Abstract
Bisphenol A (BPA) is a synthetically made chemical used in the production of polycarbonate plastics and epoxy resins. Recent studies have shown over ninety percent of humans investigated have detectable BPA concentrations. Yet, the biggest concern for BPA is exposure during early development because BPA has been shown to bind to the estrogen receptors (ER) and cause developmental and reproductive toxicity. We have investigated the potential of perinatal BPA to alter susceptibility for chemically-induced mammary cancer in rats. We demonstrate that prepubertal exposure to low concentrations of orally administered BPA given to lactating dams resulted in a significantly decreased tumor latency and increased tumor multiplicity in the dimethylbenz[a]anthracene (DMBA) model of rodent mammary carcinogenesis. Our data suggested that the mechanism of action behind this carcinogenic response was mediated through increased cell proliferation, decreased apoptosis, and centered on an up-regulation of steroid receptor coactivators (SRCs) 1–3, erbB3, and increased Akt signaling in the mammary gland.
Also, we demonstrate that prenatal exposure to BPA shifts the time of susceptibility from 50 days to 100 days for chemically-induced mammary carcinogenesis. Proteomic data suggest that prenatal BPA exposure alters the expression of several proteins involved in regulating protein metabolism, signal transduction, developmental processes, and cell cycle and proliferation. Increases in ER-alpha, SRCs 1–3, Bcl-2, epidermal growth factor–receptor (EGFR), phospho-IGF-1R, phospho-c-Raf, phospho-ERKs 1/2, phospho-ErbB2 and phospho-Akt are accompanied by increase in cell proliferation. We conclude that exposure to low concentrations of BPA during the prenatal and early postnatal periods of life can predispose for chemically-induced mammary cancer.
Keywords: bisphenol A, mammary cancer, proteomics, cell proliferation, apoptosis
Steroid hormones play a prominent role in development. This extends from procreation to senescence. Timed expression and interactions of steroids, receptors and co-regulators help to determine differentiation, development, maturation and maintenance of organs and organelles. Even subtle structural modifications in steroid molecules can result in major biological differences that can be evidenced by alterations to gene and protein expressions.
Steroid receptors have specificity, but they can also bind similar structures, including some environmental chemicals. Aberrant activation of steroid receptors by environmental chemicals during early development can lead to immediate modification of biological signaling or even to long-term effects. Some of these changes are due to direct effects while the delayed and/or permanent alterations are hypothesized to be organizational effects (1, 2). These changes in protein/enzyme expression can lead to disease manifestations.
While chemical structure is important, dose is another factor to consider. Now, we realize that not all biological responses abide by a linear dose response, whereby a low dose elicits a lesser effect than higher doses (3). Indeed, different doses of 17β-estradiol (E2) result in diverse outcomes for mammary tumors induced by dimethylbenz[a]anthracene (DMBA) in rats. Low doses have been reported to cause a marked stimulus in tumor growth, whereas much larger doses cause inhibition of tumor growth (4). Furthermore, environmental factors play a large role in cancer risk. These can be natural components of our foods or environmental chemical contaminates (5). A significant increase in cancer incidences was evidence shortly after the industrial revolution which is considered to have started in the 17th century. And it became even more frequent in the beginning of the 20th century. This may be associated with the production of environmental chemicals which can be direct acting carcinogens or even to those that are hormone mimics.
One such chemical is bisphenol A (4,4’-dihydroxy-2,2-diphenylpropane, BPA). BPA has two phenol rings that play a role in making polycarbonate plastic and epoxy resins. Polycarbonate plastics are found in the preparation of infant formula and water bottles, children’s toys, sport equipment, medical and dental devices, CDs, DVDs and household electronics. Epoxy resins of BPA are used in coatings in food and beverage cans. It is found in carbonless copy sale receipts and thermal papers. Global production of BPA was estimated to be more than 2.2 million tons in 2009. The primary route of exposure to humans occurs through the oral route, due to the leaching of BPA from incomplete polymerization of epoxy resins or degradation of the weak ester bonds that link the BPA monomers. Studies have shown that these bonds are frequently hydrolyzed during normal use, with factors such as time, elevated temperature, and pH extremes accelerating this process (6–9). Detectable concentrations of BPA have been found to leach from canned fruits, vegetables, and meat products, condensed and infant milk, canned sodas and juice, cardboard milk and juice containers, plastic food wrap, hospital intravenous tubing, and polycarbonate food and beverage containers under extreme as well as normal conditions of use (8–11).
BPA has been routinely detected in human biofluids and tissues. In a 2005 study, Calafat et al. found 95% of adults surveyed had detectable concentrations of total (free + conjugated) urinary BPA (12). A pilot study measuring the urinary concentration of a panel of environmental chemicals reported a similar proportion (90%) of girls with detectable concentrations of urinary BPA metabolites (13). Total BPA concentrations ranged from 0.3 µg BPA/L to 54.3 µg BPA/L and averaged 2.0 µg BPA/L (3.0 µg BPA/g creatinine). A recent large scale study involving over 2,000 participants supported the findings of both studies (14). They reported the average concentration of 2.6 µg BPA/L. Conservative estimates based on the values provided in these and other studies suggest that most adults are exposed to approximately 0.05–1 µg BPA/kg body weight (BW) per day, while the high-end of a biologically achievable exposure does not likely exceed 9–10 µg BPA/kg BW per day (15–18).
Once ingested, BPA is absorbed through the gastrointestinal tract and transported, via the venous circulation, to the liver. First pass metabolism results in the induction of Phase II enzymes and the subsequent conjugation of the majority of BPA absorbed (19, 20). In rodents, non-human primates, and humans, uridine diphosphate glucuronosyl transferase is reported to produce the major metabolite of BPA, BPA-glucuronide (19–21). Most studies agree that the conjugates are biologically inert and thus all downstream effects are generally attributed to the action of the remaining free, unconjugated BPA (22). Several studies have been published chronicling the pharmacodynamics of BPA in rodent models. The elimination of BPA in these studies occurs quickly, with the majority of the administered dose being eliminated within 24 hours (19, 20). Volkel et al. found that adult humans were capable of clearing a single, orally administered bolus (5 mg BPA/person or 54–94 µg BPA/kg BW) within 24 hours (20). The half-life was recorded as 5.3 hours.
This emphasis on the route of administration becomes important amid recent criticism by industry and regulatory agencies that many of the studies designed to evaluate the health hazards posed by BPA use artificial routes of exposure that bypass first pass metabolism and thus subject the animal to much higher concentrations of parental BPA (19). This criticism is not without merit. It has become increasingly apparently that orally administered BPA is subjected to first pass metabolism and undergoes rapid elimination from the body after a single dose (19–21). Other methods of administration, such as intraperitoneal or subcutaneous injections, have been shown to produce increased bioavailability, decreased time to maximum concentration, increased maximum concentration, and a difference in the metabolites produced (19).
As with estrogen (3–5), dose is also an important consideration in BPA research. The current regulations on daily BPA exposure in the United States are largely based on a study conducted by the National Toxicology Program (23). In order to define clear limits of toxicity, F433 rats were fed BPA over a two-year period, resulting in the lowest-observed-adverse-effect level (LOAEL) of 50 mg BPA/kg BW per day (23). The US Environmental Protection Agency (US EPA) applied a 1000-fold “safety factor” to this concentration to calculate a daily tolerable reference dose of 50 µg BPA/kg BW per day. It should be noted that despite this dose being significantly lower than the reported LOAEL, it still represents an exposure to BPA that is estimated to be at least five-fold greater than an exposure that can be realistically achieved through dietary intake in humans.
In the past few decades, much effort has gone into estimating daily human intake of BPA. Several estimates based on patterns of normal dietary consumption and BPA migration values currently exist. The NTP-CERHR recently reviewed this data, estimating that general population adults were exposed to 0.008–1.5 µg BPA/kg BW per day (24). The European Union estimated that most adults were exposed, at most, to 1.4 µg BPA/kg BW per day through food sources alone (25). Consuming large quantities of wines produced in vats lined with epoxy resins was estimated to result in a maximum exposure of 7.5 µg BPA/kg BW per day (25). Combined, this produced a maximum worst case scenario of normal human consumption of 9 µg BPA/kg BW per day and led to the maximum tolerated dose of 10 µg BPA/kg BW per day.
By most accounts, the deleterious actions of BPA stem from its weak ability to bind with the estrogen receptors (ERs) and induce transcription of estrogen response elements (EREs). This has been shown by multiple groups through a variety of in vitro modeling systems (22, 26). Several groups have shown the ability of BPA to compete with E2 for binding to the ERs, albeit at an affinity reported to be 2,000- to 10,000-fold less than E2 (26, 27). While it has been reported that BPA exhibits a greater affinity to ER-beta than ER-alpha and differences exist between the co-regulator proteins recruited to each of the ERs in the presence of BPA, none of these studies have shown that this translates to a greater ability of ER-beta to induce down-stream ERE-mediated gene transcription (22, 27).
While it is convenient to attribute the bulk of BPA’s deleterious effects on its ability to function as a weak ER agonist (via AF-2 activation), it has also been shown to interact with the ERs in a manner that is entirely unique from all known classes of ER ligands (weak estrogens, pure agonists, and pure antagonists) (26). This suggests that perhaps the mechanism of action of BPA is much more complicated than originally thought.
Several studies have found in vivo effects of BPA related specifically to the mammary gland and the female reproductive tract. Fetal exposure to BPA in mice has been reported to reduce the age at time of vaginal opening, and reduce time between vaginal opening and first estrus (28). In rats, perinatal exposure to BPA disrupted estrous cyclicity and decreased serum luteinizing hormone in adulthood, suggesting involvement of negative feedback (29). Whether BPA or other xenobiotics impact preadolescents and onset of puberty or menarche is uncertain, given the paucity of longitudinal studies. Perinatal exposure to 250 ng BPA/kg BW per day through a subcutaneously implanted osmotic pump was observed to cause significant alterations in the mammary gland, including an increased number of terminal end buds (TEBs), a decreased rate of apoptosis in the TEBs, increased percentage of cells expressing the progesterone receptor (PR) in the mammary gland, and increased lateral branching (30). With gestational exposure alone, BPA has been reported to increase the number of terminal ducts, TEBs, alveolar buds, and preneoplastic lesions in the mammary gland. Durando et al. have shown that prenatal exposure to BPA (via subcutaneously implanted osmotic pump) coupled to a sub-carcinogenic dose of N-nitroso-N methylurea (NMU) resulted in an increased percentage of preneoplastic and neoplastic lesions in the mammary gland (31). Recently, Murray et al. reported that fetal exposure to BPA induces mammary gland ductal hyperplasia and carcinoma in situ (32). Gestational exposure to BPA has been reported to result in reproductive and endocrine disruption in male and female rodents (33).
To investigate the potential of BPA to cause developmental toxicity and predisposition for mammary cancer, we first utilized a protocol whereby BPA exposure occurred during the early postnatal period. Since the primary route of exposure to BPA is oral, we administered BPA by gavage to lactating Sprague Dawley CD rats. We administered BPA on a daily basis to dams from day two postpartum until time of weaning on day 21 (34). We selected two BPA doses, a high dose given to the lactating dams that would not result in a change in body weight to the offspring and a second BPA dose that was one-tenth of the high dose (250 µg and 25 µg BPA/kg BW, respectively). Controls were treated with an equivalent volume of the vehicle, sesame oil, on the same schedule. In regard to potential developmental and endocrine toxicity, there were no significant alteration on body weight, puberty as assessed by vaginal opening, and circulating E2 and progesterone concentrations in 50 day old female rats (34).
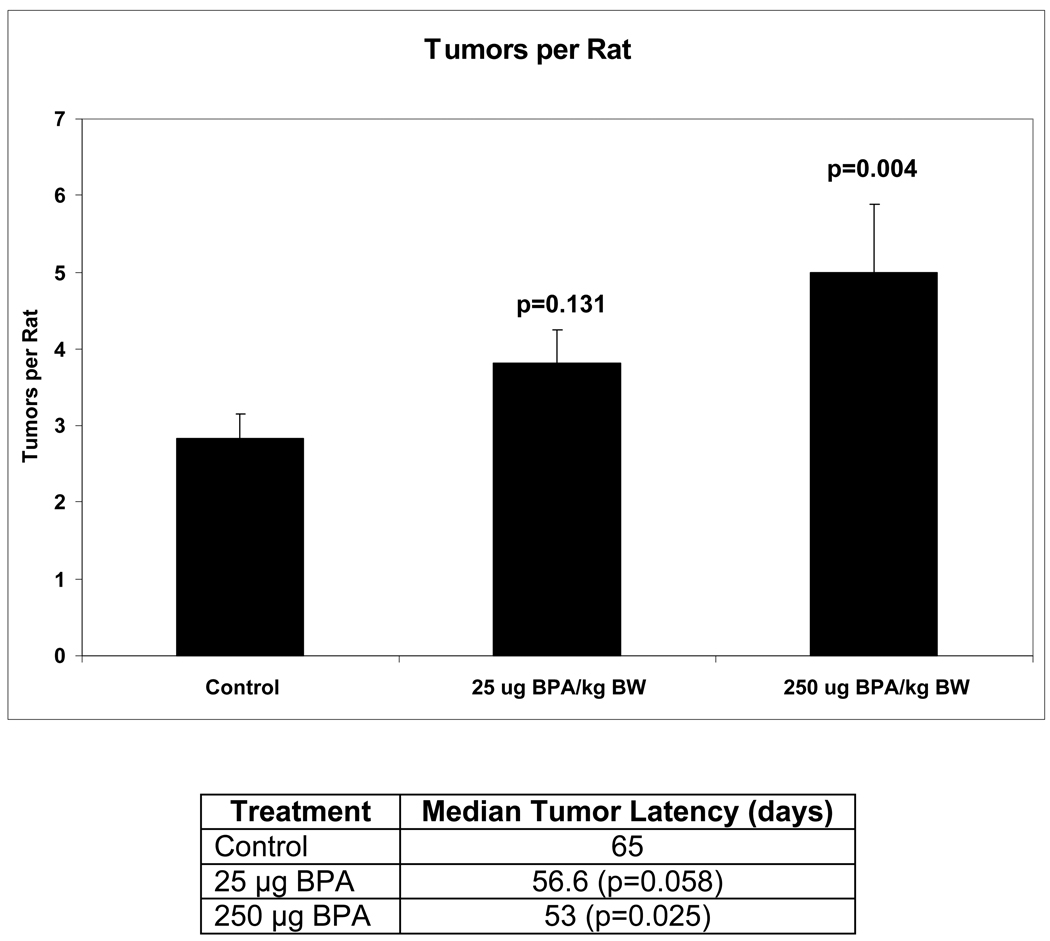
For investigating susceptibility for chemically induced mammary cancer, we used the established DMBA-induced model. At day 50 postpartum, female offspring exposed prepubertally to 0, 25 and 250 µg BPA/kg BW were treated orally with 30 mg DMBA/kg BW. Day 50 in Sprague Dawley rats is routinely used for chemically-induced mammary cancer because this is a time of high mitotic index in mammary terminal end buds (35). Rats were subsequently palpated for mammary tumors, and necropsy was carried out at 180 days post DMBA exposure. As seen in Figure 1, prepubertal BPA exposure to rats resulted in a dose dependent increase in DMBA induced mammary tumors, with the high dose causing a significant increase in the number of tumors developing per rat. Furthermore, latency (time to first palpated tumor) was significantly decreased for BPA compared to sesame oil exposure. These results demonstrate that prepubertal only exposure to BPA can result in later increased susceptibility to chemically-induced mammary cancer in rats.
Figure 1.
Tumor multiplicity and latency of DMBA induced mammary tumors in rats exposed prepubertally to bisphenol A. Lactating dams were gavaged with 0, 25, or 250 µg BPA/kg BW per day from days two through 20 postpartum. There were 32, 34, and 24 female offspring in the SO, 25 BPA, and 250 BPA groups, respectively, all derived from individual litters. At day 50, all female offspring were gavaged with a single dose of 30 mg DMBA/kg BW. For multiplicity, values are provided as mean ± SEM of tumors per rat. Latency values indicate the median time to first palpable tumor, given in days. P values greater than or equal to 0.05 were considered significant. Adapted from ref 34.
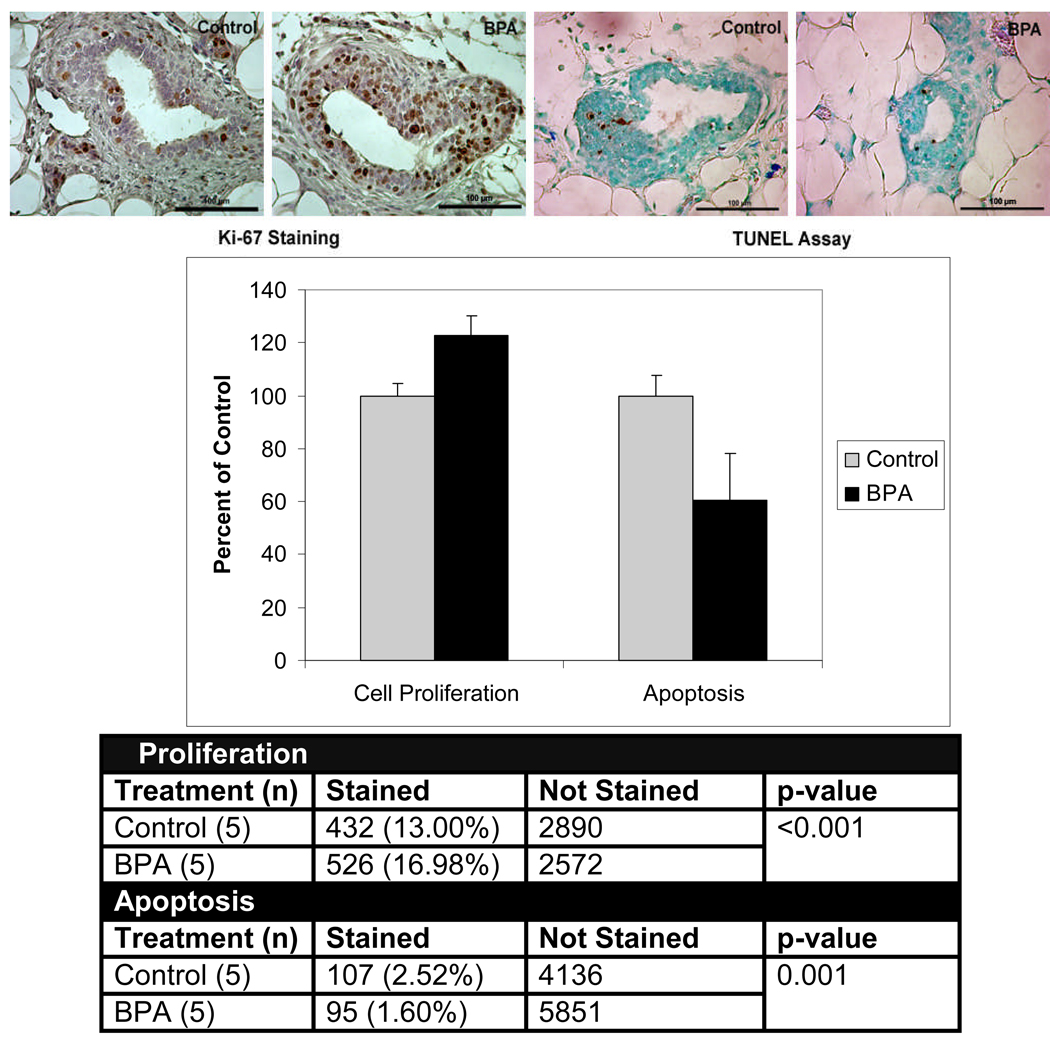
Using western blot analysis, steroid receptor co-activators (SRCs) 1–3, Akt, phospho-Akt, PR-A, and erbB3 proteins were determined to be significantly up-regulated at 50 days (34). We subsequently measured cell proliferation and apoptosis using the protein expression of Ki-67 and the TUNEL assay, respectively. At day 21, shortly after the last BPA treatment, we found no significant effect of prepubertal BPA exposure on cell proliferation and apoptosis in mammary glands of these rats. However at day 50, rate of cell proliferation was significantly increased and rate of apoptosis was significantly decreased in mammary glands of rats exposed to the high BPA dose compared to controls (Figure 2). Furthermore, the cell-proliferation-to-apoptosis ratio was over two-fold greater in the mammary glands of rats exposed prepubertally to BPA at 50 days of age (34). Since the effects on cell proliferation and apoptosis were seen at day 50, and not at day 21 (shortly after BPA treatment), we surmise that these results were not due to direct BPA action, but rather to a “permanent” developmental effect, perhaps via organizational or imprinting mechanisms (1, 2, 36).
Figure 2.
Cell proliferation and apoptosis in mammary glands of 50 day old rats exposed lactationally to dams treated with 250 µg bisphenol A (BPA)/kg BW per day. The upper panel depicts Ki-67 expression as an indicator of cell proliferation and the TUNEL assay as measure of apoptosis. Terminal end buds from five biologically distinct samples (n=5) were analyzed per treatment. The graph illustrates mean index values ± SEM as a percent of the control group. The resulting numbers were used to construct a contingency table. All images were taken at 40× magnification. The scale bar represents 100 µm. Adapted from ref. 34.
Extending our BPA studies to prenatal exposure, we treated pregnant Sprague Dawley rats with 0, 25 and 250 µg BPA/kg BW on days 2–20 postconception. In this manner, the fetuses were exposed transplacentally. At day 50 postpartum, female offspring were gavaged with 30 mg DMBA/kg BW to investigate chemically-induced mammary cancer. Interestingly, we found no difference between treated groups for mammary tumor multiplicity, latency or tumor incidence (37). Since we had previously investigated gene (38) and protein expressions (39) in mammary glands of rats exposed prenatally to BPA and found a greater number of significant changes at day 100 compared to day 50, we followed this by carrying out protein measurements at these ages.
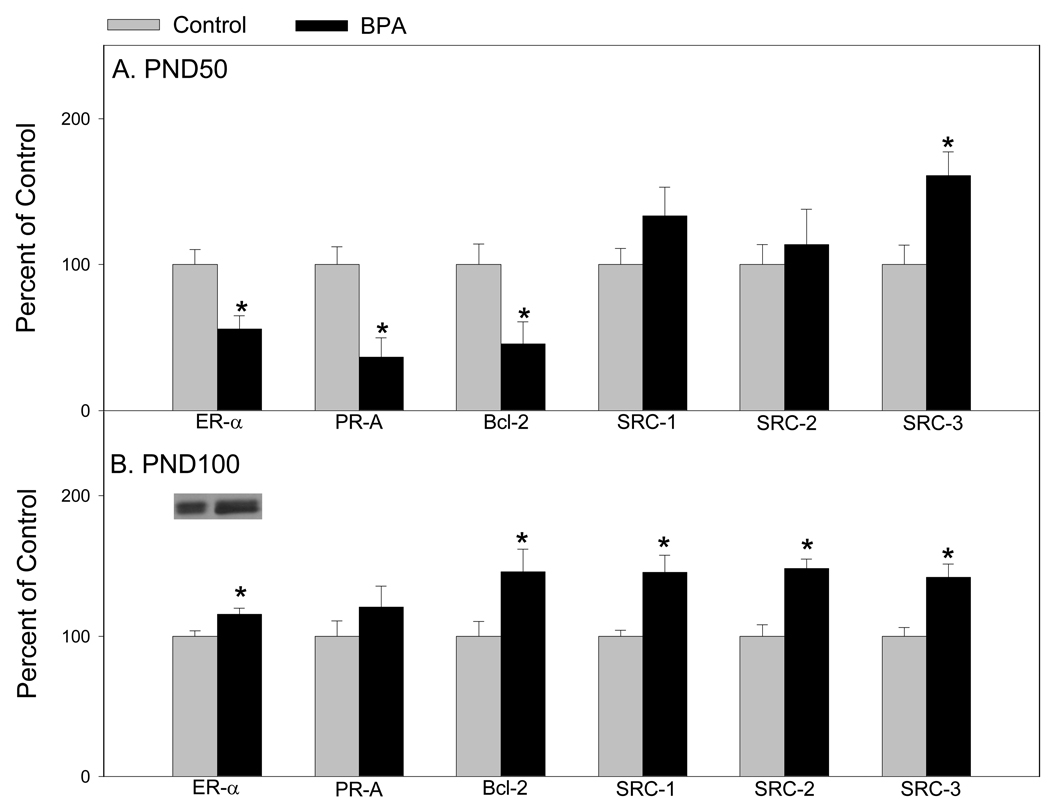
Discovery proteomic studies were carried out via two-dimensional gel electrophoresis for protein separation and enrichment and mass spectrometry for identification. We used western blot analysis from a separate set of identically treated animals for protein validation. What became evident from our proteomic studies was that there were many proteins involved in regulating protein metabolism, signal transduction, developmental processes and cell cycle and proliferation (39) (Table 1). Hence, we elected to investigate low-abundance down-stream signaling proteins in mammary glands of 50 and 100 old females in order to determine the long lasting effects of prenatal exposure to BPA. Figure 3 demonstrates that at day 50, ER-alpha, PR-A, and Bcl-2 were down-regulated and only SRC-3 was up-regulated (39). At 100 days, ER-alpha, Bcl-2 and the SRCs 1–3 were up-regulated in mammary glands of rats prenatally exposed to BPA.
Table 1.
Differentialy expressed proteins as identified by MS in mammary glands of rats exposed prenatally to BPA
| Protein Identification | Accession Number |
Fold Change |
Anova | BPA Treatment |
Age | MW KDa |
PI | Molecular Function |
|---|---|---|---|---|---|---|---|---|
| Aldose Reductase | P07943 | −2.0 | 0.040 | Low & High | 50 | 36,230 | 6.3 | Oxidoreductase |
| Tropomyosin beta chain | P58774 | −1.6 | 0.018 | Low | 50 | 32,931 | 4.5 | Cytoskeleton constituent |
| 14-3-3 Protein epsilon | P62259 | −1.6 | 0.032 | Low | 50 | 29,326 | 4.5 | Protein domain specific binding |
| Alpha-1B-glycoprotein | P04217 | −2.6 | 0.025 | High | 50 | 57,127 | 7.0 | Immunoglobulin receptor |
| Heat shock cognate 71 kDa protein |
P19378 | 1.3 | 0.035 | High | 50 | 70,989 | 5.1 | Chaperone |
| Peroxiredoxin-2 | Q61171 | 1.2 | 0.042 | High | 50 | 21,936 | 5.1 | Oxidoreductase/ peroxidase |
| Fibrinogen gamma | Q8VCM7 | −1.4 | 0.033 | High | 50 | 40,227 | 5.6 | Protein binding |
| SPARC | P07214 | −1.8 | 0.032 | High | 50 | 35,129 | 4.7 | Extracellular matrix binding |
| SH3 domain-binding glutamic acid-rich-like protein 3 |
Q91VW3 | −1.4 | 0.033 | Low | 50 | 10,527 | 4.9 | Unclassified |
| ATP synthase subunit delta | Q9D3D9 | −1.7 | 0.022 | High | 50 | 17,020 | 6.5 | Hydrogen transporter |
| Actin, cytoplasmic 1 | P60710 | −1.5 | 0.043 | High | 100 | 42,052 | 5.2 | Protein binding |
| Creatine kinase B type | Q04447 | −3.1 | 0.042 | Low & High | 100 | 42,983 | 5.3 | Kinase/ protein binding |
| Hemopexin Precursor | P02790 | 1.8 | 0.032 | High | 100 | 52,060 | 6.5 | Iron ion binding |
| Tropomyosin alpha-3 chain | Q63610 | −1.5 | 0.018 | Low | 100 | 29,217 | 4.6 | Actin binding |
| Coronin 1A | O89053 | −2.3 | 0.025 | Low & High | 100 | 51,026 | 6.2 | Actin-binding protein |
| 14-3-3 protein eta | P68510 | 1.9 | 0.012 | Low | 100 | 28,151 | 4.7 | Protein domain specific binding |
| Vimentin | P20152 | 1.8 | 0.035 | High | 100 | 53,754 | 4.9 | Structural protein/ Protein binding |
Figure 3.
Western blot analysis of ER-alpha, PR-A, Bcl-2, SRC-1, SRC-2, and SRC-3 in mammary glands of (A) 50-day-old and (B) 100-day-old rats exposed prenatally to 250 µg BPA/kg BW or an equal volume of sesame oil (controls). Values represent mean density ± SE as a percentage of the control, with densitometry values for controls set to 100; n = 6–8 samples per group. Insets are representative immunoblots for each protein per treatment. *p < 0.05 compared with corresponding controls. Adapted from reference 37.
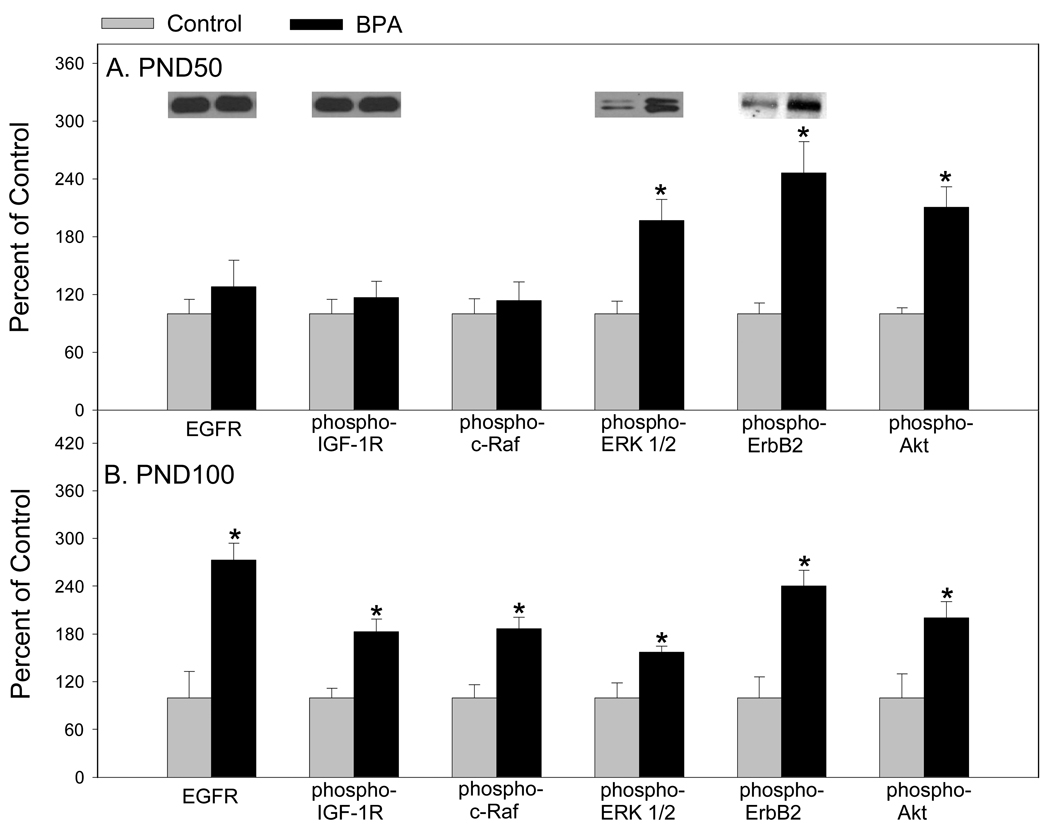
Probing further, we found that phospho-ERK-1 and 2, phospho-ErbB2 and phospho-Akt were up regulated in mammary glands of 50 day old rats prenatally to BPA (Figure 4). On the other hand, in mammary glands of 100 day old rats, epidermal growth factor receptor (EGFR), phospho-IGF-1 receptor, phospho-c-Raf, phospho-ERK-1 and 2, phospho-ErbB2 and phospho-Akt were up-regulated. Together, 11 of 12 proteins associated with cell proliferation were up-regulated in mammary glands of 100 day old rats and only five proteins that can be implicated with cell proliferation were up-regulated in mammary glands of 50 day old rats. In addition, we measured Ki-67 in the mammary epithelial cells of 100 day old rats and found a 2.25 fold increase in cell proliferation in prenatal BPA exposed rats compared to sesame oil exposed rats (31.14% and 13.84%, respectively) (37). This suggested to us that prenatal exposure to BPA may shift the timing of susceptibility for mammary cancer in rats.
Figure 4.
Western blot analysis of EGFR, phospho-IGF-1R, phospho-c-Raf, phospho-ERK 1/2, phospho-ErbB2, and phospho-Akt in mammary glands of (A) 50-day-old and (B) 100-day-old rats exposed prenatally to 250 µg BPA/kg BW or an equal volume of sesame oil (controls). Values represent mean density ± SE as a percentage of the control, with densitometry values for controls set to 100; n = 6–8 samples per group. Insets are representative immunoblots for each protein per treatment. *p < 0.05 compared with corresponding controls. Adapted from reference 37.
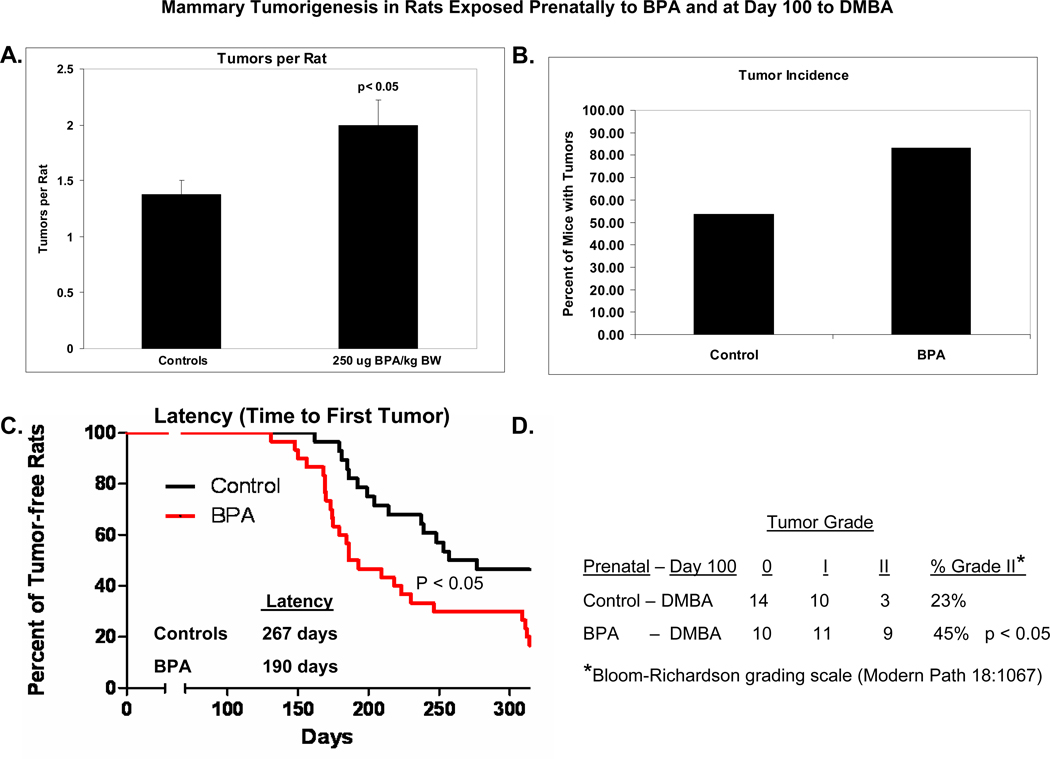
Accordingly, we investigated DMBA-induced mammary cancer in 100 day old female rats whose dams were treated orally during pregnancy with 250 µg BPA/kg BW or the vehicle, sesame oil. In contrast to the results of rats exposed on day 50 with DMBA, we recorded a significant increase in tumor incidence, a nonsignificant increase in tumor multiplicity, and a significant decrease in time to first tumor development in 100 day old rats exposed prenatally to BPA (Figure 5) (37). Furthermore, the pathology report revealed significantly increased proportion of 100 day old DMBA-induced mammary tumors classified as grade II according to the Bloom-Richardson system which takes into consideration mitotic index, nuclear grade and adenocarcinoma tubular pattern (40) in rats exposed prenatally to BPA (45%) as compared to sesame oil (23%). This suggested that prenatal BPA exposed offspring could develop more aggressive mammary cancer.
Figure 5.
(A) Tumor multiplicity, (B) tumor incidence, (C) palpable tumor latency, and (D) tumor grade in female offspring prenatally exposure to 250 µg BPA/kg BW or an equal volume of sesame oil (controls) and gavaged with a single dose of 30 mg DMBA/kg BW on postnatal day 100. Adapted from reference 37.
In summary, we have shown that prepubertal exposure to oral low concentrations of BPA resulted in a significantly decreased time to first tumor latency and increased tumor multiplicity in the DMBA model of rodent mammary carcinogenesis (34). Our data suggested that the mechanism of action behind this carcinogenic response was mediated through increased cell proliferation, decreased apoptosis, and centered on an up-regulation of SRCs 1–3, erbB3, and increased Akt signaling in the mammary gland.
Furthermore, we demonstrate that prenatal exposure to BPA shifts the time of susceptibility from 50 days to 100 days for chemically-induced mammary carcinogenesis (37). Proteomic studies prove valuable in elucidating mechanism of action (39). Increases in ER-alpha, SRCs 1–3, Bcl-2, EGFR, phospho-IGF-1R, phospho-c-Raf, phospho-ERKs 1/2, phospho-ErbB2 and phospho-Akt are accompanied by increase in cell proliferation (37).
Outlook:
Future research should investigate if prenatal and prepubertal exposures to orally administered BPA exert its long lasting effects via epigenetic mechanisms, and if populations at risk (certain phenotypes) are more likely to develop breast cancer if exposed to BPA. Finally, dose response studies (especially at low doses) and measurement of blood and urine BPA concentrations should be carried out in order to draw comparison to human exposure.
Acknowledgments
This research was supported by the Breast Cancer and the Environment Research Centers grant number U01 ES/CA ES012771 and Genes, Environment and Health Initiative grant number 1U01ES016003 from the National Institute of Environmental Health Sciences (NIEHS), and the National Cancer Institute (NCI), NIH, DHHS. Its contents are solely the responsibility of the authors and do not necessarily represent the official views of the NIEHS or NCI, NIH. SJ was supported by a Department of Defense Breast Cancer Program Traineeship Award (W81XWH-08-0777), and is currently supported through a postdoctoral fellowship from the National Cancer Institute Cancer Prevention and Control Training Program (R25 CA07888-22)
Footnotes
Competing interests: The authors do not have any conflict of interest to report.
References
- 1.Lamartiniere CA, Sloop CA, Clark J, Tilson HA, Lucier GW. Organizational effects of hormonally-active xenobiotics on postnatal development. 12th Conferences on Environmental Toxicology; Dayton, Ohio. U.S. Air Force Publication (AFAMRL-TR-81-149); p. 192. [Google Scholar]
- 2.Baylin SB, Herman JG, Graff JR, Vertino PM, Issa JP. Alterations in DNA methylation: a fundamental aspect of neoplasia. Adv Cancer Res. 1998;72:141–196. [PubMed] [Google Scholar]
- 3.Welshons WV, Nagel SC, vom Saal FS. Large effects from small exposures. III. Endocrine mechanisms mediating effects of bisphenol A at levels of human exposure. Endocrinology. 2006 Jun;147(6 Suppl):S56–S69. doi: 10.1210/en.2005-1159. [DOI] [PubMed] [Google Scholar]
- 4.Meites J, Cassell E, Clark J. Estrogen inhibition of mammary tumor growth in rats; counteraction by prolactin. Proc Soc Exp Biol Med. 1971 Sep;137(4):1225–1227. doi: 10.3181/00379727-137-35760. [DOI] [PubMed] [Google Scholar]
- 5.Barnes SaLC. The Role of Phytoestrogens as Cancer-Prevention Agents. In: Kelloff GJHE, Sigman CC, editors. Cancer Chemoprevention. Totowa, NJ: Humana Press Inc; 2003. pp. 1–11. [Google Scholar]
- 6.Brede C, Fjeldal P, Skjevrak I, Herikstad H. Increased migration levels of bisphenol A from polycarbonate baby bottles after dishwashing, boiling and brushing. Food Addit Contam. 2003 Jul;20(7):684–689. doi: 10.1080/0265203031000119061. [DOI] [PubMed] [Google Scholar]
- 7.Howdeshell KL, Peterman PH, Judy BM, Taylor JA, Orazio CE, Ruhlen RL, et al. Bisphenol A is released from used polycarbonate animal cages into water at room temperature. Environ Health Perspect. 2003 Jul;111(9):1180–1187. doi: 10.1289/ehp.5993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Joskow R, Barr DB, Barr JR, Calafat AM, Needham LL, Rubin C. Exposure to bisphenol A from bis-glycidyl dimethacrylate-based dental sealants. J Am Dent Assoc. 2006 Mar;137(3):353–362. doi: 10.14219/jada.archive.2006.0185. [DOI] [PubMed] [Google Scholar]
- 9.Olea N, Pulgar R, Perez P, Olea-Serrano F, Rivas A, Novillo-Fertrell A, et al. Estrogenicity of resin-based composites and sealants used in dentistry. Environ Health Perspect. 1996 Mar;104(3):298–305. doi: 10.1289/ehp.96104298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Calafat AM, Weuve J, Ye X, Jia LT, Hu H, Ringer S, et al. Exposure to bisphenol A and other phenols in neonatal intensive care unit premature infants. Environ Health Perspect. 2009 Apr;117(4):639–644. doi: 10.1289/ehp.0800265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Yoshida T, Horie M, Hoshino Y, Nakazawa H. Determination of bisphenol A in canned vegetables and fruit by high performance liquid chromatography. Food Addit Contam. 2001 Jan;18(1):69–75. doi: 10.1080/026520301446412. [DOI] [PubMed] [Google Scholar]
- 12.Calafat AM, Kuklenyik Z, Reidy JA, Caudill SP, Ekong J, Needham LL. Urinary concentrations of bisphenol A and 4-nonylphenol in a human reference population. Environ Health Perspect. 2005 Apr;113(4):391–395. doi: 10.1289/ehp.7534. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Wolff MS, Teitelbaum SL, Windham G, Pinney SM, Britton JA, Chelimo C, et al. Pilot study of urinary biomarkers of phytoestrogens, phthalates, and phenols in girls. Environ Health Perspect. 2007 Jan;115(1):116–121. doi: 10.1289/ehp.9488. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Calafat AM, Ye X, Wong LY, Reidy JA, Needham LL. Exposure of the U.S. population to bisphenol A and 4-tertiary-octylphenol: 2003–2004. Environ Health Perspect. 2008 Jan;116(1):39–44. doi: 10.1289/ehp.10753. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.European Food Safety Authority: Opinion of the scientific panel on food additives, flavourings, processing aids and materials in contact with food. The EFSA Journal. 2006;428:1–75. doi: 10.2903/j.efsa.2008.759. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Brock JW, Yoshimura Y, Barr JR, Maggio VL, Graiser SR, Nakazawa H, et al. Measurement of bisphenol A levels in human urine. J Expo Anal Environ Epidemiol. 2001 Jul–Aug;11(4):323–328. doi: 10.1038/sj.jea.7500174. [DOI] [PubMed] [Google Scholar]
- 17.Lakind JS, Naiman DQ, Bisphenol A. (BPA) daily intakes in the United States: estimates from the 2003–2004 NHANES urinary BPA data. J Expo Sci Environ Epidemiol. 2008 Nov;18(6):608–615. doi: 10.1038/jes.2008.20. [DOI] [PubMed] [Google Scholar]
- 18.Vandenberg LN, Hauser R, Marcus M, Olea N, Welshons WV. Human exposure to bisphenol A (BPA) Reprod Toxicol. 2007 Aug–Sep;24(2):139–177. doi: 10.1016/j.reprotox.2007.07.010. [DOI] [PubMed] [Google Scholar]
- 19.Pottenger LH, Domoradzki JY, Markham DA, Hansen SC, Cagen SZ, Waechter JM., Jr The relative bioavailability and metabolism of bisphenol A in rats is dependent upon the route of administration. Toxicol Sci. 2000 Mar;54(1):3–18. doi: 10.1093/toxsci/54.1.3. [DOI] [PubMed] [Google Scholar]
- 20.Volkel W, Colnot T, Csanady GA, Filser JG, Dekant W. Metabolism and kinetics of bisphenol a in humans at low doses following oral administration. Chem Res Toxicol. 2002 Oct;15(10):1281–1287. doi: 10.1021/tx025548t. [DOI] [PubMed] [Google Scholar]
- 21.Snyder RW, Maness SC, Gaido KW, Welsch F, Sumner SC, Fennell TR. Metabolism and disposition of bisphenol A in female rats. Toxicol Appl Pharmacol. 2000 Nov 1;168(3):225–234. doi: 10.1006/taap.2000.9051. [DOI] [PubMed] [Google Scholar]
- 22.Matthews JB, Twomey K, Zacharewski TR. In vitro and in vivo interactions of bisphenol A and its metabolite, bisphenol A glucuronide, with estrogen receptors alpha and beta. Chem Res Toxicol. 2001 Feb;14(2):149–157. doi: 10.1021/tx0001833. [DOI] [PubMed] [Google Scholar]
- 23.National Toxicology Program, Bisphenol A in F344 Rats and B6C3 Mice. 1982. [Google Scholar]
- 24.NTP-CERHR Monograph on the Potential Human Reproductive and Developmental Effects of Bisphenol A. 2008. [PubMed] [Google Scholar]
- 25.European Union Risk Assessment Report of 4,4'-Isoproplyidenediphenol (Bisphenol A) Cas No: 80-05-07. 2003. [Google Scholar]
- 26.Gould JC, Leonard LS, Maness SC, Wagner BL, Conner K, Zacharewski T, et al. Bisphenol A interacts with the estrogen receptor alpha in a distinct manner from estradiol. Mol Cell Endocrinol. 1998 Jul 25;142(1–2):203–214. doi: 10.1016/s0303-7207(98)00084-7. [DOI] [PubMed] [Google Scholar]
- 27.Routledge EJ, White R, Parker MG, Sumpter JP. Differential effects of xenoestrogens on coactivator recruitment by estrogen receptor (ER) alpha and ERbeta. J Biol Chem. 2000 Nov 17;275(46):35986–35993. doi: 10.1074/jbc.M006777200. [DOI] [PubMed] [Google Scholar]
- 28.Howdeshell KL, Hotchkiss AK, Thayer KA, Vandenbergh JG, vom Saal FS. Exposure to bisphenol A advances puberty. Nature. 1999 Oct 21;401(6755):763–764. doi: 10.1038/44517. [DOI] [PubMed] [Google Scholar]
- 29.Rubin BS, Murray MK, Damassa DA, King JC, Soto AM. Perinatal exposure to low doses of bisphenol A affects body weight, patterns of estrous cyclicity, and plasma LH levels. Environ Health Perspect. 2001 Jul;109(7):675–680. doi: 10.1289/ehp.01109675. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Munoz-de-Toro M, Markey CM, Wadia PR, Luque EH, Rubin BS, Sonnenschein C, et al. Perinatal exposure to bisphenol-A alters peripubertal mammary gland development in mice. Endocrinology. 2005 Sep;146(9):4138–4147. doi: 10.1210/en.2005-0340. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Durando M, Kass L, Piva J, Sonnenschein C, Soto AM, Luque EH, et al. Prenatal bisphenol A exposure induces preneoplastic lesions in the mammary gland in Wistar rats. Environ Health Perspect. 2007 Jan;115(1):80–86. doi: 10.1289/ehp.9282. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Murray TJ, Maffini MV, Ucci AA, Sonnenschein C, Soto AM. Induction of mammary gland ductal hyperplasias and carcinoma in situ following fetal bisphenol A exposure. Reprod Toxicol. 2007 Apr–May;23(3):383–390. doi: 10.1016/j.reprotox.2006.10.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Markey CM, Luque EH, Munoz De Toro M, Sonnenschein C, Soto AM. In utero exposure to bisphenol A alters the development and tissue organization of the mouse mammary gland. Biol Reprod. 2001 Oct;65(4):1215–1223. doi: 10.1093/biolreprod/65.4.1215. [DOI] [PubMed] [Google Scholar]
- 34.Jenkins S, Raghuraman N, Eltoum I, Carpenter M, Russo J, Lamartiniere CA. Oral exposure to bisphenol a increases dimethylbenzanthracene-induced mammary cancer in rats. Environ Health Perspect. 2009 Jun;117(6):910–915. doi: 10.1289/ehp.11751. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Russo J, Tay LK, Ciocca DR, Russo IH. Molecular and cellular basis of the mammary gland susceptibility to carcinogenesis. Environ Health Perspect. 1983 Mar;49:185–199. doi: 10.1289/ehp.8349185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Hsu PY, Hsu HK, Singer GA, Yan PS, Rodriguez BA, Liu JC, et al. Estrogen-mediated epigenetic repression of large chromosomal regions through DNA looping. Genome Res. Jun;20(6):733–744. doi: 10.1101/gr.101923.109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Betancourt AM, Eltoum IA, Desmond RA, Russo J, Lamartiniere CA. In utero exposure to Bisphenol A shifts the window of susceptibility for mammary carcinogenesis in the rat. Environ Health Perspect. 2010 doi: 10.1289/ehp.1002148. need reference. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Moral R, Wang R, Russo IH, Lamartiniere CA, Pereira J, Russo J. Effect of prenatal exposure to the endocrine disruptor bisphenol A on mammary gland morphology and gene expression signature. J Endocrinol. 2008 Jan;196(1):101–112. doi: 10.1677/JOE-07-0056. [DOI] [PubMed] [Google Scholar]
- 39.Betancourt AM, Mobley JA, Russo J, Lamartiniere CA. Proteomic Analysis in Mammary Glands of Rat Offspring Exposed In Utero to Bisphenol A J Proteomics. 2009 doi: 10.1016/j.jprot.2010.02.020. (in press) [DOI] [PubMed] [Google Scholar]
- 40.Meyer JS, Alvarez C, Milikowski C, Olson N, Russo I, Russo J, et al. Breast carcinoma malignancy grading by Bloom-Richardson system vs proliferation index: reproducibility of grade and advantages of proliferation index. Mod Pathol. 2005 Aug;18(8):1067–1078. doi: 10.1038/modpathol.3800388. [DOI] [PubMed] [Google Scholar]