Abstract
Trilateral retinoblastoma is characterized by the presence of retinoblastoma with an intracranial tumor. The incidence is low and prognosis poor. Due to the paucity of information regarding successful treatment, we report the case of a 6 month old female referred for leukocoria and found to have an associated suprasellar tumor and pineal enhancement. The patient, treated with standard infant brain tumor therapy, remains alive without signs of active disease 35 months after diagnosis; no surgery or irradiation was used. Early diagnosis of trilateral retinoblastoma may facilitate the use of less intensive therapeutic approaches and result in excellent outcomes in these patients.
Keywords: chemotherapy, pineal tumor, quadrilateral retinoblastoma, suprasellar tumor, trilateral retinoblastoma
INTRODUCTION
Trilateral retinoblastoma refers to unilateral or, most often, bilateral intraocular retinoblastoma associated with a primary intracranial neuroectodermal tumor in the pineal or suprasellar region [1]. This association was first described by Jakobiec et al. [2]; the term trilateral retinoblastoma was popularized by Bader et al. [3,4] based on the pineal gland’s role as a photosensitive organ or “third eye” in lower vertebrates. These primitive tumors display varying degrees of neuronal or photoreceptor differentiation. Most trilateral retinoblastomas occur in the pineal region and are histologically pinealoblastomas, but 20–25% occur in the suprasellar or parasellar region [5,6]. The association of bilateral retinoblastoma with a pineal tumor and another primary suprasellar tumor is termed quadrilateral retinoblastoma.
The presence of a germline mutation in the RB1 gene increases the risk of developing trilateral retinoblastoma. Children with familial retinoblastoma appear to have a higher incidence of trilateral disease than those with de novo germline mutations [6–9]. Trilateral retinoblastoma occurs in 3–9% of patients with the genetic form [8]. The disease is almost uniformly fatal and had been the primary cause of death for patients with retinoblastoma in the US [8]. Given the poor prognosis and a move to more aggressive treatment regimens, we report the case of a female child with trilateral retinoblastoma diagnosed early and successfully treated according to an infant brain tumor protocol without requiring surgery, radiation, or high-dose chemotherapy (HDC) with autologous stem cell transplant.
CASE REPORT
A 6-month-old female with no family history of retinoblastoma was diagnosed with bilateral disease after referral to the St. Jude Children’s Research Hospital for bilateral leukocoria noted during her routine well-child exam. Exam under anesthesia (EUA) revealed early multifocal bilateral disease, International Retinoblastoma Classification System group B bilaterally. MRI of the orbits and brain at presentation revealed an enhancing lesion approximately 4 mm × 6 mm in size involving the pituitary stalk/infundibular recess region, with prominent enhancement of the pineal gland (Fig. 1). Spinal MRI did not show metastatic disease. Subsequent lumbar puncture revealed no tumor cells. Bone marrow aspirate was negative for metastatic disease.
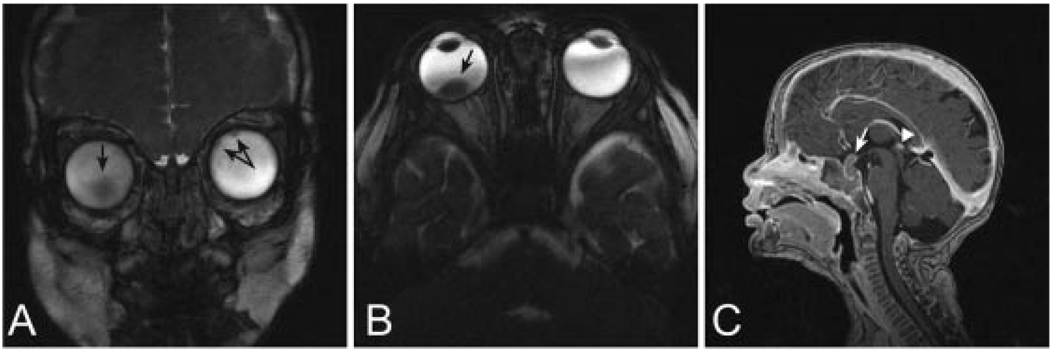
Fig. 1.
At diagnosis. A. Coronal 3D-CISS and B. Axial 3D-CISS images of the orbits show tumor foci (black arrows) within both globes, one larger on the right and at least two small ones on the left. C. Sagittal T1-weighted image of the brain reveals enhancing intra- and suprasellar mass lesion (white arrow) with prominent enhancement of the pineal gland (white arrowhead).
TREATMENT
The patient was diagnosed with synchronous trilateral retinoblastoma and was started on a regimen of chemotherapy consisting initially of four courses of vincristine (0.05 mg/kg/dose IV on days 1 and 8), cisplatin (3.5 mg/kg/dose on day 1), cyclophosphamide (65 mg/kg/dose IV on day 2), and etoposide (3.33 mg/kg/dose IV on days 2 and 3) given at 3-week intervals. These four agents have been used as standard therapy for brain tumors in infants as well as in 2 of the largest infant brain tumor trials to date—Baby POG-1 and CCG 9921 [10–12]. Both trials enrolled a wide variety of diagnoses, including atypical teratoid rhabdoid tumor and other embryonal tumors, high-grade glioma, and choroid plexus carcinoma. Doses and schedule for our patient were derived from the CCG 9921 regimen and the Head Start I and II trials [12,13]. After three cycles of chemotherapy, progressive decrease in the size of the supratentorial mass was noted, with a stable pineal gland size of 4 mm × 6 mm in the anteroposterior and craniocaudad dimensions. After the initial four courses of chemotherapy, concerns about potential ototoxicity prompted us to replace cisplatin with carboplatin at an area under the curve of 5 mg/ml min for the next eight courses of chemotherapy.
The intraocular disease also responded to chemotherapy and was further consolidated with thermo- and cryotherapy. No residual pituitary tumor was apparent 6 months after diagnosis and seven courses of chemotherapy. Subsequent MRIs of the brain and orbits conducted every 2–3 months revealed stable appearance of the pineal gland. The patient’s most recent brain MRI 34 months post diagnosis showed no evidence of intracranial tumor recurrence (Fig. 2). She continues to undergo routine EUAs and brain MRIs. Her disease remains stable 35 months after diagnosis.
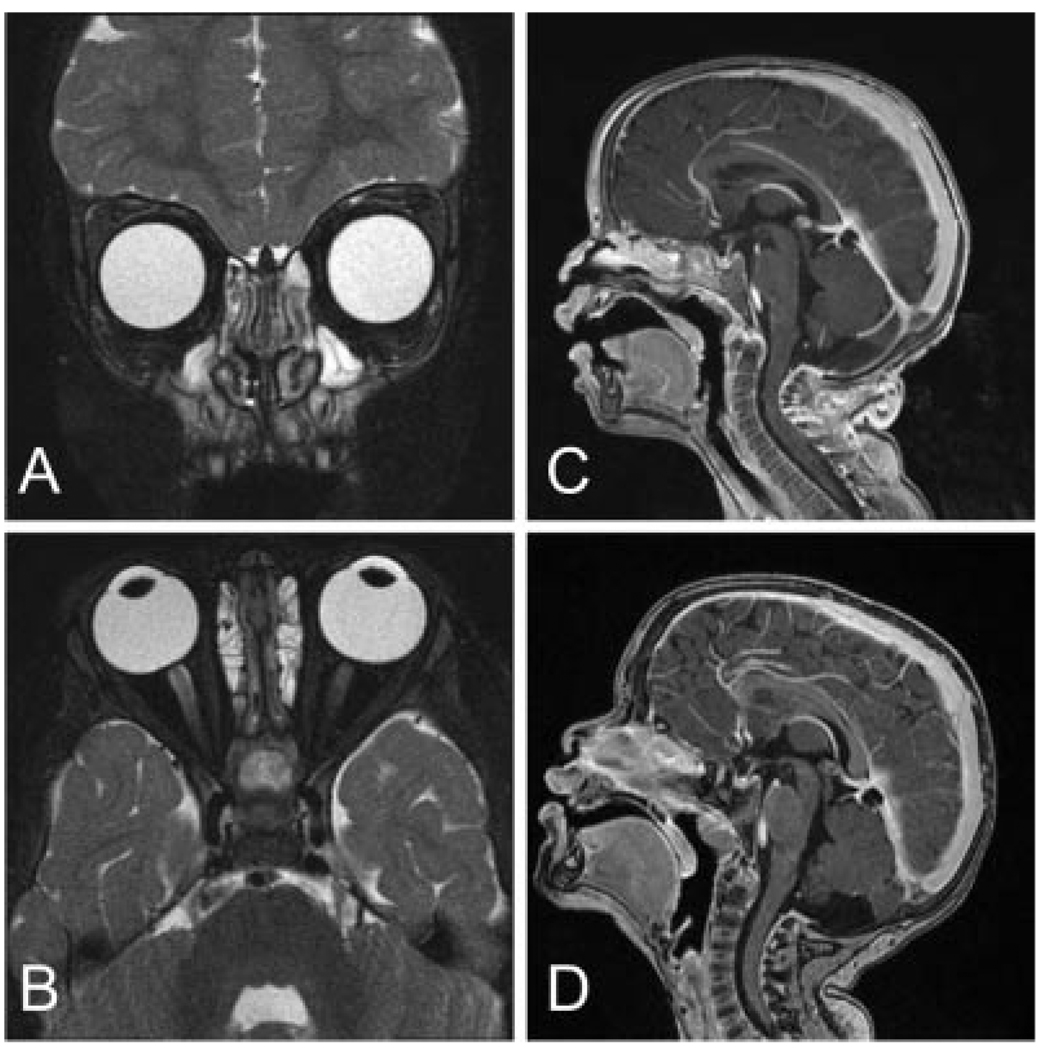
Fig. 2.
A. Coronal T2-weighted 3D fast spin-echo and B. Axial T2-weighted 3D fast spin-echo images of the orbits 10 months post diagnosis show resolution of tumor foci within both globes. C. and D. Sagittal T1-weighted images of the brain demonstrate resolution of intracranial lesion at 6 months and 34 months post diagnosis, respectively.
DISCUSSION
We report the successful treatment of a 6-month-old patient with trilateral retinoblastoma, using an infant brain tumor regimen that excluded surgery, irradiation, and high-dose chemotherapy with autologous stem cell rescue (ASCR). Our patient presented with bilateral retinoblastoma and the synchronous occurrence of a suprasellar mass and pineal enlargement, a syndrome commonly referred to as quadrilateral retinoblastoma [1,6,14]. A biopsy was not performed to confirm the disease due to the risks associated with a surgical procedure; however, the finding of a suprasellar mass on MRI was highly suggestive of trilateral retinoblastoma. Pineal enlargement and enhancement, on the other hand, could be interpreted as normal findings for a young patient with bilateral disease.
Trilateral and quadrilateral retinoblastomas are extremely rare: they occur in 1.5–5% of patients with unilateral or bilateral retinoblastoma and 2–11% in patients with bilateral retinoblastoma [9]. Trilateral retinoblastoma is rarely present at diagnosis. The median age of patients at diagnosis of bilateral retinoblastoma is 23–48 months, with an interval of approximately 21 months between diagnosis of bilateral retinoblastoma and the brain tumor [1,5,7–9][15,16]. In a study by Kivela, intracranial tumors occurred in 3 of 93 (3%) patients before, 13 of 93 (14%) cases with, and 77 of 93 (83%) cases after the diagnosis of retinoblastoma [1]. Similar results were reported by Amoaku et al. [16], intracranial tumors anteceded the diagnosis of retinoblastoma in only 15–20% of patients. Suprasellar tumors tend to be diagnosed earlier than pineal tumors after the diagnosis of intraocular tumors [15].
The prognosis for patients with trilateral retinoblastoma remains poor; death from disseminated neuroaxis disease is common [1,15]. The mean length of survival after diagnosis of the intracranial tumor for untreated patients is 1.3 months and for treated patients, 9.7–11.2 months [5,7]. Patients with midline intracranial tumors who are asymptomatic at presentation have a longer survival and overall better outcome than symptomatic patients [10,16], however, whether asymptomatic children diagnosed by screening have a better survival remains controversial [1,9,15,16]. Kivela et al. have also suggested that tumor size may play a prognostic role in trilateral retinoblastoma, with 15 mm representing the upper limit for risk of leptomeningeal spread and probability of cure [1]. Rare survivors of trilateral retinoblastoma are those diagnosed early by screening imaging and treated with intensive chemotherapy with or without craniospinal radiation [1].
In recent years, the more widespread use of chemoreduction treatment and decrease in the use of radiation for patients with bilateral retinoblastoma has been argued by some to lower the incidence of trilateral retinoblastoma, raising the controversial issue of whether patients with the genetic form of retinoblastoma are now considered to be protected to some degree against this fatal complication [17]. Several patients with bilateral disease, however, develop pineal cysts, which appear to be a forme fustre of trilateral retinoblastoma [6]. In our patient, the suprasellar mass responded completely to chemotherapy; the pineal enlargement persisted, but no cystic changes developed.
The standard treatment for intracranial tumors in patients with retinoblastoma has been surgical resection followed by chemotherapy and cranial or craniospinal radiation; more recent studies suggest improved survival for those patients treated with HDC and ASCR [18,19]. Most intracranial tumors are pinealoblastomas, which are highly chemosensitive and appear to have a steep dose–response curve for alkylating agents. Studies in older patients with primary pinealoblastoma have shown that complete resection paired with intensive alkylator- and cisplatin-based therapy, followed by craniospinal radiation and consolidation with HDC and ASCR, may improve survival in more than two-thirds of patients [20]. Similar treatment regimens applied to young children with trilateral retinoblastoma need to consider the adverse long-term toxicities of irradiation. Suitable alternative treatments for very young patients avoid the use of radiation and intensive chemotherapy followed by consolidation with ASCR, an approach similar to that used for treating brain tumors in infants. Therefore, survival rates for some patients diagnosed early or during routine screening may be improved by using a multimodal approach that includes platinum-based chemotherapy and standard infant brain tumor protocols.
Given the poor prognosis of trilateral retinoblastoma and the short interval between diagnosis of retinoblastoma and the occurrence of trilateral retinoblastoma, neuroimaging screening has become routine practice. About 25% of those cases reported by Kivela were diagnosed during routine screening methods [1]. It remains to be seen, however, whether early diagnosis through screening impacts survival. Despite this ongoing controversy, most clinicians recommend neuroimaging every 6 months until 5 years of age [1,15].
In summary, our case demonstrates that early diagnosis and the use of intensive platinum- and alkylator-based chemotherapy without HDC, ASCR and radiation therapy can be an effective cure option for children with tri- and quadrilateral retinoblastoma.
ACKNOWLEDGMENTS
This research was supported in part by grant P30 CA21765 from the National Institutes of Health, the Noyes Brain Tumor Foundation, Musicians Against Childhood Cancer (MACC) and the American Lebanese Syrian Associated Charities (ALSAC). We would also like to thank the Biomedical Communications Graphic Arts Department (Julie Groff) for preparation of the figures and the Scientific Editing Department (Vani Shanker) at St. Jude Children’s Research Hospital for review of the manuscript.
Grant sponsor: National Institutes of Health; Grant number: P30 CA21765; Grant sponsor: Noyes Brain Tumor Foundation; Grant sponsor: Musicians Against Childhood Cancer (MACC); Grant sponsor: American Lebanese Syrian Associated Charities (ALSAC).
Footnotes
Conflict of interest: Nothing to report.
REFERENCES
- 1.Kivela T. Trilateral retinoblastoma: A meta-analysis of hereditary retinoblastoma associated with primary ectopic intracranial retinoblastoma. J Clin Oncol. 1999;17:1829–1837. doi: 10.1200/JCO.1999.17.6.1829. [DOI] [PubMed] [Google Scholar]
- 2.Jakobiec FA, Tso MOM, Zimmerman LE, et al. Retinoblastoma and intracranial malignancy. Cancer. 1977;39:2048–2058. doi: 10.1002/1097-0142(197705)39:5<2048::aid-cncr2820390522>3.0.co;2-9. [DOI] [PubMed] [Google Scholar]
- 3.Bader JL, Miller RW, Meadows AT, et al. Trilateral retinoblastoma. Lancet. 1980;2:582–583. doi: 10.1016/s0140-6736(80)92009-7. [DOI] [PubMed] [Google Scholar]
- 4.Bader JL, Meadows AT, Zimmerman LE, et al. Bilateral retinoblastoma with ectopic intracranial retinoblastoma: Trilateral retinoblastoma. Cancer Genet Cytogenet. 1982;5:203–213. doi: 10.1016/0165-4608(82)90026-7. [DOI] [PubMed] [Google Scholar]
- 5.Provenzale JM, Gururangan S, Klinworth G. Trilateral retinoblastoma: Clinical and radiological progression. Am J Radiol. 2004;183:505–511. doi: 10.2214/ajr.183.2.1830505. [DOI] [PubMed] [Google Scholar]
- 6.Beck-Popovic M, Balmer A, Maeder P, et al. Benign pineal cysts in children with bilateral retinoblastoma: A new variant of trilateral retinoblastoma? Pediatr Blood Cancer. 2006;46:755–761. doi: 10.1002/pbc.20464. [DOI] [PubMed] [Google Scholar]
- 7.Holladay DA, Holladay A, Montebello JF, et al. Clinical presentation, treatment and outcome of trilateral retinoblastoma. Cancer. 1991;67:710–715. doi: 10.1002/1097-0142(19910201)67:3<710::aid-cncr2820670330>3.0.co;2-3. [DOI] [PubMed] [Google Scholar]
- 8.Blach LE, McCormick B, Abramson DH, et al. Trilateral retinoblastoma—Incidence and outcome: A decade of experience. Int J Radiat Oncol Biol Phys. 1994;29:729–733. doi: 10.1016/0360-3016(94)90560-6. [DOI] [PubMed] [Google Scholar]
- 9.De Potter P, Shields CL, Shields JA. Clinical variations of trilateral retinoblastoma: A report of 13 cases. J Pediatr Ophthalmol Strabismus. 1994;31:26–31. doi: 10.3928/0191-3913-19940101-06. [DOI] [PubMed] [Google Scholar]
- 10.Duffner PK, Horowitz ME, Krischer JP, et al. Postoperative chemotherapy and delayed radiation in children less than three years of age with malignant brain tumors. N Engl J Med. 1993;328:1725–1731. doi: 10.1056/NEJM199306173282401. [DOI] [PubMed] [Google Scholar]
- 11.Duffner PK, Horowitz ME, Krischer JP, et al. The treatment of malignant brain tumors in infants and very young children: An update of the Pediatric Oncology Group experience. Neuro-Oncology. 1999;1:152–161. doi: 10.1093/neuonc/1.2.152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Geyer JR, Sposto R, Jennings M, et al. Multiagent chemotherapy and deferred radiation in infants with malignant brain tumors: A report from the Children’s Cancer Group. J Clin Oncol. 2005;23:7621–7631. doi: 10.1200/JCO.2005.09.095. [DOI] [PubMed] [Google Scholar]
- 13.Mason WP, Grovas A, Halpern S, et al. Intensive chemotherapy and bone marrow rescue for young children with newly diagnosed malignant brain tumors. J Clin Oncol. 1998;16:210–221. doi: 10.1200/JCO.1998.16.1.210. [DOI] [PubMed] [Google Scholar]
- 14.Beck-Popovic M, Diezi M, Kuchler H, et al. Trilateral retinoblastoma with suprasellar tumor and associated pineal cyst. J Pediatr Hematol Oncol. 2007;29:53–56. doi: 10.1097/MPH.0b013e3180308782. [DOI] [PubMed] [Google Scholar]
- 15.Paulino AC. Trilateral retinoblastoma: Is the location of the intracranial tumor important? Cancer. 1999;86:135–141. [PubMed] [Google Scholar]
- 16.Amoaku WMK, Willshaw HE, Parkes SE, et al. Trilateral retinoblastoma: A report of five patients. Cancer. 1996;78:858–863. doi: 10.1002/(SICI)1097-0142(19960815)78:4<858::AID-CNCR24>3.0.CO;2-T. [DOI] [PubMed] [Google Scholar]
- 17.Shields CL, Meadows AT, Shields JA, et al. Chemoreduction for retinoblastoma may prevent intracranial neuroblastic malignancy (trilateral retinoblastoma) Arch Ophthalmol. 2003;121:1513. doi: 10.1001/archopht.119.9.1269. [DOI] [PubMed] [Google Scholar]
- 18.Dunkel IJ, Aledo A, Kernan NA, et al. Successful treatment of metastatic retinoblastoma. Cancer. 2000;89:2117–2121. doi: 10.1002/1097-0142(20001115)89:10<2117::aid-cncr12>3.0.co;2-9. [DOI] [PubMed] [Google Scholar]
- 19.Dunkel IJ, Jubran RF, Gururangan S, et al. Trilateral retinoblastoma: Potentially curable with intensive chemotherapy. Pediatr Blood Cancer. 2010;54:384–387. doi: 10.1002/pbc.22336. [DOI] [PubMed] [Google Scholar]
- 20.Gururangan S, McLaughlin C, Quinn J, et al. High-dose chemotherapy with autologous stem-cell rescue in children and adults with newly diagnosed pineoblastoma. J Clin Oncol. 2003;21:2187–2191. doi: 10.1200/JCO.2003.10.096. [DOI] [PubMed] [Google Scholar]