Abstract
Markers of hyperactive central corticotropin releasing factor (CRF) systems and CRF-related single nucleotide polymorphisms (SNPs) have been identified in patients with anxiety and depressive disorders. Designing more effective antagonists may now be guided by data showing that small molecules bind to transmembrane domains. Specifically, CRF1 receptor antagonists have been developed as novel anxiolytic and antidepressant treatments. Because CRF1 receptors become rapidly desensitized by G protein-coupled receptor kinase (GRK) and β-arrestin mechanisms in the presence of high agonist concentrations, neuronal hypersecretion of synaptic CRF alone may be insufficient to account for excessive central CRF neurotransmission in stress-induced affective pathophysiology. In addition to desensitizing receptor function, GRK phosphorylation and β-arrestin binding can shift a G protein-coupled receptor (GPCR) to signal selectively via the extracellular signal-regulated kinase/mitogen-activated protein kinase (ERK-MAPK) or Akt pathways independent of G proteins. Also, Epac-dependent CRF1 receptor signaling via the ERK-MAPK pathway has been found to potentiate brain-derived neurotrophic factor (BDNF)-stimulated TrkB signaling. Thus, genetic or acquired abnormalities in GRK and β-arrestin function may be involved in the pathophysiology of stress-induced anxiety and depression.
Keywords: corticotropin releasing factor, CRF; urocortin 2, UCN2; urocortin 3, UCN3; CRF receptor type 1, CRF1 receptor; CRF receptor type 2, CRF2 receptor; G protein-coupled receptor, GPCR; GPCR kinase, GRK; cyclic 3′,5′-adenosine monophosphate cyclic AMP; extracellular signal-regulated kinase, ERK; mitogen-activated protein kinase, MAPK; brain-derived neurotrophic factor, BDNF
Introduction
Beginning with Hippocrates’s postulate that good health was dependent on maintaining physiological function in harmonious balance to Claude Bernard’s hypothesis that the milieu interieur must be kept constant by homeostatic regulation, the detrimental effect of stress on physiological and psychological well-being has long been recognized. Although activation of brain stress systems and defensive behavior is critical for surviving threats to homeostasis, rapid counter-regulation of the stress response is equally important for re-establishing normal mood upon threat termination.1–3 Genetic abnormalities and exposure to stress early in life or unpredictable trauma at any age can increase an individual’s vulnerability to subsequent stress and reduce “resilience” in coping with adverse events.2,4 “Resilience” is a function of the threshold at which particular perturbations activate stress systems, and the rapidness with which and degree to which stress responses cease with termination of the adverse stimulus. Compelling evidence indicates that corticotropin releasing factor (CRF) activation of CRF1 receptor signaling is sufficient, and in many cases necessary, to initiate anxiety-like defensive responses. Sustained stress exposure in childhood from severe abuse and deprivation has been associated with persistent sensitization of CRF receptor–mediated stress responses during adult life.5 Genetically driven susceptibility to stress, along with early life stress and adult environmental risk factors for affective disorders, may well account for the onset and recurrence of major depressive episodes.5–7 Thus, CRF1 receptor antagonists may prove to be efficacious for treating anxiety and depressive disorders induced by highly stressful situations.1,8–10 CRF2 receptors play a more complicated role in anxiety and depressive pathophysiology. One line of research supports the concept that CRF2 receptors re-establish homeostasis by counteracting stress response–initiating effects and anxiety-like defensive behavior triggered by CRF1 receptor signaling.1,11–13 An alternative hypothesis posits that CRF1 and CRF2 receptors contribute to opposite defensive modes, with CRF1 receptors mediating active defensive responses triggered by escapable stressors, and CRF2 receptors mediating passive anxiety- and depression-like responses induced by inescapable, uncontrollable stressors.14,15 Identifying the molecular mechanisms of stress response and recovery mediated by signal transduction of both CRF receptors is a critical step in elucidating the pathophysiology of anxiety and depression precipitated by early life or adult stress.
CRF, the three urocortin peptides (UCN1, UCN2, and UCN3), and CRF1 and CRF2 receptors compose central and peripheral stress systems critical for physiological survival.9,16–19 CRF-expressing neurons are widely distributed throughout the central nervous system (CNS), with high concentrations in the hypothalamic paraventricular nucleus (PVN), neocortex, the extended amygdala—especially the central nucleus of the amygdala—bed nucleus of the stria terminalis (BNST), and brain stem, all of which are regions regulating affective state, autonomic function, and stress responses.9,16 In contrast, urocortin peptides are expressed in a highly selective pattern. Neurons expressing UCN1, which binds equally to both CRF receptors, form a discrete hindbrain projection from the Edinger Westphal nucleus to the dorsal raphe nucleus (DRN) and other brain stem nuclei, while neurons expressing the CRF2 receptor-selective ligand UCN3 project to the lateral septum (LS), cortical amygdalar nucleus, BNST, and hypothalamic nuclei as an important forebrain pathway.16–18 Neurons expressing the other CRF2 receptor–selective ligand UCN2 are discretely distributed in the hypothalamic PVN and arcuate nuclei, the locus coeruleus and several brain stem motor nuclei.16–18 While CRF1 receptors are widely distributed throughout neocortical, limbic, and brain stem regions of the CNS, like the CRF peptide, CRF2 receptors are highly expressed in a few areas, including the DRN, LS, cortical and medial amygdalar nuclei, and PVN and ventromedial hypothalamic nucleus.16–18 However, CRF1 and CRF2 receptor mRNA expression has been detected in the primate neocortex—especially in the prefrontal and cingulate cortices, which interconnect with limbic brain regions and have been implicated in anxiety, stress, and depressive disorders.
Stress-induced release of CRF from the hypothalamic PVN activates pituitary corticotroph CRF1 receptors, resulting in secretion of adrenocorticotropic hormone (ACTH), which, in turn, activates ACTH receptors in the adrenal cortex to release glucocorticoids to meet physiological demands and promote adaptational regulation.9,16 The affective, cognitive, neuroendocrine, and autonomic responses to stress controlled by coordinated activation of central CRF1 and CRF2 receptors in response to CRF and urocortins are considerably more complex.1,10–17 Because CRF has a greater affinity for the CRF1 than for the CRF2 receptor,9,16–18 low concentrations of CRF would be expected to activate CRF1 receptors but not CRF2 receptors in brain regions expressing both receptor subtypes, such as the BNST. High CRF concentrations would be required to activate both CRF receptors. In brain regions expressing CRF, UCN1, UCN3, and only CRF2 receptors, such as the LS, CRF2 receptors would signal strongly after binding either urocortin, while weaker CRF2 receptor activation would occur if only CRF were released. Binding of CRF or urocortins to the CRF2 receptor may induce distinct conformations that preferentially activate specific signaling pathways. Because urocortin-stimulated CRF2 receptor signaling in the LS elicits anxiogenic behavior especially during stress exposure,20,21 specific CRF2 receptor conformation and signaling pattern elicited by urocortins may be relevant to mood regulation. Furthermore, differential expression in brain neurons of G protein-coupled receptor kinases (GRKs) and β-arrestins,22,23 which are important regulators of CRF receptors, may greatly influence regional regulation of CRF1 and CRF2 receptor signaling.
CRF Receptor Signal Transduction Pathways
Adenylyl Cyclase–Protein Kinase A Pathway
Abnormalities in adenylyl cyclase–protein kinase A (PKA) signaling have been implicated in stress maladaptation, anxiety, and depression. Inhibition of PKA in the central amygdalar nucleus reduces CREB phosphorylation and stress-induced anxiety-like behavior24 while targeted overexpression of CREB in basolateral amygdalar neurons enhances anxiety-like responses to stress.25 Mice with a constitutive deletion of the adenylyl cyclase 5 gene exhibit a large reduction in anxiety-like defensive behavior and an “antidepressant-like” response to forced-swimming stress.26 In many endogenous and recombinant cell lines, CRF1 and CRF2 receptors preferentially signal by Gsα coupling to the third intracellular loop (IC3), resulting in the activation of adenylyl cyclase and generation of the second messenger cyclic AMP.9,16–19 Increasing cyclic AMP formation, in turn, stimulates PKA to phosphorylate downstream targets in the cytosol and CREB in the nucleus, inducing transcription of certain genes.9,16–19 In hippocampal neurons, CRF1 receptor signaling via the cyclic AMP-PKA pathway increases expression of SGK-1, a brain serine/threonine protein kinase that also upregulates during stress or glucorticoid administration and contributes to the regulation of synaptic plasticity and memory.27 In addition, SGK expression markedly increases in forebrain and limbic regions of transgenic mice with global CRF overexpression that induces excessive anxiety-like behavior and hypothalamic-pituitary-adrenal (HPA) hyper-responsiveness to stress.28 CRF overexpression transgenic mice also develop increased brain expression of FK506BP, a co-chaperone of heat-shock protein-90 that regulates glucocorticoid receptor action and has been genetically linked to the development of post-traumatic stress disorder and stress-induced depressive recurrences.28 In cerebellar granular cells, CRF1 receptor signaling via the cyclic AMP-PKA-CREB cascade robustly upregulates brain-derived neurotrophic factor (BDNF) mRNA levels.29 In locus coeruleus neurons, independent of PKA, CRF1 receptor-mediated generation of cyclic AMP activates Epac, a guanine nucleotide exchange factor for the small GTPases Rap1 and Rap2.30 CRF1 receptor Epac signaling then activates the extracellular signal-regulated kinase–mitogen-activated protein kinase (ERK-MAPK) cascade, which, in turn, potentiates BDNF-stimulated TrkB signaling, possibly by trafficking TrkB receptors to neuronal membranes.30 Consequently, Gs-coupled CRF1 receptor signaling may modulate BDNF-mediated synaptic plasticity and neurogenesis—processes that have been implicated in stress, anxiety, and depressive disorders.31–33 Indeed, postmortem studies have reported that protein levels of Epac2 are upregulated while expression of BDNF and TrkB receptors is reduced in the prefrontal cortex and hippocampus of depressed suicide victims.34,35 Potential interactions between CRF and TrkB receptors may contribute to severe anxiety and depression. Surprisingly, coupling to Gsα was recently identified to be the dominant mechanism for stimulating intracellular calcium mobilization by CRF1 and CRF2(a) receptors. Gs-coupled CRF receptor calcium signaling was mediated by activation of adenylyl cyclase and then Epac2, which, in turn, stimulated the ε isoform of phospholipase C (PLCε).36 This pattern of CRF receptor signal transduction may also be important in behavioral responses to stress. Gs-coupled CRF2 receptor signaling via the cyclic AMP-PKA cascade has also been shown to influence limbic dopaminergic neurotransmission by stimulating intracellular calcium release, which, in turn, potentiates excitation of calcium-sensitive potassium channels in midbrain dopamine neurons that are involved in limbic brain responses to stress.37
PLC–Protein Kinase C Pathway
Dysregulation of protein kinase C (PKC) isoform-specific signal transduction has been associated with severe anxiety and suicide. Stress-induced anxiety-like defensive behavior and HPA responses are abnormal in PKCε knockout mice while protein levels of α, β, and γ PKC isoforms are decreased in the prefrontal cortex and hippocampus of suicide victims.38,39 Agonist-activated CRF1 receptors generate both cyclic AMP and IP3 signals in certain peripheral cells while CRF1 receptors preferentially signal by only stimulating calcium mobilization, IP3 formation, and rapid translocation of PKC to the membrane in other peripheral cell lines.9,19,36 CRF1 receptors signal via the PKA but not PKC pathway in human retinoblastoma Y79 cells, a brain-derived cell line.40 Although both CRF1 and CRF2(a) receptors are capable of PLC-PKC signal transduction, presumably by coupling to Gqα, unknown factors may govern whether or not CRF receptors preferentially signal via the PLC-PKC or adenylyl cyclase-PKA cascade, or activate both pathways in the same cell. CRF1 or CRF2(a) receptor signaling via the PLC-PKC cascade stimulates IP3 formation and contributes to intracellular calcium mobilization in HEK293 but not brain-derived human SK-N-MC neuroblastoma cells, possibly due to expression of Epac2 and PLCε in HEK293 but not SK-N-MC cells.36 Furthermore, in HEK293 cells, coupling of both CRF receptors to Gsα and Gqα in parallel is involved in generating calcium signals.36 CRF1 and CRF2(a) receptor coupling to Gi/o contributes to a lesser extent in calcium signal transduction, although Gi/o coupling requires initial activation of Gsα.36 CRF1 receptor PLC-PKC signaling during stress has been found to prolong serotonergic regulation of GABAergic inhibitory neurotransmission in prefrontal cortical pyramidal neurons.41 CRF2 receptor PLC-PKC signaling can also potentiate NMDA receptor activation of burst firing in ventral tegmental dopamine neurons, which, in turn, stimulates dopaminergic neurotransmission in the amygdala, nucleus accumbens, and prefrontal cortex.42
ERK-MAPK Pathway
Although CRF receptors can activate ERK, p38, and Jun N-terminal kinase MAPK cascades, ERK-MAPK is the preferred signaling cascade for CRF receptors.9,19,43 Because CRF2 receptors select the ERK-MAPK signaling cascade when activated by UCN2 or UCN3 but not CRF in transfected CHO cells,44 differences in agonist-induced conformations of CRF2 receptors may govern whether or not ERK-MAPK signal transduction occurs. A PKA mechanism has been shown to regulate CRF1 receptor-mediated activation of the ERK-MAPK cascade and ERK-dependent Elk1 transcription in some but not all cells in which B-Raf expression is high.43 Paradoxically, UCN1-stimulated ERK-MAPK activation in HEK293 cells was markedly decreased by PKA phosphorylation of Ser301 in the IC3 loop of the CRF1 receptor.45 Both CRF receptors can also signal via the ERK-MAPK cascade by activating the Ras-cRaf-MEK pathway. Other research has shown that a PI-3 kinase-dependent mechanism can contribute to CRF1 and CRF2 receptor-mediated ERK activation in several brain- and peripheral-derived cell lines.44,46,47 epidermal growth factor (EGF) receptor transactivation may also play a role in CRF1 receptor ERK-MAPK signaling.46 Very little is known, however, regarding cellular and molecular factors governing the CRF1 or CRF2 receptor’s preference for a specific upstream regulator of ERK-MAPK signal transduction. Interestingly, CRF1 receptor ERK-MAPK signaling can activate Sp-1 and Ap-2 transcription, thereby upregulating expression of GRK3, which mediates homologous desensitization of CRF1 receptors.48 This process may be impaired in anxiety and depressive disorders. Excessive CRF1 receptor signaling via the ERK-MAPK cascade in the hippocampus has been found to contribute to the “depressive” phenotype of the CRF2 receptor knockout mouse.49 In mice with a targeted deletion of forebrain CRF1 receptors, anxiety-like behavior and ERK-MAPK signaling in the extended amygdala and hippocampus stimulated by central CRF administration were abolished.43 The ERK-MAPK signal transduction cascade has been shown to regulate synaptic plasticity events including dendrite stabilization; ion channel transmission; transcription of CREB and other genes; and receptor scaffolding, trafficking, and crosstalk. CRF1 receptor ERK-MAPK signaling can stimulate trafficking of ionotropic glutamate δ2 receptors to dendrites and regulate glutaminergic neurotransmission at amygdalar synapses.50 The deleterious, high-allostatic load-inducing effects of brain CRF hypersecretion in affective disorders may result from sensitized CRF1 receptor signaling via the ERK-MAPK cascade, thereby inducing “stressed synaptic function” and an imbalance between excitatory and inhibitory neurotransmission.50
GRK and Arrestin Mechanisms Regulating G Protein–Coupled Receptors
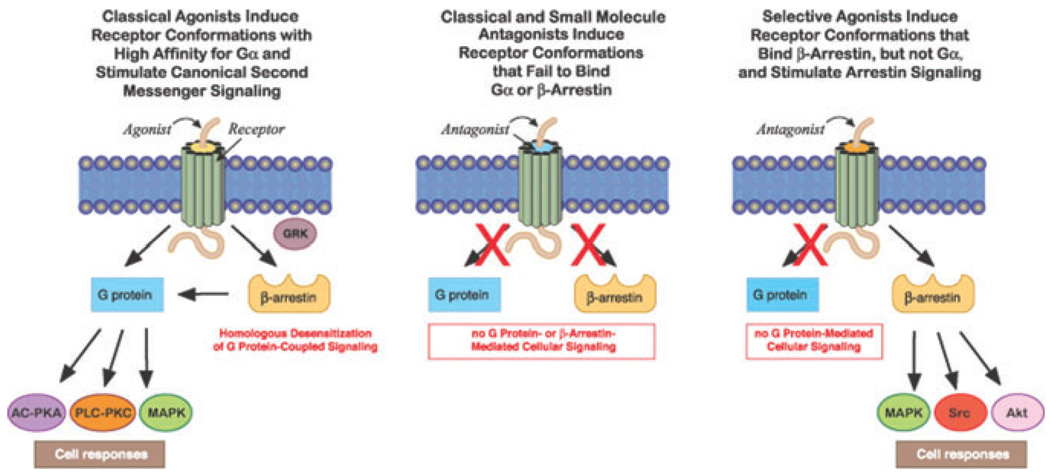
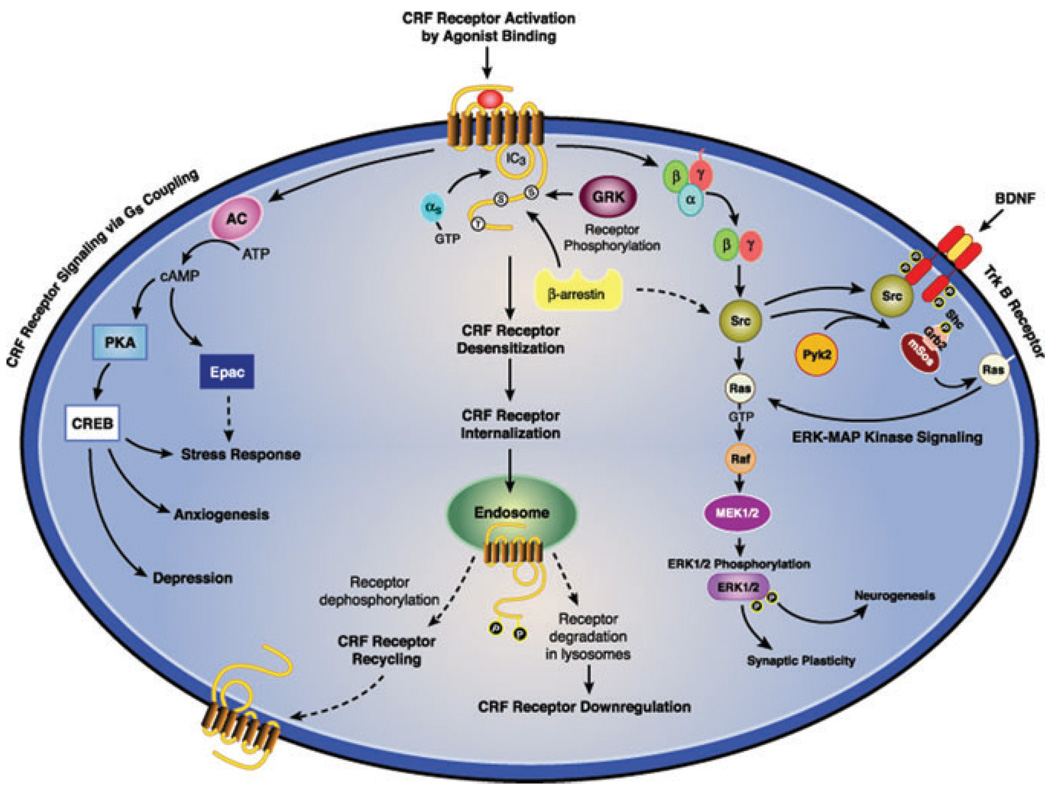
The magnitude, duration and specificity of cellular signaling by G protein–coupled receptors (GPCRs) depends upon rapid and stringent regulation to prevent deleterious effects of unrestrained, excessive GPCR signal transduction. While agonist binding causes GPCRs to assume “active” conformations, which initiate signaling by prompting receptors to bind to Gα after its dissociation from βγ subunits, specific GRKs are rapidly recruited to commence homologous desensitization by phosphorylating a specific pattern of serines and threonines in the GPCR’s IC3 and/or C terminus (Fig. 1).51–54 GRK-catalyzed phosphorylation of the receptor protein immediately increases the GPCR’s affinity for β-arrestins ~30-fold, triggering their translocation from the cytosol to the cell surface.51–56 Receptors assuming an agonist-bound conformation expose phosphorylation-independent sequences that also contribute to the binding of arrestin to GPCRs. Rapid, high-affinity binding of a single arrestin sterically uncouples the receptor from its cognate Gα protein, thereby “arresting,” or terminating, signal transduction by competition and steric hindrance.51,52
Figure 1.
Effect of agonists and antagonists on receptor conformations and G protein–coupled signal transduction. Homologous desensitization rapidly regulates signaling after classical agonists activate GPCRs. Antagonists inhibit Gsα coupling and β-arrestin recruitment. Certain agonists selectively stimulate β-arrestin-biased signal transduction. (In color in Annals online.)
In addition to terminating receptor signaling, the nonvisual arrestins β-arrestin1 and β-arrestin2 simultaneously bind to clathrin and β(2) adaptin, acting as adaptor proteins for the formation of clathrin-coated vesicles into which the ligand-receptor-arrestin complex is internalized.51–55 Arrestins do not always traffic, however, with the desensitized receptor from the cell surface into cytosolic endosomes. The temporal stability of the receptor-arrestin complex distinguishes two patterns. Class A GPCRs form transient complexes with arrestins that dissociate at or near the plasma membrane while class B GPCRs form stable complexes with arrestins that internalize as a unit into endocytic vesicles.58,59 After endocytosis occurs, internalized receptors are dephosphorylated in endosomes by specific phosphatases, and vesicles containing dephosphorylated receptors bud off from endosomes, thereby recycling resensitized GPCRs back to the plasma membrane.51–53 Differences in the stability of the receptor-arrestin interaction have been shown to regulate the rate of receptor resensitization. During very prolonged exposure to high agonist concentrations, internalized GPCRs do not recycle to the membrane but move into lysosomes, where they undergo proteolytic degradation resulting in downregulation to decrease the total number of cellular receptors.51–53
While binding of a classical agonist to a receptor initiates G protein–mediated second messenger signaling and activates GRK- and arrestin-mediated regulation, GPCRs assume a conformation after the binding of an antagonist that is incapable of coupling to Gα or recruiting GRKs or β-arrestins (Fig. 1). Although classical GPCR theory proposes that ligands activate GPCR signaling to varying degrees based on their range of intrinsic efficacies, “biased agonists” induce and stabilize distinct receptor conformations with functional selectivity for specific signal transduction pathways.55,56 Arrestins play an important role in initiating G protein–independent signaling events (Fig. 1).55,60 Specialization of GRK and β-arrestin actions has been suggested by the observation that an individual GRK selectively phosphorylates only certain serines or threonines thereby inducing a receptor–β-arrestin conformation that has specific functional consequences.51,53 In this regard, GRK2 and GRK3 phosphorylation facilitates arrestin recruitment leading to homologous desensitization while GRK5 and GRK6 can promote β-arrestin2–mediated ERK-MAPK signaling.60 These new GPCR concepts may lead to novel pharmacotherapies for medical and psychiatric illnesses that have signaling pathway–selective targeting.
Regulation of CRF Receptor Signaling
CRF1 Receptors
Homologous Desensitization of CRF1 Receptors
Endogenously expressed CRF1 receptors become desensitized in brain-derived, anteriorpituitary, and myometrial cells, and in recombinant cell lines during exposure to high concentrations of CRF or UCN1, which is a setting favoring strong GRK action.61–68 Although UCN1 binds with a slightly higher affinity than CRF, there are no significant differences in rate or magnitude of CRF1 receptor desensitization elicited by these two ligands.65
GRKs and CRF1 Receptor Desensitization
Of the five members of the GRK family expressed in stress-sensitive and CRF receptor– expressing neurons of the forebrain and extended amygdala, GRK3 and GRK6 have been shown to contribute to the homologous desensitization of CRF1 receptors.64,65,67 Reducing cellular levels of GRK3 protein by ~55% with uptake of a GRK3 antisense oligonucleotide or transfection with a GRK3 antisense construct inhibited homologous CRF1 receptor desensitization ~65% in Y79 cells.64 During prolonged exposure to high CRF, the levels of GRK3 mRNA and protein significantly increase in Y79 and CATH.a cells endogenously expressing CRF1 receptors.48,65 GRK3 upregulation may maximize phosphorylation of intracellular serines and threonines in order to more strongly desensitize the CRF1 receptor during CRF hypersecretion. In transfected HEK293 cells, cellular overexpression of GRK3 promotes a more rapid desensitization of Gs-coupled CRF1 receptor signaling during CRF stimulation (Hauger et al., unpublished data). When HEK293 cells recombinantly expressing CRF1 receptors are acutely stimulated with CRF, GRK3 and GRK6 rapidly translocate from cytosol to the cell membrane.67 In addition, pretreatment of HEK293 cells with antibodies targeting GRK3 or GRK6 suppresses homologous CRF1 receptor desensitization.67 Homologous CRF1 receptor desensitization can be inhibited, however, by transfecting the GRK2 dominant negative mutant, GRK2-K220R, into AtT-20 pituitary corticotroph tumor cells, which is a cell line expressing protein levels of GRK2 and GRK6 but not GRK3.68 Furthermore, GRK2 expression upregulates in anterior pituitary cells during prolonged CRF exposure.68 Although these findings suggest that GRK2 can desensitize corticotroph CRF1 receptors, overexpressing GRK2 in AtT-20 cells did not augment CRF-induced CRF1 receptor desensitization.68
CRF1 Receptor Phosphorylation
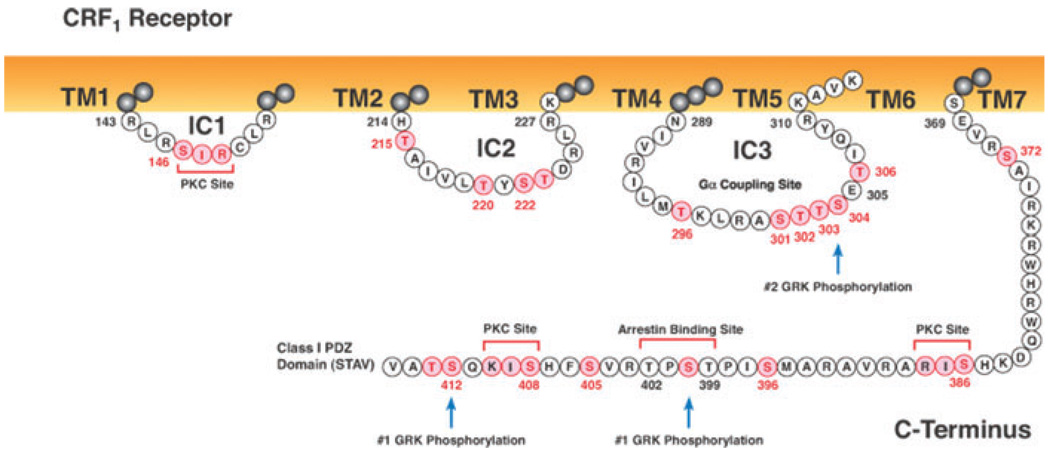
CRF1 receptors become rapidly phosphorylated in the presence of a saturating concentration of CRF during homologous desensitization.40,57,67,69,70 Determining sites in a GPCR’s ICs and C terminus that are phosphorylated by specific GRKs and then mediate β-arrestin recruitment and binding provides important information about receptor regulation. Seven serines and three threonines represent possible GRK phosphorylation sites in the CRF1 receptor’s C terminus, including a T399S400P401T402 motif that may function as a β-arrestin binding site (Fig. 2). CRF-stimulated phosphorylation of a CRF1 receptor truncated at Ser412 (Δ412 mutant) was decreased ~35% compared to that of the wild-type CRF1 receptor. CRF-induced phosphorylation was 80–90% less, however, for a CRF1 receptor truncated at Ser386 (Δ386 mutant), which deleted the TPST motif including Thr399, which may be phosphorylated by a GRK during agonist activation.57 CRF-stimulated CRF1 receptor phosphorylation and desensitization was also reduced ~50% when only Thr399 is mutated.67 These findings suggest that GRK phosphorylation sites in the CRF1 receptor C terminus are located at Ser412 and/or Thr413, Thr399, and possibly at Ser386, Ser396, Ser400,Thr402, Ser405, and/or Ser408 (Fig. 2). Because GRK2 and GRK3 are acidotropic kinases,51 the serine-threonine cluster with a glutamic acid in the CRF1 receptor IC3 (Ser301Thr302Thr303Ser304Glu305Thr306) is a potential GRK phosphorylation site. Substituting alanines for the STTSET motif (IC3– 5ST/A) caused a 75% decrease in CRF-stimulated phosphorylation of this mutant compared to that of the wild-type CRF1 receptor.57 However, reductions in CRF-stimulated phosphorylation were similar for the Δ386 mutant and a CRF1 receptor mutant with the IC3 mutation added to the Δ386 truncation. Thus, Gs-coupled CRF1 receptor signaling is regulated by hierarchical phosphorylation that first occurs in the C terminus and then occurs in the IC3’s STTSET motif.
Figure 2.
Intracellular sequences of the human CRF1 receptor. The full-length wild-type human CRF1 receptor is a 415 amino acid–long protein. Serines and threonines are highlighted in pink as potential sites for GRK or PKC phosphorylation. Important motifs in the C terminus include a potential arrestin binding site (T399S400P401T402) and a class I PDZ binding domain (S412T413A414V415) that may regulate CRF1 receptor interactions with signaling proteins. Agonist-induced phosphorylation of C-terminal motifs is required for translocation of β-arrestin2 to the membrane and hierarchical phosphorylation of the IC3’s STTSET motif. Arrestin recruitment may also be promoted by the basic His214 and the adjacent hydrophobic sequence (A216I217V218L219). (In color in Annals online.)
βarrestins and CRF1 Receptor Regulation
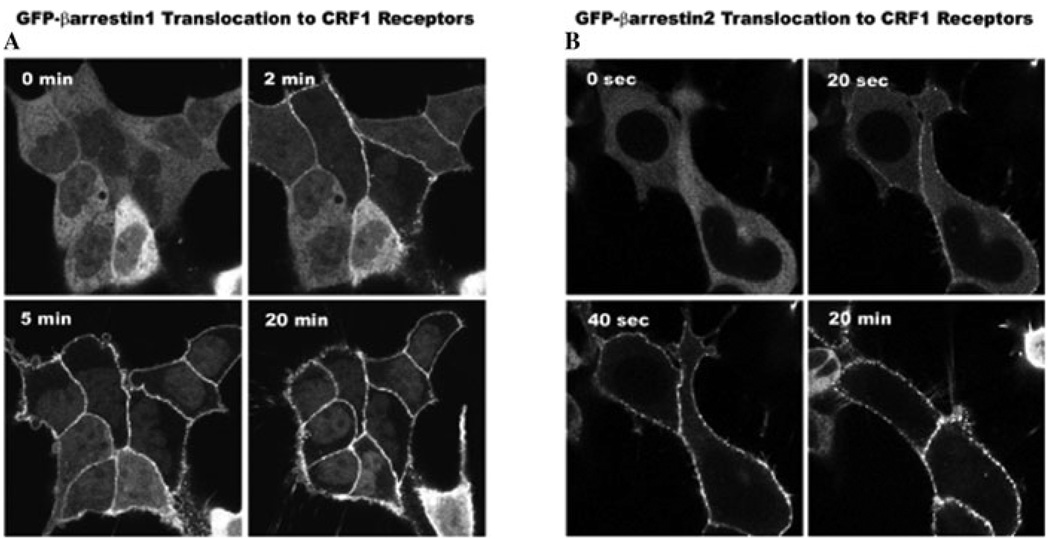
CRF1 receptors preferentially recruit β-arrestin2 over β-arrestin1 to membranes of transfected HEK293 cells (Fig. 3) and fetal mouse cortical neurons.57,71 Because neither β-arrestin isoform traffics with the receptor into cytosolic endosomes (Fig. 3), CRF1 receptor interaction with arrestins is typical of “class A” GPCRs.57–59 However, in fetal cortical neurons, a small proportion of β-arrestin2 becomes co-localized with CRF1 receptors that internalize within endocytic vesicles during prolonged CRF stimulation.71 Comparison of β-arrestin2–CRF1 receptor interactions in neuronal and non-neuronal cells requires further study. Interestingly, agonist-activated CRF1 receptors promote one of the strongest translocation responses of β-arrestin2 from cytosol to cell surface observed for studied class A GPCRs.57 A portion of phosphorylated GPCRs appear to remain at the membrane, where they can rapidly bind again to β-arrestin2 if they are re-stimulated by agonist.54 The swift return of β-arrestin2 to phosphorylated receptors after they re-bind agonist may be particularly important for brain CRF receptors during recurrent exposure to stress and, if this regulatory mechanism fails, may result in severe anxiety and possibly depression.
Figure 3.
Recruitment of β-arrestins by the agonist-activated CRF1 receptor. Confocal images depict the translocation of cytosolic β-arrestin1-GFP (A) or β-arrestin2-GFP (B) to membrane CRF1 receptors recombinantly expressed in HEK293 cells. A slower redistribution of β-arrestin1 to the cell surface occurred after (2-, 5-, 20-min) treatment with 200 nM CRF while a dramatically more rapid and robust recruitment of β-arrestin2 developed in cells after (20 sec, 40 sec, 20 min) treatment with 200 nM CRF.
While truncating the CRF1 receptor at Ser412 does not alter β-arrestin2 translocation to the membrane CRF1 receptor, truncating its C terminus at Ser386 significantly impairs β-arrestin2 recruitment due to the near absence of agonist-dependent phosphorylation.57 Because reducing cellular GRK3 expression or blocking GRK3 action inhibits homologous desensitization of CRF1 receptors,64,67 the TPST motif may be phosphorylated preferentially by GRK3, thereby transforming this sequence into an active site for β-arrestin binding. When the CRF1 receptor with a mutated STTSET in the IC3 was activated by binding CRF, β-arrestin2 translocation to the cell membrane was as robust and rapid as that produced by CRF activation of the wild-type CRF1 receptor, despite the IC3–5ST/A mutant exhibiting more than a 90% decrease in CRF-stimulated phosphorylation.57 In addition, β-arrestin2 recruitment was reduced to a similar extent in the Δ386 CRF1 receptor mutant and the CRF1 receptor with both the C-terminal Δ386 truncation and IC3 STTSET mutation.57 Thus, recruitment and binding of β-arrestin2 by the agonist-activated CRF1 receptor is mediated by at least two distinct domains: (i) a phosphorylation-dependent motif in the C-terminus, and (ii) a phosphorylation-independent motif in one or more of the ICs. Although GRK3 is involved in homologous desensitization of CRF1 receptors, GRK3 over-expression did not increase CRF1 receptor recruitment of β-arrestin2 or prevent dissociation of the CRF1 receptor–arrestin complex.57
CRF1 Receptor Internalization and Resensitization
Prominent internalization of membrane CRF1 receptors occurs in AtT-20 pituitary, fetal cortical, and recombinant cells during a 30-min treatment with a saturating concentration of CRF.57,70–72 CRF1 receptor internalization was strongly inhibited by hypertonic sucrose or the dynamin dominant negative mutant K44A.57,71 A PKA or PKC inhibitor, the caveolae disruptor filipin, or a dominant negative caveolin 1 failed to alter internalization of CRF1 receptors.70,72 CRF-stimulated CRF1 receptor internalization increased ~three-fold in COS-7 cells overexpressing β-arrestin1 or β-arrestin2.57 Consequently, both clathrin and arrestin mediate the internalization of CRF1 receptors into early endosomes. The transient interaction of β-arrestin with class A receptors can promote their more rapid recycling and resensitization by facilitating receptor dephosphorylation.59 Consistent with a class A receptor-arrestin interaction, CRF1 receptors in anterior pituitary and HEK293 cells resensitize from agonist-induced desensitized states within 1–2 h.61,67,71 A much slower time course requiring 1 day for complete restoration of CRF1 receptor cyclic AMP signaling after homologous desensitization, however, has been observed in brain-derived Y79 and IMR-32 cells.63,66 These divergent results may be explained by Y79 cells expressing β-arrestin1 but not β-arrestin2, while pituitary and HEK293 cells express both β-arrestins.57 Internalization and resensitization of CRF1 receptors may also be controlled by the Ras-like small GTP binding proteins Rab4 and Rab5.71
CRF1 Receptor Downregulation
When Y79 retinoblastoma or transfected CHO-K1 cells are exposed to CRF for 24 h, the number of membrane CRF1 receptors and the maximal response of CRF-stimulated cyclic AMP accumulation decreased 70–80%, presumably due to proteolytic degradation of receptors.63,70
Second Messenger Kinases and CRF1 Receptor Desensitization
Gs-coupled GPCRs can also be desensitized by phosphorylation of serines or threonines within intracellular PKA consensus sites.51,52 In many cells, CRF1 receptors preferentially signal by coupling to Gsα and activating the cyclic AMP-PKA pathway.9,16–19 Although Ser301 is located within a potential PKA phosphorylation site (RAS) in the IC3 where Gsα presumably binds (Fig. 2), neither maximal PKA stimulation nor PKA inhibition influenced CRF1 receptor phosphorylation and desensitization in Y79 and IMR-32 cells.63,66 CRF1 receptor signaling via the ERK-MAPK cascade, however, was markedly decreased by PKA-induced phosphorylation of Ser301.45
The CRF1 receptor has potential PKC phosphorylation sites at the IC1’s Ser146 and C terminus’s Ser386 and Ser408 (Fig. 2). In Y79 cells, PKC activation rapidly and strongly desensitized and phosphorylated CRF1 receptors, an effect that was blocked by inhibiting PKC or by downregulating α and β isoforms of PKC.40 In human myometrial cells, Gq-coupled oxytocin receptor signaling heterologously desensitized Gs-coupled CRF1 receptor signaling by a PKC mechanism.19 Since CRF1 receptors can signal via the PLC-PKC pathway, PKC may homologously desensitize CRF1 receptors in certain cells.
CRF2 Receptors
Agonist-selective CRF2 Receptor Desensitization and Internalization
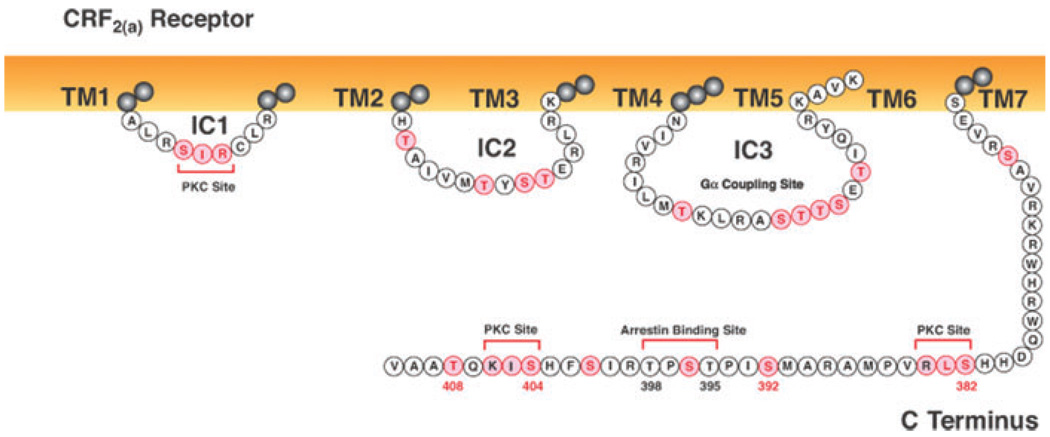
There is considerably less information about regulation of CRF2 receptor signaling. A very rapid and large (~90%) homologous desensitization of CRF2(a) receptors during brief UCN2 exposure was first reported in human retinoblastoma Y79 cells, which were found to express endogenously CRF2(a) receptors in addition to CRF1 receptors.73 Strong homologous desensitization and internalization of the human CRF2(b) receptor, the peripheral splice variant of the CRF2(a) receptor, was subsequently observed in transfected HEK293 cells stimulated with UCN2.74 Unlike the CRF1 receptor, the CRF2 receptor binds and is activated by agonists with a broad range of potencies. Desensitization of CRF2(a) receptor cyclic AMP signaling was faster and stronger when Y79 cells were exposed to UCN2 compared to UCN3, while CRF was a relatively weak desensitizing agonist.73 Similarly, recombinantly expressed CRF2(b) receptors desensitized more rapidly in transfected HEK293 cells treated with UCN2 compared to CRF.74 CRF2(b) receptor internalization was also greater when HEK293 cells were exposed to UCN2 compared to CRF.74 Knocking down clathrin heavy chain protein levels in HEK293 cells by siRNA transfection reduced UCN2-induced desensitization and internalization of CRF2(b) receptors.74 When the last four amino acids of the CRF2(b) receptor C terminus were deleted or mutated to four alanines (Fig. 4), the rate of CRF2(b) receptor internalization was accelerated during 5-min UCN2 stimulation. Interestingly, in vivo activation of CRF1 receptors by prior severe stress trafficks more CRF2(a) receptors to the membrane of DRN neurons, thereby switching from CRF1 receptor–mediated inhibition to CRF2(a) receptor–mediated stimulation of DRN sertonergic neurotransmission.75
Figure 4.
Intracellular sequences of the human CRF2(a) receptor. The full-length wild-type CRF2(a) receptor protein is 411 amino acids in length. Potential sites for GRK or PKC phosphorylation, or arrestin recruitment are highlighted in pink. The CRF2(a) receptor C-terminal tip does not contain a PDZ domain. (In color in Annals online.)
β-arrestins and CRF2 Receptor Regulation
β-arrestin2 was recruited more rapidly than β-arrestin1 by the agonist-activated CRF2(b) receptor in HEK293 cells stimulated with UCN2 or CRF for 2 min.74 High membrane levels of βarrestin2 persisted during a 15-min treatment with CRF but not UCN2.74 Similar to the CRF1 receptor, β-arrestin2 dissociated from the CRF2(b) receptor at the membrane with β-arrestin2 then remaining near the cell surface while the CRF2(b) receptor internalized characteristic of class A GPCR-arrestin interactions.74 CRF2(b) receptor desensitization and internalization decreased 50% and 80%, respectively, in HEK293 cells overexpressing the β-arrestin (319–418) dominant negative mutant.74 Conversely, decreasing cellular levels of β-arrestin1 or β-arrestin2 by siRNA transfection significantly reduced UCN2-induced desensitization and internalization of CRF2(b) receptors, although knockdown of β-arrestin1 was more effective than β-arrestin2.74 Intracellular structural determinants (Fig. 4) for GRK phosphorylation and β-arrestin recruitment remain to be determined.
Differential Regulation of CRF Receptors
Surprisingly, following binding and activation by UCN2, CRF2(a) and CRF2(b) receptors desensitize more rapidly and to a greater extent than the rate and magnitude of homologous desensitization of CRF1 receptors by CRF (Fig. 5).63,67,73,74 A more rapid homologous desensitization of neuronal CRF2(a) receptors would be expected to reduce the hypothesized CRF2(a) receptor counter-regulation of anxiogenic and stress response–promoting actions of CRF1 receptor signaling. The CRF1 and CRF2 receptors differ mostly in their N-terminal extracellular domains. Different conformational changes assumed by agonist-activated CRF1 and CRF2 receptors may influence the velocity and extent of GRK-mediated phosphorylation and desensitization. With regard to intracellular structural determinants, the ICs loops and C termini of the two CRF receptors are highly homologous (~90% sequence conservation), although the last four amino acids in their C-terminal tails differ (Figs. 2 and 4). The CRF1 receptor contains a type 1 PDZ domain but the CRF2 receptor does not. When PDZ domains are selectively phosphorylated by certain GRKs or bind PSD-95 or other regulatory proteins, GPCR signaling via the ERK-MAPK cascade or GPCR internalization and recycling can be modulated.76 Desensitization of cyclic AMP signaling and recruitment of β-arrestins were not altered, however, by deleting or mutating the last four amino acids of the CRF2(b) receptor or making a chimeric CRF2(b) receptor by replacing its TAAV motif with the CRF1 receptor’s PDZ domain (STAV).74 Deleting the STAV motif of the CRF1 receptor C terminus decreased phosphorylation by ~35%, consistent with Ser412 and/or Thr413 being GRK phosphorylation sites, but did not effect the strong recruitment of β-arrestin2 or internalization of agonist-activated CRF1 receptors.57 Since deletion or mutation of the CRF2(b) receptor’s TAAV motif inhibited signaling via the ERK- and p38 MAPK cascades but not the cyclic AMP-PKA pathway,74 the dissimilar last four amino acids of the two CRF receptors may have other important functions. Alternatively, differences in regional brain expression of GRKs and β-arrestins may mediate differential rates of homologous desensitization of CRF1 and CRF2(a) receptors. Neurons in the BNST express both CRF receptors; β-arrestin2 (but not β-arrestin1); and GRK2, GRK3, and GRK6.9,22,23 CRF2 (but not CRF1) receptors are expressed in the LS where only β-arrestin1 and high GRK5 levels are present. Basolateral amygdalar neurons express CRF1 receptors (but not CRF2 receptors); high levels of both β-arrestins; and GRK2, GRK3, and GRK6.9,22,23 Newly cloned GPCR regulators, such as ARTs (arrestin-related ubiquitin-ligase adaptors), that govern endocytosis of membrane protein cargoes77 may also control the ratio of membrane versus internalized receptor proteins, thereby promoting or inhibiting CRF1 and CRF2 receptor signaling.
Figure 5.
Major intracellular signal transduction pathways for CRF1 and CRF2 receptors. While the dominant mode of signaling for both CRF receptors signaling is Gs-coupled AC-PKA cascade, they may also signal via the PLC-PKC and ERK-MAPK cascades. CRF receptor AC-PKA signaling is stringently regulated by GRK- and arrestin-mediated homologous desensitization. Although the mechanisms regulating CRF receptor ERK-MAPK signaling are not yet fully understood, this pathway may potentiate BDNF-stimulated TrkB receptor function. (In color in Annals online.)
CRF Hypothesis of Depression
Abnormal CRF neurotransmission and CRF1 receptor signal transduction has been proposed to be a critical mechanism for stress pathophysiology that leads to major depression. Markers of hyperactive brain CRF neurotransmission, including abnormally high cerebrospinal fluid (CSF) levels of CRF and aberrant functioning of the HPA axis, are present in major depression.5,8–10 CSF CRF levels are highest in depressed suicide victims, indicating that an increasing level of CRF hypersecretion may be associated with greater illness severity.78 Postmortem studies have also detected significant increases in CRF mRNA expression and CRF-immunoreactive neurons in the hypothalamic PVN, prefrontal and frontal cortex, locus coeruleus, and median and DRN in major depression.9,10,79 HPA hyper-responsiveness may be a genetic susceptibility marker for depression based on data showing excessive secretion of cortisol following a dexamethasone-CRF challenge in 32% of first-degree relatives of patients with unipolar or bipolar depression.10 A GAG haplotype of the CRF1 receptor was found to be significantly associated with a stronger antidepressant response in major depressive patients with high anxiety.80 Another CRF1 receptor single nucleotide polymorphism (SNP), rs4792887 in the 5′ part of intron 1, has been found in depressed men who made suicide attempts during stress.81 Interestingly, individuals homozygous for alleles TT (SNP rs7209436) or AA (SNP rs242940) in the CRF1 receptor intron 1 were protected against adult major depression despite having been traumatized by childhood abuse.82 Small molecule CRF1 receptor antagonists are now being tested as novel anxiolytic and antidepressant treatments. The first clinical trial of CRF1 receptor antagonist (NBI-39775) significantly reduced depression and anxiety in patients with a major depressive episode during a small, open-label study.8,10 Treatment of nondepressed control subjects with CRF1 antagonist NBI-34041 inhibited HPA but not emotional responses to the Trier social stress test, suggesting that this compound possesses stress-protective properties.10 A recent double-blind, placebo-controlled trial failed, however, to detect an antidepressant action of a different CRF1 receptor antagonist (CP-316,211) in depressed patients.83 Difficulties with suboptimal pharmacokinetics and bioavailability as well as toxicity of highly lipophilic small molecules has complicated their effectiveness and refinement. In addition, anxiolytic and antidepressant efficacy may now be improved based on the recent finding that small molecules bind to critical sites within transmembrane-spanning domains, thereby promoting allosteric modulation of the CRF1 receptor into a stabilized conformation incapable of binding Gα to initiate signal transduction.84 Furthermore, because CRF1 receptor antagonism is most effective in reducing anxiety-like behavior in animal models with high “trait” anxiety-like behavior or involving previous exposure to stress,13 antidepressant action of CRF1 receptor antagonists may be strongest in patients with stress-induced major depression and considerable comorbid generalized anxiety. Recently, UCN3 expression was found to be strongly upregulated in the prefrontal cortex of depressed suicide victims, providing the first evidence for CRF2 receptor dysregulation in major depression.85 Abnormal signaling by brain CRF2 receptors may also contribute to the onset and recurrence of severe anxiety and depression, but bioactive small molecule CRF2 receptor antagonists have not yet been synthesized.
Controversies in Models of CRF Receptor Mechanisms in Depression
Role of CRF1 Receptors in Anxiety-like Responses to Stress
Brain CRF1 receptor signaling induces many anxiety-like defensive behaviors during stress.1,11–15,86–88 Furthermore, increased anxiety-like defensive behavior is the dominant phenotype of transgenic mice with global CRF overexpression, which also causes a Cushing’s syndrome-like phenotype.12 The high level of anxiety-like behavior in the CRF overexpression mouse can be reversed by a CRF1 receptor antagonist.12 Pharmacological antagonism or genetic knockout of brain CRF1 receptors in rodents strongly inhibits anxiety-like and HPA responses to threatening stimuli or stress.1,10–13,87,88 Anxiety-like defensive behavior is also markedly decreased in mice with a conditional knockout of brain CRF1 receptors but normal expression of anterior pituitary CRF1 receptors and HPA responses to stress.13
CRF1 Receptors and Animal Models of Depression
Animal models of depressive-like behaviors, such as the Porsolt forced swim, tail suspension, and learned helplessness tests, exhibit sensitivity to clinically effective antidepressant medications and treatments that inhibit monoamine reuptake or alter monoaminergic neurotransmission.89 These paradigms, however, have been surprisingly inconsistent in delineating the importance of CRF receptor signaling in depressive-like behaviors. The Porsolt swim test is a model of “behavioral despair” in which animals are forced to swim in an inescapable environment, with time spent immobile versus time engaged in active escape or “stress coping” behaviors considered to be an operational measure of “depressive-like” behavior. Intracerebroventricular administration of CRF1 receptor-preferring agonists cortagine and ovine CRF immediately or 1 day before testing unexpectedly reduced immobility in the forced swim test, indicating an antidepressant-like activity.90 Similarly, normal mice exposed to restraint stress, or transgenic mice with CNS- or forebrain-specific overexpression of CRF exhibit decreased immobility in the Porsolt swim test.91 Because their reduced immobility could be reversed by acute treatment with the CRF1 receptor antagonist DMP696, this phenotype was not due to chronic CRF hypersecretion. More likely, it resulted from acute activation of CRF1 receptor signaling when mice were forced to swim.91 Thus, acute release of brain CRF appears to increase escape behaviors and active “stress coping” in the Porsolt swim test.90 The CRF1 receptor antagonist CP-154,526 decreased immobility in rats subjected to the forced swim test; however, this effect was not replicated in a later study.92 Other CRF1 receptor antagonists (CRA0450, R121919) were also ineffective in reducing immobility during forced swimming.93,94 CRF1 receptor antagonists R121919 (NBI-30775) and DMP 696, but not CRA0450, have exhibited antidepressant-like activity in the tail suspension test, another paradigm of “behavioral despair” in which depressive-like behavior is measured as the duration of immobility of a mouse or rat suspended by its tail.94,95 These findings may be explained in part by differences in mouse and rat behavioral responses resulting from different evolutionary adaptations of each species.96
Another model of depression is the learned helplessness paradigm, in which animals experience a series of inescapable shocks. This experience produces a long-term reduction in escape behavior in tasks with escapable shock and increased fear learning. Learned helplessness behavior can be reversed by treatment with many antidepressants.97 CRF1 receptor antagonism by antalarmin attenuates learned helplessness–induced increases in fear learning, while blocking CRF1 receptor signaling by acute or chronic treatment with CRA0450 increases escape behavior in this paradigm.94,98 However, CRF1 receptor activation has been shown to promote consolidation of fear memories. Consequently, CRF1 receptor antagonism may attenuate induction of fear memories rather than exert direct effects on learned helplessness behavior per se.98,99 Often, interpreting positive efficacy in these animal models of antidepressant activity has been confounded by administering the CRF1 receptor antagonist before or immediately after presentation of the stressor.1,92,98 CRF1 receptor antagonism may only be effective in preventing excess CRF1 receptor activation during or immediately after stress. For example, CRF1 receptors are rapidly desensitized via internalization in the locus coeruleus, which may alter neuronal responses to subsequent CRF1 receptor activation.100 Hence, once stress has presumably triggered strong CRF1 receptor signaling, it is possible that resulting long-term depressive-like behavior switches to a CRF1 receptor-independent mechanism.
CRF2 Receptors and Animal Models of Anxiety and Depression
The role of CRF2 receptors in stress responsiveness and anxiogenesis has been controversial; however, there is mounting evidence that CRF2 receptors contribute to coordinating stress behaviors. Mice with a constitutive CRF2 receptor gene deletion exhibit either increased or normal anxiety-like defensive behaviors basally or during stress as measured by approach-avoidance conflict paradigms in the elevated plus maze and open field tests.1,9,11–13 Compensatory changes in CRF1 receptor signaling, however, may complicate the interpretation of behavioral phenotypes in CRF2 receptor knockout mice. Nonetheless, because the duration of stress-stimulated ACTH secretion is decreased in CRF2 receptor knockouts and even more reduced in mice with deletions of both CRF1 and CRF2 receptor genes,1,9,11–13 CRF2 receptor signaling may be involved in maintenance of neuroendocrine responses to stress.
Pharmacological studies have also yielded mixed results, with global pharmacological blockade of CRF2 receptors being reported to both increase and decrease anxiety-like behaviors.1,9,11–13 These data are in contrast to clear anxiogenic effects when CRF2 receptors in LS or dorsal raphe are activated.9 In contrast to nonselective CRF receptor agonism, selective agonism of CRF2 receptors with UCN2 or UCN3 has been shown to exert anxiolytic activity in some approach avoidance tasks. 9,11–13,101 However, selective CRF2 receptor activation has also been found to suppress exploration and certain locomotor behaviors that could confound interpretation of anxiety-like behavior in these experiments.9,11–13,101
A recent study has found evidence for CRF2 receptors interacting with brain systems mediating dysphoria. Mice trained to associate an odor cue with forced swimming avoided the odor in other contexts.102 This conditioned avoidance was abolished if mice were treated with a CRF2 receptor antagonist during training, while this treatment was ineffective in blocking associative learning to positive reinforcement.102 Selective CRF2 receptor agonism alone induced conditioned aversion, indicating CRF2 receptor signaling mediated this aversive or negative state.102 The dysphoric-like action of CRF2 receptors was associated with the downstream release of dynorphin, which, in turn, activated κ-opioid receptor signaling. In humans, κ-opioid receptor agonists produce significant anhedonia and depressive symptoms.102 Therefore, these data implicate a novel role for CRF2 receptor mediation of stress-induced dysphoria. Acute central injection of CRF also increases intracranial reward threshold, a model of the major depressive symptom anhedonia,103 although it is not certain whether a CRF1 or CRF2(a) receptor mechanism is involved.
In the forced swim model, CRF2 receptor– selective agonists UCN2 and UCN3 exert antidepressant-like effects while the nonselective agonist UCN1 had no effect.104 These data suggest that activation of CRF1 receptors may counteract the antidepressant-like action of CRF2 receptor signaling.104 Consistent with this hypothesis, CRF2 receptor null mutation mice exhibit increased immobility and reduced climbing behavior in the forced swim test, which is reversed by antagonizing CRF1 receptors or inhibiting CRF1 signaling via the MAPK/ERK pathway.1,49 These data are puzzling, as stress or selective pharmacological activation of CRF1 receptors in normal mice also can reduce immobility.90 It should be noted that mice with a CRF2 receptor knockout null mutation exhibit increased central CRF1 receptor expression that may result in chronically excessive signaling of brain CRF1 receptors.1 Thus, the relative balance of CRF1 and CRF2 receptor activation may be critical to the manifestation of active or passive behavior in the mouse swim stress model.
In the learned helplessness model, CRF2(a) receptor activation at the dorsal raphe is required and sufficient for the long-term effects of inescapable shock on fear learning and escape behavior, implicating this mechanism in long-term effects of uncontrollable stress.14 A possible mechanism mediating learned helplessness may be CRF2(a) receptor signaling in the dorsal raphe, exciting serotonergic neurotransmission in limbic and forebrain projections.15 Dorsal raphe CRF2 receptor activation, however, may influence fear learning rather than induce learned helplessness per se, as global pharmacological blockade of CRF2 receptors by selective antagonists, or global CRF2 receptor knockout in mice by null mutation blocks contextual fear learning.88,105 Interestingly, selective activation of septal CRF2 receptors has the opposite effect, inhibiting contextual fear memory.105 Indeed, in the last 5 years, with site-specific activation studies, behavioral effects of CRF2 receptor-selective ligands have been found to depend greatly on the brain region activated. CRF2(a) receptors in certain stress-sensitive brain regions may also interact with extended amygdalar CRF1 receptors in an additive manner to generate anxiety and depression. Thus, the pharmacology of predicting clinical effects of global CRF2 receptor activation or inhibition has become much more complex than originally anticipated. As indicated above, CRF2 receptors modulate contextual memory, learned helplessness, and conditioned aversion; thus, there is mounting evidence that CRF2 receptor activation is important for neural plasticity related to fear learning. Because of CRF2 activation effects are so critically dependent upon the specific neural circuit activated, it is difficult to predict how systemic small molecule CRF2 antagonists would affect anxiety and depression. The more recent data highlighted here support their potential to inhibit anxiety and depressive behaviors stemming from associative learning with negative reinforcers.
Perspectives and Future Directions
After the landmark work of Vale and colleagues at the Salk Institute culminated in the isolation and sequencing of CRF in 1981, subsequent studies supported the concept that CRF plays a primary role in the pathophysiology of depression.1,16 Later, the human CRF1 receptor was cloned from a pituitary corticotroph adenoma cDNA library in 1993, and then a second CRF receptor, CRF2, was cloned in 1995, establishing that both receptors belonged to the class B1 group of the GPCR superfamily.8,16 The ICs and C termini of the CRF1 and CRF2 receptors had a high sequence conservation (~90% identity) while the extracellular domains (including the ligand selectivity sites and binding pockets) were only ~60% homologous.8,16,18 At that time, studies indicated that the dominant mode of CRF1 and CRF2 receptor signaling was activation of the adenylyl cyclase-PKA pathway, although both CRF receptors were eventually found to activate the PLC-PKC and ERK-MAP kinase pathways.16–19 Soon, pharmaceutical companies worldwide began developing small molecule CRF1 receptor antagonists that blocked Gs-coupled CRF1 receptor signaling as potential antidepressant agents.8 A decade of intensive research, however, has failed to produce a small molecule CRF1 receptor antagonist that is well tolerated and efficacious in patients with major depression. Nevertheless, preclinical data shows that CRF1 receptor antagonist treatment is effective in reducing exaggerated anxiety-like behavior in animals bred for high “trait” anxiety and those previously exposed to severe stress.13 Therefore, CRF1 receptor antagonists may prove to be selectively therapeutic in patients who suffer stress-induced depressive relapses and those with major depression and co-morbid generalized anxiety with high suicide risk. They may also be useful in treating patients with panic disorder and post-traumatic stress disorder, which frequently are associated with co-morbid depression and suicide. Moreover, synthesis of more effective CRF1 receptor antagonists may nowbe possible based on the recent finding that effective small molecules bind to sites within the transmembrane spanning domains and then allosterically induce the CRF1 receptor to assume an inactive conformation that can not coupled with Gsα and initiate cyclic AMP signaling.84 The CRF2 receptor’s role in the stress response, anxiogenesis, and depressive pathophysiology is still being investigated and, to date, no small molecules targeting the CRF2 receptor have been developed.
High levels of CRF have been measured in cerebrospinal fluid of depressed patients— especially those committing suicide—and former combat soldiers experiencing severe post-traumatic stress disorder.9 These findings have led to the hypothesis that CRF hypersecretion is a critical factor in the etiology of severe depression and post-traumatic stress disorder. CRF hypersecretion alone, however, can not elicit enhanced CRF receptor signaling since receptors exposed to high agonist concentrations undergo rapid desensitization and internalization, and eventually become downregulated.51–54 In preclinical studies, molecularly inhibiting cellular expression of GRK3 or β-arrestins significantly increased CRF1 or CRF2(b) receptor cyclic AMP signaling, respectively, by reducing homologous desensitization during exposure to high agonist.64,74 In addition, substantially reducing β-arrestin expression also markedly impaired ligand-induced internalization, thereby retaining more CRF2(b) receptors on the cell surface where they would be available for agonist stimulation.74 In an animal model, mice with a deletion of the GRK5 gene exhibit a depression-like phenotype due to loss of GRK5-mediated desensitization of brain muscarinic receptors.106 Interestingly, patients with major depression were found to have abnormally low β-arrestin expression in mononuclear leukocytes. Moreover, a significant correlation existed between decreasing β-arrestin levels and increasing depression severity in these patients.107 Glucocorticoids have been shown to suppress expression of GRK2 and β-arrestins.108,109 Consequently, stress-induced hypersecretion of CRF and cortisol may result in excessive central CRF1 receptor signaling, when GRK- and β-arrestin– mediated desensitization is deficient, thereby causing severe anxiety and depression (Fig. 5). Thus, we posit a model of stress-induced depression in which a mutation or acquired deficit (possibly from severe childhood stress or adult trauma) of molecular mechanisms regulating CRF receptor signaling impairs homologous desensitization.
CRF1 receptor–β-arrestin interactions may be relevant to other stress-induced affective pathophysiology. Emerging preclinical evidence suggests that central BDNF signaling via the TrkB receptor may play a wide ranging and important role in the etiology of stress-induced affective pathophysiology through modulation of neuronal plasticity, learning, and depression-like behavior.110–113 Recently, BDNF expression has been shown to upregulate in the nucleus accumbens during chronic stress while injection of BDNF into the ventral tegmentum and nucleus accumbens increases stress-induced social defeat and depressive-like behavior.112–115 Therefore, increased BDNF-mediated synaptic plasticity in the extended amygdala may result in maladaptive learning that leads to severe anxiety and depression.113 Other studies have recently found that severe stress augments anxiety-like defensive behavior and increases dendritic spine density of basolateral amygdalar neurons.115 Furthermore, BDNF-induced phosphorylation and trafficking of TrkB receptors in the basolateral amygdala contributes to stress-induced learning, while BDNF overexpressing transgenic mice exhibit abnormally high anxiety-like defensive behavior and excessive dendritic spinogenesis of basolateral amygdalar neurons.116,117 Epac-dependent CRF1 receptor signaling via the ERK-MAPK pathway has been found to potentiate BDNF-stimulated TrkB signaling (Fig. 5).30 Recently, brain neurokinin 1 receptors have been found to regulate μ-opioid receptor signaling and trafficking via a β-arrestin2 mechanism in brain neurons.118 Consequently, CRF1 receptors may promote β-arrestin2–mediated translocation of TrkB receptors to neuronal membranes, thereby promoting excessive dendritic spinogenesis of basolateral amygdalar neurons that results in maladaptative emotional learning and severe anxiety.
Finally, phosphorylation of a GPCR by certain GRKs and binding of β-arrestin2 to the phospho-receptor forms a scaffold that can stimulate a GPCR to signal selectively via the ERK-MAPK or Akt pathways independent of Gs activation. Furthermore, “biased agonists” have been found to induce a stable receptor conformation with selectivity for β-arrestin– mediated signal transduction (Fig. 1).55,56 The β-blocker carvedilol (Coreg) can act as a “biased” ligand by stimulating a pattern of GRK-mediated phosphorylation of β1- and β2-adrenergic receptors that results in a strong, preferential recruitment of β-arrestin2, which, in turn, activates the Src-EGF receptor-ERK cascade implicated in myocyte survival and healthy left ventricular functioning.119,120 Consequently, cardevilol’s cardioprotective action and enhanced clinical efficacy in cardiovascular diseases may involve β-arrestin–regulated intracellular signaling. Interestingly, the novel antipsychotic aripiprazole (Abilify) can stimulate β-arrestin2 translocation from the cytoplasm to membrane dopamine D2 receptors, unlike classical neuroleptics or other atypical antipsychotics that inhibit dopamine-induced β-arrestin2 recruitment by D2 receptors.121 This cellular action of aripiprazole may contribute to its unique antipsychotic and mood stabilization actions. Interestingly, activation of CRF2 receptors by UCN2 and UCN3, but not CRF, promotes ERK and Akt signaling.9,44 The rapid and strong recruitment of β-arrestin2 by the CRF receptors after binding UCN2 or UCN3 may result in ERK and Akt pathway-selective signaling, which may have an important influence on stress and anxiety responses. Conceivably, development of novel ligands that direct brain CRF2(a) receptors to signal in a pathway-selective manner could provide new anxiolytic or antidepressant pharmacotherapies. Since abnormal signaling by both CRF receptors may contribute to the pathophysiology of human anxiety, stress, and depressive disorders, further research on GRK and β-arrestin mechanisms regulating neuronal signaling by CRF1 and CRF2 receptors could provide further insight into the pathogenesis of depression and stress-induced affective disorders.
NOTE ADDED IN PROOF: A new investigation replicated that the TAT haplotype formed by CRF1 receptor rs7209436, rs110402, and rs242924 SNPs confers protection in adults against the development of recurrent major depression following exposure to severe childhood stress,122 which was previously reported in two earlier studies.82,123 Another recently published study reported that CRF1 receptor rs110402 and rs242924 SNPs modulate dysregulation of adult HPA responsiveness induced by severe childhood maltreatment.124 Therefore, CRF1 receptor gene variants may influence the emotional consolidation of early life stress that contributes to the onset and recurrence of adult major depression, consistent with preclinical studies showing that neuronal signaling by both CRF receptors regulate the consolidation of aversive memories resulting from stress.88,105
Acknowledgments
R. L. Hauger was supported by a Merit Review grant from the Department of Veterans Affairs; the VA Center of Excellence for Stress and Mental Health (CESAMH) and Mental Illness Research, Education and Clinical Center (MIRECC) of VISN22; and NIH/NIA (AG022982) and NIH/NIMH (MH074697) RO1 grants. J. A. Olivares-Reyes was supported by the Center for Research and Advanced Studies of the National Polytechnic Institute (CINVESTAV-IPN) and a CONACYT-SEP research grant (CB-2005-01-48777). V. Risbrough was supported by an NIH/NIMH (MH074697) RO1 grant. R. H. Oakley was supported by the National Institute of Environmental Health Sciences. We gratefully acknowledge Susan Shew for editorial assistance.
Footnotes
Conflicts of Interest
The authors declare no conflicts of interest.
References
- 1.Bale TL, Vale WW. CRF and CRF receptors: role in stress responsivity and other behaviors. Annu. Rev. Toxicol. 2004;44:525–557. doi: 10.1146/annurev.pharmtox.44.101802.121410. [DOI] [PubMed] [Google Scholar]
- 2.Bonne O, Grillon C, Vythilingam M, et al. Adaptive and maladaptive psychobiological responses to severe psychological stress. Neurosci. Biobehav. Rev. 2004;28:65–94. doi: 10.1016/j.neubiorev.2003.12.001. [DOI] [PubMed] [Google Scholar]
- 3.McEwen BS. Physiology and neurobiology of stress and adaptation: central role of the brain. Physiol. Rev. 2007;87:873–904. doi: 10.1152/physrev.00041.2006. [DOI] [PubMed] [Google Scholar]
- 4.Leonardo ED, Hen R. Genetics of affective and anxiety disorders. Annu. Rev. Psychol. 2006;57:117–137. doi: 10.1146/annurev.psych.57.102904.190118. [DOI] [PubMed] [Google Scholar]
- 5.Heim C, Newport DJ, Mietzko T, et al. The link between childhood trauma and depression: insights from HPA axis studies in humans. Psychoneuroendocrinology. 2008;33:693–710. doi: 10.1016/j.psyneuen.2008.03.008. [DOI] [PubMed] [Google Scholar]
- 6.Kendler KS, Karkowski LM, Prescott CA. Casual relationship between stressful life events and the onset of major depression. Am. J. Psychiatry. 1999;156:837–841. doi: 10.1176/ajp.156.6.837. [DOI] [PubMed] [Google Scholar]
- 7.Kessler RC. The effects of stressful life events on depression. Annu. Rev. Psychol. 1997;48:191–214. doi: 10.1146/annurev.psych.48.1.191. [DOI] [PubMed] [Google Scholar]
- 8.Grigoriadis DE. The corticotropin-releasing factor receptor: a novel target for the treatment of depression and anxiety-related disorders. Expert Opin. Ther. Targets. 2005;9:651–684. doi: 10.1517/14728222.9.4.651. [DOI] [PubMed] [Google Scholar]
- 9.Hauger RL, Risbrough V, Brauns O, Dautzenberg FM. CRF receptor signaling in the central nervous system: new molecular targets for affective disorders. CNS Neurol. Disord. Drug Targets. 2006;5:453–479. doi: 10.2174/187152706777950684. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Holsboer F, Ising M. Central CRH system in depression and anxiety—evidence from clinical studies with CRH1 receptor antagonists. Eur. J. Pharmacol. 2008;583:350–357. doi: 10.1016/j.ejphar.2007.12.032. [DOI] [PubMed] [Google Scholar]
- 11.Heinrichs SC, Koob GF. Corticotropin-releasing factor in brain: role in activation, arousal, and affect regulation. J. Pharmacol. Exp. Therap. 2004;311:427–440. doi: 10.1124/jpet.103.052092. [DOI] [PubMed] [Google Scholar]
- 12.Coste SC, Murray SE, Stenzel-Poore SP. Animal models of CRF excess and CRH receptor deficiency display altered adaptations to stress. Peptides. 2001;22:733–741. doi: 10.1016/s0196-9781(01)00386-2. [DOI] [PubMed] [Google Scholar]
- 13.Muller MB, Holsboer F. Mice with mutations in the HPA-system as models for symptoms of depression. Biol. Psychiatry. 2006;59:1104–1115. doi: 10.1016/j.biopsych.2006.02.008. [DOI] [PubMed] [Google Scholar]
- 14.Maier SF, Watkins LR. Stressor controllability and learned helplessness: the roles of the dorsal raphe nucleus, serotonin, and CRF. Neurosci. Biobehav. Rev. 2004;29:829–841. doi: 10.1016/j.neubiorev.2005.03.021. [DOI] [PubMed] [Google Scholar]
- 15.Valentino RJ, Commons KG. Peptides that fine-tune the serotonin system. Neuropeptides. 2005;39:1–8. doi: 10.1016/j.npep.2004.09.005. [DOI] [PubMed] [Google Scholar]
- 16.Perrin MH, Vale W. Corticotropin-releasing factor receptors. Chapter 25. In: Pangalos MN, Davies CH, editors. Understanding G Protein-Coupled Receptors and Their Role in the CNS. New York, NY: Oxford University Press; 2002. pp. 505–526. [Google Scholar]
- 17.Dautzenberg FM, Hauger RL. The CRF peptide family and their receptors: yet more partners discovered. Trends Pharmacol. Sci. 2002;23:71–77. doi: 10.1016/s0165-6147(02)01946-6. [DOI] [PubMed] [Google Scholar]
- 18.Hauger RL, Grigoriadis DE, Dallman MF, et al. Current status of the nomenclature for receptors for CRF and their ligands. Pharmacol. Rev. 2003;55:21–26. doi: 10.1124/pr.55.1.3. [DOI] [PubMed] [Google Scholar]
- 19.Hillhouse EW, Grammatopoulos DK. The molecular mechanisms underlying the regulation of the biological activity of corticotropin-releasing hormone receptors: implications for physiology and pathophysiology. Endocr. Rev. 2006;27:260–286. doi: 10.1210/er.2005-0034. [DOI] [PubMed] [Google Scholar]
- 20.Henry B, Vale W, Markou A. The effect of lateral septum corticotropin-releasing factor receptor 2 activation of anxiety is modulated by stress. J. Neurosci. 2006;26:9142–9152. doi: 10.1523/JNEUROSCI.1494-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Bakshi VP, Newman SM, Smith-Roe S, et al. Stimulation of lateral septum CRF2 receptors promotes anorexia and stress-like behaviors: functional homology to CRF1 receptors in basolateral amygdala. J. Neurosci. 2007;27:10568–10577. doi: 10.1523/JNEUROSCI.3044-06.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Erdtmann-Vourliotis M, Mayer P, Ammon S, et al. Distribution of G-protein-coupled receptor kinase (GRK) isoforms 2, 3, 5, and 6 mRNA in the rat brain. Mol. Brain Res. 2001;95:129–137. doi: 10.1016/s0006-8993(01)03046-3. [DOI] [PubMed] [Google Scholar]
- 23.Gurevich EV, Benovic JL, Gurevich VV. Arrestin2 and arrestin3 are differentially expressed in the rat brain during postnatal development. Neuroscience. 2002;109:421–436. doi: 10.1016/s0306-4522(01)00511-5. [DOI] [PubMed] [Google Scholar]
- 24.Pandey SC, Roy A, Zhang H. Decreased phosphorylation of CREB in the central amygdale acts as a molecular substrate for anxiety related ethanol withdrawal. Alcohol Clin. Exp. Res. 2003;27:396–409. doi: 10.1097/01.ALC.0000056616.81971.49. [DOI] [PubMed] [Google Scholar]
- 25.Wallace TL, Stelliltano KE, Neve RL, Duman RS. Effects of CREB overexpression in the basolateral amygdala on behavioral models of depression and anxiety. Biol. Psychiatry. 2004;56:151–160. doi: 10.1016/j.biopsych.2004.04.010. [DOI] [PubMed] [Google Scholar]
- 26.Krishnan V, Graham A, Mazei-Robison MS, et al. Calcium-sensitive adenylyl cyclases in depression and anxiety: behavioral and biochemical consequences of isoform targeting. Biol. Psychiatry. 2008;64:336–343. doi: 10.1016/j.biopsych.2008.03.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Sheng H, Sun T, Cong B, et al. Corticotropin-releasing hormone stimulates SGK-1 kinase expression in culture hippocampal neurons via CRH-R1. Am. J. Physiol. 2008;295:E938–E946. doi: 10.1152/ajpendo.90462.2008. [DOI] [PubMed] [Google Scholar]
- 28.Peeters PJ, Fierens FLP, Van Den Wyngaert I, et al. Gene expression profiles highlight adaptive brain mechanisms in corticotropin releasing factor overexpressing mice. Mol. Brain Res. 2004;129:135–150. doi: 10.1016/j.molbrainres.2004.06.038. [DOI] [PubMed] [Google Scholar]
- 29.Bayatti N, Hermann H, Lutz B, Behl C. Corticotropin-releasing hormone-mediated induction of intracellular signaling pathways and brain-derived neurotrophic factor expression is inhibited by the activation of the endocannabinoid system. Endocrinology. 2005;146:1205–1213. doi: 10.1210/en.2004-1154. [DOI] [PubMed] [Google Scholar]
- 30.Traver S, Marien M, Martin E, et al. The phenotypic differentiation of locus ceruleus noradrenergic neurons mediated by brain-derived neurotrophic factor is enhanced by corticotropin releasing factor through the activation of cAMP-dependent signaling pathway. Mol. Pharmacol. 2006;70:30–40. doi: 10.1124/mol.106.022715. [DOI] [PubMed] [Google Scholar]
- 31.Duman RS, Monteggia LM. A neurotrophic model for stress-related mood disorders. Biol. Psychiatry. 2006;59:1116–1127. doi: 10.1016/j.biopsych.2006.02.013. [DOI] [PubMed] [Google Scholar]
- 32.Pittenger C, Duman RS. Stress, depression, and neuroplasticity: a convergence of mechanisms. Europsychopharmacology. 2008;33:88–109. doi: 10.1038/sj.npp.1301574. [DOI] [PubMed] [Google Scholar]
- 33.Krishnan V, Nestler EJ. The molecular neurobiology of depression. Nature. 2008;455:894–902. doi: 10.1038/nature07455. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Dwivedi Y, Mondal AC, Rizavi HS, et al. Differential and brain region-specific regulation of Rap-1 and Epac in depressed suicide victims. Arch. Gen. Psychiatry. 2006;63:639–648. doi: 10.1001/archpsyc.63.6.639. [DOI] [PubMed] [Google Scholar]
- 35.Pandey GN, Ren X, Risavi HS, et al. Brain-derived neurotrophic factor and tyrosine kinase B receptor signaling in post-mortem brain of teenage suicide victims. Int. J. Neuropsychopharmacol. 2008;11:1047–1061. doi: 10.1017/S1461145708009000. [DOI] [PubMed] [Google Scholar]
- 36.Gutknecht E, Van Der Linden I, Van Kolen K, et al. Molecular mechanisms of corticotropin-releasing factor receptor induced calcium signaling. Mol. Pharm. 2009;75:648–657. doi: 10.1124/mol.108.050427. [DOI] [PubMed] [Google Scholar]
- 37.Riegel AC, Williams JT. CRF facilitates calcium release from intracellular stores in midbrain dopamine neurons. Neuron. 2008;57:559–570. doi: 10.1016/j.neuron.2007.12.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Hodge CW, Raber J, McMahon T, et al. Decreased anxiety-like behavior, reduced stress hormones, and neurosteroid supersensitivity in mice lacking protein kinase Cε. J. Clin. Investig. 2002;110:1003–1010. doi: 10.1172/JCI15903. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Pandey GN, Diwedi Y, Risavi HS, et al. Decreased catalytic activity and expression of protein kinase C isozymes in teenage suicide victims: a postmortem brain study. Arch. Gen. Psychiatry. 2004;61:685–693. doi: 10.1001/archpsyc.61.7.685. [DOI] [PubMed] [Google Scholar]
- 40.Hauger RL, Olivares-Reyes JA, Braun S, et al. Regulation of corticotropin releasing factor type 1 receptor phosphorylation and desensitization by protein kinase C: a possible role in stress adaptation. J. Pharmacol. Exp. Ther. 2003;306:794–803. doi: 10.1124/jpet.103.050088. [DOI] [PubMed] [Google Scholar]
- 41.Tan H, Zhong P, Yan Z. Corticotropin-releasing factor and acute stress prolongs serotonergic regulation of GABA transmission in prefrontal cortical pyramidal neurons. J. Neurosci. 2004;24:5000–5008. doi: 10.1523/JNEUROSCI.0143-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Ungless MA, Signh V, Crowder TL, et al. Corticotropin-releasing factor requires CRF binding protein to potentiate NMDA receptors via CRF receptor 2 in dopamine neurons. Neuron. 39:401–407. doi: 10.1016/s0896-6273(03)00461-6. [DOI] [PubMed] [Google Scholar]
- 43.Arzt E, Holsboer F. CRF signaling: molecular specificity for drug targeting in the CNS. Trends Pharmacol. Sci. 2006;27:531–538. doi: 10.1016/j.tips.2006.08.007. [DOI] [PubMed] [Google Scholar]
- 44.Brar BK, Chen A, Perrin MH, Vale W. Specificity and regulation of ERK1/2 phosphorylation through CRF receptors 1 and 2β by the CRF/urocortin family of peptides. Endocrinology. 2004;145:1718–1729. doi: 10.1210/en.2003-1023. [DOI] [PubMed] [Google Scholar]
- 45.Papadopoulou N, Chen J, Randeva HS, et al. PKA-induced negative regulation of the CRH-R1-ERK1/2 pathway. Mol. Endocrinol. 2004;18:624–639. doi: 10.1210/me.2003-0365. [DOI] [PubMed] [Google Scholar]
- 46.Punn A, Levine MA, Grammatopoulos DK. Identification of signaling molecules mediating corticotropin-releasing hormone-R1a MAPK interactions: the critical role of PI3-kinase in regulating ERK1/2 but no p38 MAPK activation. Mol. Endocrinol. 2006;20:3179–3195. doi: 10.1210/me.2006-0255. [DOI] [PubMed] [Google Scholar]
- 47.Rossant CJ, Pinnock RD, Hughes J, et al. CRF-R1 and CRF-R2a regulate phosphorylation of calcium-CREB protein and activation of p42/p44 MAPK. Endocrinology. 1999;140:1525–1536. doi: 10.1210/endo.140.4.6656. [DOI] [PubMed] [Google Scholar]
- 48.Salim S, Hite B, Eikenburg DC. Activation of the CRF1 receptor causes ERK1/2 mediated increase in GRK3 expression in CATH.a cells. FEBS Lett. 2007;581:3204–3210. doi: 10.1016/j.febslet.2007.06.006. [DOI] [PubMed] [Google Scholar]
- 49.Todorovic C, Sherrin T, Pitts M, et al. Suppression of the MEK/ERK signaling pathway reverses depression-like behaviors of CRF2-deficient mice. Neuropsychopharmacology. 2009;34:1416–1426. doi: 10.1038/npp.2008.178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Gallagher JP, Orozco-Cabal LF, Liu J, Shinnick-Gallagher P. Synaptic physiology of central CRH system. Eur. J. Pharmacol. 2008;583:215–225. doi: 10.1016/j.ejphar.2007.11.075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Kohout TA, Lefkowitz RJ. Regulation of G protein-coupled receptor kinases and arrestins during receptor desensitization. Mol. Pharmacol. 2003;63:9–18. doi: 10.1124/mol.63.1.9. [DOI] [PubMed] [Google Scholar]
- 52.Moore ACC, Milano SK, Benovic JL. Regulation of receptor trafficking by GRKs and arrestins. Annu. Rev. Physiol. 2007;69:451–482. doi: 10.1146/annurev.physiol.69.022405.154712. [DOI] [PubMed] [Google Scholar]
- 53.Kelly E, Bailey CP, Henderson G. Agonist selective mechanism of GPCR desensitization. Br. J. Pharmacol. 2008;153:S379–S388. doi: 10.1038/sj.bjp.0707604. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Krasel C, Bunemann M, Lorenz K, Lohse MJ. Beta-arrestin binding to the beta2-adrenergic receptor requires both receptor phosphorylation and receptor activation. J. Biol. Chem. 2005;280:9528–9535. doi: 10.1074/jbc.M413078200. [DOI] [PubMed] [Google Scholar]
- 55.DeWire SM, Ahn S, Lefkowitz RJ, Shenoy SK. β-Arrestins and cell signaling. Annu. Rev. Physiol. 2007;69:483–510. doi: 10.1146/annurev.physiol.69.022405.154749. [DOI] [PubMed] [Google Scholar]
- 56.Violin JD, Lefkowitz RJ. β-Arrestinbiased ligands at seven-transmembrane receptors. Trends Pharmacol. Sci. 2007;28:416–422. doi: 10.1016/j.tips.2007.06.006. [DOI] [PubMed] [Google Scholar]
- 57.Oakley RH, Olivares-Reyes JA, Hudson CC, et al. Carboxyl terminal and intracellular loop sites for CRF1 receptor phosphorylation and βarrestin2 recruitment: a mechanism regulating stress and anxiety responses. Am. J. Physiol. 2007;293:R209–R222. doi: 10.1152/ajpregu.00099.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Oakley RH, Laporte SA, Holt JA, et al. Differential affinities of visual arrestin, β-arrestin1, and β-arrestin2 with G protein-coupled receptors delineate two major classes of receptors. J. Biol. Chem. 2000;275:17201–17210. doi: 10.1074/jbc.M910348199. [DOI] [PubMed] [Google Scholar]
- 59.Oakley RH, Laporte SA, Holt JA, et al. Association of β-arrestin with G protein-coupled receptors during clathrin-mediated endocytosis dictates the profile of receptor resensitization. J. Biol. Chem. 1999;274:32248–32257. doi: 10.1074/jbc.274.45.32248. [DOI] [PubMed] [Google Scholar]
- 60.Lefkowitz RJ, Shenoy SK. Transduction of receptor signals by β-arrestins. Science. 2005;308:512–517. doi: 10.1126/science.1109237. [DOI] [PubMed] [Google Scholar]
- 61.Hoffman AR, Ceda G, Reisine TD. Corticotropin-releasing factor desensitization of adrenocorticotropic hormone release is augmented by arginine vasopressin. J. Neurosci. 1985;5:234–242. doi: 10.1523/JNEUROSCI.05-01-00234.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Dieterich KD, Grigoriadis DE, De-Souza EB. Homologous desensitization of human corticotrophin-releasing factor1 receptor in stable transfected mouse fibroblast cells. Brain Res. Brain Res. 1996;710:287–292. doi: 10.1016/0006-8993(95)01480-2. [DOI] [PubMed] [Google Scholar]
- 63.Hauger RL, Dautzenberg FM, Flaccus A, et al. Regulation of corticotropin-releasing factor receptor function in human Y-79 retinoblastoma cells: rapid and reversible homologous desensitization but prolonged recovery. J. Neurochem. 1997;68:2308–2316. doi: 10.1046/j.1471-4159.1997.68062308.x. [DOI] [PubMed] [Google Scholar]
- 64.Dautzenberg FM, Braun S, Hauger RL. GRK3 mediates desensitization of CRF1 receptors: a potential mechanism regulating stress adaptation. Am. J. Physiol. 2001;280:R935–R946. doi: 10.1152/ajpregu.2001.280.4.R935. [DOI] [PubMed] [Google Scholar]
- 65.Dautzenberg FM, Wille S, Braun S, Hauger RL. GRK3 regulation during CRF- and urocortin-induced CRF1 receptor desensitization. Biochem. Biophys. Res. Commun. 2002;298:303–308. doi: 10.1016/s0006-291x(02)02463-4. [DOI] [PubMed] [Google Scholar]
- 66.Roseboom PH, Urben CM, Kalin N. Persistent corticotropin-releasing factor1 receptor desensitization and downregulation in the human neuroblastoma cell line IMR-32. Brain Res. Mol. Brain Res. 2001;92:115–127. doi: 10.1016/s0169-328x(01)00162-0. [DOI] [PubMed] [Google Scholar]
- 67.Teli T, Markovic D, Levine MA, et al. Regulation of corticotropin-releasing hormone (CRH) receptor type 1α signaling: structural determinants for G protein coupled receptor kinase-mediated phosphorylation and agonist-mediated desensitization. Mol. Endocrinol. 2005;19:474–490. doi: 10.1210/me.2004-0275. [DOI] [PubMed] [Google Scholar]
- 68.Kageyama K, Hanada K, Moriyama T, et al. G protein-coupled receptor kinase 2 involvement in desensitization of corticotropin-releasing facotr (CRF) receptor type 1 by CRF in murine corticotrophs. Endocrinology. 2006;147:441–450. doi: 10.1210/en.2005-0376. [DOI] [PubMed] [Google Scholar]
- 69.Hauger RL, Smith RD, Braun S, et al. Rapid agonist-induced phosphorylation of the human CRF receptor, type 1: a potential mechanism for homologous desensitization. Biochem. Biophys. Res. Comm. 2000;268:572–576. doi: 10.1006/bbrc.2000.2183. [DOI] [PubMed] [Google Scholar]
- 70.Perry SJ, Junger S, Kohout TA, et al. Distinct conformations of the corticotropin releasing factor type 1 receptor adopted following agonist and antagonist binding are differentially regulated. J. Biol. Chem. 2005;280:11560–11568. doi: 10.1074/jbc.M412914200. [DOI] [PubMed] [Google Scholar]
- 71.Holmes KD, Babwah AV, Dale LB, et al. Differential regulation of corticotropin releasing factor 1alpha receptor endocytosis and trafficking by beta-arrestins and Rab GTPases. J. Neurochem. 2006;96:934–949. doi: 10.1111/j.1471-4159.2005.03603.x. [DOI] [PubMed] [Google Scholar]
- 72.Rasmusson TN, Novak I, Nielsen SM. Internalization of the human CRF receptor 1 is independent of classical phosphorylation sites and of β-arrestin 1 recruitment. Eur. J. Biochem. 2004;271:4366–4374. doi: 10.1111/j.1432-1033.2004.04371.x. [DOI] [PubMed] [Google Scholar]
- 73.Gutknecht E, Hauger RL, Van Der Linden I, et al. Expression, binding, and signaling properties of CRF2(a) receptors endogenously expressed in human retinoblastoma Y79 cells: passage-dependent regulation of functional receptors. J. Neurochem. 2008;104:926–936. doi: 10.1111/j.1471-4159.2007.05052.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Markovic D, Punn A, Lehnert H, Grammatopoulos DK. Intracellular mechanisms regulating corticotropin-releasing hormone receptor-2b endocytosis and interaction with extracellularly regulated kinase1/2 and p38 mitogen-activated protein kinase signaling cascades. Mol. Endocrinol. 2008;22:689–706. doi: 10.1210/me.2007-0136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Waselus M, Nazzaro C, Valentino RJ, Van Bockstaele EJ. Stress-induced redistribution of corticotropin-releasing factor receptor subtypes in the dorsal raphe nucleus. Biol. Psychiatry. 2009;66:76–83. doi: 10.1016/j.biopsych.2009.02.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Drake MT, Shenoy SK, Lefkowitz RJ. Trafficking of G protein-coupled receptors. Circ. Res. 2006;99:570–582. doi: 10.1161/01.RES.0000242563.47507.ce. [DOI] [PubMed] [Google Scholar]
- 77.Lin CH, MacGurn JA, Chu T, et al. Arrestin-related ubiquitin-ligase adaptors regulate endocytosis and protein turnover at the cell surface. Cell. 2008;135:714–725. doi: 10.1016/j.cell.2008.09.025. [DOI] [PubMed] [Google Scholar]
- 78.Arborelius L, Owens MJ, Plotsky PM, Nemeroff CB. The role of corticotropin-releasing factor in depression and anxiety disorders. J. Endocrinol. 1999;160:1–12. doi: 10.1677/joe.0.1600001. [DOI] [PubMed] [Google Scholar]
- 79.Wang S-S, Kamphuis W, Huitinga I, et al. Gene expression analysis in the human hypothalamus in depression by laser microdissection and real-time PCR: the presence of multiple receptor imbalances. Mol. Psychiatry. 2008;13:786–799. doi: 10.1038/mp.2008.38. [DOI] [PubMed] [Google Scholar]
- 80.Licinio J, O’Kirwan F, Irizarry K, et al. Association of a corticotropin-releasing hormone receptor 1 haplotype and antidepressant treatment response in Mexican-Americans. Mol. Psychiatry. 2004;9:1075–1082. doi: 10.1038/sj.mp.4001587. [DOI] [PubMed] [Google Scholar]
- 81.Wasserman D, Sokolowski M, Rozanov V, Wasserman J. The CRHR1 gene: a marker for suicidality in depressed males exposed to low stress. Genes Brain Behav. 2008;7:14–19. doi: 10.1111/j.1601-183X.2007.00310.x. [DOI] [PubMed] [Google Scholar]
- 82.Bradley RG, Binder EB, Epstein MP, et al. Influence of child abuse on adult depression: moderation by the corticotropin-releasing hormone receptor gene. Arch. Gen. Psychiatry. 2008;65:190–200. doi: 10.1001/archgenpsychiatry.2007.26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Binneman B, Feltner D, Kolluri S, et al. A 6-week randomized, placebo-controlled trial of CP-316,311 (a selective CRH1 antagonist) in the treatment of major depression. Am. J. Psychiatry. 2008;165:617–620. doi: 10.1176/appi.ajp.2008.07071199. [DOI] [PubMed] [Google Scholar]
- 84.Grigoriadis DE, Hoare SRJ, Lechner SM, et al. Drugability of extracellular targets: discovery of small molecule drugs targeting allosteric, functional, and subunit-selective sites on GPCRs and ion channels. Neuropsychopharmacology. 2009;34:106–125. doi: 10.1038/npp.2008.149. [DOI] [PubMed] [Google Scholar]
- 85.Kang HJ, Adams DH, Simen A, et al. Gene expression profiling in postmortem prefrontal cortex of major depressive disorder. J. Neurosci. 2007;27:13329–13340. doi: 10.1523/JNEUROSCI.4083-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Risbrough VB, Hauger RL, Pelleymounter MA, Geyer MA. Role of CRF receptors 1 and 2 in CRF-potentiated acoustic startle in mice. Psychopharmacology. 2003;170:178–187. doi: 10.1007/s00213-003-1535-6. [DOI] [PubMed] [Google Scholar]
- 87.Risbrough VB, Hauger RL, Roberts AL, et al. Corticotropin releasing factor receptors CRF1 and CRF2 exert both additive and opposing influences on defensive startle behavior. J. Neurosci. 2004;24:6545–6552. doi: 10.1523/JNEUROSCI.5760-03.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Risbrough VB, Geyer MA, Hauger RL, et al. CRF1 and CRF2 receptors are required for potentiated startle to contextual but not discrete cues. Neuropsychopharmacology. 2009;34:1494–1503. doi: 10.1038/npp.2008.205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Cryan JF, Holmes A. The ascent of mouse: advances in modelling human depression and anxiety. Nat. Rev. Drug Discov. 2005;4:775–790. doi: 10.1038/nrd1825. [DOI] [PubMed] [Google Scholar]
- 90.Tezval H, Jahn O, Todorovic C, et al. Cortagine, a specific agonist of corticotrophin-releasing factor receptor subtype 1, is anxiogenic and antidepressive in the mouse model. Proc. Natl. Acad. Sci. USA. 2004;101:9468–9473. doi: 10.1073/pnas.0403159101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Lu A, Steiner MA, Whittle N, et al. Conditional mouse mutants highlight mechanisms of corticotropin-releasing hormone effects on stress-coping behavior. Mol. Psychiatry. 2008;13:1028–1042. doi: 10.1038/mp.2008.51. [DOI] [PubMed] [Google Scholar]
- 92.Griebel G, Simiand J, Steinberg R, et al. 4-(2-Chloro-4-methoxy-5-methylphenyl)-N-[(1S)-2-cyclopropyl-1-(3-fluoro-4-methylphenyl)ethyl]5-methyl-N-(2-propynyl)-1, 3-thiazol-2-amine hydrochloride (SSR125543A), a potent and selective corticotrophin-releasing factor(1) receptor antagonist. II. Characterization in rodent models of stress-related disorders. J. Pharmacol. Exp. Ther. 2002;301:333–345. doi: 10.1124/jpet.301.1.333. [DOI] [PubMed] [Google Scholar]
- 93.Jutkiewicz EM, Wood SK, Houshyar H, et al. The effects of CRF antagonists, antalarmin, CP154,526, LWH234, and R121919, in the forced swim test and on swim-induced increases in adreno-corticotropin in rats. Psychopharmacology. 2005;180:215–223. doi: 10.1007/s00213-005-2164-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Chaki S, Nakazato A, Kennis L, et al. Anxiolytic- and antidepressant-like profile of a new CRF1 receptor antagonist, R278995/CRA0450. Eur. J. Pharmacol. 2004;485:145–158. doi: 10.1016/j.ejphar.2003.11.032. [DOI] [PubMed] [Google Scholar]
- 95.Nielsen DM, Carey GJ, Gold LH. Antidepressant-like activity of corticotrophin-releasing factor type-1 receptor antagonists in mice. Eur. J. Pharmacol. 2004;499:135–146. doi: 10.1016/j.ejphar.2004.07.091. [DOI] [PubMed] [Google Scholar]
- 96.Swiergiel AH, Leskov IL, Dunn AJ. Effects of chronic and acute stressors and CRF on depression-like behavior in mice. Behav. Brain Res. 2008;186:32–40. doi: 10.1016/j.bbr.2007.07.018. [DOI] [PubMed] [Google Scholar]
- 97.Sherman AD, Sacquitne JL, Petty F. Specificity of the learned helplessness model of depression. Pharmacol. Biochem. Behav. 1982;16:449–454. doi: 10.1016/0091-3057(82)90451-8. [DOI] [PubMed] [Google Scholar]
- 98.Deak T, Nguyen KT, Ehrlich AL, et al. The impact of the nonpeptide corticotropin-releasing hormone antagonist antalarmin on behavioral and endocrine responses to stress. Endocrinology. 1999;140:79–86. doi: 10.1210/endo.140.1.6415. [DOI] [PubMed] [Google Scholar]
- 99.Hubbard DT, Nakashima BR, Lee I, Takahashi LK. Activation of basolateral amygdala corticotropin-releasing factor 1 receptors modulates the consolidation of contextual fear. Neuroscience. 2007;150:818–828. doi: 10.1016/j.neuroscience.2007.10.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Reyes BA, Valentino RJ, Van Bockstaele EJ. Stress-induced intracellular trafficking of corticotropin-releasing factor receptors in rat locus coeruleus neurons. Endocrinology. 2008;149:122–130. doi: 10.1210/en.2007-0705. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Zhao Y, Valdez GR, Fekete EM, et al. Subtype-selective corticotropin-releasing factor receptor agonists exert contrasting, but not opposite, effects on anxiety-related behavior in rats. J. Pharmacol. Exp. Ther. 2007;323:846–854. doi: 10.1124/jpet.107.123208. [DOI] [PubMed] [Google Scholar]
- 102.Land BB, Bruchas MR, Lemos JC, et al. The dysphoric component of stress is encoded by activation of the dynorphin kappa-opioid system. J. Neurosci. 2008;28:407–414. doi: 10.1523/JNEUROSCI.4458-07.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Macey DJ, Koob GF, Markou A. CRF and urocortin decreased brain stimulation reward in the rat: reversal by a CRF receptor antagonist. Brain Res. 2000;866:82–91. doi: 10.1016/s0006-8993(00)02229-0. [DOI] [PubMed] [Google Scholar]
- 104.Tanaka M, Telegdy G. Antidepressant-like effects of the CRF family peptides, urocortin 1, urocortin 2 and urocortin 3 in a modified forced swimming test in mice. Brain Res. Bull. 2008;75:509–512. doi: 10.1016/j.brainresbull.2007.08.009. [DOI] [PubMed] [Google Scholar]
- 105.Todorovic C, Radulovic J, Jahn O, et al. Differential activation of CRF receptor subtypes removes stress-induced memory deficit and anxiety. Eur. J. Neurosci. 2007;25:3385–3397. doi: 10.1111/j.1460-9568.2007.05592.x. [DOI] [PubMed] [Google Scholar]
- 106.Premont RT, Gainetdinov RR. Physiological roles of G protein-coupled receptor kinases and arrestins. Annu. Rev. Physiol. 2007;69:511–534. doi: 10.1146/annurev.physiol.69.022405.154731. [DOI] [PubMed] [Google Scholar]
- 107.Avissar S, Matuzany-Ruban A, Tzukert K, Schreiber G. Beta-arrestin-1 levels: reduced in leukocytes of patients weith depression and elevated by antidepressants in rat brain. Am. J. Psychiatry. 2004;161:2066–2072. doi: 10.1176/appi.ajp.161.11.2066. [DOI] [PubMed] [Google Scholar]
- 108.Mak JCW, Hisada T, Salmon M, et al. Glucocorticoids reverse IL-1β-induced impairment of b-adrenoceptor-mediated relaxation and upregulation of G-protein-coupled receptor kinases. Br. J. Pharmacol. 2002;135:987–996. doi: 10.1038/sj.bjp.0704545. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Tsutsui S, Vergote D, Shariat N, et al. Glucocorticoids regulate innate immunity in a model of multiple sclerosis: reciprocal interactions between the A1 adenosine receptor and b-arrestin-1 in monocytoid cells. FASEB J. 2008;22:786–796. doi: 10.1096/fj.07-9002com. [DOI] [PubMed] [Google Scholar]
- 110.Duman R, Monteggia LM. A neurotrophic model for stress-related mood disorders. Biol. Psychiatry. 2006;59:1116–1127. doi: 10.1016/j.biopsych.2006.02.013. [DOI] [PubMed] [Google Scholar]
- 111.Pittenger C, Duman R. Stress, depression, and neuroplasticity: a convergence of mechanisms. Neuropsychopharmacology. 2008;33:88–109. doi: 10.1038/sj.npp.1301574. [DOI] [PubMed] [Google Scholar]
- 112.Berton O, Nestler EJ. New approaches to antidepressant drug discovery: beyond monoamines. Nat. Rev. Neurosci. 2006;7:137–151. doi: 10.1038/nrn1846. [DOI] [PubMed] [Google Scholar]
- 113.Krishnan V, Nestler EJ. The molecular neurobiology of depression. Nature. 2008;455:894–902. doi: 10.1038/nature07455. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Monteggia LM, Luikart B, Barrot M, et al. Brain-derived neurotrophic factor conditional knockouts show gender differences in depression-related behaviors. Biol. Psychiatry. 2007;61:187–196. doi: 10.1016/j.biopsych.2006.03.021. [DOI] [PubMed] [Google Scholar]
- 115.Mitra R, Jadhav S, McEwen BS, et al. Stress duration modulates the spatiotemporal patterns of spine formation in the basolateral amygdale. Proc. Natl. Acad. Sci. USA. 2005;102:9371–9376. doi: 10.1073/pnas.0504011102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Rattiner LM, Davis M, French CT, Ressler KJ. Brain-derived neurotrophic factor and tyrosine kinase receptor B involvement in amygdala-dependent fear conditioning. J. Neurosci. 2004;24:4796–4806. doi: 10.1523/JNEUROSCI.5654-03.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Govindarajan A, Rao BSS, Nair D, et al. Transgenic brain-derived neurotrophic factor expression causes both anxiogenic and antidepressant effects. Proc. Natl. Acad. Sci. USA. 2006;103:13208–13213. doi: 10.1073/pnas.0605180103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Yu YJ, Arttamangkul S, Evans CJ, et al. Neurokinin 1 receptors regulate morphine-induced endocytosis and desensitization of m-opioid receptors in CNS neurons. J. Neurosci. 2009;29:222–233. doi: 10.1523/JNEUROSCI.4315-08.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Wisler JW, DeWire SM, Whalen EJ, et al. A unique mechanism of β-blocker action: carvedilol stimulates β-arrestin signaling. Proc. Natl. Acad. Sci. USA. 2007;104:16657–16662. doi: 10.1073/pnas.0707936104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Kim I-M, Tilley DG, Chen J, et al. β-blockers alprenolol and carvedilol stimulate β-arrestin-mediated EGFR transactivation. Proc. Natl. Acad. Sci. USA. 2008;105:14555–14560. doi: 10.1073/pnas.0804745105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Klewe IV, Nielsen SM, Tarpo L, et al. Recruitment of β-arrestin2 to the dopamine D2 receptor: insights into anti-psychotic and antiparkinsonian drug receptor signaling. Neuropharmacology. 2008;54:1215–1222. doi: 10.1016/j.neuropharm.2008.03.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Polanczyk G, Caspi A, Williams B, et al. Protective effect of CRHR1 gene variants on the development of adult depression following childhood maltreatment. Arch. Gen. Psychiatry. 2009;66:978–985. doi: 10.1001/archgenpsychiatry.2009.114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Liu Z, Zhu F, Wang G, et al. Association of corticotropin-releasing hormone receptor1 gene SNP and haplotype with major depression. Neurosci. Lett. 2006;404:358–362. doi: 10.1016/j.neulet.2006.06.016. [DOI] [PubMed] [Google Scholar]
- 124.Tyrka AR, Price LH, Gelernter J, et al. Interaction of childhood maltreatment with the corticotropin-releasing hormone receptor gene: effects on hypothalamic-pituitary-adrenal axis reactivity. Biol. Psychiatry. 2009 doi: 10.1016/j.biopsych.2009.05.012. in press. [DOI] [PMC free article] [PubMed] [Google Scholar]