Abstract
Perivascular adipose tissue is a local deposit of adipose tissue surrounding the vasculature. Perivascular adipose tissue is present throughout the body and has been shown to have a local effect on blood vessels. The influence of perivascular adipose tissue on the vasculature changes with increasing adiposity. This article describes the anatomy and pathophysiology of perivascular adipose tissue and the experimental evidence supporting its local adverse effect on the vasculature. Methods for quantifying perivascular adipose tissue in free-living populations will be described. Finally, the epidemiological literature demonstrating an association between perivascular adipose tissue and cardiometabolic disease will be explored.
Keywords: cardiovascular, epidemiology, imaging, mechanisms, perivascular adipose tissue
The high prevalence of obesity throughout the world has increasingly focused on the association between body weight, cardiovascular risk factors and clinical cardiovascular disease [1]. Body fat distribution may be more strongly associated with obesity-related comorbidities and cardiovascular disease compared with generalized measures of adiposity, such as BMI [2–5]. Ectopic fat, defined as the deposition of fat in nonclassical locations including the heart, kidneys and blood vessels, may contribute to the development of cardiovascular disease by exerting a local toxic effect on adjacent structures [6–8]. One such ectopic fat depot is peri-vascular adipose tissue, which is directly adherent to blood vessels. Perivascular adipose tissue includes fat surrounding large arteries, as well as organ-specific fat depots in which adipose tissue surrounds the organ’s vasculature. Periaortic fat falls into the former category, whereas epicardial, pericardial and peri-renal fat fall into the latter category.
This article will first describe the anatomy and normal function of perivascular adipose tissue. The proinflammatory changes in peri-vascular adipose tissue that are associated with the development of obesity will then be discussed. Next, the experimental evidence supporting a pathological effect of perivascular adipose tissue on the vasculature with increasing adiposity will be presented. Finally, the imaging techniques used to quantify perivascular adipose tissue and the epidemiologic literature exploring the association between perivascular adipose tissue and cardiometabolic disease will be reviewed.
Anatomy of perivascular adipose tissue
Perivascular adipose tissue is defined as adipose tissue surrounding blood vessels. Perivascular adipose tissue is present in lean animals, but the amount increases with increasing adiposity [8,9]. The composition of perivascular adipose tissue varies by blood vessel type. Resistance vessels contain predominantly white adipose tissue, whereas large vessels are characterized by both brown and white adipose tissue [10,11]. The term ‘perivascular adipose tissue’ encompasses certain organ-specific fat depots, including epicardial and perirenal fat. These fat depots are characterized by fat wrapped around the coronary and renal blood vessels, respectively. Intramuscular adipose tissue may also be a subtype of perivascular adipose tissue given its location around muscle resistance arteries in obese rats [12].
Anatomically, perivascular adipose tissue is contiguous with the adventitial layer of the blood vessel wall [13–15]. Historically, perivascular adipose tissue was believed to serve primarily as scaffolding for blood vessels. Today, it is recognized as a metabolically active endocrine organ with important effects on vascular function. The close proximity of perivascular adipose tissue with the vascular adventitia allows for the possibility of crosstalk between these two diverse cellular environments [14,16].
Normal function of perivascular adipose tissue
Perivascular adipose tissue, similarly to other fat depots, is metabolically active, secreting a wide array of bioactive substances, termed ‘adipokines’ [13,17,18]. Adipokines include cytokines, chemokines and hormones that can act in a paracrine, autocrine or endocrine fashion [19,20]. Only a minority of adipokines are secreted by adipocytes; resident immune cells, including macrophages and T cells, also produce adipokines [18,21,22]. The secretion of adipokines by perivascular adipose tissue is important for vascular regulation under normal conditions. Seminal studies on the rat aorta performed 20 years ago first demonstrated a decreased response to the vasoconstrictor norepinephrine in the presence of periaortic fat compared with aortas in which the perivascular adipose tissue had been removed [23]. This anticontractile property remained with the transfer of the culture bath to vessels without adipose tissue and suggested the action of a secreted substance [24]. The secreted substance was given the name adipocyte derived relaxing factor (ADRF). Subsequent studies have demonstrated that ADRF attenuates the vasocontrictive response of a variety of agents [24,25]. The magnitude of the anticontractile effect of ADRF and the downstream mediators of its effect in different vascular beds are still being elucidated [25,26]. Recently, angiotensin 1–7, which acts on endothelial cells to promote nitric oxide release, has been identified as an endothelium-dependent ADRF. A separate endothelium-independent anticontractile effect of perivascular adipose tissue is mediated by hydrogen peroxide [25,27]. Additional adipokines are likely to be involved in the regulation of vascular function under normal conditions, but the vasoactive properties of perivascular adipose tissue have only recently begun to be explored. The properties of perivascular adipose tissue may vary in different vascular beds and different animal species. In contrast to the anticontractile properties of perivascular adipose tissue surrounding the aorta of rats and humans [23,25,28], perivascular adipose was found to diminish endothelial nitric oxide production and coronary vasodilation in dogs, but not pigs [29,30].
Proposed mechanisms linking perivascular adipose tissue & vascular disease
A potential local pathogenic effect of perivascular adipose tissue on the vasculature may be mediated by either direct compression of the vessels themselves or changes in perivascular adipose tissue associated with disease states such as obesity, hypertension, hyperglycemia and metabolic syndrome [13,31–33]. The changes in perivascular adipose tissue in response to obesity have been the most extensively studied, and many of the other disease states studied are frequently associated with obesity. In this article, we will review the development of inflammation in perivascular adipose tissue in response to obesity. Then, we will discuss several proposed pathophysiological mechanisms linking perivascular adipose tissue to cardiovascular disease.
Obesity, inflammation & perivascular adipose tissue
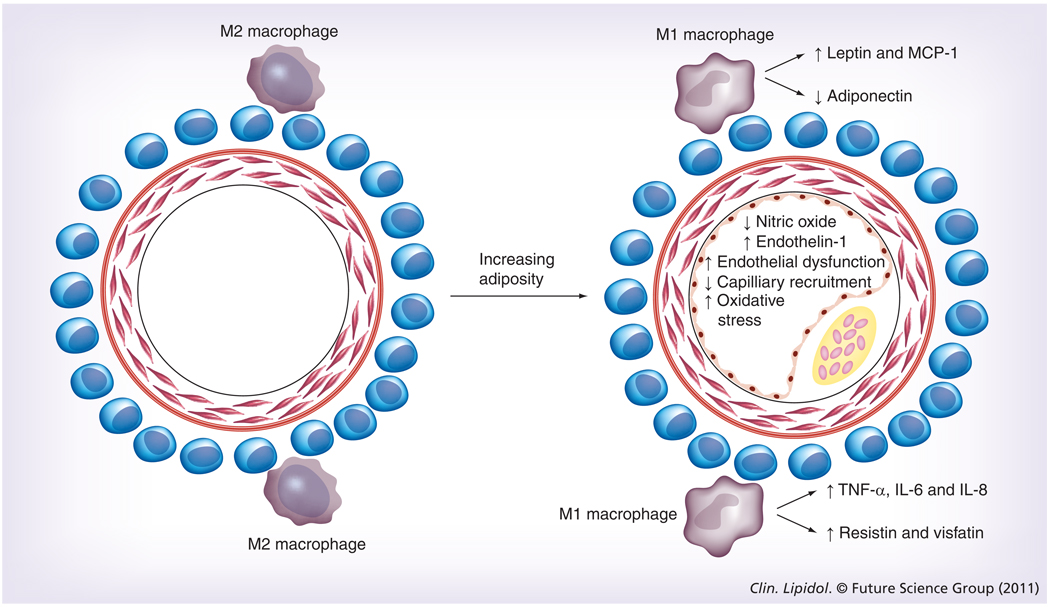
Obesity is associated with inflammation of adipose tissue [19,34]. In normal adipose tissue, the metabolic balance favors a noninflammatory state. With the development of obesity, the metabolic milieu changes, favoring the instigation and perpetuation of inflammation [19,34]. The cellular mechanisms underlying these changes are only partially understood, but involve alterations in adipokine secretion by resident cells within adipose tissue, as well as infiltration of fat by proinflammatory immune cells [18,19,34]. Adipocytes upregulate secretion of leptin and downregulate secretion of adiponectin. In addition, adipocyte hypertrophy stimulates secretion of monocyte chemoattractant protein (MCP)-1 [19,35,36], a chemokine involved in macrophage recruitment and inflammatory responses. The macrophages in obesity exhibit a proinflammatory phenotype [37]. These M1, or ‘classically activated’, macrophages are lipid-filled foam cells that demonstrate enhanced secretion of TNF-α, IL-6 and IL-8 [37,38]. By contrast, lean animals have predominantly M2, or ‘alternatively activated’, macrophages, which secrete anti-inflammatory cytokines [37].
Much of the evidence demonstrating the association of obesity with adipose inflammation comes from the study of visceral adipose tissue (VAT) [34]. However, growing evidence supports a similar process in perivascular adipose tissue [13,16,17,34]. IL-1β, IL-6, MCP-1 and TNF-α were shown to be higher in obese subjects undergoing bypass surgery in epicardial as opposed to subcutaneous fat [17]. In related studies, in vitro differentiated human perivascular adipocytes demonstrated increased levels of IL-8, IL-6 and MCP-1 compared with subcutaneous adipose tissue [13]. Gene expression studies in mice fed a high-fat diet demonstrated reductions in the expression of adiponectin, an anti-inflammatory adipokine, and increases in the expression of leptin, a proinflammatory hormone in periaortic fat after 2 weeks of the change in diet [13]. MCP-1 is also found at substantially higher levels in perivascular compared with subcutaneous adipose tissue [13]. In pigs with metabolic syndrome, perivascular adipose tissue was found to exacerbate endothelial dysfunction, and this effect appeared to be partially mediated by leptin [33].
Perivascular adipose tissue & vascular dysfunction in obesity
The growing appreciation of the proinflammatory state of adipose tissue in obesity has naturally led to speculation about the local pathologic effects of perivascular adipose tissue in obesity-mediated cardiovascular disease. The proposed mechanisms all include the actions of adipokines (Figure 1). Many of the proinflammatory adipokines upregulated in obesity are known to influence vascular function, including endothelial function [33,39], oxidative stress [40,41], vascular stiffness [42] and smooth muscle migration [43]. Adipokines also stimulate immune cell migration into the vascular wall, potentially contributing to the inflammation found in atherosclerosis [44,45]. Finally, adipokines modulate the effect of insulin on the vasculature, thereby decreasing insulin- mediated muscle glucose uptake [14,46,47]. This leads to alterations in nitric oxide signaling, insulin resistance and potentially atherogenesis [14].
Figure 1. Changes in perivascular adipokines with increasing adiposity.
Changes in vascular function that occur as a consequence of adipokine action are shown. This vascular dysfunction probably contributes to the development of atherosclerotic plaques. MCP: Monocyte chemoattractant protein.
Vasoactive properties of adipokines
As previously outlined, secretion of adipokines by healthy perivascular adipose tissue plays a role in vascular modulation, conferring a protective anticontractile effect [23]. Investigators postulated that the adipokine dysregulation inherent to obesity or other disease states may negate this effect [28,48]. Studies in both humans and animals support this theory. Rats exposed to nicotine during pregnancy and lactation had higher bodyweights and greater amounts of perivascular fat than control rats [49]. In this setting, the anticontractile properties of perivascular fat was attenuated. Similarly, in human perivascular adipose tissue biopsy samples, perivascular fat demonstrated no anticontractile effects in obese compared with healthy subjects [28]. This change in vascular function with obesity was associated and potentially mediated by inflammation. Immunohistochemistry with quantitative image analysis showed an increase in adipocyte size and increased production of TNF-α in obese patients. Furthermore, incubation of healthy perivascular adipose tissue with TNF-α caused an attenuation of the anticontractile response similar to the obese phenotype. The change in TNF-α levels appeared to be a local phenomenon, as circulating levels of this cytokine are low in obesity [28,50,51]. In addition to obesity, changes in the function of perivascular adipose tissue have been shown in animal models of hypertension. Specifically, the anticontractile properties of normal perivascular adipose tissue were attenuated with the development of hypertension. This did not appear to be mediated by the presence of obesity in the hypertensive rats as hypertension was induced over a 14-day period.
Despite the elegant experiments highlighting the influence of perivascular fat on the vasculature, a full understanding of the effect of perivascular adipose tissue in diseased states on vascular function remains incompletely understood. Incubation of perivascular adipose tissue from obese patients with an anti-TNF-α antibody did not restore the anticontractile response [28], suggesting that TNF-α is just one of many perivascular adipokines with complex and interconnected effects on vascular function.
In addition to TNF-α, vascular effects have been demonstrated for the hormones leptin and adiponectin. Physiological concentrations of leptin have been shown in vitro and in vivo to induce endothelial dysfunction [33,52]. By contrast, adiponectin, which is downregulated in obesity, has been shown to have salutary effects on the vasculature, including upregulation of nitric oxide and improvement of endothelial function [53,54]. The adipokine resistin was only described and named in 2001, but has already been associated with endothelial dysfunction through stimulation of endothelin-1 release, which fosters vasoconstriction [55].
Migration of immune cells from perivascular adipose tissue into the vasculature
In addition to their direct roles in mediating vascular function, adipokines may influence the migration of immune cells into the vascular wall and therefore promote inflammation and atherogenesis. The adipokines leptin, resistin, MCP-1 and IL-8 have all been shown to influence monocyte migration and activation into macrophages [16,55,56]. Once in the vascular space, these immune cells secrete additional proinflammatory cytokines. These include IL-1 and TNF-α, which influence interactions between leukocytes and the vasculature by increasing P-selectin-dependent leukocyte rolling and adhesion [57]. Infiltration of macrophages has been shown to be associated with endothelial dysfunction and insulin resistance in obesity [58]. Taken together, these results suggest an important role of adipokine-stimulated macrophages and T lymphocytes in the inflammatory process of atherogenesis [59].
The recognition that perivascular adipose inflammation potentially contributes to atherosclerosis has led to an ‘outside-in’ theory of vascular inflammation. Vascular inflammation was already believed to follow an ‘inside-out’ process in which intimal injury leads to expression of vascular adhesion molecules, release of inflammatory signals and subsequent homing of blood-borne immune cells to the endothelium [59]. This intimal inflammation then spreads into the media and adventitia [60]. The ‘outside-in’ theory postulates that inflammation begins in adipose tissue and then spreads inward to the vasculature [61]. This is supported by the lack of a fascial plane between the adventitia and surrounding adipose tissue, as well as the extension of the vasa vasorum into perivascular adipose tissue [62]. Consistent with this, immunostaining of atherosclerotic human aortas has shown the presence of inflammatory cells at the junction of perivascular adipose tissue and the vascular adventitia [16].
Perivascular adipokines & the vascular effects of insulin
A final link between adipokines, vascular dysfunction and cardiovascular disease involves the influence of perivascular adipokines on the vascular effects of insulin [14]. Insulin normally induces vasodilation in muscle tissue with high glucose uptake and vasoconstriction in muscle tissue with low glucose uptake [14,63]. This involves a delicate balance of the vasodilatory effects of nitric oxide and the vasoconstricting effects of endothelin-1 [64]. This insulin- mediated diversion of blood flow is a major determinant of whole-body insulin resistance. In obesity, nitric oxide production is reduced whereas endothelin-1 production is increased and capillary recruitment is reduced [14]. Adipokines secreted by perivascular adipose tissue are believed to contribute to these changes [14]. For example, TNF-α has been shown to impair insulin-induced vasodilation [46], possibly through reduced expression of the insulin receptor substrate 1. Adiponectin also influences insulin signaling, but in contrast to TNF-α and IL-6, is associated with increased sensitivity to insulin and increased glucose uptake [65,66].
In summary, increasing adiposity is associated with adipose tissue inflammation, which is initiated and perpetuated by dysregulation of adipokines. The anatomic proximity of perivascular adipose tissue to the vasculature has led researchers to investigate potential mechanisms by which dysregulation of adipokines in obesity and other disease states may have a local pathogenic effect on blood vessels. Multiple pathways have been identified through which adipokines probably contribute to the development of vascular disease. Changes in adipokine signaling are associated with inflammation, and inflammation is known to be associated with vascular disease. Adipokines stimulate immune cell migration into the vascular lumen through a potential ‘outside-in’ inflammatory cascade. However, inflammation may not be a prerequisite for changes in the vascular effects of perivascular adipose tissue. Adipokines have direct vascular effects on endothelial function and smooth muscle migration. Finally, adipokines appear to modulate the effect of insulin on the vasculature by contributing to changes in capillary recruitment. Overall, the exact biological cascade linking perivascular adipose tissue and changes in vascular function remains incompletely understood, but is probably multifaceted, with interconnected pathways.
Epidemiological studies of perivascular adipose tissue
In addition to the intriguing basic science research and small clinical studies, epidemiological studies have begun to explore associations between perivascular adipose tissue, metabolic risk factors and cardiovascular disease. Pericardial adipose tissue has been the most frequently studied perivascular fat depot from an epidemiological perspective. However, more recent work has also examined thoracic periaortic fat [8,67,68]. This section will first outline the imaging techniques used in epidemiological studies to quantitatively assess the different subtypes of perivascular adipose tissue. Then, the associations between perivascular adipose tissue and metabolic risk factors will be examined. Finally, associations between subtypes of perivascular adipose tissue and both indices of myocardial structure as well as clinical cardiovascular disease will be reviewed.
Imaging modalities used to quantify perivascular adipose tissue in human studies
The use of imaging modalities to quantitatively assess the amount of perivascular adipose tissue in specific perivascular fat depots has been fundamental to the epidemiological study of obesity and vascular disease. To date, most imaging of perivascular adipose tissue has focused on pericardial fat, which can be measured by ultrasound, multidetector computed tomography (MDCT) and MRI [7,69–71]. More recently, a reliable method for the assessment of periaortic fat volume has been developed [68]. The following section will review the advantages and disadvantages of the various imaging modalities (Table 1).
Table 1.
Advantages and disadvantages of various imaging modalities for assessing perivascular fat.
| Imaging modality |
Type(s) of perivascular fat imaged |
Advantages | Disadvantages | Ref. |
|---|---|---|---|---|
| Echocardiography | Epicardial fat | Safe Easy to perform Relatively inexpensive Frequently performed for other clinical indications |
Pericardial fat is only measured over the surface of the right ventricle, preventing volumetric assessment and not taking into account variations in fat deposition over the surface of the heart Can only assess pericardial and not other types of perivascular fat Obesity may limit image quality |
[9,63–66] |
| MDCT | Pericardial and/or epicardial fat Periaortic fat |
Easy to perform Often performed for other indications Allows volumetric assessment of adipose tissue Excellent reproducibility Can assess multiple fat depots Can simultaneously assess coronary calcium CT angiography can simultaneously assess coronary plaque |
Radiation exposure CT angiography requires iodinated contrast |
[7,59,66] |
| MRI | Pericardial and/or epicardial fat Periaortic fat |
Gold standard in assessment of visceral adipose tissue No radiation or iodinated contrast required Can simultaneously assess myocardial structure and function |
More expensive than either MDCT or echocardiography Examination is more time-consuming and less tolerated by patients |
[62,67] |
CT: Computed tomography; MDCT: Multidetector computed tomography.
Echocardiography
Echocardiography is widely performed for a variety of clinical indications, is comparatively low cost and does not involve exposure to radiation [72]. Epicardial fat can be measured as the echolucent space anterior to the right ventricle on both parasternal long- and short-axis views [9]. A major drawback of echocardiography is that measurements are based on a single point on the right ventricular free wall. This does not allow volumetric assessment and is subject to error, given the variation in adipose tissue thickness over the surface of the heart [73]. In addition, obesity may lead to poor echocardiographic image quality; indeed, the low intraobserver and interobserver variation in some studies may reflect these challenges [74]. By contrast to other imaging modalities, echocardiography only allows the assessment of epicardial and not other types of perivascular adipose tissue.
Multidetector computed tomography
Multidetector computed tomography overcomes many of the shortcomings of echocardiography. By contrast to the 2D measurements used in echocardiography, a 3D volume of adipose tissue is calculated [7,68]. Specific fat depot locations are determined by anatomic landmarks. Within these landmarks, adipose tissue pixels are identified by characteristic Hounsfield units.
On MDCT, low attenuation is specific to the identification of fat. Using a semiautomatic technique, standard software packages can quantify the low attenuation tissues and derive a measure of fat area or volume. For example, thoracic periaortic fat in the Framingham Heart Study (FHS) is defined anteriorly by the area immediately surrounding the thoracic aorta (defined by a line drawn horizontally through the esophagus, which is connected to the left costovertebral joint), and posteriorly by the right lateral border of the vertebral body and the anterior edge of the vertebral body. This results in a 6.75-cm column of fat (27 slices) surrounding the thoracic aorta [67]. These techniques have yielded excellent interobserver and intraobserver reproducibility [7,68]. Thoracic MDCT has the additional advantage of simultaneously assessing coronary calcification. The major disadvantage of MDCT is radiation exposure.
MRI
MRI assessment of adipose tissue shares many of the advantages of MDCT over echocardiography, including superior spatial resolution, volumetric assessment of adipose tissue and the ability to image multiple different types of perivascular adipose tissue [71]. Its advantages over MDCT include lack of radiation exposure and excellent assessment of the cardiac chamber and valvular structure and function [75]. However, spatial resolution of adipose tissue and imaging of the coronary vasculature with MRI is inferior to MDCT [73,75]. MRI is also more costly, more time-consuming and generally less tolerated by patients than both echocardiography and MDCT.
Epidemiological studies of epicardial & pericardial fat
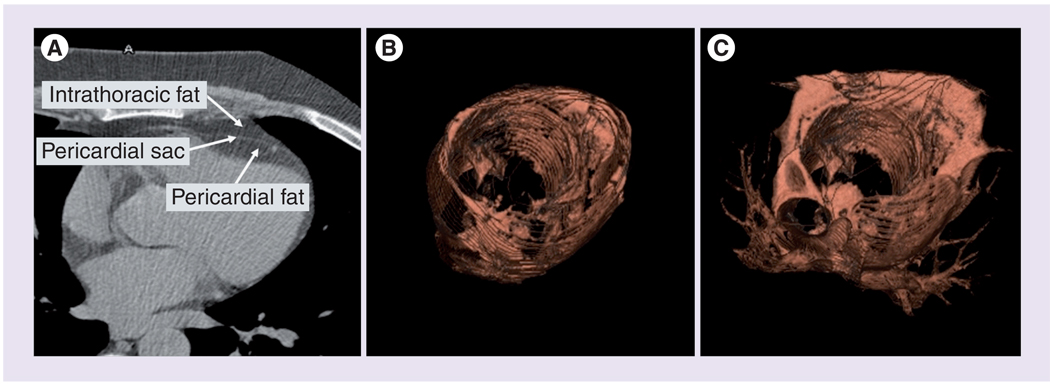
The associations of epicardial and pericardial fat with metabolic risk factors, indices of myocardial structure and function and clinical vascular disease have been assessed in epidemiological studies. These studies have used multiple imaging modalities, and the terminology differs depending on the technique used. Echocardiographic studies generally use the term ‘epicardial fat’, whereas MDCT studies generally use the term ‘pericardial fat’ [7,72]. Epicardial fat is defined anatomically as the fat located between the myocardium and visceral pericardium. Pericardial fat has been defined by MDCT in two ways. The first definition, used by the FHS, includes any fat located within the pericardial sac (Figure 2) [7]. The designation intrathoracic fat has been used by the FHS investigators to include all adipose tissue within the thoracic cavity excluding the adipose tissue within the pericardial sac [7]. The second definition of pericardial fat, used by the Multi-Ethnic Study of Atherosclerosis (MESA) and the Jackson Heart Study (JHS), includes both epicardial fat and paracardial fat. Paracardial fat includes fat covering the parietal pericardium.
Figure 2. CT imaging of pericardial and intrathoracic fat.
(A) Axial images demonstrating the anatomical landmarks, (B) 3D reconstruction of pericardial fat and (C) 3D reconstruction of total thoracic fat (pericardial plus intrathoracic fat). Pericardial fat in this figure is defined as all adipose tissue contained within the pericardium. Definitions of pericardial and epicardial fat differ in different epidemiologic studies.
Reproduced with permission from [4].
Association of epicardial & pericardial fat & metabolic risk factors
The first studies of epicardial adipose tissue and metabolic risk factors used echocardiographic assessment and analyzed relatively small numbers of patients. Associations were observed between epicardial fat and diastolic blood pressure, insulin levels and waist circumference [9]. Subsequently, MDCT was used in the FHS to examine the association of pericardial adipose tissue (defined as adipose tissue within the pericardial sac) and metabolic risk factors. This analysis included adjustments for standard cardiovascular risk factors, as well as additional adjustments for both clinical measures of adiposity (BMI and waist circumference) and volumetrically assessed VAT. In multivariable analysis, pericardial fat was associated with triglycerides, HDL, impaired fasting glucose and diabetes. After adjustment for clinical measures of adiposity, pericardial adipose tissue remained independently associated with the majority of these metabolic measurements. These results differed by gender and suggested that pericardial fat is associated with a more adverse risk factor profile in women compared with men. However, once the models were adjusted to account for VAT, pericardial fat was no longer associated with metabolic measurements. These results suggested that the association of pericardial fat and metabolic risk factors is driven primarily by its association with VAT. Similar results were observed in the JHS, a population-based study of African–Americans [76].
Pericardial fat & coronary heart disease
Multiple cohort studies have assessed the associations between pericardial fat and both sub-clinical and clinical coronary heart disease. In the FHS, pericardial fat (which is in anatomic contact with the coronary vasculature), but not intrathoracic fat (which is adherent to the pericardium but lacks direct contact with the coronary arteries), was independently associated with coronary artery calcification, even after adjustment for VAT [7]. Similar associations were observed in the FHS cohort with prevalent myocardial infarction [4]. A case–cohort study of the MESA study examined the association of pericardial fat (defined as both epicardial and paracardial fat) and incident coronary heart disease [77]. Pericardial fat was associated with coronary heart disease independently of BMI. Two additional studies examined the relationship between epicardial fat and atherosclerotic plaques. A study in The Netherlands found an association between volume of epicardial fat, assessed by computed tomography, and the degree of stenosis on conventional angiography only in individuals with a BMI less than 27.5 kg/m2 [78]. A related study found an association between pericoronary fat, defined as fat immediately surrounding the coronary arteries, and coronary plaques within the same arterial segment by MDCT in patients with suspected coronary heart disease [79]. This relationship persisted after adjustment for total pericardial adipose tissue volume. Taken together, these studies suggest a local effect of pericardial fat on both subclinical and clinical coronary heart disease.
Epicardial & pericardial fat & indices of myocardial structure & function
Epicardial and pericardial fat have also been studied in relation to cardiovascular structure and function. Early studies using echocardiography demonstrated an association between epicardial fat and left ventricular mass, left atrial dimension and abnormal diastolic filling [80,81], leading investigators to conclude that epicardial fat is an important correlate of indices of myocardial structure and function. However, an important limitation of these studies was the lack of adjustment for other fat stores, preventing an assessment of whether the observed associations were solely due to generalized adiposity. This specific question was subsequently explored in an analysis of pericardial fat and measures of myocardial structure and function as assessed by MRI [82]. Strong associations were found between pericardial fat and left ventricular mass, but these results were attenuated after additional adjustment for VAT. These findings suggest that associations between pericardial fat and myocardial function may be driven by the strong correlation between pericardial fat and VAT. One notable exception was identified: left atrial volume in men was independently associated with pericardial fat, even after additional adjustment for VAT.
Pericardial fat & atrial fibrillation
The association between pericardial fat and left atrial size may be relevant to recent literature demonstrating cross-sectional associations between pericardial fat and atrial fibrillation [83,84]. This association was independent of VAT. Taken together with the findings on myocardial structure, these findings suggest that the association of pericardial adipose tissue and atrial fibrillation may be related to a local pathologic effect of pericardial fat on the left atrium. Thus, the potential adverse local associations of pericardial fat may not be restricted to the development of atherosclerosis.
Epidemiological studies of periaortic fat
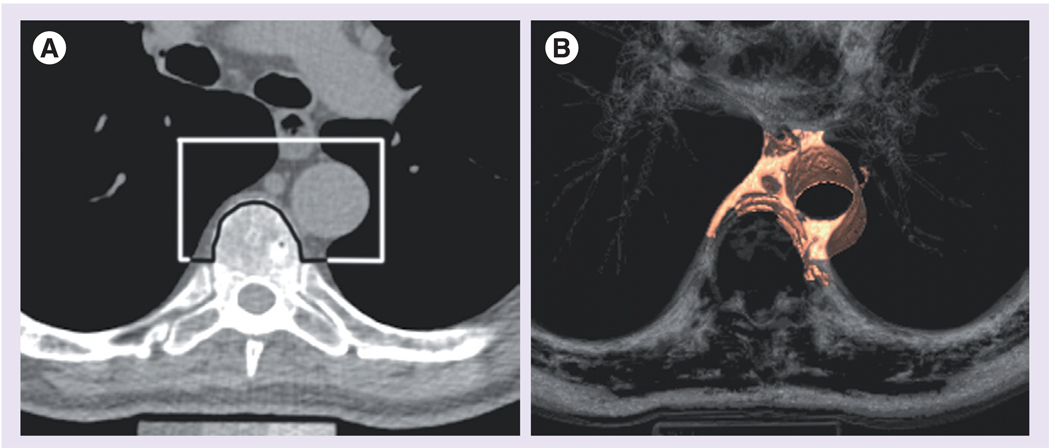
Techniques to measure periaortic fat using MDCT imaging have recently been described [68], allowing for the association of periaortic fat with metabolic risk factors and clinical vascular disease to be examined [8,67,68]. Adipose tissue is identified by characteristic Hounsfield units on MDCT [68]. Thoracic periaortic fat can be separated from surrounding anatomical structures including the esophagus, the costovertebral joints and vertebral bodies (Figure 3). By contrast, measurement of abdominal periaortic fat is more challenging due to its close relationship with aortic diameter and its contiguity with retroperitoneal fat without a clearly visualized retroperitoneal lining. For this reason, epidemiological studies have focused on thoracic periaortic fat.
Figure 3. CT imaging of thoracic periaortic fat.
(A) Axial images demonstrating the schematic border around the thoracic aorta and (B) the corresponding 3D reconstruction.
Reproduced with permission from [67].
Thoracic periaortic fat & metabolic risk factors
Associations of thoracic periaortic fat with metabolic risk factors have been examined using MDCT [8]. As has been observed in other fat depots, correlations between thoracic periaortic fat and cardiometabolic risk factors tend to be stronger in women. Thoracic periaortic fat was also strongly correlated with VAT (r = 0.75; p < 0.001) in both sexes. Similar to pericardial fat, thoracic periaortic fat was associated with fasting glucose and triglycerides in multivariable regression analysis, even after adjustment for BMI and waist circumference. Findings after adjustment for VAT were also similar to pericardial fat. Specifically, thoracic periaortic fat was no longer associated with metabolic risk factors, suggesting that the association of periaortic fat and risk factors is driven primarily by its association with VAT.
Periaortic fat & vascular disease
Given the suggestion of a local effect of pericardial fat on the coronary vasculature, related studies have assessed whether a similar association exists for thoracic periaortic fat and subclinical and clinical vascular diseases [8,67]. Unlike the findings with pericardial fat and coronary calcium, an independent association was not observed between thoracic periaortic fat and thoracic calcification after adjustment for cardiovascular disease risk factors. This may be due to a biologically different association of periaortic fat with the vasculature compared with pericardial fat. However, independent associations were found between thoracic periaortic fat and both coronary and abdominal aortic calcification. The inability of the protocol to determine aortic arch calcification may have limited the thoracic periaortic fat analysis and may explain the lack of association. This is supported by the finding of an independent association between thoracic periaortic fat and clinical vascular disease, specifically peripheral artery disease, even after adjustment for both cardiovascular risk factors and VAT [67]. A similar association was not observed between BMI, waist circumference or VAT and peripheral artery disease. Although causality can not be inferred due to the cross-sectional nature of the study, these findings suggest the possibility of a local influence of periaortic fat on the vasculature. Given the difference in the associations between thoracic periaortic fat and subclinical as opposed to clinical vascular disease, additional research will hopefully clarify the complex biology of perivascular adipose tissue and vascular disease.
Future directions
Since the initial recognition that adipose tissue is a highly active endocrine organ, there has been a substantial body of research devoted to this topic, much of which is directly applicable to perivascular adipose tissue. In addition, an array of basic science, clinical and epidemiological research is now devoted specifically to perivascular adipose tissue. From these studies, a growing appreciation of the unique role of perivascular adipose tissue as a pathogenic fat depot with local effects on the vasculature has emerged.
The current body of work related to perivascular adipose tissue has uncovered areas of uncertainty and provides a foundation for future studies. Most of the basic science and small clinical studies to date have been limited to animal models. These have been crucial to the current understanding of the biology of perivascular adipose tissue and will continue to provide important insights. Additional investigations in human subjects will also be essential. A more detailed characterization of the metabolic activity of perivascular adipose tissue, both in states of health and disease, is required. Further research is necessary in order to better understand the changes in perivascular adipose tissue with the development of obesity and clinical vascular disease, including atherosclerosis and hypertension. Comparisons are needed between perivascular adipose tissue and both subcutaneous adipose tissue and VAT, as well as between different subtypes of perivascular adipose tissue. Differences may exist depending on the size of the arteries the fat surrounds or the organs those arteries supply.
Any differences may have important implications in terms of the influence of perivascular adipose tissue on the vasculature. In addition, any effect of weight loss on the biology of perivascular adipose tissue and vascular function has important implications for the prevention of obesity-mediated vascular disease.
Further epidemiological studies are equally important. Volumetric assessment of other organ-specific perivascular adipose tissue depots might be developed in the future. Further epidemiological studies can continue to clarify the similar and different associations between various perivascular adipose tissue depots and cardiometabolic disease. In addition, the majority of current studies of perivascular adipose tissue are cross-sectional, and longitudinal analyses are necessary. Epidemiological studies might also enable an understanding of the genetic correlates of perivascular fat. The genetics of body fat distribution is a nascent but growing field. A recent publication identifying 14 loci for body fat distribution independent of BMI is proof-of-principle that such loci exist [85]. Current work is now focusing on the genetics of perivascular fat including periaortic fat and pericardial fat.
Conclusion
Obesity will remain one of the most important worldwide public health challenges. Additional research to understand the adverse consequences of excess body weight is critical, particularly in relation to the development of clinical vascular disease. The growing understanding of the biology of perivascular adipose tissue and its potential role in vascular morbidity and mortality is an exciting new avenue of investigation with important advances anticipated in the coming years.
Future perspective
As research continues in this field, the understanding of the biology of perivascular adipose tissue and its effect on the vasculature, both in states of health and disease, will rapidly expand. As this occurs, the next step will be the crossover of the science from experimental and epidemiological studies into clinical applications. Weight management will certainly remain the cornerstone of therapy for obesity. However, the amount of perivascular adipose tissue may provide additional prognostic information for patients beyond that of generalized adiposity. In addition, increasing knowledge about perivascular adipose tissue may eventually be used to develop therapies that are aimed at ameliorating obesity-mediated vascular disease.
Executive summary
Perivascular adipose tissue anatomy
Perivascular adipose tissue surrounds blood vessels.
Perivascular adipose tissue and the adventitial layer of blood vessels are in direct contact with each other.
Healthy perivascular adipose tissue secretes adipokines and regulates vascular function.
Further understanding of the mediators and effects of healthy perivascular adipokines on vascular function is necessary.
Proposed mechanisms linking perivascular adipose tissue with vascular disease
Obesity is associated with changes in adipokine secretion and the resultant inflammation of perivascular adipose tissue.
The dysregulation of adipokines changes the effect of perivascular adipose tissue on the vasculature.
Changes in perivascular adipokine secretion in obesity appear to contribute to the development of obesity-mediated vascular disease.
Mechanisms by which perivascular adipose tissue negatively influences the vasculature in obesity include direct effects on the vasculature, stimulation of immune cell migration into the vascular lumen and alterations in insulin-mediated capillary recruitment.
An understanding of the differences in adipokine secretion between perivascular adipose tissue and other fat depots, as well as between subtypes of perivascular adipose tissue, is needed.
Additional research is necessary to fully understand the multitude of vascular effects of perivascular adipose tissue.
Epidemiology of perivascular adipose tissue
Imaging modalities including echocardiography, multidetector computed tomography and MRI have been essential in the study of perivascular adipose tissue in epidemiological studies.
Epidemiological studies have primarily focused on two subtypes of perivascular adipose tissue: pericardial fat and periaortic fat.
Associations between perivascular adipose tissue and metabolic risk factors and subclinical and clinical cardiovascular disease have been demonstrated in epidemiological studies.
Additional longitudinal studies will be helpful to further elucidate the association of varying types of perivascular adipose tissue with cardiovascular disease.
Footnotes
Financial & competing interests disclosure
Caroline Fox is a member of the NIH Intramural Research Program. The authors have no other relevant affiliations or financial involvement with any organization or entity with a financial interest in or financial conflict with the subject matter or materials discussed in the manuscript apart from those disclosed.
No writing assistance was utilized in the production of this manuscript.
Bibliography
Papers of special note have been highlighted as:
▪ of interest
▪▪ of considerable interest
- 1.Caterson I, Hubbard V, Bray G, et al. Prevention Conference VII: Obesity, a worldwide epidemic related to heart disease and stroke: Group III: worldwide comorbidities of obesity. Circulation. 2004;110:E476–E483. doi: 10.1161/01.CIR.0000140114.83145.59. [DOI] [PubMed] [Google Scholar]
- 2.Canoy D. Distribution of body fat and risk of coronary heart disease in men and women. Curr. Opin. Cardiol. 2008;23:591–598. doi: 10.1097/HCO.0b013e328313133a. [DOI] [PubMed] [Google Scholar]
- 3.Fox CS, Massaro JM, Hoffmann U, et al. Abdominal visceral and subcutaneous adipose tissue compartments: association with metabolic risk factors in the Framingham Heart Study. Circulation. 2007;116:39–48. doi: 10.1161/CIRCULATIONAHA.106.675355. [DOI] [PubMed] [Google Scholar]
- 4. Mahabadi AA, Massaro JM, Rosito GA, et al. Association of pericardial fat, intrathoracic fat, and visceral abdominal fat with cardiovascular disease burden: the Framingham Heart Study. Eur. Heart J. 2009;30:850–856. doi: 10.1093/eurheartj/ehn573.▪ Demonstrates a cross-sectional association between increasing amounts of pericardial fat and prevalent cardiovascular disease
- 5.Wang Y, Rimm EB, Stampfer MJ, Willett WC, Hu FB. Comparison of abdominal adiposity and overall obesity in predicting risk of Type 2 diabetes among men. Am. J. Clin. Nutr. 2005;81:555–563. doi: 10.1093/ajcn/81.3.555. [DOI] [PubMed] [Google Scholar]
- 6.Montani JP, Carroll JF, Dwyer TM, Antic V, Yang Z, Dulloo AG. Ectopic fat storage in heart, blood vessels and kidneys in the pathogenesis of cardiovascular diseases. Int. J. Obes. Relat. Metab. Disord. 2004;28:S58–S65. doi: 10.1038/sj.ijo.0802858. [DOI] [PubMed] [Google Scholar]
- 7. Rosito GA, Massaro JM, Hoffmann U, et al. Pericardial fat, visceral abdominal fat, cardiovascular disease risk factors, and vascular calcification in a community-based sample: the Framingham Heart Study. Circulation. 2008;117:605–613. doi: 10.1161/CIRCULATIONAHA.107.743062.▪▪ Demonstrates a cross-sectional association between pericardial fat and certain metabolic risk factors after adjustment for clinical measures of adiposity, as well as an association between pericardial fat and coronary calcium that remains after adjustment for cardiovascular risk factors and visceral adipose tissue, suggesting a potential local toxic effect of pericardial fat on the coronary vasculature.
- 8.Lehman SJ, Massaro JM, Schlett CL, O’Donnell CJ, Hoffmann U, Fox CS. Peri-aortic fat, cardiovascular disease risk factors, and aortic calcification: the Framingham Heart Study. Atherosclerosis. 2010;210:656–661. doi: 10.1016/j.atherosclerosis.2010.01.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Iacobellis G, Ribaudo MC, Assael F, et al. Echocardiographic epicardial adipose tissue is related to anthropometric and clinical parameters of metabolic syndrome: a new indicator of cardiovascular risk. J. Clin. Endocrinol. Metab. 2003;88:5163–5168. doi: 10.1210/jc.2003-030698. [DOI] [PubMed] [Google Scholar]
- 10.Gao YJ. Dual modulation of vascular function by perivascular adipose tissue and its potential correlation with adiposity/lipoatrophy-related vascular dysfunction. Curr. Pharm. Des. 2007;13:2185–2192. doi: 10.2174/138161207781039634. [DOI] [PubMed] [Google Scholar]
- 11.Sacks HS, Fain JN, Holman B, et al. Uncoupling protein-1 and related messenger ribonucleic acids in human epicardial and other adipose tissues: epicardial fat functioning as brown fat. J. Clin. Endocrinol. Metab. 2009;94:3611–3615. doi: 10.1210/jc.2009-0571. [DOI] [PubMed] [Google Scholar]
- 12. Yudkin JS, Eringa E, Stehouwer CDA. ‘Vasocrine’ signaling from perivascular fat: a mechanism linking insulin resistance to vascular disease. Lancet. 2005;365:1817–1820. doi: 10.1016/S0140-6736(05)66585-3.▪▪ Describes the ‘vasocrine’ signaling hypothesis of perivascular fat. Speculates that perivascular fat may modulate the effect of insulin on the vasculature.
- 13. Chatterjee TK, Stoll LL, Denning GM, et al. Proinflammatory phenotype of perivascular adipocytes: influence of high-fat feeding. Circ. Res. 2009;104:541–549. doi: 10.1161/CIRCRESAHA.108.182998.▪▪ Demonstrates that human pericoronary adipose tissue has a proinflammatory adipokine profile and that high-fat feeding leads to upregulation of proinflammatory gene expression in murine perivascular adipose tissue
- 14.Eringa EC, Bakker W, Smulders YM, Serné EH, Yudkin JS, Stehouwer CDA. Regulation of vascular function and insulin sensitivity by adipose tissue: focus on perivascular adipose tissue. Microcirculation. 2007;14:389–402. doi: 10.1080/10739680701303584. [DOI] [PubMed] [Google Scholar]
- 15.Vela D, Buja LM, Madjid M, et al. The role of periadventitial fat in atherosclerosis. Arch. Pathol. Lab. Med. 2007;131:481–487. doi: 10.5858/2007-131-481-TROPFI. [DOI] [PubMed] [Google Scholar]
- 16.Henrichot E, Juge-Aubry CE, Pernin A, et al. Production of chemokines by perivascular adipose tissue: a role in the pathogenesis of atherosclerosis? Arterioscler. Thromb. Vasc. Biol. 2005;25:2594–2599. doi: 10.1161/01.ATV.0000188508.40052.35. [DOI] [PubMed] [Google Scholar]
- 17.Mazurek T, Zhang L, Zalewski A, et al. Human epicardial adipose tissue is a source of inflammatory mediators. Circulation. 2003;108:2460–2466. doi: 10.1161/01.CIR.0000099542.57313.C5. [DOI] [PubMed] [Google Scholar]
- 18.Weisberg SP, McCann D, Desai M, Rosenbaum M, Leibel RL, Ferrante AW. Obesity is associated with macrophage accumulation in adipose tissue. J. Clin. Invest. 2003;112:1796–1808. doi: 10.1172/JCI19246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Karastergiou K, Mohamed-Ali V. The autocrine and paracrine roles of adipokines. Mol. Cell. Endocrinol. 2010;318:69–78. doi: 10.1016/j.mce.2009.11.011. [DOI] [PubMed] [Google Scholar]
- 20.Halberg N, Wernstedt I, Scherer PE. The adipocyte as an endocrine cell. Endocrinol. Metab. Clin. North Am. 2008;37:753–768. doi: 10.1016/j.ecl.2008.07.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Kintscher U, Hartge M, Hess K, et al. T-lymphocyte infiltration in visceral adipose tissue: a primary event in adipose tissue inflammation and the development of obesity-mediated insulin resistance. Arterioscler. Thromb. Vasc. Biol. 2008;28:1304–1310. doi: 10.1161/ATVBAHA.108.165100. [DOI] [PubMed] [Google Scholar]
- 22.Moos MPW, John N, Grabner R, et al. The lamina adventitia is the major site of immune-cell accumulation in standard chow-fed apolipoprotein E-deficient mice. Arterioscler. Thromb. Vasc. Biol. 2005;25:2386–2391. doi: 10.1161/01.ATV.0000187470.31662.fe. [DOI] [PubMed] [Google Scholar]
- 23.Soltis E, Cassis L. Influence of perivascular adipose tissue on rat aortic smooth muscle responsiveness. Clin. Exp. Hypertens. A. 1991;13:277–296. doi: 10.3109/10641969109042063. [DOI] [PubMed] [Google Scholar]
- 24.Lohn M, Dubrovska G, Lauterbach B, Luft FC, Gollasch M, Sharma AM. Periadventitial fat releases a vascular relaxing factor. FASEB J. 2002;16:1057–1063. doi: 10.1096/fj.02-0024com. [DOI] [PubMed] [Google Scholar]
- 25.Gao YJ, Lu C, Su LY, Sharma AM, Lee RMKW. Modulation of vascular function by perivascular adipose tissue: the role of endothelium and hydrogen peroxide. Br. J. Pharmacol. 2007;151:323–331. doi: 10.1038/sj.bjp.0707228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Verlohren S, Dubrovska G, Tsang S-Y, et al. Visceral periadventitial adipose tissue regulates arterial tone of mesenteric arteries. Hypertension. 2004;44:271–276. doi: 10.1161/01.HYP.0000140058.28994.ec. [DOI] [PubMed] [Google Scholar]
- 27.Schleifenbaum J, Kohn C, Voblova N, et al. Systemic peripheral artery relaxation by KCNQ channel openers and hydrogen sulfide. J. Hypertens. 2010;28:1875–1882. doi: 10.1097/HJH.0b013e32833c20d5. [DOI] [PubMed] [Google Scholar]
- 28. Greenstein AS, Khavandi K, Withers SB, et al. Local inflammation and hypoxia abolish the protective anticontractile properties of perivascular fat in obese patients. Circulation. 2009;119:1661–1670. doi: 10.1161/CIRCULATIONAHA.108.821181.▪▪ Demonstrates a vasodilatory effect of healthy human perivascular adipose tissue. This effect was lost and accompanied by immunohistochemical evidence of inflammation in obese subjects. The application of TNF-α to healthy perivascular fat was shown to reduce vasodilator activity
- 29.Payne GA, Borbouse L, Bratz IN, et al. Endogenous adipose-derived factors diminish coronary endothelial function via inhibition of nitric oxide synthase. Microcirculation. 2008;15:417–426. doi: 10.1080/10739680701858447. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Payne GA, Bohlen HG, Dincer UD, Borbouse L, Tune JD. Periadventitial adipose tissue impairs coronary endothelial function via PKC-β-dependent phosphorylation of nitric oxide synthase. Am. J. Physiol. Heart Circ. Physiol. 2009;297:H460–H465. doi: 10.1152/ajpheart.00116.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Lee RMKW, Ding L, Lu C, Su L-Y, Gao Y-J. Alteration of perivascular adipose tissue function in angiotensin II-induced hypertension. Can. J. Physiol. Pharmacol. 2009;87:944–953. doi: 10.1139/y09-088. [DOI] [PubMed] [Google Scholar]
- 32.Lee RMKW, Lu C, Su L-Y, Werstuck G, Gao Y-J. Effects of hyperglycemia on the modulation of vascular function by perivascular adipose tissue. J. Hypertens. 2009;27:118–131. doi: 10.1097/HJH.0b013e3283163cc9. [DOI] [PubMed] [Google Scholar]
- 33.Payne GA, Borbouse L, Kumar S, et al. Epicardial perivascular adipose-derived leptin exacerbates coronary endothelial dysfunction in metabolic syndrome via a protein kinase C-β pathway. Arterioscler. Thromb. Vasc. Biol. 2010;30:1711–1717. doi: 10.1161/ATVBAHA.110.210070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Galic S, Oakhill JS, Steinberg GR. Adipose tissue as an endocrine organ. Mol. Cell. Endocrinol. 2010;316:129–139. doi: 10.1016/j.mce.2009.08.018. [DOI] [PubMed] [Google Scholar]
- 35.Neels JG, Olefsky JM. Inflamed fat: what starts the fire? J. Clin. Invest. 2006;116:33–35. doi: 10.1172/JCI27280. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Sartipy P, Loskutoff DJ. Monocyte chemoattractant protein 1 in obesity and insulin resistance. Proc. Natl Acad. Sci. USA. 2003;100:7265–7270. doi: 10.1073/pnas.1133870100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Lumeng CN, Bodzin JL, Saltiel AR. Obesity induces a phenotypic switch in adipose tissue macrophage polarization. J. Clin. Invest. 2007;117:175–184. doi: 10.1172/JCI29881. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Wellen KE, Hotamisligil GKS. Obesity-induced inflammatory changes in adipose tissue. J. Clin. Invest. 2003;112:1785–1788. doi: 10.1172/JCI20514. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Wimalasundera R, Fexby S, Regan L, Thom S, Hughes A. Effect of tumor necrosis factor-α and interleukin-1β on endothelium-dependent relaxation in rat mesenteric resistance arteries in vitro. Br. J. Pharmacol. 2003;138:1285–1294. doi: 10.1038/sj.bjp.0705168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Salgado-Somoza A, Teijeira-Fernandez E, Fernandez AL, Gonzalez-Juanatey JR, Eiras S. Proteomic analysis of epicardial and subcutaneous adipose tissue reveals differences in proteins involved in oxidative stress. Am. J. Physiol. Heart Circ. Physiol. 2010;299:H202–H209. doi: 10.1152/ajpheart.00120.2010. [DOI] [PubMed] [Google Scholar]
- 41.DeMarco V, Johnson M, Whaley-Connell A, Sowers J. Cytokine abnormalities in the etiology of the cardiometabolic syndrome. Curr. Hypertens. Rep. 2010;12:93–98. doi: 10.1007/s11906-010-0095-5. [DOI] [PubMed] [Google Scholar]
- 42.Tsioufis C, Dimitriadis K, Selima M, et al. Low-grade inflammation and hypoadiponectinaemia have an additive detrimental effect on aortic stiffness in essential hypertensive patients. Eur. Heart J. 2007;28:1162–1169. doi: 10.1093/eurheartj/ehm089. [DOI] [PubMed] [Google Scholar]
- 43.Barandier C, Montani J-P, Yang Z. Mature adipocytes and perivascular adipose tissue stimulate vascular smooth muscle cell proliferation: effects of aging and obesity. Am. J. Physiol. Heart Circ. Physiol. 2005;289:H1807–H1813. doi: 10.1152/ajpheart.01259.2004. [DOI] [PubMed] [Google Scholar]
- 44.Zhang H, Zhang C. Regulation of microvascular function by adipose tissue in obesity and Type 2 diabetes: evidence of an adipose-vascular loop. Am. J. Biomed. Sci. 2009;1:133. doi: 10.5099/aj090200133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Guzik TJ, Hoch NE, Brown KA, et al. Role of the T cell in the genesis of angiotensin II-induced hypertension and vascular dysfunction. J. Exp. Med. 2007;204:2449–2460. doi: 10.1084/jem.20070657. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Eringa EC, Stehouwer CDA, Walburg K, et al. Physiological concentrations of insulin induce endothelin-dependent vasoconstriction of skeletal muscle resistance arteries in the presence of tumor necrosis factor-α dependence on c-Jun N-terminal kinase. Arterioscler. Thromb. Vasc. Biol. 2006;26:274–280. doi: 10.1161/01.ATV.0000198248.19391.3e. [DOI] [PubMed] [Google Scholar]
- 47.Eringa EC, Stehouwer CDA, Roos MH, Westerhof N, Sipkema P. Selective resistance to vasoactive effects of insulin in muscle resistance arteries of obese zucker (fa/fa) rats. Am. J. Physiol. Endocrinol. Metab. 2007;293:E1134–E1139. doi: 10.1152/ajpendo.00516.2006. [DOI] [PubMed] [Google Scholar]
- 48.Guzik T, Mangalat D, Korbut R. Adipocytokines – novel link between inflammation and vascular function? J. Physiol. Pharmacol. 2007;54:505–528. [PubMed] [Google Scholar]
- 49.Gao Y-J, Holloway AC, Zeng Z-H, et al. Prenatal exposure to nicotine causes postnatal obesity and altered perivascular adipose tissue function. Obesity. 2005;13:687–692. doi: 10.1038/oby.2005.77. [DOI] [PubMed] [Google Scholar]
- 50.Hotamisligil GS, Arner P, Caro JF, Atkinson RL, Spiegelman BM. Increased adipose tissue expression of tumor necrosis factor-α in human obesity and insulin resistance. J. Clin. Invest. 1995;95:2409–2415. doi: 10.1172/JCI117936. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Mohamed-Ali V, Goodrick S, Rawesh A, et al. Subcutaneous adipose tissue releases interleukin-6, but not tumor necrosis factor-α, in vivo. J. Clin. Endocrinol. Metab. 1997;82:4196–4200. doi: 10.1210/jcem.82.12.4450. [DOI] [PubMed] [Google Scholar]
- 52.Tune JD, Considine R. Effects of leptin on cardiovascular physiology. J. Am. Soc. Hypertens. 2007;1:231–241. doi: 10.1016/j.jash.2007.04.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Chen H, Montagnani M, Funahashi T, Shimomura I, Quon MJ. Adiponectin stimulates production of nitric oxide in vascular endothelial cells. J. Biol. Chem. 2003;278:45021–45026. doi: 10.1074/jbc.M307878200. [DOI] [PubMed] [Google Scholar]
- 54.Gustafsson S, Lind L, Soderberg S, Ingelsson E. Associations of circulating adiponectin with measures of vascular function and morphology. J. Clin. Endocrinol. Metab. 2010;95:2927–2934. doi: 10.1210/jc.2009-2685. [DOI] [PubMed] [Google Scholar]
- 55.Knudson JD, Dick GM, Tune JD. Adipokines and coronary vasomotor dysfunction. Exp. Biol. Med. 2007;232:727–736. [PubMed] [Google Scholar]
- 56.Weber C, Schober A, Zernecke A. Chemokines: key regulators of mononuclear cell recruitment in atherosclerotic vascular disease. Arterioscler. Thromb. Vasc. Biol. 2004;24:1997–2008. doi: 10.1161/01.ATV.0000142812.03840.6f. [DOI] [PubMed] [Google Scholar]
- 57.Thorlacius H, Lindbom L, Raud J. Cytokine-induced leukocyte rolling in mouse cremaster muscle arterioles in P-selectin dependent. Am. J. Physiol. Heart Circ. Physiol. 1997;272:H1725–H1729. doi: 10.1152/ajpheart.1997.272.4.H1725. [DOI] [PubMed] [Google Scholar]
- 58.Apovian CM, Bigornia S, Mott M, et al. Adipose macrophage infiltration is associated with insulin resistance and vascular endothelial dysfunction in obese subjects. Arterioscler. Thromb. Vasc. Biol. 2008;28:1654–1659. doi: 10.1161/ATVBAHA.108.170316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Libby P, Ridker PM, Maseri A. Inflammation and atherosclerosis. Circulation. 2002;105:1135–1143. doi: 10.1161/hc0902.104353. [DOI] [PubMed] [Google Scholar]
- 60.Moreno PR, Purushothaman KR, Fuster V, O’Connor WN. Intimomedial interface damage and adventitial inflammation is increased beneath disrupted atherosclerosis in the aorta: implications for plaque vulnerability. Circulation. 2002;105:2504–2511. doi: 10.1161/01.cir.0000017265.52501.37. [DOI] [PubMed] [Google Scholar]
- 61.Maiellaro K, Taylor WR. The role of the adventitia in vascular inflammation. Cardiovasc. Res. 2007;75:640–648. doi: 10.1016/j.cardiores.2007.06.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Chaldakov GN, Tonchev AB, Stankulov IS, et al. Periadventitial adipose tissue (tunica adiposa): enemy or friend around? Arch. Pathol. Lab. Med. 2007;131:1766–1768. doi: 10.5858/2007-131-1766a-PATTAE. [DOI] [PubMed] [Google Scholar]
- 63.Clark M, Wallis M, Barrett E, et al. Blood flow and muscle metabolism: a focus on insulin action. Am. J. Physiol. Endocrinol. Metab. 2003;284:E241–E258. doi: 10.1152/ajpendo.00408.2002. [DOI] [PubMed] [Google Scholar]
- 64.Cardillo C, Nambi SS, Kilcoyne CM, et al. Insulin stimulates both endothelin and nitric oxide activity in the human forearm. Circulation. 1999;100:820–825. doi: 10.1161/01.cir.100.8.820. [DOI] [PubMed] [Google Scholar]
- 65.Hopkins TA, Ouchi N, Shibata R, Walsh K. Adiponectin actions in the cardiovascular system. Cardiovasc. Res. 2007;74:11–18. doi: 10.1016/j.cardiores.2006.10.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Yamauchi T, Kamon J, Minokoshi Y, et al. Adiponectin stimulates glucose utilization and fatty-acid oxidation by activating AMP-activated protein kinase. Nat. Med. 2002;8:1288–1295. doi: 10.1038/nm788. [DOI] [PubMed] [Google Scholar]
- 67.Fox CS, Massaro JM, Schlett CL, et al. Peri-aortic fat deposition is associated with peripheral arterial disease: the Framingham Heart Study. Circ. Cardiovasc. Imaging. 2010;3:515–519. doi: 10.1161/CIRCIMAGING.110.958884. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Schlett CL, Massaro JM, Lehman SJ, et al. Novel measurements of periaortic adipose tissue in comparison to anthropometric measures of obesity, and abdominal adipose tissue. Int. J. Obes. 2009;33:226–232. doi: 10.1038/ijo.2008.267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Pietrobelli A, Boner AL, Tato L. Adipose tissue and metabolic effects: new insight into measurements. Int. J. Obes. Relat. Metab. Disord. 2005;29:S97–S100. doi: 10.1038/sj.ijo.0803079. [DOI] [PubMed] [Google Scholar]
- 70.Maurovich-Horvat P, Massaro J, Fox CS, Moselewski F, O’Donnell CJ, Hoffmann U. Comparison of anthropometric, area- and volume-based assessment of abdominal subcutaneous and visceral adipose tissue volumes using multidetector computed tomography. Int. J. Obes. 2007;31:500–506. doi: 10.1038/sj.ijo.0803454. [DOI] [PubMed] [Google Scholar]
- 71.Fluchter S, Haghi D, Dinter D, et al. Volumetric assessment of epicardial adipose tissue with cardiovascular magnetic resonance imaging. Obesity. 2007;15:870–878. doi: 10.1038/oby.2007.591. [DOI] [PubMed] [Google Scholar]
- 72.Iacobellis G, Assael F, Ribaudo MC, et al. Epicardial fat from echocardiography: a new method for visceral adipose tissue prediction. Obesity. 2003;11:304–310. doi: 10.1038/oby.2003.45. [DOI] [PubMed] [Google Scholar]
- 73.Abbara S, Desai JC, Cury RC, Butler J, Nieman K, Reddy V. Mapping epicardial fat with multidetector computed tomography to facilitate percutaneous transepicardial arrhythmia ablation. Eur. J. Radiol. 2006;57:417–422. doi: 10.1016/j.ejrad.2005.12.030. [DOI] [PubMed] [Google Scholar]
- 74.Saura D, Oliva MJ, Rodríguez D, et al. Reproducibility of echocardiographic measurements of epicardial fat thickness. Int. J. Cardiol. 2008;141:311–313. doi: 10.1016/j.ijcard.2008.11.127. [DOI] [PubMed] [Google Scholar]
- 75.Pennell DJ. Cardiovascular magnetic resonance. Circulation. 2010;121:692–705. doi: 10.1161/CIRCULATIONAHA.108.811547. [DOI] [PubMed] [Google Scholar]
- 76.Liu J, Fox CS, Hickson D, et al. Pericardial adipose tissue, atherosclerosis, and cardiovascular disease risk factors. Diabetes Care. 2010;33:1635–1639. doi: 10.2337/dc10-0245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77. Ding J, Hsu F-C, Harris TB, et al. The association of pericardial fat with incident coronary heart disease: the Multi-Ethnic Study of Atherosclerosis (MESA) Am. J. Clin. Nutr. 2009;90:499–504. doi: 10.3945/ajcn.2008.27358.▪ Demonstrates an association between pericardial fat and incident coronary heart disease after adjustment for BMI and cardiovascular risk factors in a case-cohort study
- 78.Gorter PM, de Vos AM, van der Graaf Y, et al. Relation of epicardial and pericoronary fat to coronary atherosclerosis and coronary artery calcium in patients undergoing coronary angiography. Am. J. Cardiol. 2008;102:380–385. doi: 10.1016/j.amjcard.2008.04.002. [DOI] [PubMed] [Google Scholar]
- 79. Mahabadi AA, Reinsch N, Lehmann N, et al. Association of pericoronary fat volume with atherosclerotic plaque burden in the underlying coronary artery: a segment analysis. Atherosclerosis. 2010;211:195–199. doi: 10.1016/j.atherosclerosis.2010.02.013.▪ Demonstrates a cross-sectional association between pericoronary fat and atherosclerotic plaque in the same coronary segment by computed tomography angiography
- 80.Iacobellis G, Leonetti F, Singh N, Sharma A. Relationship of epicardial adipose tissue with atrial dimensions and diastolic function in morbidly obese subjects. Int. J. Cardiol. 2007;115:272–273. doi: 10.1016/j.ijcard.2006.04.016. [DOI] [PubMed] [Google Scholar]
- 81.Iacobellis G, Ribaudo MC, Zappaterreno A, Iannucci CV, Leonetti F. Relation between epicardial adipose tissue and left ventricular mass. Am. J. Cardiol. 2004;94:1084–1087. doi: 10.1016/j.amjcard.2004.06.075. [DOI] [PubMed] [Google Scholar]
- 82.Fox CS, Gona P, Hoffmann U, et al. Pericardial fat, intrathoracic fat, and measures of left ventricular structure and function: the Framingham Heart Study. Circulation. 2009;119:1586–1591. doi: 10.1161/CIRCULATIONAHA.108.828970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Thanassoulis G, Massaro JM, O’Donnell CJ, et al. Pericardial fat is associated with prevalent atrial fibrillation: the Framingham Heart Study. Circ. Arrhythm. Electrophysiol. 2010;3:345–350. doi: 10.1161/CIRCEP.109.912055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Al Chekakie MO, Welles CC, Metoyer R, et al. Pericardial fat is independently associated with human atrial fibrillation. J. Am. Coll. Cardiol. 2010;56:784–788. doi: 10.1016/j.jacc.2010.03.071. [DOI] [PubMed] [Google Scholar]
- 85.Heid IM, Jackson AU, Randall JC, et al. Meta-analysis identifies 13 new loci associated with waist-hip ratio and reveals sexual dimorphism in the genetic basis of fat distribution. Nat. Genet. 2010;42:949–960. doi: 10.1038/ng.685. [DOI] [PMC free article] [PubMed] [Google Scholar]