Abstract
Six experiments were performed to determine the role of mediodorsal thalamus (MD) in the devaluation task, varying the type of contingencies (Pavlovian or operant), the number of reinforcers (one versus two) and the order of experiments (in naïve or experimentally experienced rats). MD lesioned rats were impaired in devaluation performance when switched between Pavlovian and operant devaluation tasks, but not when switched from one Pavlovian devaluation task to another Pavlovian devaluation task. MD lesions caused no devaluation impairment in a multiple reinforcer Pavlovian devaluation task. These results suggest that MD lesions impair performance in devaluation tasks as a result of an inability to switch the form of associations made from one type of outcome-encoding association to another. This is in accord with previous literature suggesting that MD is needed for strategy set shifting. The results further suggest that MD is a necessary part of devaluation circuits only in cases in which previous associations need to be suppressed in order for new associations to be learned and control behavior, and otherwise the devaluation circuit does not require MD.
Keywords: Mediodorsal thalamus, devaluation, set-shifting, reward
Devaluation tasks involve training a relationship that earns or predicts a valuable outcome, such as a lever press that earns a type of food or a light that predicts the delivery of food (Adams & Dickinson, 1981; Holland & Rescorla, 1975). The value of this outcome is then reduced by motivational (e.g., satiation) or associative methods (e.g., a food aversion is established by food-toxin pairings). After such outcome devaluation, responding based on the relationship that predicts or earns food is typically reduced. Similarities in task demands to human decision making, as well as similarities in neural underpinnings, suggests that the devaluation task provides a model of goal expectancy-guided behavior (as reviewed in Pickens & Holland, 2004).
Many reports have examined the neural circuitry involved in acting according to the current value of goals. For example, lesions of amygdala, or specific lesions of basolateral complex of amygdala (BLA), cause impairments in many versions of the devaluation task (Balleine, Killcross, & Dickinson, 2003; Blundell, Hall & Killcross, 2003; Hatfield, Han, Conley, Gallagher, & Holland, 1996; Malkova, Gaffan & Murray, 1997). Several laboratories have also found that bilateral orbitofrontal cortex (OFC) lesions (Gallagher, McMahan & Schoenbaum, 1999; Izquierdo, Suda & Murray, 2004; but see Ostlund & Balleine, 2007) or lesions that disconnect amygdala and OFC (Baxter, Parker, Lindner, Izquierdo, & Murray, 2000) impair performance in devaluation tasks.
Another brain area that may be needed for devaluation is mediodorsal thalamus (MD). MD, BLA and OFC are all interconnected (Carmichael & Price, 1995; Ghashghaei & Barbas, 2002; Groenewegen, 1988; Ray & Price, 1992) and the three brain areas share some reward functions (Chudasama, Bussey, & Muir, 2001; Gaffan & Murray, 1990; Gaffan, Murray, & Fabre-Thorpe, 1993; Schoenbaum, Setlow, Nugent, Saddoris, & Gallagher, 2003). MD lesions impair devaluation in operant devaluation tasks in rats (Corbit, Muir & Balleine, 2003) and macaques (Mitchell, Browning, & Baxter, 2007), and disconnections of MD from BLA and OFC impair the performance of macaques in an operant devaluation experiment (Izquierdo & Murray, 2004b).
The goal of the present experiments was to determine the role of MD in devaluation task performance. Although devaluation performance is impaired by MD lesions when rats lever press for food (Corbit et al., 2003) or macaques displace objects for food (Izquierdo & Murray, 2004b; Mitchell et al., 2007), initial data presented in Abstract format suggested that rats’ performance in a Pavlovian devaluation task was not affected (Pickens, Gallagher, & Holland, 2006). However, that study differed from the previous experiments not only in the training contingencies (Pavlovian vs. operant), but also in the number of reinforcers used (one vs. two) and the devaluation method (lithium chloride (LiCl)-based taste aversion training vs. selective satiation).
This series of experiments began with an investigation of the effects of MD lesions in the single-reinforcer Pavlovian devaluation task used extensively in our lab. This task has previously been shown to be sensitive to BLA and OFC lesions (Gallagher et al., 1999; Hatfield et al., 1996). This experiment was followed with a single reinforcer operant devaluation task, using the same animals. This task used the same method of reducing the reward’s value (taste aversion training) and the same number of reinforcers (one) to compare the effects of MD lesions on Pavlovian and operant devaluation more specifically. The results of Experiments 1 and 2 suggested that MD was not part of the circuit needed for all devaluation tasks and four more experiments were performed to determine the factors that determine the role of MD in devaluation. These experiments suggested that the role of MD may be in switching associations needed for devaluation between tasks rather than a role in particular types of devaluation tasks.
Methods
Experiment 1
Subjects
The subjects were 65 male Long-Evans rats (Charles River Laboratories, Raleigh, NC), which weighed 300–325 g when they arrived in the laboratory vivarium. The rats were maintained for 1 week with free access to food and water in individual cages before they were food deprived to 85% of their ad lib weights. The vivarium was illuminated from 7 a.m. to 7 p.m.
Apparatus
The behavioral training apparatus consisted of the eight individual chambers (22.9 × 20.3 × 20.3 cm) described in Pickens and colleagues (2003). Each chamber was enclosed in a soundproof shell. A 6 W lamp, which served as the source of the visual conditioned stimulus (CS), was mounted on the inside wall of the shell, 10 cm above the experimental chamber and even with the end wall opposite the food cup. A speaker, which was used to present an 80-dB white noise in another experiment, was mounted next to the house light. A dimmed food cup was recessed in the center of one end wall; a jeweled lamp that could be illuminated with a 6 W bulb (not used in this study) was located 5 cm above that recess. An infrared photocell placed just inside the food cup was polled (1 kHz) by computer circuitry. A 10 cm chain could be suspended from the ceiling to within 10 cm of the floor, 3.5 cm to the left of the food cup, but it was not present in this experiment.
Surgical procedures
Before behavioral training, aseptic surgery was performed under isoflurane inhalation anaesthesia (Isovet; Mallinckrodt, Mundelein, IL). Bilateral MD lesions were made by infusing 0.36 μl of 0.12 M NMDA (Sigma, St. Louis, MO) to remove neurons in a target area that focused on medial and central portions of MD, centered around 2.8 mm posterior to bregma. In 43 animals (25 lesion, 18 sham), lesions were performed using procedures used by Corbit and colleagues (2003). Rats were placed with the incisor bar set at +5.0. A single injection in each hemisphere was made at coordinates 1.8 mm posterior to bregma, 0.8 mm lateral to bregma and 6.2 mm ventral to the dura. A large number of lesions using these coordinates were found to be caudal to the target area, so new coordinates were used for the remaining animals in this and all subsequent experiments. In 22 animals (15 lesion, 7 sham), new lesion coordinates were utilized. The incisor bar was adjusted so that the skull surface was flat and injections were made at coordinates 2.9 mm posterior, 0.8 mm lateral and 5.5 ventral from bregma. The needle was moved ventrally down to 6.7, left there for 10 s, then pulled up to 5.5 and left there for 1 min before infusing. In the sham surgeries, the injector was lowered to the corresponding depths and PBS vehicle was injected in a manner comparable to lesion injections. The NMDA or vehicle was infused at a rate of 0.1 μl/min using a 2.0 μl Hamilton syringe.
Behavioral training procedures
An outline of the experimental procedures can be found in the top of Table 1. The rats were first trained to eat from the recessed food cup in two 64-min sessions which each included 16 deliveries of the unconditioned stimulus (US), two 45-mg Noyes grain food pellets (Research Diets, New Brunswick, NJ). Next, in each of eight daily 64-min sessions, the rats received sixteen 10-s presentations of the house light, each followed immediately by the delivery of two 45-mg food pellets.
Table 1.
Experimental designs for experiments 1 and 2
| Group | Surgery | Conditioning | Aversion | Test |
|---|---|---|---|---|
| Experiment 1: | ||||
| Paired | Neurotoxic or sham MD lesion | House light → Grain pellets | Grain pellets → LiCl | House Light? |
| Unpaired | Neurotoxic or sham MD lesion | House light → Grain pellets | Grain pellets, LiCl | House Light? |
|
| ||||
| Experiment 2: | ||||
| Paired | Lever press → Fruit punch pellet | Fruit punch pellets → LiCl, Grain pellets | Lever press? | |
| Unpaired | Lever press → Fruit punch pellet | Grain pellets → LiCl, Fruit punch pellets | Lever press? | |
Note. LiCl-lithium chloride; → - paired present at ions;, -unpaired presentation
The rats were then assigned to Paired (n=31) and Unpaired (n=27) groups for taste aversion training, which took place in beddingless cages in the rats’ colony room. All rats received three exposures to food pellets and three injections of lithium chloride (LiCl), but the Paired groups received the injections immediately following the food consumption and the Unpaired group received the LiCl and food 24 hr apart. This equated both groups for the number of exposures to the food pellets and the LiCl while giving only the Paired group a taste aversion. On each of the two days before aversion training began, all animals received 1-hour exposure to the white ceramic bowls and the beddingless cages to be used in the aversion training to minimize neophobia to the bowls and cages. On the first, third and fifth days of aversion training, the rats in the Paired group received, in the beddingless cages, 20-min access to a ceramic bowl containing 100 food pellets (identical to those delivered in the acquisition chambers). Immediately following food consumption by the Paired animals, rats in both the Paired and Unpaired groups received a 3 ml injection (i.p.) of 0.3 M LiCl solution. On the second, fourth and sixth days of aversion training, the rats in the Unpaired group received 20-min access to a ceramic bowl containing 100 food pellets (identical to those delivered in the acquisition chambers), but these were not followed by injection. All rats then received two 64-min test sessions in the experimental chamber on successive days. These sessions each included 16 presentations of the 10-s house light, but no food pellets were delivered. Data will only be presented for the first test session, because extinction reduced food cup responding in the sham rats such that devaluation could not be detected. The following day, the rats received 20-min access to 50 45-mg food pellets (those used as the US) placed in the food cup of the experimental chamber, in order to assess the level of generalization of the taste aversion from the homecage to the experimental chamber.
Response measures
The primary measure of appetitive conditioning to the house light CS during training was the percentage of time the rat spent with its head in the food cup during the last 5 s of the 10-s CS, and during the 5-s empty interval immediately before each CS, as indicated by disruption of the photocell beam. Previous data (Holland, 1977) show that food cup behaviors during 10 second visual CSs are concentrated in the last 5 s of the cue. For the analysis of the test session, I report this measure as well as the percentage of time the rat spent with its head in the food cup in the 5 s after the light ended, focusing on the first half of the session, as in a previous study (Pickens, Saddoris, Gallagher, & Holland, 2005). Consumption of food pellets in the beddingless cage was determined by counting the number of pellets in the bowl after 20 min. Consumption in the experimental chamber test was determined by subtracting the number of pellets in the food cup and tray beneath the floor after 20 min from the number initially given (100).
Experiment 2
Subjects
This experiment used the same rats as Experiment 1 and was performed immediately afterwards. The rats were maintained at 85% of their free feeding weights in a vivarium illuminated from 7 a.m. to 7 p.m.
Apparatus
This experiment briefly utilized the chambers from Experiment 1 and thereafter was performed in new chambers very similar to these chambers.
The behavioral training apparatus for Experiment 2 consisted of four individual chambers very similar to the ones used in Experiment 1. A liquid cup was recessed in the center of the opposite wall from the food cup in a 5 cm × 5 cm opening. A retractable 2 × 2 cm lever could be positioned 5 cm above the floor, 2.5 cm to the left of the food cup.
Behavioral procedures
An outline of the behavioral procedures can be found at the bottom of Table 1. The rats were initially given 3 exposures to the reinforcers for the second experiment in the chambers from Experiment 1. For each exposure, the rats received20-min access to 50 45-mg fruit punch food pellets (Research Diets, New Brunswick, N.J.) placed in the food cup of the experimental chamber. They were given one session on the first of two days and then one session the next morning and the other the next afternoon.
Rats then began training in the context for Experiment 2. They received a single 32-min magazine training session, with the levers retracted, in which there were 16 reward deliveries, each containing 2 fruit punch pellets. On the next day, the rats began lever press training, in which each lever press earned a food pellet. Rats were removed once they had earned 50 pellets or when an hour had passed, whichever came first. Rats that did not earn 50 pellets were given an extra session that evening. They then received one session in which lever pressing was reinforced on a variable-interval (VI) 15 second schedule, followed by three daily sessions in which lever pressing was reinforced on a VI 30 second schedule.
Taste aversion training was next performed in the experimental chamber, with the lever retracted, over 10 days broken into 5 2-day cycles. Each day, each rat received 60 pellets delivered one at a time over 32 min. This was done to approximate the number and temporal distribution of the rewards earned during the VI 30 operant training. All rats received five injections of LiCl, five sessions in which fruit punch pellets (the operant reinforcer) were delivered and five sessions in which grain pellets (the reinforcer from Experiment 1) were delivered. The Paired rats (n=30) received fruit punch pellets on the first, third, fifth, seventh and ninth day and then received an injection of 0.3 M LiCl solution (5 ml/kg i.p.). The Unpaired rats (n=28) received grain pellets on these days and then an injection of LiCl as in the Paired animals. On the alternate days, each group received the other type of pellet. On days 2, 4, 6, 8 and 10, the Paired rats received 60 grain pellets and the Unpaired rats received 60 fruit punch pellets, but no injections were given on these days.
The day after taste aversion ended, all of the rats were given a 33-min test extinction session with the lever. Rats were able to lever press but no pellets were delivered. The following day, all rats received a consumption test in the experimental chamber in which 60 fruit punch pellets were delivered over 32 min.
Response measures
The primary measure of operant conditioning was the rate of lever pressing per min. This was calculated for the entire session during training and for the first 4-min block in the test. Test responding was normalized for responding on the last day of acquisition by using the formula:
Consumption in the taste aversion sessions and experimental chamber test was determined by counting the number of pellets in the food cup and tray beneath the floor after the 32-min sessions and subtracting from the number delivered (60).
Histological procedures
After completion of behavioral testing, rats were deeply anaesthetized with isoflurane and perfused with 0.1-M phosphate buffered saline (PBS), followed by 10% (v/v) formalin. The brains were removed and stored in 0.1-M PBS with 20% (w/v) sucrose and 1% (w/v) DMSO at 40 C for 24–48 hr. Sections (40-μm) were taken from each brain, and every third section was mounted on slides and Nissl-stained to verify lesions.
Results
Histology for Experiments 1 and 2
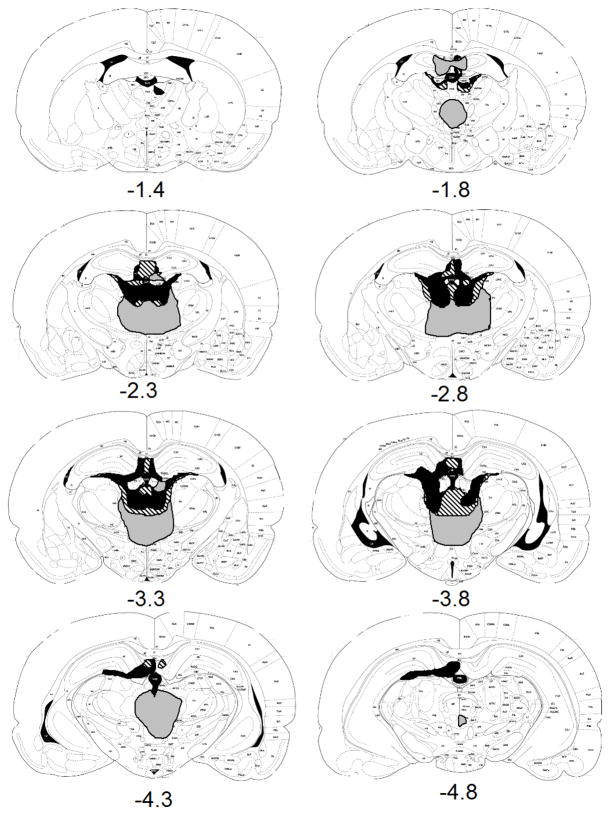
Six of the lesioned rats and one of the sham rats died, all of which had surgeries with the coordinates taken from Corbit and colleagues (2003). Two rats had unacceptable lesions (which spared the thalamus unilaterally or entirely), one which had surgery done with the Corbit and colleagues’ (2003) coordinates and one which had surgery using flat skull coordinates. One sham rat had thalamic damage due to an infection and was excluded. There were 23 sham rats included in these 2 experiments. Acceptable lesions (n=17) required complete damage to the medial and central areas of MD thalamus on at least one histological section between 2.3 and 2.8 mm caudal to bregma and included complete damage to the lateral region of MD in all but two cases, in which the damage was complete on only one side. Oyoshi and colleagues (1996) found that most neurons responsive to conditioned cues or conditioned responses were found at or rostral to −2.8 from bregma. No lesions were limited to areas of MD that were rostral to −2.3 from bregma. Figure 1 shows the largest, smallest and representative lesions. All lesions included at least some damage to the centrolateral and intermediodorsal thalamic nuclei as well as the dorsal surface of the dorsomedial portion of laterodorsal thalamic nucleus and mediorostral portion of lateral posterior thalamic nucleus. Most animals also had some damage to the lateral habenula and the thalamic nuclei that surround MD. Finally, there were five animals that had damage that extended far beyond MD, all done with the tilted skull coordinates. This damage often included the rhomboid, reunions, gustatory thalamic, subparafascicular and precommissural nuclei. There was also damage to the most medial part of the hippocampus and the dentate in most animals, with the damage often negatively correlated with the amount of excess thalamic damage. A previous experiment has found that lesions of the surrounding areas damaged in the representative lesions have a mild effect on working memory but do not impair reward learning (Mitchell & Dalrymple-Alford, 2005), so they probably did not affect the devaluation results. There is a small amount of evidence linking lateral habenula to reward processes (Matsumoto & Hikosaka, 2006; Tronel & Sara, 2002). However, there is no previous evidence of damage to this area causing devaluation deficits and this area does not appear to be interconnected with any of the areas involved in devaluation other than MD (Sutherland, 1982), such as BLA, OFC, PLC or DMS (Felton, Linton, Rosenblatt, & Morell, 1999; Klemm, 2004; Sutherland, 1982), so damage to lateral habenula probably did not affect the results.
Figure 1.
Extents of minimum (black), maximum (gray), and representative (stripes) MD lesions at various distances posterior to bregma for rats in Experiments 1 and 2.
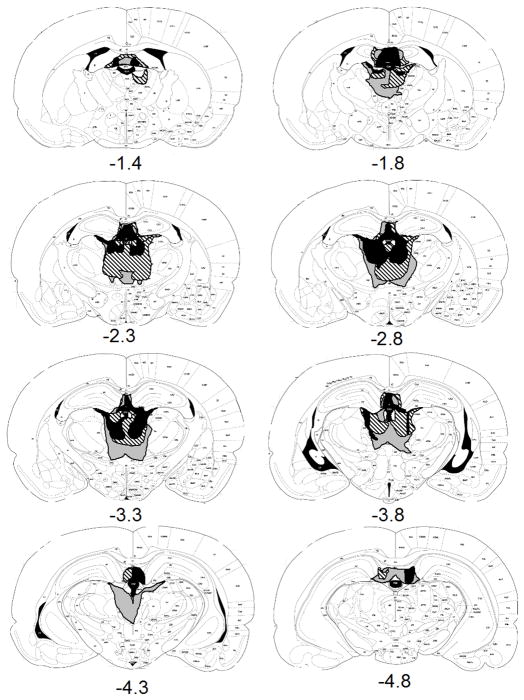
The role of non-MD damage in the deficits was examined in several ways. First, analyses of experiments that demonstrated lesion effects found no effect of the amount of damage to the lateral habenula, hippocampus or thalamus beyond MD. Separate ANOVAs of test responding were performed for lateral habenula damage, hippocampal damage and for thalamic damage beyond MD. Second, Experiments 1 and 2 had a Control Thalamic Lesion to control for effects of damage to other thalamic areas, in particular the areas caudal to MD. These Control thalamic lesions did not impair devaluation performance. The Control lesions were made using the same lesion coordinates as the MD lesions, although most of them resulted from use of the tilted skull coordinates. The majority of these 15 lesions were at the correct dorsal/ventral and medial/lateral coordinates but were caudal to the lesions which met the criteria. In fact, the large number of caudal lesions with tilted skull surgical procedures was the reason we switched lesion coordinates. These lesions tended to damage the caudal MD and parafascicular (PF) nucleus of the thalamus as well as other caudal areas damaged in the large MD lesions. These also included two animals in which the damage was ventral to the MD, in areas that were also damaged by the larger lesions that met the MD criteria. Rats were not excluded from this group for MD damage between 2.3 and 2.8 caudal to bregma as long as it was not complete. Figure 2 gives the extent of the largest, smallest and representative lesions that fit these criteria. As can be seen in Figure 2, the majority of these animals had some incomplete damage to the target areas and this damage was not in any uniform position. However, although the three nonoverlapping lesions represented in Figure 2 combine to appear to include all of MD, none of the lesions had complete or acceptable damage to MD.
Figure 2.
Extents of minimum (black), maximum (gray), and representative (stripes) Control thalamic lesions at various distances posterior to bregma for rats in Experiments 1 and 2.
Experiment 1
Seven rats were excluded for insufficient conditioning to the light in Pavlovian training (4 sham, 3 MD lesions) (less than 25 percent time in the food cup during the light on the last 2 days of conditioning). One sham rat was excluded for insufficient taste aversion learning. The following data are from 47 rats: 8 MD-Paired, 6 MD-Unpaired, 10 Control Lesion-Paired, 5 Control Lesion-Unpaired, 9 Sham-Paired and 9 Sham-Unpaired.
Pavlovian acquisition
All groups increased their food cup responses during the light (Figure 3a, large symbols), but not their baseline level of responding (Figure 3a, small symbols), over the course of training. Performance on the final two conditioning sessions during either the pre-CS or light CS periods did not differ as a function of Lesion status or assignment to Paired or Unpaired taste aversion training. A Lesion × Taste Aversion × Session ANOVA of acquisition of responding during the CS found a significant effect of Session (F(7,287) = 90.23, p < 0.01). No other effects or interactions were significant (all p > 0.10). A Lesion × Taste Aversion × Session ANOVA of acquisition of responding during the pre-CS period found no significant effects or interactions (all p > 0.05). Similarly, there were no significant differences or interactions on the last 2 days of acquisition, in either the pre-CS or CS periods (all p > 0.05).
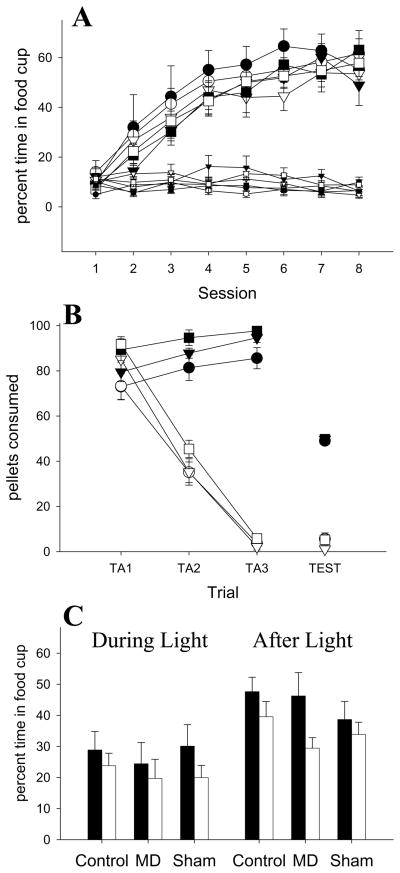
Figure 3.
Experiment 1 behavioral performance. A: Acquisition of food cup responding prior to (small symbols) and during the light (large symbols) B: Acquisition of taste aversion. Taste aversion training in the beddingless cage is shown on the left with the connected lines. The taste aversion test in the experimental chamber is shown with the free-floating symbols on the right. On each training trial, 100 pellets were available, but only 50 pellets were available in the experimental chamber test. C: Responding in the Pavlovian devaluation test. Responding during the light is on the left and responding in the period after the light is on the right. Black symbols and bars represent Unpaired groups. White symbols and bars represent Paired groups. Circles represent animals with Control thalamic lesions, triangles represent animals with MD thalamic lesions and squares represent those with sham lesions.
Taste aversion learning
Taste aversion training reduced food consumption in the Paired groups but not in the Unpaired groups, and this reduction was unaffected by Lesion condition. There was slightly greater overall consumption in the sham group than in the lesioned groups. A Lesion × Taste Aversion × Trial ANOVA for the three training trials (left side of Figure 3b) found significant main effects of Lesion (F(2,41) = 6.78, p < 0.01), Taste Aversion (F(1,41) = 267.65, p < 0.01) and Trial (F(2,82) = 167.87, p < 0.01) and a significant interaction of Taste Aversion × Trial (F(2,82) = 309.76, p < 0.01). The main effect of Lesion seemed to reflect greater overall consumption in rats with sham lesions than those with Control thalamic lesions. A Tukey’s Honestly Significant Difference (HSD) post-hoc test found that the Sham group differed from the Control Lesion group (p < 0.01). No other comparisons were significant (p > 0.10). Most important, none of the interactions involving Lesion were significant (all p > 0.10).
The taste aversion transferred readily from the homecage to the experimental chamber and this transfer was unaffected by the lesions. A Lesion × Taste Aversion ANOVA for the experimental chamber consumption test (right side of Figure 3b) found a main effect of Taste Aversion (F(1,41) = 861.29, p < 0.01), but no effect of Lesion or an interaction (all F < 1).
Light devaluation test
Figure 3c shows the primary data from this experiment, the results of the devaluation test. Neither lesion impaired the rats’ ability to display devaluation performance. Responding during the last 5-s of the light (left part of Figure 3c) failed to show a significant devaluation effect, perhaps due to small sample size in some groups. A Lesion × Taste Aversion ANOVA for this measure found no significant effects or interactions (all p > 0.10), including Taste Aversion (p = 0.17). Responding in the 5-s after the light was also analyzed, as in Pickens, Saddoris and colleagues’ (2005) study. Responding after the light (right side of Figure 3c) exhibited lower responding in each Paired group compared to its respective Unpaired group. A Lesion × Taste Aversion ANOVA found a significant effect of Taste Aversion (F(1,41) = 5.21, p < 0.05). Neither the effect of Lesion nor its interactions were significant (all p > 0.10). There were no differences in responding in the pre-CS period (percent time in food cup: MD-Paired −3.6 ± 1.7, MD-Unpaired −2.8 ± 1.1, Control-Paired −7.2 ± 2.4, Control-Unpaired −4.0 ± 1.2, Sham-Paired −2.5 ± 1.1, Sham-Unpaired −4.0 ± 1.2). A Lesion × Taste Aversion ANOVA found no effects or interaction on pre-CS responding (all p > 0.10).
It appears that neither lesion had an effect on Pavlovian devaluation. This outcome differs from the results found by Corbit and colleagues (2003), Izquierdo & Murray (2004b) and Mitchell and colleagues (2007), using operant procedures. Therefore, it would be valuable to assess the performance of the same rats in an operant procedure (Experiment 2).
Experiment 2
Nine rats were excluded for insufficient taste aversion learning in Experiment 2 (6 sham, 3 MD). The following data are from 46 rats: 7 MD-Paired,7 MD-Unpaired, 6 Control-Paired, 9 Control-Unpaired, 5 Sham-Paired and 12 Sham-Unpaired. Some aspects of the results of Experiment 2 were affected by the rats’ taste aversion assignment in Experiment 1. Thus, that variable was included in the analyses. The number of animals in each group was: MD-Paired-Previously Paired: 4, MD-Paired-Previously Unpaired: 3, MD-Unpaired-Previously Paired: 3, MD-Unpaired-Previously Unpaired: 4, Control-Paired-Previously Paired: 3, Control-Paired-Previously Unpaired: 3, Control-Unpaired-Previously Paired: 7, Control-Unpaired-Previously Unpaired: 2, Sham-Paired-Previously Paired: 2, Sham-Paired-Previously Unpaired: 3, Sham-Unpaired-Previously Paired: 6, Sham-Unpaired-Previously Unpaired: 6.
Operant acquisition
All groups increased lever pressing over the course of training. No impairment of lever press acquisition was found in rats with either lesion. A Lesion × Taste Aversion × Conditioning Session × Previous Taste Aversion ANOVA of lever press acquisition (Figure 4a) found a significant effect of Conditioning Session (F(3,102) = 33.06, p < 0.01). There also was an interaction of Taste Aversion × Conditioning Session × Previous Taste Aversion (F(3,102) = 3.32, p < 0.05). No other effects or interactions were significant (all p > 0.05). A Lesion × Taste Aversion × Previous Taste Aversion ANOVA of responding on the last day of acquisition found no significant effects or interactions (all p > 0.05).
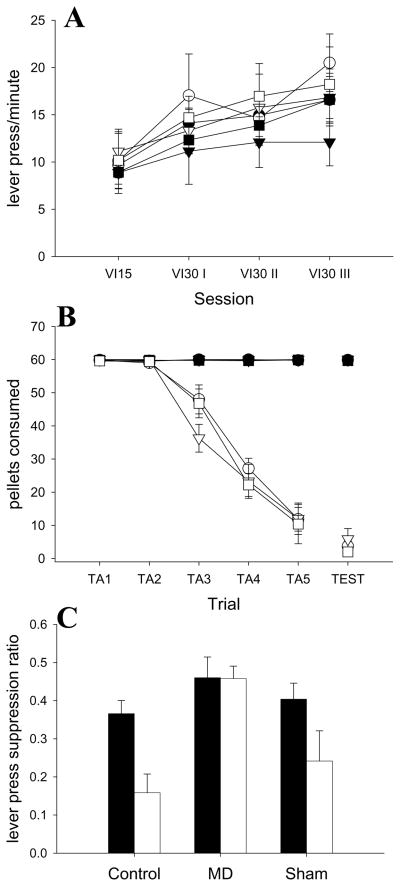
Figure 4.
Experiment 2 behavioral performance A: Acquisition of lever pressing. B: Consumption of the operant reinforcer, fruit punch pellets, during taste aversion. Taste aversion training prior to the devaluation test is shown on the left with the connected lines. The taste aversion test, performed after the devaluation test, is shown with the free-floating symbols on the right. Sixty pellets were delivered in each training and test trial. C: Responding in the operant devaluation test in Experiment 2. The lever press suppression ratio is: Responding in test/(Responding in test + Responding on last day of acquisition) White symbols represent the animals that received taste aversion to the fruit punch pellets that served as the operant reinforcer, black symbols represent the animals which received taste aversions to grain pellets and no taste aversion to the fruit punch pellets. Circles represent animals with Control thalamic lesions, triangles represent animals with MD thalamic lesions and squares represent those with sham lesions.
Taste aversion learning
The course of taste aversion learning was very different depending on whether the food involved was the relatively novel fruit punch pellets used in operant training or the grain pellet that was used in the Pavlovian and taste aversion phases of Experiment 1. The consumption of fruit punch pellets, which exhibited a fairly typical pattern of change over the course of taste aversion learning, will be addressed first. Notably, fruit punch pellets were the operant reinforcer in Experiment 2, and thus the acquisition of taste aversions to this food is the focus of this study.
All Paired groups reduced consumption of the operant fruit punch pellet reward over the course of taste aversion, regardless of lesion status. This taste aversion persisted to the consumption test performed after the operant test. A Lesion × Taste Aversion × Trial × Previous Taste Aversion ANOVA of the taste aversion acquisition trials (left side of Figure 4b) found significant effects of Taste Aversion (F(1,34) = 337.03, p < 0.01) and Trial (F(4,136) = 281.40, p < 0.01) and significant interactions of Taste Aversion × Trial (F(4,136) = 283.61, p < 0.01) and Previous Taste Aversion × Trial (F(4,136) = 2.49, p < 0.05). No other main effects or interactions were significant (all p > 0.05). The Previous Taste Aversion × Trial interaction appears to be due to greater consumption of fruit punch pellets on Trial 3 in the Paired groups that had not received taste aversion in the previous experiment. This greater consumption disappeared on Trials 4 and 5. A Lesion × Taste Aversion × Previous Taste Aversion ANOVA of the consumption test (right side of Figure 4b) found a significant effect of Taste Aversion (F(1,34) = 2149.49, p < 0.01). No other main effects or interactions were significant (all p > 0.10).
The pattern of consumption of grain pellets was more complex. Rats that had received taste aversions to grain pellets in Experiment 1 had low consumption at the beginning of the taste aversion phase in Experiment 2 and either increased consumption in the Paired group (which had grain pellets unpaired with LiCl in Experiment 2) or maintained this low consumption in the Unpaired group (which received grain pellets paired with LiCl in Experiment 2). By contrast, rats that had received food and LiCl Unpaired in Experiment 1 started with consumption of the grain pellets high, and were unable to form taste aversions to these pellets in Experiment 2. Nevertheless, as noted before, the previous taste aversion experience of these rats appeared to have little effect on the target fruit punch pellet taste aversion. Therefore, the rats in the Unpaired groups that failed to form taste aversions to the grain pellets were not excluded. It is important to note that the primary purpose of the grain pellet-LiCl pairings in group Unpaired was to equate groups Paired and Unpaired for the number of LiCl injections and food presentations. Thus, the extent of grain pellet aversions obtained here was not critical to the interpretation of the establishment of a fruit punch pellet aversion. Only rats in the Paired groups that showed an insufficient taste aversion to the fruit punch pellets were excluded.
Lever press devaluation test
Figure 4c shows the primary data from this experiment, the results of the devaluation test. Formation of a taste aversion to fruit punch pellets reduced lever pressing in the Control and sham animals, but not MD animals. A Lesion × Taste Aversion × Previous Taste Aversion ANOVA found significant effects of Lesion (F(2,34) = 10.56, p < 0.01), Taste Aversion (F(1,34) = 8.54, p < 0.01) and Previous Taste Aversion (F(1,34) = 5.16, p < 0.05). Interestingly enough, it was the groups that were not given taste aversions in Experiment 1 that had lower responding in this experiment (Table 2). None of the interactions were significant (all p > 0.10). Tukey’s HSD post-hoccomparisons found that the MD groups were significantly different from both the Control and sham groups (all p< 0.05), but the Control and sham groups did not differ from one another (p > 0.10). Surprisingly, there was no significant Taste Aversion × Lesion interaction (p= 0.16). Since there was an effect of Lesion, however, planned comparisons were performed. The analyses showed that taste aversion caused a reduction in lever pressing in the Control group (F(1,34) = 6.42, p < 0.05) and the sham group (F(1,34) = 5.69, p< 0.05) but not in the MD group (F(1,34) = 0.01, p= 0.93). Comparing the sham-Paired group to the other Paired groups, the analyses showed that the sham-Paired rats differed significantly from the MD-Paired groups (F(1,34) = 8.80, p< 0. 01) but not the Control-Paired rats (F(1,34) = 1.32, p > 0.10). Neither of the lesion-Unpaired groups differed significantly from the sham-Unpaired group (all p> 0.10). It is worth noting that the devaluation impairment was not due to excess damage in the MD-Paired animals. Analysis of the MD-Paired group found no effects of external damage to thalamus (F < 1), lateral habenula (F < 1) or hippocampus (F < 1) on test performance when divided into subgroups based on presence or absence of damage to external area. Each subgroup had an average suppression ratio between 0.44 and 0.48, which was similar to the MD-Paired group mean (0.46) and was not similar to the group mean for the Sham-Paired group (0.24).
Table 2.
Adjusted rates of responding in the test in Experiment 2 separated according to previous taste aversion status
| Previous TA Operant TA |
Previously Paired | Previously Unpaired | ||
|---|---|---|---|---|
| Paired | Unpaired | Paired | Unpaired | |
| MD | 0.43 ± 0.03 | 0.53 ± 0.06 | 0.50 ± 0.07 | 0.41 ± 0.08 |
| Control | 0.25 ± 0.05 | 0.39 ± 0.03 | 0.06 ± 0.02 | 0.29 ± 0.12 |
| Sham | 0.26 ± 0.18 | 0.48 ± 0.05 | 0.23 ± 0.10 | 0.33 ± 0.06 |
Discussion
The results of Experiments 1 and 2 show that MD lesions impaired performance in an operant, but not a Pavlovian, devaluation task. Lesions of other areas of the thalamus that did not completely damage the target area had no effect on performance in either devaluation task. These findings are in accord with the data of Corbit and colleagues (2003), Izquierdo and Murray (2004b) and Mitchell and colleagues (2007), which suggest that MD is needed for successful operant devaluation. It is possible that there could be separate circuits sub serving performance in operant and Pavlovian devaluation tasks, with MD apart of the circuit or circuits for operant devaluation.
An alternative explanation is that the absence of discrete signals to indicate when to respond made MD necessary for the operant devaluation task. If this explanation were correct, then the discrete trials in the Pavlovian experiment made MD unnecessary for devaluation performance. This account, however, is not supported by previous evidence. MD was important for operant devaluation when objects or visual stimuli were placed in front of macaques as discrete signals to signal the beginning of a new trial (Izquierdo & Murray, 2004b; Mitchell et al., 2007), as well as in experiments in which discrete trials were absent and rats were allowed to lever press at will (Corbit et al., 2003).
There is, however, another possibility. The same animals were utilized for Experiments 1 and 2 in order to attempt to show Pavlovian and operant devaluation in the same animals. This was done with the Pavlovian devaluation training first, followed by the operant devaluation second, in all animals. As a result there is an order effect confound. One basis for an order effect is that different reinforcers were used in the two experiments. Thus, in Experiment 1 the animals had been exposed to one reinforcer (grain pellets) and in Experiment 2 the animals had been exposed to two reinforcers (grain pellets previously and fruit punch pellets in the operant training). It might, therefore, be supposed that the rats treated Experiment 1 as a one-reinforcer experiment and Experiment 2 as a multiple-reinforcer experiment, even though they only received one type of reinforcer in each experiment, and that lesions of MD impair multiple reinforcer, but not single reinforcer, devaluation. This account would also be able to explain the lesion effects seen by Corbit and colleagues (2003), Izquierdo and colleagues (2004b) and Mitchell and colleagues (2007), which all utilized multiple reinforcers.
Experiments 3 and 4 were performed to determine whether operant versus Pavlovian differences or the number of reinforcers utilized can explain the results of Experiments 1 and 2. These experiments used the same designs as Experiments 1 and 2, except with the order of the Pavlovian and operant experiments reversed. The operant training and testing was performed first, followed by the Pavlovian training and testing with a new reinforcer. Experiment 2 performed the taste aversion in the experimental chamber with the reinforcers delivered into the food cup to attempt to make the taste aversion similar to the operant reinforcer as much as possible. However, this did not seem appropriate for the Pavlovian Experiments 4, 5 and 6 since the target response was food cup entries, rather than lever pressing, and food cup entries would precede the illness if the taste aversion was performed in the experimental chamber. Likewise, the taste aversions were performed in a beddingless cage for the operant Experiment 3 so that Experiment 1 and Experiment 3 could be directly compared without differences in taste aversion method. Therefore, the remaining experiments in this paper all perform the taste aversions in the beddingless cages.
If the MD-lesioned rats were impaired on operant but not Pavlovian devaluation, even when the operant training and testing was done first, then it is likely that the different outcomes of Experiments 1 and 2 are attributable to the differences in procedural contingencies. However, if MD rats were impaired on Pavlovian, but not operant devaluation, in Experiments 3 and 4, then a role for MD in processing multiple reinforcers, changes in contingencies or some other order effect would be implied.
Methods
Experiment 3
Subjects
The subjects were 40 rats similar to those used in Experiments1 and 2.
Apparatus
The apparatus was the same one used in Experiment 1 except with the chain inserted on operant training and test days (3 cm from the side wall and 3 cm in front of the food cup).
Surgical procedures
All surgeries were performed using the flat skull method from Experiment 1. Twenty rats received neurotoxic lesions of MD and twenty rats received sham lesions.
Behavioral training procedures
The rats were first trained in two 64-min sessions, each with 16 deliveries of the US, two 45-mg grain food pellets (Bioserv, Frenchtown, N.J.). The chain was not present on these sessions. The rats then received a session of continuous reinforcement in which the chain was available and each chain pull earned a food pellet. A sugary paste consisting of sugar, water and the rats’ ground food chow was smeared on the chain to encourage chain manipulation. The rats were removed once they had earned 50 pellets or when an hour had passed, whichever occurred first. Rats that did not earn 50 pellets were given up to two additional sessions with the paste on the chain to acquire the response. The rats then began VI chain pull training. They received one session in which chain pulling was reinforced on a VI 15 second schedule followed by three daily sessions in which chain pulling was reinforced on a VI 30 second schedule.
Taste aversion
Taste aversion training proceeded as in Experiment 1. On each of the two days before aversion training began, all animals received 1-hour exposure to the white ceramic bowls and the beddingless cages to minimize neophobia to the bowls and cages. All rats received three exposures to 100 grain food pellets contained in a bowl in a beddingless cage and three 3ml injections (i.p.) of 0.3 M lithium chloride (LiCl). The Paired animals (n=20) received the injections immediately following the food consumption and the Unpaired animals (n=20) received the LiCl and food 24 hr apart. Some rats refused to eat out of the bowls on the first trial (n=4) and were given 20 additional min with 30 of the pellets out of the bowl in the cage. If the rats only ate the pellets outside the bowl (n=3), they received the pellets in the cage with no bowl on subsequent trials. The rats that received extra time in the cages were not included in the analysis of the taste aversion trials, but were included in the analysis of the final consumption test.
The rats then received a single 33-min operant test in extinction in which the chain was available but no food pellets were delivered. The following day, the rats received 20-min access to 50 45-mg grain food pellets (those used as the US) placed in the food cup of the experimental chamber in order to assess the level of generalization of the taste aversion from the homecage to the experimental chamber.
Response measures
The primary measure of operant conditioning was the rate of chain pulling per min. This was calculated for the entire session during training and for the first 4-min block in the test, with test performance normalized for responding at the end of acquisition, as in Experiment 2. The taste aversion measures were the same as in Experiment 1. The rats that refused to eat out of the bowl had their consumption measured by counting the pellets left in the beddingless cage at the end of 20 min and subtracting from the number given (100).
Experiment 4
Subjects
The subjects were the same rats used in Experiment 3.
Apparatus
The apparatus was the same one used in Experiment 3, except without the chain inserted.
Behavioral training procedures
The rats were given two exposures to the reinforcers for the second experiment in the chambers from Experiment 3 in two daily sessions. The rats received 40-min access to 50 45-mg fruit punch food pellets (Research Diets, New Brunswick, N.J.) placed in the food cup of the experimental chamber on each day. The rats were then trained in one 64-min session with 16 unsignaled deliveries of the US, two fruit punch food pellets.
Next, the rats received eight daily 64-min sessions, each containing sixteen 10-s presentations of the house light, each followed immediately by the delivery of 2 45-mg fruit punch food pellets.
A discriminative taste aversion procedure was performed over 6 days, broken into three 2-day cycles. Each day, each rat received 100 pellets in a bowl in abeddingless cage for 20 minutes. All rats received three injections of LiCl, three sessions in which fruit punch pellets (the Pavlovian reinforcer) were delivered and three sessions in which grain pellets (the reinforcer from Experiment 3) were delivered. The Paired rats (n=17) received fruit punch pellets on the first, third and fifth day, followed immediately by a 3 ml injection (i.p.) of 0.3 M LiCl solution. The Unpaired rats (n=18) received grain pellets on these days and then an injection of LiCl as in the Paired animals. On the alternate days, each group received the other type of pellet without LiCl injection. Thus, on days 2, 4 and 6 the Paired rats received 100 grain pellets and the Unpaired rats received 100 fruit punch pellets. Rats that refused to eat the Pavlovian reinforcer on the first trial (n=2) were given 20 extra min with 70 pellets inside the bowl and 30 outside and were given their next set of trials with 70 pellets inside the bowl and 30 outside and the final set of trials with all 100 outside the bowl. One rat did not eat enough pellets to form a taste aversion. The other rat was not included in the analysis of the taste aversion trials, but was included in analysis of the consumption test.
The rats then received a single 64-min test session in the experimental chamber. This session included 16 presentations of the 10-s house light, but no food pellets were delivered. The following day, the rats received 20-min access to 50 45-mg fruit punch food pellets (those used as the US) placed in the food cup of the experimental chamber, in order to assess the level of generalization of the taste aversion from the homecage to the experimental chamber.
Response measures
The primary measure of appetitive conditioning to the house light CS during training was the percentage of time the rat spent with its head in the food cup during the last 5 s of the 10-s CS. Rapid extinction of responding obscured devaluation performance in some cases, such that devaluation was more reliably found in the sham animals if analysis was confined to the first 4 test trials. Therefore, for this experiment and the remaining experiments in this paper, responding is reported for the first 4 trials of the test session. Consumption during the taste aversion trials and tests was measured as in Experiment 3.
Histological procedures
The histology was the same as in Experiment 2.
Results
Histology for Experiments 3 and 4
All lesions except one appeared to be in the proper place and to damage the entire medial and central MD at some point between 2.3 and 2.8 mm caudal to bregma. In Experiments 3 and 4, all of the accepted lesions also destroyed lateral MD bilaterally. Figure 5 shows the biggest, smallest and representative lesions. These lesions were similar to those in Experiments 1 and 2 in most ways. However, the lesions in Experiments 3 and 4 tended to be more rostral, damaging more anterior thalamic areas and fewer caudal ones. All lesions had at least some damage to the anterodorsal, centrolateral and paraventricular nuclei, the dorsal surface of the dorsomedial portion of laterodorsal thalamic nucleus, the mediorostral portion of lateral posterior thalamic nucleus and the most medial portions of hippocampus. Most animals also had some damage to the lateral habenula and the paracentral and centromedial nuclei, parafascicular, posteromedian and paratenial thalamic nuclei. However, for reasons described in Experiments 1 and 2, it is believed that the deficits seen are due to MD lesions rather than damage to surrounding areas. Furthermore, analyses of the lesions showed that thalamic damage beyond MD did not cause greater impairments. There was no substantial mechanical damage in the sham lesioned animals.
Figure 5.
Extents of minimum (black), maximum (gray), and representative (stripes) MD lesions at various distances posterior to bregma for rats in Experiments 3 and 4.
Experiment 3
All rats showed adequate chain pull acquisition and taste aversion learning, so no rats were excluded for these reasons. There were 10 rats included in each of the 4 groups except for MD-Paired, which had 9.
Operant acquisition
All groups increased chain pulling over the course of training. No impairment of chain pull acquisition was found in rats with MD lesions. A Lesion × Taste Aversion × Conditioning Session ANOVA of chain pull acquisition found a significant effect of Conditioning Session (F(3,105) = 31.60, p< 0.01). No other effects or interactions were significant (all p > 0.10). On the last day of acquisition, responding for the groups was (chain pullper min): MD Paired = 16.21 ± 1.70, MD-Unpaired = 14.99 ± 1.52, Sham-Paired = 13.20 ± 1.40, and Sham-Unpaired = 13.45 ± 1.13. A Lesion × Taste Aversion ANOVA of responding on the last day of acquisition found no significant effects or interactions (all p> 0.10).
Taste aversion learning
Both Paired groups reduced consumption of the grain pellet reward over the course of taste aversion, regardless of lesion status. This taste aversion persisted to the consumption test performed after the operant test. A Lesion × Taste Aversion × Trial ANOVA of the taste aversion acquisition trials found significant effects of Taste Aversion (F(1,31) = 1498.72, p< 0.01) and Tri al (F(2,62) = 462.14, p< 0.01) and a significant interaction of Taste Aversion × Trial (F(2,62) = 550.27, p< 0.01). No other main effects or interactions were significant (all p> 0.10). In the consumption test, every rat in the Paired groups ate 0 pellets and every rat in the Unpaired group ate 50 pellets, regardless of Lesion condition.
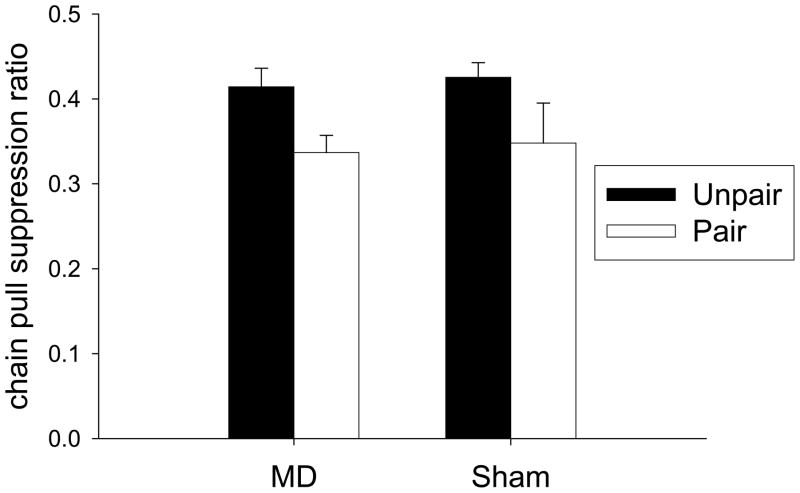
Chain pull devaluation test
Figure 6 shows the primary data from this experiment, the results of the devaluation test. The formation of a taste aversion to grain pellets in Paired rats caused a reduction in chain pulling in both sham and MD Lesion rats. A Lesion × Taste Aversion ANOVA found significant effects of Taste Aversion (F(1,35) = 6.81, p< 0.05). Neither the effect of Lesion nor the interaction was significant (all F< 1). Thus, it appears that in contrast to the results of Experiment 2, no effect of MD lesions was found in the operant task if it was performed as the first experiment and the rats had not been exposed to any other reinforcers previously. This result also differs from the results found by Corbit and colleagues (2003), Izquierdo & Murray (2004b) and Mitchell and colleagues (2007), in which MD lesions were found to have an effect on performance in multiple reinforcer operant devaluation tasks. Therefore, the results of Experiments 2 and 3 support the hypothesis that MD is necessary for operant devaluation tasks if subjects have been trained with multiple reinforcers, but not if the subjects have only been trained with a single reinforcer. The results of Experiment 1 suggests that MD is not needed for single reinforcer Pavlovian devaluation experiments in naïve rats. Therefore, it is important to determine if MD is needed for Pavlovian devaluation experiments in which rats are not naïve and have experienced multiple reinforcers.
Figure 6.
Responding in the operant devaluation test in Experiment 3. The chain pull suppression ratio is:
Responding in test/(Responding in test + Responding on last day of acquisition) Black bars represent Unpaired groups and white bars represent Paired groups.
Experiment 4
Five rats (3 MD, 2 Sham) were excluded for poor conditioning to the light (less than 25 percent time in the food cup during the light on the last 2 days of conditioning). during acquisition and 1 sham rat was excluded for a poor taste aversion to fruit punch pellets. The data reported here are from 33 rats: 8 MD-Paired, 8 MD-Unpaired, 8 Sham-Paired, and 9 Sham-Unpaired. The number of rats in each group, apportioned according to their taste aversion treatment in Experiment 3, were MD-Paired-Previously Paired: 4, MD-Paired-Previously Unpaired: 4, MD-Unpaired-Previously Paired: 5, MD-Unpaired-Previously Unpaired: 3, Sham-Paired-Previously Paired: 4, Sham-Paired-Previously Unpaired: 4, Sham-Unpaired-Previously Paired: 4, Sham-Unpaired-Previously Unpaired: 5. In Experiment 4, none of the ANOVAs for acquisition, the fruit punch pellet taste aversion training or test performance found any significant main effects or interactions of Previous Taste Aversion (all p > 0.05), so this variable was dropped from the analyses.
Pavlovian acquisition
All groups increased their food cup responses during the light over the course of training. Performance during the light in the final two conditioning sessions did not differ as a function of lesion status or subsequent assignment to Paired or Unpaired taste aversion training (right side of Table 3). However, MD lesioned rats exhibited higher baseline responding than sham rats by the end of training. A Lesion × Taste Aversion × Session ANOVA of responding during the light throughout conditioning found a significant effect of Session (F(7,175) = 92.15, p< 0.01). No other effects or interactions were significant (all p > 0.05). A Lesion × Taste Aversion × Session ANOVA of pre-CS responding throughout conditioning also found a significant effect of Session (F(7,175) = 3.52, p< 0.01). No other effects or interactions were significant (all p> 0.10). A Lesion × Taste Aversion ANOVA of responding during the CS on the last 2 days of conditioning found no significant main effects or interactions (all F < 1). A Lesion × Taste Aversion ANOVA of pre-CS responding on the last 2 days of conditioning (left side of Table 3) found a significant effect of Lesion (F(1,29) = 4.77, p< 0.05). No other main effects or interactions were significant (all F< 1).
Table 3.
Rates of responding on last 2 days of training in Experiment 4:
| Responding before light presentation | Responding at end of light presentation | |||
|---|---|---|---|---|
| Paired | Unpaired | Paired | Unpaired | |
| MD | 20.9 ± 5.0 | 17.8 ± 2.4 | 54.8 ± 4.2 | 58.8 ± 2.7 |
| Sham | 11.3 ± 3.0 | 12.0 ± 3.2 | 52.3 ± 7.1 | 52.4 ± 4.2 |
Taste aversion learning
As in Experiment 2, conditioning of an aversion to the target Pavlovian reinforcer, fruit punch pellets, proceeded normally, but taste aversion training of the grain pellets, which had participated in taste aversion training in Experiment 3, was unsuccessful. As noted in Experiment 2, the discriminative taste aversion procedure used here was designed simply to equate the rats’ exposure to each pellet and illness across the Paired and Unpaired groups, and thus acquisition of aversions in the Unpaired rats was not critical. All Paired groups reduced consumption of the Pavlovian fruit punch pellet reward over the course of taste aversion, regardless of lesion status. This taste aversion persisted to the consumption test performed after the Pavlovian test. A Lesion × Taste Aversion × Trial ANOVA of the taste aversion acquisition trials found significant effects of Taste Aversion (F(1,28) = 216.95, p < 0.01) and Trial (F(2, 56) = 107.43, p< 0.01) and a significant interaction of Taste Aversion × Trial (F(2,56) = 140.34, p< 0.01). No other main effects or interactions were significant (all p> 0.05). The taste aversion generalized to the experimental chamber test (pellets consumed): MD-Paired = 2.5 ± 0.8, MD-Unpaired = 49.4 ± 0.4, Sham-Paired = 1.5 ± 0.8 and Sham-Unpaired = 49.9 ± 0.1. A Lesion × Taste Aversion × Previous Taste Aversion ANOVA of the consumption test found a significant effect of Taste Aversion (F(1,25) = 6340.88, p < 0.01). No other main effects or interactions were significant (all p> 0.10).
Rats that had received taste aversions to grain pellets in Experiment 3 exhibited low consumption of grain pellets at the beginning of the taste aversion training, and none of the rats ate enough pellets to have the opportunity to learn that consumption of the pellets did not cause illness in this experiment. Therefore, all rats maintained their taste aversions to grain pellets regardless of whether the rats had presentation of grain pellets Paired or Unpaired in Experiment 4. Rats that had received food and LiCl unpaired in the operant experiment started with consumption of the grain pellets high. It appeared that rats that had the grain pellets unpaired in Experiment 3 were resistant to formation of taste aversions to these pellets in Experiment 4. Although there was some hint of a taste aversion to grain pellets on the last trial in the Sham-Paired rats that had grain pellets Unpaired in Experiment 3, this was not a large effect (they ate 71.2 ± 14.0 pellets). None of the other groups showed any hint of acquiring a taste aversion. As noted before, the previous taste aversion experience of these rats appeared to have little effect on the taste aversion to the fruit punch pellets reinforcer in Experiment 4, therefore the rats that failed to form taste aversions to the grain pellets were not excluded.
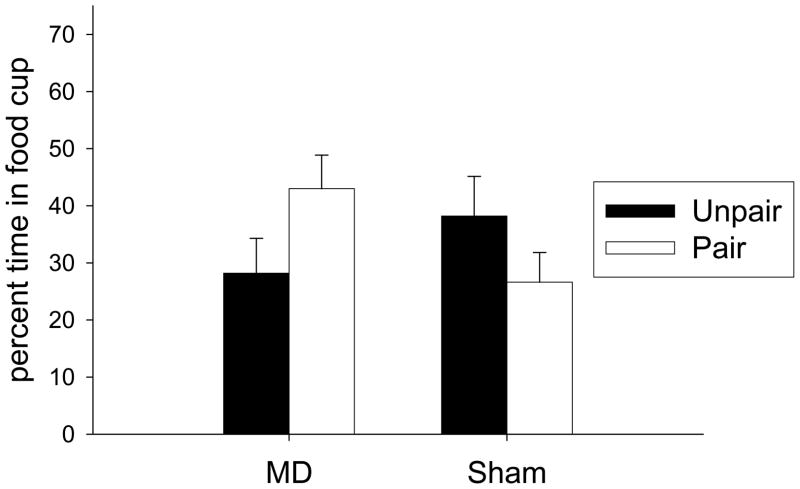
Light devaluation test
Figure 7 shows the primary data from this experiment, the results of the devaluation test. Sham rats showed a devaluation effect in responding during the light, but there was a reversal of this effect in the MD lesioned animals. There were no differences in responding in the pre-CS period (MD-Paired −9.2 ± 3.4, MD-Unpaired −9.5 ± 4.9, Sham-Paired −7.5 ± 5.5, Sham-Unpaired −3.0 ± 1.6). A Lesion × Taste Aversion ANOVA of pre-CS responding found no effects or interaction (all p> 0.10). A Lesion × Taste Aversion ANOVA on responding during the light found a significant interaction of Lesion × Taste Aversion (F(1,29) = 4.62, p< 0.05). This interaction reflects the lower responding in the sham-Paired animals than the sham-Unpaired animals, and the higher responding of the MD-Paired animals than the MD-Unpaired animals. Neither of the main effects was significant (all F< 1).
Figure 7.
Responding during the light in the Pavlovian devaluation test in Experiment 4. Black bars represent Unpaired groups and white bars represent Paired groups.
It does not appear to be the case that damage outside MD contributed to these results. Analyses of the reduction in responding from acquisition to test found no effect of presence or absence of external thalamic damage (p > 0.10) on MD-Paired animals. The same analysis was also run on the MD-Unpaired animals, since they also showed responding that differed from their corresponding Sham group. There was no effect of the size of external thalamic damage (F < 1) on responding in the MD-Unpaired animals, nor was there any effect when the rats were divided based on presence or absence of damage to lateral habenula (p > 0.10). There was no trend for either group to show greater test impairment with larger lesions. Responding was slightly higher for more discrete thalamic lesions in the MD-Paired group and was slightly lower in the MD-Unpaired subgroup with smaller thalamic lesion or spared lateral habenula, although none of these differences approached significance (all p > 0.10).
Discussion
Experiments 3 and 4 found that when rats were trained in an operant devaluation experiment before they participated in a Pavlovian devaluation experiment, MD lesions impaired the later Pavlovian devaluation performance but did not affect the earlier operant devaluation. These results can help to rule out several possible explanations for the results of Experiments 1 and 2. First, it is clear from the results of Experiments 3 and 4 that MD lesions do not always impair operant devaluation and spare Pavlovian devaluation. Second, it appears that MD lesions do not always impair devaluation in tasks without discrete cues that signal when to respond and spare devaluation in tasks with discrete cues to signal reward availability. Third, the results of Experiment 4 suggest that the different taste aversion training method used in Experiment 2 was not the sole determinant of the role of MD, since Experiment 4 presented the pellets 100 at a time in a beddingless cage as in Experiments 1 and 3 and MD impaired devaluation.
Two alternative explanations remain to be explored. First, as noted earlier, perhaps the reinforcer change involved in Experiments 2 and 4 caused the rats to code them as multiple reinforcer experiments, and MD is needed for successful performance in multiple reinforcer devaluation experiments. This possibility is consistent with the previous results of Corbit and colleagues (2003), Izquierdo and Murray (2004b) and Mitchell and colleagues (2007). Alternatively, another type of order effect, for example, the changing contingencies, responses or cues related to the rewards, may have been responsible. This is a possibility, since the effects of MD on devaluation were found in macaques that had been tested in other types of experiments previously (Izquierdo and Murray,2004b; Mitchell and colleagues, 2007), although MD effects on devaluation have been seen in naïve rats (Corbit and colleagues, 2003).
Experiment 5 directly investigated whether MD lesions impair devaluation performance in multiple reinforcer tasks. A multiple reinforcer Pavlovian devaluation experiment, in naïve rats, was performed that was as similar as possible to the designs in Experiments 1 and 4. If MD lesioned animals showed a devaluation impairment in this experiment, it would suggest that the previous MD lesion deficits were due to the experience with multiple reinforcers that the rats had in Experiments 2 and 4. If there were no devaluation deficits in MD lesioned animals in Experiment 5, it would suggest that the impaired devaluation in Experiments 2 and 4 was related to the rats’ prior participation in previous devaluation tasks in these experiments.
Experiment 5
Methods
Subjects
The subjects were 24 rats similar to those used in Experiments 1 and 2.
Apparatus
The apparatus was the same one used in Experiment 1, and also utilized the jeweled light above the food cup.
Surgical procedures
The surgical procedures were the same as the flat skull surgical method in Experiment 1. 13 rats received neurotoxic MD lesions and 11 rats received sham lesions.
Behavioral training procedures
The rats were first trained in two 64-min sessions each of which included 16 deliveries of one of the USs, two 45-mg fruit punch food pellets (Test Diets, Richmond, IN), or two 45-mg grain pellets (Bioserv, Frenchtown, N.J.), with each rat receiving one session with each reinforcer. One session occurred in the morning and the second session occurred later that afternoon. Half of the rats received grain pellets in the morning and fruit punch pellets in the afternoon and the other half of the rats received the reverse.
Next, the rats received eight days of training consisting of 16 twice-daily 64-min sessions in which the rats received sixteen 10-s presentations of the steady house light or the flashing (3 Hz) panel light, each followed immediately by the delivery of 2 45-mg fruit punch food pellets or grain pellets. For each rat, one of the two lights was consistently paired with one of the reinforcers, and the other light was consistently paired with the other reinforcer, throughout training (the identity of which light was paired with which pellet was counterbalanced across rats). Half of the sessions contained the steady light and half contained the flashing light. There were alternations in which light and food was delivered in the morning session, with each rat receiving sessions with one light-food arrangement in the morning on days 1, 4, 6 and 7 and receiving the other light-food arrangement in the morning on days 2, 3, 5, and 8.
Taste aversion
On each of the two days before aversion training began, all animals received 1-hour exposure to the white ceramic bowls and the beddingless cages to be used in the aversion training to minimize neophobia to the bowls and cages. Taste aversion training occurred with all rats presented with 100 of one of the two pellets presented in a ceramic bowl in a beddingless cage for 20 min followed by 3 ml injections (i.p.) of 0.3 M LiCl on days 1, 3 and 5. On days 2, 4 and 6, the rats received 100 of the other pellets as on the Paired days but with no injections afterward. On days 1 and 2, the rats received 70 of the pellets in the bowls and 30 outside. Rats which refused to eat out of the bowls (n = 4) were given an extra 20 min with 50 pellets in the bowl and the rest outside to induce them to eat more. Rats that refused to eat from the bowls (n = 3) received subsequent trials with the pellets in the cage without the bowls. The remaining rats were given all 100 pellets in the bowls. The rats which were given extra time with the pellets were included in the analysis of the consumption tests, but not the taste aversion trials.
The rats then received a 64-min Pavlovian test session in the experimental chamber in which there were 8 presentations of each of the visual stimuli with no food following them. The trials were presented in the following order with A representing one light and B representing the other: ABBABAABBAABABBA. Which light came first was counterbalanced. Next, the rats received two consumption tests in the experimental chamber, each with 20-min access to 50 pellets (those used as the US’s) placed in the food cup of the experimental chamber in order to assess the level of generalization of the taste aversion from the homecage to the experimental chamber. Fruit punch pellets were provided in the morning and grain pellets were provided in the afternoon.
Response measures
The response measures were the same as in Experiment 4.
Results
Four MD lesion rats were not included in the final analysis: one died during surgery, one had an insufficient lesion, and two failed to show sufficient conditioning to the lights (average of less than 35 percent time in the food cup during both lights on the last 2 days of conditioning). The following data are from 20 rats: 9 MD and 11 sham. The lesions in this experiment were very similar to those in Experiments 3 and 4. There was one lesion that fit the criteria of our smallest lesions in Experiments 3, one lesion in which the damage extended to the mammillothalamic tract and the rest of the lesions were of intermediate size. Most lesions damaged lateral habenula and the most medial part of hippocampus.
Behavioral data analysis
There were two counterbalancing variables that were involved in interactions. To keep the cell size above 1 for all of the analyses, it was necessary to limit the number of counterbalancing variables to a maximum of one in each ANOVA. A variable of Box Assignment was involved in significant interactions, such that the test data in one set of 4 boxes was different from that of another set of boxes. This variable will be kept in all of the analyses. The identity of the food that the rats had paired with LiCl was also involved in some interactions in the taste aversion trials. However, this factor had no effect on responding at the end of acquisition or during the test, so it will be left out of the primary analyses of the data. The numbers of animals broken down according to each counterbalancing variable were: MD-fruit punch aversion-Boxes A: 2, MD-fruit punch aversion-Boxes B: 3, MD-grain aversion-Boxes A: 2, MD-grain aversion-Boxes B: 2, Sham-fruit punch aversion-Boxes A: 3, Sham-fruit punch aversion-Boxes B: 2, Sham-grain aversion-Boxes A: 3, Sham-grain aversion-Boxes B: 3,
Pavlovian acquisition
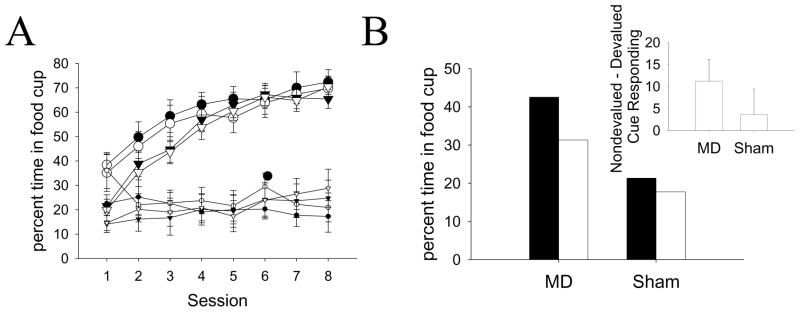
All groups increased their food cup responses during the light over the course of training, although the MD lesioned rats showed greater responding early in training (Figure 8a, large symbols). Performance during the lights in the final two conditioning sessions did not differ as a function of lesion status or the future taste aversion training for the food presented with each light. Performance in the baseline period (Figure 8a, small symbols) showed a small but persistent effect of Devalued/Nondevalued Cue, with rats responding more in the baseline periods in sessions where the reinforcer delivered was to be devalued later. In contrast to Experiments 1–4, the level of responding to the lights that predict the to-be-devalued and to-be-nondevalued foods can be determined in the same rats and analyzed with the within-subjects variable of Devalued/Nondevalued Cue. A Lesion × Box × Devalued/Nondevalued Cue × Session ANOVA of acquisition of responding in the pre-CS baseline period found a significant effect of Devalued/Nondevalued Cue (F(1, 16) = 16.66, p< 0.01) and a significant interaction of Lesion × Session (F(7, 112) = 3.72, p< 0.01). No other effects or interactions were significant (all p> 0.05). A Lesion × Box × Devalued/Nondevalued Cue × Session ANOVA of acquisition of responding during the lights found a significant effect of Session (F(7, 112) = 49.60, p< 0.01) and a significant interaction of Lesion × Session (F(7, 112) = 2.66, p< 0.05), reflecting higher responding in MD animals early in acquisition. No other effects or interactions were significant. A Lesion × Box × Devalued/Nondevalued Cue ANOVA of the baseline period during the last2 sessions (top left of Table 4, Figure 8a) found a significant effect of Devalued/Nondevalued Cue (F(1, 16) = 14.30, p < 0.01). No other effects or interactions were significant (all p> 0.10). A Lesion × Box × Devalued/Nondevalued Cue ANOVA of responding during the lights in the last 2 sessions (top right of Table 4, Figure 8a) found no significant effects or interactions (all p> 0.10).
Figure 8.
Experiment 5 behavioral performance. A: Acquisition of food cup responding prior to (small symbols) and during the light (large symbols). B: Responding during the lights in the Pavlovian devaluation test. Responding is presented separately for responding during the light that led to the to-be-devalued food and the light that led to the to-be-nondevalued food. The inset shows difference scores for the two groups with standard errors of the difference. Black symbols and bars represent responding to the light that predicts the Unpaired food. White symbols and bars represent responding to the light that predicts the Paired food. Circles represent animals with MD lesions, triangles represent animals with sham lesions.
Table 4.
Rates of responding on last 2 days of training and testing in Experiment 5
| Responding before light presentation | Responding at end of light presentation | |||
|---|---|---|---|---|
| Paired | Unpaired | Paired | Unpaired | |
| End of Acquisition | ||||
| Boxes A: | ||||
| MD | 12.8 ± 7.9 | 8.7 ± 5.0 | 63.1 ± 6.1 | 66.8 ± 10.6 |
| Sham | 25.6 ± 11.1 | 23.0 ± 11.7 | 66.0 ± 5.8 | 63.2 ± 3.7 |
|
| ||||
| Boxes B: | ||||
| MD | 28.6 ± 6.2 | 24.5 ± 6.3 | 73.0 ± 5.3 | 74.7 ± 6.8 |
| Sham | 30.0 ± 8.9 | 25.7 ± 8.2 | 69.6 ± 6.5 | 68.5 ± 7.5 |
| Test | ||||
|
| ||||
| Boxes A: | ||||
| MD | 21.6 ± 11.1 | 0.4 ± 0.4 | 5.1 ± 2.9 | 28.2 ± 4.1 |
| Sham | 9.5 ± 5.4 | 13.6 ± 6.6 | 25.5 ± 4.4 | 22.5 ± 7.0 |
|
| ||||
| Boxes B: | ||||
| MD | 13.9 ± 9.3 | 7.6 ± 6.7 | 52.3 ± 9.9 | 54.0 ± 5.6 |
| Sham | 5.4 ± 4.1 | 2.5 ± 2.0 | 8.4 ± 2.2 | 19.9 ± 9.2 |
Taste aversion learning
All groups reduced their consumption of the Paired food and maintained consumption of the Unpaired food. A Lesion × Box × Devalued/Nondevalued Food × Trial ANOVA found significant effects of Devalued/Nondevalued Food (F(1, 12) = 126.89, p< 0.01) and Trial (F(2, 24) = 64.30, p < 0.01) and significant interactions of Devalued/Nondevalued Food × Trial (F(2, 24) = 125.38, p< 0.01). No other effects or interactions were significant (all p> 0.10). The taste aversions generalized to the experimental chamber consumption tests (consumption in pellets): MD-Averted Reinforcer = 0.1 ± 0.1, MD-Nonaverted Reinforcer = 50.0 ± 0.0, Sham-Averted Reinforcer = 0.3 ± 0.1, and Sham-Nonaverted Reinforcer = 50.0 ± 0.0. A Lesion × Box × Devalued/Nondevalued Food ANOVA for the consumption test found a significant effect of Devalued/Nondevalued Food (F(1, 16) = 274,474.19, p < 0.01). No other effects or interactions were significant (all p > 0.10).
Light devaluation test
The MD lesions did not appear to impair devaluation, if anything, the MD lesion rats showed a larger devaluation effect than the shams, although this superiority was not statistically significant (Figure 8b). Likewise, there was greater responding in the MD lesion animals, which was driven primarily by the MD lesioned animals in one set of boxes. A Lesion × Box × Devalued/Nondevalued Cue ANOVA of baseline responding (bottom left of Table 4) found no significant effects or interaction (all p> 0.05). A Lesion × Box × Devalued/Nondevalued Cue ANOVA of responding during the light (bottom right of Table 7, Figure 8b) found significant effects of Devalued/Nondevalued Cue (F(1, 16) = 5.52, p< 0.05), Lesion (F(1, 16) = 8.21, p< 0.05) and Box (F(1, 16) = 5.85, p< 0.05), and significant interactions of Lesion × Box (F(1, 16) = 17.69, p< 0.01) and Lesion × Box × Devalued/Nondevalued Cue (F(1, 16) = 6.41, p< 0.05). No other effects or interactions were significant (all p> 0.10).
Discussion
MD lesions appeared to have no detrimental effect on multiple-reinforcer Pavlovian devaluation. Rats with MD lesions showed devaluation effects at least as large as those seen in sham rats. This outcome provides evidence that MD is not needed for multiple reinforcer devaluation. This evidence suggests that an intact MD may be more involved in preventing a previous task’s information from interfering with present goal expectancy guided action than in coding multiple reinforcers within a given experiment. However, more evidence will be needed in order to evaluate that possibility further.
It is unclear why there was a main effect of Lesion in this experiment. MD rats may have learned more rapidly or had a bias to respond more than the sham rats (as seen in the responding in early acquisition sessions) and this initial difference might have been obscured as the rats reached their behavioral asymptotes. Reductions in responding due to devaluation or nonreinforcement in the test session may have caused these differences to reemerge. However, no such effect was observed in the previous experiments.
Regardless, there was no impairment of multiple-reinforcer Pavlovian devaluation caused by MD lesions. It appears that the MD lesion effects in Experiments 2 and 4 were due to an order effect unrelated to the number of reinforcers. Experiment 6 evaluated whether the second task has to be different from the first task for MD to be needed. Experiments 1 and 2 involved a switch from Pavlovian to operant contingencies and Experiments 3 and 4 involved the opposite switch. In Experiment 6, the rats from Experiment 5 were trained with another Pavlovian devaluation task. A single reinforcer task was utilized to make Experiment 6 similar to Experiments 1 and 4. If MD lesions impaired performance in this devaluation task, it would suggest that simple order effects are responsible for the role of MD in devaluation tasks, with MD lesions having no effect on devaluation in naïve rats and causing deficits after a rat has been exposed to previous tasks. If no effect of MD lesions was found, it would suggest that a switch between tasks that have different types of stimuli, responses or task contingencies is required for MD to be needed for devaluation performance.
Experiment 6
Methods
Subjects
The 22 rats with acceptable sham or MD lesions from Experiment 5 (including the two rats that were excluded from Experiment 5 for a failure to show Pavlovian conditioning) were used in this experiment.
Apparatus
The apparatus was the same one used in Experiment 5. The speaker was used in this experiment to present an 80-dB white noise and no chains were available in the experimental chamber.
Behavioral training procedures
The rats were first given 40 min in the experimental chamber with 50 sucrose food pellets (Test Diets, Richmond, IN). Any pellets that were left after this 40 min were placed in their homecage with them overnight. The following day, all rats were placed in the experimental chamber with 50 sucrose pellets and were left there for 20 min or until they ate at least 45 of the 50 pellets, whichever occurred last.
Next, the rats received four daily 64-min sessions in which the rats received sixteen 10-s presentations of the white noise, each followed immediately by the delivery of 2 45-mg sucrose food pellets.
Taste aversion
Taste aversion proceeded as in Experiment 1, except it was performed over 8 days rather than 6. All rats received four exposures to 100 sucrose food pellets in a beddingless cage and four 3-ml injections (i.p.) of 0.3 M lithium chloride (LiCl). The four rats that refused to eat out of the bowls in Experiment 5 were given their pellets with no bowl, the remaining rats received their pellets in a bowl. All rats received the LiCl on days 1, 3, 5 and 7, immediately following food consumption by the Paired animals (n=11). The Unpaired animals (n=11) received the food on days 2, 4, 6, and 8, at the same time of day that the food was given to the Paired animals. Some rats refused to eat more than 20 pellets (n=3) on their first day with the food and were given 20 additional min with the remaining pellets. If the pellets had been given in a bowl, 30 pellets were placed outside the bowl and the rest were left in the bowl. These rats that received the extra 20 min were not included in the analysis of the taste aversion trials, but were included in the analysis of the consumption test.
The rats then received a 64-min Pavlovian test session in the experimental chamber in which there were 16 presentations of the noise with no food following them. On the next day, the rats received a consumption test in the experimental chamber with 20-min access to 50 sucrose pellets placed in the food cup of the experimental chamber in order to assess the level of generalization of the taste aversion from the homecage to the experimental chamber.
Response measures
The response measures were the same as in Experiment 4.
Histological procedures
The histology was the same as in Experiment 2.
Results
Two rats were excluded for insufficient conditioning (1 MD, 1 sham) (less than 35 percent time in the food cup during the noise on the last 2 days of conditioning). This left 6 MD-Paired, 4 MD-Unpaired, 5 Sham-Paired and 5 Sham-Unpaired rats in the experiment. The rats were approximately evenly split according to which food had been devalued and what their Box Assignment had been in Experiment 5. The MD-Unpaired group had only one rat that had one of the Box Assignments and only one rat that had one of the previous reinforcers devalued, so it was impossible to have a fully replicated design and the data couldn’t be analyzed using either of these previous experiment counterbalancing variables.
Pavlovian acquisition
All groups increased their responding to the noise over the 4 days of acquisition. The groups were equated on their level of responding across training. A Lesion × Taste Aversion × Session ANOVA of responding during the noise found a significant effect of Session (F(3, 48) = 14.85, p< 0.01). No other effects or interactions were significant (all p> 0.05). A comparable ANOVA examining the baseline period found no significant effects or interactions (all p> 0.10). Table 5 shows the levels of responding in the baseline and noise periods on the last 2 days of acquisition. Separate Lesion × Taste Aversion ANOVAs for the baseline and noise period responding on the last 2 days of acquisition found no significant effects or interactions (all p> 0.10).
Table 5.
Rates of responding on last 2 days of training in Experiment 6
| Responding before light presentation | Responding at end of light presentation | |||
|---|---|---|---|---|
| Paired | Unpaired | Paired | Unpaired | |
| MD | 14.9 ±5.9 | 14.4 ±3.3 | 77.3 ±3.5 | 74.1 ±4.7 |
| Sham | 17.3 ±5.3 | 15.2 ±3.9 | 83.9 ±5.7 | 81.3 ±5.5 |
Taste aversion learning
Both Paired groups reduced their consumption of the food regardless of Lesion status and both Unpaired groups maintained their consumption regardless of Lesion Status. These patterns were maintained in the consumption test regardless of Lesion condition. A Lesion × Taste Aversion × Trial ANOVA of the taste aversion trials found significant effects of Taste Aversion (F(1, 13) = 45.55, p< 0.01), Trial (F(3, 39) = 9.03, p < 0.01) and a significant interaction of Taste Aversion × Trial (F(3, 39) = 34.13, p< 0.01). None of the other effects or interactions were significant (all F< 1). The taste aversion generalized to the experimental chamber consumption test (consumption in pellets): MD-Paired = 0.2 ± 0.2, MD-Unpaired = 49.8 ± 0.3, Sham-Paired = 0.0 ± 0.0, Sham-Unpaired = 50.0 ± 0.0. A Lesion × Taste Aversion ANOVA of the consumption test found a significant effect of Taste Aversion (F(1, 16) = 122,708.61, p< 0.01). No other effects or interactions were significant (all p> 0.10).
Noise devaluation test
Both MD and sham rats reduced responding if they had a taste aversion to the Pavlovian reinforcer. There was no interaction between the lesion and taste aversion treatment. Baseline responding was MD-Paired = 3.6 ± 1.0, MD-Unpaired = 3.3 ± 1.7, Sham-Paired = 5.9 ± 3.2, and Sham-Unpaired = 13.6 ± 5.9. A Lesion × Taste Aversion ANOVA of baseline responding found no significant effects or interactions (all p > 0.05). A Lesion × Taste Aversion ANOVA of responding during the noise (Figure 9) found a significant effect of Taste Aversion (F(1, 16) = 11.25, p< 0.01). No other effects or interactions were significant (all F< 1).
Figure 9.
Responding during the noise in the Pavlovian devaluation test in Experiment 6. Black bars represent Unpaired groups and white bars represent Paired groups.
Discussion
The present results suggest that MD lesions are not always critical to performance in devaluation tasks that follow other devaluation tasks. There does not appear to be a simple dichotomy in which the first task a rat learns is sensitive to outcome devaluation regardless of whether MD is intact and all subsequent tasks are sensitive to devaluation only in rats with MD intact. It appears that there may be a requirement for a change in associative contingencies in order for MD to be required for devaluation.
The results from multiple reinforcer Pavlovian devaluation tasks in these studies are consistent with the data in the macaque literature, with devaluation performance impaired by MD lesions in monkeys which have been previously exposed to different reward contingencies (Izquierdo & Murray, 2004b; Mitchell et al., 2007), although neither macaque experiment used an explicitly Pavlovian task prior to operant devaluation. The present data are in conflict, however, with those of Corbit and colleagues (2003), who found that naïve rats are impaired in a multiple reinforcer operant devaluation task.
General Discussion
This series of experiments was performed to determine the role of MD in the devaluation task. The results eliminated as the critical factor several of the obvious procedural differences between Experiment 1 and previous experiments that had found an effect of MD lesions in devaluation tasks (Corbit et al., 2003; Izquierdo & Murray, 2004b; Mitchell et al., 2007). The factors of Pavlovian versus operant contingencies, one versus multiple reinforcers and taste aversion versus selective satiation appeared to all be possible determinants of MD involvement. Experiments 2 through 6 eliminated each of these factors as the key determinant that would determine whether a task required MD.
Operant devaluation was impaired by MD lesions in Experiment 2, but not in Experiment 3, and Pavlovian devaluation was impaired by MD lesions in Experiment 4, but not in Experiments 1, 5 and 6. Therefore, the role of MD is not determined solely by whether a devaluation task utilizes Pavlovian or operant contingencies. The role of MD is not determined solely by whether a task devalues the reinforcer using taste aversion or selective satiation since all of the experiments in this paper utilized taste aversions for devaluation and there were different results based on other task demands. Finally, the lack of devaluation deficit in Experiments 1, 3 and 6, which had a single reinforcer, and Experiment 5, which had multiple reinforcers, eliminated the reinforcer number as the sole determinant of the role of MD lesions in the devaluation task.
These results suggest an explanation involving proactive interference, in which learning, memory or performance based on some information is disrupted by learning that precedes it (Bouton, 1993). In these experiments, it appears that MD is needed in the second task in which there is a switch in the type of associations or responses from the first task. This possibility has some parallels in the conditioning literature with procedures in which original training interferes with learning or retrieval of subsequent contradictory information (Bouton &Peck, 1992; Lubow & De la Casa, 2002; Wheeler, Stout, & Miller, 2004). This suggests that the second task must involve a shift in “learning contingencies”, because there was no effect of MD lesions on devaluation in Experiment 6, which used a Pavlovian task following a previous Pavlovian task.
Perhaps the best precursor to the data in this paper is a report that MD is needed for task set shifting (Block, Dhanji, Thompson-Tardif, & Floresco, 2007). In this experiment, rats were trained to solve a T-maze using either a response (e.g. always turn left) or cued (e.g. always turn onto the black arm) strategy. Rats were unimpaired in learning an initial strategy, regardless of whether they were trained to always turn in a particular direction or turn onto a particular arm. However, the rats with MD lesions were impaired in shifting to the opposite strategy later on in the task. This result suggests that rats were not impaired in learning the first task but were impaired in switching their strategies or “task sets” when the task changed. Likewise, an increase in the hemodynamic response was seen in MD in a human fMRI experiment when people received negative feedback that indicated that the task relevant stimulus dimension had changed (Monchi, Petrides, Petre, Worsley, & Dagher, 2001). Thus, MD has been implicated in tasks in several species in changing response strategies.
In order to be sensitive to devaluation, Pavlovian tasks should use stimulus-outcome (S-O) associations and operant tasks should use response-outcome (R-O) associations. However, there is a long history of attributing conditioning to stimulus-response (S-R) associations (Hull, 1943). S-R associations could control responding in both operant and Pavlovian tasks, but neither involves an outcome representation and responding controlled by these associations would not be sensitive to reinforcer devaluation. The results reported in this paper could be explained if MD was required to switch between strategies involving S-O and R-O associations and, when rats were unable switch between S-O and R-O associations, the rats relied on a S-R strategy to acquire responding in the second task. Kimble and Perlmuter (1970) have suggested that learning to habitually respond to stimuli with S-R associations is less cognitively demanding than processing information to determine what to do. Thus, a MD lesioned rat that was not able to easily switch to a different form of outcome-encoding associations, or was having trouble using them to motivate behavior in acquisition, could useless demanding S-R associations. Thus, the rat would be able to acquire the appropriate response at the same rate as rats with MD intact, but would be unable to adjust responding when the value of the reinforcer changes.
This hypothesis is supported by previous literature that implicates MD in switching strategy set (Block et al., 2007; Monchi et al., 2001). However, it has some difficulties in explaining the effect of MD lesions on operant devaluation tasks in naïve rats (Corbit et al., 2003) and macaques that had been previously exposed to other operant tasks and fearful stimuli but no explicit Pavlovian stimuli (Izquierdo & Murray, 2004b; Mitchell et al.,2007). However, the macaques in Izquierdo and Murray (2004b) had been trained on a large number of previous tasks including serial reversals (Izquierdo & Murray, 2004a), which could represent a different type of change in contingencies. In addition, some of the macaques in Mitchell and colleagues’ (2007) experiment had been previously trained on serial reversals and all had been trained on the object-in-place scene task (Gaffan, 1994), which involves several shifts in which dimensions of the stimulus should guide behavior, so this may have taxed the animals with MD damage in the study as in strategy set shifting.
A related form of order effect could result from the change in responses between the operant and Pavlovian experiments. Beyond the switch between S-O and R-O associations, these experiments also required a switch from head entries in response to a light that predicted food to lever pressing for food (Experiments 1 and 2) or a switch from chain pulling in order to earn food to head cup entries in response to a light (Experiments 3 and 4). The response in Experiment 6, however, was a head entry response that was the same as the response in Experiment 5, which could explain the lack of a lesion effect. It is unclear why this factor would cause MD lesions to cause impairments in devaluation tasks. At this time, however, it cannot be ruled out that switching responses, rather than a requirement to switch from S-O to R-O associations, causes the formation of S-R associations. This might also be able explain how MD lesions can cause devaluation deficits in multiple reinforcer/multiple response operant experiments. Learning each response could interfere with learning the other response via R-O associations, even when they are trained in the same or interleaved sessions. Thus, a task that requires multiple responses would be learned via S-R associations. This would be able to explain all of the present data (Experiments 1–6), as well as the effects of MD lesions in the experiments of Corbit and colleagues (2003), Izquierdo & Murray (2004b) and Mitchell and colleagues (2007), which all used tasks with two levers (Corbit et al., 2003) or responses made toward one of two objects or visual stimuli (Izquierdo & Murray, 2004b; Mitchell et al., 2007). It would be possible to distinguish between the possibilities that MD is needed for switching between S-O and R-O associations or for switching between responses by using a switch between two operant tasks, with lever press responses needed for the first task and chain pull responses needed for the second task.
Summary and Conclusions
The role of MD seems to depend on the task parameters. The present data suggest that MD is needed in cases in which there is a switch between operant and Pavlovian devaluation tasks (or a switch in responses). It is possible, therefore, that in rats that have been previously trained in an operant task, MD is needed in order to reduce the impact of the existing R-O associations or initiate the switch in reinforcer specific associations so that new reinforcer specific S-O associations can be formed, rather than defaulting to an S-R strategy in Pavlovian conditioning. MD could receive reward information directly from BLA (Oyoshi et al., 1996) and facilitate the formation of new S-O associations, and/or the storage of those associations in OFC, with which MD shares reciprocal connections (Giguere & Goldman-Rakic, 1988; Groenewegen, 1988; Negyessy, Hamori, & Bentivoglio, 1998; Ray & Price, 1992), and which may control Pavlovian behavior.
MD could assist in the formation and/or storage of these new associations or it might reduce the impact of the old associations so other brain areas such as BLA and OFC, via their direct connections, could form the new associations without interference. In strategy set shifting tasks, MD lesions increase perseverative responding based on the old rule rather than errors committed once responding has reached chance level (Block et al., 2007; but see Chudasama, Bussey, & Muir, 2001). This suggests that the role of MD is to reduce the impact of previous associations so that new associations can be formed, rather than in the formation of the new associations themselves. Also, multiple reinforcer free operant devaluation is impaired by MD lesions made before (Corbit et al., 2003), but not after (Ostlund & Balleine, 2005) operant training, suggesting that MD is needed at the time of learning, rather than at the time of performance. Thus, the previous data supports a role for MD in suppressing the impact of previously-acquired associations, rather than a role in the formation, maintenance or usage of the new associations themselves.
MD may be able to suppress the impact of previously-acquired associations due to its connectivity as part of a basal ganglia-thalamocortical loop along with PLC and DMS. Lesions or inactivations of all three areas have been found to cause impairments in devaluation and in a task in which knowledge about the contingencies associated with two lever-reward contingencies is tested (Balleine & Dickinson, 1998; Corbit et al., 2003; Corbit & Balleine, 2003; Killcross & Coutureau, 2003; Yin, Ostlund, Knowlton, & Balleine, 2005), as well as in strategy set shifting (Block et al., 2007; Ragozzino, Detri ck, & Kesner, 1999; Ragozzino, Ragozzino, Mizumori, & Kesner, 2002). This suggests that the role of this loop may be in suppressing old associations so that new associations can be learned in tasks with new contingencies or responses, and that this can account for previous findings that have suggested a role for this circuit in “goal-directed action”. Thus, it is possible that a “devaluation circuit” of BLA and OFC converges in MD with a basal ganglia-thalamocortical loop for strategy shifting, although other inputs and output between this loop and BLA/OFC are possible (Kelley, Domesick, & Nauta, 1982; Ray & Price, 1992; Vertes, 2004). More work will be needed to investigate this possibility.
Acknowledgments
The work reported here comprised a portion of a dissertation submitted to Johns Hopkins University in partial fulfillment of the requirements for the doctoral degree. In addition, preliminary versions of Experiments 1 and 2 were presented at the 2006 Society for Neuroscience conference. The research was supported by NIH grants MH53667 and MH65879 to Peter C. Holland and NSF Predoctoral Fellowship DGE0202743 to CLP.
Many thanks are due my advisorPeter C. Holland for his advice and support throughout this project. I am also grateful to Michela Gallagher for her advice and support throughout this project. I also thank Gregory F. Ball, Michael P. Saddoris, Jean-Marie Maddux and Jennifer M. Bossert for many helpful comments and suggestions, Gorica D. Petrovich for assistance in judging lesion size and Weidong Hu for technical assistance.
References
- Adams CD, Dickinson A. Instrumental responding following reinforcer devaluation. Quarterly Journal of Experimental Psychology: Comparative & Physiological Psychology. 1981;33B:109–121. [Google Scholar]
- Balleine BW, Dickinson A. Goal-directed instrumental action: contingency and incentive learning and their cortical substrates. Neuropharmacology. 1998;37:407–419. doi: 10.1016/s0028-3908(98)00033-1. [DOI] [PubMed] [Google Scholar]
- Balleine BW, Killcross AS, Dickinson A. The effect of lesions of the basolateral amygdala on instrumental conditioning. Journal of Neuroscience. 2003;23:666–675. doi: 10.1523/JNEUROSCI.23-02-00666.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baxter MG, Parker A, Lindner CC, Izquierdo AD, Murray EA. Control of response selection by reinforcer value requires interaction of amygdala and orbital frontal cortex. Journal of Neuroscience. 2000;20:4311–4319. doi: 10.1523/JNEUROSCI.20-11-04311.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Block AE, Dhanji H, Thompson-Tardif SF, Floresco SB. Thalamic-Prefrontal Cortical-Ventral Striatal Circuitry Mediates Dissociable Components of Strategy Set Shifting. Cerebral Cortex. 2007;17:1625–1636. doi: 10.1093/cercor/bhl073. [DOI] [PubMed] [Google Scholar]
- Blundell P, Hall G, Killcross S. Preserved sensitivity to outcome value after lesions of the basolateral amygdala. Journal of Neuroscience. 2003;23:7702–7709. doi: 10.1523/JNEUROSCI.23-20-07702.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bouton ME. Context, time, and memory retrieval in the interference paradigms of Pavlovian learning. Psychological Bulletin. 1993;114:80–99. doi: 10.1037/0033-2909.114.1.80. [DOI] [PubMed] [Google Scholar]
- Bouton ME, Peck CA. Spontaneous recovery in cross-motivational transfer (counterconditioning) Animal Learning and Behavior. 1992;20:313–321. [Google Scholar]
- Carmichael ST, Price JL. Limbic connections of the orbital and medial prefrontal cortex in macaque monkeys. Journal of Comparative Neurology. 1995;363:615–641. doi: 10.1002/cne.903630408. [DOI] [PubMed] [Google Scholar]
- Chudasama Y, Bussey TJ, Muir JL. Effects of selective thalamic and prelimbic cortex lesions on two types of visual discrimination and reversal learning. European Journal of Neuroscience. 2001;14:1009–1020. doi: 10.1046/j.0953-816x.2001.01607.x. [DOI] [PubMed] [Google Scholar]
- Corbit LH, Balleine BW. The role of prelimbic cortex in instrumental conditioning. Behavioural Brain Research. 2003;146:145–157. doi: 10.1016/j.bbr.2003.09.023. [DOI] [PubMed] [Google Scholar]
- Corbit LH, Muir JL, Balleine BW. Lesions of mediodorsal thalamus and anterior thalamic nuclei produce dissociable effects on instrumental conditioning in rats. European Journal of Neuroscience. 2003;18:1286–1294. doi: 10.1046/j.1460-9568.2003.02833.x. [DOI] [PubMed] [Google Scholar]
- Felton TM, Linton L, Rosenblatt JS, Morell JI. First and second order maternal behavior related afferents of the lateral habenula. Neuroreport. 1999;10:883–887. doi: 10.1097/00001756-199903170-00039. [DOI] [PubMed] [Google Scholar]
- Gaffan D. Scene-specific memory for objects: a model of episodic memory impairment in monkeys with fornix transection. Journal of Cognitive Neuroscience. 1994;6:305–320. doi: 10.1162/jocn.1994.6.4.305. [DOI] [PubMed] [Google Scholar]
- Gaffan D, Murray EA. Amygdalar interaction with the mediodorsal nucleus of the thalamus and the ventromedial prefrontal cortex in stimulus-reward associative learning in the monkey. Journal of Neuroscience. 1990;10:3479–3793. doi: 10.1523/JNEUROSCI.10-11-03479.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gaffan D, Murray EA, Fabre-Thorpe M. Interaction of the amygdala with the frontal lobe in reward memory. European Journal of Neuroscience. 1993;5:968–975. doi: 10.1111/j.1460-9568.1993.tb00948.x. [DOI] [PubMed] [Google Scholar]
- Gallagher M, McMahan RW, Schoenbaum G. Orbitofrontal cortex and representation of incentive value in associative learning. Journal of Neuroscience. 1999;19:6610–6614. doi: 10.1523/JNEUROSCI.19-15-06610.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ghashghaei HT, Barbas H. Pathways for emotion: interactions of prefrontal and anterior temporal pathways in the amygdala of the rhesus monkey. Neuroscience. 2002;115:1261–1279. doi: 10.1016/s0306-4522(02)00446-3. [DOI] [PubMed] [Google Scholar]
- Giguere M, Goldman-Rakic PS. Mediodorsal nucleus: areal, laminar, and tangential distribution of afferents and efferents in the frontal lobe of rhesus monkeys. Journal of Comparative Neurology. 1988;277:195–213. doi: 10.1002/cne.902770204. [DOI] [PubMed] [Google Scholar]
- Groenewegen HJ. Organization of the afferent connections of the mediodorsal thalamic nucleus in the rat, related to the mediodorsal-prefrontal topography. Neuroscience. 1988;24:379–431. doi: 10.1016/0306-4522(88)90339-9. [DOI] [PubMed] [Google Scholar]
- Hatfield T, Han JS, Conley M, Gallagher M, Holland P. Neurotoxic lesion of the basolateral, but not central, amygdala interfere with Pavlovian second-order conditioning and reinforcer-devaluation effects. Journal of Neuroscience. 1996;16:5256–5265. doi: 10.1523/JNEUROSCI.16-16-05256.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Holland PC. The conditioned stimulus as a determinant of the form of the Pavlovian conditioned response. Journal of Experimental Psychology: Animal Behavior Processes. 1977;3:77–104. doi: 10.1037//0097-7403.3.1.77. [DOI] [PubMed] [Google Scholar]
- Holland PC, Rescorla RA. The effect of two ways of devaluing the unconditioned stimulus after first- and second-order appetitive conditioning. Journal of Experimental Psychology: Animal Behavior Processes. 1975;1:355–363. doi: 10.1037//0097-7403.1.4.355. [DOI] [PubMed] [Google Scholar]
- Hull CL. Principles of Behavior. New York: Appleton-Century-Crofts; 1943. [Google Scholar]
- Izquierdo A, Murray EA. Combined unilateral lesions of the amygdala and orbital prefrontal cortex impair affective processing in rhesus monkeys. Journal of Neurophysiology. 2004a;91:2023–2039. doi: 10.1152/jn.00968.2003. [DOI] [PubMed] [Google Scholar]
- Izquierdo A, Murray EA. Mediodorsal nucleus of the thalamus contributes to reinforcer devaluation effects in rhesus monkeys [Abstract] Society for Neuroscience Abstracts. 2004b:84.9. (Program No. 84.9) [Google Scholar]
- Izquierdo A, Suda RK, Murray EA. Bilateral orbital prefrontal cortex lesions in rhesus monkeys disrupt choices guided by both reward value and reward contingency. Journal of Neuroscience. 2004;24:7540–7548. doi: 10.1523/JNEUROSCI.1921-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kelley AE, Domesick VB, Nauta WJ. The amygdalostriatal projection in the rat – an anatomical study by anterograde and retrograde tracing methods. Neuroscience. 1982;7:615–630. doi: 10.1016/0306-4522(82)90067-7. [DOI] [PubMed] [Google Scholar]
- Killcross S, Coutureau E. Coordination of actions and habits in the medial prefrontal cortex of rats. Cerebral Cortex. 2003;13:400–408. doi: 10.1093/cercor/13.4.400. [DOI] [PubMed] [Google Scholar]
- Kimble GA, Perlmuter LC. The problem of volition. Psychological Review. 1970;77:361–384. doi: 10.1037/h0029782. [DOI] [PubMed] [Google Scholar]
- Klemm WR. Habenular and interpeduncularis nuclei: shared components in multiple-function networks. Medical Science Monitor. 2004;10:RA261–73. [PubMed] [Google Scholar]
- Lubow RE, De la Casa LG. Superlatent inhibition and spontaneous recovery: differential effects of pre-and postconditioning CS-alone presentations after long delays in different contexts. Animal Learning and Behavior. 2002;30:376–386. doi: 10.3758/bf03195962. [DOI] [PubMed] [Google Scholar]
- Malkova L, Gaffan D, Murray EA. Excitotoxic lesions of the amygdala fail to produce impairment in visual learning for auditory secondary reinforcement but interfere with reinforcer devaluation effects in rhesus monkeys. Journal of Neuroscience. 1997;17:6011–6020. doi: 10.1523/JNEUROSCI.17-15-06011.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matsumoto M, Hikosaka O. Society for Neuroscience Abstracts. 2006. Role of the primate habenula in reward processing. I. Prediction of negative reward value [Abstract] p. 670.16. [Google Scholar]
- Mitchell AS, Browning PGF, Baxter MG. Neurotoxic lesions of the medial mediodorsal nucleus of the thalamus disrupt reinforcer devaluation effects in rhesus monkeys. Journal of Neuroscience. 2007 doi: 10.1523/JNEUROSCI.1914-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mitchell AS, Dalrymple-Alford JC. Dissociable memory effects after medial thalamus lesions in the rat. European Journal of Neuroscience. 2005;22:973–985. doi: 10.1111/j.1460-9568.2005.04199.x. [DOI] [PubMed] [Google Scholar]
- Monchi O, Petrides M, Petre V, Worsley K, Dagher A. Wisconsin Card Sorting revisited: distinct neural circuits participating in different stages of the task identified by event-related functional magnetic resonance imaging. Journal of Neuroscience. 2001;21:7733–7741. doi: 10.1523/JNEUROSCI.21-19-07733.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Negyessy L, Hamori J, Bentivoglio M. Contralateral cortical projection to the mediodorsal thalamic nucleus: origin and synaptic organization in the rat. Neuroscience. 1998;84:741–753. doi: 10.1016/s0306-4522(97)00559-9. [DOI] [PubMed] [Google Scholar]
- Ostlund SB, Balleine BW. Society for Neuroscience Abstracts. 2005. Lesions of the orbitofrontal cortex disrupt pavlovian, but not instrumental, outcome-encoding. [Abstract] p. 71.20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ostlund SB, Balleine BW. Orbitofrontal cortex mediates outcome encoding in Pavlovian but not instrumental conditioning. Journal of Neuroscience. 2007;27:4819–4825. doi: 10.1523/JNEUROSCI.5443-06.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oyoshi T, Nishijo H, Asakura T, Takamura Y, Ono T. Emotional and behavioral correlates of mediodorsal thalamic neurons during associative learning in rats. Journal of Neuroscience. 1996;16:5812–5829. doi: 10.1523/JNEUROSCI.16-18-05812.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pickens CL, Holland PC. Conditioning and cognition. Neuroscience and Biobehavioral Reviews. 2004;28:651–661. doi: 10.1016/j.neubiorev.2004.09.003. [DOI] [PubMed] [Google Scholar]
- Pickens CL, Gallagher M, Holland CL. Mediodorsal thalamus lesions impair performance of rats in an operant, but not Pavlovian, devaluation task [Abstract] Society for Neuroscience Abstracts. 2006:70.6. [Google Scholar]
- Pickens CL, Saddoris MP, Setlow B, Gallagher M, Holland PC, Schoenbaum G. Different roles for orbitofrontal cortex and basolateral amygdala in a reinforcer devaluation task. Journal of Neuroscience. 2003;23:11078–11084. doi: 10.1523/JNEUROSCI.23-35-11078.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pickens CL, Saddoris MP, Gallagher M, Holland PC. Orbitofrontal lesions impair use of cue-outcome associations in a devaluation task. Behavioral Neuroscience. 2005;119:317–322. doi: 10.1037/0735-7044.119.1.317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ragozzino ME, Detrick S, Kesner RP. Involvement of the prelimbic-infralimbic areas of the rodent prefrontal cortex in behavioral flexibility for place and response learning. Journal of Neuroscience. 1999;19:4585–4594. doi: 10.1523/JNEUROSCI.19-11-04585.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ragozzino ME, Ragozzino KE, Mizumori SJ, Kesner RP. Role of the dorsomedial striatum in behavioral flexibility for response and visual cue discrimination learning. Behavioral Neuroscience. 2002;116:105–115. doi: 10.1037//0735-7044.116.1.105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ray JP, Price JL. The organization of the thalamocortical connections of the mediodorsal thalamic nucleus in the rat, related to ventral forebrain-prefrontal cortex topography. The Journal of Comparative Neurology. 1992;323:167–197. doi: 10.1002/cne.903230204. [DOI] [PubMed] [Google Scholar]
- Schoenbaum G, Setlow B, Nugent SL, Saddoris MP, Gallagher M. Lesions of orbitofrontal cortex and basolateral amygdala complex disrupt acquisition of odor-guided discriminations and reversals. Learning and Memory. 2003;10:129–140. doi: 10.1101/lm.55203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sutherland RJ. The dorsal diencephalic conduction system: a review of the anatomy and functions of the habenular complex. Neuroscience and Biobehavioral Reviews. 1982;6:1–13. doi: 10.1016/0149-7634(82)90003-3. [DOI] [PubMed] [Google Scholar]
- Tronel S, Sara SJ. Mapping of olfactory memory circuits: region-specific c-fos activation after odor-reward associative learning or after its retrieval. Learning and Memory. 2002;9:105–11. doi: 10.1101/lm.47802. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Uwano T, Nishijo H, Ono T, Tamura R. Neuronal responsiveness to various sensory stimuli, and associative learning in the rat amygdala. Neuroscience. 1995;68:339–361. doi: 10.1016/0306-4522(95)00125-3. [DOI] [PubMed] [Google Scholar]
- Vertes RP. Differential projections of the infralimbic and prelimbic cortex in the rat. Synapse. 2004;51:32–58. doi: 10.1002/syn.10279. [DOI] [PubMed] [Google Scholar]
- Wheeler DS, Stout SC, Miller RR. Interaction of retention interval with CS-preexposure and extinction treatments: symmetry with respect to primacy. Learning and Behavior. 2004;32:335–347. doi: 10.3758/bf03196032. [DOI] [PubMed] [Google Scholar]
- Yin HH, Ostlund SB, Knowlton BJ, Balleine BW. The role of the dorsomedial striatum in instrumental conditioning. European Journal of Neuroscience. 2005;22:513–523. doi: 10.1111/j.1460-9568.2005.04218.x. [DOI] [PubMed] [Google Scholar]