Abstract
Regioisomeric spiropyrazolines were synthesized through a tandem intramolecular cyclization/methylation reaction of a functionalized 5,5-disubstituted pyrazoline in one reaction vessel. The 5,5-pyrazolines were constructed through a 1,3-dipolar cycloaddition reaction of aromatic ring containing nitrile imines and a disubstituted geminal alkene. An evaluation of the relative location of the nucleophilic and electrophilic functional groups on the pyrazoline was performed in order to ascertain the best pyrazoline system for the intramolecular cyclization/methylation reaction. Higher spiropyrazoline isolated yields were realized from pyrazolines with the electrophilic ester located further away from the pyrazoline when compared to pyrazolines with a directly bonded ester.
Keywords: Spiropyrazoline, Intramolecular Cyclization, Cycloaddition, Regioselectivity, Heterocycles, Spirocycles
Introduction
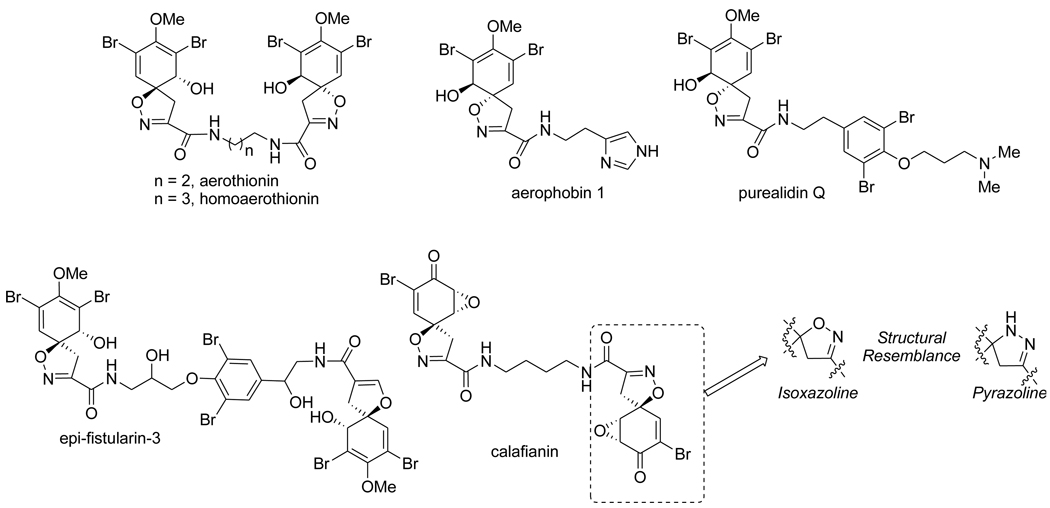
The spirocyclic molecular framework is a prevalent feature for a number of biologically active heterocyclic natural products,1,2 and among the FDA approved pharmaceutically relevant heterocycles, the pyrazole and isoxazole moieties are found in the COX II inhibitors, celebrex and bextra.3,4 Furthermore, as the syntheses of isoxazoles and pyrazoles flourished,5,6 the corresponding synthetic methodologies targeting spiroisoxazolines and spiropyrazolines were also developing.7,8 While the major structural feature of a number of biologically active bromotyrosine derived heterocyclic marine natural products is the spiroisoxazoline,1,2,7 spiropyrazolines have an extra nitrogen, in place of isoxazoline oxygen, and offer the feasibility to construct structurally relevant analogues for the exploration of potential biological activity. (Figure 1) In general spiropyrazolines8 contain the common basic molecular architecture derived from the corresponding pyrazoline and are characterized by a unique spiro fusion at the C-5 position of a pyrazoline ring. These spiropyrazoline heterocycles are interesting targets for synthesis based upon the interesting spirocyclic skeleton that is often associated with potential bioactive properties.
Figure 1.
Naturally occurring spiroisoxazolines and spiropyrazoline structural resemblance.
A variety of methods exists for the synthesis of functionalized spiropyrazolines. General and classical syntheses of pyrazoles and pyrazoline derivatives rely on the 1,3-dipolar cycloaddition9 of diazoalkanes or nitrile imines with alkynes,5d,6c–g,j,l–n alkyne surrogates,6h,q or alkenes,10 closely related [2+3] cycloadditions,6d,r,11 and other methods.12 Special attention is warranted toward the synthetic design and development of pyrazoles, pyrazolines, and their derivatives because of their high demand in academic and pharmaceutical sectors. Our recent report on spiroisoxazoline synthesis utilized intramolecular alkylation/methylation of a 5,5-disubstituted isoxazoline as the central spiroisoxazoline synthetic pathway.13 In this paper, we extend the application of this practical synthetic methodology toward the synthesis of functionalized unsaturated carbocyclic spiropyrazolines while also determining the best pyrazoline system for the intramolecular cyclization/methylation reaction.
Results and Discussion
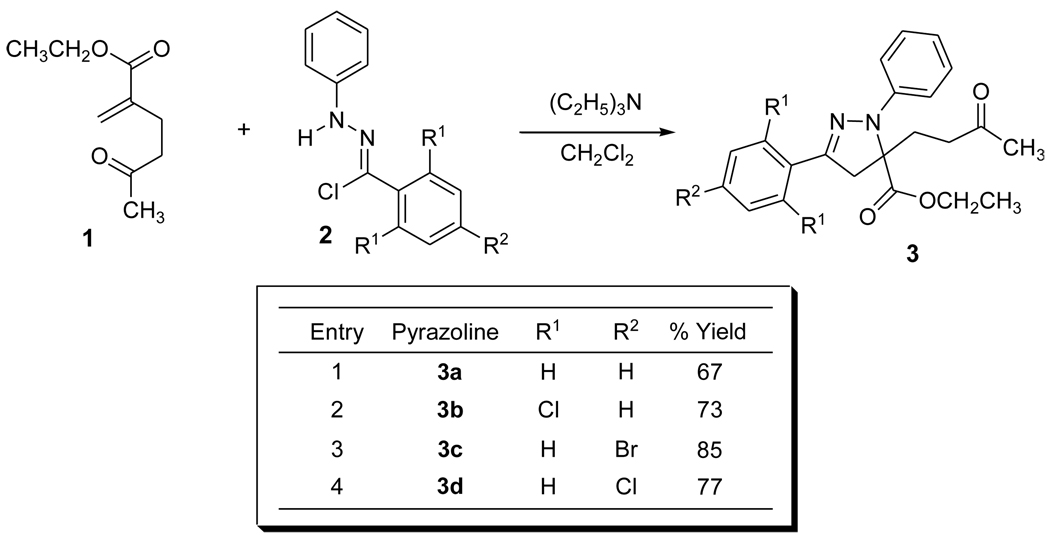
Synthesis of the spiropyrazoline precursor 3a was initiated by the regioselective 1,3-dipolar cycloaddition of the readily available alkene dipolarophile 1.14 The reaction of α-chlorohydrazone 2 with triethylamine results in the in situ generation of the corresponding nitrile imine6h,i,8a which combines with 1 in a 1,3-dipolar fashion to afford the highly functionalized 5,5-disubstituted pyrazoline in moderate to good yields as a single regioisomer.15 (Scheme 1) The 1,3-dipolar cycloaddition of 1 with a variety of substituted aromatic nitrile imines was then pursued in order to investigate the scope and limitations of 5,5-disubstituted pyrazolines as precursors to spiropyazolines via the intramolecular cyclization/methylation synthetic methodology. The 1,3-dipolar cycloaddition results are shown in Scheme 1. All of these cycloadditions occurred with complete regiochemical intergrity and in reasonable to good isolated yields.
Scheme 1.
Synthesis of highly functionalized pyrazoline 3.
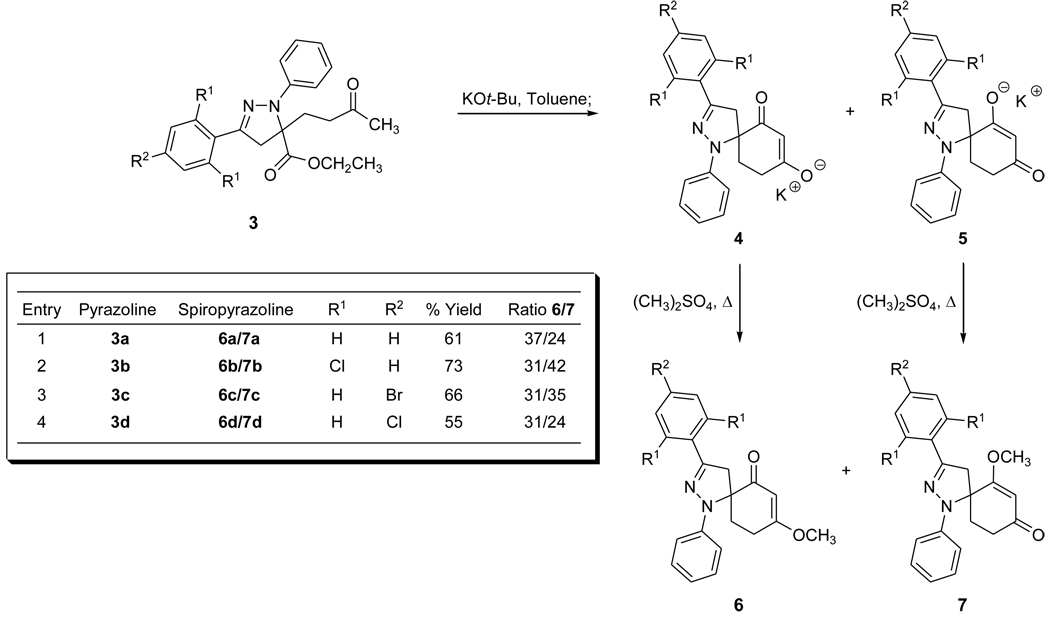
Suitable bases for the intramolecular cyclization process were evaluated in conjunction with solvent compatibility and temperature. Optimal reaction conditions were realized for the intramolecular cyclization/methylation reaction by employing potassium t-butoxide as the base in anhydrous toluene.16 The intramolecular cyclization/methylation was achieved in one reaction vessel, and each step was monitored via TLC through the consumption of the starting materials. Spiropyrazoline 6a was isolated in 37% yield, and its regioisomer, 7a, was isolated in 24% yield. (Scheme 2) The isolation of the two spiropyrazoline regioisomers results from the O-methylation of both spiropyrazoline enolates. (Scheme 2)
Scheme 2.
Synthesis of spiropyrazoline regioisomers 6 and 7.
Based upon the positive results obtained from the intramolecular cyclization/methylation of 3a, the application of this synthetic methodology was applied toward a variety of pyrazoline derivatives for spiropyrazoline construction. The isolated yields of various spiropyrazolines arising from pyrazoline intramolecular cyclization/methylation are shown in Scheme 2. The overall yields for the isolation of the spiropyrazoline regioisomers range from 55–73% for the two step one pot process. Each spirocyclic regioisomer was identified based upon the unique nature of the 13C chemical shift of the quaternary carbon of the pyrazoline. Generally, when the pyrazoline quaternary carbon is located alpha to the carbonyl, as found in compound 6, the 13C chemical shift is greater than 70 ppm. However, due to the non-availability of carbonyl’s deshielding effect, the pyrazoline quaternary carbon of the alternate regioisomer, such as compound 7, gives rise to a 13C chemical shift that is less than 70 ppm. These spectroscopic observations are in correlation with previous results reported from our research laboratories.13 Even though the isolated yields for the aforementioned spiropyrazolines were good, we decided to investigate the potential of improving the intramolecular cyclization/methylation yields by repositioning the electrophilic ester to a more remote location relative to the pyrazoline ring thereby potentially decreasing the steric environment of the ester.
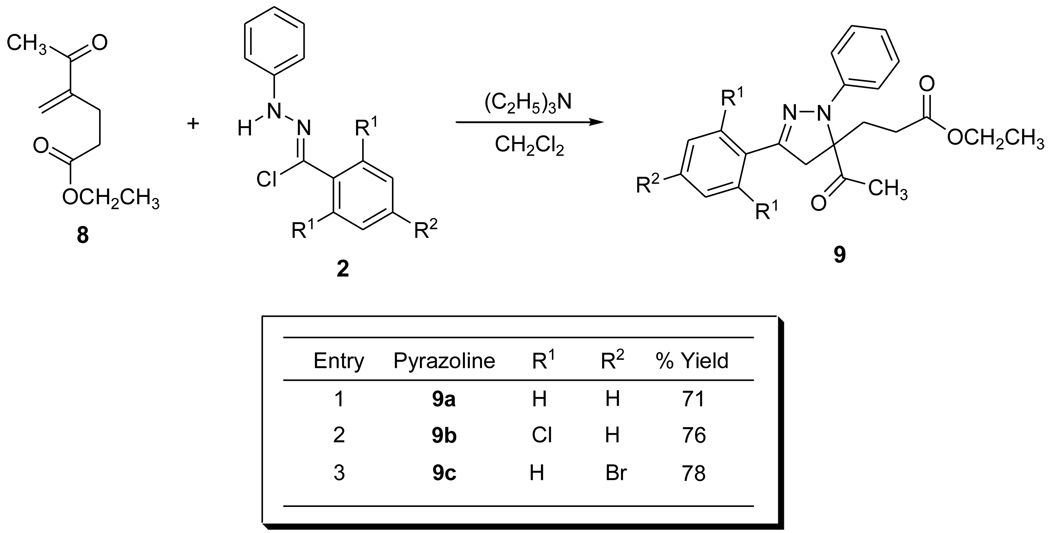
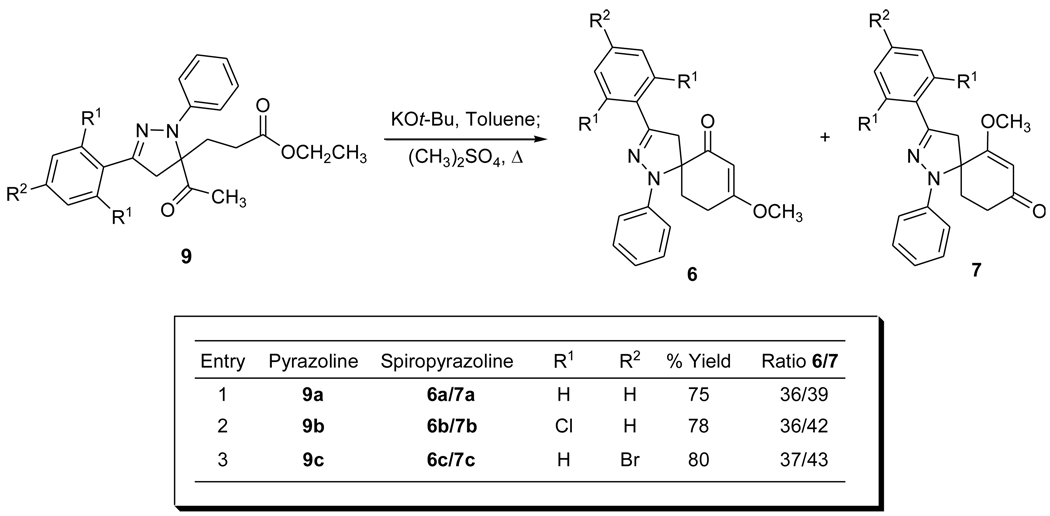
The cycloaddition of the nitrile imine arising from 2a with 817 affords functionalized 5,5-disubstituted pyrazoline 9a in high yield as a single regioisomer. Scheme 3 illustrates the products and the isolated yields of the pyrazolines derived from 1,3-dipolar cycloaddition reactions of 8 with the corresponding α-chloro hydrazone precursor. Intramolecular cyclization/methylation of 9a was achieved to afford spiropyrazolines 6a and 7a in 36% and 39% respective isolated yields which represents an overall 15% increase for the isolation of 6a and 7a arising from pyrazoline 3a.16 Improved overall isolated yields were realized for the syntheses of both regioisomers for other spiropyrazolines as shown in Scheme 4. Higher isolated yields for these spiropyrazolines likely result from decreasing the steric environment of the pyrazoline ester.13b
Scheme 3.
5,5-Disubstituted pyrazolines isolated from the 1,3-dipolar cycloaddition reaction.
Scheme 4.
Spiropyrazoline regioisomers 6 and 7 arising from the intramolecular cyclization/methylation reactions of 9.
Conclusions
In conclusion, we have developed a synthetic methodology to construct unsaturated spiropyrazolines through an intramolecular cyclization/methylation process. 1,3-Dipolar cycloaddition of the functionalized alkene with nitrile imines led to the formation of the 5,5-disubstituted pyrazolines. The latter was used as the precursor to prepare the corresponding spiropyrazolines as two regioisomers through sequential intramolecular cyclization/methylation reactions. Future studies directed towards the synthesis of spiropyrazoline derivatives and other natural product analogues through intramolecular cyclization/methylation are currently in progress.
Acknowledgements
The project described was supported by Award Number SC3GM094081, G12RR13459 (NMR and Analytical CORE facilities) from the National Institute of General Medical Sciences, and HRD-0734645 (HBCU-RISE) from the National Science Foundation. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institute of General Medical Sciences, the National Institutes of Health, or the National Science Foundation.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.(a) Berquist PR, Wells RJ. Chemotaxonomy of the Porifera: The Development and Current Status of the Field. In: Scheuer PJ, editor. Marine Natural Products: Chemical and Biological Perspectives. Vol. 5. New York: Academic Press; 1993. pp. 1–50. [Google Scholar]; (b) Faulkner DJ. Nat. Prod. Rep. 1998:113. doi: 10.1039/a815113y. [DOI] [PubMed] [Google Scholar]; (c) Faulkner DJ. Nat. Prod. Rep. 1997:259. [Google Scholar]; (d) Berquist PR, Wells RJ. Chapter 1. In: Scheuer PJ, editor. Marine Natural Products. Vol. V. New York: Academic Press; 1983. [Google Scholar]
- 2.(a) Faulkner J. Nat. Prod. Rep. 2001;18:1. doi: 10.1039/b006897g. [DOI] [PubMed] [Google Scholar]; (b) Boehlow TR, Spilling CD. Nat. Prod. Lett. 1995;7:1. [Google Scholar]; (c) Faulkner JD. Nat. Prod. Rep. 2002;19:1. doi: 10.1039/b009029h. [DOI] [PubMed] [Google Scholar]; (c) Encarnacion RD, Sandoval E, Malmstrom J, Christophersen C. J. Nat. Prod. 2000;63:874. doi: 10.1021/np990489d. [DOI] [PubMed] [Google Scholar]; (d) Kernan MR, Canbie RC, Bergquist PR. J. Nat. Prod. 1990;53:615. [Google Scholar]; (e) Acosta AL, Rodri´guez AD. J. Nat. Prod. 1992;55:1007. doi: 10.1021/np50085a031. [DOI] [PubMed] [Google Scholar]; (f) Grunasekera SP, Cross SS. J. Nat. Prod. 1992;55:509. doi: 10.1021/np50082a020. [DOI] [PubMed] [Google Scholar]; (g) Compagnone RS, Avila R, Suarez AI, Abrams OV, Rangel HR, Arvelo F, Pina IC, Merentes E. J. Nat. Prod. 1999;62:1443. doi: 10.1021/np9901938. [DOI] [PubMed] [Google Scholar]; (h) Turnbull D, Maron SH. J. Am. Chem. Soc. 1943;65:212. [Google Scholar]
- 3.(a) Talley JJ, Brown DL, Carter JS, Graneto MJ, Koboldt CM, Masferrer JL, Perkins WE, Rogers RS, Shaffer AF, Zhang YY, Zweifel BS, Seibert K. J. Med. Chem. 2000;43:775. doi: 10.1021/jm990577v. [DOI] [PubMed] [Google Scholar]; (b) Talley JJ. 5,859,257. US Patent. 1999; Chem. Abstr. 1999;130:110269. [Google Scholar]; (c) Talley JJ, Bertenshaw SR, Brown DL, Carter JS, Graneto MJ, Kellogg MS, Koboldt CM, Yuan J, Zhang YY, Seibert K. J. Med. Chem. 2000;43:1661. doi: 10.1021/jm000069h. [DOI] [PubMed] [Google Scholar]; (c) Talley JJ. Selective Inhibitors of COX-2. In: King FD, Oxford A, editors. Progress in Medicinal Chemistry 36. Amsterdam: Elsevier; 1999. pp. 201–234. [DOI] [PubMed] [Google Scholar]
- 4.(a) Toyokuni T, Dileep Kumar JS, Walsh JC, Shapiro A, Talley JJ, Phelps ME, Herschman HR, Barrio JR, Satyamurthy N. Bioorg. Med. Chem. Lett. 2005;15:4699. doi: 10.1016/j.bmcl.2005.07.065. [DOI] [PubMed] [Google Scholar]; (b) Sandanayaka VP, Youjun Y. Org. Lett. 2000;2:3087. doi: 10.1021/ol006256r. [DOI] [PubMed] [Google Scholar]; (c) Dadiboyena S, Nefzi A. Eur. J. Med. Chem. 2010;45:4697. doi: 10.1016/j.ejmech.2010.07.045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.For the construction of isoxazoles, see: Waldo JP, Larock RC. Org. Lett. 2005;23:5203. doi: 10.1021/ol052027z. Waldo JP, Larock RC. J. Org. Chem. 2007;72:9643. doi: 10.1021/jo701942e. Bourbeau MP, Rider JT. Org. Lett. 2006;8:3679. doi: 10.1021/ol061260+. Moore JE, Davies MW, Goodenough KM, Wybrow RAJ, York M, Johnson CN, Harrity JPA. Tetrahedron. 2005;61:6707. Moore JE, Goodenough KM, Spinks D, Harrity JPA. Synlett. 2002:2071. Cecchi L, De Sarlo F, Machetti F. Eur. J. Org. Chem. 2006:4852. Dadiboyena S, Xu J, Hamme AT., II Tetrahedron Lett. 2007;48:1295. doi: 10.1016/j.tetlet.2006.12.005. Xu J, Hamme AT., II Synlett. 2008:919. Grecian S, Fokin VV. Angew. Chem., Int. Ed. 2008;47:8285. doi: 10.1002/anie.200801920. Crossley JA, Browne DL. J. Org. Chem. 2010;75:5414. doi: 10.1021/jo1011174. Easton CJ, Hughes CM, Tiekink ERT, Lubin CE, Savage GP, Simpson GW. Tetrahedron Lett. 1994;35:3589. Lee CC, Fitzmaurice RJ, Caddick S. Org. Biomol. Chem. 2009;7:4349. doi: 10.1039/b911098d.
- 6.For the construction of Pyrazoles, see: Shen D-M, Shu M, Chapman T. Org. Lett. 2000;2:2789. doi: 10.1021/ol006197h. Mansour AK, Eid MM, Khalil NSAM. Molecules. 2003;8:744. Penning TD, Talley JJ, Bertenshaw SR, Carter JS, Collins PW, Doctor S, Graneto MJ, Lee LF, Malecha JW, Miyashiro JM, Rogers RS, Rogier DJ, Yu SS, Anderson GD, Burton EG, Cogburn JN, Gregory SA, Koboldt CM, Perkins WE, Seibert K, Veenhuizen AW, Zhang YY, Isakson PC. J. Med. Chem. 1997;40:1347. doi: 10.1021/jm960803q. Deng X, Mani NS. Org. Lett. 2008;10:1307. doi: 10.1021/ol800200j. Delaney PM, Moore JE, Harrity JPA. Chem. Commun. 2006:3323. doi: 10.1039/b607322k. Moore JE, York M, Harrity JPA. Synlett. 2005:860. Browne DL, Helm MD, Plant A, Harrity JPA. Angew. Chem. Int. Ed. 2007;46:8656. doi: 10.1002/anie.200703767. Dadiboyena S, Valente EJ, Hamme AT., II Tetrahedron Lett. 2010;51:1341. doi: 10.1016/j.tetlet.2010.01.017. Sibi PM, Stanley ML, Jasperse PC. J. Am. Chem. Soc. 2005;127:8276. doi: 10.1021/ja051650b. Browne Dl, Viva JF, Plant A, Gomez-Bengoa E, Harrity JPA. J. Am. Chem. Soc. 2009;131:7762. doi: 10.1021/ja902460n. Bhat GA, Montero JL, Panzica RP, Wotring LL, Townsend LB. J. Med. Chem. 1981;24:1165. doi: 10.1021/jm00142a009. Helm MD, Moore JE, Plant A, Harrity JPA. Angew. Chem. 2005;117:3957. doi: 10.1002/anie.200500288. Helm MD, Moore JE, Plant A, Harrity JPA. Angew. Chem. Int. Ed. 2005;44:3889. doi: 10.1002/anie.200500288. Browne DL, Taylor JB, Plant A, Harrity JPA. J. Org. Chem. 2010;75:984. doi: 10.1021/jo902514v. Browne DL, Harrity JPA. Tetrahedron. 2010;66:553. Stauffer SR, Coletta CJ, Tedesco R, Nishigushi G, Carlson KE, Sun J, Katzenellenebogen BS, Katzenellenbogen JA. J. Med. Chem. 2000;43:4934. doi: 10.1021/jm000170m. Oh L. Tetrahedron Lett. 2006;47:7943. Deng X, Mani NS. Org. Lett. 2006;8:3505. doi: 10.1021/ol061226v.
- 7.(a) Goldenstein K, Fendert T, Proksch P, Winterfeldt E. Tetrahedron. 2000;56:4173. [Google Scholar]; (b) Adamo MFA, Chimichi S, De Sio F, Donati D, Sarti-Fantoni P. Tetrahedron Lett. 2002;43:4157. [Google Scholar]; (c) Harburn JJ, Rath NP, Spilling CD. J. Org. Chem. 2005;70:6398. doi: 10.1021/jo050846r. [DOI] [PubMed] [Google Scholar]; (d) Adamo MFA, Donati D, Duffy EF, Sarti-Fantoni P. J. Org. Chem. 2005;70:8395. doi: 10.1021/jo051181w. [DOI] [PubMed] [Google Scholar]; (e) Marsini MA, Huang Y, Van De Water R, Pettus TRR. Org. Lett. 2007;9:3229. doi: 10.1021/ol0710257. [DOI] [PMC free article] [PubMed] [Google Scholar]; (f) Wasserman HH, Wang J. J. Org. Chem. 1998;63:5581. [Google Scholar]; (g) Boehlow TR, Harburn JJ, Spilling CD. J. Org. Chem. 2001;66:3111. doi: 10.1021/jo010015v. [DOI] [PubMed] [Google Scholar]; (h) Forrester AR, Thomson RH, Woo S-O. Liebigs Ann. Chem. 1978:66. [Google Scholar]; (i) Murakata M, Yamada K, Hoshino O. J. Chem. Soc., Chem. Commun. 1994:443. [Google Scholar]; (j) Masatoshi M, Yamada K, Hoshino O. Tetrahedron. 1996:14713. [Google Scholar]; (k) Murakata M, Yamada K, Hoshino O. Heterocycles. 1998;47:921. [Google Scholar]; (l) Ogamino T, Nishiyama S. Tetrahedron. 2003;59:9419. [Google Scholar]; (m) Kumar HM, Anjaneyulu S, Yadav JS. Synth. Commun. 1999;29:877. [Google Scholar]; (n) Bardhan S, Schmitt DC, Porco JA., Jr Org. Lett. 2006;8:927. doi: 10.1021/ol053115m. [DOI] [PubMed] [Google Scholar]; (o) Singh V, Yadav GP, Maulik PR, Batra S. Tetrahedron. 2008;64:2979. [Google Scholar]
- 8.(a) Dadiboyena S, Valente EJ, Hamme AT., II Tetrahedron Lett. 2009;50:291. doi: 10.1016/j.tetlet.2008.10.145. [DOI] [PMC free article] [PubMed] [Google Scholar]; (b) Dawood K, Fuchigami T. J. Org. Chem. 2005;70:7537. doi: 10.1021/jo0507587. [DOI] [PubMed] [Google Scholar]; (d) Dawood K. Tetrahedron. 2005;61:5229. [Google Scholar]; (d) Kerbel A, Vebrel J, Roche M, Laude B. Tetrahedron Lett. 1990;31:4145. [Google Scholar]; (e) Shanmugasundaram M, Raghunathan R, Malar EJP. Heteroatom Chem. 1998;9:517. [Google Scholar]; (f) Mernyak E, Kozma E, Hetenyi A, Mark L, Schneider G, Wolfling J. Steroids. 2009;74:520. doi: 10.1016/j.steroids.2009.02.001. [DOI] [PubMed] [Google Scholar]; (g) Jedlovska E, Levai A, Toth G, Balazs B, Fisera LJ. Heterocyclic Chem. 1999;36:1087. [Google Scholar]; (h) Albar HA, Makki MSI, Faidallah HM. J. Chem. Res. (S) 1997:40. [Google Scholar]; (i) Singh V, Singh V, Batra S. Eur. J. Org. Chem. 2008:5446. [Google Scholar]
- 9.(a) Huisgen R. Angew. Chem. Int. Ed. Engl. 1963;2:565. [Google Scholar]; (b) Huisgen R. Angew. Chem. Int. Ed. Engl. 1963;2:633. [Google Scholar]
- 10.(a) Lan R, Liu Q, Fan P, Lin S, Fernando SR, McCallion D, Pertwee R, Makriyannis A. J. Med. Chem. 1999;42:769. doi: 10.1021/jm980363y. [DOI] [PubMed] [Google Scholar]; (b) Naoum F, Kasiotis KM, Magiatis P, Haroutounian SA. Molecules. 2007;12:1259. doi: 10.3390/12071259. [DOI] [PMC free article] [PubMed] [Google Scholar]; (c) Jachak MN, Avhale AB, Tantak CD, Toche RB. J. Heterocycl. Chem. 2005;42:1311. [Google Scholar]; (d) Hanefeld U, Rees CW, White AJP, Williams DJ. J. Chem. Soc., Perkin Trans 1. 1996:1545. [Google Scholar]; (e) Chun YS, Lee KK, Ko YO, Shin H, Lee S. Chem. Commun. 2008:5098. doi: 10.1039/b813369g. [DOI] [PubMed] [Google Scholar]; (f) Devery JJ, III, Mohanta PK, Casey BM, Flowers RA. Synlett. 2009:1490. doi: 10.1055/s-0029-1217163. [DOI] [PMC free article] [PubMed] [Google Scholar]; (g) Lee KY, Kim JM, Kim JN. Tetrahedron Lett. 2003;44:6737. [Google Scholar]; (h) Han Y, Huynh HV. Chem. Commun. 2007:1089. doi: 10.1039/b615441g. [DOI] [PubMed] [Google Scholar]; (i) Hahn FE. Angew. Chem., Int. Ed. 2006;45:1348. doi: 10.1002/anie.200503858. [DOI] [PubMed] [Google Scholar]; (j) Huynh HV, Han Y, Ho JHH, Tan GK. Organometallics. 2006;25:3267. [Google Scholar]
- 11.(a) Abunada NM, Hassaneen HM, Kandile NG, Miqdad OA. Molecules. 2008;13:1501. doi: 10.3390/molecules13071501. [DOI] [PMC free article] [PubMed] [Google Scholar]; (b) Liu B, Wang Z, Huang Z. Tetrahedron Lett. 1999;40:7399. [Google Scholar]
- 12.(a) Chakravarty M, Bhuvan Kumar NN, Sajna KV, Kumara Swamy KC. Eur. J. Org. Chem. 2008:4500. doi: 10.1021/jo102240u. [DOI] [PubMed] [Google Scholar]; (b) Chakravarty M, Kumara Swamy KC. J. Org. Chem. 2006;71:9128. doi: 10.1021/jo061525y. [DOI] [PubMed] [Google Scholar]
- 13.(a) Xu J, Wang J, Ellis ED, Hamme AT., II Synthesis. 2006:3815. [Google Scholar]; (b) Ellis ED, Xu J, Valente EJ, Hamme AT., II Tetrahedron Lett. 2009;50:5516. doi: 10.1016/j.tetlet.2009.07.095. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Ramon F, Degueil-Castaing M, Maillard B. European Polymer Journal. 2000;36:315. [Google Scholar]
- 15.General Procedure for the Synthesis of [2+3]-Cycloaddition Products: A solution of the functionalized alkene (3.0 mmol) and the hydrazonyl chloride (3.0 mmol) in 10 mL of either dry chloroform or dichloromethane was treated with triethylamine (0.463 mL, 3.3 mmol). The reaction mixture was stirred at rt until the disappearance of the starting materials, as evidenced by TLC. After the reaction was complete, the crude reaction mixture was concentrated, and the solvent was evaporated under reduced pressure. The crude products were purified by flash column chromatography over silica gel by using the appropriate hexanes-ethyl acetate ratio as an eluant system.
- 16.General Procedure for the intramolecular cyclization/alkylation: To a solution of the cycloaddition product (1.0 mmol) in 15 mL of anhydrous toluene was added potassium tert-butoxide (6-8 mmol). The reaction mixture was then allowed to stir under anhydrous conditions until the disappearance of the starting materials, as evidenced by TLC. To this mixture was added 2 equivalents of dimethyl sulfate (2 mmol, 0.186 mL), and the reaction mixture was heated to reflux temperature for 8 hrs under anhydrous conditions. The reaction mixture was later quenched with saturated ammonium chloride and extracted with EtOAc. The crude reaction mixture was concentrated, and the solvent was evaporated under reduced pressure. The crude products were purified by flash column chromatography over silica gel by using the appropriate hexanes-ethyl acetate ratio as an eluant system.
- 17.(a) Ayed TB, Amri H. Synth Commun. 1995;25:3813. [Google Scholar]; (b) Kalaus G, Juhasz I, Greiner I, Kajtar-Peredy M, Brlik J, Szabo L, Szantay C. J. Org. Chem. 1997;62:9188. doi: 10.1021/jo020386r. [DOI] [PubMed] [Google Scholar]