Abstract
An understanding of how axons elongate is needed to develop rational strategies to treat neurological diseases and nerve injury. Growth cone-mediated neuronal elongation is currently viewed as occurring through cytoskeletal dynamics involving the polymerization of actin and tubulin subunits at the tip of the axon. However, recent work suggests that axons and growth cones also generate forces (through cytoskeletal dynamics, kinesin, dynein, and myosin), forces induce axonal elongation, and axons lengthen by stretching. This review highlights results from various model systems (Drosophila, Aplysia, Xenopus, chicken, mouse, rat, and PC12 cells), supporting a role for forces, bulk microtubule movements, and intercalated mass addition in the process of axonal elongation. We think that a satisfying answer to the question, “How do axons grow?” will come by integrating the best aspects of biophysics, genetics, and cell biology.
1. Introduction: How Do Axons Grow?
Despite a century of investigation since the pioneering work of Ramón y Cajal, a satisfying answer to the question “How do axons grow?” still eludes us. This question is interesting for at least two reasons: Firstly, understanding the mechanism of axonal elongation is essential for acquiring a better picture of what happens during the development of the nervous system (Lowery and Van Vactor, 2009). Secondly and perhaps more practically, if we have a better understanding of how axons grow, we can devise rational strategies to overcome intrinsic limitations to regrowth and to accelerate regeneration following injury and disease (Chen et al., 2007). Enormous gains have recently been made in our understanding of the cell biology of axonal growth: force generation by molecular motors and microtubule dynamics have emerged as crucial processes for both axonal guidance and lengthening (Conde and Caceres, 2009; Vallee et al., 2009). Yet most studies to date have used relatively simple read-outs, such as measuring changes in the rates of axonal elongation, rather than characterizing and quantifying the underlying behavior of individual axonal and growth cone components. Thus, in order to achieve a better understanding of the mechanisms of axonal elongation, quantitative biophysical and imaging methods are needed to analyze the relationship between (1) forces acting on neurons, (2) the bulk movement of cytoskeletal elements/organelles in response to forces, and (3) cytoskeletal assembly/disassembly dynamics inside living neurons. In this review, we discuss the underlying cytoskeletal mechanisms of tension-induced axonal elongation. In the first part, we highlight recent key studies providing evidence for the role of tension in driving axonal elongation. This has been an understudied problem, but we think it is very important because it offers new insights into the process of axonal elongation. We then follow with a discussion of how forces could affect microtubule polymerization/translocation dynamics during axonal elongation and growth cone advance. Lastly, we discuss potential mechanisms of how forces could translate into changes of cellular physiology and signaling as well as the question whether a universal mechanism of axonal elongation exists across different species.
2. Forces and Axonal Elongation
2.1 Forces Cause Axons to Grow
It is utterly remarkable that neurons can grow to the length of 30 m in blue whales and more than 1 m in humans (Smith, 2009), perhaps even more so when considering that most of this growth happens after synapse formation and is driven by the increase in body size of the animal. This mechanism of elongation has long been recognized: Harrison (1935) called it “passive stretching” and Weiss (1941) called it “towed growth”. Stretch growth of axons likely begins during embryogenesis. As the animal's body grows, the distances between neuronal cell bodies and synapses steadily increase, thereby exerting tensile forces on the axons. In a series of innovative in vitro studies, the growth cones of cultured chick sensory axons were attached to glass needles to examine their response to forces (Bray, 1984; Lamoureux et al., 1989). Axons could be stretched up to 100 μm over a few hours without apparent thinning or disruption of the cytoskeleton. More recent studies have confirmed that externally applied forces potently induce axonal elongation. In the context of this problem the work from Smith's group is particularly interesting. In an effort to design strategies for improved axonal regeneration following injury, they developed a specialized chamber system in which neurons are cultured on two initially contiguous platforms that are pulled apart by a stepper motor (Pfister et al., 2006; Pfister et al., 2004). The axons of neurons plated onto these platforms can be elongated to lengths of 10 cm at a sustained rate of 8 mm/d (330 μm/h). This is approximately ten times faster than typical growth-cone mediated axonal outgrowth rates (See Table 1.1 in Gordon-Weeks, 2000) and can be continued for many days. Furthermore, these neurons tend to increase in diameter (Pfister et al., 2004) and are functionally normal in their electrophysiology (Pfister et al., 2006). In a separate study, the effect of leg lengthening on axonal stretching was examined in vivo (Abe et al., 2004). As an indirect way to determine if axons stretch, the distance between nodes of Ranvier was measured as an assay. In myelinated axons in the peripheral nervous system, Schwann cells are wrapped tightly around axons. Stretching of the underlying axon causes lengthening of the Schwann cells and the internodal distance. Using orthopedic leg-lengthening procedures in adult rats, it was found that applied forces could double inter-nodal distances without significant axonal thinning. An important aspect of all the studies discussed above is that forces were applied at low levels over long time periods: hours to days. Notably, acute stretching resulting in high tension, as it occurs clinically when large nerve gaps are directly joined, impairs axonal regeneration (Sunderland et al., 2004; Yi and Dahlin, 2010). Together these results indicate that forces, when carefully controlled, are powerful stimulators of axonal elongation.
2.2 Neurons Generate Forces
With the advent of nanotechnology and sophisticated software to track microscopic movements, there has been a surge of interest in neuronal biomechanics. Several recent reviews focused on biophysical properties of neurons. Ayali (2010) discusses the role of forces in neuronal morphology, network formation, and the effects of substrate stiffness. Franze et al., (2009) have written an excellent book chapter covering the foundations of rheology, measurement techniques, and the viscoelastic properties of neurons and the brain. Bueno and Shah (2008) discuss the effects of tensile loading on neurons and the nervous system. Lastly, Franze and Guck (2010) recently published a comprehensive review on the biophysics of neuronal growth and the susceptibility of neurons to physical cues. In brief, the methods used to study the physical properties of neurons have innovatively utilized nanowires (Hallstrom et al., 2010), force calibrated glass needles (Bernal et al., 2007), microfabricated silicon-based micromechanical force sensors (Siechen et al., 2009), optical stretchers (Lu et al., 2006), stretchable polydimethylsiloxane (PDMS) substrates (Ahmed et al., 2010), and polyacrylamide gel-based compliant substrates (Chan and Odde, 2008). Using these approaches the significant findings have been that (1) tension generation by growth cones is higher on softer (i.e. < ~ 1 kiloPascal) substrates (Chan and Odde, 2008), (2) glial cells provide a soft substrate that may facilitate axonal elongation (Lu et al., 2006), (3) active force generation in neurons causes them to shorten when slackened (Ahmed et al., 2010; Bernal et al., 2007), and (4) the rest tension of axons both in vivo and in vitro is in the range of 1–10 nN (Hallstrom et al., 2010; Rajagopalan et al., 2010; Siechen et al., 2009). We think there is great promise in the application of these approaches to longstanding problems in the field of molecular cell biology, not only of neurons but cells in general. Specifically, these techniques will provide qualitative and quantitative information about the relationships between forces, molecular motors, cytoskeletal dynamics, signaling pathways, substrate adhesion and stiffness contributing to axonal elongation.
2.3 Cytoskeletal Assembly is Integral to Axonal Elongation
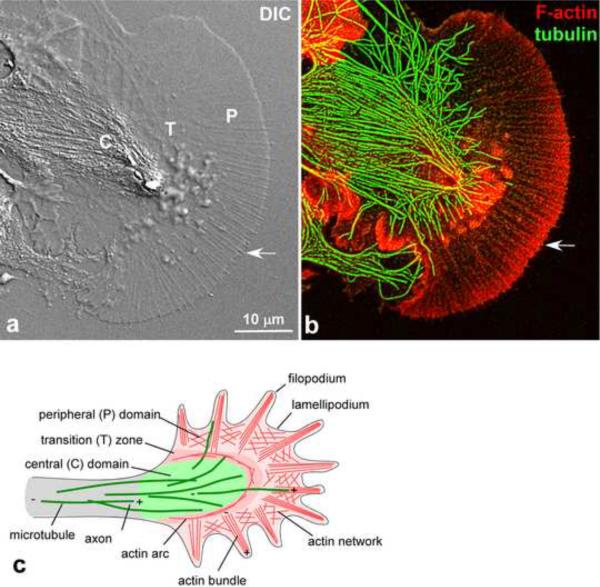
Most diagrams of axonal elongation are, in essence, models of microtubule and actin dynamics occurring in the growth cone as it grows in response to extracellular cues (Conde and Caceres, 2009; Dent and Gertler, 2003; Lowery and Van Vactor, 2009). The actin cytoskeleton in the peripheral (P) domain and transition (T) zone of the growth cone is highly dynamic and is constantly turning over, while the less well-characterized F-actin structures in the central (C) domain appear to be more stable (Fig. 1). Actin filaments are assembled at the filopodial tips and at the leading edge of the intervening lamellipodial veils, retrogradely transported by a process referred to as retrograde flow and then recycled and disassembled in the T zone (Forscher and Smith, 1988; Lin and Forscher, 1995; Schaefer et al., 2002). The majority of the retrograde actin flow is powered by myosin II in the T zone, while actin assembly push against the plasma membrane appears to contribute to flow as well (Lin et al., 1996; Medeiros et al., 2006). While this process results in a relatively simple and regular turnover of the actin cytoskeleton in growth cones, the dynamics of microtubules is much more complex, particularly in the growth cone P domain (Fig. 1) (Dent and Kalil, 2001; Schaefer et al., 2002; Suter et al., 2004; Tanaka and Kirschner, 1991).
Figure 1.
Cytoplasmic Domain Organization and Cytoskeletal Structures in Neuronal Growth Cones.
(a) Differential interference contrast image of an Aplysia bag cell neuronal growth cone on poly-L-lysine substrate. The C domain is rich in organelles, the T zone has ruffling or intrapodia activity, the P domain contains alternating filopodia (arrow) and lamellipodial veils. (b) Corresponding cytoskeletal labeling of the growth cone shown in (a) following fixation. F-actin was labeled with fluorescently labeled phalloidin, while microtubules were detected by tubulin immunofluorescence. Images in (a) and (b) were acquired by Aih Cheun Lee in the Suter lab. (c) Schematic of growth cone with cytoplasmic domains and cytoskeletal structures indicated. Actin arcs surround the C domain. Plus and minus ends of microtubules and actin filaments are indicated.
Early studies using pharmacological inhibitors of microtubule dynamics demonstrated that microtubule assembly is critical for axonal elongation (Bamburg et al., 1986; Letourneau and Ressler, 1984), but gave the impression that microtubule polymerization in the growth cone is a mechanism of axonal elongation as discussed by Mitchison and Kirschner (1988) over 20 years ago. Taken together, most current models of axonal elongation suggest that dynamic exploratory microtubules (Conde and Caceres, 2009; Sabry et al., 1991; Tanaka and Kirschner, 1991) are important for axonal guidance, and that microtubule assembly in the growth cone (as the end result of slow axonal transport) is critical for axonal lengthening (Conde and Caceres, 2009; Dent and Gertler, 2003; Lowery and Van Vactor, 2009) (Fig 2a).
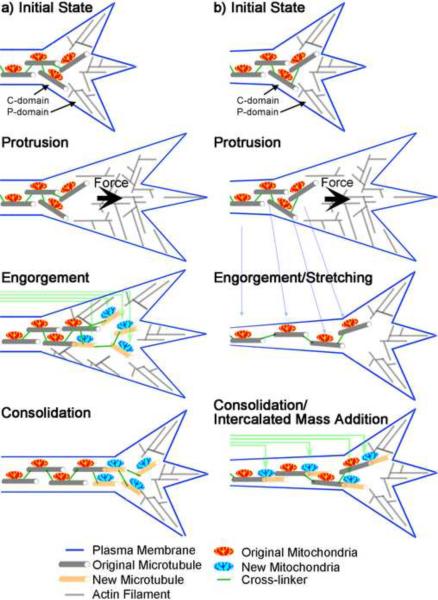
Figure 2.
Two Models of Axonal Elongation
(a) The Standard Protrusion, Engorgement, and Consolidation Model. The actin cytoskeleton protrudes forward through polymerization at the leading edge of the cell. Forces generated at the interface of the C and P domains clear a corridor for the assembly of microtubules. Engorgement occurs as microtubule polymerization and the delivery of organelles by fast transport adds new material at the tip of the C domain. Consolidation occurs as microtubules in the neck of the growth cone are bundled and actin filaments are disassembled. (b) The Stretch and Intercalated Growth Model. Protrusion occurs by assembly as in the previous model, but Engorgement differs in that forces generated in the growth cone pull the C domain and the rest of the axon forward and stretch the axon. Consolidation occurs as pulling forces bundle the microtubules and new mass is added along the length of the axon in an intercalated fashion to prevent thinning (green arrows). In this model, the growth cone is not assembled at the leading edge and disassembled at the neck; instead the entire growth cone advances as a coherent unit. Time progresses from top to bottom. Panel B is used with permission from Lamoureux et al., “Growth and Elongation Within and Along the Axon” Developmental Neurobiology, Wiley Periodicals, Inc. Copyright 2009 Wiley.
2.4 Slow Axonal Transport and Axonal Elongation
Historically, slow axonal transport and axonal elongation are conceptually linked (Reinsch et al., 1991). Both occur at about the same rate (~ 1 mm/d), and it is intuitive that an axon can only elongate at the rate of its most slowly transported essential components (e.g. tubulin). A series of recent experiments now suggests that the addition of new cytoskeletal mass to the axon involves the transport of individual microtubule polymers as well as the transport of soluble tubulin subunits. In brief, it is agreed that close to the cell body the axonal framework is stationary relative to the substrate, whereas microtubule-based motors such as kinesin transport either soluble cytoskeletal elements or polymers (Baas et al., 2006; Jung and Brown, 2009; Kuznetsov et al., 2010; Miller and Joshi, 1996; Roy et al., 2008; Terada et al., 2010; Terada et al., 2000) to generate slow axonal transport (Miller and Heidemann, 2008). Nonetheless, there is a paradox as to how axons can elongate at a rate faster than slow axonal transport. In particular, Pfister et al. have reported rates of elongation at fast as 8 mm/day for stretch grown neurons, whereas the commonly cited rate for slow tubulin transport is 0.3 – 3 mm/day (Brown, 2000). One possible explanation for this apparent discrepancy is that local protein synthesis in the axon supplies the extra protein (Hengst et al., 2009; Jung and Holt, 2011; Lin and Holt, 2008; Roche et al., 2009). Along these lines, one could envision a mechanotransduction pathway that is activated by axonal stretching and increases protein synthesis both in the cell body and along the axon similarly to stretch-activated protein translation in muscle cells (Kijima et al., 1996). A second possibility envisions a mechanism whereby stretching itself increases the rate of slow axonal transport. A mathematical analysis of Pfister's work (Pfister et al., 2004) suggests that almost half of the total axonal tubulin is being transported by stretching when the axons are elongating rapidly (O'Toole and Miller, 2011). This raises the possibility that the rate of slow axonal transport is not fixed, but varies with the rate of axonal elongation.
2.5 Forces Generated at the Growth Cone Cause Axons to Stretch
Challenges in studying bulk microtubule movements in axons quantitatively are that microtubules are difficult to mark (typically requiring microinjection of fluorescently labeled tubulin), the axons tend to stop growing under the intense imaging conditions needed to see the marks, and the marks disappear in less than an hour because of dynamic instability. To study bulk movement in chick sensory neurons, Miller and colleagues monitored the movement of docked mitochondria, axonal branch points, and beads bound to the axons (Lamoureux et al., 2010a; Miller and Sheetz, 2006; O'Toole et al., 2008a). As was previously reported, the marks close to the cell body were stationary (Hirokawa et al., 1997). In contrast, the marks close to the growth cone advanced. Because the distance between marks increased over time, the data suggested that the slow movement was a result of stretching of the axonal framework caused by tension generated by the growth cone (Fig. 2b). To better understand this problem a mathematical model was developed that incorporates force generation at the growth cone, the viscoelastic properties of the axon, and adhesions between the axon and substrate (O'Toole et al., 2008a). Using force-calibrated needles to apply and measure forces at the growth cone, coherent low velocity axonal transport was identified that decreased away from the growth cone. Additional studies by Shah's group, performed with rat dorsal root ganglion neurons stretched on silicone sheets, revealed that globally the axon behaves as a viscoelastic continuum. Below a characteristic length, though, it appears to behave as a series of independent linked elements that may be microtubules, actin filaments, or neurofilaments (Chetta et al., 2010). To determine if mass addition is coupled to stretching, the addition of new mitochondria to Drosophila axons was examined in vivo and changes in axonal diameter during stretching were analyzed in vitro (Lamoureux et al., 2010a; O'Toole et al., 2008b). In both cases, clear evidence suggests that new material is added along the lengths of axons (Fig. 2b). Nonetheless, this only sharpens the question of how new microtubules are added to growing axons.
3. Force Generation by Growth Cones
3.1 The Role of Actin and Microtubules in Advancing Growth Cones
The early work by Paul Letourneau has clearly shown that both “push” by microtubules and “pull” by actomyosin in the neuronal growth cone play a role in axonal elongation (Letourneau et al., 1987). A number of follow up studies using several neuronal systems have shown that actin-microtubule interactions are essential for axonal elongation, pathfinding, and branching (Buck and Zheng, 2002; Challacombe et al., 1996; Dent and Kalil, 2001; Geraldo and Gordon-Weeks, 2009; Lee and Suter, 2008; Schaefer et al., 2008; Zhou et al., 2002). Significant insights into the organization, dynamics, and interaction of these two cytoskeletal structures have emerged from studies conducted on Aplysia growth cones, which are 5–10× larger than growth cones from other frequently used model systems. Their large size, well-organized cytoplasmic regions, and relative slow movement rate on poly-lysine substrates allow a detailed quantitative analysis of both actin and microtubule dynamics at high spatiotemporal resolution using fluorescent speckle microscopy (FSM) (Schaefer et al., 2002; Waterman-Storer et al., 1998). Actin and microtubule FSM revealed that microtubules use filopodial actin bundles as polymerization guides. Yet at the same time, they are swept back through coupling to the retrograde actin flow (Burnette et al., 2007; Schaefer et al., 2002) (Fig. 3a). These two mechanisms result in a relatively low density of highly dynamic and oriented, co-linear with actin bundles, microtubules in the growth cone periphery (Fig. 1b). This observation raises interesting questions: 1. What is the purpose of retrograde actin flow and dynamic microtubules for directional growth cone movement? 2. How does the growth cone build up pulling force in substrate-mediated growth?
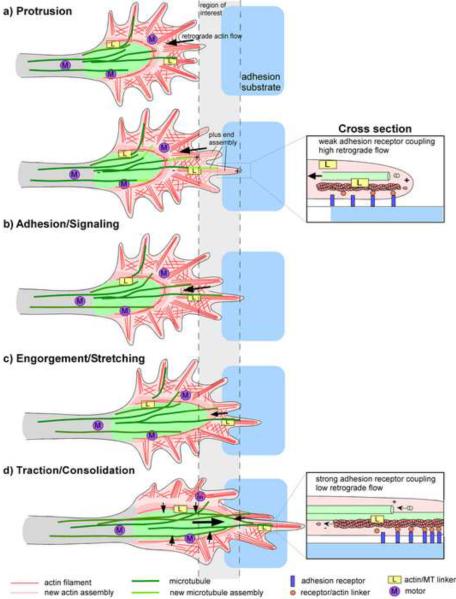
Figure 3.
Cytoskeletal Rearrangements in Adhesion-Mediated Growth Cone Advance.
(a) Protrusion: F-actin is assembled along the leading edge and turned over by the actin flow mechanism. Dynamic microtubules explore the periphery by assembly and coupling to the retrogradely moving actin filaments via actin/microtubule linkers. Cross-section shows weak adhesion receptor coupling. (b) Adhesion/signaling: Growth cone makes contact with adhesive substrate, stimulating adhesion receptor clustering and signaling followed by preferential exploration of the adhesion site by microtubules that uncouple from the actin cytoskeleton. These microtubules could support signaling and receptor-cytoskeletal coupling. (c and d) Engorgement/Stretching/Traction/Consolidation: Strong adhesion receptor cytoskeleton coupling results in actin flow attenuation and forward movement of actin recycling zone in the P domain, see inset. Actin arcs surrounding the C domain direct microtubules towards adhesion sites. Coordinated forward movement of C domain and T zone microtubules together with F-actin structures could be mediated by microtubule motors and actin/microtubule linkers. “M” stands for any actin or microtubule based motor; “L” stands for any actin/microtubule linker. Time progresses from top to bottom.
3.2 Retrograde Flow of Actin is Linked with Force Generation in the Growth Cone
There are at least two possibilities for how myosin-driven actin flow can support guided growth cone advance. One role could be a delivery mechanism for signals generated upon guidance cue detection by filopodial tips or at the leading edge. The highly oriented actin flow would provide an effective directional transport system for signaling molecules to the microtubules in the C domain in order to guide the microtubules to the site of cue detection. While this is an interesting hypothesis, there is no experimental evidence to support it so far. On the other hand, a biophysical role of actin flow for growth cone translocation is well established. When growth cones (or other motile cells) form weak interactions between substrate molecules (e.g. cell adhesion proteins and extracellular matrix proteins), the actomyosin machinery runs in idle state, resulting in slow forward movement, high retrograde flow rate and no tension build up at adhesion sites (Fig. 3a). On the other hand, when adhesion receptors strongly couple extracellular substrates to the moving actin cytoskeleton, growth cones use actomyosin-mediated force for pulling themselves forward (Fig. 3b–d). This is the basis of the substrate-cytoskeletal model, originally postulated by Mitchison and Kirschner over 20 years ago as a mechanism for substrate-mediated growth cone movements (Mitchison and Kirschner, 1988; Suter and Forscher, 1998; Suter and Forscher, 2000) and cell migration in general (Hu and Chien, 2007; Jurado et al., 2005). Strong support for substrate-cytoskeletal coupling and force transduction at adhesion sites in growth cones came from studies that used substrate-coated microbeads which were physically restrained on the surface of Aplysia growth cones with a micropipette to prevent their retrograde movement (Suter et al., 1998). In this so-called “restrained bead interaction” assay, beads coated with apCAM, the Aplysia homolog of NCAM, induced cytoskeletal rearrangements typical of growth cone responses to cellular substrates. These include actin accumulation at the contact site, attenuation of actin flow specifically along the interaction axis, accompanied with tension build up, and microtubule extension to the bead site (Suter et al., 1998). The deflection of the micropipette towards the C domain indicates that the growth cone pulls on the substrate (Fig. 3d). This tension is dissipated quickly upon bead release (Lee and Suter, 2008). Additional forward pulling of the restrained bead results in an increased rate of growth cone advance and anterograde movement of organelles in concert with the bead suggesting a tight coupling between apCAM, actin, and microtubules in the growth cone (Daniel Suter and Paul Forscher, unpublished observation). The coupling between apCAM and the actin cytoskeleton is regulated by the tyrosine kinase Src, which in turn is regulated by tension and microtubule dynamics (Suter and Forscher, 2001; Suter et al., 2004). All major adhesion receptor families use the substrate-coupling mechanism to transduce myosin-driven actin flow into forward growth cone movement. In addition to the immunoglobulin superfamily molecule apCAM (Suter et al., 1998), N-cadherin and integrins have also been demonstrated to mediate coupling between extracellular substrates and intracellular cytoskeleton to control growth cone motility (Bard et al., 2008; Chan and Odde, 2008).
3.3 Actin-Microtubule Interactions in the Growth Cone
Two interesting questions refer to the microtubule extension during attractive growth cone steering events: (1) By which mechanisms do microtubules extend to the adhesion site during contact-mediated growth and (2) how could microtubules regulate substrate-cytoskeletal coupling? Two recent studies combining the restrained bead interaction assay with actin/microtubule FSM showed that coordinated actin-microtubule interactions are the main regulators of microtubule rearrangements during adhesion-evoked growth (Lee and Suter, 2008; Schaefer et al., 2008). These studies have shown that actin arcs in the T zone, as well as C domain actin structures, undergo forward translocation together with microtubules, resulting in focusing the C domain towards the adhesion site (Fig. 3c). These actin and microtubule structures appear to be highly coupled and pulled forward towards the bead during the “traction” phase of restrained bead interactions (Lee and Suter, 2008; Schaefer et al., 2008) (Fig. 3d). The bulk of P domain microtubules in the bead interaction corridor extend into the actin-free zone due to the forward shift of the actin recycling zone. These microtubule movements occur during the late “traction” phase of adhesive interactions when coupling and tension are already high, supporting the stretch-mediated growth model (Fig. 2b). Furthermore, Rho/Rho kinase/myosin II-mediated actin arc contractility regulates the microtubule forward movement during adhesion-evoked neurite growth (Schaefer et al., 2008), as well as the microtubule bundling during the consolidation phase when the C domain transforms into the axon (Burnette et al., 2008) (Fig. 3d). How could microtubules regulate signaling between adhesion receptors and the actin cytoskeleton during the earlier “latency” phase? Indeed, early microtubules preferentially explore the adhesion site before actin flow attenuation occurs (Lee and Suter, 2008) (Fig. 3b). Quantitative analysis of microtubule dynamics revealed that these early microtubules spend less time in retrograde actin flow coupling and depolymerization. Thus, a partial uncoupling of these highly dynamic microtubules in the P domain as well as stabilization results in higher microtubule presence at the adhesion site (Fig. 3b). These early microtubules could deliver signaling molecules to strengthen the coupling, for example Src itself or an activator for Src. In agreement with this idea, a recent study showed that microtubules mediate redistribution of endoplasmic reticulum-bound tyrosine phosphatase PTB1B (a Src activator) to cell-cell contacts in hippocampal neurons (Fuentes and Arregui, 2009). In summary, these findings indicate that dynamic microtubules are required for adhesion- and tension-mediated axonal elongation. Indeed, application of low doses of microtubule drugs, that dampen dynamic instability, compromise the ability of neurons to elongate axons in response to applied tension (Suter et al., 2004; Zheng et al., 1993).
While it is well accepted that forces modulate microtubule assembly in non-neuronal cells (Kaverina et al., 2002), especially during mitosis (Dumont and Mitchison, 2009), we are just beginning to understand the links between forces, microtubule dynamics, and axonal elongation. Recent work using fluorescently labeled microtubule plus-end tracking (+TIP) proteins (Akhmanova and Steinmetz, 2008) allows full visualization of the pattern of microtubule assembly in neurons (Ma et al., 2004; Morrison et al., 2002; Rolls et al., 2007). While these studies show microtubule polymerization occurs at an elevated level over the last 50 μm of the distal axon (Kollins et al., 2009a), which confirms earlier findings (Brown et al., 1992), polymerization is by no means restricted to the growth cone. Odde's group has thought deeply about axonal microtubules in axonal specification (Dotti et al., 1988; Seetapun and Odde, 2010). Based on modeling and experimental data, they suggest that during the process of neuronal polarization, the longest neurite becomes the axon not because microtubules have different dynamic properties at the tip of different processes, but because microtubules have more room to polymerize (Seetapun and Odde, 2010). Conceptually, this is important because it shifts the focus of neuronal growth from the growth cone to the whole axon.
Obviously, an interesting question remains which linker and motor molecules mediate actin and microtubule interactions in the various growth cone and axonal domains during adhesion-mediated growth (Fig. 2 and 3). A number of potential actinmicrotubule crosslink mechanisms have been proposed in the growth cone so far, involving either 1) a single protein that binds both cytoskeletal filaments; 2) an interaction complex that involves multiple proteins; 3) a signaling mechanism between the two cytoskeletal structures, although it is difficult to envision how such mechanism alone could build up the tension observed between the P and C domain (Conde and Caceres, 2009; Geraldo and Gordon-Weeks, 2009; Lowery and Van Vactor, 2009). Both microtubule-based motors (dynein, kinesin) and actin-based motors (myosin) have been implicated in axonal outgrowth and turning (Grabham et al., 2007; Myers et al., 2006; Nadar et al., 2008; Turney and Bridgman, 2005). However, most of these studies have been conducted in systems that are less suitable for high-resolution quantitative analysis of actin and microtubule dynamics and coupling compared to the Aplysia system. Thus, the combination of molecular tools for these candidate linker molecules and quantitative fluorescent speckle microscopy will be needed to ultimately understand “who is pulling whom by which motor” during growth cone advance.
3.4 Two Pools of Myosin?
Given the role of actomyosin in the substrate-cytoskeletal coupling, one would expect that reducing myosin II activity should decrease the rate of axonal elongation. Indeed myosin II inhibition by knockdown approaches or treatment with the myosin inhibitor blebbistatin reduced the rate of neuronal outgrowth and turning on laminin substrates (Bridgman et al., 2001; Ketschek et al., 2007; Tullio et al., 2001; Turney and Bridgman, 2005). On the other hand on poly-lysine substrate, myosin II inhibition increases the rate of axonal elongation as well as of leading edge protrusion (Ketschek et al., 2007; Kollins et al., 2009b; Lin et al., 1996; Medeiros et al., 2006; Rosner et al., 2007). Detailed analysis of different neuronal cell types and myosin II isoforms in the above mentioned studies indicate that the regulation of axonal elongation by myosin motors may involve two different (Rochlin et al., 1995) functional pools of myosin. One appears to be primarily associated with retrograde actin flow in the growth cone (Medeiros et al., 2006), corresponds with myosin IIB (Bridgman et al., 2001; Brown and Bridgman, 2003), and is not regulated by Rho/Rho kinase (Zhang et al., 2003). The second pool is enriched along the axon and the actin arcs that surround the central domain of the growth cone (Burnette et al., 2008; Burnette et al., 2007). This pool corresponds to myosin IIA (Kubo et al., 2008), is regulated by Rho/Rho kinase, and leads to axonal retraction upon activation (Gallo, 2004; Zhang et al., 2003). Together this raises the possibility that forces generated by axonal and growth cone myosin serve different functions and are selectively regulated by guidance cues such as myelin-associated inhibitors (e.g. Nogo) (Alabed et al., 2006; Gross et al., 2007), semaphorin 3A (Brown et al., 2009; Gallo, 2006), slit, and netrin-1 (Fritz and VanBerkum, 2002; Guan et al., 2007; Kim et al., 2002; Murray et al., 2010). At this point, it is well agreed that myosin is important for force generation during axonal elongation. What is notably lacking is a coherent model to explain how myosin is involved in axonal elongation in different types of neurons grown on different substrates.
4. Mechanical Signaling in Axonal Elongation
As mentioned above, application of forces to axons can induce rapid elongation without thinning of the axon (Abe et al., 2004; Lamoureux et al., 2010a; Pfister et al., 2004). This implies that neurons somehow sense when they are stretched and respond by increasing protein synthesis and transport (Hengst et al., 2009; Jung and Holt, 2011; Lin and Holt, 2008; O'Toole and Miller, 2011). Between the force stimulus and the elongation response, mechanotransduction in neurons occurs through unknown but presumably conserved intracellular signaling pathways (Janmey and Miller, 2011; Vogel and Sheetz, 2006). The two best understood systems are mechanotransduction in vascular physiology (reviewed in Hahn and Schwartz, 2009) and force sensing during cell migration (Chen, 2008).
In migrating cells, it is well accepted that cells generate and sense forces. A key question is how forces are transduced into biochemical signaling events. Stretch-activated calcium channels leading to increased calcium concentrations during cell migration provide a possible answer (Lee et al., 1999). Other mechanisms may involve the activation of tyrosine phosphorylation (Giannone and Sheetz, 2006), specifically of Src family tyrosine kinases (Wang et al., 2005), focal adhesion kinase (FAK) (Wang et al., 2001), protein-tyrosine phosphatase Shp2 (von Wichert et al., 2003a), and receptor protein tyrosine phosphatase alpha (RPTPα) (von Wichert et al., 2003b). Stretch-induced protein unfolding of p130Cas allows Src-mediated phosphorylation, providing a well-described example for force-induced changes in protein conformation (Sawada et al., 2006). Recently discovered additional examples of force sensors include talin (del Rio et al., 2009), filamin (Byfield et al., 2009), and integrins (Friedland et al., 2009; Roca-Cusachs et al., 2009). As these proteins are broadly expressed and well conserved they are exciting candidates for molecules involved in mechanotransduction in neurons.
In neurons, traction force can be developed by growth cones in response to adhesion molecules and guidance cues such as apCAM and netrin-1 when immobilized on beads (Moore et al., 2009; Suter et al., 1998). As is seen in mechanotransduction in non-neuronal cells, both apCAM- and netrin-1-induced signaling pathways also involve Src tyrosine kinase activity (Li et al., 2004; Liu et al., 2004; Meriane et al., 2004; Suter and Forscher, 2001). Furthermore, tension-dependent tyrosine phosphorylation suggested a positive feed-back between force development and Src kinase signaling in adhesion-mediated growth (Suter and Forscher, 2001). A recent study showed that membrane stretch can activate a thermosensitive TRP channel and thereby enhance axonal elongation of developing sensory neurons (Shibasaki et al., 2010). Jacques-Fricke and colleagues also provide evidence that Ca2+ influx through mechanosensitive channels inhibits neurite outgrowth (Jacques-Fricke et al., 2006). However, the role of such Ca2+ influx has not been investigated in the context of forces during axonal elongation. While there is mounting evidence that mechanotransduction plays a role in axonal growth and guidance, the identities of the critical mediators are still largely unknown.
5. Is there a Universal Mechanism of Axonal Elongation?
It may be an understatement to merely say that the fields of slow axonal transport and axonal elongation have a contentious history. One plausible reason is that different types of neurons have unique mechanisms of elongation. Alternatively, there may be a highly conserved mechanism that is sensitive to position along the axon (Lim et al., 1990; Miller and Sheetz, 2006), the substrate of growth (Chang et al., 1998; O'Toole et al., 2008a), developmental stage (Jones et al., 2006; Lamoureux et al., 2010b), variation in axonal diameter (O'Toole et al., 2008a), and other factors yet undiscovered. Axonal elongation has been examined in at least a dozen types of neurons. Key systems include chicken, rat, and mouse dorsal root ganglion neurons (Chetta et al., 2010; Gallo et al., 1997; Lamoureux et al., 2010a; Lamoureux et al., 2010b; Lim et al., 1990; Okabe and Hirokawa, 1990); rat hippocampal neurons (Goslin and Banker, 1990), grasshopper neurons (Sabry et al., 1995), Drosophila neurons (Ahmed et al., 2010; Sanchez-Soriano et al., 2010); Aplysia neurons (Goldberg and Burmeister, 1986; Lee and Suter, 2008; Schaefer et al., 2008), Xenopus spinal cord neurons (Jacques-Fricke et al., 2006), and PC12 cells (Bernal et al., 2007; Keith, 1987; Lim et al., 1989). The introduction of the paper by Chang et al., (1998) illustrates that once Xenopus neurons were thought to be the only type of neuron that grew by stretching. Over the past five years, PC12 cells, chick sensory, rat sensory, and Aplysia neurons have been shown to lengthen by stretching (Bernal et al., 2007; Chetta et al., 2010; Lee and Suter, 2008; Miller and Sheetz, 2006; Schaefer et al., 2008). Consistently these newer studies confirm the older experimental findings that the axonal cytoskeleton does not move in bulk out of the cell body, but demonstrate stretching of the distal axon; especially when neurons are grown on laminin (Fig. 2b). This raises the possibility of a conserved mechanism for axonal elongation and suggests that previous conflicting reports could result from variations in experimental technique (e.g. the location of bulk transport assessment and the growth substrate). Importantly, it is clear that different neuronal cell types have unique morphologies and may respond in opposite ways to disruption of the cytoskeleton. For example, cytochalasin increases (Ruthel and Hollenbeck, 2000) or has no effect on the rate of elongation of hippocampal neurons (Bradke and Dotti, 1999), whereas it decreases the rate of elongation of sensory neurons (Jones et al., 2006; Letourneau et al., 1987). We suspect that a systematic analysis will show that the core mechanism of axonal elongation is highly conserved across neuronal subtypes and species, but that quantitative and qualitative differences exist, in particular between neurons from the central and peripheral nervous system.
6. Conclusions/Perspectives
Based on innovative approaches at the intersection of biophysics and molecular cell biology, new models for the role of forces in axonal elongation are emerging. There is increasing evidence that not only microtubule assembly but also forces on microtubules play a key role in axonal elongation. A number of questions remain to be addressed: 1. Which motors are responsible for force generation in axons and growth cones? 2. Which cytoskeletal structures do these motors move and against which substrates? 3. What are the directions and magnitudes of the forces these motors produce? 4. Which linker proteins couple microtubules to actin filaments in the P and C domains? 5. Which signaling pathways regulate force generation? 6. How do microtubules regulate signaling in the growth cone?
To address these questions and distinguish between different models of axonal elongation, there is a pressing need to better visualize cytoskeletal dynamics while monitoring and applying forces and disrupting gene function. At present, the molecular tools to study axonal elongation in Xenopus, chicken, and Aplysia neurons particularly in vivo are somewhat limited, but the cell biology is exquisite. Likewise, the development of nanotechnology and mathematical modeling provide powerful tools to analyze and interpret patterns of force generation, but have rarely been coupled with the best tools for live cell imaging and genetic manipulation. Finally, while genetic model systems such as Drosophila melanogaster and C. elegans have provided deep insights into the molecular players important for axonal elongation, cell biologists and biophysicists rarely use these systems. We think that satisfying answers to the question, “How do axons grow?” can be found by integrating the best aspects of biophysics, genetics, and cell biology.
Acknowledgements
We thank Steven Heidemann, Matthew O'Toole, and members of the Miller lab for helpful comments on the manuscript. We acknowledge the contribution of images in Fig. 1a–b taken by Aih Cheun Lee in the Suter lab. This work was partially supported by Startup Funds from Michigan State University (KEM), NSF grant IOS 0951019 (KEM), NIH grant R01 NS049233 (DMS) and the Bindley Bioscience Center at Purdue University (DMS). The authors declare that there are no conflicts of interest related to this manuscript.
Abbreviation List
- m
meter
- μm
micron
- mm
millimeter
- d
day
- h
hour
- PDMS
polydimethylsiloxane
- nN
nanoNewton
- P
peripheral
- T
transition
- C
central
- pN
picoNewton
- FSM
fluorescent speckle microscopy
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Abe I, Ochiai N, Ichimura H, Tsujino A, Sun J, Hara Y. Internodes can nearly double in length with gradual elongation of the adult rat sciatic nerve. J Orthop Res. 2004;22:571–577. doi: 10.1016/j.orthres.2003.08.019. [DOI] [PubMed] [Google Scholar]
- Ahmed WW, Kural MH, Saif TA. A novel platform for in situ investigation of cells and tissues under mechanical strain. Acta Biomater. 2010;6:2979–2990. doi: 10.1016/j.actbio.2010.02.035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Akhmanova A, Steinmetz MO. Tracking the ends: a dynamic protein network controls the fate of microtubule tips. Nat Rev Mol Cell Biol. 2008;9:309–322. doi: 10.1038/nrm2369. [DOI] [PubMed] [Google Scholar]
- Alabed YZ, Grados-Munro E, Ferraro GB, Hsieh SH, Fournier AE. Neuronal responses to myelin are mediated by rho kinase. J Neurochem. 2006;96:1616–1625. doi: 10.1111/j.1471-4159.2006.03670.x. [DOI] [PubMed] [Google Scholar]
- Ayali A. The function of mechanical tension in neuronal and network development. Integr Biol (Camb) 2010;2:178–182. doi: 10.1039/b927402b. [DOI] [PubMed] [Google Scholar]
- Baas PW, Vidya Nadar C, Myers KA. Axonal transport of microtubules: the long and short of it. Traffic. 2006;7:490–498. doi: 10.1111/j.1600-0854.2006.00392.x. [DOI] [PubMed] [Google Scholar]
- Bamburg JR, Bray D, Chapman K. Assembly of microtubules at the tip of growing axons. Nature. 1986;321:788–790. doi: 10.1038/321788a0. [DOI] [PubMed] [Google Scholar]
- Bard L, Boscher C, Lambert M, Mege RM, Choquet D, Thoumine O. A molecular clutch between the actin flow and N-cadherin adhesions drives growth cone migration. J Neurosci. 2008;28:5879–5890. doi: 10.1523/JNEUROSCI.5331-07.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bernal R, Pullarkat PA, Melo F. Mechanical properties of axons. Phys Rev Lett. 2007;99:018301. doi: 10.1103/PhysRevLett.99.018301. [DOI] [PubMed] [Google Scholar]
- Bradke F, Dotti CG. The role of local actin instability in axon formation. Science. 1999;283:1931–1934. doi: 10.1126/science.283.5409.1931. [DOI] [PubMed] [Google Scholar]
- Bray D. Axonal growth in response to experimentally applied mechanical tension. Dev Biol. 1984;102:379–389. doi: 10.1016/0012-1606(84)90202-1. [DOI] [PubMed] [Google Scholar]
- Bridgman PC, Dave S, Asnes CF, Tullio AN, Adelstein RS. Myosin IIB is required for growth cone motility. J Neurosci. 2001;21:6159–6169. doi: 10.1523/JNEUROSCI.21-16-06159.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brown A. Slow axonal transport: stop and go traffic in the axon. Nat Rev Mol Cell Biol. 2000;1:153–156. doi: 10.1038/35040102. [DOI] [PubMed] [Google Scholar]
- Brown A, Slaughter T, Black MM. Newly assembled microtubules are concentrated in the proximal and distal regions of growing axons. J Cell Biol. 1992;119:867–882. doi: 10.1083/jcb.119.4.867. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brown JA, Wysolmerski RB, Bridgman PC. Dorsal root ganglion neurons react to semaphorin 3A application through a biphasic response that requires multiple myosin II isoforms. Mol Biol Cell. 2009;20:1167–1179. doi: 10.1091/mbc.E08-01-0065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brown ME, Bridgman PC. Retrograde flow rate is increased in growth cones from myosin IIB knockout mice. J Cell Sci. 2003;116:1087–1094. doi: 10.1242/jcs.00335. [DOI] [PubMed] [Google Scholar]
- Buck KB, Zheng JQ. Growth cone turning induced by direct local modification of microtubule dynamics. J Neurosci. 2002;22:9358–9367. doi: 10.1523/JNEUROSCI.22-21-09358.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bueno FR, Shah SB. Implications of tensile loading for the tissue engineering of nerves. Tissue Eng Part B Rev. 2008;14:219–233. doi: 10.1089/ten.teb.2008.0020. [DOI] [PubMed] [Google Scholar]
- Burnette DT, Ji L, Schaefer AW, Medeiros NA, Danuser G, Forscher P. Myosin II activity facilitates microtubule bundling in the neuronal growth cone neck. Dev Cell. 2008;15:163–169. doi: 10.1016/j.devcel.2008.05.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burnette DT, Schaefer AW, Ji L, Danuser G, Forscher P. Filopodial actin bundles are not necessary for microtubule advance into the peripheral domain of Aplysia neuronal growth cones. Nat Cell Biol. 2007;9:1360–1369. doi: 10.1038/ncb1655. [DOI] [PubMed] [Google Scholar]
- Byfield FJ, Wen Q, Levental I, Nordstrom K, Arratia PE, Miller RT, Janmey PA. Absence of filamin A prevents cells from responding to stiffness gradients on gels coated with collagen but not fibronectin. Biophys J. 2009;96:5095–5102. doi: 10.1016/j.bpj.2009.03.046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Challacombe JF, Snow DM, Letourneau PC. Actin filament bundles are required for microtubule reorientation during growth cone turning to avoid an inhibitory guidance cue. J Cell Sci. 1996;109(Pt 8):2031–2040. doi: 10.1242/jcs.109.8.2031. [DOI] [PubMed] [Google Scholar]
- Chan CE, Odde DJ. Traction dynamics of filopodia on compliant substrates. Science. 2008;322:1687–1691. doi: 10.1126/science.1163595. [DOI] [PubMed] [Google Scholar]
- Chang S, Rodionov VI, Borisy GG, Popov SV. Transport and turnover of microtubules in frog neurons depend on the pattern of axonal growth. J Neurosci. 1998;18:821–829. doi: 10.1523/JNEUROSCI.18-03-00821.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen CS. Mechanotransduction - a field pulling together? J Cell Sci. 2008;121:3285–3292. doi: 10.1242/jcs.023507. [DOI] [PubMed] [Google Scholar]
- Chen ZL, Yu WM, Strickland S. Peripheral regeneration. Annu Rev Neurosci. 2007;30:209–233. doi: 10.1146/annurev.neuro.30.051606.094337. [DOI] [PubMed] [Google Scholar]
- Chetta J, Kye C, Shah SB. Cytoskeletal dynamics in response to tensile loading of mammalian axons. Cytoskeleton (Hoboken) 2010;67:650–665. doi: 10.1002/cm.20478. [DOI] [PubMed] [Google Scholar]
- Conde C, Caceres A. Microtubule assembly, organization and dynamics in axons and dendrites. Nat Rev Neurosci. 2009;10:319–332. doi: 10.1038/nrn2631. [DOI] [PubMed] [Google Scholar]
- del Rio A, Perez-Jimenez R, Liu R, Roca-Cusachs P, Fernandez JM, Sheetz MP. Stretching single talin rod molecules activates vinculin binding. Science. 2009;323:638–641. doi: 10.1126/science.1162912. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dent EW, Gertler FB. Cytoskeletal dynamics and transport in growth cone motility and axon guidance. Neuron. 2003;40:209–227. doi: 10.1016/s0896-6273(03)00633-0. [DOI] [PubMed] [Google Scholar]
- Dent EW, Kalil K. Axon branching requires interactions between dynamic microtubules and actin filaments. J Neurosci. 2001;21:9757–9769. doi: 10.1523/JNEUROSCI.21-24-09757.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dotti CG, Sullivan CA, Banker GA. The establishment of polarity by hippocampal neurons in culture. J Neurosci. 1988;8:1454–1468. doi: 10.1523/JNEUROSCI.08-04-01454.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dumont S, Mitchison TJ. Force and length in the mitotic spindle. Curr Biol. 2009;19:R749–761. doi: 10.1016/j.cub.2009.07.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Forscher P, Smith SJ. Actions of cytochalasins on the organization of actin filaments and microtubules in a neuronal growth cone. J Cell Biol. 1988;107:1505–1516. doi: 10.1083/jcb.107.4.1505. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Franze K, Guck J. The biophysics of neuronal growth. Rep Prog Phys. 2010;73 [Google Scholar]
- Franze K, Reichenbach A, Kas J. Biomechanics of the CNS. In: Kamkin A, Kiseleva I, editors. Mechanosensitivity of the Nervous System. Springer; Netherlands: 2009. pp. 173–213. [Google Scholar]
- Friedland JC, Lee MH, Boettiger D. Mechanically activated integrin switch controls alpha5beta1 function. Science. 2009;323:642–644. doi: 10.1126/science.1168441. [DOI] [PubMed] [Google Scholar]
- Fritz JL, VanBerkum MF. Regulation of rho family GTPases is required to prevent axons from crossing the midline. Dev Biol. 2002;252:46–58. doi: 10.1006/dbio.2002.0842. [DOI] [PubMed] [Google Scholar]
- Fuentes F, Arregui CO. Microtubule and cell contact dependency of ER-bound PTP1B localization in growth cones. Mol Biol Cell. 2009;20:1878–1889. doi: 10.1091/mbc.E08-07-0675. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gallo G. Myosin II activity is required for severing-induced axon retraction in vitro. Exp Neurol. 2004;189:112–121. doi: 10.1016/j.expneurol.2004.05.019. [DOI] [PubMed] [Google Scholar]
- Gallo G. RhoA-kinase coordinates F-actin organization and myosin II activity during semaphorin-3A-induced axon retraction. J Cell Sci. 2006;119:3413–3423. doi: 10.1242/jcs.03084. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gallo G, Lefcort FB, Letourneau PC. The trkA receptor mediates growth cone turning toward a localized source of nerve growth factor. J Neurosci. 1997;17:5445–5454. doi: 10.1523/JNEUROSCI.17-14-05445.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Geraldo S, Gordon-Weeks PR. Cytoskeletal dynamics in growth-cone steering. J Cell Sci. 2009;122:3595–3604. doi: 10.1242/jcs.042309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Giannone G, Sheetz MP. Substrate rigidity and force define form through tyrosine phosphatase and kinase pathways. Trends Cell Biol. 2006;16:213–223. doi: 10.1016/j.tcb.2006.02.005. [DOI] [PubMed] [Google Scholar]
- Goldberg DJ, Burmeister DW. Stages in axon formation: observations of growth of Aplysia axons in culture using video-enhanced contrast-differential interference contrast microscopy. J Cell Biol. 1986;103:1921–1931. doi: 10.1083/jcb.103.5.1921. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gordon-Weeks PR. Neuronal growth cones. Cambridge University Press; Cambridge, UK ; New York: 2000. [Google Scholar]
- Goslin K, Banker G. Rapid changes in the distribution of GAP-43 correlate with the expression of neuronal polarity during normal development and under experimental conditions. J Cell Biol. 1990;110:1319–1331. doi: 10.1083/jcb.110.4.1319. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grabham PW, Seale GE, Bennecib M, Goldberg DJ, Vallee RB. Cytoplasmic dynein and LIS1 are required for microtubule advance during growth cone remodeling and fast axonal outgrowth. J Neurosci. 2007;27:5823–5834. doi: 10.1523/JNEUROSCI.1135-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gross RE, Mei Q, Gutekunst CA, Torre E. The pivotal role of RhoA GTPase in the molecular signaling of axon growth inhibition after CNS injury and targeted therapeutic strategies. Cell Transplant. 2007;16:245–262. doi: 10.3727/000000007783464740. [DOI] [PubMed] [Google Scholar]
- Guan CB, Xu HT, Jin M, Yuan XB, Poo MM. Long-range Ca2+ signaling from growth cone to soma mediates reversal of neuronal migration induced by slit-2. Cell. 2007;129:385–395. doi: 10.1016/j.cell.2007.01.051. [DOI] [PubMed] [Google Scholar]
- Hahn C, Schwartz MA. Mechanotransduction in vascular physiology and atherogenesis. Nat Rev Mol Cell Biol. 2009;10:53–62. doi: 10.1038/nrm2596. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hallstrom W, Lexholm M, Suyatin DB, Hammarin G, Hessman D, Samuelson L, Montelius L, Kanje M, Prinz CN. Fifteen-piconewton force detection from neural growth cones using nanowire arrays. Nano Lett. 2010;10:782–787. doi: 10.1021/nl902675h. [DOI] [PubMed] [Google Scholar]
- Harrison RG. On the origin and development of the nervous system studied by the methods of experimental embryology. Proc. Roy. Soc. (Lond.) B. 1935;118:155–196. [Google Scholar]
- Hengst U, Deglincerti A, Kim HJ, Jeon NL, Jaffrey SR. Axonal elongation triggered by stimulus-induced local translation of a polarity complex protein. Nat Cell Biol. 2009;11:1024–1030. doi: 10.1038/ncb1916. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hirokawa N, Terada S, Funakoshi T, Takeda S. Slow axonal transport: the subunit transport model. Trends in Cell Biology. 1997;7:384–388. doi: 10.1016/S0962-8924(97)01133-1. [DOI] [PubMed] [Google Scholar]
- Hu YL, Chien S. Dynamic motion of paxillin on actin filaments in living endothelial cells. Biochem Biophys Res Commun. 2007;357:871–876. doi: 10.1016/j.bbrc.2007.04.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jacques-Fricke BT, Seow Y, Gottlieb PA, Sachs F, Gomez TM. Ca2+ influx through mechanosensitive channels inhibits neurite outgrowth in opposition to other influx pathways and release from intracellular stores. J Neurosci. 2006;26:5656–5664. doi: 10.1523/JNEUROSCI.0675-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Janmey PA, Miller RT. Mechanisms of mechanical signaling in development and disease. J Cell Sci. 2011;124:9–18. doi: 10.1242/jcs.071001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jones SL, Selzer ME, Gallo G. Developmental regulation of sensory axon regeneration in the absence of growth cones. J Neurobiol. 2006;66:1630–1645. doi: 10.1002/neu.20309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jung H, Holt CE. Local translation of mRNAs in neural development. Wiley Interdisciplinary Reviews: RNA. 2011;2:153–165. doi: 10.1002/wrna.53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jung P, Brown A. Modeling the slowing of neurofilament transport along the mouse sciatic nerve. Phys Biol. 2009;6:046002. doi: 10.1088/1478-3975/6/4/046002. [DOI] [PubMed] [Google Scholar]
- Jurado C, Haserick JR, Lee J. Slipping or gripping? Fluorescent speckle microscopy in fish keratocytes reveals two different mechanisms for generating a retrograde flow of actin. Mol Biol Cell. 2005;16:507–518. doi: 10.1091/mbc.E04-10-0860. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaverina I, Krylyshkina O, Beningo K, Anderson K, Wang YL, Small JV. Tensile stress stimulates microtubule outgrowth in living cells. J Cell Sci. 2002;115:2283–2291. doi: 10.1242/jcs.115.11.2283. [DOI] [PubMed] [Google Scholar]
- Keith CH. Slow transport of tubulin in the neurites of differentiated PC12 cells. Science. 1987;235:337–339. doi: 10.1126/science.2432662. [DOI] [PubMed] [Google Scholar]
- Ketschek AR, Jones SL, Gallo G. Axon extension in the fast and slow lanes: substratum-dependent engagement of myosin II functions. Dev Neurobiol. 2007;67:1305–1320. doi: 10.1002/dneu.20455. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kijima K, Matsubara H, Murasawa S, Maruyama K, Mori Y, Ohkubo N, Komuro I, Yazaki Y, Iwasaka T, Inada M. Mechanical stretch induces enhanced expression of angiotensin II receptor subtypes in neonatal rat cardiac myocytes. Circ Res. 1996;79:887–897. doi: 10.1161/01.res.79.4.887. [DOI] [PubMed] [Google Scholar]
- Kim YS, Fritz JL, Seneviratne AK, VanBerkum MF. Constitutively active myosin light chain kinase alters axon guidance decisions in Drosophila embryos. Dev Biol. 2002;249:367–381. doi: 10.1006/dbio.2002.0768. [DOI] [PubMed] [Google Scholar]
- Kollins KM, Bell RL, Butts M, Withers GS. Dendrites differ from axons in patterns of microtubule stability and polymerization during development. Neural Dev. 2009a;4:26. doi: 10.1186/1749-8104-4-26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kollins KM, Hu J, Bridgman PC, Huang YQ, Gallo G. Myosin-II negatively regulates minor process extension and the temporal development of neuronal polarity. Dev Neurobiol. 2009b;69:279–298. doi: 10.1002/dneu.20704. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kubo T, Endo M, Hata K, Taniguchi J, Kitajo K, Tomura S, Yamaguchi A, Mueller BK, Yamashita T. Myosin IIA is required for neurite outgrowth inhibition produced by repulsive guidance molecule. J Neurochem. 2008;105:113–126. doi: 10.1111/j.1471-4159.2007.05125.x. [DOI] [PubMed] [Google Scholar]
- Kuznetsov AV, Avramenko AA, Blinov DG. Effect of diffusion on slowing the velocity of a bell-shaped wave in slow axonal transport. International Communications in Heat and Mass Transfer. 2010;37:770–774. [Google Scholar]
- Lamoureux P, Buxbaum RE, Heidemann SR. Direct evidence that growth cones pull. Nature. 1989;340:159–162. doi: 10.1038/340159a0. [DOI] [PubMed] [Google Scholar]
- Lamoureux P, Heidemann SR, Martzke NR, Miller KE. Growth and elongation within and along the axon. Dev Neurobiol. 2010a;70:135–149. doi: 10.1002/dneu.20764. [DOI] [PubMed] [Google Scholar]
- Lamoureux PL, O'Toole MR, Heidemann SR, Miller KE. Slowing of axonal regeneration is correlated with increased axonal viscosity during aging. BMC Neurosci. 2010b;11:140. doi: 10.1186/1471-2202-11-140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee AC, Suter DM. Quantitative analysis of microtubule dynamics during adhesion-mediated growth cone guidance. Dev Neurobiol. 2008;68:1363–1377. doi: 10.1002/dneu.20662. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee J, Ishihara A, Oxford G, Johnson B, Jacobson K. Regulation of cell movement is mediated by stretch-activated calcium channels. Nature. 1999;400:382–386. doi: 10.1038/22578. [DOI] [PubMed] [Google Scholar]
- Letourneau PC, Ressler AH. Inhibition of neurite initiation and growth by taxol. J Cell Biol. 1984;98:1355–1362. doi: 10.1083/jcb.98.4.1355. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Letourneau PC, Shattuck TA, Ressler AH. “Pull” and “push” in neurite elongation: observations on the effects of different concentrations of cytochalasin B and taxol. Cell Motil Cytoskeleton. 1987;8:193–209. doi: 10.1002/cm.970080302. [DOI] [PubMed] [Google Scholar]
- Li W, Lee J, Vikis HG, Lee SH, Liu G, Aurandt J, Shen TL, Fearon ER, Guan JL, Han M, Rao Y, Hong K, Guan KL. Activation of FAK and Src are receptor-proximal events required for netrin signaling. Nat Neurosci. 2004;7:1213–1221. doi: 10.1038/nn1329. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lim SS, Edson KJ, Letourneau PC, Borisy GG. A test of microtubule translocation during neurite elongation. J Cell Biol. 1990;111:123–130. doi: 10.1083/jcb.111.1.123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lim SS, Sammak PJ, Borisy GG. Progressive and spatially differentiated stability of microtubules in developing neuronal cells. J Cell Biol. 1989;109:253–263. doi: 10.1083/jcb.109.1.253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lin AC, Holt CE. Function and regulation of local axonal translation. Curr Opin Neurobiol. 2008;18:60–68. doi: 10.1016/j.conb.2008.05.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lin CH, Espreafico EM, Mooseker MS, Forscher P. Myosin drives retrograde F-actin flow in neuronal growth cones. Neuron. 1996;16:769–782. doi: 10.1016/s0896-6273(00)80097-5. [DOI] [PubMed] [Google Scholar]
- Lin CH, Forscher P. Growth cone advance is inversely proportional to retrograde F-actin flow. Neuron. 1995;14:763–771. doi: 10.1016/0896-6273(95)90220-1. [DOI] [PubMed] [Google Scholar]
- Liu G, Beggs H, Jurgensen C, Park HT, Tang H, Gorski J, Jones KR, Reichardt LF, Wu J, Rao Y. Netrin requires focal adhesion kinase and Src family kinases for axon outgrowth and attraction. Nat Neurosci. 2004;7:1222–1232. doi: 10.1038/nn1331. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lowery LA, Van Vactor D. The trip of the tip: understanding the growth cone machinery. Nat Rev Mol Cell Biol. 2009;10:332–343. doi: 10.1038/nrm2679. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lu YB, Franze K, Seifert G, Steinhauser C, Kirchhoff F, Wolburg H, Guck J, Janmey P, Wei EQ, Kas J, Reichenbach A. Viscoelastic properties of individual glial cells and neurons in the CNS. Proc Natl Acad Sci USA. 2006;103:17759–17764. doi: 10.1073/pnas.0606150103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ma Y, Shakiryanova D, Vardya I, Popov SV. Quantitative analysis of microtubule transport in growing nerve processes. Curr Biol. 2004;14:725–730. doi: 10.1016/j.cub.2004.03.061. [DOI] [PubMed] [Google Scholar]
- Medeiros NA, Burnette DT, Forscher P. Myosin II functions in actin-bundle turnover in neuronal growth cones. Nat Cell Biol. 2006;8:215–226. doi: 10.1038/ncb1367. [DOI] [PubMed] [Google Scholar]
- Meriane M, Tcherkezian J, Webber CA, Danek EI, Triki I, McFarlane S, Bloch-Gallego E, Lamarche-Vane N. Phosphorylation of DCC by Fyn mediates Netrin-1 signaling in growth cone guidance. J Cell Biol. 2004;167:687–698. doi: 10.1083/jcb.200405053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miller KE, Heidemann SR. What is slow axonal transport? Exp Cell Res. 2008;314:1981–1990. doi: 10.1016/j.yexcr.2008.03.004. [DOI] [PubMed] [Google Scholar]
- Miller KE, Joshi HC. Tubulin transport in neurons. J Cell Biol. 1996;133:1355–1366. doi: 10.1083/jcb.133.6.1355. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miller KE, Sheetz MP. Direct evidence for coherent low velocity axonal transport of mitochondria. J Cell Biol. 2006;173:373–381. doi: 10.1083/jcb.200510097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mitchison T, Kirschner M. Cytoskeletal dynamics and nerve growth. Neuron. 1988;1:761–772. doi: 10.1016/0896-6273(88)90124-9. [DOI] [PubMed] [Google Scholar]
- Moore SW, Biais N, Sheetz MP. Traction on immobilized netrin-1 is sufficient to reorient axons. Science. 2009;325:166. doi: 10.1126/science.1173851. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morrison EE, Moncur PM, Askham JM. EB1 identifies sites of microtubule polymerisation during neurite development. Brain Res Mol Brain Res. 2002;98:145–152. doi: 10.1016/s0169-328x(01)00290-x. [DOI] [PubMed] [Google Scholar]
- Murray A, Naeem A, Barnes SH, Drescher U, Guthrie S. Slit and Netrin-1 guide cranial motor axon pathfinding via Rho-kinase, myosin light chain kinase and myosin II. Neural Dev. 2010;5:16. doi: 10.1186/1749-8104-5-16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Myers KA, Tint I, Nadar CV, He Y, Black MM, Baas PW. Antagonistic forces generated by cytoplasmic dynein and myosin-II during growth cone turning and axonal retraction. Traffic. 2006;7:1333–1351. doi: 10.1111/j.1600-0854.2006.00476.x. [DOI] [PubMed] [Google Scholar]
- Nadar VC, Ketschek A, Myers KA, Gallo G, Baas PW. Kinesin-5 is essential for growth-cone turning. Curr Biol. 2008;18:1972–1977. doi: 10.1016/j.cub.2008.11.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- O'Toole M, Lamoureux P, Miller KE. A physical model of axonal elongation: force, viscosity and adhesions govern the mode of outgrowth. Biophys J. 2008a;94:2610–2620. doi: 10.1529/biophysj.107.117424. [DOI] [PMC free article] [PubMed] [Google Scholar]
- O'Toole M, Latham R, Baqri RM, Miller KE. Modeling mitochondrial dynamics during in vivo axonal elongation. J Theor Biol. 2008b;255:369–377. doi: 10.1016/j.jtbi.2008.09.009. [DOI] [PubMed] [Google Scholar]
- O'Toole M, Miller KE. The Role of Stretching in Slow Axonal Transport. Biophys J. 2011;100:351–360. doi: 10.1016/j.bpj.2010.12.3695. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Okabe S, Hirokawa N. Turnover of fluorescently labelled tubulin and actin in the axon. Nature. 1990;343:479–482. doi: 10.1038/343479a0. [DOI] [PubMed] [Google Scholar]
- Pfister BJ, Bonislawski DP, Smith DH, Cohen AS. Stretch-grown axons retain the ability to transmit active electrical signals. FEBS Lett. 2006;580:3525–3531. doi: 10.1016/j.febslet.2006.05.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pfister BJ, Iwata A, Meaney DF, Smith DH. Extreme stretch growth of integrated axons. J Neurosci. 2004;24:7978–7983. doi: 10.1523/JNEUROSCI.1974-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rajagopalan J, Tofangchi A, Saif T. Drosophila Neurons Actively Regulate Axonal Tension In Vivo. Biophys J. 2010;99:3208–3215. doi: 10.1016/j.bpj.2010.09.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reinsch SS, Mitchison TJ, Kirschner M. Microtubule polymer assembly and transport during axonal elongation. J Cell Biol. 1991;115:365–379. doi: 10.1083/jcb.115.2.365. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roca-Cusachs P, Gauthier NC, Del Rio A, Sheetz MP. Clustering of alpha(5)beta(1) integrins determines adhesion strength whereas alpha(v)beta(3) and talin enable mechanotransduction. Proc Natl Acad Sci USA. 2009;106:16245–16250. doi: 10.1073/pnas.0902818106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roche FK, Marsick BM, Letourneau PC. Protein synthesis in distal axons is not required for growth cone responses to guidance cues. J Neurosci. 2009;29:638–652. doi: 10.1523/JNEUROSCI.3845-08.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rochlin MW, Itoh K, Adelstein RS, Bridgman PC. Localization of myosin II A and B isoforms in cultured neurons. J Cell Sci. 1995;108(Pt 12):3661–3670. doi: 10.1242/jcs.108.12.3661. [DOI] [PubMed] [Google Scholar]
- Rolls MM, Satoh D, Clyne PJ, Henner AL, Uemura T, Doe CQ. Polarity and intracellular compartmentalization of Drosophila neurons. Neural Dev. 2007;2:7. doi: 10.1186/1749-8104-2-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rosner H, Moller W, Wassermann T, Mihatsch J, Blum M. Attenuation of actinomyosinII contractile activity in growth cones accelerates filopodia-guided and microtubule-based neurite elongation. Brain Res. 2007;1176:1–10. doi: 10.1016/j.brainres.2007.07.081. [DOI] [PubMed] [Google Scholar]
- Roy S, Winton MJ, Black MM, Trojanowski JQ, Lee VM. Cytoskeletal requirements in axonal transport of slow component-b. J Neurosci. 2008;28:5248–5256. doi: 10.1523/JNEUROSCI.0309-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ruthel G, Hollenbeck PJ. Growth cones are not required for initial establishment of polarity or differential axon branch growth in cultured hippocampal neurons. J Neurosci. 2000;20:2266–2274. doi: 10.1523/JNEUROSCI.20-06-02266.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sabry J, O'Connor TP, Kirschner MW. Axonal transport of tubulin in Ti1 pioneer neurons in situ. Neuron. 1995;14:1247–1256. doi: 10.1016/0896-6273(95)90271-6. [DOI] [PubMed] [Google Scholar]
- Sabry JH, O'Connor TP, Evans L, Toroian-Raymond A, Kirschner M, Bentley D. Microtubule behavior during guidance of pioneer neuron growth cones in situ. J Cell Biol. 1991;115:381–395. doi: 10.1083/jcb.115.2.381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sanchez-Soriano N, Goncalves-Pimentel C, Beaven R, Haessler U, Ofner-Ziegenfuss L, Ballestrem C, Prokop A. Drosophila growth cones: a genetically tractable platform for the analysis of axonal growth dynamics. Dev Neurobiol. 2010;70:58–71. doi: 10.1002/dneu.20762. [DOI] [PubMed] [Google Scholar]
- Sawada Y, Tamada M, Dubin-Thaler BJ, Cherniavskaya O, Sakai R, Tanaka S, Sheetz MP. Force sensing by mechanical extension of the Src family kinase substrate p130Cas. Cell. 2006;127:1015–1026. doi: 10.1016/j.cell.2006.09.044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schaefer AW, Kabir N, Forscher P. Filopodia and actin arcs guide the assembly and transport of two populations of microtubules with unique dynamic parameters in neuronal growth cones. J Cell Biol. 2002;158:139–152. doi: 10.1083/jcb.200203038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schaefer AW, Schoonderwoert VT, Ji L, Mederios N, Danuser G, Forscher P. Coordination of actin filament and microtubule dynamics during neurite outgrowth. Dev Cell. 2008;15:146–162. doi: 10.1016/j.devcel.2008.05.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seetapun D, Odde DJ. Cell-length-dependent microtubule accumulation during polarization. Curr Biol. 2010;20:979–988. doi: 10.1016/j.cub.2010.04.040. [DOI] [PubMed] [Google Scholar]
- Shibasaki K, Murayama N, Ono K, Ishizaki Y, Tominaga M. TRPV2 enhances axon outgrowth through its activation by membrane stretch in developing sensory and motor neurons. J Neurosci. 2010;30:4601–4612. doi: 10.1523/JNEUROSCI.5830-09.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Siechen S, Yang S, Chiba A, Saif T. Mechanical tension contributes to clustering of neurotransmitter vesicles at presynaptic terminals. Proc Natl Acad Sci USA. 2009;106:12611–12616. doi: 10.1073/pnas.0901867106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith DH. Stretch growth of integrated axon tracts: extremes and exploitations. Prog Neurobiol. 2009;89:231–239. doi: 10.1016/j.pneurobio.2009.07.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sunderland IR, Brenner MJ, Singham J, Rickman SR, Hunter DA, Mackinnon SE. Effect of tension on nerve regeneration in rat sciatic nerve transection model. Ann Plast Surg. 2004;53:382–387. doi: 10.1097/01.sap.0000125502.63302.47. [DOI] [PubMed] [Google Scholar]
- Suter DM, Errante LD, Belotserkovsky V, Forscher P. The Ig superfamily cell adhesion molecule, apCAM, mediates growth cone steering by substrate-cytoskeletal coupling. J Cell Biol. 1998;141:227–240. doi: 10.1083/jcb.141.1.227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Suter DM, Forscher P. An emerging link between cytoskeletal dynamics and cell adhesion molecules in growth cone guidance. Curr Opin Neurobiol. 1998;8:106–116. doi: 10.1016/s0959-4388(98)80014-7. [DOI] [PubMed] [Google Scholar]
- Suter DM, Forscher P. Substrate-cytoskeletal coupling as a mechanism for the regulation of growth cone motility and guidance. J Neurobiol. 2000;44:97–113. [PubMed] [Google Scholar]
- Suter DM, Forscher P. Transmission of growth cone traction force through apCAM-cytoskeletal linkages is regulated by Src family tyrosine kinase activity. J Cell Biol. 2001;155:427–438. doi: 10.1083/jcb.200107063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Suter DM, Schaefer AW, Forscher P. Microtubule dynamics are necessary for SRC family kinase-dependent growth cone steering. Curr Biol. 2004;14:1194–1199. doi: 10.1016/j.cub.2004.06.049. [DOI] [PubMed] [Google Scholar]
- Tanaka EM, Kirschner MW. Microtubule behavior in the growth cones of living neurons during axon elongation. J Cell Biol. 1991;115:345–363. doi: 10.1083/jcb.115.2.345. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Terada S, Kinjo M, Aihara M, Takei Y, Hirokawa N. Kinesin-1/Hsc70-dependent mechanism of slow axonal transport and its relation to fast axonal transport. EMBO J. 2010;29:843–854. doi: 10.1038/emboj.2009.389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Terada S, Kinjo M, Hirokawa N. Oligomeric tubulin in large transporting complex is transported via kinesin in squid giant axons. Cell. 2000;103:141–155. doi: 10.1016/s0092-8674(00)00094-5. [DOI] [PubMed] [Google Scholar]
- Tullio AN, Bridgman PC, Tresser NJ, Chan CC, Conti MA, Adelstein RS, Hara Y. Structural abnormalities develop in the brain after ablation of the gene encoding nonmuscle myosin II-B heavy chain. J Comp Neurol. 2001;433:62–74. doi: 10.1002/cne.1125. [DOI] [PubMed] [Google Scholar]
- Turney SG, Bridgman PC. Laminin stimulates and guides axonal outgrowth via growth cone myosin II activity. Nat Neurosci. 2005;8:717–719. doi: 10.1038/nn1466. [DOI] [PubMed] [Google Scholar]
- Vallee RB, Seale GE, Tsai JW. Emerging roles for myosin II and cytoplasmic dynein in migrating neurons and growth cones. Trends Cell Biol. 2009;19:347–355. doi: 10.1016/j.tcb.2009.03.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vogel V, Sheetz M. Local force and geometry sensing regulate cell functions. Nat Rev Mol Cell Biol. 2006;7:265–275. doi: 10.1038/nrm1890. [DOI] [PubMed] [Google Scholar]
- von Wichert G, Haimovich B, Feng GS, Sheetz MP. Force-dependent integrin-cytoskeleton linkage formation requires downregulation of focal complex dynamics by Shp2. Embo J. 2003a;22:5023–5035. doi: 10.1093/emboj/cdg492. [DOI] [PMC free article] [PubMed] [Google Scholar]
- von Wichert G, Jiang G, Kostic A, De Vos K, Sap J, Sheetz MP. RPTP-alpha acts as a transducer of mechanical force on alphav/beta3-integrin-cytoskeleton linkages. J Cell Biol. 2003b;161:143–153. doi: 10.1083/jcb.200211061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang HB, Dembo M, Hanks SK, Wang Y. Focal adhesion kinase is involved in mechanosensing during fibroblast migration. Proc Natl Acad Sci U S A. 2001;98:11295–11300. doi: 10.1073/pnas.201201198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang Y, Botvinick EL, Zhao Y, Berns MW, Usami S, Tsien RY, Chien S. Visualizing the mechanical activation of Src. Nature. 2005;434:1040–1045. doi: 10.1038/nature03469. [DOI] [PubMed] [Google Scholar]
- Waterman-Storer CM, Desai A, Bulinski JC, Salmon ED. Fluorescent speckle microscopy, a method to visualize the dynamics of protein assemblies in living cells. Curr Biol. 1998;8:1227–1230. doi: 10.1016/s0960-9822(07)00515-5. [DOI] [PubMed] [Google Scholar]
- Weiss P. Nerve Pattern: The mechanics of nerve growth. Growth (Suppl. Third Growth Symp.) 1941;5:163–203. [Google Scholar]
- Yi C, Dahlin LB. Impaired nerve regeneration and Schwann cell activation after repair with tension. Neuroreport. 2010;21:958–962. doi: 10.1097/WNR.0b013e32833e787f. [DOI] [PubMed] [Google Scholar]
- Zhang XF, Schaefer AW, Burnette DT, Schoonderwoert VT, Forscher P. Rho-dependent contractile responses in the neuronal growth cone are independent of classical peripheral retrograde actin flow. Neuron. 2003;40:931–944. doi: 10.1016/s0896-6273(03)00754-2. [DOI] [PubMed] [Google Scholar]
- Zheng J, Buxbaum RE, Heidemann SR. Investigation of microtubule assembly and organization accompanying tension-induced neurite initiation. J Cell Sci. 1993;104(Pt 4):1239–1250. doi: 10.1242/jcs.104.4.1239. [DOI] [PubMed] [Google Scholar]
- Zhou FQ, Waterman-Storer CM, Cohan CS. Focal loss of actin bundles causes microtubule redistribution and growth cone turning. J Cell Biol. 2002;157:839–849. doi: 10.1083/jcb.200112014. [DOI] [PMC free article] [PubMed] [Google Scholar]