Abstract
Introduction:
Concentrations of the total pool of fibronectin in plasma (TFN), and the subset of this pool that contains the alternatively spliced EDA segment (A+FN), are both affected by disease processes, and the latter pool has gained a reputation as a biomarker for vascular injury. We therefore wished to determine if changes in either FN pool correlate with clinical outcomes in critically ill individuals.
Methods:
We analyzed a database for 57 patients with major trauma (n = 33) or sepsis syndrome (n = 24) in which plasma levels of TFN and A+FN had been measured at intervals, along with clinical parameters. Logistic regression analysis was performed to detect associations between predictive variables and three clinical outcomes: 1) the acute respiratory distress syndrome (ARDS), 2) milder acute lung injury designated acute hypoxemic respiratory failure (AHRF), and 3) survival to hospital discharge.
Results:
An increase in plasma TFN during the first 24 hours of intensive care unit (ICU) observation was negatively associated with progression to ARDS (odds ratio 0.98 per 1 microgram (μg)/ml increase, 95% CI (0.97, 1.00)) and AHRF (OR 0.97 per 1 μg/ml increase, (0.95, 0.99)), whereas an increase in A+FN over the first 24 hours was positively associated with progression to AHRF (OR 1.65 per 1 μg/ml increase, (1.04, 2.62)). Additionally, the ratio of the partial pressure of oxygen in arterial blood (PaO2) to the percentage of oxygen in inspired air (FIO2) after 24 hours was positively associated with survival (OR 1.01 per 1 unit increase in ratio, (1.00, 1.03)), along with change in A+FN (OR 1.30 per 1 μg/ml increase, (0.90, 1.88)).
Conclusions:
Different FN isoforms may constitute predictive covariate markers for distinct clinical outcomes in critically ill patients. The data also suggest that early TFN accumulation in the circulation may confer a clinical benefit to patients at risk for acute lung injury.
Keywords: fibronectin, extracellular matrix, alternative splicing, acute lung injury, clinical outcomes
Introduction
Fibronectins (FNs) are a family of large multifunctional adhesion glycoproteins that exist both in soluble and insoluble forms. Plasma FN (pFN) is the designation for the pool of soluble circulating FNs, which collectively constitute a major component of blood (∼0.6 micromolar in plasma), whereas insoluble extracellular matrix (ECM) FNs are concentrated in dense tissue structures, including basement membranes and blood vessel walls.1 FNs are involved in the regulation of a wide variety of cellular functions, including adhesion, migration, proliferation, and apoptosis.1 Much of this regulation is mediated through interactions between FNs and cell surface integrin receptors.2,3 The capacity of FNs to regulate apoptosis is probably clinically relevant, based upon the observation that pFN restricts infarction size in a mouse model of cerebral ischemia by penetrating the zone of tissue injury and triggering α5β1 integrin-mediated neuronal upregulation of the anti-apoptosis gene Bcl-2.4
Although derived from a single gene, FN isoforms differ as a consequence of alternative splicing at three major sites. Two of these are ∼90 amino acid type III repeating modules that may be completely included or excluded from the FN molecule. Accordingly, they are designated “extra type III repeats” A (called “EDA”, for “extra domain A”, or EIIIA) and B (called EDB or EIIIB). Although no function has yet been assigned to EDB, a recognition site for α4β1 and α9β1 integrins is present in the EDA segment.5,6 Since the vast bulk of pFN is produced by hepatocytes, which do not normally express EDA or EDB,1 pFN in healthy subjects is nearly devoid of these segments. 7 In contrast, endothelial cells synthesize and secrete a mixture of FN isoforms in which EDA and EDB segments are included in ∼15%–40% of total synthesized FN monomers.8,9 Such production may contribute to the low concentrations of EDA+ FN (A+FN) and EDB+ FN (B+FN) that have been detected in the plasma of healthy individuals (accounting <∼1% of the total circulating pool of FN).10,11
Total levels of pFN (TFN) fall rapidly after surgery, trauma, burns, and sepsis.12–17 Patients who recover from such insults demonstrate a rebound in TFN, whereas those who die have been observed to exhibit persistently low TFN.1,13,17 These findings, in addition to observations that circulating dimeric pFN (440–500 kDa) is in an equilibrium with the insoluble multimeric FN in capillary walls, and stimulates opsonization of particles within the bloodstream, have long suggested that pFN could exert an adaptive role in acute vascular injury.1,18–20
In contrast to TFN, levels of A+FN increase after major trauma or the onset of sepsis.10 In an earlier descriptive analysis of the same patient database that we examined for clinical outcomes in this study, we found that, compared to levels in healthy subjects, average TFN concentrations were significantly reduced for sepsis or trauma patients at the time of ICU admission and for at least 24 hours thereafter.10 In contrast, in comparison to levels in healthy subjects, A+FN levels were significantly increased for sepsis or trauma patients upon ICU admission and for the subsequent 72 hours, exhibiting a peak mean value at 48 hours for the sepsis group and 72 hours for the trauma group.10 Plasma levels of B+FN were also observed to increase significantly above normal levels at 24, 48, and 72 hours following ICU admission in patients with major trauma.11
Several potential predictors of survival in critically-ill patients, including severity-of-illness measures,21,22 levels of intestinal platelet trapping,23 as well as blood concentrations of cytokines,24 cytokine receptors,25 hormones,26 and coagulation-modulating proteins27 have been studied. Sets of variables have also been considered in the prediction of clinical outcome in critically ill patients, eg, an association has been detected between APACHE III score, gas exchange measured by the ratio of PaO2 to FIO2 on the third ICU day, and mortality in trauma patients with ARDS.28
To determine if particular species among the FN family of alternatively spliced adhesion proteins constitute biomarkers for clinical outcomes in patients with acute systemic illness—possibly in combination with other putative markers of microvascular dysfunction such as the PaO2/FIO2 ratio28—and to look for clues to the physiologic significance of changes in plasma levels of TFN and A+FN, we performed logistic regression analysis to seek associations between the changes in such levels and three outcomes: 1) severe acute lung injury, fulfilling criteria for ARDS,29 2) a milder form of acute lung injury, designated “acute hypoxemic respiratory failure” (AHRF), which is clinically similar to the currently widely accepted acute lung injury (ALI) designation,30 and 3) survival. We have found that the change in plasma TFN levels between 0 and 24 hours of ICU observation (ΔTFN) constitutes a predictive covariate for progression to acute lung injury. Specifically, increases in TFN during this period were associated with reduced odds of progression to ARDS. Among six candidate covariates, ΔTFN alone was the best predictor of ARDS, using a model selection criterion. For prediction of AHRF, three variables in combination were selected: ΔTFN, ΔA+FN (the change in A+FN levels between 0 and 24 hours of ICU observation) and an indicator for risk-group.
Materials and Methods
Study design
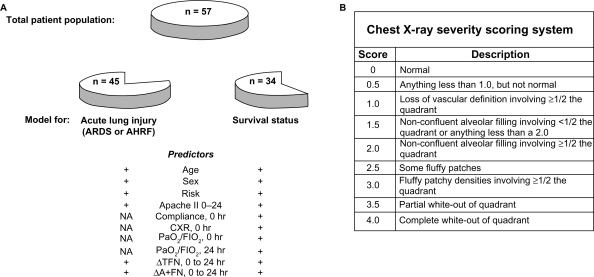
We retrospectively analyzed a clinical database that had originally been collected for a cohort of 59 patients (34 with acute major trauma and 25 with sepsis syndrome) who were admitted to the ICUs of Harborview Medical Center in Seattle, WA in 1985–1986. The research was approved by the institutional review board at the University of Washington, conducted according to the principles of the Declaration of Helsinki, and informed consent was obtained in all instances. During the current study, two of the 59 original patients were eliminated from analysis: 1) a patient originally included in the trauma group10 was retrospectively determined to also have suffered Pneumocystis carinii pneumonia in association with the acquired immune deficiency syndrome, and 2) a patient in the sepsis group was determined to have developed ARDS before admission to the ICU. We have therefore retrospectively analyzed data from the remaining 57 patients (33 with trauma, and 24 with sepsis) (Fig. 1, panel A, “total patient population”), none of whom exhibited either AHRF or ARDS upon induction into the study.
Figure 1.
Experimental schema. A) Patient groups. A total population of 57 patients (33 with trauma and 24 with sepsis) was studied via logistic regression analysis of data collected shortly after admission to the ICU. No patient within this group, designated by the intact “pie” at the top of the panel, had ARDS or AHRF upon ICU admission. For the purpose of determining logistic regression models for progression to acute lung injury (ARDS or AHRF), a subsample of 45 patients was studied (pie remnant to the lower left). For analysis of survival, a group of 34 patients, each of whom was also included in the 45 patient subsample cited above, was analyzed (pie remnant to the lower right) (inclusion criteria for each patient subsample are described in Methods). Demographic and physiologic parameters, as well as changes in concentrations of TFN and A+FN are included in the list of potential predictors available for inclusion in logistic regression models for the prediction of acute lung injury or survival. B) Chest x-ray scoring system. Each chest x-ray was divided into quadrants, each of which was scored according to criteria described in the right panels. The resulting quadrant scores were summed and divided by 4 to yield a composite chest x-ray severity score.
Two or more of the following criteria were required for inclusion in the major trauma group: 1) pelvic or long bone fracture (n = 26), 2) intra-abdominal injury requiring laparotomy (n = 21), 3) pulmonary contusion, flail chest, or intrathoracic injury requiring thoracotomy (n = 20), or 4) transfusion of >8 units of whole blood and/or packed red cells in a 12 hour period (n = 20); whereas sepsis syndrome was defined as systemic infection with a deleterious systemic response.31 The data set includes serial plasma levels of TFN and A+FN, which we measured in samples collected via indwelling arterial catheters at 24 hour intervals, up to a maximum of 72 hours, as reported previously.10 Methods used in the preparation and storage of plasma samples, the quantitative immunoassays for measurement of TFN and A+FN, and descriptive statistics regarding levels of the two categories of FN in the two groups of patients have been published.10
The following demographic variables had been recorded for each patient at the time of the original study: age in years, sex, and risk group (trauma or sepsis, as defined above) (Fig. 1, panel A, “predictors”). In addition, the following physiologic parameters were measured and recorded at 0, 24, 48 and 72 hours following ICU admission: total static thoracic compliance (mls/cm H2O), PaO2, FIO2, and a composite chest x-ray score. The latter was derived from a 4-quadrant analysis of each chest radiograph in which right and left lungs were each divided into upper and lower quadrants by a horizontal line 2 centimeters below the carina. Each quadrant was then scored on a 0–4 point scale, and the composite score was calculated by adding the individual quadrant scores and dividing by 4 (Fig. 1, panel B). For example, a score of 0 indicated an absolutely clear chest x-ray, while a score of 4 indicated total opacification of all lung fields. Chest x-ray scoring was performed at the time of initial patient accrual by a chest radiologist and a pulmonary physician. The ratio of PaO2 to FIO2 (PaO2/FIO2 ratio) was calculated as an indicator of severity of hypoxemia for each patient at each time point. Finally, an APACHE II score32 was calculated and recorded for each patient, based on the worst values over the course of the first 24 hours (“APACHE II 0–24” in Fig. 1, panel A).
Although ARDS29 was the sole accepted clinical designation for acute hypoxemic lung injury at the time of the original study, we recognized at that time that acute lung injury represents a continuum of abnormalities, as reflected by our incremental radiographic scoring system (Fig. 1, panel B), Nevertheless, the American European Consensus Conference (AECC) definition of acute lung injury (ALI)30 had not yet been developed, and our original composite CXR scores do not permit retrospective application of the ALI definition. However, the scoring system allows us to identify a group of patients with a milder degree of hypoxemic respiratory failure approximating what many would consider to be ALI. We define this condition, designated “acute hypoxemic respiratory failure” (AHRF), as both a PaO2/FIO2 ratio <300 and a composite CXR score >1.5. Survival is defined as discharge from the hospital, regardless of the total duration of hospitalization.
Statistical analysis
Several patients died or were transferred out of the ICU before 72 hours, leading to progressive attrition in the subject population at successive time points. For example, FN levels were measured for 57 (33 trauma and 24 sepsis), 45 (26 trauma and 19 sepsis), 42 (24 trauma and 18 sepsis), and 37 (22 trauma and 15 sepsis) patients at 0, 24, 48, and 72 hours. We therefore limited our analysis to the 0 and 24 hour time points (for which data are available for 45 patients) in order to focus on the time period containing the maximum density of data, while permitting study of clinical time courses.
Our goal was to determine a reduced set of covariates which have high utility in predicting clinical outcome. For each of the binary outcomes (ARDS, AHRF, and survival), model selection was performed by successively eliminating predictors from a full model, using the Akaike Information Criterion (AIC).33,34 We used the “step AIC” function, available in the MASS library of the statistical software package R,35 to carry out the elimination steps. Goodness of fit was assessed using measures of deviance, 2-by-2 cross-classifications of predicted versus observed outcomes, and (on a patient-by-patient basis) residuals and influence values. We used Agresti36 as a general reference on logistic regression.
Prediction of ARDS and AHRF
Among the total population of 57 patients, logistic regression models for ARDS or AHRF status were estimated using the subsample of 45 patients who had complete data at admission for age, sex, risk group (trauma or sepsis), APACHE, and plasma concentrations of TFN and A+FN at the 0 and 24 hour time points (Fig. 1, panel A). Static thoracic compliance, chest x-ray score, and PaO2/FIO2 ratios were excluded from this analysis, as these are primary variables used in the diagnosis of acute lung injury,37 and their inclusion as predictors of ARDS or AHRF would lead to logical circularities.
ΔTFN and ΔA+FN, calculated by subtracting the levels of TFN and A+FN at 0 hours from their respective levels at 24 hours, are entered as predictors in a model for acute lung injury, along with age, sex, risk group, APACHE II 0–24 and an intercept. We call this the “full” model for acute lung injury (Fig. 1, panel A).
Prediction of survival
A logistic regression model for survival status was estimated using the subsample of n = 34 patients who had complete data for age in years, sex, risk-group (trauma or sepsis), APACHE II 0–24, thoracic compliance at 0 hours, chest x-ray score at 0 hours, and complete data for PaO2 and FIO2, as well as concentrations of TFN and A+FN, at the 0 and 24 hour time points (Fig. 1, panel A). The PaO2/FIO2 ratios after 0 and 24 hours of ICU observation are entered as predictors into a model for survival status, along with an intercept, ΔTFN, ΔA+FN and the other variables listed immediately above. We call this the “full” model for survival status.
Results
Subject characteristics
Sepsis patients were older (average age 49 versus 38), and had a higher rate of acute lung injury (ARDS or AHRF), and a lower rate of survival, than the trauma group (Table 1). Nevertheless, the time interval analyzed in this study—the first 24 hours following ICU admission (time = 0 to 24 hours)—was similar, relative to clinical course, for the two groups. For example, the “risk-to-draw time” (time of diagnosis of sepsis syndrome or major trauma to the time = 0 hour blood draw), which was available for 50 patients, did not differ significantly for 19 sepsis patients (16.8 ± 12.1 hours) versus 31 trauma patients (mean 17.6 ± 7.0 hours; two-tailed t-test, P = n.s.). Within the subsample of 45 patients analyzed for progression to ARDS or AHRF (Fig. 1, panel A), the risk-to-draw time also did not differ significantly for the 19 with sepsis (16.8 ± 12.1 hours) versus the 26 with trauma (17.2 ± 6.9 hours). Similarly, among the subsample of 34 patients analyzed for survival (Fig. 1, panel A), this value also did not differ significantly for the 13 with sepsis (17.8 ± 12.7 hours) versus the 21 with trauma (18.3 ± 6.3 hours) (Table 1). Of note, however, is that the time from hospital admission to the first blood draw (“admit-to-draw time”, available for 50 patients) was significantly greater for the sepsis group (97.6 ± 135 hours) than for the trauma group (15.0 ± 8.1 hours; two-tailed t-test, P = 0.003) (Table 1).
Table 1.
Subject characteristics.1
| Sepsis (n = 24) | Trauma (n = 33) | Combined group (n = 57) | |
|---|---|---|---|
| Age | 49.0 ± 16.5 | 38.4 ± 18.2 | 42.8 ± 18.1 |
| % male | 62.5 | 60.6 | 61.4 |
| % ARDS | 66.7 | 45.5 | 54.4 |
| % AHRF | 87.5 | 63.6 | 73.7 |
| % survival | 62.5 | 66.7 | 64.9 |
| Lung compliance at 0 h | 43.7 ± 12.0 | 45.0 ± 19.9 | 44.6 ± 17.4 |
| APACHE II 0–24 h | 23.8 ± 8.5 | 21.8 ± 9.3 | 22.6 ± 8.9 |
| CXR score 0 h | 2.0 ± 1.0 | 1.2 ± 1.1 | 1.5 ± 1.1 |
| PaO2/FiO2 at 0 h | 158.7 ± 92.3 | 254.5 ± 103.0 | 218.1 ± 108.8 |
| PaO2/FiO2 at 24 h | 181.3 ± 111.2 | 254.4 ± 97.6 | 225.5 ± 108.2 |
| ΔTFN 0–24 h | −14.5 ± 38.5 | 18.8 ± 62.5 | 4.7 ± 55.7 |
| ΔEIIIA + FN 0–24 h | 2.0 ± 1.9 | 6.8 ± 3.1 | 4.7 ± 3.6 |
| Risk-to-draw, all patients (h)2 | 16.8 ± 12.1 | 17.6 ± 7.0 | 17.3 ± 9.1 |
| Admit-to-draw, all patients (h)3 | 97.6 ± 135.0 | 15.0 ± 8.1 | 46.4 ± 91.5 |
| Risk-to-draw, patients analyzed for progression to ARDS or AHRF (n = 45) (h)4 | 16.8 ± 12.1 | 17.2 ± 6.9 | 17.0 ± 9.3 |
| Risk-to-draw, patients analyzed for survival (n = 34) (h)5 | 17.8 ± 12.7 | 18.3 ± 6.3 | 18.1 ± 9.1 |
Notes:
Data are expressed as the average ± standard deviation;
Risk-to-draw = the time in hours from initial diagnosis of either major trauma or sepsis syndrome to the time of the 0 h (first) blood draw. Data were available for 50 (19 sepsis and 31 trauma) patients;
Admit-to-draw = the time in hours from hospital admission to the 0 h (first) blood draw. Data were available for 50 (19 sepsis and 31 trauma) patients;
In this subgroup, data were available for 19 sepsis patients and 26 trauma patients;
In this subgroup, data were available for 13 sepsis patients and 21 trauma patients.
Logistic regression for ARDS
Of the predictors in the full model, only ΔTFN, along with an intercept, remains in the best sub-model chosen by backwards elimination with the AIC (Table 2, top). The odds ratio for a unit (1 μg/ml) increase in TFN at 24 hours, as compared to 0 hours, is 0.98, with 95% confidence interval (0.97, 1.00). The odds ratio for a 20-unit (20 μg/ml) increase in TFN is 0.73, with 95% CI (0.55, 0.96), suggesting an average 27% reduction in the odds of an ARDS diagnosis for a change in TFN typical of the trauma sample (see Table 1). Table 3 (top) gives a cross-classification of observed versus predicted ARDS status using the logistic regression model of Table 2. As shown, of the 19 patients predicted by the model to develop ARDS, 12 did.
Table 2.
Logistic regression models for ARDS, AHRF, and survival.
| Predictor | Coefficient | Std. error | z-value | P-value |
|---|---|---|---|---|
| ARDS | ||||
| Intercept | 0.040 | 0.326 | 0.122 | 0.902 |
| ΔTFN | −0.016 | 0.007 | −2.408 | 0.016 |
| AHRF | ||||
| Intercept | −5.645 | 2.931 | −1.926 | 0.054 |
| Risk = sepsis | 3.348 | 1.523 | 2.199 | 0.028 |
| ΔTFN | −0.030 | 0.011 | −2.687 | 0.007 |
| ΔA+FN | 0.503 | 0.235 | 2.139 | 0.032 |
| Survival | ||||
| Intercept | −6.680 | 3.415 | −1.956 | 0.050 |
| Risk = sepsis | 2.214 | 1.393 | 1.590 | 0.111 |
| PaO2/FIO2(24) | 0.014 | 0.006 | 2.344 | 0.019 |
| ΔA+FN | 0.263 | 0.189 | 1.392 | 0.164 |
Notes: Estimated coefficients and associated statistics for the best submodel chosen by backwards elimination with the AIC. The coefficient “risk = sepsis” gives the difference in the log odds of the outcome for the sepsis group, as compared to the trauma group, with a positive coefficient indicating that the outcome is more likely within the sepsis group, other variables being equal. The P-values are for two-sided tests.
Table 3.
Observed versus predicted lung injury (ARDS or AHRF) and survival status.
| Predicted status: | |||
|---|---|---|---|
| ARDS | |||
| ARDS | No ARDS | ||
| Observed status: | ARDS | 12 | 10 |
| No ARDS | 7 | 16 | |
| AHRF | |||
| AHRF | No AHRF | ||
| Observed status: | AHRF | 28 | 3 |
| No AHRF | 5 | 9 | |
| Survival | |||
| Survival | Death | ||
| Observed status: | Survived | 17 | 4 |
| Died | 4 | 9 | |
Notes: Observed ARDS and AHRF (using study criteria for diagnosis of ARDS and AHRF) and survival status, cross-classified with ARDS, AHRF, and survival status as predicted by the logistic regression models of Table 2, for the n = 45 cases used to fit the acute lung injury model and the n = 34 cases used to fit the survival model. Predicted ARDS, AHRF, or survival status is based on assigning patients with estimated probabilities of ARDS, AHRF, or survival greater than 0.5 to the “predicted ARDS”, “predicted AHRF”, or “predicted survival” groups.
Logistic regression for AHRF
Of the predictors in the “full” model: age, sex, risk-group, APACHE II 0–24, ΔTFN, and ΔA+FN, four variables (ΔTFN, ΔA+FN, risk group, and an intercept) remain in the best sub-model (Table 2, middle). The odds ratio for a unit (1 μg/ml) increase in TFN at 24 hours, as compared to 0 hours, is 0.97, with 95% confidence interval (0.95, 0.99). Contrary to TFN, an increase in A+FN between 0 and 24 hours is associated with an increase in the odds of an AHRF diagnosis (OR 1.65 per1 μg/ml, 95% CI (1.04, 2.62)). Finally, risk-group remains in the predictive set of variables. Table 3 (middle) gives a cross-classification of observed versus predicted AHRF status using the logistic regression model of Table 2. As can be seen, of the 33 patients predicted by the model to have AHRF, 28 actually developed AHRF. Similarly of the 12 patients not predicted by the model to have AHRF, 9 actually did not have AHRF.
Logistic regression for survival
The predictors included in the “full” model were age, sex, risk-group, APACHE II 0–24, lung compliance at 0 hours, chest x-ray score at 0 hours, PaO2/FIO2 ratios at 0 and 24 hours, ΔA+FN, ΔTFN, and an intercept term. The aim is to predict survival status using clinical variables, separately from AHRF or ARDS status. However, as PaO2/FIO2 ratios constitute an essential part of AHRF or ARDS diagnoses,30,37 they may be viewed as proxies for lung injury in a model for survival. Of the variables in the full model, only the PaO2/FIO2 ratio at 24 hours (PaO2/FIO2(24)), ΔA+FN and risk-group, along with an intercept, remain in the best sub-model (Table 2). The odds ratio for a unit increase in PaO2/FIO2 ratio (24) is 1.01, with 95% CI (1.00, 1.03). Although ΔA+FN and risk-group remain in the best sub-model, they are non-significant predictors using conventional thresholds (see P-values in Table 2). Table 3 (bottom) gives a cross-classification of observed versus predicted survival status using the logistic regression model of Table 2. As can be seen, the predictive accuracy of the model for survival is relatively good, with 26 out of 34 patients correctly classified for outcome.
Associations between AHRF, ARDS and survival
Table 4 gives a cross-classification of ARDS or AHRF diagnoses and survival among the 57 patients in the study sample. It is clear that the patients with ARDS or AHRF were significantly less likely to survive, and consequently ARDS or AHRF are important predictors of survival in this sample.
Table 4.
ARDS or AHRF diagnoses and survival.
| Observed survival status: | |||
|---|---|---|---|
| ARDS | |||
| Died | Survived | ||
| Observed ARDS status: | ARDS | 18 (10.9) | 13 (20.1) |
| No ARDS | 2 (9.1) | 24 (16.9) | |
| AHRF | |||
| Observed AHRF status: | AHRF | 20 (14.7) | 22 (27.3) |
| No AHRF | 0 (5.3) | 15 (9.7) | |
Notes: Observed ARDS or AHRF status cross-classified with observed survival status, for the n = 57 cases in the total study sample. Expected counts under the null-hypothesis of no association between ARDS or AHR F status and survival are given in parentheses. For ARDS, the chi-square test-statistic is X2 = 15.6 on 1 degree of freedom, with P-value approximately 8 × 10−5. For AHRF, the chi-square test-statistic is X2 = 11.0 on 1 degree of freedom, with P-value approximately 9 × 10−4.
Discussion
Using logistic regression analysis, we have observed that early changes in TFN constitute covariate predictors for acute lung injury in ICU patients with major trauma or sepsis syndrome. Specifically, increases in TFN during the first 24 hours of ICU observation following diagnosis correlated negatively with progression either to the more severe ARDS or a milder presentation of acute lung injury, designated here as AHRF. ΔTFN (negative correlation) stood alone as a predictive variable in the best model for ARDS and was included with ΔA+FN (positive correlation) and risk-group in the best subset of variables for prediction of AHRF. The predictive accuracy of the AHRF model was relatively good, with 37 out of 45 patients correctly classified for clinical outcome, whereas the model for prediction of ARDS exhibited a lower predictive accuracy, with 28 out of 45 patients correctly classified (Table 3). Of note, the time from diagnosis of either major trauma or sepsis syndrome to initiation of analysis did not differ, indicating that the study population was homogeneous for timing of analysis relative to onset of risk for acute lung injury or death.
Although mean initial plasma TFN levels have been observed to be higher in medical ICU patients who survive than in those who die,16 and lower in critically ill patients who progress to acute lung injury than in those who do not,38 measurements of plasma TFN at a single time point have not proven to be either sensitive or specific in the prediction of acute lung injury.38 In contrast to these earlier studies, we have analyzed changes in TFN levels shortly after the recognition of major trauma or sepsis syndrome, and found that these correlate negatively with the development of either ARDS or AHRF. Since development of ARDS or AHRF increases the likelihood of death (Table 4), these observations are consistent with reports showing that a “rebound” in the circulating level of FN is associated with increased survival in patients with trauma, sepsis and other critical illnesses.1,13,17
Why should rapid early accumulation of FN within the circulation correlate with maintenance of lung function in patients with vascular tissue injury resulting from trauma or sepsis? Since soluble pFN is in equilibrium with insoluble FN in the ECM of basement membranes and blood vessel walls,1,18 the rate of accumulation of circulating FN after lung microvascular injury could potentially correlate with rates of FN incorporation into pulmonary capillary walls which, in turn, could: 1) inhibit apoptosis of cells involved in maintenance of the alveolar-capillary barrier, as previously observed for neurons in an in vivo model of brain tissue injury,4 and 2) reinforce the ECM in pulmonary capillary basement membranes, thereby increasing barrier function towards solutes.18,20 FN also promotes reticuloendothelial clearance of particulate matter from the circulation, and could therefore potentially protect the microcirculation of vital organs, including the lungs, from microembolization during times of tissue injury.1,19
A+FN expression is triggered at sites of tissue injury, where such upregulated expression appears to facilitate wound healing.1,39 The association between increased A+FN expression and adaptation to tissue injury could potentially underlie the observed positive associations between ΔA+FN and two disparate clinical outcomes in critically ill patients: AHRF and survival (Table 2). For example, the association between the accumulation of circulating A+FN and AHRF is consistent with previous observations that A+FN is released into the circulation at sites of experimental pulmonary vascular injury/dysfunction in vitro and in vivo.40,41 The magnitude of such responses might be expected to reflect the extent of pulmonary tissue injury, and therefore the relative risk for progression to organ dysfunction. Conversely, the positive association between ΔA+FN and survival that we have observed, although not significant by conventional standards, could reflect the necessity of A+FN for healing of injured tissue.39
Among the strengths of this study is that TFN and A+FN were measured simultaneously using a common standard antigen.10 Among the several limitations is the substantial interval that has passed between the analysis reported here and the original work upon which it is based. Clinical data collection and the assays for TFN and A+FN were performed in the late 1980’s, as described.10 While the widely accepted designation for acute lung injury at the time of the original study was ARDS, the focus of lung injury research has subsequently shifted toward ALI, an earlier stage in the continuum of injury that is likely to be more amenable to molecular characterization and treatment.30 In an attempt to identify and analyze a subset of patients whose condition approximates ALI, it was necessary for us to rely on a surrogate designation (AHRF) that incorporates parenchymal opacities on chest radiograph and the same level of hypoxemia as the currently accepted classification of ALI.30 We have also studied a relatively small number of patients, and our focus on those who survived for at least 24 hours in the ICU may have biased our sample toward subjects who were more likely to survive.
Additionally, several centers have reported an increase in survival of ARDS patients since the time of our original study,42 potentially influencing the usefulness of our data. The improvement in ARDS outcome has been commonly attributed to the use of lung protective ventilation, although other enhancements in ICU care may also play a role. Despite this, the causes and timing of ARDS deaths have not changed substantially over time, with most deaths occurring later in the course of illness.43 Since the focus of our study is on the much earlier phase of vascular injury preceding ARDS onset, we feel that the findings presented here may still be broadly applicable and provide a foundation for future mechanistic and clinical lung injury research.
Finally, our observations do not elucidate a specific mechanism for the changes in circulating levels of TFN and A+FN that follow major trauma or sepsis syndrome. A myriad of physiologic processes and cell types could potentially contribute to such changes in critically ill patients, including vascular fluid shifts associated with disease and treatments, altered FN synthesis, nutritional status, incorporation of FN into blood clots, and altered liver function.1
Conclusions
The data presented in this pilot study suggest that early TFN accumulation within the circulation of patients with trauma or sepsis correlates with reduced odds of acute lung injury. Conversely, the data also suggest a correlation between the rate of early accumulation of A+FN isoforms and development of clinical signs of mild lung injury. Although these observations are correlative and, as such, do not demonstrate cause and effect, they suggest that TFN could play a role in protection against acute lung injury in patients with sepsis or major trauma. This conjecture is not new, as attempts were made in the 1980s to protect critically ill patients from acute lung injury and death through infusion of pFN or pFN-containing cryoprecipitate. Although these research experiments were not successful, they may have been confounded by difficulties inherent in the isolation and preparation of FN and in clinical use of protein mixtures such as cryoprecipitate.1,19,44 With the advent of gene-targeted mice that have been rendered null for hepatocyte-derived (EDA−/EDB−) pFN,4 A+FN,39,45 or B+FN,46 the effects of specific species of circulating FN upon the progression of acute and chronic forms of tissue injury and inflammation can now be tested without the necessity for infusion of soluble FNs obtained from blood or other external sources.
Acknowledgments
We thank Charles G. Cochrane for leading us to study the role of extracellular matrix molecules in acute lung injury. Supported by a Merit Review award from the Department of Veterans Affairs, a gift from the Charles See Foundation, and a grant from the Nora Eccles Treadwell Foundation (JHP).
List of Abbreviations
- FN
fibronectin
- TFN
total circulating FN
- EDA
the alternatively spliced extra domain A of FN
- A+FN
the subset of FN containing the alternatively spliced EDA segment
- ARDS
acute respiratory distress syndrome
- AHRF
acute hypoxemic respiratory failure
- ICU
intensive care unit
- CI
confidence interval
- OR
odds ratio
- ECM
extracellular matrix
- pFN
plasma FN
- EDB
the alternatively spliced extra domain B of FN
- APACHE
Acute Physiology and Chronic Health Evaluation
- PaO2
partial pressure of oxygen in arterial blood
- FIO2
percentage of oxygen in inspired air
Footnotes
Authors’ Contributions
Dr. Peters contributed to the study design, acquisition of data, analysis of data and writing of the manuscript. Dr. Grote performed the statistical analyses and also contributed to the writing of the manuscript. Dr. Lane contributed to the interpretation/analysis of the data and to writing the manuscript. Dr. Maunder contributed to the study design, analysis of data, writing the manuscript, and was the supervisor of the original clinical study.
Disclosure
This manuscript has been read and approved by all authors. This paper is unique and is not under consideration by any other publication and has not been published elsewhere. The authors and peer reviewers of this paper report no conflicts of interest. The authors confirm that they have permission to reproduce any copyrighted material.
Key Messages
In critically ill patients with acute major trauma or sepsis syndrome, early accumulation of the adhesion protein fibronectin within the circulation correlates with reduced odds of progression to acute lung injury, manifest either as the Acute Respiratory Distress Syndrome (ARDS), or a milder injury designated acute hypoxemic respiratory failure (AHRF).
In critically ill patients with acute major trauma or sepsis syndrome, early accumulation within the circulation of the subcategory of fibronectin isoforms containing the alternatively spliced EDA segment correlates with increased odds of progression to AHRF.
Different plasma fibronectin isoforms convey different clinical information and may be useful in the prediction of distinct clinical outcomes in critically ill patients.
References
- 1.Hynes R. Fibronectins. New York: Springer-Verlag Inc.; 1990. [Google Scholar]
- 2.Matter ML, Ruoslahti E. A signaling pathway from the alpha5 beta1 and alpha(v)beta3 integrins that elevates bcl-2 transcription. J Biol Chem. 2001;276(30):27757–63. doi: 10.1074/jbc.M102014200. [DOI] [PubMed] [Google Scholar]
- 3.Han SW, Roman J. Fibronectin induces cell proliferation and inhibits apoptosis in human bronchial epithelial cells: pro-oncogenic effects mediated by PI3-kinase and NF-kappaB. Oncogene. 2006;25(31):4341–9. doi: 10.1038/sj.onc.1209460. [DOI] [PubMed] [Google Scholar]
- 4.Sakai T, Johnson KJ, Murozono M, et al. Plasma fibronectin supports neuronal survival and reduces brain injury following transient focal cerebral ischemia but is not essential for skin-wound healing and hemostasis. Nat Med. 2001;7(3):324–30. doi: 10.1038/85471. [DOI] [PubMed] [Google Scholar]
- 5.Liao YF, Gotwals PJ, Koteliansky VE, Sheppard D, Van De Water L. The EIIIA segment of fibronectin is a ligand for integrins alpha9 beta1 and alpha4 beta1 providing a novel mechanism for regulating cell adhesion by alternative splicing. J Biol Chem. 2002;277(17):14467–74. doi: 10.1074/jbc.M201100200. [DOI] [PubMed] [Google Scholar]
- 6.Shinde AV, Bystroff C, Wang C, et al. Identification of the peptide sequences within the EIIIA (EDA) segment of fibronectin that mediate integrin alpha9beta1-dependent cellular activities. J Biol Chem. 2008;283(5):2858–70. doi: 10.1074/jbc.M708306200. [DOI] [PubMed] [Google Scholar]
- 7.Peters JH, Greasby T, Lane N, Woolf A. Correlations between plasma levels of a fibronectin isoform subpopulation and C-reactive protein in patients with systemic inflammatory disease. Biomarkers. 2009;14(4):250–7. doi: 10.1080/13547500902836032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Peters JH, Sporn LA, Ginsberg MH, Wagner DD. Human endothelial cells synthesize, process, and secrete fibronectin molecules bearing an alternatively spliced type III homology (ED1) Blood. 1990;75(9):1801–8. [PubMed] [Google Scholar]
- 9.Peters JH, Trevithick JE, Johnson P, Hynes RO. Expression of the alternatively spliced EIIIB segment of fibronectin. Cell Adhes Commun. 1995;3(1):67–89. doi: 10.3109/15419069509081278. [DOI] [PubMed] [Google Scholar]
- 10.Peters JH, Maunder RJ, Woolf AD, Cochrane CG, Ginsberg MH. Elevated plasma levels of ED1+ (“cellular”) fibronectin in patients with vascular injury. J Lab Clin Med. 1989;113(5):586–97. [PubMed] [Google Scholar]
- 11.Peters JH, Loredo GA, Chen G, et al. Plasma levels of fibronectin bearing the alternatively spliced EIIIB segment are increased after major trauma. J Lab Clin Med. 2003;141(6):401–10. doi: 10.1016/S0022-2143(03)00042-8. [DOI] [PubMed] [Google Scholar]
- 12.Cembrowski GS, Mosher DF. Plasma fibronectin concentration in patients with acquired consumptive coagulopathies. Thromb Res. 1984;36(5):437–45. doi: 10.1016/0049-3848(84)90300-1. [DOI] [PubMed] [Google Scholar]
- 13.Richards WO, Scovill WA, Shin B. Opsonic fibronectin deficiency in patients with intra-abdominal infection. Surgery. 1983;94(2):210–7. [PubMed] [Google Scholar]
- 14.Eriksen HO, Molke-Jensen F, Clemmensen I. Plasma fibronectin concentration in patients admitted to intensive care unit. Haematologia (Budap) 1984;17(1):93–100. [PubMed] [Google Scholar]
- 15.Rubli E, Bussard S, Frei E, Lundsgaard-Hansen P, Pappova E. Plasma fibronectin and associated variables in surgical intensive care patients. Ann Surg. 1983;197(3):310–7. doi: 10.1097/00000658-198303000-00011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.O’Connell MT, Becker DM, Steele BW, Peterson GS, Hellman RL. Plasma fibronectin in medical ICU patients. Crit Care Med. 1984;12(6):479–82. doi: 10.1097/00003246-198406000-00001. [DOI] [PubMed] [Google Scholar]
- 17.Brodin B, Von Schenck H, Schildt B, Liljedahl SO. Low plasma fibronectin indicates septicaemia in major burns. Acta Chir Scand. 1984;150(1):5–11. [PubMed] [Google Scholar]
- 18.Oh E, Pierschbacher M, Ruoslahti E. Deposition of plasma fibronectin in tissues. Proc Natl Acad Sci U S A. 1981;78(5):3218–21. doi: 10.1073/pnas.78.5.3218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Saba TM, Blumenstock FA, Shah DM, et al. Reversal of opsonic deficiency in surgical, trauma, and burn patients by infusion of purified human plasma fibronectin. Correlation with experimental observations. Am J Med. 1986;80(2):229–40. doi: 10.1016/0002-9343(86)90014-8. [DOI] [PubMed] [Google Scholar]
- 20.Seeger W, Walmrath D, Heimburger N, Neuhof H. Fibronectin decreases pulmonary vascular permeability under baseline conditions and after administration of arachidonic acid in rabbit lungs. Thromb Res. 1986;44(2):135–46. doi: 10.1016/0049-3848(86)90129-5. [DOI] [PubMed] [Google Scholar]
- 21.Vassar MJ, Lewis FR, Jr, Chambers JA, et al. Prediction of outcome in intensive care unit trauma patients: a multicenter study of Acute Physiology and Chronic Health Evaluation (APACHE), Trauma and Injury Severity Score (TRISS), and a 24-hour intensive care unit (ICU) point system. J Trauma. 1999;47(2):324–9. doi: 10.1097/00005373-199908000-00017. [DOI] [PubMed] [Google Scholar]
- 22.Kuhls DA, Malone DL, McCarter RJ, Napolitano LM. Predictors of mortality in adult trauma patients: the physiologic trauma score is equivalent to the Trauma and Injury Severity Score. J Am Coll Surg. 2002;194(6):695–704. doi: 10.1016/s1072-7515(02)01211-5. [DOI] [PubMed] [Google Scholar]
- 23.Sigurdsson GH, Christenson JT, el-Rakshy MB, Sadek S. Intestinal platelet trapping after traumatic and septic shock. An early sign of sepsis and multiorgan failure in critically ill patients? Crit Care Med. 1992;20(4):458–67. doi: 10.1097/00003246-199204000-00005. [DOI] [PubMed] [Google Scholar]
- 24.Holzheimer RG, Capel P, Cavaillon JM, et al. Immunological surrogate parameters in a prognostic model for multi-organ failure and death. Eur J Med Res. 2000;5(7):283–94. [PubMed] [Google Scholar]
- 25.Parsons PE, Matthay MA, Ware LB, Eisner MD. Elevated Plasma Levels of Soluble TNF Receptors Are Associated with Morbidity and Mortality in Patients with Acute Lung Injury. Am J Physiol Lung Cell Mol Physiol. 2004. [DOI] [PubMed]
- 26.Ray DC, Macduff A, Drummond GB, Wilkinson E, Adams B, Beckett GJ. Endocrine measurements in survivors and non-survivors from critical illness. Intensive Care Med. 2002;28(9):1301–8. doi: 10.1007/s00134-002-1427-y. [DOI] [PubMed] [Google Scholar]
- 27.Matthay MA, Ware LB. Plasma protein C levels in patients with acute lung injury: prognostic significance. Crit Care Med. 2004;32(5 Suppl):S229–32. doi: 10.1097/01.ccm.0000126121.56990.d3. [DOI] [PubMed] [Google Scholar]
- 28.Navarrete-Navarro P, Ruiz-Bailen M, Rivera-Fernandez R, Guerrero-Lopez F, Pola-Gallego-de-Guzman MD, Vazquez-Mata G. Acute respiratory distress syndrome in trauma patients: ICU mortality and prediction factors. Intensive Care Med. 2000;26(11):1624–9. doi: 10.1007/s001340000683. [DOI] [PubMed] [Google Scholar]
- 29.Hudson LD, Milberg JA, Anardi D, Maunder RJ. Clinical risks for development of the acute respiratory distress syndrome. Am J Respir Crit Care Med. 1995;151(2 Pt 1):293–301. doi: 10.1164/ajrccm.151.2.7842182. [DOI] [PubMed] [Google Scholar]
- 30.Bernard GR, Artigas A, Brigham KL, et al. The American-European Consensus Conference on ARDS. Definitions, mechanisms, relevant outcomes, and clinical trial coordination. Am J Respir Crit Care Med. 1994;149(3 Pt 1):818–24. doi: 10.1164/ajrccm.149.3.7509706. [DOI] [PubMed] [Google Scholar]
- 31.Pepe PE, Potkin RT, Reus DH, Hudson LD, Carrico CJ. Clinical predictors of the adult respiratory distress syndrome. Am J Surg. 1982;144(1):124–30. doi: 10.1016/0002-9610(82)90612-2. [DOI] [PubMed] [Google Scholar]
- 32.Knaus WA, Draper EA, Wagner DP, Zimmerman JE. APACHE II: a severity of disease classification system. Crit Care Med. 1985;13(10):818–29. [PubMed] [Google Scholar]
- 33.Akaike H. A new look at statistical model identification. IEEE Transactions on Automatic Control. 1974;AU-19:716–22. [Google Scholar]
- 34.Venables WN, Ripley BD. Modern Applied Statistics with S. 4th ed. New York: Springer-Verlag; 2002. [Google Scholar]
- 35.Venables WN, Ripley BD. MASS version 7.1–11. 2003. In.
- 36.Agresti A. Categorical Data Analysis. 2nd ed. New York: Wiley Interscience; 2002. [Google Scholar]
- 37.Atabai K, Matthay MA. The pulmonary physician in critical care. 5: Acute lung injury and the acute respiratory distress syndrome: definitions and epidemiology. Thorax. 2002;57(5):452–8. doi: 10.1136/thorax.57.5.452. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Maunder RJ, Harlan JM, Pepe PE, Paskell S, Carrico CJ, Hudson LD. Measurement of plasma fibronectin in patients who develop the adult respiratory distress syndrome. J Lab Clin Med. 1984;104(4):583–90. [PubMed] [Google Scholar]
- 39.Muro AF, Chauhan AK, Gajovic S, et al. Regulated splicing of the fibronectin EDA exon is essential for proper skin wound healing and normal lifespan. J Cell Biol. 2003;162(1):149–60. doi: 10.1083/jcb.200212079. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Peters JH, Ginsberg MH, Bohl BP, Sklar LA, Cochrane CG. Intravascular release of intact cellular fibronectin during oxidant-induced injury of the in vitro perfused rabbit lung. J Clin Invest. 1986;78(6):1596–603. doi: 10.1172/JCI112752. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Peters JH, Ginsberg MH, Case CM, Cochrane CG. Release of soluble fibronectin containing an extra type III domain (ED1) during acute pulmonary injury mediated by oxidants or leukocytes in vivo. Am Rev Respir Dis. 1988;138(1):167–74. doi: 10.1164/ajrccm/138.1.167. [DOI] [PubMed] [Google Scholar]
- 42.Zambon M, Vincent JL. Mortality rates for patients with acute lung injury/ARDS have decreased over time. Chest. 2008;133(5):1120–7. doi: 10.1378/chest.07-2134. [DOI] [PubMed] [Google Scholar]
- 43.Stapleton RD, Wang BM, Hudson LD, Rubenfeld GD, Caldwell ES, Steinberg KP. Causes and timing of death in patients with ARDS. Chest. 2005;128(2):525–32. doi: 10.1378/chest.128.2.525. [DOI] [PubMed] [Google Scholar]
- 44.Grossman JE. Plasma fibronectin and fibronectin therapy in sepsis and critical illness. Rev Infect Dis. 1987;9(Suppl 4):S420–30. doi: 10.1093/clinids/9.supplement_4.s420. [DOI] [PubMed] [Google Scholar]
- 45.Tan MH, Sun Z, Opitz SL, Schmidt TE, Peters JH, George EL. Deletion of the alternatively spliced fibronectin EIIIA domain in mice reduces atherosclerosis. Blood. 2004;104(1):11–8. doi: 10.1182/blood-2003-09-3363. [DOI] [PubMed] [Google Scholar]
- 46.Fukuda T, Yoshida N, Kataoka Y, et al. Mice lacking the EDB segment of fibronectin develop normally but exhibit reduced cell growth and fibronectin matrix assembly in vitro. Cancer Res. 2002;62(19):5603–10. [PubMed] [Google Scholar]