Abstract
Background
Both the United States and Canada offer government-financed health insurance for the elderly, but few studies have compared care at the end of life for cancer patients between the two systems.
Methods
We identified care for non–small cell lung cancer (NSCLC) patients who died of cancer at age 65 years and older during 1999–2003. Patients were identified from US Surveillance, Epidemiology, and End Results (SEER)–Medicare data (N = 13 533) and the Ontario Cancer Registry (N = 8100). Health claims during the last 5 months of life identified chemotherapy and emergency room use, hospitalizations, and supportive care. We estimated rates per person-months (PM) for short-term survivors (died <6 months after diagnosis) and longer-term survivors (died ≥6 months after diagnosis), adjusting for demographic differences. To test whether monthly rates in Ontario were statistically significantly different from the United States, standardized differences were computed, and a 99% confidence interval (CI) was constructed to account for the multiple tests performed. All statistical tests were two-sided.
Results
Rates of chemotherapy use were statistically significantly higher for SEER–Medicare patients than Ontario patients in every month before death (short-term survivors at 5 months before death: SEER–Medicare, 33.2 patients per 100 PM vs Ontario, 9.5 per 100 PM, rate difference = 23.7 per 100 PM, 99% CI = 18.3 to 29.1 per 100 PM, P < .001; longer-term survivors at 5 months before death: SEER–Medicare, 24.4 patients per 100 PM vs Ontario, 14.5 per 100 PM, rate difference = 9.9 per 100 PM, 99% CI = 7.7 to 12.1 per 100 PM, P <. 001). During the last 30 days of life, fewer SEER–Medicare than Ontario patients were hospitalized (short-term survivors, 49.9 vs 78.6 patients per 100 PM, rate difference = 28.6 per 100 PM, 95% CI = 22.9 to 34.4 per 100 PM, P <. 001; longer-term survivors, 44.1 vs 67.1 patients per 100 PM, rate difference = 23.0 per 100 PM, 95% CI = 18.5 to 27.5 per 100 PM, P < .001).
Conclusions
NSCLC patients in both Ontario and the United States used extensive end-of-life care. Limited availability of hospice care in Ontario and differing attitudes between the United States and Ontario regarding end-of-life care may explain the differences in practice patterns.
CONTEXTS AND CAVEATS
Prior knowledge
Government-financed health care covers elderly patients in both Canada and the United States, but the organization of care at the end of life differs between the two countries.
Study design
Care at the end of life was compared for non–small cell lung cancer patients aged 65 years and older identified from US Surveillance, Epidemiology, and End Results (SEER)–Medicare data and the Ontario Cancer Registry. Data on chemotherapy, emergency room use, hospitalizations, and supportive care were collected from health claims for both short-term (<6 months) and longer-term survivors (≥6 months).
Contribution
Although hospital and emergency room services were used more extensively in Ontario, chemotherapy rates were statistically significantly higher among SEER–Medicare patients than among Ontario patients, for both short- and longer-term survivors. There was extensive use of home health and hospice care by SEER–Medicare patients, whereas in Ontario, palliative care was more likely to be administered in the hospital.
Implications
The lack of a formal hospice program in Ontario during the study period may account for some of the differences in hospital and emergency room use between the two systems. However, treatment differences may reflect differing attitudes between the United States and Ontario regarding end-of-life care.
Limitations
Administrative data on treatment did not include information about noncovered services or patients’ treatment choices. Palliative care offered to Medicare patients could not be identified because there was no billing code for palliative services. Medicare does not cover care in long-term care facilities, and nursing home stays are not covered in Ontario, so the use of palliative care in hospital and nursing home settings between lung cancer patients in the United States and Ontario could not be compared.
From the Editors
Canada and the United States differ substantially in the structure of their health-care systems. In Canada, health care is paid for by the provincial governments and is universally provided to all permanent residents and citizens. In the United States, health insurance is primarily employer-based for people of working age. The US Medicare program provides government-sponsored health care to 97% of people aged 65 years and older and a limited group of people younger than age 65 years who have long-term disability or selected medical conditions (1). The availability of health insurance affects the structure of health-care delivery and decisions that physicians and patients make regarding treatment.
The organization of care at the end of life differs between the United States and Canada. Medicare coverage includes a hospice program for patients who are certified by their physician as being terminally ill with a life expectancy of 6 months or less. Under Medicare, hospice services are primarily home-based health care with the goal of symptom control and psychosocial support to dying patients and their families (2). Medicare patients who choose to enter hospice must consent to forgo life-prolonging therapy such as chemotherapy and hospitalization. For Medicare patients not in a hospice program, home health-care services are available. In Canada, the types of end-of-life care vary by province (3). Ontario, the most populous province, has no hospice program comparable to that available through the US Medicare program. End-of-life care in Ontario is provided through a range of services delivered by physicians and other health personnel. Palliative care is delivered in inpatient acute care units and at home through visits from physicians, nurses, and home-health aides.
Published studies comparing health-care delivery between Canada and the United States have reported that in the United States, physicians have a more aggressive attitude toward treatment (4) and provide more intensive testing and treatment for myocardial infarctions (5) and cancers of the bladder, glottis, prostate, and ovary (6–10). Comparisons of end-of-life care between Canada and the United States have not been reported, although end-of-life care has received increased attention because of uncertainty related to the appropriate level of treatment. For patients diagnosed with advanced cancer, as death approaches, optimal health care would shift from life-prolonging therapy to supportive care and symptom control. There are also concerns about the cost of care for cancer patients at the end of life because cancer treatment with curative intent can be costly and may not substantially extend survival. Recent studies of the costs of cancer care in the United States and Ontario reported that from the time of a cancer diagnosis until death, costs were highest at the end of life (11,12).
For this study, we compared care at the end of life between the United States and Ontario for elderly non–small cell lung cancer (NSCLC) patients. We selected patients aged 65 years and older to have two similar cohorts with government-sponsored health insurance. We chose NSCLC because it is the leading cause of cancer deaths in the United States (13) and Ontario (14) and the majority of patients diagnosed with NSCLC present with advanced disease (15) and die of their cancer.
Methods
Data Sources
Data for this study were obtained from health-care systems in the United States and Ontario for the period 1999–2003. We evaluated health care in the United States using the National Cancer Institute Surveillance, Epidemiology and End Results (SEER)–Medicare data, a linkage of patient records from the SEER cancer registries with their Medicare enrollment and claims files (1). The population-based SEER registries collect demographic and clinical information for each patient. The clinical information included data for each occurrence of a primary incident cancer, month and year of diagnosis, follow-up vital status, and date and cause of death for patients who died. The SEER areas included in our study represent about 14% of the US population. For persons reported to a SEER registry who were aged 65 years or older, 94% have been linked to Medicare’s master enrollment file. The Medicare data, collected by the Center for Medicare and Medicaid Services (CMS), included claims for each beneficiary with fee-for-service coverage, with information about all inpatient hospitalizations, outpatient and emergency room (ER) visits, physician services, hospice, and home health care. All files included specific dates of service and codes for specific diagnoses and procedures using either International Classification of Diseases, Ninth Revision, Clinical Modification (ICD-9-CM) codes (16) or Health Care Procedure Codes (HCPCS) (17,18). In addition, these data have been linked with the Area Resource File (19) by county of residence to determine the level of urbanicity/rurality of the area where the patient resided. Proxy variables for the patient’s median household income were estimated from Census data.
The second data source included linked health utilization data for Ontario, a Canadian province with 13 million people. Ontario data are housed at the Institute for Clinical Evaluative Sciences (ICES), Toronto, Canada. ICES maintains linkable health records for all Ontario residents, including cancer registry data, hospitalization data, physician services, and home care records. NSCLC patients were identified from the Ontario Cancer Registry. The Ontario Cancer Registry includes date of initial cancer diagnosis, site of cancer, age at diagnosis, and date and cause of death for patients who died. The frequency of hospital admissions, associated procedure and diagnosis codes, inpatient palliative care, and in-hospital deaths were determined from the Discharge Abstract Database (20), maintained by the Canadian Institute for Health Information (CIHI). Information about physician services, including house calls, nursing home visits, palliative care consults, intensive care unit (ICU) use, and chemotherapy administration was obtained from the Physician’s Claims History Database of the Ontario Health Insurance Plan (OHIP) (21). ER use was available from CIHI National Ambulatory Care Reporting System Data (22) only from 2002 onwards. Before 2002, frequency of ER use was obtained from the Physicians Claims History Database of OHIP. We determined if patients had received home care using the Ontario Home Care Administrative System database (23).
Study Populations
The study included persons diagnosed with pathologically confirmed NSCLC who died of any cancer between January 1, 1999, and December 31, 2003, the years of data that were available when this study was initiated. We included Medicare beneficiaries who died at age 65 years and 6 months, or older, to include at least 6 months of data before death. We initially identified 62 356 lung cancer patients in the SEER–Medicare files and 33 510 lung cancer patients from the Ontario data. Patients were excluded if they had more than one cancer (SEER–Medicare n = 6825 and Ontario n = 4183) or died before reaching the age threshold (SEER–Medicare n = 3571 and Ontario n = 8056). We eliminated lung cancer patients who did not have NSCLC (SEER–Medicare n = 11 351 and Ontario n = 7660). The study was focused on patients with advanced disease at the time of diagnosis because their physicians are likely to consider them to have short life expectancy. Because the Ontario Cancer Registry has limited staging information for the period of this study, we used the absence of cancer-directed surgery as a proxy for advanced stage at diagnosis. Surgery is usually performed in patients with early-stage disease but not in patients with more advanced disease. Among NSCLC patients in the SEER–Medicare data, 86% of staged cancer patients without cancer-directed surgery had stage IIIB or IV disease. Therefore, we excluded patients who underwent cancer-directed surgery within 1 year after lung cancer diagnosis (SEER–Medicare n = 10 089 and Ontario n = 2092). We also excluded patients who died within 30 days of diagnosis (SEER–Medicare n = 7901 and Ontario n = 1135) or who had a condition other than the cancers listed as the cause of death (SEER–Medicare n = 2744 and Ontario n = 908). Patients in the Ontario sample were excluded if they died outside of Ontario (n = 63) or had an invalid OHIP number (n = 1313). Patients were excluded from the SEER–Medicare sample if they did not have continuous fee-for-service coverage and Part A and B enrollment in Medicare for the entire period of the study (n = 6342). The final cohort included 13 533 patients from the SEER–Medicare data and 8100 patients from the Ontario data.
Although all patients in our sample were considered to have been diagnosed with advanced disease, the time between diagnosis and death varied, with approximately half of SEER–Medicare and Ontario patients dying within 6 months following diagnosis. For patients who died within 6 months of diagnosis, care captured in our analysis may be a mixture of primary treatment for a newly diagnosed cancer and care at the end of life. For patients who lived more than 6 months, care at the end of life would be less likely to include peri-diagnostic services. Therefore, we reported patterns of care separately for patients who were short-term survivors (survived <180 days following diagnosis) and those who were longer-term survivors (patients who survived ≥180 days).
Identification of Specific Types of Health Care
Health care received by NSCLC patients was identified by reviewing all records for each patient on or before the date of death retrospectively up to 5 months before death or the day of diagnosis, whichever came first. We examined trends in use of acute and supportive care in the last 5 months of life and also examined in more detail the use of hospital and ER services in the last 30 days of life, including ICU admissions and in-hospital deaths.
Acute care included chemotherapy, ER visits, and inpatient hospitalizations. Chemotherapy was identified from any inpatient or outpatient claim for administration of an intravenous chemotherapeutic agent. Chemotherapy use was counted for every month for which a claim was found. Hospitalizations were assigned to the month in which the first day of hospital admission occurred.
The measures of supportive care were not directly comparable between the United States and Ontario, reflecting differences in the organization of health care. There is no formal program of palliative care covered by Medicare. In the United States, supportive care was measured through home health care and hospice use. Most hospice care provided to Medicare beneficiaries is home-based and includes home health services and physician visits. SEER–Medicare patients were considered to have received hospice or home health care if there was any Medicare claim for either service during each month. Home health claims and hospice claims were mutually exclusive, and patients could have either service or both within each month. Supportive care in Ontario included all home care services, house calls by physicians, and palliative care consultations in the outpatient setting. Home care was identified from claims in the Ontario Home Care Administrative System database. Codes on physician claims were used to identify palliative care services from physicians and house calls, excluding those for pronouncement of death. We created mutually exclusive categories to identify Ontario patients who received one or more palliative care services (palliative care consultation, physician house call, or home care services) during each month. For Ontario patients, admissions for inpatient palliative care were included in the estimates of hospitalization rates because these admissions capture patients in need of care for which there was no home-based alternative.
Rates of Health Care by Month During the Last 5 Months of Life
We calculated monthly rates of patients being treated, defined as 30-day intervals up to 5 months before death. We stratified patients into short-term survivors and longer-term survivors based on the length of time from diagnosis to death. To accurately calculate monthly rates of service use for the patients surviving less than 180 days, we estimated services per person-day. Within each 30-day interval before death, we computed the number of days that each patient was alive and diagnosed with lung cancer. Person-days were summed during the 30-day interval and for all patients and used as the denominator, person-months (PM). The numerator was the number of patients who used a service in the same period. The result of the numerator to denominator ratio was the rate of patients with the service per PM. Rates are reported as numbers of patients treated per 100 PM. When calculating the rate of ER visits, hospitalizations, and home health care, we modified the denominators to exclude those patients who were already in-hospital, because they would not be at risk for any of these events. We also modified denominators for Ontario to exclude the days that patients were hospitalized from estimates of home care or palliative care received at home or in the outpatient setting. These approaches to calculating person-days denominators also helped to ensure that potential differences in hospitalization rates between SEER–Medicare and Ontario did not affect comparisons of rates for ER and home health-care use.
For the monthly rates of patients receiving health care, we only reported health care received during the last 5 months of life. By definition, all of the short-term survivors who had any observation time in the sixth month before death were diagnosed during that 30-day period. Few short-term survivors contributed the full 30 days of observation to this 30-day period. Thus the PM denominator in the sixth month before death was incomplete for short-term survivors. As a result, during the sixth month before death, rates of patients with service use per 100 PM were not an appropriate measure when so few short-term survivors were observed for a full 30 days.
Health Care Received in the Last 30 Days of Life
Assessment of ER use during the last 30 days of life included the percent of all patients with any ER visit, the rate of ER use (number of patients with ER visits per 100 PM, excluding days when patients were in-hospital), the mean number of ER visits per patient, and the percent of ER visits that resulted in an inpatient hospitalization. Hospital use during the last 30 days of life was measured by estimating the percent of all patients who were hospitalized, the rate of hospitalization (number of patients with hospital admissions per 100 PM, excluding days when patients were already in hospital), the mean number of hospitalizations per patient, and the percent of patients who died in hospital. We also assessed utilization among only the patients who were hospitalized, which included estimates of the mean length of stay per hospitalization, the percent of hospitalized patients who were treated in an ICU, the mean length of ICU stay for patients admitted to an ICU, and the percent of hospitalized patients dying in hospital.
Statistical Analysis
We compared demographic characteristics of NSCLC patients between the SEER–Medicare and Ontario data with χ2 statistics. Median household income was measured for the Census tract of residence. Because of variation in income levels in the SEER areas within the United States and Ontario, income quintiles were calculated within each SEER registry and within each Census Metropolitan Area in Ontario. We reported the number of patients in each income quintile within each area. Income amounts were not compared between the United States and Ontario; therefore no adjustment for differing standards of living was required.
To account for any demographic differences between the SEER–Medicare and Ontario populations, all rates of service use, percentages, and means were adjusted to the US cohort based on age (65–69, 70–79, or ≥80 years), sex, income quintile (two highest quintiles or other), and urbanicity (big metropolitan, metropolitan, or other). The adjustment was calculated as a weighted average of the crude rates, percentages, or means for each subgroup, where the weights were the proportions of persons in the corresponding subgroups of the US cohort. Given the large sample sizes, standard errors and 95% confidence intervals (CI) for proportions and means related to patient demographic characteristics (Table 1), and use of ER and hospital services in the last 30 days of life (Table 2) were calculated assuming normal distributions. For Ontario patients, monthly estimates of rates of service use for each of the last 5 months of life were adjusted to the US population. To test whether monthly rates in Ontario were statistically significantly different from the United States, standardized differences were computed, and a 99% confidence interval was constructed to account for the multiple tests performed (Figures 1 and 2). Inclusion of zero in the 99% confidence interval for the rate differences was taken as evidence that there was no difference between the Ontario and US rates. When the 99% confidence interval did not include zero, we rejected the hypothesis of the same rate for Ontario and the US.
Table 1.
Characteristics of patients diagnosed with late stage non–small cell lung cancer who died of cancer at age 65 or older, 1999–2003*
| Characteristic | Survived <6 mo |
Survived ≥6 mo following diagnosis |
||||
| SEER–Medicare, No. (%)† | Ontario No. (%)‡ | P | SEER–Medicare No. (%)§ | Ontario No. (%)‖ | P | |
| Age at death, y | <.001 | <.001 | ||||
| 65–69 | 1118 (17.8) | 919 (25.2) | 1423 (19.6) | 1136 (25.5) | ||
| 70–79 | 3181 (50.7) | 2007 (55.0) | 3677 (50.7) | 2399 (53.9) | ||
| ≥80 | 1978 (31.5) | 726 (19.9) | 2156 (29.7) | 913 (20.5) | ||
| Sex | <.001 | <.001 | ||||
| Men | 3501 (55.8) | 2207 (60.4) | 3871 (53.4) | 2631 (59.2) | ||
| Women | 2776 (44.2) | 1445 (39.6) | 3385 (46.7) | 1817 (40.9) | ||
| Urban/rural residence at time of diagnosis | <.001 | <.001 | ||||
| Big metro | 3770 (60.1) | 1634 (44.7) | 4330 (59.7) | 1865 (41.9) | ||
| Metro | 1558 (24.8) | 1376 (37.7) | 1825 (25.2) | 1567 (35.2) | ||
| Others | 949 (15.1) | 642 (17.6) | 1101 (15.2) | 1016 (22.8) | ||
| Median census tract household income relative to national/provincial income quintiles | <.001 | <.001 | ||||
| High income (top 2 quintiles) | 2246 (35.8) | 1247 (34.2) | 2466 (34.0) | 1390 (31.3) | ||
| Low income | 4031 (64.2) | 2405 (65.9) | 4790 (66.0) | 3058 (68.8) | ||
| Time from diagnosis to death, mo | <.001 | <.001 | ||||
| <6 | 6277 (100.0) | 3652 (100.0) | 0 (0.0) | 0 (0.0) | ||
| 6 to <12 | 0 (0.0) | 0 (0.0) | 2778 (38.3) | 1783 (40.1) | ||
| 12 to <18 | 0 (0.0) | 0 (0.0) | 1791 (24.7) | 890 (20.0) | ||
| 18 to <24 | 0 (0.0) | 0 (0.0) | 898 (12.4) | 470 (10.6) | ||
| 24 to <36 | 0 (0.0) | 0 (0.0) | 934 (12.9) | 442 (9.9) | ||
| ≥36 | 0 (0.0) | 0 (0.0) | 855 (11.8) | 863 (19.4) | ||
P values were calculated by the two-sided χ2 test. SEER = Surveillance, Epidemiology, and End Results database.
N = 6277.
N = 3652.
N = 7256.
N = 4448.
Table 2.
Emergency room and inpatient services during the last 30 days of life among non–small cell lung cancer patients who died of cancer at age 65 and older, 1999–2003*
| Services | Survived <6 mo following diagnosis |
Survived ≥6 mo following diagnosis |
||||
| SEER–Medicare | Ontario | P | SEER–Medicare | Ontario | P | |
| Estimate (95% CI) | Estimate (95% CI) | Estimate (95% CI) | Estimate (95% CI) | |||
| ER use | ||||||
| % of patients with any ER visit† | 38.3 (36.2 to 40.4) | 50.9 (48.5 to 53.3) | <.001 | 35.9 (34.3 to 37.6) | 47.9 (45.7 to 50.1) | <.001 |
| Rate of ER admissions‡ | 43.9 (42.6 to 45.2) | 67.3 (66.7 to 70.4) | <.001 | 40.1 (38.9 to 41.3) | 58.9 (57.9 to 61.2) | <.001 |
| Mean ER visits per patient among those with any ER visit | 1.34 (1.29 to 1.39) | 1.34 (1.30 to 1.38) | .959 | 1.31 (1.28 to 1.34) | 1.29 (1.26 to 1.32) | .033 |
| % of ER visits that resulted in hospitalization | 69.4 (66.2 to 72.6) | 64.0 (61.2 to 66.7) | .011 | 69.3 (66.6 to 71.9) | 66.4 (64.6 to 68.1) | .066 |
| Inpatient utilization and events, all patients | ||||||
| % of patients with hospital admissions† | 43.5 (40.7 to 46.3) | 59.3 (56.6 to 62.1) | <.001 | 39.5 (37.4 to 41.6) | 54.6 (52.5 to 56.6) | <.001 |
| Rate of hospital admissions§ | 49.9 (48.5 to 51.2) | 78.6 (78.0 to 81.7) | <.001 | 44.1 (42.9 to 45.3) | 67.1 (67.0 to 70.3) | <.001 |
| % of patients dying in hospital | 20.4 (18.7 to 22.1) | 48.5 (45.9. to 51.0) | <.001 | 19.0 (17.4 to 20.5) | 44.3 (42.1 to 46.6) | <.001 |
| Inpatient utilization and events, hospitalized patients only | ||||||
| Mean hospitalizations per person | 1.20 (1.17 to 1.23) | 1.17 (1.14 to 1.19) | .081 | 1.18 (1.16 to 1.19) | 1.14 (1.12 to 1.16) | .013 |
| Mean length of stay/hospitalization, days | 6.50 (6.23 to 6.75) | 8.26 (7.83 to 8.68) | <.001 | 6.22 (5.96 to 6.47) | 8.18 (7.9 to 8.5) | <.001 |
| % hospitalized patients treated in the ICU | 15.6 (13.6 to 17.7) | 7.1 (5.8 to 8.4) | <.001 | 14.7 (13.0 to 16.3) | 5.8 (4.7 to 6.9) | <.001 |
| Mean length of ICU stay in days per patient admitted to ICU | 4.58 (4.09 to 5.06) | 3.85 (3.08 to 4.62) | .100 | 4.64 (4.12 to 5.16) | 4.10 (3.46 to 4.74) | .182 |
| % hospitalized patients dying in hospital | 46.4 (43.9 to 48.8) | 81.3 (79.2 to 83.4) | <.001 | 47.7 (45.2 to 50.1) | 80.6 (78.6 to 82.6) | <.001 |
Adjusted for age (65–69, 70–79, ≥80 years), sex, income (2 highest quintiles, other), and urbanicity (big metropolitan, metropolitan, other). The P values for the rates in Table 2 were calculated by creating 95% confidence intervals for the difference between the two standardized rates. The P value reported is such that the (1−P)% confidence interval just includes zero. The other P values in Table 2 were calculated using a two-sample t test of the weighted data. All statistical tests were two-sided. CI = confidence interval; ER = emergency room; ICU = intensive care unit; PM = person-months; SEER = Surveillance, Epidemiology, and End Results database.
Excludes patients who were hospitalized for the entire 30-day period.
No. of patients with ER visits/100 PM. Excludes patients on days that they were in hospital.
No. of patients with hospital admission/100 PM. Excludes patients on days that they were in hospital.
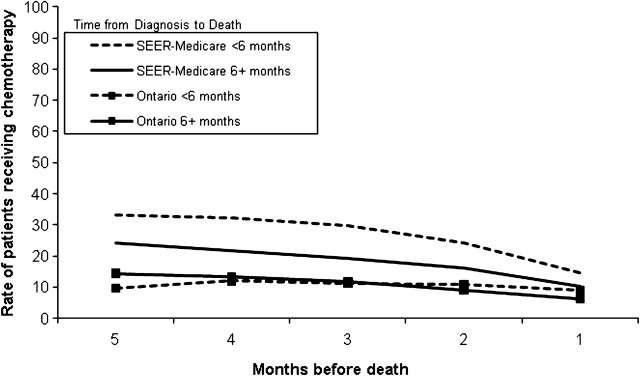
Figure 1.
Adjusted rates of use of chemotherapy (number of patients per 100 person-months) in the last 5 months of life of non–small cell lung cancer patients who died of cancer at age 65 years and older, 1999–2003. Monthly rates are adjusted for age group (65–69, 70–79,and ≥80 years), sex, income (two highest quintiles, other), and urbanicity (big metropolitan, metropolitan, other). Standardized monthly rates were compared using 99% confidence intervals. P values for differences in all rates for SEER–Medicare patients vs Ontario patients surviving less than 6 months and 6 months or more were less than .001. The P values for the monthly rates were calculated by creating confidence intervals for the difference between the two standardized rates. The P value reported is such that the (1−P)% confidence interval just includes zero. All statistical tests were two-sided. SEER = Surveillance, Epidemiology, and End Results database.
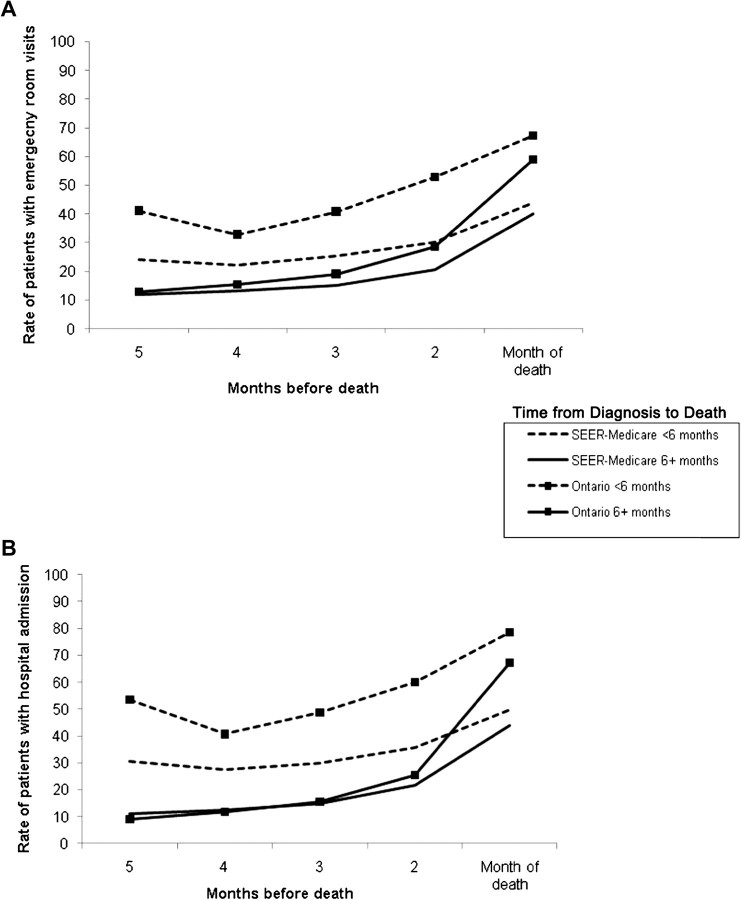
Figure 2.
Adjusted rates of emergency room (ER) and hospital use in the last 5 months of life for non–small cell lung cancer patients who died of cancer at age 65 years and older, 1999–2003. A) Rates of ER admissions (No. of patients admitted per 100 person-months [PM]). B) Rates of inpatient hospital admissions (No. of patients admitted per 100 PM). Monthly rates were adjusted for age (65–69, 70–79, and ≥80 years), sex, income (two highest quintiles, other), and urbanicity (big metropolitan, metropolitan, other). Standardized monthly rates were compared using 99% confidence intervals. For ER visits, P values for differences in all rates for SEER–Medicare patients vs Ontario patients surviving less than 6 months were less than .001. For patients surviving 6 or more months, P values were .152 at 5 months before death, .003 for 4 months before death, and less than.001 for 3–1 month(s) before death. For hospital admissions, P values for differences in all rates for SEER–Medicare patients vs Ontario patients surviving less than 6 months were less than .001. For patients surviving 6 or more months, P values were .222 at 4 months before death, .366 for 3 months before death, and less than.001 for months 5, 2, and 1 before death. The P values for the monthly rates were calculated by creating confidence intervals for the difference between the two standardized rates. The P value reported is such that the (1−P) % confidence interval just includes zero. All statistical tests were two-sided. SEER = Surveillance, Epidemiology, and End Results database.
Results
NSCLC patients in the SEER–Medicare data were statistically significantly older, more likely to be women, and to reside in large metropolitan areas compared with Ontario patients (Table 1). This was true for both short-term and longer-term survivors. Approximately 40% of the longer-term survivors died between 6 and 12 months after diagnosis.
Rates of Health Care During the Last 5 Months of Life
In each of the 5 months before death, chemotherapy rates were statistically significantly higher among SEER–Medicare patients than the adjusted rates among Ontario patients, for both short- and longer-term survivors (Figure 1). At 5 months before death, among short-term survivors the chemotherapy rate was 33.2 patients per 100 PM for SEER–Medicare vs 9.5 patients per 100 PM for Ontario (rate difference = 23.7 per 100 PM, 99% CI = 18.3 to 29.1 per 100 PM, P < .001) For longer-term survivors, in the fifth month before death, the rate of patients using chemotherapy was 24.4 patients per 100 PM for SEER–Medicare, contrasted with 14.5 patients per 100 PM for Ontario (rate difference = 9.9 per 100 PM, 99% CI = 7.7 to 12.1 per 100 PM, P < .001). By the last month of life, chemotherapy rates for all groups had declined, although rates were statistically significantly higher for SEER–Medicare patients than Ontario patients (short-term survivors rate per 100 PM: 14.8 for SEER–Medicare vs 9.0 Ontario, rate difference = 5.8 per 100 PM, 99% CI = 4.0 to 7.7 per 100 PM, P < .001 longer-term survivors rate per 100 PM: 10.2 for SEER–Medicare vs 6.4 Ontario, rate difference = 3.8 per 100 PM, 99% CI = 2.4 to 5.2 per 100 PM, P < .001).
Use of ER services for SEER–Medicare and Ontario rose appreciably as the time of death approached (Figure 2, A). For SEER–Medicare longer-term survivors, the number of patients per 100 PM with an ER visit increased from 11.8 at 5 months before death to 40.1 in the last month of life, whereas for Ontario longer-term survivors, the adjusted rates were 12.8 and 58.9 patients per 100 PM (5 month difference in ER use between SEER–Medicare and Ontario rate = 1.0 per 100 PM, 99% CI = –.8 to 2.8 per 100 PM, P = .15; 1 month difference between SEER–Medicare and Ontario rate = 18.8 per 100 PM, 99% CI = 14.5 to 23.1 per 100 PM, P < .001). Short-term survivors had a different pattern of ER use. At 5 months before death the adjusted rate was 24.0 patients per 100 PM with an ER visit for SEER–Medicare and 41.5 patients per 100 PM for Ontario (rate difference = 17.4 per 100 PM, 99% CI = 7.4 to 27.5 per 100 PM, P < .001) For both groups, these rates declined in the fourth month before death before increasing steadily. By the last month of life, the number of patients per 100 PM with an ER visit was 43.9 for SEER–Medicare patients and 67.2 for Ontario patients (rate difference = 23.3 per 100 PM, 99% CI = 17.9 to 28.6 per 100 PM, P < .001).
Hospitalization rates increased for all patients shortly before death (Figure 2, B). For short-term survivors, Ontario rates of hospitalization were statistically significantly higher than SEER–Medicare rates in all months. In the fifth month before death, the adjusted rate of patients with a hospital admission was 30.5 patients per 100 PM for SEER–Medicare and 53.6 patients for Ontario (rate difference = 23.1 per 100 PM, 99% CI = 13.1 to 34.5 per 100 PM, P < .001). By the last month of life, the adjusted hospital admission rates for short-term survivors (Table 2, Figure 2, B) were 49.9 patients per 100 PM for SEER–Medicare and 78.6 per 100 PM for Ontario (rate difference = 28.6 per 100 PM, 99% CI = 22.9 to 34.4 per 100 PM, P < .001). For longer-term survivors, the number of patients with a hospital admission increased markedly in the last month of life (Table 2, Figure 2, B) with a rate of 44.1 patients per 100 PM for SEER–Medicare and 67.1 per 100 PM for Ontario (rate difference = 23.0 per 100 PM, 99% CI = 18.5 to 27.5, P < .001).
There was extensive use of home health and hospice care in the SEER–Medicare patients (Figure 3). Use of hospice increased steadily as death neared and was equivalent between short- and longer-term survivors in all months, except for the last month of life where the rate was 57.4 patients per 100 PM for short-term survivors and 66.8 per 100 PM for longer-term survivors. Fifty-eight percent (n = 7883) of patients received hospice services during the last 6 months of life; 17% (n = 665) of patients admitted to hospice in the last month of life (n = 3997) had a length of stay less than 3 days. Twelve percent (n = 949) of hospice patients were in a nursing home for at least 1 day during the last 30 days of life (data not shown).
Figure 3.
Rates of use (No. of patients per 100 person-months) of hospice and home health during the last 6 months of life among Surveillance, Epidemiology, and End Results–Medicare patients who died of non–small cell lung cancer by length of survival, 1999–2003.
Among Ontario patients, use of outpatient supportive care during the last 5 months of life was comparable between short- and longer-term survivors (Figure 4). The rate of persons receiving community-based supportive care rose from 40 patients per 100 PM at 5 months before death to more than 88 patients per 100 PM in the last month of life. By the last month of life, most patients were receiving more than one type of supportive care, including physician house calls, outpatient palliative care, and home care.
Figure 4.
Rates of use (No. of patients per 100 person-months) of community-based supportive care services during the last 5 months of life among Ontario patients dying of lung cancer, 1999–2003, by length of survival.
Acute Care in the Last 30 Days of Life
During the last 30 days of life, the percentage of SEER–Medicare patients with an ER visit was about 12% lower, on average, than for Ontario patients, for both short- and longer-term survivors (Table 2). Hospital admissions during the last 30 days of life were frequent for SEER–Medicare and Ontario patients, although the percentage of patients with a hospital admission in the last 30 days was statistically significantly lower among SEER–Medicare patients than for patients in Ontario (short-term survivors: SEER–Medicare, 43.5%, 95% CI = 40.7% to 46.3%; Ontario, 59.3%, 95% CI = 56.6% to 62.1%, Pdifference < .001; longer-term survivors: SEER–Medicare, 39.5%, 95% CI = 37.4% to 41.6%; Ontario, 54.6%, 95% CI = 52.5% to 56.6%, Pdifference < .001, Table 2). Among hospitalized patients, more SEER–Medicare than Ontario patients were treated in an ICU (short-term survivors: SEER–Medicare, 15.6%, 95% CI = 13.6% to 17.7%; Ontario, 7.1%, 95% CI = 5.8% to 8.4%, Pdifference < .001; longer-term survivors: SEER–Medicare, 14.7%, 95% CI = 13.0% to 16.3%; Ontario, 5.8%, 95% CI = 4.7% to 6.9%, Pdifference < .001, Table 2). More than 60% (n = 2613) of hospitalized patients in Ontario received inpatient palliative care during the last month of life (data not shown). SEER–Medicare patients were much less likely to die in-hospital compared with Ontario patients (short-term survivors: SEER–Medicare, 20.4% (n = 1262), 95% CI = 18.7% to 22.1%; Ontario, 48.5% (n=1586), 95% CI = 45.9% to 51.0%, Pdifference < .001; longer-term survivors: SEER–Medicare, 19.0% (n = 1367), 95% CI = 17.4% to 20.5%; Ontario, 44.3% (n = 1866), 95% CI = 42.1% to 46.6%, Pdifference < .001, Table 2). Among patients hospitalized in the last 30 days, 46.4% (n = 1262) of SEER–Medicare short-term survivors died in hospital, compared with 81.3% (n = 1586.) of Ontario short-term survivors. Similar percentages were found for longer-term survivors, 47.7% (n = 1367) for SEER–Medicare patients vs 80.6% (n = 1866) for Ontario patients.
Discussion
In this study, we compared care at the end of life for elderly NSCLC patients who died of cancer in the United States and Ontario. Both groups used health-care services extensively in the last 5 months of life, particularly during the last month of life. These findings are consistent with earlier studies that have reported high levels of health-care use by cancer patients at the end of life (11,24,25).
Chemotherapy was given to statistically significantly more SEER–Medicare patients than Ontario patients, with the differences greatest among short-term survivors. The lower rate of chemotherapy use among Ontario patients may reflect differences between Ontario and the United States in patients’ or physicians’ perceptions regarding the benefit of chemotherapy for elderly patients with advanced lung cancer. The US physicians in our study may have been more willing to administer chemotherapy in response to professional societies’ recommendations in the mid-1990s that chemotherapy may benefit a select number of NSCLC patients with advanced disease (26,27). In addition, oncologists paid by Medicare can profit from the administration of specific chemotherapy agents whereas oncologists in Ontario do not have a financial incentive to prescribe chemotherapy.
During the last month of life, SEER–Medicare patients had statistically significantly lower rates of hospitalization than Ontario patients. This difference may be attributable to no formal hospice program being available to Ontario patients. More than 58% of SEER–Medicare patients were in hospice during their last month of life. Medicare pays hospices a daily-capitated payment to cover all care related to the patient’s terminal illness and will only provide additional reimbursement for inpatient hospitalizations if the patient’s acuity of care exceeds what can be provided in another setting or for short-term respite care (28). Because hospices are financially responsible for all other hospital care, they have a strong incentive to keep patients out of ERs and limit hospital use. A previous study of SEER–Medicare colorectal and lung cancer patients reported that only 4% of patients had a hospitalization following enrollment in hospice (2). We observed that SEER–Medicare patients who were admitted to hospital had ICU admission rates more than double those of Ontario patients. These patients were likely not in hospice because hospice programs would make efforts to avoid expensive ICU services for their patients.
Although there is no formal hospice program in Ontario, we found extensive use of palliative care, especially in the last 30 days when the number of patients per 100 PM receiving community supportive services exceeded 85. Despite the high use of community palliative services, 55% of Ontario patients had a hospital admission during the last month of life. It appears that most Ontario patients who were hospitalized in the last 30 days of life were admitted for supportive care. More than half of all Ontario patients died in hospital, 2.5-fold more than observed among SEER–Medicare patients. A study that evaluated the use of hospital care at the end of life for lung cancer patients in Ontario reported that 90% of Ontario lung cancer decedents who were hospitalized in the 2 weeks before death had a “do not resuscitate” order on their chart (15).
The disparity in the number of in-hospital deaths between Ontario and the SEER–Medicare patients may reflect differences in health systems or patient preference regarding care at the end of life. Data from surveys of terminal cancer patients in Canada demonstrate that up to 80% of respondents would prefer to die at home (29), a perspective shared by their families. However, moving care for dying patients to the home setting can be demanding for family members in terms of physical needs and loss of time from work. Surveys of patients and community-based palliative care providers in Ontario have found that dying patients have experienced unmet needs and challenges in coordinating palliative care (30,31). In 2005, the Ontario Ministry of Health established networks to improve the coordination of end-of-life care and shift palliative care from the hospital to home. The impact of the initiative is still being assessed, although it appears that the number of in-hospital deaths and ER visits in Ontario has not changed following implementation of these new programs (32).
The type and patterns of health-care use also varied for short- and longer-term survivors. In both SEER–Medicare and Ontario cohorts, rates of patients with ER visits and hospitalizations were statistically significantly higher for short-term survivors than for longer-term survivors, perhaps reflecting a blend of health care for a newly diagnosed lung cancer and services related to the end of life for these recently diagnosed patients. The higher rates of ER visits and hospitalizations observed across all months for Ontario short-term survivors relative to Medicare short-term survivors may reflect differences in the two groups in terms of the time it takes to access the needed care. This explanation is supported by the fact that rates of ER visits and hospitalizations were similar for Ontario and SEER–Medicare longer-term survivors in months 3–5 before death. Longer-term survivors may have had time to organize support services, arrange for experienced caregivers, and be referred to palliative care. A recent study that examined the reasons that terminal cancer patients in Ontario went to the ER concluded that many of the visits may have been avoided with comprehensive and coordinated palliative care and better symptom control at home (33).
The differences we observed in patterns of health-care use between elderly lung cancer patients in the United States and Ontario are likely to extend to the total costs of care. Because of many differences in coverage policies, payment of physicians, and submission of claims, comparing costs of care is exceedingly complex and was not included in our analysis. Despite these challenges, efforts to systematically measure comparable costs of end-of-life care in these two health-care systems are needed to provide a better understanding of the complete burden of the different structures of care.
Our study had several important strengths. The population-based data used in the analysis included large numbers of NSCLC patients with government-funded health care. We were able to evaluate the trajectory of care that NSCLC patients received across multiple care settings (home, ER, and hospital) during the last 5 months of life. The comprehensiveness of these data provided a detailed picture of much of the care given at the end of life. The NSCLC patients in our analysis had very high rates of hospitalization and poor survival following diagnosis. By using person-day estimates, we limited the analysis to only those patients who were “at risk” of receiving that type of care. This approach resulted in a more accurate estimate of the rate of service use than if we had included all patients. Finally, we limited our analysis to specific types of health care that were comparable between the SEER–Medicare and Ontario data.
Our study also had several limitations. We used administrative data to capture patients’ treatment. Administrative data do not include information about noncovered services or patients’ treatment choices. We could not identify palliative care offered to Medicare patients because there was no billing code to identify palliative services. There were challenges to identifying end-of-life care provided to patients in nursing homes. We could capture care for Medicare patients who are in skilled nursing facilities, but these facilities are limited to patients who require skilled services such as intravenous medications or physical therapy. Medicare does not cover care in long-term care facilities, and as a result, patients receiving supportive services in long-term care facilities were not included. In Ontario, nursing home stays are not covered by OHIP and there are no bills from these facilities. As a result of these limitations in the data, it is not possible to compare the use of palliative care in hospital and nursing home settings between lung cancer patients in the United States and Ontario.
We used a retrospective approach, identifying patients who died and evaluating their care in the last 5 months before death. The retrospective approach to evaluating care at the end of life has been debated because it may capture patients who do not appear to their physicians to be likely to die in the immediate future, and these patients may have received care that was different from patients who are clearly in the last months of life (34). Others have suggested that end-of-life studies based on prospective cohorts are also limited because it is difficult to identify with certainty which patients are dying (35). To reduce the likelihood that patients in our study included those not dying of cancer, we restricted our samples to patients with advanced disease at diagnosis who had cancer reported as the cause of death.
In conclusion, we compared health care during the last 5 months of life between Ontario and the United States for elderly patients with advanced NSCLC. In both countries, patients used a large amount of health-care resources. Our study revealed marked differences in the patterns of service delivery at the end of life in Ontario and the United States, likely reflecting differences between their health systems in the organization of end-of-life care. Our findings related to the use of chemotherapy and the ICU support commonly held perceptions that patients in the United States tend to receive more intensive health-care services than in Canada. However, we found that use of hospital and ER services were statistically significantly higher in Ontario. The findings from this study will inform health planners and policy makers in each country regarding current patterns of end-of-life care, and where there may be opportunities for changing practice patterns or programs. This information will help enlighten the current public debate regarding the intensity and benefit of health care for treatments of patients at the end of life.
Funding
There was no external funding for the SEER–Medicare work. Funding for the Ontario arm of the study was provided by research grants from Cancer Care Ontario, the Ontario Institute for Cancer Research, and a career support award through the F. Norman Hughes Chair in Pharmacoeconomics, Faculty of Pharmacy, University of Toronto. This study was also supported by the Institute for Clinical Evaluative Sciences. Institute for Clinical Evaluative Sciences is funded by an annual grant from the Ontario Ministry of Health and Long-Term Care.
Footnotes
The opinions, results and conclusions reported in this article are those of the authors and are independent from the funding sources. No endorsement by Institute for Clinical Evaluative Sciences or the Ontario Ontario Ministry of Health and Long-Term Care is intended or should be inferred. The funders did not have any involvement in the design of the study; the collection, analysis, and interpretation of the data; the writing of the article; or the decision to submit the article for publication. The SEER–Medicare portion of the study was performed by federal researchers at the US National Cancer Institute.
References
- 1.Warren JL, Klabunde CN, Schrag D, Bach PB, Riley GF. Overview of the SEER-Medicare data: content, research applications, and generalizability to the United States elderly population. Med Care. 2002 Aug;40(8 suppl):IV-3–IV-18. doi: 10.1097/01.MLR.0000020942.47004.03. [DOI] [PubMed] [Google Scholar]
- 2.Cintron A, Hamel MB, Davis RB, Burns RB, Phillips RS, McCarthy EP. Hospitalization of hospice patients with cancer. J Palliat Med. 2003;6(5):757–768. doi: 10.1089/109662103322515266. [DOI] [PubMed] [Google Scholar]
- 3.Quality End of Life Coalition. Hospice Palliative Home Care in Canada: A Progress Report. May 2008. www.qelccc.ca/uploads/files/hphc-progress_Report/Hospice_Palliative_Home_Care_Progress_Report_final.pdf. Accessed April 15, 2010. [Google Scholar]
- 4.Koeck CM, Hemenway D, Donelan K, Lipsitz S. Using a hypothetical case to measure differences in treatment aggressiveness among physicians in Canada, Germany and the United States. Wien Klin Wochenschr. 1998;110(22):783–788. [PubMed] [Google Scholar]
- 5.Batchelor WB, Peterson ED, Mark DB, et al. A comparison of U.S. and Canadian cardiac catheterization practices in detecting severe coronary artery disease after myocardial infarction: efficiency, yield and long-term implications. J Am Coll Cardiol. 1999;34(1):12–19. doi: 10.1016/s0735-1097(99)00174-6. [DOI] [PubMed] [Google Scholar]
- 6.Chung D, Hersey K, Fleshner N. Differences between urologists in United States and Canada in approach to bladder cancer. Urology. 2005;65(5):919–925. doi: 10.1016/j.urology.2004.11.028. [DOI] [PubMed] [Google Scholar]
- 7.Groome PA, O’Sullivan B, Irish JC, et al. Management and outcome differences in supraglottic cancer between Ontario, Canada, and the Surveillance, Epidemiology, and End Results areas of the United States. J Clin Oncol. 2003;21(3):496–505. doi: 10.1200/JCO.2003.10.106. [DOI] [PubMed] [Google Scholar]
- 8.Groome PA, O’Sullivan B, Irish JC, et al. Glottic cancer in Ontario, Canada and the SEER areas of the United States. Do different management philosophies produce different outcome profiles? J Clin Epidemiol. 2001;54(3):301–315. doi: 10.1016/s0895-4356(00)00295-x. [DOI] [PubMed] [Google Scholar]
- 9.Fleshner N, Rakovitch E, Klotz L. Differences between urologists in the United States and Canada in the approach to prostate cancer. J Urol. 2000;163(5):1461–1466. [PubMed] [Google Scholar]
- 10.LoCoco S, Covens A, Carney M, et al. Does aggressive therapy improve survival in suboptimal stage IIIc/IV ovarian cancer? A Canadian-American comparative study. Gynecol Oncol. 1995;59(2):194–199. doi: 10.1006/gyno.1995.0007. [DOI] [PubMed] [Google Scholar]
- 11.Yabroff KR, Lamont EB, Mariotto A, et al. Cost of care for elderly cancer patients in the United States. J Natl Cancer Inst. 2008;100(9):630–641. doi: 10.1093/jnci/djn103. [DOI] [PubMed] [Google Scholar]
- 12.Krahn MD, Zagorski B, Laporte A, et al. Healthcare costs associated with prostate cancer: estimates from a population-based study. BJU Int. 2010;105(3):338–346. doi: 10.1111/j.1464-410X.2009.08758.x. [DOI] [PubMed] [Google Scholar]
- 13.Horner MJ, RiesL AG, Krapcho M. SEER Cancer Statistics Review, 1975–2006, Based on November 2008 SEER Data Submission, Posted to the SEER web site, 2009. Bethesda, MD: National Cancer Institute; http://seer.cancer.gov/csr/1975_2006/. Accessed April 15, 2010. [Google Scholar]
- 14.Cancer Care Ontario. Lung Cancer Continues to be the Biggest Cancer Killer in Ontario (Jan. 2008). http://www.cancercare.on.ca/cms/one.aspx?pageId=9763. Accessed April 15, 2010. [Google Scholar]
- 15.Barbera L, Paszat L, Qiu F. End-of-life care in lung cancer patients in Ontario: aggressiveness of care in the population and a description of hospital admissions. J Pain Symptom Manage. 2008;35(3):267–274. doi: 10.1016/j.jpainsymman.2007.04.019. [DOI] [PubMed] [Google Scholar]
- 16.U.S. Public Health Service. International Classification of Diseases, Ninth Revision. Washington, DC: U.S. Government Printing Office; 2003. [Google Scholar]
- 17.Centers for Medicare & Medicaid Services. Healthcare Common Procedure Codes System (HCPCS). http://www.cms.hhs.gov/MedHCPCSGenInfo/. Accessed April 15, 2010. [Google Scholar]
- 18.Chicago, IL: American Medical Association; 2004. Current Procedural Terminology 2005. [Google Scholar]
- 19.Area Resource File (ARF) U.S.Department of Health and Human Services. Rockville, MD: Health Resources and Services Administration, Bureau of Health Professions; 2005. [Google Scholar]
- 20.Canadian Institute for Health Information. Discharge Abstract Database. http://secure.cihi.ca/cihiweb/dispPage.jsp?cw_page=services_dad_e. Accessed April 15, 2010. [Google Scholar]
- 21.Ministry of Health and Long-Term Care. Ontario Health Insurance Program. http://www.health.gov.on.ca/en/public/programs/ohip/. Accessed April 15, 2010. [Google Scholar]
- 22.Canadian Institute for Health Information. National Ambulatory Care Reporting System. http://secure.cihi.ca/cihiweb/dispPage.jsp?cw_page=services_nacrs_e. Accessed April 15, 2010. [Google Scholar]
- 23.Ministry of Health and Long-Term Care. Homecare Database CCAC Guidelines. http://www.mohltcfim.com/cms/upload/a_9996/HCD_GuidelinesV3_4.pdf. Accessed April 15, 2010. [Google Scholar]
- 24.Riley GF, Potosky AL, Lubitz JD, Kessler LG. Medicare payments from diagnosis to death for elderly cancer patients by stage at diagnosis. Med Care. 1995;33(8):828–841. doi: 10.1097/00005650-199508000-00007. [DOI] [PubMed] [Google Scholar]
- 25.Brown ML, Riley GF, Schussler N, Etzioni R. Estimating health care costs related to cancer treatment from SEER-Medicare data. Med Care. 2002;40(8) suppl:IV-104–IV-117. doi: 10.1097/00005650-200208001-00014. [DOI] [PubMed] [Google Scholar]
- 26.American Society of Clinical Oncology. Clinical practice guidelines for the treatment of unresectable non-small-cell lung cancer. J Clin Oncol. 1997;15(8):2996–3018. doi: 10.1200/JCO.1997.15.8.2996. [DOI] [PubMed] [Google Scholar]
- 27.Non-small Cell Lung Cancer Collaborative Group. Chemotherapy in non-small cell lung cancer: a meta-analysis using updated data on individual patients from 52 randomised clinical trials. BMJ. 1995;311(7010):899–909. [PMC free article] [PubMed] [Google Scholar]
- 28.Medicare Payment Advisory Commission. Hospice Services Payment System: Payment Basics. May 2008. http://www.medpac.gov/documents/MedPAC_Payment_Basics_08_hospice.pdf. Accessed April 15, 2010. [Google Scholar]
- 29.Burge F, Lawson B, Johnston G. Trends in the place of death of cancer patients, 1992-1997. CMAJ. 2003;168(3):265–270. [PMC free article] [PubMed] [Google Scholar]
- 30.Heyland DK, Groll D, Rocker G, et al. End-of-life care in acute care hospitals in Canada: a quality finish? J Palliat Care. 2005;21(3):142–150. [PubMed] [Google Scholar]
- 31.Brazil K, Bainbridge D, Sussman J, Whelan T, O’Brien MA, Pyette N. Coordination of palliative cancer care in the community: “unfinished business”. Support Care Cancer. 2009;17(7):819–828. doi: 10.1007/s00520-009-0582-x. [DOI] [PubMed] [Google Scholar]
- 32.Seow H, Barbera L, Howell D, Dy SM. Did Ontario’s end-of-life care strategy reduce acute care service use? The need to use quality indicators for improvement. Healthc Q. 2010;1(13):93–100. doi: 10.12927/hcq.2013.21620. [DOI] [PubMed] [Google Scholar]
- 33.Barbera L, Taylor C, Dudgeon D. Why do patients with cancer visit the emergency department near the end of life? CMAJ. 2010;182(6):563–568. doi: 10.1503/cmaj.091187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Bach PB, Schrag D, Begg CB. Resurrecting treatment histories of dead patients: a study design that should be laid to rest. JAMA. 2004;292(22):2765–2770. doi: 10.1001/jama.292.22.2765. [DOI] [PubMed] [Google Scholar]
- 35.Earle CC, Ayanian JZ. Looking back from death: the value of retrospective studies of end-of-life care. J Clin Oncol. 2006;24(6):838–840. doi: 10.1200/JCO.2005.03.9388. [DOI] [PubMed] [Google Scholar]