Abstract
Background:
Hallmarks of the pathogenesis of rotator cuff disease (RCD) include an abnormal immune response, angiogenesis, and altered variables of vascularity. Degenerative changes enhance production of pro-inflammatory, anti-inflammatory, and vascular angiogenesis-related cytokines (ARC) that play a pivotal role in the immune response to arthroscopic surgery and participate in the pathogenesis of RCD. The purpose of this study was to evaluate the ARC profile, ie, interleukin (IL): IL-1β, IL-6, IL-8, IL-10, vascular endothelial growth factor (VEGF), basic fibroblast growth factor (bFGF), and angiogenin (ANG), in human peripheral blood serum and correlate this with early degenerative changes in patients with RCD.
Methods:
Blood specimens were obtained from 200 patients with RCD and 200 patients seen in the orthopedic clinic for nonrotator cuff disorders. Angiogenesis imaging assays was performed using power Doppler ultrasound to evaluate variables of vascularity in the rotator cuff tendons. Expression of ARC was measured by commercial Bio-Plex Precision Pro Human Cytokine Assays.
Results:
Baseline concentrations of IL-1β, IL-8, and VEGF was significantly higher in RCD patients than in controls. Significantly higher serum VEGF levels were found in 85% of patients with RCD, and correlated with advanced stage of disease (r = 0.75; P < 0.0005), average microvascular density (r = 0.68, P < 0.005), and visual analog score (r = 0.75, P < 0.0002) in RCD patients. ANG and IL-10 levels were significantly lower in RCD patients versus controls. IL-1β and ANG levels were significantly correlated with degenerative tendon grade in RCD patients. No difference in IL-6 and bFGF levels was observed between RCD patients and controls. Patients with degenerative changes had markedly lower ANG levels compared with controls. Power Doppler ultrasound showed high blood vessel density in patients with tendon rupture.
Conclusion:
The pathogenesis of RCD is associated with an imbalance between pro-inflammatory, anti-inflammatory, and vascular ARC.
Keywords: rotator cuff disease, tendon degeneration, angiogenesis-related cytokines, biomarkers, power Doppler ultrasound
Introduction
Rotator cuff tear is one of the most common disorders in the shoulder joint and causes pain, stiffness, and functional disability. Approximately 16% of the general population is believed to have rotator cuff disease (RCD) at any given time.1 This rises to 21% in elderly hospitalized patients and community populations. 2 The hallmarks of RCD pathogenesis include pro-inflammatory and anti-inflammatory processes, an abnormal immune response, angiogenesis, and altered variables of vascularity.3 Angiogenesis is a fundamental process that dominates diseases in many branches of medicine and surgery.4 The relationship between angiogenesis and degenerative changes in RCD patients is attracting increasing attention.
The tendons to supraspinatus, biceps brachii (long head), and upper parts of the infraspinatus muscles have a zone in which there are no blood vessels, and it is in this area that signs of degeneration, including cell death, calcium deposits, and microscopic ruptures, are predominantly located. When blood circulation is impaired, such as through compression and static load on the shoulder tendons, degeneration can be accelerated because the normal mechanisms for tissue function will not be optimal. Avascularity is considered to be a key factor in the etiology of degenerative tendon disease.5,6 The capacity for repair after repetitive microtrauma is strongly compromised in the avascular tissue of gliding tendons. Reduced vascularity is not a specific feature of gliding tendons, and several studies have shown that the number and size of blood vessels are shortened considerably in the waist of the tendon. Angiogenesis is mediated by angiogenic factors, and recent studies have shown that vascular endothelial growth factor (VEGF) is highly expressed in degenerative tendons, whereas VEGF expression is almost completely downregulated in healthy tendons. Several factors are able to upregulate VEGF expression in tenocytes, including hypoxia, inflammatory cytokines, and mechanical load. Because VEGF has the potential to stimulate the expression of matrix metalloproteinases and inhibit the expression of tissue inhibitors of matrix metalloproteinases in various cell types (ie, endothelial cells, fibroblasts, chondrocytes), this cytokine might have a significant role in the pathological process of degenerative tendon disease. The neovessels are accompanied by small glutamate-positive neural structures. This finding suggests that angiogenesis also plays an important role in the pain associated with degenerative tendon disease. In the tendon, degenerative changes mostly occur in regions that are hypovascular or avascular. Avascular zones of tendons are predisposed to degenerative changes and spontaneous rupture in RCD.7 Angiogenesis is mediated by angiogenic factors, and recent studies have shown that growth factors are highly expressed in degenerative tendons.8,9 Alterations in vascularity might also be involved in the pathogenesis of degenerative tendon disease.10
Many extrinsic (impingement, trauma) and intrinsic (degeneration, inflammation, hypovascularity) factors have been proposed as possible etiologies for the wide disparity in progression of RCD between individuals. In healthy individuals, the synthesis of cytokines in tendons increases in response to physical activity, which results in stronger skeletal muscle and more resistant connective tissue.11 Cytokines signal the normal processes of inflammation and repair in all organs, yet the aberrant expression of these peptide mediators is associated with significant organ dysfunction. Because cytokines play important roles in cell chemotaxis, proliferation, matrix synthesis, and cell differentiation, these molecules have the potential to improve rotator cuff tendon healing.12 Angiogenesis-related cytokines (ARC) play an important role in initiating the early nonhealing response in tendon tissue. ARC and their activity in the early/late stages of tendon degeneration are largely unknown. Abnormalities in the ARC network of importance in the clinical signs and symptoms of RCD will be the focus of this paper.
Accurate and sensitive methods for measurement and detection of cytokines are important for the understanding of cytokine biology and biochemistry, and for the assessment of cytokine involvement in pathophysiological processes.13–15 Various types of assays are available for estimation of cytokine levels in biological fluids.16 Blood still represents an ideal clinical source of potential markers because of its minimally invasive and standardized acquisition methods, and because of its known role in reflecting systemic changes associated with RCD. Serum and plasma are composed of highly complex protein/peptide mixtures resulting from the systemic monitoring of every biological process occurring in a living organism.
Changes in cytokine levels in peripheral blood serum are now recognized as potentially useful markers of disease, indicating stage, severity, and prognosis. Measurement of cytokine levels and/or soluble markers of immune activation can provide reliable information in disease regarding diagnosis, staging, prognosis, and evaluation of therapy. Serological methods for detecting ARC as surrogate markers of the processes of neovascularization, infection, and inflammation in RCD have great clinical potential, and signal a significant paradigm shift in the clinical management of orthopedic disease. The quality of laboratory data can impact on their clinical relevance. The future of serological markers, such as ARC in RCD, requires close cooperation between the public and private sectors, including government, the pharmaceutical and biotechnology industries, and academia.
Degenerative changes enhance production of pro-inflammatory, anti-inflammatory, and vascular ARC that play a pivotal role in the immune response to surgical intervention and take part in the pathogenesis of RCD.17,18 Investigating the dynamic changes in ARC following RCD is important for both understanding the pathophysiologic mechanisms of RCD and for exploring new methods for therapy, ie, reconstructive techniques using angiogenic factors.
The purpose of this study was to evaluate the ARC profile, ie, IL-1β, IL-6, IL-8, IL-10, VEGF, bFGF, and ANG, in human peripheral blood serum and correlate this with early degenerative changes in patients with RCD.
Methods
Patients
The experimental RCD group consisted of 200 patients with significant inflammation, tendon degeneration, and partial or full thickness rotator cuff tears. Patients were followed with clinical measures, ie, (University of California, Los Angeles shoulder score, Constant score, and Western Ontario Rotator Cuff Index). The Constant score19 and visual analog score20 were used to perform clinical assessments. The Constant score is based on two subjective measurements, ie, pain and the relationship between pain and activities of daily living (score, 0–35), and two objective measurements, ie, strength and range of motion as assessed by a physician (score, 0–65). The sum of the two scores ranges from 0 (total shoulder impairment) to 100 (non-impaired shoulder). The visual analog score is a 10 cm graduated scale with scores ranging from 0 (no pain) to 10 (unbearable pain) whereby patients report the score that they believe corresponds to their pain.
The comparative group consisted of 200 patients seen in the orthopedic clinic for nonrotator cuff disorders. Nonrotator cuff disorders include synovial disorders (such as adhesive capsulitis and synovial osteochondromatosis), degenerative disorders (such as osteoarthritis, amyloid arthropathy, hemarthrosis, and chondrocalcinosis), infectious disorders (such as septic arthritis and bursitis), and space-occupying lesions. All patients with RCD and nonrotator cuff disorders provided their written informed consent, and the study was approved by the ethics committee of the National Institute of Rehabilitation, Mexico City. Patient follow-up information was entered into a computerized database.
The control group consisted of 200 age- and sex-matched healthy individuals with no medical history of RCD.
Serum collection
For the cytokine assays, 10 mL of venous blood sample was drawn aseptically into Vacutainer® tubes (Becton Dickinson, Franklin Lakes, NJ) and immediately centrifuged at 3000 rpm for 10 minutes. Thereafter, the serum was separated and aliquoted in 1.0 mL fractions and stored at −80 °C until the assays were performed.
Multiplex assay
A Bio-Plex human cytokine assay for simultaneous quantitation of ARC was run according to the recommended procedure. In brief, the premixed standards were reconstituted in 0.5 mL of culture medium, generating a stock concentration of 50,000 pg/mL for each cytokine. The standard stock was serially diluted in the same culture medium to generate eight points for the standard curve. The assay was performed in a 96-well filtration plate supplied with the assay kit. Premixed beads (50 μL) coated with target capture antibodies were transferred to each well of the filter plate (5000 beads per well per cytokine) and washed twice with Bio-Plex wash buffer. Premixed standards or samples (50 μL) were added to each well containing washed beads. The samples were used directly without further dilution. The plate was shaken for 30 seconds and then incubated at room temperature for 30 minutes with low-speed shaking (300 rpm). After incubation and washing, premixed detection antibodies (50 μL, final concentration of 2 μg/mL) were added to each well. The incubation was terminated after shaking for 10 minutes at room temperature. After three washes, the beads were resuspended in 125 μL of Bio-Plex assay buffer. Beads were read on the Bio-Plex suspension array system, and the data were analyzed using Bio-Plex Manager™ software (version 3.0) with 5PL curve fitting.
Standards and control
The ARC was measured using commercial immunoassays, ie, the Bio-Plex human cytokine assay kit (Bio-Rad Inc, Hercules, CA) as per the manufacturer’s instructions, the International Standards of the National Institute for Biological Standards and Control, and the recommendations of the Expert Committee on Biological Standardization of the World Health Organization for cytokine measurements. All samples were masked for subject identity and analyzed using a standard range of 0–3200 pg/mL and a sample dilution of 1:4, as recommended by the manufacturer.
Purification of human ARC
Serum samples donated by normal human volunteers and RCD patients were collected through the Blood Transfusion Service of the National Institute of Rehabilitation. Human ARC was purified in an ARC affinity column (Amersham Pharmacia Biotech, Piscataway, NJ) and AKTAFPLC (Amersham Pharmacia Biotech). Procedures were carried out according to the manufacturer’s instructions. Briefly, serum samples were first prepared with ammonium sulfate until the final concentration was 0.8 M. The prepared sera were applied to the affinity column which had been precalibrated. ARC affinity binding buffer [20 nM sodium phosphate and 0.8 M (NH4)2SO4, pH 7.5] was then applied to wash out unbound factors; bound ARC was eluted by 20 nM sodium phosphate, pH 7.5. The flow rate of the overall purification procedure was 1 mL/min.
Specimen preparation
Rotator cuff samples were immediately fixed in 4% formaldehyde for 24 hours, dehydrated in graded alcohol solution and cedarwood oil, and embedded in paraffin. Sections were cut at 5 μm with a RM2055 microtome (Leica, Bensheim, Germany) 40 °C stainless steel knife. Standard histological staining was performed using a Masson-Goldner staining kit (Merck, Darmstadt, Germany) according to the manufacturer’s instructions.
Histology
Histological analysis was performed at a primary objective lens magnification of 5× using a Leica DM5000 and Quips analysis software (Leica). Biopsies of the superficial part of the supraspinatus tendon in patients with RCD were analyzed using histological techniques.
Ultrasound imaging
Ultrasound imaging studies were performed on each shoulder. All scans were performed using a Siemens Acuson Antares (Siemens, Erlangen, Germany) machine with a 7.5–15 mHz linear array transducer. The shoulder scoring system assessed elements of inflammation, as well as structural tendinous and bony damage. Rotator cuff tendons were investigated for the presence of total or partial tears in the longitudinal and transverse planes in static and dynamic positions. The synovial structures of the shoulder, including the subacromial-subdeltoid bursa, sheath of the long biceps tendon, and axillary and posterior recess of the glenohumeral joint, were examined for the presence of effusions and synovial hypertrophy. The humeral head was examined for the presence of erosions. Power Doppler assessment of selected synovial sites, including the biceps sheath, subacromial-subdeltoid bursa, and axillary and posterior recesses was carried out with settings standardized to a pulse repetition frequency of 400–500 Hz and low wall filters. The power Doppler gain was adjusted to a level just below the disappearance of color signs under the bony cortex as recommended by Rubin et al. OMERACT (Outcome Measures in Rheumatoid Arthritis Clinical Trials) definitions for joint effusion, synovial hypertrophy, tenosynovitis, and bone erosions were adhered to. The following definitions for the classification of ultrasonographic findings were used: cortical irregularities >2 mm were considered as erosions; a hypoechoic area of at least >3 mm around the long head of the biceps tendon as tenosynovitis of the long biceps tendon; bursal thickness >3 mm or effusion as effusion/synovial hypertrophy of the subacromial-subdeltoid bursa; >3 mm effusion/synovial hypertrophy at the posterior recess superior to the glenoid labrum as synovitis; and >3 mm effusion/synovial hypertrophy at the axillary recess as synovitis. No ultrasonographic distinction was made between effusions and synovial hypertrophy, and these abnormalities were taken together for the analyses.
Magnetic resonance imaging
Magnetic resonance imaging studies (MRI) were performed on each shoulder. Assessment of the affected shoulder by MRI took place within five working days prior to the ultrasound investigation in all patients. MRI was performed with a 1.5-T unit (Signa Excite, General Electric, Milwaukee, WI) using a flexible wraparound coil. The following sequences were used: T1-weighted fast spin echo sequence with repetition time of 500 msec, echo time of 13.6 msec, slice thickness of 4 mm, and fields of view of 140–160 mm in the axial, transverse, and oblique coronal slice orientations parallel to the course of the tendon of the supraspinatus; T2-weighted fat-suppressed images in the coronal and sagittal planes with a repetition time of 3300 msec and an echo time of 71 msec. After selection of a suitable slice in which abnormal changes were visualized, a dynamic contrast-enhanced study with intravenous injection of gadolinium diethylenetriaminepentaacetic acid 0.1 mmol/kg body weight was performed using axial and coronal T1-weighted (repetition time 500 msec, echo time 13.6 msec, and fields of view of 140–160 mm) fat-suppressed sequences T1-weighted and coronal oblique contrast-enhanced fat-suppressed T1-weighted sequences were performed. The MRI scans were evaluated by two radiologists who were in agreement and had no knowledge of the results of ultrasonography. The MRI scans were analyzed for the presence or absence of the same structures that were visualized by ultrasonography. The MRI criterion for effusion was an intra-articular or intrabursal area with a high signal on T2-weighted sequences without contrast enhancement on fat-suppressed T1-weighted sequences. The criterion for synovitis was enhancing signals seen on the fat-suppressed T1-weighted sequences.
Statistical analysis
Statistical analyses were done using the Wilcoxon rank sum test without any assumption about the distribution of data. Standard statistical software (SAS 6.12 version, SAS Inc, Cary, NC) was used for all statistical analyses. Data are reported as the means ± standard error of measurements. Statistical significance was defined as P < 0.05.
Results
Patient characteristics
The baseline characteristics of the study RCD patients are shown in Table 1.
Table 1.
Baseline characteristics.
| RCD patients | Controls | |
|---|---|---|
| Characteristic | ||
| Patients (n) | 200 | 200 |
| Mean (SD) age (years) | 40.3 ± 10.9 | 43.3 ± 11.5 |
| Range (years) | 29–72 | 31–75 |
| Male | 112 | 107 |
| Female | 88 | 93 |
| Mean (SD) height mass (kg) | 169.0 ± 9.1 | 161.2 ± 10.2 |
| Mean (SD) body mass (kg) | 79.1 ± 13.5 | 74.9 ± 11.8 |
| Mean (SD) body mass index (kg/m2) | 27.8 ± 4.4 | 26.4 ± 5.1 |
Abbreviations: RCD, rotator cuff disease; SD, standard deviation.
ARC profiles
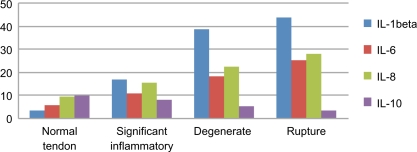
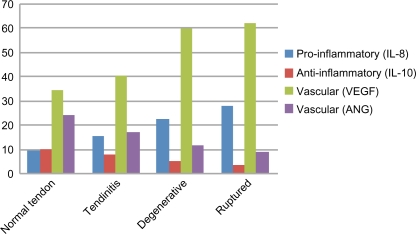
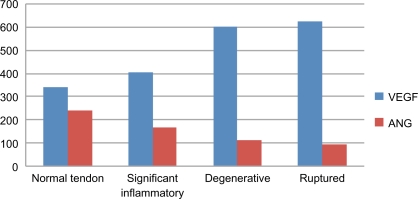
Following the Bio-Plex assay procedure, we examined variations in ARC levels in the sera from controls and RCD patients (Table 2). ARC were detected using the Bio-Plex assay in the sera of RCD patients, as well as in the sera of controls (Figs. 1–3). IL-1β, IL-8, and VEGF levels were significantly higher in RCD patients than in controls. Serum ANG and IL-10 levels were significantly lower in RCD patients than in controls. Serum IL-10 levels are a diagnostic indicator of the anti-inflammatory phase. The anti-inflammatory action of this cytokine consists of inhibition of inflammatory mediators, such as IL-6 and IL-8. Anti-inflammatory IL-10 exerts a protective effect, but protection is incomplete. Patients with degenerative changes had considerably lower serum angiogenin levels compared with controls. A combination of high VEGF and low ANG concentrations in the sera have been considered as a predisposing factor for patients with ruptures of rotator cuff tendons. No difference in bFGF levels was observed between RCD patients and controls. Levels of IL-1β and angiogenin were significantly correlated with degenerative tendon grade in RCD patients.
Table 2.
Angiogenesis-related cytokine expression.
| ARC (pg/mL) | The Control group | The Comparative group |
RCD patients |
||
|---|---|---|---|---|---|
| Significant inflammatory | Degenerative | Ruptured | |||
| Pro-inflammatory ARC | |||||
| Local inflammatory | |||||
| IL-1 | 3.33 ± 0.69 | 5.21 ± 1.10 | 16.17 ± 6.71 | 38.52 ± 10.32 | 43.71 ± 8.91 |
| IL-6 | 5.37 ± 0.93 | 6.11 ± 0.87 | 10.45 ± 2.92 | 18.21 ± 0.71 | 25.11 ± 3.11 |
| Chronic inflammatory | |||||
| IL-8 | 9.11 ± 0.98 | 9.78 ± 2.31 | 15.31 ± 0.85 | 22.41 ± 0.92 | 27.81 ± 1.11 |
| Anti-inflammatory ARC | 9.53 ± 1.21 | 10.21 ± 1.45 | 7.64 ± 1.11 | 5.15 ± 0.65 | 3.11 ± 1.91 |
| IL-10 | |||||
| Vascular ARC | |||||
| VEGF | 339.67 ± 74.65 | 349.21 ± 55.78 | 402.11 ± 88.11 | 598.03 ± 298.25 | 621.24 ± 301.11 |
| bFGF | 8.14 ± 2.91 | 7.54 ± 1.84 | 12.34 ± 2.37 | 16.82 ± 3.41 | 19.91 ± 5.24 |
| ANG | 239.51 ± 58.4 | 265.21 ± 77.11 | 166.45 ± 44.90 | 111.31 ± 34.21 | 89.39 ± 40.19 |
Abbreviations: ANG, angiogenin; ARC, angiogenesis-related cytokines; bFGF, basic fibroblast growth factor; IL, interleukin; RCD, rotator cuff disease; VEGF, vascular endothelial growth factor.
Figure 1.
Pro-/anti-inflammatory ARC in RCD.
Figure 3.
ARC profiles of tendon.
Correlations
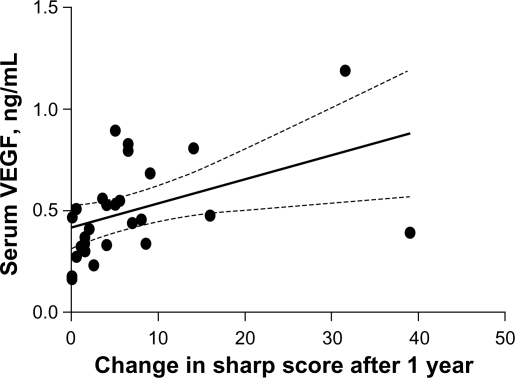
We examined the affected shoulder with ultrasound according to guidelines issued by the European Society of Musculoskeletal Radiology.21 For each patient, the tendon was recorded (Fig. 4). Power Doppler ultrasound examination revealed high blood vessel density in patients with tendon ruptures.5 We found a significant correlation between power Doppler ultrasound and VEGF expression in patients with RCD and controls. There was a significant correlation between vessel density and VEGF (Figs. 5 and 6). Overexpression of VEGF correlated with advanced disease (r = 0.75; P < 0.0005), average microvascular density (r = 0.68, P < 0.005), and visual analog score (r = 0.75, P < 0.0002) in patients with RCD. We found a correlation between VEGF and IL-8 in the sera of patients with RCD (Table 3).
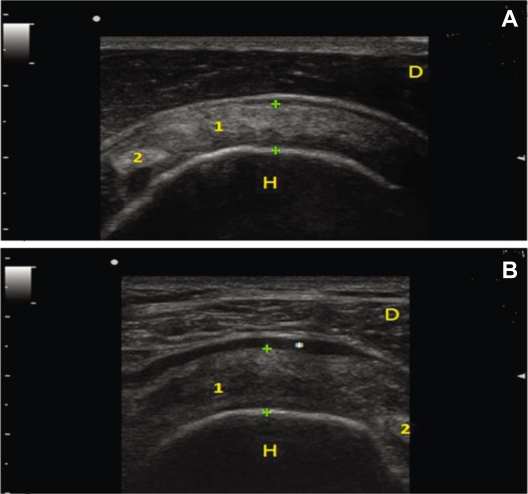
Figure 4.
Ultrasound appearance of a normal and a degenerative supraspinatus tendon. Panel A) Transverse scan: Normal fibrillar pattern and thickness of Supraspinatus Tendon is observed (between calipers). Panel B) Supraspinatus tendon is thickened (calipers), hypoechoic, non-homogeneous, with loss of the normal fibrillar pattern. In this picture, an effusion in the subacromial bursa is present*. 1 supraspinatus tendon, 2 biceps tendon, H humeral head and D deltoid muscle.
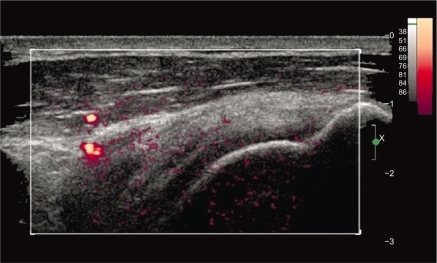
Figure 5.
Baseline long-axis power Doppler ultrasound image of supraspinatus tendon shows two peribursal vessels and no intratendinous vessels. Low level of background noise (color) is evident. The large vessels (far left) were persistent findings when observed in real time. The remaining tiny flecks of orange, likely representing noise, were inconsistent during real-time observation. Redder hues correspond to greater intensity of the Doppler signal.
Figure 6.
Relationship between vasular endothelial growth factor concentrations, measured at first presentation in a cohort of patients with early rotator cuff disease, and tendon damage over the following year, expressed as change in the Van der Heijde modification of the Sharp score.
Table 3.
Correlation between serum vascular and pro-inflammatory/anti-inflammatory cytokines in patients with rotator cuff disease.
| ARC | IL-1β (pg/mL) | IL-6 (pg/mL) | IL-8 (pg/mL) | IL-10 (pg/mL) |
|---|---|---|---|---|
| ANG (pg/mL) | NS | NS | –0.22 P < 0.0005 | –0.37 P < 0.0005 |
| bFGF (pg/mL) | NS | NS | 0.13 P < 0.03 | 0.38 P < 0.0005 |
| VEGF (pg/mL) | 0.34 P < 0.0005 | 0.44 P < 0.0005 | 0.73 P < 0.0005 | 0.54 P < 0.0005 |
Abbreviations: ANG, angiogenin; ARC, angiogenesis-related cytokines; bFGF, basic fibroblast growth factor; IL, interleukin; NS, not significant; RCD, rotator cuff disease; VEGF, vascular endothelial growth factor.
Vascularity
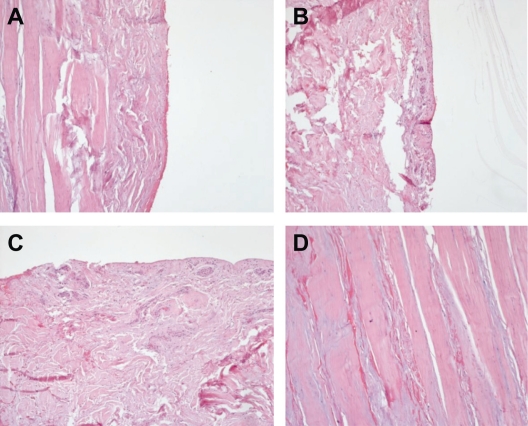
Histological biopsies from degenerative tendons and Doppler flow examinations revealed a high blood vessel density in patients with degenerative tendon disease. Figure 7 shows examples for different tendon sections and staining. Vascularity analyses support our hypothesis that tendon degeneration is closely associated with abnormalities in angiogenesis.
Figure 7.
Histology of zonal differences in the supraspinatus tendon. A) Zone without blood vessels, (B) Zone with low number of blood vessels, (C) Vascular zone, (D) Degenerative evidence.
Discussion
The applications for biomarkers is a growing field, particularly in RCD.22–24 Combining markers is intuitively the next step for a number of reasons, and is a strategy being adopted in a number of fields, not just in biomarkers, and it makes good financial as well as scientific sense to use what has already been identified. 25 Biomarkers have a number of uses, eg, selection of participants in drug trials, and for early diagnosis, prognosis, and assessment of response to treatment. Because of the chronic and variable nature of RCD, new methods are needed to optimize the selection of patients for trials and as outcome measures, in short, any route by which the chronic course of RCD can be shortened into a more cost-effective time frame.
The etiology of RCD is still not entirely understood. Tendons are metabolically active tissues requiring a blood supply,26 compromise of which may cause degeneration.27,28 Tendon vascularization is the most important factor in the pathogenesis of RCD.29,30 Some authors ascribe mechanical damage to subacromial impingement, while others suspect a primary process of tendon degeneration.31,32 In a recent literature review, Nho et al concluded that hypovascularity contributes minimally to cuff tears, which supports our finding that hypervascularization occurs late in the course of rotator cuff tendinopathy.14,33–35
Cytokine and chemokine networks play a key role in the pathogenesis of RCD. Several cytokines are expressed in tissues from primary frozen shoulders. However, little research has been done to investigate the molecular mechanism and cytokine expression in rotator cuff lesions with shoulder stiffness. Therefore, we hypothesized that shoulder pain and joint stiffness in RCD is caused by the ARC network. Therefore, the present study focused on ARC expression and its relationship to clinical features in patients with RCD.
This study evaluated ARC concentrations extensively, correlated these with degenerative tendons in RCD, provides a novel standardized analytic approach for assessing changes in ARC levels, and may improve strategies for monitoring the immune response in patients with RCD. We used the Bio-Plex assay to determine ARC responses in RCD patients successfully, showing that it can be used for serological diagnosis of RCD and as a basis for further laboratory tests.
Our results suggest a significant role for ARC in the development of RCD. Most patients present a typical course of inflammatory response, which runs in two phases, ie, pro-inflammatory and anti-inflammatory. Increased serum levels of cytokines, including IL-1β, IL-6, and IL-8 in RCD patients are a diagnostic indicator of the inflammatory phase. IL-8 is one of the most important inflammation markers. Its pro-inflammatory activity is associated with stimulation of lymphocytes B and T, as well as mesangial cell proliferation.36 In the present study, a significant increase in serum IL-1β and IL-8 levels was demonstrated in RCD patients. The IL-6 level was also higher in RCD patients. Ko et al demonstrated that expression of IL-8 in joint fluid from patients with rotator cuff tears and stiffness was higher than that from patients with rotator cuff tears without shoulder stiffness. As for IL-8 expression, our findings are consistent with those of Ko et al, but also indicate that the levels of IL-1β and TNF-α in joint fluid are higher in patients with stiffness. In our study, there was no significant difference in IL-6 level between RCD patients and controls.
Increased serum IL-10 is also a diagnostic indicator of the anti-inflammatory phase.37 The anti-inflammatory action of this cytokine consists of inhibition of inflammatory mediators, including IL-6 and IL-8. Furthermore, IL-10 stops the synthesis of tumor necrosis factor alpha and reduces the effector functions of both monocytes and natural killer cells.38 An intensified anti-inflammatory phase of the inflammatory response in RCD patients may be protective against the development of a significant inflammatory stage. In this study, our patients had low serum IL-10 levels during development of RCD.
Neovascularization in rotator cuff and tendon tissues is mediated by vascular ARC. While VEGF-mediated angiogenesis contributes to repair and remodeling of degenerative tendons, invasion by endothelial cells may also weaken the mechanical stability of the tendon.10,39,40 We assume that increasing vessel density weakens the tendons due to the accretive nature of tendon retraction. This has been supported by the recently published theoretical model of Peers et al, showing that chronic tendon loading causes mechanical trauma, with multiple microruptures of the tendon microvasculature.18,41 These microruptures initiate a VEGF-mediated cascade of vascular remodeling that becomes chronically pathological.42,43 Hypervascularity has been demonstrated in degenerative human and rabbit Achilles tendons, and in diseased patellae and long head of biceps tendons.44,45 Chronic tendon pathology appears to be a highly active process of ongoing neovascularization.46,47 VEGF expression and neovascularization could be used in the clinical setting to monitor tendon degeneration in patients with rotator cuff injuries. A hypervascularized zone has been identified in the rotator cuff, and coincides with the most common location for tears.17,26,48,49 Our findings are supported by studies showing increased Doppler flow in RCD, likely reflecting a revascularization process.
RCD causes considerable shoulder pain, and this is one of the most uncomfortable symptoms for patients. An inflammatory ARC condition might be responsible for the shoulder pain, although the relationship between ARC and the pain of a cuff tear has received little attention in previous reports. Our data indicate a correlation between VEGF levels and visual analog score. Thus, as for the VEGF concentration in the affected shoulder joint, there might be a relationship to the shoulder pain of RCD patients.
Our data demonstrate that the use of a multiple ARC assay platform is suitable for identifying distinct ARC profiles associated with the clinical manifestations and severity of RCD. The pathogenesis of RCD is associated with an imbalance between pro-inflammatory, anti-inflammatory, and vascular cytokines. The dynamic changes in ARC levels in patients with RCD suggest that angiogenesis factors might play a role in the pathogenesis of RCD, such that RCD disorders could be classified together as “angiogenesis-dependent diseases”. ARC expression in the serum of patients with RCD may provide useful prognostic information for orthopedic surgeons. Moreover, assessment of an ARC panel may help monitor rehabilitation after arthroscopic RCD, and assist in future planning of treatment and/or surgical intervention.
Measurement of ARC levels can provide reliable information regarding diagnosis, staging, prognosis, and evaluation of therapy. However, methodological difficulties and inaccuracies have been reported, and a number of factors have been shown to affect the validity and quality of such measurements.50 Bio-Plex assays are the most widely used measurement technique, although pitfalls and limitations are known. Differences in levels of measured analytes for identical samples in the range of 10–100-fold have been reported.51 Thus, much research, including international collaborative studies organized by the World Health Organization for standardization of cytokine measurements, have been conducted.
Conclusion
The Bio-Plex assay is the measurement of choice for endogenous ARC levels in biologic fluids, and normal ranges for cytokines must be established if they are to have clinical applications in the future. ARC play an important role in initiating the early response to tendon degeneration. The role of ARC in the pathogenesis of RCD creates an imbalance between degeneration and repair. Early tendon degeneration is ARC-mediated. Degenerative changes enhance production of pro-inflammatory, anti-inflammatory, and vascular ARC that play a pivotal role in the immune response. The discovery of the importance of IL-8, IL-10, VEGF, and angiogenin in the pathogenesis of RCD has greatly altered views about the role of ARC in RCD.
Figure 2.
Vascular ARC in RCD.
Acknowledgments
The authors are grateful to Dr. Luis Guillermo Ibarra Ibarra (National Institute of Rehabilitation, Mexico City, Mexico) for his generous support and invaluable advice. This work was support by a grant from FONSEC SSA/IMSS/ISSSTE–CONACyT SALUD-2010-01-138883.
Footnotes
Disclosure
This manuscript has been read and approved by all authors. This paper is unique and is not under consideration by any other publication and has not been published elsewhere. The authors and peer reviewers of this paper report no conflicts of interest. The authors confirm that they have permission to reproduce any copyrighted material.
References
- 1.Erstad S. Rotator cuff repair. Review Arizona Orthop Associates. 2008.
- 2.Beasley L, Faryniarz DA, Hannafin JA. Multidirectional instability of the shoulder in the female athlete. Clin Sports Med. 2000 Apr;19(2):331–49. doi: 10.1016/s0278-5919(05)70207-6. [DOI] [PubMed] [Google Scholar]
- 3.Löhr JF, Uhthoff HK. Epidemiology and pathophysiology of rotator cuff tears. Orthopade. 2007 Sep;36(9):788–95. doi: 10.1007/s00132-007-1146-8. [DOI] [PubMed] [Google Scholar]
- 4.Folkman J. Clinical applications of research on angiogenesis. New Engl J Med. 1995;333:1750–7. doi: 10.1056/NEJM199512283332608. [DOI] [PubMed] [Google Scholar]
- 5.Gamradt S. Vascularity of the rotator cuff after arthroscopic repair: Characterization using contrast-enhanced ultrasound. AANA J. 2008:SS36. [Google Scholar]
- 6.Hegedus EJ. Vascularity and tendon pathology in the rotator cuff: A review of literature and implications for rehabilitation and surgery. Br J Sports Med. 2010;44:838–47. doi: 10.1136/bjsm.2008.053769. [DOI] [PubMed] [Google Scholar]
- 7.Petersen W, Pufe T, Zantop T, Paulsen F. Blood supply of the flexor hallucis longus tendon with regard to dancer’s tendinitis: injection and immunohistochemical studies of cadaver tendons. Foot Ankle Int. 2003 Aug;24(8):591–6. doi: 10.1177/107110070302400804. [DOI] [PubMed] [Google Scholar]
- 8.Lohr JF. The microvascular pattern of the supraspinatus tendon. Clin Orthop Relat Res. 1990;254:35–8. [PubMed] [Google Scholar]
- 9.Papatheodorou A. US of the shoulder: Rotator cuff and non-rotator cuff disorders. Radiographics. 2006;26:e23. doi: 10.1148/rg.e23. [DOI] [PubMed] [Google Scholar]
- 10.Perry SM. Inflammatory and angiogenic mRNA levels are altered in a supraspinatus tendon overuse animal model. J Shoulder Elbow Surg. 2005;14:S79–83. doi: 10.1016/j.jse.2004.09.020. [DOI] [PubMed] [Google Scholar]
- 11.Pufe T. The role of vasculature and angiogenesis for the pathogenesis of degenerative tendons disease. Scand J Med Sci Sports. 2005;15:211–2. doi: 10.1111/j.1600-0838.2005.00465.x. [DOI] [PubMed] [Google Scholar]
- 12.Petersen W. Overload damage to the Achilles tendon: The importance of vascularization and angiogenesis. Orthopade. 2005;34:533–42. doi: 10.1007/s00132-005-0808-7. German. [DOI] [PubMed] [Google Scholar]
- 13.Bauer TW. Are there biological markers of wear? J Am Acad Orthop Surg. 2008;16:S68–71. doi: 10.5435/00124635-200800001-00014. [DOI] [PubMed] [Google Scholar]
- 14.Molloy T. The roles of growth factors in tendon and ligament healing. Sports Med. 2003;33:381–94. doi: 10.2165/00007256-200333050-00004. [DOI] [PubMed] [Google Scholar]
- 15.Norwood LA. Clinical presentation of complete tears of the rotator cuff. J Bone Joint Surg Am. 1989;17IA:499–505. [PubMed] [Google Scholar]
- 16.Reid DB. The clinical value of three-dimensional intravascular ultrasound imaging. J Endovasc Surg. 1995;2:356–64. doi: 10.1583/1074-6218(1995)002<0356:TCVOTD>2.0.CO;2. [DOI] [PubMed] [Google Scholar]
- 17.Blevins FT. Rotator cuff pathology in athletes. Sports Med. 1997;24:205–20. doi: 10.2165/00007256-199724030-00009. [DOI] [PubMed] [Google Scholar]
- 18.Fealy S. Patterns of vascular and anatomical response after rotator cuff repair. Am J Sports Med. 2006;34:120–7. doi: 10.1177/0363546505280212. [DOI] [PubMed] [Google Scholar]
- 19.Constant CR, Murley AH. A Clinical method of functional assessment of the shoulder. Clin Orthop Relat Res. 1987;214:160–4. [PubMed] [Google Scholar]
- 20.Langley GB, Sheppeard H. The visual analogue scale: Its use in pain measurement. Rheumatol Int. 1985;5:145–8. doi: 10.1007/BF00541514. [DOI] [PubMed] [Google Scholar]
- 21.Beggs I, Bianchi S, Bueno A. Musculoskeletal ultrasound technical guidelines. I. The shoulder. European Society of Musculoskeletal Radiology. Available at: http://www.essr.org/html/img/pool/shoulder.pdf. Accessed December 15, 2008.
- 22.Lawrence V. What’s new in orthopaedic research? J Bone Joint Surg Am. 2007;108:1417–22. [Google Scholar]
- 23.Lo KY. Arthroscopic revision of failed rotator cuff repairs: Technique and results. Arthroscopy. 2007;20:250–67. doi: 10.1016/j.arthro.2004.01.006. [DOI] [PubMed] [Google Scholar]
- 24.Petersen W, Pufe T, Kurz B, Mentlein R, Tillmann B. Angiogenesis in fetal tendon development: Spatial and temporal expression of the angiogenic peptide vascular endothelial cell growth factor. Anat Embryol (Berl) 2002;205:263–70. doi: 10.1007/s00429-002-0241-1. [DOI] [PubMed] [Google Scholar]
- 25.Heinegard D. Macromolecular markers in joint disease. J Rheumatol Suppl. 1991;27:27–9. [PubMed] [Google Scholar]
- 26.Chansky HA. The vascularity of the rotator cuff. Clin Sports Med. 1991;10:807–22. [PubMed] [Google Scholar]
- 27.Cofield RH. Degenerative and arthritic problems of the glenohumeral joint. In: Rockwood CA, Matsen FA, editors. The Shoulder. Philadelphia, PA: WB Saunders; 1990. [Google Scholar]
- 28.Djurasovic M. Revision rotator cuff repair: Factors influencing results. J Bone Joint Surg Am. 2001;83A:1849–55. doi: 10.2106/00004623-200112000-00013. [DOI] [PubMed] [Google Scholar]
- 29.Krishnan SG. Rotator cuff and impingement lesions in adult and adolescent athletes. In: DeLee JC, Drez D, editors. Orthopaedic Sports Medicine, Principles and Practice. Philadelphia, PA: WB Saunders; 2003. [Google Scholar]
- 30.Payne LZ. Arthroscopic treatment of partial rotator cuff tears in young athletes: A preliminary report. Am J Sports Med. 1997;25:229–305. doi: 10.1177/036354659702500305. [DOI] [PubMed] [Google Scholar]
- 31.Nho SJ, Yadav H, Shindle MK, Macgillivray JD. Rotator cuff degeneration: Etiology and pathogenesis. Am J Sports Med. 2008;36:987–3. doi: 10.1177/0363546508317344. [DOI] [PubMed] [Google Scholar]
- 32.Somerset MF. Angiogenesis: An organizing principle for drug discovery? Nat Rev Drug Discov. 2007;6:273–86. doi: 10.1038/nrd2115. [DOI] [PubMed] [Google Scholar]
- 33.Baumgartner RW. Transcranial color-coded duplex sonography, magnetic resonance angiography, and computed tomography angiography: Methods, applications, advantages, and limitations. J Clin Ultrasound. 1995;23:89–111. doi: 10.1002/jcu.1870230205. [DOI] [PubMed] [Google Scholar]
- 34.Handelsman H. Magnetic resonance angiography: Vascular and flow imaging. Health Technol Assess (Rockv) 1994;3:1–20. [PubMed] [Google Scholar]
- 35.Healy DA. Current applications of duplex ultrasonography and color Doppler imaging. J Cardiovasc Surg. 1994;35:403–12. [PubMed] [Google Scholar]
- 36.Le JM, Vilcek J. Interleukin-6: A multifunctional cytokine regulating immune reactions and acute phase protein response. Lab Invest. 1989;61:588–602. [PubMed] [Google Scholar]
- 37.Gotoh M. Interleukin-1 induced subacromial synovitis and shoulder pain in rotator cuff diseases. Rheumatology. 2001;40:995–1001. doi: 10.1093/rheumatology/40.9.995. [DOI] [PubMed] [Google Scholar]
- 38.Kishimoto K. The biology of interleukin-1. Blood. 1989;74:1–10. [PubMed] [Google Scholar]
- 39.Badet J. Angiogenin. In: Bikfalvi A, editor. Vascular Biology and Pathology: An Encyclopedic Reference. New York, NY: Springer-Verlag; 2000. [Google Scholar]
- 40.Ostanin AA, Leplina OY, Shevela CY, Kozhevnikov VS, Chernykh HR. Inflammatory Syndromes (SIRS, MARS, CARS) in Patients with Surgical Infection. Russ J Immunol. 2000 Oct;5(3):289–300. [PubMed] [Google Scholar]
- 41.Shaw M. Clinical biomarkers forum: Harnessing the potential of biomarkers from translational research throughout clinical trials. Expert Rev Mol Diagn. 2007;7:759–60. doi: 10.1586/14737159.7.6.759. [DOI] [PubMed] [Google Scholar]
- 42.Gladstone JN. Fatty infiltration and atrophy of the rotator cuff do not improve after rotator cuff repair and correlate with poor functional outcome. Am J Sports Med. 2007;35:719–28. doi: 10.1177/0363546506297539. [DOI] [PubMed] [Google Scholar]
- 43.Levy O. Measurement of blood flow in the rotator cuff using laser Doppler flowmetry. J Bone Joint Surg Br. 2008;90:893–8. doi: 10.1302/0301-620X.90B7.19918. [DOI] [PubMed] [Google Scholar]
- 44.Yanagisawa K. Vascular endothelial growth factor (VEGF) expression in the subacromial bursa is increased in patients with impingement syndrome. J Orthop Res. 2001;19:448–55. doi: 10.1016/S0736-0266(00)90021-4. [DOI] [PubMed] [Google Scholar]
- 45.Paleolog EM. Angiogenesis in rheumatoid arthritis. Arthritis Res. 2002;4:S81–90. doi: 10.1186/ar575. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Gaitini D. Sonographic evaluation of vascular injuries. J Ultrasound Med. 2008;21:95–107. doi: 10.7863/jum.2008.27.1.95. [DOI] [PubMed] [Google Scholar]
- 47.Phelps EA. Update of therapeutic vascularization strategies. Regen Med. 2009;4:65–80. doi: 10.2217/17460751.4.1.65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Blevins FT. Rotator cuff injury in contact athletes. Am J Sports Med. 1996;24:263–7. doi: 10.1177/036354659602400303. [DOI] [PubMed] [Google Scholar]
- 49.Candiotto S. Surgical treatment of the impingement syndrome and the rotator cuff tears: Personal experience in 134 cases. Reumatismo. 2002;54:308–15. doi: 10.4081/reumatismo.2002.308. Italian. [DOI] [PubMed] [Google Scholar]
- 50.Wang CJ. Shock wave therapy induces neovascularization at the tendon-bone junction: A study in rabbits. J Orthop Res. 2003;21:984–9. doi: 10.1016/S0736-0266(03)00104-9. [DOI] [PubMed] [Google Scholar]
- 51.Strunk J. Doppler sonographic findings in the long bicipital tendon sheath in patients with rheumatoid arthritis as compared with patients with degenerative diseases of the shoulder. Arthritis Rheum. 2003;48:1828–32. doi: 10.1002/art.11039. [DOI] [PubMed] [Google Scholar]