Abstract
Background
Human immunodeficiency virus (HIV)–infected persons have a high incidence of pneumonia and pneumococcal disease. Benefits of vaccination with the 23-valent pneumococcal polysaccharide vaccine (PPV) among these patients continue to be debated.
Methods
The impact of PPV vaccination on the incidence of pneumonia events (i.e., the composite of pneumococcal pneumonia and pneumonia due to nonspecified organisms) was examined among participants in the Veterans Aging Cohort 5-Site Study, an ongoing prospective study of HIV-infected patients matched to an HIV-uninfected control group. Dates of PPV vaccination and pneumonia were determined by retrospective review of electronic medical records. Time to events was measured for up to 2 years from PPV vaccination or from enrollment for vaccinated and unvaccinated patients, respectively. Kaplan-Meier and Cox proportional hazards regression methods were used to examine the incidence of pneumonia by HIV infection and PPV vaccination status.
Results
Among 692 HIV-uninfected and 934 HIV-infected study participants, 59% were vaccinated with PPV. The 2-year incidence of pneumonia was 6% (97 participants developed pneumonia). HIV-infected patients had a higher rate of pneumonia (hazard ratio, 5.81; 95% confidence interval, 3.15–10.71); overall, vaccinated patients showed a trend toward lower risk of pneumonia (hazard ratio, 0.75; 95% confidence interval, 0.50–1.13). Among HIV-infected patients, after controlling for HIV-specific and other variables, vaccination significantly reduced the risk of pneumonia (hazard ratio, 0.65; 95% confidence interval, 0.42–1.00); current smoking, low hemoglobin level, and low CD4 cell count significantly increased such risk. The effect of PPV vaccination among HIV-uninfected patients was not significant.
Conclusions
Among HIV-infected patients, PPV vaccination offered protection against pneumonia. Smoking cessation needs to be pursued as an additional strategy for preventing pneumonia.
Streptococcus pneumoniae is the most frequently documented cause of bacterial pneumonia [1]. In the developed and the developing world, the rate of pneumonia due to all causes and the rate of invasive pneumococcal disease is several-fold higher among HIV-infected persons than among the general population [2]. The incidence of invasive pneumococcal disease among HIV-infected patients has decreased since the introduction of HAART [3–5], but it continues to be substantially higher than that observed among HIV-uninfected matched control subjects [2, 6–8]. Black HIV-infected patients represent a group with one of the highest incidences of disease [8].
Vaccination with 23-valent pneumococcal polysac charide vaccine (PPV) is the main strategy pursued in the United States to prevent morbidity and mortality related to pneumococcal infections [9, 10], and PPV vaccination is indicated for adults with risk factors for pneumococcal disease. In the population at highest risk, PPV vaccination offers protection against bacteremia, but protection against pneumonia has not been clearly established [11, 12].
Among HIV-infected persons, the current recommendations for vaccination with PPV are largely based on expert opinion [13]. The only prospective study evaluating the efficacy of vaccination among HIV-infected persons was performed in Uganda and revealed a significantly increased rate of pneumococcal disease during the first 6 months after vaccination [14]. In contrast, retrospective studies from North America have shown that PPV protects against pneumococcal disease among certain groups of HIV-infected patients, specifically among those vaccinated while their CD4 cell count was ≥500 cells/mm3 [15] or >200 cells/mm3 [16] or while they were receiving HAART [4, 15]. These studies have not controlled for the presence of other medical comorbidities in their cohorts.
The Veterans Aging Cohort 5-Site Study (VACS 5) is an ongoing prospective study consisting of HIV-infected and HIV-uninfected patients. The VACS 5 HIV-infected group is composed of older participants, compared with other HIV-infected cohorts [17]; the group consists of a high proportion of black individuals, has a high rate of smoking, and has a high prevalence of comorbidities. As such, this cohort is well suited to evaluate the protective effect of PPV vaccination among HIV-infected persons, while controlling for other factors that can increase this group’s risk of pneumonia and pneumococcal disease. With this objective, we evaluated the incidence of pneumonia and pneumococcal disease following vaccination with PPV among HIV-infected and HIV-uninfected participants in VACS 5.
METHODS
VACS 5 is an ongoing prospective study consisting of HIV-infected patients who were block-matched to HIV-uninfected patients on the basis of 5-year increments of age, ethnicity, and location and who were enrolled during 2001–2002 from the outpatient infectious diseases and general medicine clinics at 5 Veterans Affairs (VA) medical centers. At study entry, participants completed a comprehensive self-administered survey (available at http://www.vacohort.org). The institutional review boards at all locations approved the study, and all participants provided written informed consent. This study is fully described elsewhere [17].
VACS 5 participants were stratified by PPV vaccination status. Dates for vaccination were established by retrospective review of the VA electronic administrative and medical records. If a patient had received PPV at any time during the 3 years before and 2 years after the survey date, they were considered to be vaccinated. Patients who had not been vaccinated during that time were considered to be unvaccinated. All HIV-infected patients received PPV after the diagnosis of HIV infection was made. The PPV vaccination date and the survey date were established as the index date (date at which follow-up for the primary outcome started) for the vaccinated and unvaccinated patients, respectively. Exclusion criteria were (1) <1 year of observation after the index date, (2) hospitalization for pneumonia during the year before the index date (because these patients are at increased risk of subsequent pneumonia and the diagnosis of pneumonia may have prompted the vaccination) [18, 19], (3) and female sex (n = 52; because of the small numbers). The maximal extent of follow-up for clinical events was established at 2 years after the index date.
Our primary outcome was the incidence of pneumonia. International Classification of Diseases, Ninth Revision (ICD-9) codes for pneumonia and dates of occurrence were obtained from patient treatment files in the VA electronic medical records. A pneumonia event was defined as an outpatient or inpatient ICD-9 code for pneumonia due to a nonspecified organism or for a pneumococcal-specific diagnosis. If no other diagnoses were specified, cases of pneumococcal bacteremia and sepsis were considered as pneumonia events. Specific nonpneumococcal pneumonia diagnoses (e.g., Pneumocystis carinii pneumonia, pulmonary tuberculosis, and Haemophilus influenza pneumonia) were excluded.
Additional variables were as follows: age, ethnicity, Alcohol Use Disorder Identification Test result, drug use, and current smoking status (all data were obtained from the patient’s survey); the underlying conditions of interest, alcohol and drug dependence, chronic obstructive pulmonary disease and/or asthma, coronary artery disease and/or congestive heart failure, diabetes mellitus, lung cancer, and other malignancies (all were determined by ICD-9 codes, as described elsewhere [20, 21]); hemoglobin level, CD4 cell count, and HIV load (value closest to the survey date within 6 months; obtained from the laboratory package); and antiretroviral use (HAART vs. non- HAART; obtained from the pharmacy package). The reference date for the above variables was the date of survey completion, which, for unvaccinated patients, was the index date; for vaccinated patients, the survey and index dates (vaccine date) were different.
Statistical analysis
The primary outcome was defined as the occurrence of a first pneumonia event during the observation time. The time to event was calculated as the difference in days between the event date and the index date. Because the present analytic study focused on the association between vaccination and risk of an event, neither of which was part of the sampling scheme, the sampling weights were not used in the analysis, and those variables used in the sample design were included as covariates to control for potential residual confounding. Comparisons of continuous variables were performed using Student’s t tests and analysis of variance, and comparisons between categorical variables were performed using χ2 tests. The risk for the event was compared by PPV vaccination status and HIV infection status. Cox proportional hazards regression models were used to determine the hazard ratio (HR), controlling for age; ethnicity; tobacco use; alcohol use; drug use; diagnosis of chronic obstructive pulmonary disease and/or asthma, coronary artery disease and/or congestive heart failure, diabetes, or cancer; and hemoglobin level; for HIV-infected patients, these models were used, controlling for CD4 cell count and viral load. Results are expressed as HRs with 95% CIs. The proportionality assumption was met for all analyses. All analyses were conducted using SAS, version 9.1 (SAS Institute).
RESULTS
The VACS 5 sample included 1719 male patients. Fifty patients (10% of whom were vaccinated and 80% of whom were infected with HIV) were excluded because they died within 1 year after study entry; more HIV-infected patients than HIV-uninfected patients died within 1 year after study entry (4% vs. 1%; P < .01). An additional 43 patients were excluded because they had experienced pneumonia events in the year before the index date; more HIV-infected patients than HIV-uninfected patients experienced such events (4% vs. 1%; P < .01). There were 1626 patients remaining in the analytic sample (692 HIV-uninfected patients and 934 HIV-infected patients). Seventy-five percent of the vaccinated patients received PPV before the survey date, and 50% of all vaccinated patients received PPV within 1 year of the survey date.
Characteristics of HIV-infected patients, compared with HIV-uninfected patients
Compared with HIV-uninfected patients, HIV-infected patients were younger (49.2 vs. 55.4 years; P < .01), more likely to be African American (55% vs. 44%; P < .01), more likely to smoke (44% vs. 34%; P < .01), and more likely to have received an ICD-9 diagnosis of alcohol dependence (24% vs. 17%; P < .01) or drug dependence (30% vs. 15%; P < .01). HIV-uninfected patients were more likely than HIV-infected patients to have received diagnoses of coronary artery disease and/or congestive heart failure (17% vs. 6%; P < .01), diabetes (24% vs. 14%; P < .01), and cancer (19% vs. 14%; P < .05) and had a higher mean number of medical comorbidities (1.8 vs. 1.4; P < .01). HIV-infected patients had lower hemoglobin levels than did HIV-uninfected patients (14 g/dL vs. 14.2 g/dL; P < .05).
Characteristics of vaccinated patients, compared with unvaccinated patients
Overall, 59% of patients had been vaccinated; HIV-infected patients were more likely than HIV-uninfected patients to have been vaccinated (69% vs. 46%; P < .01). When all vaccinated patients were compared with all unvaccinated patients, the vaccinated patients were older (52.7 vs. 50.4 years; P < .05); were more likely to have received diagnoses of drug disorders (25% vs. 21%; P < .05), coronary artery disease and/or congestive heart failure (12% vs. 8%; P < .05), diabetes (22% vs. 12%; P < .01), and cancer (18% vs. 13%; P < .01); and had a higher number of comorbid conditions (1.7 vs. 1.3; P < .01). Vaccinated and unvaccinated patients did not differ with regard to ethnicity, smoking status, alcohol use at the time of the survey, or hemoglobin level. The incidence of pneumonia events during the observation period was similar among all vaccinated and all unvaccinated patients (6% vs. 6%; P = .964).
Characteristics of patients stratified by HIV infection status and vaccination status
Vaccinated HIV-uninfected patients were significantly older than unvaccinated HIV-uninfected patients and both vaccinated and unvaccinated HIV-infected patients (table 1). Unvaccinated HIV-infected patients were more likely to be black or Hispanic than either vaccinated HIV-infected patients or vaccinated HIV-uninfected patients.
Table 1.
Demographics, clinical characteristics, and outcome data for patients stratified by HIV infection status and pneumococcal polysaccharide vaccine (PPV) vaccination status.
| HIV-uninfected patients | HIV-infected patients | ||||
|---|---|---|---|---|---|
| Characteristic | Not vaccinated (n = 373) |
Vaccinated (n = 319) |
Not vaccinated (n = 294) |
Vaccinated (n = 640) |
Pa |
| Age, mean years | 52.97 | 58.28b | 48.78 | 49.37 | <.01 |
| Ethnicity | |||||
| Black | 172 (46.11) | 135 (42.31) | 167 (56.80) | 356 (54.06) | <.01 |
| White | 128 (34.31) | 131 (41.06) | 75 (25.51) | 186 (29.06) | |
| Hispanic | 52 (13.94) | 40 (12.53) | 44 (14.96) | 79 (12.34) | |
| Other | 21 (5.63) | 13 (4.07) | 8 (2.72) | 29 (4.53) | |
| Clinical characteristic | |||||
| Current smoker | 136 (36.46) | 110 (34.48) | 129 (43.87) | 287 (44.84) | <.01 |
| Alcohol dependence or abuse | 53 (14.20) | 67 (21.00)b | 72 (24.48) | 149 (23.28) | <.01 |
| Mean AUDIT score | 3.9 | 3.7 | 3.8 | 3.8 | .96 |
| Drug disorder | 44 (11.79) | 60 (18.80)b | 93 (31.63) | 184 (28.75) | <.01 |
| COPD and/or asthma | 32 (8.57) | 52 (16.30)b | 45 (15.30) | 83 (12.96) | <.05 |
| CAD and/or CHF | 42 (11.26) | 77 (24.13)b | 14 (4.76) | 40 (6.25) | <.01 |
| Diabetes | 46 (12.33) | 121 (37.93)b | 37 (12.58) | 94 (14.68) | <.01 |
| Any cancer diagnosis | 49 (13.13) | 79 (24.76)b | 35 (11.90) | 95 (14.84) | <.01 |
| Mean no. of comorbid conditions | 1.31 | 2.37b | 1.37 | 1.43 | <.01 |
| Hemoglobin level, mean g/dL | 14.33 | 14.02b | 13.89 | 14.06 | <.01 |
| HIV load, mean log RNA copies/mL | … | … | 3.19 | 2.88 | <.01 |
| HIV RNA level <500 copies/mm3, % | … | … | 24.82 | 39.84 | .09 |
| CD4 cell count, mean cells/mm3 | … | … | 406.3 | 422 | .42 |
| Square root of the CD4 cell count | … | … | 18.93 | 19.44 | .28 |
| Receiving HAART | … | … | 207 (70.4) | 478 (74.7) | .17 |
| Duration of follow-up, mean days ± SD | 725 ± 57 | 673 ± 134 | 683 ± 149 | 673 ± 145 | <.05 |
| Pneumonia events | |||||
| Total | 5 (1.34) | 7 (2.19) | 35 (11.90) | 50 (7.81)b | <.01 |
| During the 0–6-month period | 1 (0.26) | 2 (0.62) | 11 (3.74) | 16 (2.50) | <.01 |
| During the 6–12-month period | 3 (0.80) | 2 (0.62) | 9 (3.06) | 15 (2.34) | <.05 |
| During the 12–24-month period | 1 (0.26) | 3 (0.94) | 15 (5.10) | 19 (2.96) | <.01 |
NOTE. Data are no. (%) of patients, unless otherwise indicated. Age, smoking status, alcohol use, Alcohol Use Disorder Identification Test (AUDIT) score, comorbid conditions, and laboratory values for all patients correspond to those at the time of the survey. The index date for initiation of follow-up for pneumonia events was the date of vaccination and the survey date for vaccinated and unvaccinated patients, respectively. CAD, coronary artery disease; CHF, congestive heart failure; COPD, chronic obstructive pulmonary disease.
CAD, coronary artery disease; CHF, congestive heart failure; COPD, chronic obstructive pulmonary disease.
Overall comparison with the χ2 test.
Compared with the respective unvaccinated group, P < .05, by the χ2 test.
Vaccinated HIV-infected patients were significantly more likely than patients in the other groups to be current smokers. Compared with the other groups, unvaccinated HIV-infected patients were significantly more likely to have received a diagnosis of alcohol abuse or dependence or drug use disorders and to have lower hemoglobin levels. Vaccinated HIV-uninfected patients had a significantly higher mean number of comorbid conditions (2.37 comorbid conditions), compared with the other groups. There was no significant difference in CD4 cell count between vaccinated and unvaccinated HIV-infected patients; however, unvaccinated patients had a significantly higher mean log viral load (P < .01).
Effects of vaccination status, HIV infection status, and clinical characteristics on pneumonia events
Ninety-seven pneumonia events (in 6% of the patients) occurred during the study period. Fourteen of the events (14%) were related to pneumococcal-specific diagnoses (1 septicemia and pneumonia, 3 pneumonia, and 10 bacteremia). The remaining events were associated with pneumonia due to nonspecified organisms. HIV-infected patients were more likely than HIV-uninfected patients to have experienced a pneumonia event during the observation period (9.1% vs. 1.7%; P < .01) and during the 0–6-month (2.9% vs. 0.4%), 6–12-month (2.6% vs. 0.7%), and 12–24-month (3.6% vs. 0.6%) periods. There was no significant difference in the age of patients with and without pneumonia. However, patients with pneumonia were more likely to be current smokers (49.5% vs. 38.9%; P < .05); to have received a diagnosis of an alcohol disorder (30.9% vs. 20.3%; P < .05), a drug disorder (36.1% vs. 22.6%; P < .01), or chronic obstructive pulmonary disease (22.7% vs. 12.4%; P < .01); to have a lower hemoglobin level (13.4 g/dL vs. 14.1 g/dL; P < .01); and to have more comorbid conditions (2.09 vs. 1.54; P < .01). HIV-infected patients who had pneumonia had significantly lower CD4 cell counts (330.9 cells/mm3 vs. 425.8 cells/mm3; P < .01) and higher log viral loads (3.4 vs. 2.9; P < .01) than HIV-infected patients who did not have pneumonia.
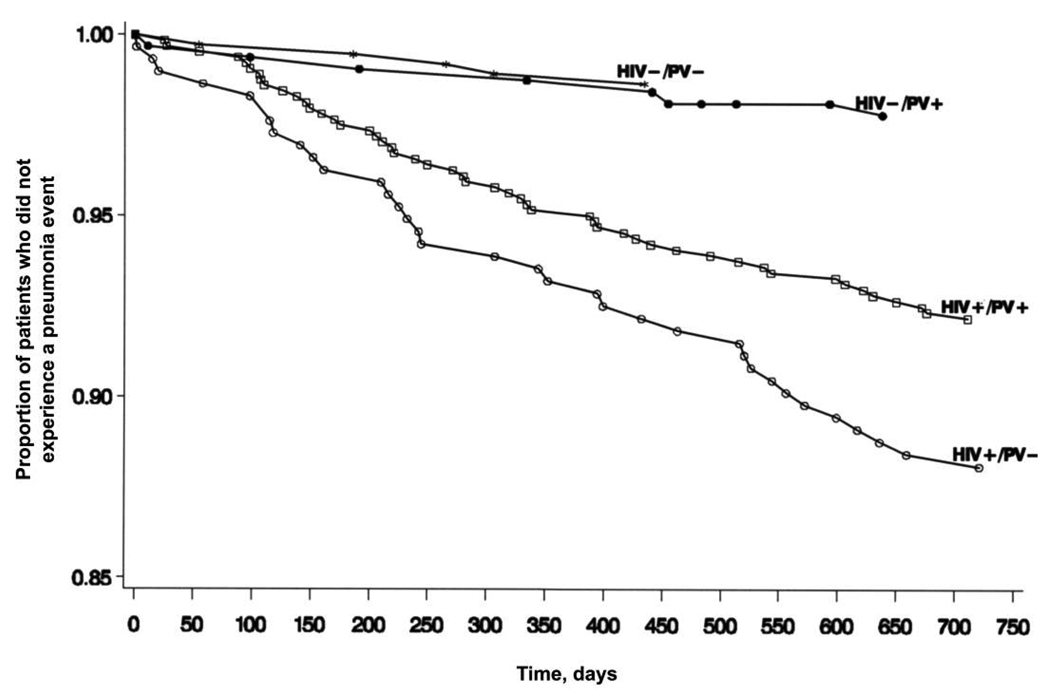
In an analysis of time to a pneumonia event (figure 1), HIV-infected patients had a higher risk of pneumonia, compared with HIV-uninfected patients (HR, 5.81; 95% CI, 3.15–10.71), and overall, vaccinated patients had a nonsignificant trend toward less risk for pneumonia (HR, 0.75; 95% CI, 0.50–1.13) (table 2). The interaction of HIV infection status and vaccination status was not significant, indicating that, prior to controlling for HIV-specific and other variables, the effect of vaccination did not vary by HIV infection status (P = .13). In the full model (table 2), having HIV infection, having received a diagnosis of chronic obstructive pulmonary disease and/or asthma, and having a low hemoglobin level were significant risk factors for pneumonia, and vaccination yielded a trend toward protection (P = .08). Additional nested models, performed to reassess the effects after deleting nonsignificant terms one at a time, yielded no substantial changes.
Figure 1.
Time of occurrence of pneumonia events for patients stratified by HIV infection status and pneumococcal vaccination (PV) status. +, condition is present; −, condition is absent.
Table 2.
Results of proportional hazards regression models predicting risk of pneumonia among all patients and among patients stratified by HIV infection status.
| Variable | HR (95% CI) |
|||
|---|---|---|---|---|
| Limited model |
Full model |
|||
| All patients n = 1628 |
All patients n = 1628 |
HIV-uninfected patients n = 692 |
HIV-infected patients n = 934 |
|
| HIV infection | 5.81a (3.15–10.71) | 6.26a (3.28–11.93) | NA | NA |
| PPV vaccination | 0.75 (0.50–1.13) | 0.69 (0.46–1.05) | 0.85 (0.25–2.95) | 0.65a (0.42–1.00) |
| Age | … | 1.01 (0.99–1.04) | 1.03 (0.99–1.10) | 1.01 (0.99–1.04) |
| Black | … | 0.84 (0.27–2.58) | 0.46 (0.06–3.82) | 1.12 (0.31–4.05) |
| White | … | 0.96 (0.30–3.09) | 0.37 (0.04–3.37) | 1.30 (0.34–4.99) |
| Hispanic | … | 0.61 (0.18–2.06) | 0.28 (0.02–4.26) | 0.83 (0.22–3.16) |
| Current smoker | … | 1.48 (0.97–2.26) | 1.15 (0.30–4.41) | 1.62a (1.03–2.55) |
| Alcohol disorder | … | 1.18 (0.64–2.17) | 1.05 (0.20–5.55) | 1.20 (0.62–2.30) |
| Drug disorder | … | 1.10 (0.60–2.02) | 1.80 (0.33–9.90) | 1.02 (0.54–1.95) |
| Diabetes | … | 1.45 (0.88–2.39) | 2.19 (0.63–7.56) | 1.39 (0.78–2.46) |
| CAD and/or CHF | … | 1.78 (0.98–3.23) | 2.02 (0.59–6.87) | 1.59 (0.78–3.25) |
| COPD and/or asthma | … | 1.66a (1.01–2.72) | 2.68 (0.71–10.16) | 1.43 (0.83–2.46) |
| Cancer | … | 0.91 (0.50–1.65) | 0.99 (0.25–4.02) | 0.89 (0.46–1.73) |
| Hemoglobin level | … | 0.78a (0.69–0.89) | 0.70 (0.48–1.04) | 0.85a (0.74–0.97) |
| Log viral load | … | … | … | 1.18b (1–1.4) |
| Square root of the CD4 cell count | … | … | … | 0.96a (0.93–1.00) |
| HAART | … | … | … | 1.20 (0.71–2.04) |
NOTE. Data are presented as a limited model, which includes only HIV status and pneumococcal polysaccharide vaccine (PPV) vaccination status as variables, and as a full model, which includes all of the specified variables.
CAD, coronary artery disease; CHF, congestive heart failure; COPD, chronic obstructive pulmonary disease; HR, hazard ratio; NA, not applicable.
P < .05.
P = .06.
Among HIV-infected patients, after controlling for HIV-specific variables, vaccination significantly reduced the risk of pneumonia (HR, 0.65; 95% CI, 0.42–1.00; P < .05) (table 2 and figure 1). Among this group, smoking, low hemoglobin level, and low CD4 cell count at the time of the survey were risk factors for pneumonia, and low viral load yielded a trend toward protection (P = .06).
Among HIV-uninfected patients, vaccination was not significantly associated with a reduction in risk of pneumonia (HR, 0.85; 95% CI, 0.25–2.95), possibly because of lack of power to detect an effect. The HR for the medical comorbidities known to be associated with pneumonia was not statistically significant. However, a lower hemoglobin level at the time of the survey was associated with a trend for an increased risk (P = .07).
DISCUSSION
In this study, we examined the effect of vaccination on the incidence of pneumonia events in a cohort of HIV-infected and HIV-uninfected patients with a high frequency of medical and substance use comorbidities. We found that HIV-infected patients had a significantly higher frequency of pneumonia events, compared with their HIV-uninfected counterparts, and that HIV-infected patients experienced a protective effect from PPV vaccination after controlling for HIV-related variables (CD4 cell count and viral load) and other medical comorbidities. Among the HIV-uninfected patients, we found no statistically significant benefit from vaccination, possibly because of lack of power to detect an effect.
The VACS 5 HIV-infected group is composed of older participants, compared with other HIV-infected cohorts [17], and the group has a high proportion of black patients, a high rate of smoking, and a high prevalence of comorbidities, all of which are factors associated with increased risk of pneumococcal disease [2, 8, 22, 23]. Furthermore, black persons may obtain less protection from pneumococcal vaccination [22]. Yet, despite the numerous risk factors for pneumonia in our population, PPV vaccination was protective among the HIV-infected patients.
We did not find an increased risk of pneumonia events in the first 6 months following vaccination; the rate of pneumonia events was similar during the first and second 6-month periods and during the second year of observation. More than 70% of our HIV-infected patients were receiving HAART at the time of the survey. Use of HAART may explain the discrepant results between the Ugandan study [14] and studies from Europe and North America [4, 15, 16]. Both CD4 cell count and HIV viremia affect B cell responses among HIV-infected individuals, and HAART-induced improvements in either parameter translate into improvements of B cell function [24, 25]. In addition, HAART may induce improved qualitative and quantitative antibody responses to pneumococcal antigens [26, 27]. In our multivariate analysis, CD4 cell count but not viral load or use of HAART had a significant effect on the incidence of pneumonia among our patients.
Most observational studies show that PPV is effective in preventing invasive pneumococcal disease among older adults and adults with underlying medical conditions; however, its effectiveness in preventing pneumococcal pneumonia or pneumonia of any cause has been more difficult to demonstrate [28]. In our study, the lack of effectiveness of PPV among the HIV-uninfected group may have explanations other than the potential lack of vaccine efficacy. Among veterans receiving treatment, PPV is given routinely to those who have an indication for vaccination [9]. Consequently, the unvaccinated HIV-uninfected patients were younger and had a lower mean number of comorbid conditions, compared with the vaccinated HIV-uninfected patients. If the unvaccinated patients had been a matched group with similar risk for pneumonia, the effect of vaccination might have been evident.
We elected as an outcome the composite of specific pneumococcal diagnoses and diagnoses of pneumonia due to non-specified organisms. We chose a broad definition, because in clinical practice, an etiologic diagnosis of pneumonia is rarely made [29]. Indeed, 86% of the pneumonia events that we examined were cases of pneumonia without a microbiologic diagnosis, and only 14% were specific pneumococcal disease diagnoses.
Our study has a number of strengths when examining the effect of vaccination on the incidence of pneumonia. The rates of vaccination and documentation of vaccination status within the VA system are high (driven by electronic clinical reminders and electronic documentation of all vaccinations, even if received outside the VA setting) [30]; thus, the risk of biasing the results on the effectiveness of vaccination because of incorrect assignment of vaccination status was low. However, this bias is differential, and it could bias the estimate of effect in either direction. HIV-infected and HIV-uninfected participants in VACS 5 report 72% and 71% of their inpatient care and 82% and 83% of their outpatient care, respectively, from the VA [29]. In addition, all visits to outpatient clinics or emergency departments, as well as hospital admissions, that occur at any VA facility are documented through electronic medical records. Furthermore, within VACS 5, data collected from surveys complement the electronic chart information [17]. We included as an outcome both inpatient and outpatient diagnoses to maximize the chances of observing an effect and to eliminate the potential bias caused by clinicians being more inclined to admit to the hospital HIV-infected patients who present with pneumonia. Thus, the risk of biasing results because of inadequate detection of an event was also low and unlikely to be asymmetric by HIV infection or PPV vaccination status. There may still be other potential biases that we cannot account for, such as differential care-seeking behavior by HIV infection status among patients with symptoms suggestive of pneumonia.
One weakness of our analysis is that the survey information, laboratory data, and comorbidity data were linked to the survey date. For 50% of the vaccinated patients, the vaccine date and the survey date were >1 year apart; thus, conclusions from the analysis that take into consideration these parameters need to be interpreted with caution. In addition, patients classified as unvaccinated (no vaccination during the 3 years before the survey date) could have received PPV before that date. This misclassification would have biased our results toward the null. Because not all of the VACS 5 participants receive their inpatient and outpatient care at the VA, it is possible that we did not capture all of the clinical events of interest; however, because the percentage of VA use was similar between both HIV-infected and HIV-uninfected groups, the results should not have been differentially affected.
Consistent with prior studies, risk factors for pneumonia among HIV-infected patients were smoking and lower CD4 cell count [2, 16, 22, 31]. We also found that lower hemoglobin level was a risk factor for a pneumonia event. After adjusting for comorbidities and other variables, lower hemoglobin level remained significantly associated with the risk of pneumonia events among the whole group and among HIV-infected patients. This potential association has been described for pneumococcal [32] and other bacterial infections [33] and deserves further exploration.
In summary, we found that, among HIV-infected patients, PPV vaccination offered protection against pneumonia events. Because vaccination with PPV has minimal apparent clinical deleterious effect and, despite all of the caveats described above, may have a beneficial effect among this group at high risk for pneumonia, our findings support the current recommendation of vaccination of HIV-infected patients. In addition to immune preservation and reconstitution through HAART and pneumococcal vaccination, smoking cessation needs to be more aggressively implemented as a strategy for preventing pneumonia among HIV-infected patients.
Acknowledgments
Financial support. National Institute on Alcohol and Alcohol Abuse (U01 AA 13566 and U10 AA 13566), National Institute of Aging (NIA; K23 AG00826), Robert Wood Johnson Generalist Faculty Scholar Award, and an interagency agreement between the NIA and the National Institute of Mental Health.
Footnotes
Potential conflicts of interest. All authors: no conflicts.
References
- 1.Musher DM. Streptococcus pneumoniae. In: Bennett JB, Mandell GL, Dolin R, editors. Principles and practice of infectious diseases. Philadelphia: Elsevier; 2005. pp. 2392–2411. [Google Scholar]
- 2.Feikin DR, Feldman C, Schuchat AJ, Janoff EN. Global strategies to prevent bacterial pneumonia in adults with HIV disease. Lancet Infect Dis. 2004;4:445–455. doi: 10.1016/S1473-3099(04)01060-6. [DOI] [PubMed] [Google Scholar]
- 3.Heffernan RT, Barrett NL, Gallagher KM, et al. Declining incidence of invasive Streptococcus pneumoniae infections among persons with AIDS in an era of highly active antiretroviral therapy, 1995–2000. J Infect Dis. 2005;191:2038–2045. doi: 10.1086/430356. [DOI] [PubMed] [Google Scholar]
- 4.López-Palomo C, Martín-Zamorano M, Benítez E, et al. Pneumonia in HIV-infected patients in the HAART era: incidence, risk, and impact of the pneumococcal vaccination. J Med Virol. 2004;72:517–524. doi: 10.1002/jmv.20045. [DOI] [PubMed] [Google Scholar]
- 5.Paul S, Gilbert HM, Ziecheck W, Jacobs J, Sepkowitz KA. The impact of potent antiretroviral therapy on the characteristics of hospitalized patients with HIV infection. AIDS. 1999;13:415–418. doi: 10.1097/00002030-199902250-00015. [DOI] [PubMed] [Google Scholar]
- 6.Nuorti JP, Butler JC, Gelling L, Kool JL, Reingold AL, Vugia DJ. Epidemiologic relation between HIV and invasive pneumococcal disease in San Francisco County, California. Ann Intern Med. 2000;132:182–190. doi: 10.7326/0003-4819-132-3-200002010-00003. [DOI] [PubMed] [Google Scholar]
- 7.Jordano Q, Falcó V, Almirante B, et al. Invasive pneumococcal disease in patients infected with HIV: still a threat in the era of highly active antiretroviral therapy. Clin Infect Dis. 2004;38:1623–1628. doi: 10.1086/420933. [DOI] [PubMed] [Google Scholar]
- 8.Kyaw MH, Rose CE, Jr, Fry AM, et al. The influence of chronic illnesses on the incidence of invasive pneumococcal disease in adults. J Infect Dis. 2005;192:377–386. doi: 10.1086/431521. [DOI] [PubMed] [Google Scholar]
- 9.Centers for Disease Control and Prevention. Prevention of pneumococcal disease: recommendations of the Advisory Committee on Immunization Practices (ACIP) MMWR Recomm Rep. 1997;46:1–24. [PubMed] [Google Scholar]
- 10.Whitney CG, Schaffner W, Butler JC. Rethinking recommendations for use of pneumococcal vaccines in adults. Clin Infect Dis. 2001;33:662–675. doi: 10.1086/322676. [DOI] [PubMed] [Google Scholar]
- 11.Jackson LA, Neuzil KM, Yu O, et al. Effectiveness of pneumococcal polysaccharide vaccine in older adults. N Engl J Med. 2003;348:1747–1755. doi: 10.1056/NEJMoa022678. [DOI] [PubMed] [Google Scholar]
- 12.Musher DM, Rueda-Jaimes AM, Graviss EA, Rodriguez-Barradas MC. Effect of pneumococcal vaccination: a comparison of vaccination rates in patients with bacteremic and nonbacteremic pneumococcal pneumonia. Clin Infect Dis. 2006;43:1004–1008. doi: 10.1086/507699. [DOI] [PubMed] [Google Scholar]
- 13.Masur H, Kaplan JE, Holmes KK. Guidelines for preventing opportunistic infections among HIV-infected persons—2002: recommendations of the U.S. Public Health Service and the Infectious Diseases Society of America. Ann Intern Med. 2002;137:435–478. doi: 10.7326/0003-4819-137-5_part_2-200209031-00002. [DOI] [PubMed] [Google Scholar]
- 14.French N, Nakiyingi J, Carpenter LM, et al. 23-valent pneumococcal polysaccharide vaccine in HIV-1–infected Ugandan adults: double-blind, randomised and placebo controlled trial. Lancet. 2000;355:2106–2111. doi: 10.1016/s0140-6736(00)02377-1. [DOI] [PubMed] [Google Scholar]
- 15.Dworkin MS, Ward JW, Hanson DL, Jones JL, Kaplan JE Adult and Adolescent Spectrum of HIV Disease Project. Pneumococcal disease among human immunodeficiency virus–infected persons: incidence, risk factors, and impact of vaccination. Clin Infect Dis. 2001;32:794–800. doi: 10.1086/319218. [DOI] [PubMed] [Google Scholar]
- 16.Gebo KA, Moore RD, Keruly JC, Chaisson RE. Risk factors for pneumococcal disease in human immunodeficiency virus–infected patients. J Infect Dis. 1996;173:857–862. doi: 10.1093/infdis/173.4.857. [DOI] [PubMed] [Google Scholar]
- 17.Justice AC, Dombrowski E, Conigliaro J, et al. Veterans Aging Cohort Study (VACS): overview and description. Med Care. 2006;44 Suppl 2:13–24. doi: 10.1097/01.mlr.0000223741.02074.66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Navin TR, Rimland D, Lennox JL, et al. Risk factors for community-acquired pneumonia among persons infected with human immunodeficiency virus. J Infect Dis. 2000;181:158–164. doi: 10.1086/315196. [DOI] [PubMed] [Google Scholar]
- 19.Fedson DS, Baldwin JA. Previous hospital care as a risk factor for pneumonia: implications for immunization with pneumococcal vaccine. JAMA. 1982;248:1989–1995. [PubMed] [Google Scholar]
- 20.Justice AC, Lasky E, McGinnis KA, et al. Medical disease and alcohol use among veterans with human immunodeficiency infection: a comparison of disease measurement strategies. Med Care. 2006;44 Suppl 2:52–60. doi: 10.1097/01.mlr.0000228003.08925.8c. [DOI] [PubMed] [Google Scholar]
- 21.Crothers K, Griffith TA, McGinnis KA, et al. The impact of cigarette smoking on mortality, quality of life, and comorbid illness among HIV-positive veterans. J Gen Intern Med. 2005;20:1142–1145. doi: 10.1111/j.1525-1497.2005.0255.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Breiman RF, Keller DW, Phelan MA, et al. Evaluation of effectiveness of the 23-valent pneumococcal capsular polysaccharide vaccine for HIV-infected patients. Arch Intern Med. 2000;160:2633–2638. doi: 10.1001/archinte.160.17.2633. [DOI] [PubMed] [Google Scholar]
- 23.Burns DN, Hillman D, Neaton JD, et al. Cigarette smoking, bacterial pneumonia, and other clinical outcomes in HIV-1 infection. Terry Beirn Community Programs for Clinical Research on AIDS. J Acquir Immune Defic Syndr Hum Retrovirol. 1996;13:374–383. doi: 10.1097/00042560-199612010-00012. [DOI] [PubMed] [Google Scholar]
- 24.Moir S, Ogwaro KM, Malaspina A, et al. Perturbations in B cell responsiveness to CD4+ T cell help in HIV-infected individuals. Proc Natl Acad Sci U S A. 2003;100:6057–6062. doi: 10.1073/pnas.0730819100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Moir S, Malaspina A, Ogwaro KM, et al. HIV-1 induces phenotypic and functional perturbations of B cells in chronically infected individuals. Proc Natl Acad Sci U S A. 2001;98:10362–10367. doi: 10.1073/pnas.181347898. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Subramaniam KS, Segal R, Lyles RH, Rodriguez-Barradas MC, Pirofski LA. Qualitative change in antibody responses of human immunodeficiency virus–infected individuals to pneumococcal capsular polysaccharide vaccination associated with highly active antiretroviral therapy. J Infect Dis. 2003;187:758–768. doi: 10.1086/368331. [DOI] [PubMed] [Google Scholar]
- 27.Rodriguez-Barradas MC, Alexandraki I, Nazir T, et al. Response of human immunodeficiency virus–infected patients receiving highly active antiretroviral therapy to vaccination with 23-valent pneumococcal polysaccharide vaccine. Clin Infect Dis. 2003;37:438–447. doi: 10.1086/375841. [DOI] [PubMed] [Google Scholar]
- 28.David S, Fedson DMM. Pneumococcal polysaccharide vaccine. In: Orenstein WA, Plotkin SA, Offit PA, editors. Vaccines. Philadelphia: Saunders; 2004. pp. 529–588. [Google Scholar]
- 29.Goulet JL, Fultz SL, Rimland D, et al. Do patterns of comorbidity vary by HIV status, age, and HIV severity? Clin Infect Dis. 2007;45:1593–1601. doi: 10.1086/523577. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Chi RC, Reiber GE, Neuzil KM. Influenza and pneumococcal vaccination in older veterans: results from the behavioral risk factor surveillance system. J Am Geriatr Soc. 2006;54:217–223. doi: 10.1111/j.1532-5415.2005.00577.x. [DOI] [PubMed] [Google Scholar]
- 31.Guerrero M, Kruger S, Saitoh A, et al. Pneumonia in HIV-infected patients: a case-control survey of factors involved in risk and prevention. AIDS. 1999;13:1971–1975. doi: 10.1097/00002030-199910010-00021. [DOI] [PubMed] [Google Scholar]
- 32.Musher DM, Alexandraki I, Graviss EA, et al. Bacteremic and non-bacteremic pneumococcal pneumonia: a prospective study. Medicine (Baltimore) 2000;79:210–221. doi: 10.1097/00005792-200007000-00002. [DOI] [PubMed] [Google Scholar]
- 33.Musher DM, Lamm N, Darouiche RO, Young EJ, Hamill RJ, Landon GC. The current spectrum of Staphylococcus aureus infection in a tertiary care hospital. Medicine (Baltimore) 1994;73:186–208. doi: 10.1097/00005792-199407000-00002. [DOI] [PubMed] [Google Scholar]