Abstract
Pathfinding by growing axons in the developing or regenerating nervous system may be guided by gradients of molecular guidance cues. The neuronal growth cone, at the ends of axons, uses surface receptors to sense these cues and transduce guidance information to cellular machinery that mediates growth and turning responses. Cytoplasmic Ca2+ signals can play key roles in regulating this motility. Global growth cone Ca2+ signals may regulate cytoskeletal elements and membrane dynamics to control elongation, whereas Ca2+ signals localized to one side of the growth cone may cause asymmetric activation of effector enzymes to steer the growth cone. Modulating growth cone Ca2+ levels may overcome inhibitory signals that normally prevent regeneration in the central nervous system.
The neuronal growth cone, first described by Ramon y Cajal in 1890, is a conical expansion at the tips of developing axons and dendrites, with finger-like filopodial protrusions (< 1 μm in diameter and up to 10 μm long) that actively extend and retract during neurite outgrowth and axon pathfinding. During neuronal development or regeneration, the growth cone actively transduces extracellular guidance signals through a cascade of intracellular events, leading to the motility required for steering extending neurites. A host of specific surface receptors for molecular guidance cues have now been identified that are linked to a common set of signaling pathways in the cytoplasm [1]. Notably, cytoplasmic Ca2+ participates in transducing many guidance signals that regulate growth cone motility and steering [2,3], both of which depend on a concerted regulation of cytoskeletal structures [4,5] and trafficking of membrane precursor vesicles [6], either locally or across the entire growth cone. In this review, we first describe findings that showed the critical role of Ca2+ signals in controlling growth cone motility and steering. This is followed by discussions on how Ca2+ signals are regulated in the growth cone and the potential downstream effector proteins and their functions in regulating cytoskeletal and membrane dynamics. A model for Ca2+ signaling in growth cone steering is presented to illustrate current hypotheses and missing links in our understanding.
Importance of Ca2+ in regulating neurite growth and motility
The concentration of cytoplasmic or intracellular Ca2+ ([Ca2+]i) has long been implicated as an important regulator of neurite growth. Depending on the neuronal type and experimental preparation, diverse responses ranging from Ca2+-induced promotion of growth cone motility [7–9] to inhibition of neurite growth [10–14] have been reported [3]. These results led to the Ca2+ set-point hypothesis [15]: Normal growth cone motility depends on an optimal range of [Ca2+]i; above or below this optimal range neurite growth stops. Table I summarizes many results consistent with this hypothesis [9,12,13,16–24]. These findings also suggest that two distinct Ca2+-dependent processes might regulate growth cone motility - moderate Ca2+ signals might initiate or promote motility and extension, whereas a higher level of [Ca2+]i might inhibit growth cone motility and arrest growth. In many cases, excessively low levels of [Ca2+]i also inhibit motility.
Table 1.
Range of [Ca2+]i for optimal neurite growth
| [Ca2+]i Range (nM) | Basal [Ca2+]i (nM) | Neuron Type | Ref |
|---|---|---|---|
| 200 – 1000 | 30 – >200 | Rat embryonic CNS | [9] |
| 100 – 300 | 45 – 130 | Helisoma CNS | [19] |
| 60 | 55 – 225 | N1E-115 neuroblastoma | [12] |
| >100 | 65 – >100 | Grasshopper embryonic CNS (in situ) | [20] |
| 200 – 300 | 40 – 1340 | Chick DRG | [13] |
| 35 | ND | Rat DRG | [21] |
| 100 – 350 | 100 | Rat superior cervical ganglion (SCG) | [17] |
| *175 – 335 | 100 – 120 | Helisoma CNS | [22] |
| <500 | 50 | Xenopus embryonic CNS | [18] |
| *300 – 800 | 60 | Grasshopper CNS | [16] |
| ~60 | 45 – 130 | Xenopus embryonic CNS | [23,24] |
Range of [Ca 2+]i optimal for promoting extension of filopodia and growth cone motility. Abbreviations: Ref, references; CNS, central nervous system; ND, not determined; DRG, dorsal root ganglion.
The bimodal nature of Ca2+-regulated motility is supported by more recent findings [3]. For example, inositol 1,4,5-trisphospate (IP3)-mediated Ca2+ release from internal stores is necessary to maintain [Ca2+]i within the optimum range for neurite growth [25]. On the other hand, Ca2+ spikes within growth cones apparently inhibit growth cone extension [3,18,26–28]. Thus, different [Ca2+]i appear to regulate distinct cellular events that lead to diametrically opposite effects on growth cone motility.
Although the Ca2+ set-point hypothesis is well supported by experimental evidence, there is additional complexity in Ca2+-dependent mechanisms. For example, there is the process of “accommodation”, whereby a high Ca2+ signal that normally inhibits motility becomes conducive to growth of a neurite that is chronically exposed to it [10]. Other studies have shown that the attractive and repulsive turning response of a growth cone in a gradient of many guidance cues depends on Ca2+ signaling [2] (see Figure 1). These turning responses can be switched between attraction and repulsion, depending on the level of cyclic nucleotide signaling [2,29,30]. Cyclic nucleotides may act through modulation of Ca2+ channels [31], resulting in distinct patterns of Ca2+ signals underlying attractive or repulsive growth cone steering. Thus Ca2+ regulation of growth cone motility depends on the spatiotemporal patterns of Ca2+ signals as well as the internal state of the neuron, which is modulated by other signals received by the neuron.
Figure 1.
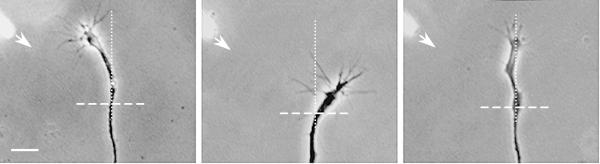
Growth cone turning induced by diffusible factors.
Phase-contrast images show Xenopus spinal neuron growth cones actively extending in culture during a 1-hr exposure to an external gradient (arrow) of netrin-1 (left) or MAG (middle and right). Assays were done in normal saline (left and middle) or low Ca2+ saline (with EGTA) after preloading the neuron with BAPTA-AM (right) to suppress Ca2+ signals. Cross hairs indicate the position of the growth cone central domain before the onset of the gradient. Assays were performed as previously reported [44]. Scale bar, 25 μm.
Steering of Growth Cones by Localized Ca2+ Signals
How the growth cone steers its course in the nervous system has been an outstanding question since the discovery of the growth cone. Ramon y Cajal's suggestion [32] that growth cones undergo chemotactic migration under the guidance of diffusible factors secreted by their target tissues has received much recent attention, thanks to the discovery of many secreted protein factors that induce chemotropic or chemorepulsive growth cone responses in cultured preparations [1,33]. Early studies also provided evidence that neurite outgrowth from tissue explants and isolated neurons in culture can be modulated by extracellular electric fields [34–36], with increased neurite initiation, faster elongation rates and steering toward the cathode of the applied fields. Although the steering effect might be attributed in part to an electrophoretic redistribution of surface receptors in the plasma membrane [36], asymmetric Ca2+ influx induced by the applied field may be an important cause. Indeed, electric fields applied either uniformly [8] or focally [37] can trigger localized Ca2+ increases, resulting in the protrusion of filopodia and growth cone turning. Electrical activity in neurons leads to global elevations in [Ca2+]i but can also trigger hotspots of [Ca2+]i in the growth cone [38,39], which correlate with the protrusion of new growth cone membrane and filopodia [38]. Similarly, in the presence of Ca2+ ionophore, neurites grow towards the source of elevated Ca2+ delivered from a micropipette, which presumably causes a gradient of Ca2+ influx across the growth cone [7]. These findings support the notion that global growth cone Ca2+ elevations can regulate neurite growth, whereas local Ca2+ elevations can have a steering effect. This is further confirmed by the findings that extracellular application of neurotransmitters, which increases [Ca2+]i to high levels globally within the growth cone, inhibits neurite growth [11,40,41], whereas an extracellular gradient of glutamate [42] or acetylcholine (ACh) [24] can induce attractive turning responses of neuronal growth cones. These turning responses are abolished when extracellular Ca2+ is depleted, implicating the participation of Ca2+ influx through Ca2+ channels in the plasma membrane, which can trigger a cytoplasmic gradient of Ca2+ in the growth cone [24].
Recent studies on axon guidance by diffusible chemoattractants and chemorepellants have provided further insights into the role of localized Ca2+ signals in steering growth cones. Extracellular gradients of nerve growth factor [2,7], brain-derived neurotrophic factor [2,30], netrin-1 [2,43] and myelin-associated glycoprotein (MAG) [2,44,45] all induce growth cone turning responses that depend on Ca2+ signaling. Furthermore, gradients of netrin-1 [43] or MAG [45], which normally induce either attractive or repulsive growth cone turning, respectively, can trigger increases in [Ca2+]i that are highest on the side of the growth cone facing the source of the factor (see Figure 2). Thus, both attractive and repulsive growth cone steering can be triggered by Ca2+ gradients that are highest on the same side of the growth cone. What is the difference in the Ca2+ signals? An important distinction seems to be the amplitude of the Ca2+ signal, which appears to be higher in response to netrin-1 than to MAG [43,45]. The higher amplitude Ca2+ signal triggered by netrin-1 is mediated by both Ca2+ influx through plasma membrane Ca2+ channels and Ca2+-induced Ca2+ release from internal stores [43]. In contrast, the lower amplitude Ca2+ signal triggered by MAG is mediated by release from internal stores alone [45]. Importantly, treatments that reduce Ca2+ signals convert netrin-1 induced growth cone steering from attraction to repulsion [43], whereas MAG-induced repulsive steering can be converted to attraction under conditions that raise [Ca2+]i [29,45]. These results support the notion that a gradient of [Ca2+]i across the growth cone can mediate steering responses, with higher amplitude Ca2+ signals mediating attraction and lower amplitude Ca2+ signals mediating repulsion. Consistent with this, an extracellular gradient of a low concentration of ryanodine, which triggers an elevation of Ca2+ in the growth cone by stimulating Ca2+ release from internal stores, can induce attractive steering [43]. This attraction induced by a Ca2+ gradient is reminiscent of an earlier finding that dorsal root ganglion neurites grow toward the source of elevated Ca2+ in the presence of Ca2+ ionophore [7]. These results are supported and expanded by recent experiments on cultured neurons subjected to either focal photolysis of caged Ca2+[23] or exposure to a gradient of Ca2+ ionophore in the presence of defined concentrations of extracellular Ca2+ ([Ca2+]o) [45]. The latter work directly supports the idea that gradients of high and low amplitude Ca2+ signals across the growth cone can mediate attractive and repulsive growth cone steering, respectively.
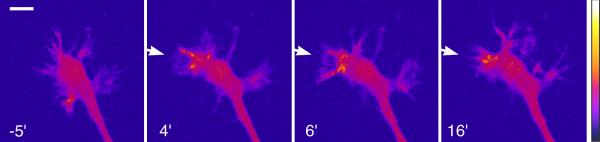
Figure 2.
Localized Ca2+ signals in the growth cone induced by an extracellular gradient of the guidance cue netrin-1.
Confocal images depict [Ca2+]i in the growth cone of a cultured Xenopus spinal neuron after injection with the Ca2+-sensitive fluorescence indicator Oregon Green BAPTA-dextran. In this pseudocolor scheme, blue and white represent the lowest and highest [Ca2+]i, respectively, and time (in min) before and after the onset of the netrin-1 gradient (arrows) is indicated. Imaging was done as previously published [43]. Scale bar, 10 μm. The full-length movie can be accessed by the internet (http://www.sciencedirect.com/science/journal/09628924).
Localized transient elevations of [Ca2+]i have also been observed in filopodia of motile growth cones extending in culture [46]. These Ca2+ signals, which are substrate-dependent, can propagate back to the growth cone central domain and stimulate more global [Ca2+]i elevations. Importantly, these Ca2+ signals appeared to slow neurite growth by reducing filopodial motility and promoted repulsive steering when stimulated locally within filopodia on one side of the growth cone. The repulsive steering may result from a gradient of low-level Ca2+ signals across the growth cone as described above. However, previous Ca2+ imaging studies done on growth cones and isolated filopodia have indicated that [Ca2+]i regulation in filopodia differs from that at the growth cone central domain [47]. One possible mechanism by which filopodial Ca2+ transients can regulate growth cone motility and guidance is through local activation of calpain [48]. Calpain is a protease that can cleave certain enzymes (e.g. the calpain-catalyzed activation of protein kinase C; PKC) and numerous cytoskeletal, membrane and receptor proteins [49]. Localized Ca2+ signals induced by focally applied electric fields [37] or photolysis of caged Ca2+ [16,50] can also cause filopodial elongation, which depends on the function of calmodulin (CaM) and Ca2+-dependent phosphatase 2B (calcineurin) [50].
Differential Actions of Ca2+ on Growth Cone Extension and Steering
Treatment of cultured dorsal root ganglion neurons with cytochalasin B causes the depolymerization of actin filaments (F-actin) and retraction of filopodia, which can inhibit neurite growth [4,51]. Later experiments have shown that axons can still grow in the presence of cytochalasin B-induced actin depolymerization, but axon pathfinding is defective in cell cultures [4,42] and in vivo [52–54]. In contrast, disruption of microtubules with colchicine does not immediately affect filopodia, but results in neurite retraction and causes protrusion of new lamellipodia and filopodia along the axon [55]. Thus an important distinction can be made between the mechanisms of growth cone extension versus steering - steering depends upon intact filopodia and F-actin, whereas extension requires intact microtubules. More recent evidence has implicated microtubules in growth cone steering as well [4,5,56–58].
Growth cone extension and steering can also have distinct Ca2+ requirements [12,14]. For example, neurites of cultured Xenopus spinal neurons grow faster when intracellular Ca2+ is reduced to low levels [3,18,24,26,30,42] but similar treatment abolishes growth cone steering induced by a variety of guidance cues, including growth factors, neurotransmitters, and MAG [2,24,30,42,45]. Intriguingly, inhibition of CaM-kinase II (CaMKII) in these neurons abolishes attractive turning induced by gradients of ACh [24], yet still permits substantial neurite extension. Thus it appears that growth cone steering requires higher level activity of Ca2+ effectors, e.g., CaMKII, than that for growth cone extension. The differential requirement of microtubules and F-actin for extension and steering, respectively, also suggest that distinct signaling cascades and different cytoskeletal machinery participate in these two processes.
Cytoplasmic Regulation of Growth Cone Ca2+
Many cellular processes participate in regulating [Ca2+]i, including Ca2+ influx via voltage- and ligand-gated ion channels in the plasma membrane, extrusion through the plasma membrane Ca2+ pump and Na+/Ca2+ exchanger, and Ca2+ release from intracellular stores through ryanodine- and IP3-sensitive channels [39,59–61]. Also, modulation of Ca2+ uptake into internal stores (i.e. mitochondria and ER) present in the growth cone can affect the spatiotemporal profile of Ca2+ signals [39,59]. Another important aspect of Ca2+ regulation is buffering by endogenous Ca2+-binding proteins, such as calbindin-D28k, parvalbumin and calretinin, which are expressed at different levels in various types of neurons [59,62]. In addition to a buffering role to modulate cytosolic Ca2+ transients, Ca2+ binding proteins, e.g. CaM and calpain, may directly regulate the activity of downstream effectors.
Cytoplasmic Ca2+ signals can be local as well as global [8,37–39]. Activation of Ca2+ channels in the plasma membrane or the ER can result in high level [Ca2+]i only near the channels [63,64]. Due to slow diffusion of Ca2+, resulting from binding to relatively immobile binding proteins, local Ca2+ transients produced by asymmetric activation of Ca2+ channels may establish a longer lasting global Ca2+ gradient across the growth cone, leading to differential regulation of downstream effectors and growth cone motility.
There is increasing evidence for the importance of Ca2+ channels in regulating growth cone motility. For example, blocking plasma membrane Ca2+ channels can inhibit growth cone motility and neurite extension [14], and abolishes growth cone steering in a gradient of netrin-1 [43]. Conversely, enhancing the activity of voltage-gated Ca2+ channels in the growth cone promotes attractive steering [29,31]. In Caenorhabditis elegans, loss of function mutations in the egl-19 allele, which encodes a voltage-gated Ca2+ channel, leads to axon pathfinding errors [65]. Other plasma membrane Ca2+ channels [18,27], e.g., TRPC5 [66], may also participate in regulating neurite extension and growth cone motility. In the cytoplasm, blocking the function of IP3 receptors in the ER also inhibits neurite growth [25]. Thus modulation of Ca2+ channels in the plasma membrane and ER provides a natural basis for the regulation of Ca2+ signals, which in turn induce growth cone extension or steering.
Finally, recent evidence [67] has suggested that Rho GTPase activity can regulate [Ca2+]i. Specific inhibitors of Rho, but not Rac or Cdc42, inhibited the migration of hematopoietic stem cells induced by the chemokine stromal derived factor (SDF)-1α by inhibiting SDF-1 induced Ca2+ transients. It remains to be determined whether Rho-GTPases also regulate [Ca2+]i in neurons and if so, how this affects growth cone extension and steering.
Ca2+ Effectors and Cytoskeletal Dynamics
Cytoskeletal dynamics during growth extension and steering
The most prominent component of the force-generating machinery for growth cone extension and steering is the actin-myosin cytoskeleton. The growth cone peripheral domain, which is largely devoid of cytoplasmic organelles, includes filopodia and lamellipodia-flattened veils between filopodia. A thicker central domain makes up the palm of the growth cone and is loaded with vesicles and organelles. Neurites extend by first protruding filopodia and lamellipodia and then subsequent engorgement of the initial peripheral domain with vesicles and organelles to advance the central domain [68]. At the core of filopodia are bundles of polarized F-actin, which often extend to the edge of the growth cone central domain [69] (see Figures 3 and 4). Furthermore, a dense meshwork of interconnecting F-actin is prominent in lamellipodia and a cortical actin network is located just beneath the plasma membrane [4,69]. The second prominent cytoskeletal component within the growth cone are microtubules, which are arranged as parallel bundles in the neurite shaft but splay apart and turn as they enter the growth cone central domain [4,5,69] (see Figures 3 and 4). Microtubules may also penetrate into the growth cone peripheral domain and interact with F-actin [4,5,58]. It seems imperative that any signal mediating growth cone turning responses must regulate these actin and microtubule assemblies. Tables 2 and 3 summarizes various Ca2+-dependent effectors that may regulate cytoskeletal rearrangements either directly or by acting at intermediate events.
Figure 3.
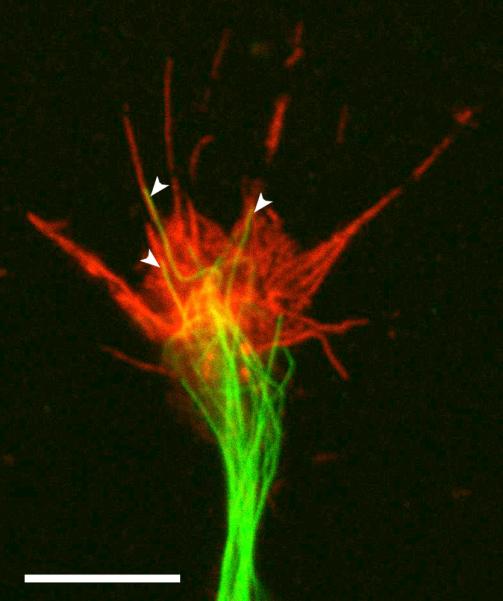
The growth cone actin and microtubule cytoskeleton.
Fluorescence image shows the distribution of F-actin (red) and microtubules (green) in the growth cone of a Xenopus spinal neuron that was extending in culture before being fixed and processed for immunofluorescence microscopy. Actin filaments (stained by rhodamine-phalloidin) are predominant in the peripheral domain, whereas bundles of microtubules (labeled with a tubulin antibody) localize to the central domain. Free microtubules may also penetrate into filopodia and lamellipodia (arrowheads). Scale bar, 10 μm. (Courtesy of Xiaobin Yuan and Ming Jin.)
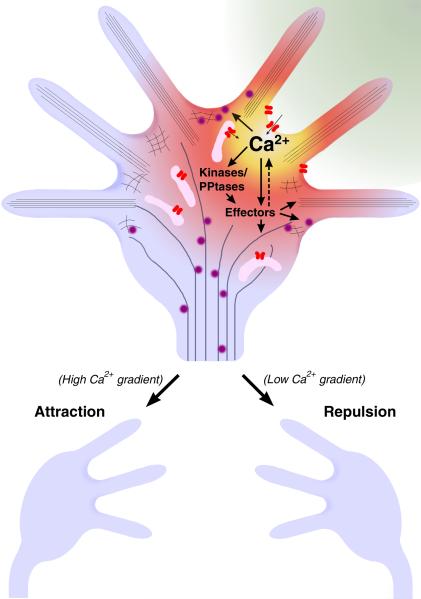
Figure 4.
Model for growth cone steering mediated by localized Ca2+ signals. An extracellular gradient of guidance cue (green) can induce localized Ca2+ signals on one side of the growth cone. The [Ca2+]i is highest near open Ca2+ channels (red) in the plasma membrane and ER (pink), establishing a Ca2+ gradient across the growth cone. These localized Ca2+ signals activate Ca2+-sensitive kinases (e.g. CaMKII and PKC), phosphatases (e.g. calcineurin) and other effectors (e.g. Rho-family GTPases, see text and Tables 2 and 3), which in turn regulate the dynamics of actin- and microtubule-elements and membrane-bound vesicles (purple) to steer the growth cone. Higher amplitude Ca2+ signals trigger a local increase in cytoskeletal dynamics and filopodial protrusions to mediate attractive steering, whereas lower amplitude Ca2+ signals reduce filopodial activity, leading to repulsive steering.
Table 2.
Ca2+ -dependent Effector Proteins in the Growth Cone: Part I
| Protein | Regulation / Function | References |
|---|---|---|
| Calmodulin (CaM) | Activated by Ca2+, regulates many Ca2+-dependent effectors | [70] |
| Regulates growth cone extension and guidance | [71,72] | |
| CaMKII | Activated by Ca2+/CaM; phosphorylates many substrates | [70,74] |
| Regulates growth cone motility | [24,72,75–78] | |
| Calcineurin | Protein phosphatase activated by Ca2+/CaM | [70] |
| Regulates neurite extension | [84,85,120] | |
| Regulates filopodial and lamellipodial protrusions | [50,84] | |
| PKC | Activated by Ca2+ and phospholipids | [70] |
| Activity is catalyzed by calpain | [49] | |
| Regulates microtubule assembly | [5] | |
| Regulates growth cone shape and motility | [86,87] | |
| Adenylyl cyclase | Activated by Ca2+/CaM (type 1 & 8) and PKC (type 1, 2 & 5) | [2] |
| Modulates growth cone steering and pattern formation in the brain. | ||
| Tiam1 | GEF for Rac1; regulates growth cone motility | [96] |
| Regulated by CaMKII | [95] | |
| Ras-GRF 1 & 2 | Activated by Ca2+/CaM, in turn activates Ras and Rac | [97,98] |
| MLCK | Activated by Ca2+/CaM; regulates myosin-II motor activity | [4] |
| Inactivated by Rac/Cdc42-dep PAK | [108] | |
| Regulates growth cone motility and axon pathfinding | [113,115] | |
| Myosin II | Activated by Ca2+/CaM, MLCK- or ROCK-dep LC phosphoryl; inactivated by myosin-II-LC phosphatase | [4] |
| Filaments destabilized by Ca2+- and Rac-dep HC phosphoryl | [107] | |
| Regulates growth cone shape, motility and extension | [4] | |
| Myosin I & V | Regulated by Ca2+/CaM and by PAK-dep HC phosphoryl | [103] |
| Growth cone extension (myosin Ic) | [117] | |
| Filopodial extension (myosin V) | [118] | |
| Gelsolin | Ca2+-dependent F-actin severing protein, localizes to the growth cone and initiates filopodial retraction | [119] |
Table 3.
Ca2+-dependent Effector Proteins in the Growth Cone: Part II
| Protein | Regulation / Function | References |
|---|---|---|
| ADF/Cofilin | Depolymerizes F-actin; inactivated by LIM kinases/phosphoryl | [94] |
| Activated by calcineurin/dephosphoryl; regulates neurite growth | [120] | |
| GAP-43 | Binds and localizes CaM; regulated by PKC | [4,88,132] |
| Regulates the actin cytoskeleton | [89] | |
| Regulates neurite growth and axon guidance | [132] | |
| CAP23 | Binds CaM; regulates sub-PM actin cytoskeleton | [132] |
| Regulates neurite growth | [132] | |
| MARCKS | F-actin cross-linking protein regulated by PKC and Ca+/CaM | [91,92] |
| α-actinin | Ca2+/CaM-binding and F-actin cross-linking protein | [133] |
| Concentrated in filopodia | [102] | |
| Regulates F-actin depolymerization and growth cone shape | [133] | |
| Spectrin (calspectin/fodrin) | Binds Ca2+/CaM; cross-links F-actin and integral PM proteins | [133] |
| Cleaved by Ca2+-activated calpain | [49] | |
| Regulates growth cone adhesion | [133] | |
| Calpain | Ca2+-dependent protease | [49] |
| Catalyzes PKC activation | [49] | |
| Regulates growth cone motility and extension | [48] | |
| MAP2 | Binds CaM in a Ca2+-dependent manner | [121] |
| Inhibits microtubule assembly when phosphoryl by CaMKII | [121] | |
| Can bind to microtubules and F-actin | [121] | |
| Tau | Binds CaM in a Ca2+-dependent manner | [121] |
| Inhibits microtubule assembly when phosphoryl by CaMKII | [121] | |
| Dephosphoryl by calcineurin | [121] | |
| Regulates growth cone motility and elongation | [5,121] | |
| Synaptotagmin I | Ca +-dependent regulator of exocytosis | [127] |
| Regulates neurite outgrowth | [134] |
Abbreviations: dep, dependent; CaMKII, Ca2+/CaM-dependent kinase II; MAP2, microtubule-associated protein 2; PKC, protein kinase C; GEF, guanine nucleotide exchange factor; PM, plasma membrane; MLCK, myosin light chain kinase; PAK, p21-activated kinases; ROCK, Rho-associated protein kinase; LC, light chain; phosphoryl, phosphorylation; HC, heavy chain; ADF, actin depolymerizing factor; dephosphoryl, dephosphorylation; MARCKS, myristoylated, alanine-rich C kinase substrate.
Calmodulin, CaM-kinase II and calcineurin
A Ca2+ rise in the growth cone activates numerous target proteins and cellular machinery. One key Ca2+-binding protein is CaM, which is abundant in growth cone filopodia and central domains and binds Ca2+ at concentrations only slightly above basal [Ca2+]i [70]. Severe axon pathfinding errors occur in Drosophila mutants expressing a CaM inhibitory peptide [71]. Furthermore, treatment with an irreversible Ca2+/CaM inhibitor abolishes attractive turning of growth cones towards laminin-coated beads [72]. Thus CaM seems to be a key regulator of the steering machinery. Interestingly, Ca2+ interaction with CaM can persist for approximately one minute following brief electrical activity in the neuron [73], thus allowing a relatively long-lasting activation of downstream effectors.
Binding of Ca2+ to CaM leads to the activation of CaMKII α and β isoforms, which are highly enriched in neurons and phosphorylate a host of substrates [70,74]. In some cell culture systems, inhibition of CaMKII leads to decreased neurite growth [72], whereas overexpression of CaMKII induces neurite growth [75]. In optic tectal neurons of Xenopus tadpoles, CaMKII activity was found to inhibit the growth of neuronal processes [76]. More recent studies have shown that transient high [Ca2+]i elevations induced by gangliosides can activate CaMKII, leading to a reorganization of the actin cytoskeleton and the formation of new filopodia in neurons [77], and it appears to be the CaMKIIβ isoform that specifically participates in growth cone extension and steering [78]. These effects of CaMKII were mediated by the activation of Cdc42, a member of the Rho-family GTPases [79], which are known to regulate growth cone morpholgy and steering [54,80–83].
Binding of Ca2+ to CaM also directly activates the phosphatase calcineurin. Pharmacolocigal inhibition of calcineurin has been reported to inhibit [84] or promote [3,85] neurite outgrowth, depending on the culture system. In Xenopus spinal neurons, inhibiting calcineurin activity with a specific autoinhibitory peptide prevents the Ca2+-induced reduction of neurite growth, whereas expression of a constitutively active calcineurin inhibits neurite growth [85]. However, focal inactivation of calcineurin in regions of the growth cone by chromophore-assisted laser inactivation causes localized retraction of filopodia, which in turn influences the direction of subsequent neurite growth [84]. Thus, asymmetric calcineurin activity within the growth cone can regulate growth cone steering, whereas a more global activation may regulate neurite elongation.
Protein kinase C
Members of the Ca2+ and phospholipid-dependent PKC family are enriched in growth cones and are important for neurite growth and growth cone steering [86,87]. For example, pharmacological inhibition of PKC can switch the steering of cerebellar granule cell growth cones induced by a gradient of SDF-1 from repulsion to attraction [87]. In the growth cone, CaM associates with GAP-43, which targets it to the inner plasma membrane [88]. Activated PKC can phosporylate GAP-43, which then induces dissociation of the CaM/GAP-43 complex, leaving CaM free to bind Ca2+ [88]. Phosphorylated GAP-43 also stabilizes the formation of long actin filaments in the growth cone and dephosphorylation of GAP-43 by calcineurin leads to the destabilization of F-actin [89]. This regulation of the actin cytoskeleton is likely to influence growth cone motility [13,90].
Another major substrate of PKC in the growth cone is the myristoylated, alanine-rich C kinase substrate (MARCKS), which moves from the plasma membrane to the cytoplasm when phosphorylated and participates in the regulation of cell motility [91]. The F-actin crosslinking activity of MARCKS is inhibited by PKC-mediated phosphorylation and by binding to CaM [92]. This CaM- and PKC-mediated regulation of MARCKS, together with the PKC-mediated regulation of free CaM levels, exemplify how the Ca2+/CaM and PKC signal transduction pathways can converge to regulate the actin cytoskeleton.
Rho GTPases
Although the mechanism remains to be determined, it is intriguing that Ca2+ signals can regulate the activity of Cdc42 in neurons [77], which in turn interacts with downstream effectors to regulate the organization of the actin cytoskeleton [83,93,94]. Rho, Rac and Cdc42, the prototypical Rho-GTPases [79], are regulated by guanine nucleotide exchange factors (GEFs) that promote activation and by GTPase activating proteins (GAPs), which tend to keep them in the inactive GDP-bound state. Phosphorylation of specific GEFs is one of the potential mechanisms by which Ca2+ signals can regulate Rho-GTPase activity. Significantly, the Rac-specific exchange factor Tiam1, which is phosphorylated by both PKC and CaMKII [95], has been localized to the growth cone and can regulate growth cone motility and neurite extension [96]. The activity of other GEFs, such as Ras-GRF 1 and 2, which are specific for both Ras and Rac [97], are enhanced by raised Ca2+ due to a Ca2+/CaM binding site within these proteins [98]. Taken together, these results indicate that nucleotide exchange on Rho-GTPases may be regulated by changes in [Ca2+]i.
In the cytoplasm, inactive Rac is found in a complex with RhoGDI, which masks the Rac C-terminal tail and prevents targeting to the plasma membrane and subsequent interactions with membrane-associated activators [83,99]. Elevation of [Ca2+ ]i in fibroblasts triggers the PKC-mediated phosphorylation of RhoGDI, which then induces the translocation of Rac to the plasma membrane [100]. Active membrane-associated Rac then induces changes in the actin cytoskeleton, leading to the formation of lamellipodia. In vascular smooth muscle, the activity of RhoA and its effector Rho kinase (ROCK) is stimulated by Ca2+, which leads to muscle contraction in response to depolarization or agonists [101]. In this system, membrane depolarization induced a sustained increase in the amount of active GTP-bound RhoA and activation was suppressed by a CaM inhibitor.
The evidence indicates that [Ca2+]i elevations can regulate the intracellular localization and activation of multiple Rho-GTPases. Although some of these studies were done in non-neuronal cells, it is likely that similar mechanisms operate in the growth cone to regulate the activity of Rho-GTPases and induce cytoskeletal changes in response to receptor stimulation or membrane depolarization.
Myosin
Members of Rho-family GTPases, along with Ca2+/CaM, also regulate actinomyosin-based contraction. Immunolocalization studies have shown that myosin is present in the growth cone, distributed in spots throughout the central and peripheral domains and also concentrated at the base of filopodia [69,102]. Myosin II, the conventional non-muscle myosin, is found in the peripheral region of growth cones close to the plasma membrane, and has been implicated in regulating neurite outgrowth and growth cone morphology. In response to extracellular signals, myosin II can be rapidly phosphorylated to regulate both its motor activity and assembly into filaments, leading to shape changes associated with motility [4,103].
Actinomyosin-based contraction is stimulated by Ca2+/CaM and by phosphorylation of the myosin II regulatory light chain, which is a substrate for Ca2+/CaM-dependent myosin-light-chain kinase (MLCK) and myosin-II-light-chain phosphatase [4]. The Rho-activated kinase ROCK can also activate myosin II either by directly phosphorylating the light chain [104] or by blocking the activity of the myosin-II-light-chain phosphatase [105]. Thus, Ca2+/CaM and activated Rho both increase myosoin light chain phosphorylation and stimulate the contraction of actin-myosin filaments.
The Rho-mediated stimulation of myosin contractility induces cell rounding and neurite retraction in neuronal cell lines [83,106]. In contrast, Rac activation causes a loss of contractility associated with cell spreading and the extension of neurites, and appears to interfere with Rho-dependent processes [83,107]. Activation of Rac in neuronal cell lines promotes Ca2+-dependent phosphorylation of the myosin-II heavy chain [107], which appears to destabilize the actinomyosin cytoskeleton [4,83] and antagonizes Rho-mediated contraction [107]. The myosin-II heavy chain thus appears to be a target of Ca2+-dependent kinase pathways that are regulated by Rac.
Activated Rac or Cdc42 can also reduce actinomyosin contractility by decreasing myosin light chain phosphorylation. This is because activated Rac and Cdc42 can stimulate p21-activated serine/threonine kinases (PAK), which in turn phosphorylate and thereby inactivate MLCK [83,108]. It is known that PAK, a critical regulator of axon guidance in Drosophila [109], is also an important regulator of the actin cytoskeleton that, along with ROCK [110], can phosphorylate LIM kinase [83,111]. LIM kinase directly phosphorylates and thereby inactivates ADF/cofilin, leading to reduced disassembly of F-actin [94] and growth cone collapse [112].
Myosin II also appears to regulate the bundling of F-actin in growth cones [4]. Inhibition of myosin II by pharmacological inhibition of MLCK causes a reversible loss of filopodia and lamellipodia, resulting in growth cone arrest [113], and can cause F-actin bundles to merge, leading to growth cone collapse [114]. Furthermore, local application of a MLCK inhibitor (ML-7) to one side of the growth cone causes repulsive steering [58], whereas attractive steering is induced by an extracellular gradient of a specific myosin ATPase inhibitor (BDM) [81]. Interestingly, overexpression of a constitutively active MLCK impedes both attractive and repulsive axon guidance in Drosophila CNS neurons in vivo [115].
Unconventional myosins (e.g. classes I and V), which can be activated by the PAK-related myosin I heavy chain kinase, can also associate with Ca2+/CaM and translocate actin filaments in vitro in a Ca2+-regulated manner [103]. Many of these myosins (e.g. classes I, V and VI) localize within the growth cone [116] where they have been implicated in such processes as coupling the retrograde flow of F-actin to growth cone advance [117] and filopodial extension [118].
Actin Filaments
Inhibition of retrograde actin flow can result from Ca2+-induced changes in the actin cytoskeleton [90]. Also, transient focal elevation of [Ca2+]i by photolysis of caged Ca2+ has been found to induce the formation of filopodia from nascent axons near growth cones in situ [16]. In neurons coinjected with rhodamine-phalloidin, F-actin was observed in dynamic cortical patches along nascent axons and new filopodia often emerged from these patches following the [Ca2+]i elevation. These results indicate that local transient [Ca2+]i elevations can trigger polymerization and/or rearrangement of F-actin to extend filopodia. Conversely, several studies suggest that [Ca2+]i elevations can lead to collapse of the growth cone by disrupting F-actin bundles [13,90,114]. It is likely that this collapsing effect is mediated by distinct effectors that are activated by higher [Ca2+]i elevations than those triggering filopodial extension. One candidate for coupling membrane events to neurite retraction is the actin-associated protein gelsolin, which localizes to the growth cone and severs F-actin in its Ca2+-activated state [119].
If Ca2+ is sufficient to mediate growth cone turning then it seems likely that the activity of actin-binding proteins [93,94] could be regulated either by direct binding to Ca2+, by Ca2+-sensitive adaptor proteins, or by Ca2+-sensitive kinases and phosphatases. One example is ADF [94], which sequesters actin monomers and therefore favors the depolymerization of F-actin. This protein is concentrated in growth cones and its activity, which is regulated by many signals that affect growth cone motility, causes an increase in neurite extension [94,120]. Phosphorylation of ADF by ROCK and LIM kinases downstream of Rho GTPase signaling makes it inactive at depolymerizing F-actin and favors the assembly of F-actin [83,94]. Such a signal highly localized to one side of the growth cone could lead to protrusion of filopodia and favor turning of the growth cone toward that side. ADF becomes dephosphorylated and activated downstream of signaling pathways that activate PI-3 kinase, cAMP, or increased [Ca2+]i [120].
Microtubules
Net assembly of microtubules occurs within the growth cone [5]. Ca2+/CaM can bind directly to certain microtubule-associated protiens (e.g. MAP1B, MAP2, tau) and compete for their association with tubulin, thereby inhibiting the assembly of microtubules [4,121]. Furthermore, Ca2+/CaM-dependent phosphorylation of many MAPs can lead to microtubule disassembly [4,121]. Elevated Ca2+ levels also inhibit casein kinase II activity, leading to decreased MAP1B phosphorylation and decreased binding to tubulin and thereby favoring disassembly of microtubules.
Ca2+ and Membrane Dynamics
Neurite extension at the growth cone eventually requires insertion of new membrane material, a process that presumably depends on exocytotic fusion of membrane precursor vesicles. Furthermore, preferential addition of membrane on one side of the growth cone may contribute to growth cone turning responses (see Figure 4). The findings that growth cones of cultured neurons undergo Ca2+-dependent exocytosis [122,123] of the neurotransmitter ACh suggest that asymmetric membrane insertion may be triggered by Ca2+ signals associated with growth cone steering. Growth cone secretion may also provide “autocrine” actions on growth cone motility and steering. For example, application of a nicotinic ACh receptor (AChR) antagonist inhibits the electric field-induced re-orientation of neurites toward the cathode in cutured Xenopus spinal neurons [124], which suggests that ACh released from the growth cone may act in an autocrine fashion to activate nicotinic AChRs. The finding of protease secretion from the growth cone [125] further supports the notion that exocytosis at the growth cone may play important functions for growth cone motility and extension.
The growth cone central doman contains numerous synaptic vesicles, which are thought to be preferentially inserted at the growth cone of actively extending neurites [6,126]. Importantly, the synaptic vesicle protein synaptotagmin I, which triggers exocytosis when its C2A domain binds Ca2+ [127], has also been implicated in regulating neurite growth. Recently, synaptic vesicles have been visualized moving within individual filopodia [128]. These vesicles can fuse with the plasma membrane after focal depolarization with a high K+ solution [128], suggesting the possibility that regulation of vesicle transport and fusion participates in steering the growth cone. Using surface-attached latex beads to monitor membrane flow, Dai and Sheetz [129] observed a rapid membrane flow from growth cone to cell body during neurite growth in cultured dorsal root ganglion neurons. When artificial flow is induced to the center of the neurite by using laser tweezers, the primary source of the membrane is from the growth cone. This study suggests that in an actively growing neurite, there is excess membrane added at the growth cone. Given this excess membrane, the formation of new filopodia and lamellipodia associated with the turning response may not require immediate addition of new membrane. Thus whether preferential exocytotic addition of membrane plays any role in growth cone steering remains an outstanding question.
Concluding Remarks—a Model for Growth Cone Steering
The formation of specific connections in the developing nervous system depends on the pathfinding of growing axons to reach their target cells. For long-range guidance by diffusible cues, the growth cone must first detect small gradients of chemoattractants and chemorepellants and then respond reliably by activating the appropriate cellular machinery. One way that guidance signals can be transduced and amplified is through cytoplasmic Ca2+ signals. Furthermore, as the growth cone extends, it may need to readjust its sensitivity to a guidance cue by a process referred to as adaptation [130]. Potential mechanisms by which this adaptation could be achieved are by modulating Ca2+ signals or resetting the sensitivity of downstream Ca2+ effectors.
The results discussed herein implicate Ca2+ as a regulator of actin and microtubule dynamics to provide a mechanism by which [Ca2+]i can influence growth cone extension and steering. Given this evidence, one may conclude that axon pathfinding requires two different but overlapping ranges of Ca2+, one optimal for neurite growth and the other for growth cone steering. Furthermore, within the Ca2+ range that supports growth cone steering, two distinct levels of Ca2+ signals localized on one side of the growth cone can mediate attractive and repulsive steering. A low-amplitude Ca2+ signal triggers a local inhibition/collapse of filopodia, resulting in repulsive steering. On the other hand, a higher amplitude Ca2+ signal causes a local initiation/stabilization of filopodial protrusions, resulting in attractive steering (see Figure 4). These focal Ca2+ signals, which are limited by diffusion, arise by asymmetric activation of Ca2+ channels in the plasma membrane and/or internal stores. Distinct Ca2+ signals then trigger a gradient of activity of effector proteins, e.g., kinases and phosphatases, which in turn modulate downstream effectors, e.g., different Rho GTPases, leading to cytoskeletal rearrangements required for growth cone steering. This bimodal regulation of growth cone steering and the participation of Ca2+-dependent kinases and phosphatases exhibit interesting parallels to Ca2+-dependent synaptic modifications induced by neuronal activity [70]. Low-amplitude Ca2+ signals that trigger repulsive growth cone turning may be similar to those that induce long-term depression (e.g. activate calcineurin), and high-amplitude Ca2+ signals that trigger attractive growth cone turning may be similar to those that induce long-term potentiation (e.g. leading to the activation of CaMKII and PKC). The exact identity of these Ca2+-effector proteins and the spatiotemporal profiles of their activities during growth cone steering remain to be elucidated. The use of recently developed fluorescence resonance energy transfer-based probes to monitor enzyme activity [131] is likely to provide crucial spatiotemporal information necessary for understanding the growth cone steering mechanism. Finally, although it seems likely that addition of new membrane material to the growth cone surface membrane [6,126] is necessary to support continuous growth cone extension, the role of Ca2+-dependent exocytotic insertion and endocytic uptake of membrane in the growth cone steering process remains to be determined.
Supplementary Material
Box 1. Actin dynamics in the growth cone.
Actin monomers are assembled onto the barbed ends of F-actin near the plasma membrane of the growth cone. Actin filaments are transported by a myosin motor-driven process from the peripheral domain toward the central domain of the growth cone, resulting in retrograde actin flow [4]. Actin filaments are then severed and depolymerized by several proteins including gelsolin and actin depolymerizing factor (ADF)/cofilin at the boundary of the peripheral and central domain [94]. This retrograde actin flow occurs in both filopodia and lamellipodia [4]. Retrograde flow is prevented when F-actin becomes immobilized by attachment to adhesion sites of the growth cone membrane, allowing myosin motors to exert the traction force for forward protrusive activity. Regulation of either the assembly of the actin-myosin cytoskeleton, myosin motor activity, or attachment of F-actin to adhesion sites may all influence growth cone extension and steering. Localized F-actin based protrusive activity appears to be an initial step in steering the growth cone, since asymmetry in the protrusion of filopodia precedes growth cone turning [42,52]. However, turning of the growth cone shaft requires asymmetric extension and stabilization of microtubule bundles towards the new direction [4,5].
Acknowledgements
We thank Scott Wong for helpful discussions, Kyonsoo Hong and Makoto Nishiyama for assistance with confocal microscopy while collecting the images for the movie and Figure 2, and Kuo-hua Huang and Dennis Wang for technical service. The fluorescence image for Figure 3 was kindly provided courtesy of Xiaobin Yuan and Ming Jin. Finally, we would like to acknowledge the work of many investigators who could not be cited due to space constraints. We apologize for the omissions.
References
- 1.Dickson BJ. Molecular mechanisms of axon guidance. Science. 2002;298(5600):1959–1964. doi: 10.1126/science.1072165. [DOI] [PubMed] [Google Scholar]
- 2.Song HJ, Poo MM. Signal transduction underlying growth cone guidance by diffusible factors. Curr Opin Neurobiol. 1999;9(3):355–363. doi: 10.1016/s0959-4388(99)80052-x. [DOI] [PubMed] [Google Scholar]
- 3.Gomez TM, Spitzer NC. Regulation of growth cone behavior by calcium: new dynamics to earlier perspectives. J Neurobiol. 2000;44(2):174–183. [PubMed] [Google Scholar]
- 4.Dent EW, Gertler FB. Cytoskeletal dynamics and transport in growth cone motility and axon guidance. Neuron. 2003;40(2):209–227. doi: 10.1016/s0896-6273(03)00633-0. [DOI] [PubMed] [Google Scholar]
- 5.Gordon-Weeks PR. Microtubules and growth cone function. J Neurobiol. 2004;58(1):70–83. doi: 10.1002/neu.10266. [DOI] [PubMed] [Google Scholar]
- 6.Craig AM, et al. Preferential addition of newly synthesized membrane protein at axonal growth cones. Nature. 1995;375(6532):592–594. doi: 10.1038/375592a0. [DOI] [PubMed] [Google Scholar]
- 7.Gundersen RW, Barrett JN. Characterization of the turning response of dorsal root neurites toward nerve growth factor. J Cell Biol. 1980;87(3 Pt 1):546–554. doi: 10.1083/jcb.87.3.546. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Bedlack RS, Jr., et al. Localized membrane depolarizations and localized calcium influx during electric field-guided neurite growth. Neuron. 1992;9(3):393–403. doi: 10.1016/0896-6273(92)90178-g. [DOI] [PubMed] [Google Scholar]
- 9.Connor JA. Digital imaging of free calcium changes and of spatial gradients in growing processes in single, mammalian central nervous system cells. Proc Natl Acad Sci U S A. 1986;83(16):6179–6183. doi: 10.1073/pnas.83.16.6179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Fields RD, et al. Accommodation of mouse DRG growth cones to electrically induced collapse: kinetic analysis of calcium transients and set-point theory. J Neurobiol. 1993;24(8):1080–1098. doi: 10.1002/neu.480240807. [DOI] [PubMed] [Google Scholar]
- 11.Haydon PG, et al. Serotonin selectively inhibits growth cone motility and synaptogenesis of specific identified neurons. Science. 1984;226(4674):561–564. doi: 10.1126/science.6093252. [DOI] [PubMed] [Google Scholar]
- 12.Silver RA, et al. Elevated cytosolic calcium in the growth cone inhibits neurite elongation in neuroblastoma cells: correlation of behavioral states with cytosolic calcium concentration. J Neurosci. 1989;9(11):4007–4020. doi: 10.1523/JNEUROSCI.09-11-04007.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Lankford KL, Letourneau PC. Roles of actin filaments and three second-messenger systems in short-term regulation of chick dorsal root ganglion neurite outgrowth. Cell Motil Cytoskeleton. 1991;20(1):7–29. doi: 10.1002/cm.970200103. [DOI] [PubMed] [Google Scholar]
- 14.Mattson MP, Kater SB. Calcium regulation of neurite elongation and growth cone motility. J Neurosci. 1987;7(12):4034–4043. doi: 10.1523/JNEUROSCI.07-12-04034.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Kater SB, Mills LR. Regulation of growth cone behavior by calcium. J Neurosci. 1991;11(4):891–899. doi: 10.1523/JNEUROSCI.11-04-00891.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Lau PM, et al. Induction of filopodia by direct local elevation of intracellular calcium ion concentration. J Cell Biol. 1999;145(6):1265–1275. doi: 10.1083/jcb.145.6.1265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Garyantes TK, Regehr WG. Electrical activity increases growth cone calcium but fails to inhibit neurite outgrowth from rat sympathetic neurons. J Neurosci. 1992;12(1):96–103. doi: 10.1523/JNEUROSCI.12-01-00096.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Gu X, et al. Spontaneous neuronal calcium spikes and waves during early differentiation. J Neurosci. 1994;14(11 Pt 1):6325–6335. doi: 10.1523/JNEUROSCI.14-11-06325.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Cohan CS, et al. Electrically and chemically mediated increases in intracellular calcium in neuronal growth cones. J Neurosci. 1987;7(11):3588–3599. doi: 10.1523/JNEUROSCI.07-11-03588.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Bentley D, et al. Calcium ion distribution in nascent pioneer axons and coupled preaxonogenesis neurons in situ. J Neurosci. 1991;11(5):1300–1308. doi: 10.1523/JNEUROSCI.11-05-01300.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.al-Mohanna FA, et al. A narrow window of intracellular calcium concentration is optimal for neurite outgrowth in rat sensory neurones. Dev Brain Res. 1992;70(2):287–290. doi: 10.1016/0165-3806(92)90209-f. [DOI] [PubMed] [Google Scholar]
- 22.Rehder V, Kater SB. Regulation of neuronal growth cone filopodia by intracellular calcium. J Neurosci. 1992;12(8):3175–3186. doi: 10.1523/JNEUROSCI.12-08-03175.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Zheng JQ. Turning of nerve growth cones induced by localized increases in intracellular calcium ions. Nature. 2000;403(6765):89–93. doi: 10.1038/47501. [DOI] [PubMed] [Google Scholar]
- 24.Zheng JQ, et al. Turning of nerve growth cones induced by neurotransmitters. Nature. 1994;368(6467):140–144. doi: 10.1038/368140a0. [DOI] [PubMed] [Google Scholar]
- 25.Takei K, et al. Regulation of nerve growth mediated by inositol 1,4,5-trisphosphate receptors in growth cones. Science. 1998;282(5394):1705–1708. doi: 10.1126/science.282.5394.1705. [DOI] [PubMed] [Google Scholar]
- 26.Gomez TM, Spitzer NC. In vivo regulation of axon extension and pathfinding by growth-cone calcium transients. Nature. 1999;397(6717):350–355. doi: 10.1038/16927. [DOI] [PubMed] [Google Scholar]
- 27.Gomez TM, et al. Characterization of spontaneous calcium transients in nerve growth cones and their effect on growth cone migration. Neuron. 1995;14(6):1233–1246. doi: 10.1016/0896-6273(95)90270-8. [DOI] [PubMed] [Google Scholar]
- 28.Tang F, et al. Spontaneous calcium transients in developing cortical neurons regulate axon outgrowth. J Neurosci. 2003;23(3):927–936. doi: 10.1523/JNEUROSCI.23-03-00927.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Ming G, et al. Electrical activity modulates growth cone guidance by diffusible factors. Neuron. 2001;29(2):441–452. doi: 10.1016/s0896-6273(01)00217-3. [DOI] [PubMed] [Google Scholar]
- 30.Song HJ, et al. cAMP-induced switching in turning direction of nerve growth cones. Nature. 1997;388(6639):275–279. doi: 10.1038/40864. [DOI] [PubMed] [Google Scholar]
- 31.Nishiyama M, et al. Cyclic AMP/GMP-dependent modulation of Ca2+ channels sets the polarity of nerve growth-cone turning. Nature. 2003;424(6943):990–995. doi: 10.1038/nature01751. [DOI] [PubMed] [Google Scholar]
- 32.Ramon y Cajal S. Histology of the nervous system. Oxford Univ. Press; 1995. [Google Scholar]
- 33.Tessier-Lavigne M, Goodman CS. The molecular biology of axon guidance. Science. 1996;274(5290):1123–1133. doi: 10.1126/science.274.5290.1123. [DOI] [PubMed] [Google Scholar]
- 34.Jaffe LF, Poo MM. Neurites grow faster towards the cathode than the anode in a steady field. J Exp Zool. 1979;209(1):115–128. doi: 10.1002/jez.1402090114. [DOI] [PubMed] [Google Scholar]
- 35.Hinkle L, et al. The direction of growth of differentiating neurones and myoblasts from frog embryos in an applied electric field. J Physiol. 1981;314:121–135. doi: 10.1113/jphysiol.1981.sp013695. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Patel N, Poo MM. Orientation of neurite growth by extracellular electric fields. J Neurosci. 1982;2(4):483–496. doi: 10.1523/JNEUROSCI.02-04-00483.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Davenport RW, Kater SB. Local increases in intracellular calcium elicit local filopodial responses in Helisoma neuronal growth cones. Neuron. 1992;9(3):405–416. doi: 10.1016/0896-6273(92)90179-h. [DOI] [PubMed] [Google Scholar]
- 38.Silver RA, et al. Calcium hotspots caused by L-channel clustering promote morphological changes in neuronal growth cones. Nature. 1990;343(6260):751–754. doi: 10.1038/343751a0. [DOI] [PubMed] [Google Scholar]
- 39.Lipscombe D, et al. Spatial distribution of calcium channels and cytosolic calcium transients in growth cones and cell bodies of sympathetic neurons. Proc Natl Acad Sci U S A. 1988;85(7):2398–2402. doi: 10.1073/pnas.85.7.2398. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Mattson MP, et al. Outgrowth-regulating actions of glutamate in isolated hippocampal pyramidal neurons. J Neurosci. 1988;8(6):2087–2100. doi: 10.1523/JNEUROSCI.08-06-02087.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.McCobb DP, et al. Dopamine and serotonin inhibition of neurite elongation of different identified neurons. J Neurosci Res. 1988;19(1):19–26. doi: 10.1002/jnr.490190104. [DOI] [PubMed] [Google Scholar]
- 42.Zheng JQ, et al. Essential role of filopodia in chemotropic turning of nerve growth cone induced by a glutamate gradient. J Neurosci. 1996;16(3):1140–1149. doi: 10.1523/JNEUROSCI.16-03-01140.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Hong K, et al. Calcium signalling in the guidance of nerve growth by netrin-1. Nature. 2000;403(6765):93–98. doi: 10.1038/47507. [DOI] [PubMed] [Google Scholar]
- 44.Wong ST, et al. A p75(NTR) and Nogo receptor complex mediates repulsive signaling by myelin-associated glycoprotein. Nat Neurosci. 2002;5(12):1302–1308. doi: 10.1038/nn975. [DOI] [PubMed] [Google Scholar]
- 45.Henley JR, Poo M.-m. Cyclic AMP-dependent calcium signaling mediates the conversion of neuronal growth cone responses from repulsion to attraction. Mol Biol Cell Supp. 2003;14:406a. [Google Scholar]
- 46.Gomez TM, et al. Filopodial calcium transients promote substrate-dependent growth cone turning. Science. 2001;291(5510):1983–1987. doi: 10.1126/science.1056490. [DOI] [PubMed] [Google Scholar]
- 47.Davenport RW, et al. Distinct calcium signaling within neuronal growth cones and filopodia. J Neurobiol. 1996;31(1):1–15. doi: 10.1002/(SICI)1097-4695(199609)31:1<1::AID-NEU1>3.0.CO;2-6. [DOI] [PubMed] [Google Scholar]
- 48.Robles E, et al. Filopodial calcium transients regulate growth cone motility and guidance through local activation of calpain. Neuron. 2003;38(4):597–609. doi: 10.1016/s0896-6273(03)00260-5. [DOI] [PubMed] [Google Scholar]
- 49.Glading A, et al. Cutting to the chase: calpain proteases in cell motility. Trends Cell Biol. 2002;12(1):46–54. doi: 10.1016/s0962-8924(01)02179-1. [DOI] [PubMed] [Google Scholar]
- 50.Cheng S, et al. Local calcium changes regulate the length of growth cone filopodia. J Neurobiol. 2002;50(4):263–275. doi: 10.1002/neu.10027. [DOI] [PubMed] [Google Scholar]
- 51.Yamada KM, et al. Ultrastructure and function of growth cones and axons of cultured nerve cells. J Cell Biol. 1971;49(3):614–635. doi: 10.1083/jcb.49.3.614. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Bentley D, Toroian-Raymond A. Disoriented pathfinding by pioneer neurone growth cones deprived of filopodia by cytochalasin treatment. Nature. 1986;323(6090):712–715. doi: 10.1038/323712a0. [DOI] [PubMed] [Google Scholar]
- 53.Chien CB, et al. Navigational errors made by growth cones without filopodia in the embryonic Xenopus brain. Neuron. 1993;11(2):237–251. doi: 10.1016/0896-6273(93)90181-p. [DOI] [PubMed] [Google Scholar]
- 54.Kaufmann N, et al. Drosophila Rac1 controls motor axon guidance. Development. 1998;125(3):453–461. doi: 10.1242/dev.125.3.453. [DOI] [PubMed] [Google Scholar]
- 55.Bray D, et al. Growth cone formation in cultures of sensory neurons. Proc Natl Acad Sci U S A. 1978;75(10):5226–5229. doi: 10.1073/pnas.75.10.5226. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Buck KB, Zheng JQ. Growth cone turning induced by direct local modification of microtubule dynamics. J Neurosci. 2002;22(21):9358–9367. doi: 10.1523/JNEUROSCI.22-21-09358.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Sabry JH, et al. Microtubule behavior during guidance of pioneer neuron growth cones in situ. J Cell Biol. 1991;115(2):381–395. doi: 10.1083/jcb.115.2.381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Zhou FQ, et al. Focal loss of actin bundles causes microtubule redistribution and growth cone turning. J Cell Biol. 2002;157(5):839–849. doi: 10.1083/jcb.200112014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Clapham DE. Calcium signaling. Cell. 1995;80(2):259–268. doi: 10.1016/0092-8674(95)90408-5. [DOI] [PubMed] [Google Scholar]
- 60.Blaustein MP, et al. Physiological roles of the sodium-calcium exchanger in nerve and muscle. Ann N Y Acad Sci. 1991;639:254–274. doi: 10.1111/j.1749-6632.1991.tb17315.x. [DOI] [PubMed] [Google Scholar]
- 61.Strehler EE, Zacharias DA. Role of alternative splicing in generating isoform diversity among plasma membrane calcium pumps. Physiol Rev. 2001;81(1):21–50. doi: 10.1152/physrev.2001.81.1.21. [DOI] [PubMed] [Google Scholar]
- 62.Baimbridge KG, et al. Calcium-binding proteins in the nervous system. Trends Neurosci. 1992;15(8):303–308. doi: 10.1016/0166-2236(92)90081-i. [DOI] [PubMed] [Google Scholar]
- 63.Monck JR, et al. Pulsed laser imaging of rapid Ca2+ gradients in excitable cells. Biophys J. 1994;67(2):505–514. doi: 10.1016/S0006-3495(94)80554-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Klingauf J, Neher E. Modeling buffered Ca2+ diffusion near the membrane: implications for secretion in neuroendocrine cells. Biophys J. 1997;72(2 Pt 1):674–690. doi: 10.1016/s0006-3495(97)78704-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Tam T, et al. Voltage-gated calcium channels direct neuronal migration in Caenorhabditis elegans. Dev Biol. 2000;226(1):104–117. doi: 10.1006/dbio.2000.9854. [DOI] [PubMed] [Google Scholar]
- 66.Greka A, et al. TRPC5 is a regulator of hippocampal neurite length and growth cone morphology. Nat Neurosci. 2003;6(8):837–845. doi: 10.1038/nn1092. [DOI] [PubMed] [Google Scholar]
- 67.Henschler R, et al. SDF-1alpha-induced intracellular calcium transient involves Rho GTPase signalling and is required for migration of hematopoietic progenitor cells. Biochem Biophys Res Commun. 2003;311(4):1067–1071. doi: 10.1016/j.bbrc.2003.10.112. [DOI] [PubMed] [Google Scholar]
- 68.Harris WA, et al. Retinal axons with and without their somata, growing to and arborizing in the tectum of Xenopus embryos: a time-lapse video study of single fibres in vivo. Development. 1987;101(1):123–133. doi: 10.1242/dev.101.1.123. [DOI] [PubMed] [Google Scholar]
- 69.Bridgman PC, Dailey ME. The organization of myosin and actin in rapid frozen nerve growth cones. J Cell Biol. 1989;108(1):95–109. doi: 10.1083/jcb.108.1.95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Zucker RS. Calcium- and activity-dependent synaptic plasticity. Curr Opin Neurobiol. 1999;9(3):305–313. doi: 10.1016/s0959-4388(99)80045-2. [DOI] [PubMed] [Google Scholar]
- 71.VanBerkum MF, Goodman CS. Targeted disruption of Ca(2+)-calmodulin signaling in Drosophila growth cones leads to stalls in axon extension and errors in axon guidance. Neuron. 1995;14(1):43–56. doi: 10.1016/0896-6273(95)90239-2. [DOI] [PubMed] [Google Scholar]
- 72.Kuhn TB, et al. Laminin directs growth cone navigation via two temporally and functionally distinct calcium signals. J Neurosci. 1998;18(1):184–194. doi: 10.1523/JNEUROSCI.18-01-00184.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Milikan JM, et al. Integration of calcium signals by calmodulin in rat sensory neurons. Eur J Neurosci. 2002;15(4):661–670. doi: 10.1046/j.1460-9568.2002.01900.x. [DOI] [PubMed] [Google Scholar]
- 74.Hanson PI, Schulman H. Neuronal Ca2+/calmodulin-dependent protein kinases. Annu Rev Biochem. 1992;61:559–601. doi: 10.1146/annurev.bi.61.070192.003015. [DOI] [PubMed] [Google Scholar]
- 75.Goshima Y, et al. Overexpression of Ca2+/calmodulin-dependent protein kinase II in Neuro2a and NG108-15 neuroblastoma cell lines promotes neurite outgrowth and growth cone motility. J Neurosci. 1993;13(2):559–567. doi: 10.1523/JNEUROSCI.13-02-00559.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Zou DJ, Cline HT. Postsynaptic calcium/calmodulin-dependent protein kinase II is required to limit elaboration of presynaptic and postsynaptic neuronal arbors. J Neurosci. 1999;19(20):8909–8918. doi: 10.1523/JNEUROSCI.19-20-08909.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Chen N, et al. Ganglioside/calmodulin kinase II signal inducing cdc42-mediated neuronal actin reorganization. Neuroscience. 2003;120(1):163–176. doi: 10.1016/s0306-4522(03)00259-8. [DOI] [PubMed] [Google Scholar]
- 78.Fink CC, et al. Selective regulation of neurite extension and synapse formation by the beta but not the alpha isoform of CaMKII. Neuron. 2003;39(2):283–297. doi: 10.1016/s0896-6273(03)00428-8. [DOI] [PubMed] [Google Scholar]
- 79.Hall A. Rho GTPases and the actin cytoskeleton. Science. 1998;279(5350):509–514. doi: 10.1126/science.279.5350.509. [DOI] [PubMed] [Google Scholar]
- 80.Ruchhoeft ML, et al. The neuronal architecture of Xenopus retinal ganglion cells is sculpted by rho-family GTPases in vivo. J Neurosci. 1999;19(19):8454–8463. doi: 10.1523/JNEUROSCI.19-19-08454.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Yuan XB, et al. Signalling and crosstalk of Rho GTPases in mediating axon guidance. Nat Cell Biol. 2003;5(1):38–45. doi: 10.1038/ncb895. [DOI] [PubMed] [Google Scholar]
- 82.Ng J, et al. Rac GTPases control axon growth, guidance and branching. Nature. 2002;416(6879):442–447. doi: 10.1038/416442a. [DOI] [PubMed] [Google Scholar]
- 83.Lundquist EA. Rac proteins and the control of axon development. Curr Opin Neurobiol. 2003;13(3):384–390. doi: 10.1016/s0959-4388(03)00071-0. [DOI] [PubMed] [Google Scholar]
- 84.Chang HY, et al. Asymmetric retraction of growth cone filopodia following focal inactivation of calcineurin. Nature. 1995;376(6542):686–690. doi: 10.1038/376686a0. [DOI] [PubMed] [Google Scholar]
- 85.Lautermilch NJ, Spitzer NC. Regulation of calcineurin by growth cone calcium waves controls neurite extension. J Neurosci. 2000;20(1):315–325. doi: 10.1523/JNEUROSCI.20-01-00315.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Cheng S, et al. Filopodial behavior is dependent on the phosphorylation state of neuronal growth cones. Cell Motil Cytoskeleton. 2000;47(4):337–350. doi: 10.1002/1097-0169(200012)47:4<337::AID-CM7>3.0.CO;2-B. [DOI] [PubMed] [Google Scholar]
- 87.Xiang Y, et al. Nerve growth cone guidance mediated by G protein-coupled receptors. Nat Neurosci. 2002;5(9):843–848. doi: 10.1038/nn899. [DOI] [PubMed] [Google Scholar]
- 88.Liu YC, Storm DR. Regulation of free calmodulin levels by neuromodulin: neuron growth and regeneration. Trends Pharmacol Sci. 1990;11(3):107–111. doi: 10.1016/0165-6147(90)90195-e. [DOI] [PubMed] [Google Scholar]
- 89.He Q, et al. Modulation of actin filament behavior by GAP-43 (neuromodulin) is dependent on the phosphorylation status of serine 41, the protein kinase C site. J Neurosci. 1997;17(10):3515–3524. doi: 10.1523/JNEUROSCI.17-10-03515.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Welnhofer EA, et al. Calcium influx alters actin bundle dynamics and retrograde flow in Helisoma growth cones. J Neurosci. 1999;19(18):7971–7982. doi: 10.1523/JNEUROSCI.19-18-07971.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Aderem A. The MARCKS brothers: a family of protein kinase C substrates. Cell. 1992;71(5):713–716. doi: 10.1016/0092-8674(92)90546-o. [DOI] [PubMed] [Google Scholar]
- 92.Hartwig JH, et al. MARCKS is an actin filament crosslinking protein regulated by protein kinase C and calcium-calmodulin. Nature. 1992;356(6370):618–622. doi: 10.1038/356618a0. [DOI] [PubMed] [Google Scholar]
- 93.Higgs HN, Pollard TD. Regulation of actin filament network formation through ARP2/3 complex: activation by a diverse array of proteins. Annu Rev Biochem. 2001;70:649–676. doi: 10.1146/annurev.biochem.70.1.649. [DOI] [PubMed] [Google Scholar]
- 94.Sarmiere PD, Bamburg JR. Regulation of the neuronal actin cytoskeleton by ADF/cofilin. J Neurobiol. 2004;58(1):103–117. doi: 10.1002/neu.10267. [DOI] [PubMed] [Google Scholar]
- 95.Fleming IN, et al. Ca2+/calmodulin-dependent protein kinase II regulates Tiam1 by reversible protein phosphorylation. J Biol Chem. 1999;274(18):12753–12758. doi: 10.1074/jbc.274.18.12753. [DOI] [PubMed] [Google Scholar]
- 96.Leeuwen FN, et al. The guanine nucleotide exchange factor Tiam1 affects neuronal morphology; opposing roles for the small GTPases Rac and Rho. J Cell Biol. 1997;139(3):797–807. doi: 10.1083/jcb.139.3.797. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Fan WT, et al. The exchange factor Ras-GRF2 activates Ras-dependent and Rac-dependent mitogen-activated protein kinase pathways. Curr Biol. 1998;8(16):935–938. doi: 10.1016/s0960-9822(07)00376-4. [DOI] [PubMed] [Google Scholar]
- 98.Farnsworth CL, et al. Calcium activation of Ras mediated by neuronal exchange factor Ras-GRF. Nature. 1995;376(6540):524–527. doi: 10.1038/376524a0. [DOI] [PubMed] [Google Scholar]
- 99.Stam JC, et al. Targeting of Tiam1 to the plasma membrane requires the cooperative function of the N-terminal pleckstrin homology domain and an adjacent protein interaction domain. J Biol Chem. 1997;272(45):28447–28454. doi: 10.1074/jbc.272.45.28447. [DOI] [PubMed] [Google Scholar]
- 100.Price LS, et al. Calcium signaling regulates translocation and activation of Rac. J Biol Chem. 2003;278(41):39413–39421. doi: 10.1074/jbc.M302083200. [DOI] [PubMed] [Google Scholar]
- 101.Sakurada S, et al. Ca2+-dependent activation of Rho and Rho kinase in membrane depolarization-induced and receptor stimulation-induced vascular smooth muscle contraction. Circ Res. 2003;93(6):548–556. doi: 10.1161/01.RES.0000090998.08629.60. [DOI] [PubMed] [Google Scholar]
- 102.Letourneau PC, Shattuck TA. Distribution and possible interactions of actin-associated proteins and cell adhesion molecules of nerve growth cones. Development. 1989;105(3):505–519. doi: 10.1242/dev.105.3.505. [DOI] [PubMed] [Google Scholar]
- 103.Mooseker MS, Cheney RE. Unconventional myosins. Annu Rev Cell Dev Biol. 1995;11:633–675. doi: 10.1146/annurev.cb.11.110195.003221. [DOI] [PubMed] [Google Scholar]
- 104.Amano M, et al. Phosphorylation and activation of myosin by Rho-associated kinase (Rho-kinase) J Biol Chem. 1996;271(34):20246–20249. doi: 10.1074/jbc.271.34.20246. [DOI] [PubMed] [Google Scholar]
- 105.Kimura K, et al. Regulation of myosin phosphatase by Rho and Rho-associated kinase (Rho-kinase) Science. 1996;273(5272):245–248. doi: 10.1126/science.273.5272.245. [DOI] [PubMed] [Google Scholar]
- 106.Hirose M, et al. Molecular dissection of the Rho-associated protein kinase (p160ROCK)-regulated neurite remodeling in neuroblastoma N1E-115 cells. J Cell Biol. 1998;141(7):1625–1636. doi: 10.1083/jcb.141.7.1625. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.van Leeuwen FN, et al. Rac regulates phosphorylation of the myosin-II heavy chain, actinomyosin disassembly and cell spreading. Nat Cell Biol. 1999;1(4):242–248. doi: 10.1038/12068. [DOI] [PubMed] [Google Scholar]
- 108.Sanders LC, et al. Inhibition of myosin light chain kinase by p21-activated kinase. Science. 1999;283(5410):2083–2085. doi: 10.1126/science.283.5410.2083. [DOI] [PubMed] [Google Scholar]
- 109.Hing H, et al. Pak functions downstream of Dock to regulate photoreceptor axon guidance in Drosophila. Cell. 1999;97(7):853–863. doi: 10.1016/s0092-8674(00)80798-9. [DOI] [PubMed] [Google Scholar]
- 110.Maekawa M, et al. Signaling from Rho to the actin cytoskeleton through protein kinases ROCK and LIM-kinase. Science. 1999;285(5429):895–898. doi: 10.1126/science.285.5429.895. [DOI] [PubMed] [Google Scholar]
- 111.Edwards DC, et al. Activation of LIM-kinase by Pak1 couples Rac/Cdc42 GTPase signalling to actin cytoskeletal dynamics. Nat Cell Biol. 1999;1(5):253–259. doi: 10.1038/12963. [DOI] [PubMed] [Google Scholar]
- 112.Aizawa H, et al. Phosphorylation of cofilin by LIM-kinase is necessary for semaphorin 3A-induced growth cone collapse. Nat Neurosci. 2001;4(4):367–373. doi: 10.1038/86011. [DOI] [PubMed] [Google Scholar]
- 113.Ruchhoeft ML, Harris WA. Myosin functions in Xenopus retinal ganglion cell growth cone motility in vivo. J Neurobiol. 1997;32(6):567–578. [PubMed] [Google Scholar]
- 114.Zhou FQ, Cohan CS. Growth cone collapse through coincident loss of actin bundles and leading edge actin without actin depolymerization. J Cell Biol. 2001;153(5):1071–1084. doi: 10.1083/jcb.153.5.1071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Kim YS, et al. Constitutively active myosin light chain kinase alters axon guidance decisions in Drosophila embryos. Dev Biol. 2002;249(2):367–381. doi: 10.1006/dbio.2002.0768. [DOI] [PubMed] [Google Scholar]
- 116.Suter DM, et al. Localization of unconventional myosins V and VI in neuronal growth cones. J Neurobiol. 2000;42(3):370–382. [PubMed] [Google Scholar]
- 117.Diefenbach TJ, et al. Myosin 1c and myosin IIB serve opposing roles in lamellipodial dynamics of the neuronal growth cone. J Cell Biol. 2002;158(7):1207–1217. doi: 10.1083/jcb.200202028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Wang FS, et al. Function of myosin-V in filopodial extension of neuronal growth cones. Science. 1996;273(5275):660–663. doi: 10.1126/science.273.5275.660. [DOI] [PubMed] [Google Scholar]
- 119.Lu M, et al. Delayed retraction of filopodia in gelsolin null mice. J Cell Biol. 1997;138(6):1279–1287. doi: 10.1083/jcb.138.6.1279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Meberg PJ, et al. Actin depolymerizing factor and cofilin phosphorylation dynamics: response to signals that regulate neurite extension. Cell Motil Cytoskeleton. 1998;39(2):172–190. doi: 10.1002/(SICI)1097-0169(1998)39:2<172::AID-CM8>3.0.CO;2-8. [DOI] [PubMed] [Google Scholar]
- 121.Dehmelt L, Halpain S. Actin and microtubules in neurite initiation: are MAPs the missing link? J Neurobiol. 2004;58(1):18–33. doi: 10.1002/neu.10284. [DOI] [PubMed] [Google Scholar]
- 122.Hume RI, et al. Acetylcholine release from growth cones detected with patches of acetylcholine receptor-rich membranes. Nature. 1983;305(5935):632–634. doi: 10.1038/305632a0. [DOI] [PubMed] [Google Scholar]
- 123.Young SH, Poo MM. Spontaneous release of transmitter from growth cones of embryonic neurones. Nature. 1983;305(5935):634–637. doi: 10.1038/305634a0. [DOI] [PubMed] [Google Scholar]
- 124.Erskine L, McCaig CD. Growth cone neurotransmitter receptor activation modulates electric field-guided nerve growth. Dev Biol. 1995;171(2):330–339. doi: 10.1006/dbio.1995.1285. [DOI] [PubMed] [Google Scholar]
- 125.Krystosek A, Seeds NW. Plasminogen activator release at the neuronal growth cone. Science. 1981;213(4515):1532–1534. doi: 10.1126/science.7197054. [DOI] [PubMed] [Google Scholar]
- 126.Pfenninger KH, Maylie-Pfenninger MF. Lectin labeling of sprouting neurons. II. Relative movement and appearance of glycoconjugates during plasmalemmal expansion. J Cell Biol. 1981;89(3):547–559. doi: 10.1083/jcb.89.3.547. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Fernandez-Chacon R, et al. Synaptotagmin I functions as a calcium regulator of release probability. Nature. 2001;410(6824):41–49. doi: 10.1038/35065004. [DOI] [PubMed] [Google Scholar]
- 128.Sabo SL, McAllister AK. Mobility and cycling of synaptic protein-containing vesicles in axonal growth cone filopodia. Nat Neurosci. 2003;6(12):1264–1269. doi: 10.1038/nn1149. [DOI] [PubMed] [Google Scholar]
- 129.Dai J, Sheetz MP. Axon membrane flows from the growth cone to the cell body. Cell. 1995;83(5):693–701. doi: 10.1016/0092-8674(95)90182-5. [DOI] [PubMed] [Google Scholar]
- 130.Ming GL, et al. Adaptation in the chemotactic guidance of nerve growth cones. Nature. 2002;417(6887):411–418. doi: 10.1038/nature745. [DOI] [PubMed] [Google Scholar]
- 131.Zacharias DA, et al. Recent advances in technology for measuring and manipulating cell signals. Curr Opin Neurobiol. 2000;10(3):416–421. doi: 10.1016/s0959-4388(00)00101-x. [DOI] [PubMed] [Google Scholar]
- 132.Frey D, et al. Shared and unique roles of CAP23 and GAP43 in actin regulation, neurite outgrowth, and anatomical plasticity. J Cell Biol. 2000;149(7):1443–1454. doi: 10.1083/jcb.149.7.1443. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Sobue K. Actin-based cytoskeleton in growth cone activity. Neurosci Res. 1993;18(2):91–102. doi: 10.1016/0168-0102(93)90012-f. [DOI] [PubMed] [Google Scholar]
- 134.Kabayama H, et al. Functional involvement of synaptotagmin I/II C2A domain in neurite outgrowth of chick dorsal root ganglion neuron. Neuroscience. 1999;88(4):999–1003. doi: 10.1016/s0306-4522(98)00547-8. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.