Abstract
Heat shock proteins (HSP) are essential for intracellular protein folding during stress and protect cells from denaturation and aggregation cascades that can lead to cell death. HSP genes are regulated at the transcriptional level by heat shock transcription factor 1 (HSF1) that is activated by stress and binds to heat shock elements in HSP genes. The activation of HSF1 during heat shock involves conversion from an inert monomer to a DNA binding trimer through a series of intramolecular folding rearrangements. However, the trigger for HSF1 at the molecular level is unclear and hypotheses for this process include reversal of feedback inhibition of HSF1 by molecular chaperones and heat-induced binding to large non-coding RNAs. Heat shock also causes a profound modulation in cell signaling pathways that lead to protein kinase activation and phosphorylation of HSF1 at a number of regulatory serine residues. HSP genes themselves exist in an accessible chromatin conformation already bound to RNA polymerase II. The RNA polymerase II is paused on HSP promoters after transcribing a short RNA sequence proximal to the promoter. Activation by heat shock involves HSF1 binding to the promoter and release of the paused RNA polymerase II followed by further rounds of transcriptional initiation and elongation. HSF1 is thus involved in both initiation and elongation of HSP RNA transcripts. Recent studies indicate important roles for histone modifications on HSP genes during heat shock. Histone modification occurs rapidly after stress and may be involved in promoting nucleosome remodeling on HSP promoters and in the open reading frames of HSP genes. Understanding these processes may be key to evaluating mechanisms of deregulated HSP expression that plays a key role in neurodegeneration and cancer.
Keywords: heat shock proteins, cell signaling, RNA
Introduction: The Heat Shock Response
The heat shock response (HSR) is a cellular mechanism for responding to a range of stresses, epitomized by heat shock, that result in protein inactivation.1,2 Activation of the HSR leads to cellular resistance to stress, a phenomenon termed thermotolerance.1,3 The molecular events that underlie thermotolerance have been characterized and involve the coordinated synthesis of heat shock proteins (HSP).1,4 HSPs are a group of molecular chaperones that are induced by stress and inhibit the changes in stressed proteins that lead to protein denaturation, aggregation and cell death.5 They have further desirable properties including the ability to directly antagonize death pathways including caspase-dependent apoptosis and replicative senescence.6,7 HSP thus play a pivotal role in the thermotolerance phenotype by directly and indirectly blocking stress-induced death. Understanding thermotolerance is important in the effective deployment of hyperthermia and thermal ablation in the clinic.1,8–11 However, probably of greater significance, these properties of HSPs are thought to underlie some of the pathophysiological changes of cancer and aging.12,13 For reasons that are not currently clear, HSP levels rise in cancer and decline during aging, and these changes are significant in disease etiology12,14 Further understanding of signaling in the HSR may clarify such mechanisms. The HSR is regulated at the transcriptional level by heat shock transcription factor 1 (HSF1).15,16
Heat Shock Factor 1-Regulation by Monomer-Trimer Transition
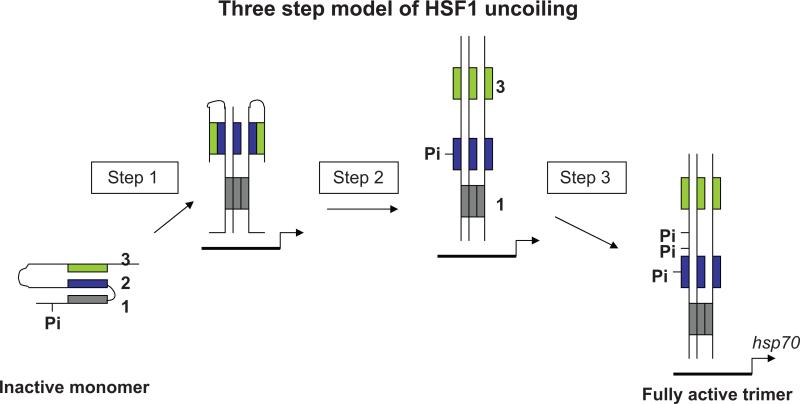
HSF1 binds to the promoters of all significant HSP genes after stress and orchestrates the upregulation of HSP mRNA synthesis.15,16 Understanding HSF1 is thus the key to comprehending the role of HSPs in thermotolerance, aging and cancer. HSF family proteins are conserved and homologous proteins are expressed in S. cerevisiae and S. pombe each binding as trimers to heat shock elements (HSE) in HSP promoters. In mammalian cells HSF1 exists under resting conditions as a monomer kept in an inactive form through intramolecular binding between three leucine zipper (LZ) domains that combine to form a triple stranded coiled coil16,17 (Fig. 1). Activation involves the uncoiling of this dormant protein and unmasking of an array of regulatory domains. Stress causes the breaking of the bond between leucine zipper 1 (LZ1) and the remaining LZ domains and formation of trimers through interactions at LZ1 which become capable of binding to DNA17–19 (Fig. 1). Interestingly some agents such as salicylic acid (at the lower concentration of 20mM), oxidants and high Calcium (Ca2+) levels can lead to the production of HSF1 arrested in this form capable of binding to DNA but unable to activate transcription.20–22
Figure 1.
Model for multi-step HSF1 activation. We envisage at least three stages in the transition from inactive HSF1 maintained as a monomer by intramolecular triple-stranded coiled-coil interactions. Leucine zipper 1 (grey) binds to leucine zipper 2 (blue) and leucine zipper 3 (green). In step1, heat shock leads to severing the bond between LZ1 and LZ2, causing partial uncoiling and permitting DNA binding. In Step 2, the inactive trimers are then further activated by breaking the bond between LZ2 and LZ3, an event that appears to involve phosphorylation at S195 in the center of LZ2 (Fig. 2). This step is essential but not sufficient for trans-activation, which requires further steps including HSF1 phosphorylation at Step3. I the figure a single monomer of the activated trimer is depicted as being phosphorylated. however, the exact stoichiometry of HSF1 phosphorylation at S195 or indeed at any of its phosphorylation sites is not currently known.
The next stage in HSF1 activation appears to involve further intramolecular unfolding reactions that may involve the severing of another of the LZ-LZ interactions and opening up of the C-terminal activation domains (Fig. 1). It is not clear whether this step involves phosphorylation.17,19 However we have shown that a serine residue in leucine zipper 2 (S195) is required for full activity of HSF1 suggesting a role for this site in uncoiling responses (Fig. 2). Previous studies have shown that HSF1 activation is regulated by leucine zipper 2 in the N-terminus of HSF1 and that regulation does not involve trimerization or DNA binding.23 We have found a serine residue in the human LZ2 that is conserved between all HSF1 molecules as well in Drosophila and S. cereviseae HSF at the serine 195 residue of human HSF1. We have examined the potential effects of this serine (S195) on the function of HSF1 during the stress response in vivo. To study the influence of S195 on trans activation, we constructed chimeras between the GAL4 DNA binding and dimerization domain and the C-terminus of HSF1 containing (amino acids 120–529) in order to distinguish between the effects of mutated HSF1 and wild-type HSF1. Trans activating effects of S195 containing mutations were assessed using a reporter construct containing the GAL4 DNA binding domain coupled to luciferase (pFR-Luc) in HeLa cells. The data are summarized in Figure 2.
Figure 2.
Role of serine 195 phosphorylation in HSF1 activation. A Effect of S195 mutation on heat shock induced HSF1 trans-activation. Experiments involve wild-type Gal-HSF1, Gal-HSF1 S195S/A modification and Gal-HSF1 S/D modification. B Role of overexpression of wild-type HSF1, HSF1 S195S/A and HSF1 S/D in the repression of non-HSP target genes c-fos (four columns on left) and c-fms (four columns on right). Data are expressed as mean luciferase activity +/-SD after correction by transfection efficiency control (pCMV-lacZ). experiments were each performed at least 3 times.
Constructs containing the C-terminus of wt HSF1 as well as alanine and aspartate mutations of HSF1 (S195A and S195D) failed to activate pFR-Luc under control, non-stress conditions (Fig. 2A) Heat shock led to the activation of the wt HSF1 chimera and induction of the pFR-Luc reporter. S195A mutation blocked trans-activation while S195D mutation was permissive for trans activation (Fig. 2A). These experiments thus indicate that the generation of negative charge at S195 is required for trans-activation of HSF1. The fact that S195D mutation does not activate HSF1 at 37 °C suggest that this modification is necessary but not sufficient for trans-activation. As the GAL4-HSF1 fusion proteins do not require heat shock to bind DNA, these experiments suggest that S195 mediates changes induced by stress downstream from the trimerization and events in step 1. In addition, previous studies have shown that the majority of LZ2 can be deleted from HSF1 without affecting ability to trimerize and bind to DNA.23 These experiments prompted us to propose a 3 step model for HSF1 activation, in which S195 mediates step 2, the breaking of intramolecular interactions between LZ2 and LZ3. S195 would regulate a generic unmasking step required to expose later events needed for trans-activation. This hypothesis is supported by the experiments on gene repression by HSF1. We have shown that HSF1 functions as a transcriptional repressor of a range of proteins including c-fms and c-fos.24,25 S195A mutation inhibits the repressive effects of HSF1 on the c-fms and c-fos promoters while S195D mutation is supportive of such an interaction (Fig. 2 B). As the activation domains of HSF1 are not required for trans repression of non-heat shock genes25 these experiments further support the role of S195 in regulating an uncoiling step in HSF1 activation needed for both trans-activation and trans-repression.
The final step in the remodeling of HSF1 may include further modifications and one residue in particular, serum 326 appears to play a key role in achieving the fully active trimer and transcriptional activation.26
Triggers for HSF1 Activation
A number of mechanisms for activation of HSF1 have been proposed. The first is the reversal of feedback inhibition by products of HSF1 dependent transcription, such as HSP70 and HSP90 (de-repression mechanism).16 Indeed convincing evidence for HSF1 regulation by each of these molecular chaperones has been produced and elevated levels of either HSP70 or HSP90 inhibit the HSR and HSP90 activation, while HSP90 inhibitors activated the HSR.16,27 HSF1 activation is considered to stem from HSP90 sequestration in aggregates of unfolded proteins.16 Whether this mechanism can account for the almost instantaneous activation of HSF1-mediated transcription is not clear. However, HSF1 activation has also been linked to a second mechanism, the heat-induced binding of HSF1 to a large non-coding RNA (HSR1) complexed to eukaryotic elongation 1α (eEF1α), a major cell protein that mediates translational elongation.28,29 Large non-coding RNAs are involved in both positive and negative regulation of transcription of a wide range of genes.30,31 It is not clear at this stage which mechanism is the primary trigger for the HSR. In addition HSF1 is rapidly phosphorylated on serine 326 after stress and phosphorylation at this site correlates with the onset of HSF gene transcription, suggesting a role for serine phosphorylation at this site in HSR regulation. HSF1 is then rapidly recruited to the promoters of HSF gene.26 It is evident therefore that much more needs to be determined regarding HSF1 triggering and progression to full activation.
Signal Cascades Activated by Heat Shock
A number of protein kinases are activated by heat shock and can profoundly influence the HSR in positive or negative ways (Fig. 3). The stress kinases p38-MAP kinase (p38-MK) and c-jun kinase (junK) are strongly activated by stresses including heat shock and sodium arsenite exposure.32–36 Activation of p38-MK appears to induce cellular effects through a bifurcating cascade response involving induction of the downstream kinase MK2 that phosphorylates HSP27 to cause enhancement of its molecular chaperone function as well as independently phosphorylating HSF1 on serine 121 (see below).33,37,38 Induction of junK by heat shock appears to mediate apoptosis.39,40 However, other components of the HSR including Hsp70 expression and Hsp27 phosphorylation were shown to antagonize the pro-apoptotic effects of stress kinases and increase cell survival.12,39,40 In addition, a third member of the MAP kinase family ERK1 also modulates the HSR. Heat activation of this pathway may have a bifurcating effect as ERK1 binds to HSF1 and leads to transcriptional inhibition as well as causing downstream activation of RSK kinases that can also repress HSF1.41,42 Activation of the HSR may also involve induction of the phosphatidylinositol-3 kinase pathway. Heat shock activates PI-3K leading to accumulation of phosphatidylinositol-1, 4-bisphosphate, phosphatidylinositol-1, 4, 5-trisphosphate and PI-3 K activation.43 Activation of PI-3-K leads to activation of the downstream kinase Akt which, although not directly phosphorylating HSF1 can cause indirect activation through inhibition of glycogen synthase kinase 3 (GSK3).44 GSK 3 is an inhibitor of HSF1 and its inhibition by Akt can promote HSF1 activation.45 Finally, HSF1 is strongly activated by cyclic AMP (PKA)-dependent protein kinase during heat shock by an as-yet-unknown mechanism.46
Figure 3.
Signaling kinase cascades activated by heat shock and their downstream targets.
Role of Phosphorylation in HSF1 Activation and Repression
Early studies showed that HSF1 activation involves a profound retardation in electrophoretic mobility that appears to be caused by phosphorylation.47 HSF1 hyperphosphorylation in mammalian cells is envisaged as mediating steps in HSF1 activation downstream of the DNA binding step and in some way coupling promoter occupation to trans-activation as discussed above (Figs. 1, 2, 4).22,48
Figure 4.
Functional domains and phosphorylation motifs in HSF1. HSF1 contains four functional domains: the DNA binding, leucine zipper trimerization domain, a regulatory region and two trans-activation domains arranged in tandem. HSF1 contains both activating and inhibitory phosphorylation sites located mostly in the regulatory domain. Heat-induced phosphorylation at these sites may reflect the activation of kinases by heat (as in Fig. 3) or unmasking of cryptic sites by the unfolding changes during heat shock (Figs. 1, 2). On activation, the DNA binding domain contacts heat shock elements (HSE) on HSP promoters recruiting a range of activating molecules such as general transcription factors, mediator, elongation factors, chromatin remodeling proteins and histone modifying proteins such as CBP/p300. Some of these proteins as well as RNA polymerase II (Pol II) are depicted in (b).
Analysis of HSF1 by phosphoaminoacid analysis and 2 D phosphopeptide mapping indicated multiple sites of phosphorylation largely on serine, perhaps to be expected in a protein containing 20%–25% serine and threonine residues.44 Characterization of sites with regulatory activity by analytical chemistry approaches has begun (Fig. 4). The first physiologically significant sites to be characterized were in the proline-rich regions of the protein and we have shown that HSF1 contains inhibitory phosphorylation sites at serines 303, 307 and 363 that are targeted by, respectively the kinases GSK3, ERK1 and PKC and these studies have been confirmed in part by others44,49–51 (Fig. 4). These effects are thought to permit down-regulation of HSF1 in non-stress conditions and preferable expression of housekeeping or pro-growth genes under these conditions. In the case of phosphoserines 303 and 307, at least a portion of these inhibitory effect is due to recruitment of the phosphoserine binding protein 14-3-3 to HSF1-phospho-S303, phospho-S307 and nuclear export of the modified HSF1.52,53 Interestingly, although heat shock causes ERK1 to bind stably to HSF1 and thus mediates 14-3-3 association, no effect on stress-induced HSF1 activation is seen and HSP transcription proceeds.52,53 In addition, S303 phosphorylation observed during heat shock can promote another inhibitory modification- the sumoylation of lysine 298 in HSF1.54,55 Heat shock can evidently override a number of inhibitory signaling events that can cause powerful regulation of HSF1 under non-stress conditions and HSF1 becomes activated by stress despite phosphorylation at S303 (Chou, S and Calderwood SK, in preparation). Similarly, we have observed HSF1 phosphorylation on S121 by the pro-inflammatory kinase MK2.38 HSF1 phosphorylation on S121 likely reduces the ability of HSF1 to repress pro-inflammatory genes in the acute phase response by inhibiting its nuclear uptake and transcriptional activation.38 Our studies suggest that MK2 phosphorylation promotes HSF1–HSP90 binding and in this way represses activity. However, as with the 14-3-3 studies, heat shock overrides the effects of MK2, even when the kinase is overexpressed and leads to S121 dephosphorylation, dissociation of HSF1 from HSP90 and binding to heat shock elements.38 The p38/MK2 pathway activates the HSR during stress by phosphorylation of Hsp27 a modification that enhances the chaperone properties of this protein (Fig. 3). The effects of MK2 on HSF1 and Hsp27 appear to be independent at least during heat shock.
Positively acting phosphorylation sites in HSF1, that stimulate HSP gene transcription, are now beginning to be analyzed and serines 230 and 326 in the regulatory domain are required for trans activation of HSF genes.56 The studies above also suggest a role for S195 in transactivation (Fig. 2). Understanding of how HSF1 phosphorylation is coupled to trans activation of HSF promoters is currently incomplete and much still remains to be learned regarding such signaling events. It is still not known how upstream signaling events triggered by heat shock are coupled to HSF1 phosphorylation and in most cases, how HSF1 phosphorylation affects the transition from inactive monomer to fully active trimer. However, HSF1 gene transcription mediated by stress requires the activation of uncharacterized tyrosine kinases that act upstream of HSF122 As HSF1 is not phosphorylated on tyrosine before or after heat shock, direct effects of tyrosine kinases on HSF1 seem unlikely.44 One candidate is the HER3/HER2 heterodimer. Heregulin, a potent activator of these cell surface tyrosine kinases induces HSF1 by triggering the PI-3K/Akt cascade and inhibiting GSK3.45 However the full significance of this pathway in regulation of the HSR is not yet clear. In addition early studies indicate that HSF1 is activated by cyclic AMP-dependent protein kinase (PKA) and our unpublished studies confirm this (A. Murshid, S.K. Calderwood, in preparation).
HSF Gene Promoters-Events in the Nucleus and the Influence of Chromatin Modification
HSP gene promoters all possess one or more arrays of heat shock elements (HSE), with each array containing three repeats of the sequence nGAAn—reviewed by15 (Figs. 4, 5). Arrays of HSE provide binding sites for HSF trimers and confer stress/HSF inducibility on the promoter.15 Detailed studies have been carried out on stress regulation of the Drosophila HSP70 promoter which resembles the human and murine HSP70.1 promoters.57 In uninduced Drosophila cells, HSP promoters are poised for a rapid activation of transcription by assembly in an accessible chromatin structure.58 At least two factors bind constitutively to the promoter, GAGA factor and TATA binding protein of the TFIID complex and these maintain accessibility of factors to the promoter- reviewed-.59 In addition to the binding of these factors in unstressed cells, each HSP70 gene promoter binds RNA polymerase II after initiating a short transcript from the HSP70 gene.59 The HSP26 and HSP27 genes are also regulated by promoter proximal pausing of bound RNA polymerase II.60
Figure 5.
Chromatin modifications on HSP genes after binding HSF1 and TAC1 in heat shocked cells. Heat shock-induced modification of lysines within tail residues of histones H3 and H4, within nucleosomes (each containing histones H1, H2A, H2B, H3, H4) on chromatin may alter the accessibility of factors to HSP genes, provide binding sites for nucleasome modeling proteins and lead to transcriptional initiation and elongation. Histone acetylases and methyltransferases may be recruited during stress by the binding of HSF1 to HSE on the promoter as well as TAC1 association with downstream residues 3’ to the promoter. In the hsp70 promoter, histone H3 may be targeted by HATs of the SAGA protein complex family and H4 by CREB binding protein (CBP). Histone acetylation may permit binding of the SWI/SNF complex to the promoter and subsequent nucleosome remodeling. In the downstream ORF region, binding of the TAC1 complex after heat shock leads to methylation of histone H3 on lysine 4 (MeK4) and acetylation of H4 on lysine 9 (AcK9). This may facilitate the release of promoter proximal paused PolII in a process that involves interaction of Pol II with P-TEFb, phosphorylation and transcriptional elongation. We depict the activated Pol II complex negotiating a nucleosomes during elongation, a process that may be facilitated by lysine modifications induced by heat shock.
Following stress, HSF is rapidly targeted to HSE in the promoter and relieves transcriptional pausing of bound RNA polymerase II, leading to multiple rounds of HSP gene transcription.45,59 Promoter proximal pausing is also observed in the human HSP70 gene and other rapidly induced genes including c-fos, c-myc and igk.61–63 One striking change in RNA polymerase II as it progresses from its promoterentry state to its elongation-competent mode is the phosphorylation of the C-terminal domain (CTD) of its largest subunit.64,65 The CTD contains a series of 52 heptapeptide repeats with a consensus sequence YSPTSPS and transcriptional66 elongation is correlated with a switch from a hypophosphorylated form to a hyperphosphorylated form.65 The major phosphoacceptor sites in the CTD are Ser-2 and Ser-5 within the consensus and a number of kinases interact with these sites.65 These sites are preferentially phosphorylated by, respectively, on Ser-2 by the TFII complex, (containing cdk7) and, on Ser-5, by the pTEFb complex containing cyclinT/cdk9 complexes.65 Interestingly, pTEFb is recruited to promoter proximal paused genes and interacts with heat shock loci in polytene chromosomes from Drosophila salivary gland preparations.67 Indeed direct recruitment of a Gal4-binding domain-P-PTEFb hybrid protein to the HSP70 promoter is sufficient to activate transcription in the absence of heat shock, implying that one role of HSF1 in stressed cells is to recruit P-TEFb, phosphorylate the CTD of RNA polymerase II leading to transcription of HSP70.67 In addition to P-TEFb, full activation of HSP70 in Drosophila requires the cdk7 gene, indicating functions for both canonical serine residues in HSF1/CTD activation.66 Drosophila HSF also directly engages the Mediator complex on the HSP70 promoter and thus initiates signal transfer from enhancer-bound HSF to the basal transcription activity.68 Mediator engagement by HSF is involved in activation of CTD phosphorylation.69 In addition to the activation of Pol II, HSP promoters also require the resolution of chromatin structures during elongation. Covalent modifications of histones may also be involved in HSP70 activation and histone phosphorylation and acetylation increases globally in heat shock genes on activation by heat.70,71 The activation of HSP70 transcription in mammalian cells appears to involve similar mechanisms.
Studies in yeast have recently indicated the importance of histone modification and nucleosome remodeling in responses to stress. Heat shock promoters undergo wholesale clearance of nucleosomes during elevated temperatures.72 This is an extremely rapid response and occurs within seconds of exposure.72 The initial trigger for this response appears to be acetylation on the tails of histones H3 and H472 (Fig. 5). There appears to be consensus that H3 acetylation is due to recruitment of the SAGA complex containing the histone acetylase GCN5.73–75 However, histone H4 acetylation is also a rapid response to heat and is also observed in mammalian HSF genes.76 There is interplay between histone modification by acetylation and ATP dependent chromatin remodeling by the SWI/SNF complex and inactivation of SWI/SNF decreases HSF expression and H4 acetylation.77 HSF1 appears to be instrumental in recruiting SWI/SNF to HSF promoters, although stable binding may require histone acetylation to provide a binding site for the bromodomain of SNF.77 The transcriptional activation domains of human HSF1 have however, been shown to recruit human BRG1, the ATPase domain of SWI/SNF complexes involved in ATP dependent remodeling of chromatin.78,79 The SWI/ SNF complex may be involved in resolving the nucleosome structures in front of the elongating Pol II on the HSP70 promoter, overcoming the transcriptional pause and stimulating Hsp70 RNA synthesis.78 Whether or not there are requirements for SWI/SNF in Pol II CTD phosphorylation or engagement of the mediator complex are involved in signaling from HSF1 to HSP gene transcription is currently under study.
Recent studies have shown heat shock dependent alterations in histone H4 acetylation in the mouse HSP70 gene indicating potentially similar complexity in mammalian HSP70 transcription compared with Drosophila HSP70.76 These studies indicate that histone H3 is minimally acetylated during heat shock but activated by sodium arsenite indicating stimulus specific acetylation of histone on HSF genes. We have shown recently that histone H3 is acetylated on the lysine 9 (K9) residue in human cells after heat shock (Y. Zhang and SK Calderwood, in preparation) a finding that might suggest a role for a SAGA-like complex in human HSP transcription as GCN5 commonly acetylates Histone H3 on the activating K9 residue. Histone methylation might also play a significant role in HSP expression. Studies in Drosophila show that the trithorax-containing complex TAC1 binds to heat shock loci leading to enhanced HSP expression.71,80 The Drosophila studies showed that the location of the Trithorax complex in HSP loci was partially coincident with HSF in the heat shock activated loci.71,80 The trithorax complex is responsible for the trimethylation of histone H3 at the lysine 4 (K4) residue, a mark that is associated with gene activation. Interestingly, histone H3K4 trimethylation by the trithorax complex occurs in the 5’ portion of the coding regions of genes and may be particularly important in RNA elongation during transcription, a key event for transcriptionally paused genes such as HSP.71,80 Other mechanisms of activation in addition to HSF recruitment may thus be involved. Trithorax genes are regulated during development in vivo by the binding of non-transcribed large RNA species.81 This mechanism is reminiscent of HSF1 regulation by non-coding RNA during heat shock.28,29 In addition, Trithorax binds to Hsp90 and requires Hsp90 binding on chromatin for its function in promoting transcript elongation.82 Both mechanisms—non-coding RNA binding as well as interaction with Hsp90 are thus shared in common between regulation of HSF1 and Trithorax.16,28,29,81,82 HSP gene activation may involve direct regulation of Trithorax by HSF1 and/or independent recruitment during stress. Trithorax homologs MLL1 and MLL2 are expressed in mammalian cells and further study will be required to ascertain role for these molecules.
We have in this section attempted to provide a coherent narrative with regard to processes on the chromatin adjacent to HSP genes transcribing during heat shock. Due to the relative paucity of data in this area, we have pieced together data from different species largely yeast, Drosophila and mammalian, which may not always utilize the same regulatory processes. Although we believe the mechanisms proposed to be largely correct, further studies are required to provide a reliable picture of changes on chromatin in human HSP genes.
Potential Mechanisms for Altered HSF Activity in Aging, Neurodegeneration and Cancer
HSF1 and HSP expression are required not only in the response to acute stress but also to maintain cellular proteins during cell division, stationary phase existence and to resist programmed cell death and tissue destruction. The levels of protein aggregation increase in tissues due to progressive accumulation of insoluble protein products with time, the decreased ability of the molecular chaperone/protein degradation system to deal with them or, more likely a combination of both processes.14 Most studies indicate a decrease in HSP gene transcription and HSF1 activity in neurones and degeneration of HSF1 activity during aging seems a feature of most tissues.28,83 This may be due to a reduction in HSF1 expression at the protein level during aging that might place the response beneath a key threshold for activation.84,85 However, HSF1 is not activated in cultured neuronal cells even under conditions of HSF1 overexpression.86 Loss of ability to mount the HSR might be construed as a major catastrophe for the aging cell as HSF1 is closely associated with the pathways of longevity. HSF1 appears to be an intermediate in the pathways of starvation-induced longevity and HSF1 overexpression promotes longevity while knockout abrogates the HSF1 mediated longevity pathways.14 The activity of HSF1 in this regard has been ascribed to induction of HSP, particularly the small HSP family.14 It is unclear how HSF1 is activated in pro-longevity pathways, although Sirtuin-1 a key longevity pathway intermediate has been shown to deacetylate HSF1 on inhibitory sites and foster activity.87 Further understanding of HSF1 regulation will be required to decipher the interplay between pro-longevity pathways and degenerative changes in aging tissues in regulation of HSF genes.
Curiously, in another disease associated with advancing age, cancer, HSF1 becomes activated and its activity is associated with transformation and maintenance of the malignant phenotype.45,88,89 How HSF1 is activated in cancer is not entirely clear although our studies indicated that HSF1 is induced downstream of the HER2 pathway, a major route for cell transformation.45,90 One effect of the HER2 pathway was to activate phosphatidyl-inositol-3-kinase and initiate a cascade response leading to activation of protein kinase Akt and inhibition of GSK3.45 As GSK3 is inhibitory for HSF1, HER2/Akt signaling can then activate HSF1 by blocking inhibitory signaling45 (Fig. 3). In addition, induction of the HER2 pathway activates another transcriptional property of HSF1- the ability to repress anti-metastatic gene expression. This effect appears to be due to HER2 dependent association of HSF1 with the co-repressor complex NuRD.91 HSF1 association with NuRD promotes the anchorage independent growth of cancer cells and pro-metastatic transcription in such cells. Gene repression by HSF1 has only been briefly studied. However in yeast, HSF is known to repress a wide spectrum of genes through a pathway dependent on the presence of active SWI/SNF chromatin remodeling complexes.75
Further studies will be required to decipher this conundrum regarding the opposite changes in HSF1 in aging and in cancer which may involve multiple signal inputs into HSF1 and both activating and inhibitory effects of HSF1 on gene transcription.
Acknowledgement
This work was supported by National Institutes of Health grant, CA47407.
Footnotes
Disclosures
This manuscript has been read and approved by all authors. This paper is unique and is not under consideration by any other publication and has not been published elsewhere. The authors and peer reviewers of this paper report no conflicts of interest.
References
- 1.Calderwood SK, Ciocca DR. Heat shock proteins: stress proteins with Janus-like properties in cancer. Int J Hyperthermia. 2008;24:31–9. doi: 10.1080/02656730701858305. [DOI] [PubMed] [Google Scholar]
- 2.Schlessinger MJ. How the cell copes with stress and the function of heat shock proteins. Pediatric Research. 1994;36:1–6. doi: 10.1203/00006450-199407001-00001. [DOI] [PubMed] [Google Scholar]
- 3.Li GC, Hahn GM. A proposed operational model for thermotolerance. Cancer Research. 1981;40:4501–8. [PubMed] [Google Scholar]
- 4.Sekhar KR, Sonar VN, Muthusamy V, et al. Novel chemical enhancers of heat shock increase thermal radiosensitization through a mitotic catastrophe pathway. Cancer Res. 2007;67:695–701. doi: 10.1158/0008-5472.CAN-06-3212. [DOI] [PubMed] [Google Scholar]
- 5.Lindquist S, Craig EA. The heat shock proteins. Ann Rev Genet. 1988;22:631–7. doi: 10.1146/annurev.ge.22.120188.003215. [DOI] [PubMed] [Google Scholar]
- 6.Gabai VL, Sherman MY. Hsp72 and cell Signaling. Cambdridge Univ. Press; Cambridge: 2005. [Google Scholar]
- 7.Garrido C, Brunet M, Didelot C, Zermati Y, Schmitt E, Kroemer G. Heat Shock Proteins 27 and 70: Anti-Apoptotic Proteins with Tumorigenic Properties. Cell Cycle. 2006:5. doi: 10.4161/cc.5.22.3448. [DOI] [PubMed] [Google Scholar]
- 8.Batuello CN, Kelley MR, Dynlacht JR. Role of Ape1 and base excision repair in the radioresponse and heat-radiosensitization of HeLa Cells. Anti-cancer Res. 2009;29:1319–25. [PMC free article] [PubMed] [Google Scholar]
- 9.Issels RD. Hyperthermia adds to chemotherapy. Eur J Cancer. 2008;44:2546–54. doi: 10.1016/j.ejca.2008.07.038. [DOI] [PubMed] [Google Scholar]
- 10.Lencioni R, Crocetti L. Radiofrequency ablation of liver cancer. Tech Vasc Interv Radiol. 2007;10:38–46. doi: 10.1053/j.tvir.2007.08.006. [DOI] [PubMed] [Google Scholar]
- 11.Pauly KB, Diederich CJ, Rieke V, et al. Magnetic resonance-guided high-intensity ultrasound ablation of the prostate. Top Magn Reson Imaging. 2006;17:195–207. doi: 10.1097/RMR.0b013e31803774dd. [DOI] [PubMed] [Google Scholar]
- 12.Calderwood SK, Khaleque MA, Sawyer DB, Ciocca DR. Heat shock proteins in cancer: chaperones of tumorigenesis. Trends Biochem Sci. 2006;31:164–72. doi: 10.1016/j.tibs.2006.01.006. [DOI] [PubMed] [Google Scholar]
- 13.Ciocca DR, Calderwood SK. Heat shock proteins in cancer: diagnostic, prognostic, predictive, and treatment implications. Cell Stress Chaperones. 2005;10:86–103. doi: 10.1379/CSC-99r.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Calderwood SK, Murshid A, Prince T. The Shock of Aging: Molecular Chaperones and the Heat Shock Response in Longevity and Aging—A Mini-Review. Gerontology. 2009 doi: 10.1159/000225957. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Wu C. Heat shock transcription factors: structure and regulation. Ann Rev Cell Dev Biol. 1995;11:441–69. doi: 10.1146/annurev.cb.11.110195.002301. [DOI] [PubMed] [Google Scholar]
- 16.Zou J, Guo Y, Guettouche T, Smith DF, Voellmy R. Repression of heat shock transcription factor HSF1 activation by HSP90 (HSP90 complex) that forms a stress-sensitive complex with HSF1. Cell. 1998;94:471–80. doi: 10.1016/s0092-8674(00)81588-3. [DOI] [PubMed] [Google Scholar]
- 17.Zuo J, Baler R, Dahl G, Voellmy R. Activation of the DNA-binding ability of human heat shock transcription factor 1 may involve the transition from an intramolecular to an intermolecular triple-stranded coiled-coil structure. Mol Cell Biol. 1994;14:7557–68. doi: 10.1128/mcb.14.11.7557. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Westwood T, Wu C. Activation of drosophila heat shock factor: conformational changes associated with monomer-to-trimer transition. Mol Cell Biol. 1993;13:3481–6. doi: 10.1128/mcb.13.6.3481. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Zuo J, Rungger D, Voellmy R. Multiple layers of regulation of human heat shock transcription factor 1. Mol Cell Biol. 1995;15:4319–30. doi: 10.1128/mcb.15.8.4319. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Bruce JL, Price BD, Coleman CN, Calderwood SK. Oxidative injury rapidly activates the heat shock transcription factor but fails to increase levels of heat shock proteins. Cancer Research. 1993;53:12–15. [PubMed] [Google Scholar]
- 21.Housby JN, Cahill CM, Chu B, et al. Non steroidal antiinflammatory drugs inhibit the expression of cytokines and induce HSP70 in human monocytes. Cytokine. 1999;11:347–58. doi: 10.1006/cyto.1998.0437. [DOI] [PubMed] [Google Scholar]
- 22.Price BD, Calderwood SK. Calcium is essential for multistep activation of the heat shock factor in permeabilized cells. Mol Cell Biol. 1991;11:3365–8. doi: 10.1128/mcb.11.6.3365. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Zuo J, Rungger D, Voellmy R. Activation of the DNA-binding form of human heat shock factor 1 may involve the transition from an intramolecular to an intermolecular triple-stranded coiled -coil structure. Mol Cell Biol. 1995;15:4319–30. doi: 10.1128/mcb.14.11.7557. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Xie Y, Chen C, Stevenson MA, Auron PE, Calderwood SK. Heat shock factor 1 represses transcription of the IL-1b gene through physical interaction with nuclear factor of interleukin 6. J Biol Chem. 2002;277:11802–10. doi: 10.1074/jbc.M109296200. [DOI] [PubMed] [Google Scholar]
- 25.Xie Y, Zhong R, Chen C, Calderwood SK. Heat Shock factor 1 contains two functional domains that mediate transcriptional repression of the c-fos and c-fms genes. J Biol Chem. 2003;278:4687–98. doi: 10.1074/jbc.M210189200. [DOI] [PubMed] [Google Scholar]
- 26.Guettouche T, Boellmann F, Lane WS, Voellmy R. Analysis of phosphorylation of human heat shock factor 1 in cells experiencing a stress. BMC Biochem. 2005;6:4. doi: 10.1186/1471-2091-6-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Abravaya K, Myers MP, Murphy SP, Morimoto RI. The human heat shock protein hsp70 interacts with HSF, the transcription factor that regulates heat shock protein expression. Genes Dev. 1992;6:1153–64. doi: 10.1101/gad.6.7.1153. [DOI] [PubMed] [Google Scholar]
- 28.Shamovsky I, Gershon D. Novel regulatory factors of HSF-1 activation: facts and perspectives regarding their involvement in the age-associated attenuation of the heat shock response. Mech Ageing Dev. 2004;125:767–75. doi: 10.1016/j.mad.2004.07.006. [DOI] [PubMed] [Google Scholar]
- 29.Shamovsky I, Ivannikov M, Kandel ES, Gershon D, Nudler E. RNA-mediated response to heat shock in mammalian cells. Nature. 2006;440:556–60. doi: 10.1038/nature04518. [DOI] [PubMed] [Google Scholar]
- 30.Costa FF. Non-coding RNAs: lost in translation? Gene. 2007;386:1–10. doi: 10.1016/j.gene.2006.09.028. [DOI] [PubMed] [Google Scholar]
- 31.Mercer TR, Dinger ME, Mattick JS. Long non-coding RNAs: insights into functions. Nat Rev Genet. 2009;10:155–9. doi: 10.1038/nrg2521. [DOI] [PubMed] [Google Scholar]
- 32.Adler V, Schaffer A, Kim J, Dolan L, Ronai Z. UV irradiation and heat shock mediate JNK activation via alternate pathways. J Biol Chem. 1995;270:26071–7. doi: 10.1074/jbc.270.44.26071. [DOI] [PubMed] [Google Scholar]
- 33.Landry J, Huot J. Modulation of actin dynamics during stress and physiological stimulation by a signaling pathway involving p38 MAP kinase and heat-shock protein 27. Biochem Cell Biol. 1995;73:703–7. doi: 10.1139/o95-078. [DOI] [PubMed] [Google Scholar]
- 34.Medicherla S, Reddy M, Ying J, et al. p38alpha-selective MAP kinase inhibitor reduces tumor growth in mouse xenograft models of multiple myeloma. Anticancer Res. 2008;28:3827–33. [PubMed] [Google Scholar]
- 35.Sanchez I, Hughes RT, Mayer BJ, et al. Role of SAPK/ERK kinase-1 in the stress-activated pathway regulating transcription factor c-Jun. Nature. 1994;372:794–8. doi: 10.1038/372794a0. [DOI] [PubMed] [Google Scholar]
- 36.Waskiewicz AJ, Cooper JA. Mitogen and stress response pathways: MAP kinase cascades and phosphatase regulation in mammals and yeast. Curr Opin Cell Biol. 1995;7:798–805. doi: 10.1016/0955-0674(95)80063-8. [DOI] [PubMed] [Google Scholar]
- 37.Kostenko S, Moens U. Heat shock protein 27 phosphorylation: kinases, phosphatases, functions and pathology. Cell Mol Life Sci. 2009;66:3289–307. doi: 10.1007/s00018-009-0086-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Wang X, Khaleque MA, Zhao MJ, Zhong R, Gaestel M, Calderwood SK. Phosphorylation of HSF1 by MAPK-activated protein kinase 2 on serine 121, inhibits transcriptional activity and promotes HSP90 binding. J Biol Chem. 2006;281:782–91. doi: 10.1074/jbc.M505822200. [DOI] [PubMed] [Google Scholar]
- 39.Gabai VL, Mabuchi K, Mosser DD, Sherman MY. Hsp72 and stress kinase c-jun N-terminal kinase regulate the bid-dependent pathway in tumor necrosis factor-induced apoptosis. Mol Cell Biol. 2002;22:3415–24. doi: 10.1128/MCB.22.10.3415-3424.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Yaglom JA, Gabai VL, Meriin AB, Mosser DD, Sherman MY. The function of HSP72 in suppression of c-Jun N-terminal kinase activation can be dissociated from its role in prevention of protein damage. J Biol Chem. 1999;274:20223–8. doi: 10.1074/jbc.274.29.20223. [DOI] [PubMed] [Google Scholar]
- 41.Wang X, Asea A, Xie Y, Kabingu E, Stevenson MA, Calderwood SK. RSK2 represses HSF1 activation during heat shock. Cell Stress Chaperones. 2000;5:432–7. doi: 10.1379/1466-1268(2000)005<0432:rrhadh>2.0.co;2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Wang X, Grammatikakis N, Siganou A, Stevenson MA, Calderwood SK. Interactions between extracellular signal-regulated protein kinase 1, 14-3-3 epsilon, and heat shock factor 1 during stress. J Biol Chem. 2004;279:49460–9. doi: 10.1074/jbc.M406059200. [DOI] [PubMed] [Google Scholar]
- 43.Calderwood SK, Stevenson MA, Hahn GM. Heat stress stimulates inositol trisphosphate release and phosphorylation of phosphoinositides in CHO and Balb C 3T3 cells. J Cell Physiol. 1987;130:369–76. doi: 10.1002/jcp.1041300309. [DOI] [PubMed] [Google Scholar]
- 44.Chu B, Soncin F, Price BD, Stevenson MA, Calderwood SK. Sequential phosphorylation by mitogen-activated protein kinase and glycogen synthase kinase 3 represses transcriptional activation by heat shock factor-1. J Biol Chem. 1996;271:30847–57. doi: 10.1074/jbc.271.48.30847. [DOI] [PubMed] [Google Scholar]
- 45.Khaleque MA, Bharti A, Sawyer D, et al. Induction of heat shock proteins by heregulin beta1 leads to protection from apoptosis and anchorage-independent growth. Oncogene. 2005;24:6564–73. doi: 10.1038/sj.onc.1208798. [DOI] [PubMed] [Google Scholar]
- 46.Choi HS, Li B, Lin Z, Huang E, Liu AYC. cAMP and cAMP-dependent protein kinase regulate the human heat shock protein 70 gene promoter activity. J Biol Chem. 1991;266:11858–65. [PubMed] [Google Scholar]
- 47.Hensold JO, Hunt CR, Calderwood SK, Houseman DE, Kingston RE. DNA binding of heat shock factor to the heat shock element is insufficient for transcriptional activation in murine erythroleukemia cells. Mol Cell Biol. 1990;10:1600–8. doi: 10.1128/mcb.10.4.1600. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Voellmy R. Transduction of the stress signal and mechanisms of transcriptional regulation of heat shock/stress protein expression in higher eukaryotes. Crit Rev Eukaryotic Gene Expr. 1994;4:357–401. [PubMed] [Google Scholar]
- 49.Chu B, Zhong R, Soncin F, Stevenson MA, Calderwood SK. Transcriptional activity of heat shock factor 1 at 37 °C is repressed through phosphorylation on two distinct serine residues by glycogen synthase kinase 3 and protein kinase C α and Cζ. J Biol Chem. 1998;273:18640–6. doi: 10.1074/jbc.273.29.18640. [DOI] [PubMed] [Google Scholar]
- 50.Kline MP, Morimoto RI. Repression of the heat shock factor1 transcriptional activation domain is modulated by constitutive phosphorylation. Mol Cell Biol. 1997;17:2107–15. doi: 10.1128/mcb.17.4.2107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Knauf U, Newton EM, Kyriakis J, Kingston RE. Repression of heat shock factor 1 activity at control temperature by phosphorylation. Genes Dev. 1996;10:2782–93. doi: 10.1101/gad.10.21.2782. [DOI] [PubMed] [Google Scholar]
- 52.Wang X, Grammatikakis N, Siganou A, Calderwood SK. Regulation of molecular chaperone gene transcription involves the serine phosphorylation, 14-3-3 epsilon binding, and cytoplasmic sequestration of heat shock factor 1. Mol Cell Biol. 2003;23:6013–26. doi: 10.1128/MCB.23.17.6013-6026.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Wang X, Grammatikakis N, Siganou A, Stevenson MA, Calderwood SK. Interactions between extracellular signal regulated protein kinase 1 (ERK1), 14-3-3 epsilon and heat shock factor 1 during stress. J Biol Chem. 2004 doi: 10.1074/jbc.M406059200. [DOI] [PubMed] [Google Scholar]
- 54.Anckar J, Hietakangas V, Denessiouk K, Thiele DJ, Johnson MS, Sistonen L. Inhibition of DNA binding by differential sumoylation of heat shock factors. Mol Cell Biol. 2006;26:955–64. doi: 10.1128/MCB.26.3.955-964.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Hietakangas V, Ahlskog JK, Jakobsson AM, et al. Phosphorylation of serine 303 is a prerequisite for the stress-inducible SUMO modification of heat shock factor 1. Mol Cell Biol. 2003;23:2953–68. doi: 10.1128/MCB.23.8.2953-2968.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Holmberg CI, Hietakangas V, Mikhailov A, et al. Phosphorylation of serine 230 promotes inducible transcriptional activity of heat shock factor 1. EMBO J. 2001;20:3800–10. doi: 10.1093/emboj/20.14.3800. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Hunt C, Calderwood SK. Characterization and sequence of a mouse HSP70 gene and its expression in mouse cell lines. Gene. 1990;87:199–204. doi: 10.1016/0378-1119(90)90302-8. [DOI] [PubMed] [Google Scholar]
- 58.Wu C. Activating protein factor binds in vitro to upstream control sequences in heat shock gene chromatin. Nature. 1984;311:81–4. doi: 10.1038/311081a0. [DOI] [PubMed] [Google Scholar]
- 59.Lis JT, Wu C. Protein traffic on the heat shock promoter: parking, stalling, and trucking along. Cell. 1993;74:1–4. doi: 10.1016/0092-8674(93)90286-y. [DOI] [PubMed] [Google Scholar]
- 60.Rasmussen EB, Lis JT. In vivo transcriptional pausing and cap formation on three drosophila heat shock genes. Proc Natl Acad Sci U S A. 1993;90:7923–7. doi: 10.1073/pnas.90.17.7923. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Brown SA, Kingston RE. Disruption of downstream chromatin by a transcriptional activator. Genes Dev. 1997;11:3116–21. doi: 10.1101/gad.11.23.3116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Fivaz J, Bassi MC, Pinaud S, Mirkovitch J. RNA polymerase II promoter-proximal pausing upregulates c-fos gene expression. Gene. 2000;255:185–94. doi: 10.1016/s0378-1119(00)00340-1. [DOI] [PubMed] [Google Scholar]
- 63.Schneider EE, Albert T, Wolf DA, Eick D. Regulation of c-Myc and immunoglobulin kappa gene transcription by promoter proximal pausing of RNA polymerase II. Curr Top Microbiol Immunol. 1999;246:225–31. doi: 10.1007/978-3-642-60162-0_28. [DOI] [PubMed] [Google Scholar]
- 64.Dahmus ME. The role of multisite phosphorylation in the regulation of RNA polymerase II activity. Progress in Nucleic Acids Research and Molecular Biology. 1994;48:143–79. doi: 10.1016/s0079-6603(08)60855-7. [DOI] [PubMed] [Google Scholar]
- 65.Kobor MS, Greenblatt J. Regulation of transcriptional elongation by phosphorylation. Biochim Biophys Acta. 2002;1577:261–75. doi: 10.1016/s0167-4781(02)00457-8. [DOI] [PubMed] [Google Scholar]
- 66.Schwartz BE, Werner JK, Lis JT. Indirect immunofluorescent labeling of Drosophila polytene chromosomes: visualizing protein interactions with chromatin in vivo. Methods Enzymol. 2004;376:393–404. doi: 10.1016/S0076-6879(03)76026-1. [DOI] [PubMed] [Google Scholar]
- 67.Lis JT, Mason P, Peng J, Price DH, Werner J. p-TEFb kinase recruitement and function at heat shock loci. Genes Dev. 2000;14:792–803. [PMC free article] [PubMed] [Google Scholar]
- 68.Park JM, Werner J, Kim JM, Lis JT, Kim YJ. Mediator, not holoenzyme, is directly recruited to the heat shock promoter by HSF upon heat shock. Mol Cell. 2001;8:9–19. doi: 10.1016/s1097-2765(01)00296-9. [DOI] [PubMed] [Google Scholar]
- 69.Guidi BW, Bjornsdottir G, Hopkins DC, et al. Mutual targeting of mediator and the TFIIH kinase Kin28. J Biol Chem. 2004;279:29114–20. doi: 10.1074/jbc.M404426200. [DOI] [PubMed] [Google Scholar]
- 70.Nowak SJ, Corces VG. Phosphorylation of histone H3 correlates with transcriptionally active loci. Genes Dev. 2000;14:3003–13. doi: 10.1101/gad.848800. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Smith ST, Petruk S, Sedkov Y, et al. Modulation of heat shock gene expression by the TAC1 chromatin-modifying complex. Nat Cell Biol. 2004;6:162–7. doi: 10.1038/ncb1088. [DOI] [PubMed] [Google Scholar]
- 72.Han Q, Lu J, Duan J, et al. Gcn5- and Elp3-induced histone H3 acetylation regulates hsp70 gene transcription in yeast. Biochem J. 2008;409:779–88. doi: 10.1042/BJ20070578. [DOI] [PubMed] [Google Scholar]
- 73.Kremer SB, Gross DS. The SAGA and Rpd3 chromatin modification complexes dynamically regulate heat shock gene structure and expression. J Biol Chem. 2009 doi: 10.1074/jbc.M109.058610. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Kurshakova MM, Krasnov AN, Kopytova DV, et al. SAGA and a novel Drosophila export complex anchor efficient transcription and mRNA export to NPC. Embo J. 2007;26:4956–65. doi: 10.1038/sj.emboj.7601901. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Zanton SJ, Pugh BF. Full and partial genome-wide assembly and disassembly of the yeast transcription machinery in response to heat shock. Genes Dev. 2006;20:2250–65. doi: 10.1101/gad.1437506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Thomson S, Hollis A, Hazzalin CA, Mahadevan LC. Distinct stimulus-specific histone modifications at hsp70 chromatin targeted by the transcription factor heat shock factor-1. Mol Cell. 2004;15:585–94. doi: 10.1016/j.molcel.2004.08.002. [DOI] [PubMed] [Google Scholar]
- 77.Shivaswamy S, Iyer VR. Stress-dependent dynamics of global chromatin remodeling in yeast: dual role for SWI/SNF in the heat shock stress response. Mol Cell Biol. 2008;28:2221–34. doi: 10.1128/MCB.01659-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Corey LL, Weirich CS, Benjamin IJ, Kingston RE. Localized recruitment of a chromatin-remodeling activity by an activator in vivo drives transcriptional elongation. Genes Dev. 2003;17:1392–401. doi: 10.1101/gad.1071803. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Sullivan EK, Weirich CS, Guyon JR, Sif S, Kingston RE. Transcriptional activation domains of human heat shock factor 1 recruit human SWI/SNF. Mol Cell Biol. 2001;21:5826–37. doi: 10.1128/MCB.21.17.5826-5837.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Sanchez-Elsner T, Sauer F. The heat is on with TAC1. Nat Cell Biol. 2004;6:92–3. doi: 10.1038/ncb0204-92. [DOI] [PubMed] [Google Scholar]
- 81.Hekimoglu B, Ringrose L. Non-coding RNAs in polycomb/trithorax regulation. RNA Biol. 2009:6. doi: 10.4161/rna.6.2.8178. [DOI] [PubMed] [Google Scholar]
- 82.Tariq M, Nussbaumer U, Chen Y, Beisel C, Paro R. Trithorax requires Hsp90 for maintenance of active chromatin at sites of gene expression. Proc Natl Acad Sci U S A. 2009;106:1157–62. doi: 10.1073/pnas.0809669106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Walker GA, Thompson FJ, Brawley A, Scanlon T, Devaney E. Heat shock factor functions at the convergence of the stress response and developmental pathways in Caenorhabditis elegans. Faseb J. 2003;17:1960–2. doi: 10.1096/fj.03-0164fje. [DOI] [PubMed] [Google Scholar]
- 84.Brown IR, Rush SJ. Cellular localization of the heat shock transcription factors HSF1 and HSF2 in the rat brain during postnatal development and following hyperthermia. Brain Res. 1999;821:333–40. doi: 10.1016/s0006-8993(99)01087-2. [DOI] [PubMed] [Google Scholar]
- 85.Marcuccilli CJ, Mathur SK, Morimoto RI, Miller RJ. Regulatory differences in the stress response of hippocampal neurons and glial cells after heat shock. J Neurosci. 1996;16:478–85. doi: 10.1523/JNEUROSCI.16-02-00478.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Batulan Z, Shinder GA, Minotti S, et al. High threshold for induction of the stress response in motor neurons is associated with failure to activate HSF1. J Neurosci. 2003;23:5789–98. doi: 10.1523/JNEUROSCI.23-13-05789.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Westerheide SD, Anckar J, Stevens SM, Jr, Sistonen L, Morimoto RI. Stress-inducible regulation of heat shock factor 1 by the deacetylase SIRT1. Science. 2009;323:1063–6. doi: 10.1126/science.1165946. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Dai C, Whitesell L, Rogers AB, Lindquist S. Heat shock factor 1 is a powerful multifaceted modifier of carcinogenisis. Cell. 2007;130:1005–18. doi: 10.1016/j.cell.2007.07.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Wang Y, Theriault JR, He H, Gong J, Calderwood SK. Expression of a dominant negative heat shock factor-1 construct inhibits aneuploidy in prostate carcinoma cells. J Biol Chem. 2004;279:32651–9. doi: 10.1074/jbc.M401475200. [DOI] [PubMed] [Google Scholar]
- 90.Ciocca DR, Gago FE, Fanelli MA, Calderwood SK. Co-expression of steroid receptors (estrogen receptor alpha and/or progesterone receptors) and Her-2/neu: Clinical implications. J Steroid Biochem Mol Biol. 2006;102:32–40. doi: 10.1016/j.jsbmb.2006.09.008. [DOI] [PubMed] [Google Scholar]
- 91.Khaleque MA, Bharti A, Gong J, et al. Heat shock factor 1 represses estrogen-dependent transcription through association with MTA1. Oncogene. 2008;27:1886–93. doi: 10.1038/sj.onc.1210834. [DOI] [PubMed] [Google Scholar]