by stimulation of any known ER and that these antioxidant properties are dependent on the presence of at least one phenol ring in the molecular structure. Because there is a simple genetic test that can be done on the children of known FRDA allele carriers, it is possible to determine the presence of FRDA in newborns, years before the cardio- and neurodegeneration and clinical symptoms begin, a time window during which estrogens and other antioxidants could potentially be clinically useful. This study presents the first data supporting estrogens and nonfeminizing estrogens as a potential drug for the treatment and prevention of the symptoms of clinical FRDA.
Acknowledgments
We thank Dr. Robert Luedtke for all of his help with ChemDraw software, Amanda Yu for all of her help with FRDA cell cultures, David Julovich for his help with EC50 calculations, and Ethan Poteet for his help with Calcien AM imaging. The authors contributed to this work in the following: T.E.R. and J.W.S. participated in research design; T.E.R. conducted the experiments; T.E.R., J.W.S., and Y.W. contributed new reagents or analytic tools; T.E.R. and J.W.S. performed data analysis; and T.E.R., J.W.S., and S.-H.Y. wrote or contributed to the writing of the manuscript.
This work was supported by National Institutes of Health Grants P01 AG100485, P01 AG22550, and P01 AG027956.
Disclosure Summary: The authors have no potential conflicts of interest.
Footnotes
- BSO
- l-Buthionine (S,R)-sulfoximine
- DMSO
- dimethyl sulfoxide vehicle control
- DPN
- diarylpropiolnitrile
- E2
- 17β-estradiol
- ER
- estrogen receptor
- FRDA
- Friedreich's ataxia
- GPR30
- G- protein coupled receptor 30
- LDH
- lactate dehydrogenase
- mER
- membrane ER
- PPT
- 4,4′,4′-(4-propyl-[1H]-pyrazole-1,3,5-triyl)trisphenol
- ROS
- reactive oxygen species
- ZYC-26
- 2-adamantyl, 4 methyl estrone
- ZYC-23
- 2-adamantyl, 3–0-methyl estradiol.
References
- 1. Bradley JL, Blake JC, Chamberlain S, Thomas PK, Cooper JM, Schapira AH. 2000. Clinical, biochemical and molecular genetic correlations in Friedreich's ataxia. Hum Mol Genet 9:275–282 [DOI] [PubMed] [Google Scholar]
- 2. Leone M, Brignolio F, Rosso MG, Curtoni ES, Moroni A, Tribolo A, Schiffer D. 1990. Friedreich's ataxia: a descriptive epidemiological study in an Italian population. Clin Genet 38:161–169 [DOI] [PubMed] [Google Scholar]
- 3. Pandolfo M. 1998. Molecular genetics and pathogenesis of Friedreich ataxia. Neuromuscul Disord 8:409–415 [DOI] [PubMed] [Google Scholar]
- 4. Schulz JB, Boesch S, Bürk K, Dürr A, Giunti P, Mariotti C, Pousset F, Schöls L, Vankan P, Pandolfo M. 2009. Diagnosis and treatment of Friedreich ataxia: a European perspective. Nat Rev Neurol 5:222–234 [DOI] [PubMed] [Google Scholar]
- 5. Harding AE. 1983. Classification of the hereditary ataxias and paraplegias. Lancet 1:1151–1155 [DOI] [PubMed] [Google Scholar]
- 6. Campuzano V, Montermini L, Moltò MD, Pianese L, Cossée M, Cavalcanti F, Monros E, Rodius F, Duclos F, Monticelli A, Zara F, Cañizares J, Koutnikova H, Bidichandani SI, Gellera C, Brice A, Trouillas P, De Michele G, Filla A, De Frutos R, Palau F, Patel PI, Di Donato S, Mandel JL, Cocazza S, Koenig M, Pandolfo M. 1996. Friedreich's ataxia: autosomal recessive disease caused by an intronic GAA triplet repeat expansion. Science 271:1423–1427 [DOI] [PubMed] [Google Scholar]
- 7. Isnard R, Kalotka H, Dürr A, Cossée M, Schmitt M, Pousset F, Thomas D, Brice A, Koenig M, Komajda M. 1997. Correlation between left ventricular hypertrophy and GAA trinucleotide repeat length in Friedreich's ataxia. Circulation 95:2247–2249 [DOI] [PubMed] [Google Scholar]
- 8. Lodi R, Tonon C, Calabrese V, Schapira AH. 2006. Friedreich's ataxia: from disease mechanisms to therapeutic interventions. Antioxid Redox Signal 8:438–443 [DOI] [PubMed] [Google Scholar]
- 9. Montermini L, Andermann E, Labuda M, Richter A, Pandolfo M, Cavalcanti F, Pianese L, Iodice L, Farina G, Monticelli A, Turano M, Filla A, De Michele G, Cocozza S. 1997. The Friedreich ataxia GAA triplet repeat: permutation and normal alleles. Hum Mol Genet 6:1261–1266 [DOI] [PubMed] [Google Scholar]
- 10. Al-Mahdawi S, Pinto RM, Varshney D, Lawrence L, Lowrie MB, Hughes S, Webster Z, Blake J, Cooper JM, King R, Pook MA. 2006. GAA repeat expansion mutation mouse models of Friedreich ataxia exhibit oxidative stress leading to progressive neuronal and cardiac pathology. Genomics 88:580–590 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Babcock M, de Silva D, Oaks R, Davis-Kaplan S, Jiralerspong S, Montermini L, Pandolfo M, Kaplan J. 1997. Regulation of mitochondrial iron accumulation by Yfh1p, a putative homolog of frataxin. Science 276:1709–1712 [DOI] [PubMed] [Google Scholar]
- 12. Jauslin ML, Meier T, Smith RA, Murphy MP. 2003. Mitochondria-targeted antioxidants protect Friedreich ataxia fibroblasts from endogenous oxidative stress more effectively than untargeted antioxidants. FASEB J 17:1972–1974 [DOI] [PubMed] [Google Scholar]
- 13. Jauslin ML, Wirth T, Meier T, Shoumacher F. 2002. A cellular model for Friedreich ataxia reveals small-molecule glutathione peroxidase mimetics as novel treatment strategy. Hum Mol Genet 11:3055–3063 [DOI] [PubMed] [Google Scholar]
- 14. Rötig A, de Lonlay P, Chretien D, Foury F, Koenig M, Sidi D, Munnich A, Rustin P. 1997. Aconitase and mitochondrial iron-sulfur protein deficiency in Friedreich ataxia. Nat Genet 17:215–217 [DOI] [PubMed] [Google Scholar]
- 15. Gibson GE, Sheu KF, Blass JP. 1998. Abnormalities of mitochondrial enzymes in Alzheimer disease. J Neural Transm 105:855–870 [DOI] [PubMed] [Google Scholar]
- 16. Lenaz G, Baracca A, Barbero G, Bergamini C, Dalmonte ME, Del Sole M, Faccioli M, Falasca A, Fato R, Genova ML, Sgarbi G, Solaini G. 2010. Mitochondrial respiratory chain super-complex I-III in physiology and pathology. Biochim Biophys Acta 1797:633–640 [DOI] [PubMed] [Google Scholar]
- 17. Lenaz G, Baracca A, Fato R, Genova ML, Solaini G. 2006. Mitochondrial complex I: structure, function, and implications in neurodegeneration. Ital J Biochem 55:232–253 [PubMed] [Google Scholar]
- 18. Mizuno Y, Ohta S, Tanaka M, Takamiya S, Suzuki K, Sato T, Oya H, Ozawa T, Kagawa Y. 1989. Deficiencies in complex I subunits of the respiratory chain in Parkinson's disease. Biochem Biophys Res Commun 163:1450–1455 [DOI] [PubMed] [Google Scholar]
- 19. Parker WD, Jr, Boyson SJ, Parks JK. 1989. Abnormalities of the electron transport chain in idiopathic Parkinson's disease. Ann Neurol 26:719–723 [DOI] [PubMed] [Google Scholar]
- 20. Simpkins JW, Dykens JA. 2008. Mitochondrial mechanisms of estrogen neuroprotection. Brain Res Rev 57:421–430 [DOI] [PubMed] [Google Scholar]
- 21. Dürr A, Cossée M, Agid Y, Campuzano V, Mignard C, Penet C, Mandel JL, Brice A, Koenig M. 1996. Clinical and genetic abnormalities in patients with Friedreich's ataxia. N Engl J Med 335:1169–1175 [DOI] [PubMed] [Google Scholar]
- 22. Geoffroy G, Barbeau A, Breton G, Lemieux B, Aube M, Leger C, Bouchard JP. 1976. Clinical description and roentgenologic evaluation of patients with Friedreich's ataxia. Can J Neurol Sci 3:279–286 [DOI] [PubMed] [Google Scholar]
- 23. Dutka DP, Donnelly JE, Palka P, Lange A, Nuñez DJ, Nihoyannopoulous P. 2000. Echocardiographic characterization of cardiomyopathy in Friedreich's ataxia with tissue Doppler echocardiographically derived myocardial velocity gradients. Circulation 102:1276–1282 [DOI] [PubMed] [Google Scholar]
- 24. Harding AE. 1981. Friedreich's ataxia: a clinical and genetic study of 90 families with an analysis of early diagnostic criteria and intrafamilial clustering of clinical features. Brain 104:589–620 [DOI] [PubMed] [Google Scholar]
- 25. Bishop J, Simpkins JW. 1994. Estradiol treatment increases viability of glioma and neuroblastoma cells in vitro. Mol Cell Neurosci 5:303–308 [DOI] [PubMed] [Google Scholar]
- 26. Behl C, Widmann M, Trapp T, Holsboer F. 1995. 17-β estradiol protects neurons from oxidative stress induced cell death in vitro. Biochem Biophys Res Commun 216:473–482 [DOI] [PubMed] [Google Scholar]
- 27. Goodman Y, Bruce AJ, Cheng B, Mattson MP. 1996. Estrogens attenuate and corticosterone exacerbates excitotoxicity, oxidative injury and amyloid β-peptide toxicity in hippocampal neurons. J Neurochem 66:1836–1844 [DOI] [PubMed] [Google Scholar]
- 28. Singer CA, Rogers KL, Strickland TM, Dorsa DM. 1996. Estrogen protects primary cortical neurons from glutamate toxicity. Neurosci Lett 212:13–16 [DOI] [PubMed] [Google Scholar]
- 29. Simpkins JW, Yang SH, Sarkar SN, Pearce V. 2008. Estrogen actions on mitochondria-physiological and pathological implications. Mol Cell Endocrinol 290:51–59 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Simpkins JW, Yi KD, Yang SH. 2009. Role of protein phosphatases and mitochondria in the neuroprotective effects of estrogens. Front Neuroendocrinol 30:93–105 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Perez E, Cai ZY, Covey DF, Simpkins JW. 2006. Neuroprotective effects of estratriene analogs: structure-activity relationships and molecular optimization. Drug Dev Res 66:78–92 [Google Scholar]
- 32. Prokai L, Prokai-Tatrai K, Perjesi P, Zharikova AD, Perez EJ, Liu R, Simpkins JW. 2003. Quinol-based cyclic antioxidant mechanism in estrogen neuroprotection. Proc Natl Acad Sci USA 100:11741–11746 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Prokai-Tatrai K, Perjesi P, Rivera-Portalatin NM, Simpkins JW, Prokai L. 2008. Mechanistic investigation on the antioxidant action of a neuroprotective estrogen derivative. Steroids 73:280–288 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Wang J, Green PS, Simpkins JW. 2001. Estradiol protects against ATP depletion, mitochondrial membrane potential decline and the generation of reactive oxygen species induced by 3-nitroproprionic acid in SK-N-SH human neuroblastoma cells. J Neurochem 77:804–811 [DOI] [PubMed] [Google Scholar]
- 35. Wang X, Simpkins JW, Dykens JA, Cammarata PR. 2003. Oxidative damage to human lens epithelial cells in culture: estrogen protection of mitochondrial potential, ATP, and cell viability. Invest Ophthalmol Vis Sci 44:2067–2075 [DOI] [PubMed] [Google Scholar]
- 36. Sarkar SN, Huang RQ, Logan SM, Yi KD, Dillon GH, Simpkins JW. 2008. Estrogens directly potentiate neuronal L-type Ca2+ channels. Proc Natl Acad Sci USA 105:15148–15153 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Jayachandran M, Preston CC, Hunter LW, Jahangir A, Owen WG, Korach KS, Miller VM. 2010. Loss of estrogen receptor β decreases mitochondrial energetic potential and increases thrombogenicity of platelets in aged female mice. Age (Dordr) 32:109–121 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Dykens JA, Moos WH, Howell N. 2005. Development of 17α-estradiol as a neuroprotective therapeutic agent: rationale and results from a phase I clinical study. Ann NY Acad Sci 1052:116–135 [DOI] [PubMed] [Google Scholar]
- 39. Yi KD, Cai ZY, Covey DF, Simpkins JW. 2008. Estrogen receptor-independent neuroprotection via protein phosphatase preservation and attenuation of persistent extracellular signal-regulated kinase 1/2 activation. J Pharmacol Exp Ther 324:1188–1195 [DOI] [PubMed] [Google Scholar]
- 40. de Cupis A, Noonan D, Pirani P, Ferrera A, Clerico L, Favoni RE. 1995. Comparison between novel steroid-like and conventional nonsteroidal antioestrogens in inhibiting oestradiol- and IGF-I-induced proliferation of human breast cancer-derived cells. Br J Pharmacol 116:2391–2400 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Simpkins JW, Rajakumar G, Zhang YQ, Simpkins CE, Greeenwald D, Yu CJ, Bodor N, Day AL. 1997. Estrogens may reduce mortality and ischemic damage caused by middle cerebral artery occlusion in the female rat. J Neurosurg 87:724–730 [DOI] [PubMed] [Google Scholar]
- 42. Haczynski J, Tarkowski R, Jarzabek K, Slomczynska M, Wolczynski S, Magoffin DA, Jakowiki JA, Jakimiuk AJ. 2002. Human cultured skin fibroblasts express estrogen receptor α and β. Int J Mol Med 10:149–153 [PubMed] [Google Scholar]
- 43. Madeo A, Maggiolini M. 2010. Nuclear alternate estrogen receptor GPR30 mediates 17β-estradiol-induced gene expression and migration in breast cancer-associated fibroblasts. Cancer Res 70:6036–6046 [DOI] [PubMed] [Google Scholar]
- 44. Jauslin ML, Vertuani S, Durini E, Buzzoni L, Ciliberti N, Verdecchia S, Palozza P, Meier T, Manfredini S. 2007. Protective effects of Fe-Aox29, a novel antioxidant derived from a molecular combination of Idebenone and vitamin E, in immortalized fibroblasts and fibroblasts from patients with Friedreich ataxia. Mol Cell Biochem 302:79–85 [DOI] [PubMed] [Google Scholar]
- 45. Li K, Besse EK, Ha D, Kovtunovych G, Rouault TA. 2008. Iron-dependent regulation of frataxin expression: implications for treatment of Friedreich ataxia. Hum Mol Genet 17:2265–2273 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Ku S, Soragni E, Campau E, Thomas EA, Altun G, Laurent LC, Loring JF, Napierala M, Gottesfeld JM. 2010. Friedreich's ataxia induced pluripotent stem cells model intergenerational GAA-TTC triplet repeat instability. Cell Stem Cell 7:631–637 [DOI] [PMC free article] [PubMed] [Google Scholar]