Abstract
A recent clinical report suggested that kappa opioids such as nalbuphine and butorphanol produced greater pain relief in women than in men. However, both compounds have been characterized as partial agonists with mixed mu/kappa opioid actions in animal studies. The aim of this study was to evaluate whether there is a sex difference in antinociception caused by nalbuphine and butorphanol as well as more selective kappa opioid agonists including U50,488 and Cl-977 in mice. In the acid-induced writhing assay, all compounds (U50,488: 1–10 mg/kg; Cl-977: 0.01–0.1 mg/kg; nalbuphine: 1–320 mg/kg; butorphanol: 0.032–0.32 mg/kg) dose-dependently inhibited writhing, but there were no sex-related differences found when comparing ED50 values in male and female mice. In the warm water (48°C) tail withdrawal assay, U50,488 (10–100 mg/kg) and Cl-977 (0.1–3.2 mg/kg) also dose-dependently produced antinociception, although there were no sex-related differences observed. Nalbuphine (10–320 mg/kg) did not have antinociceptive effects under this condition. On the other hand, butorphanol (0.32–32 mg/kg) produced greater antinociception in male (50% MPE) than female mice (20% MPE). Further antagonist studies revealed that butorphanol is a mixed mu/kappa opioid with low efficacy. In summary, there were no sex-related differences in response to more selective kappa opioid agonists on antinociception in mice under these conditions.
Keywords: Kappa opioids, Antinociception, Sex difference, Visceral pain, Somatic pain
Introduction
With regard to treatment of pain, there has been much evidence on which gender receives greater antinociception from various opioid drugs. Many rodent studies have shown that females receive less pain relief than males from morphine, which acts predominantly at mu opioid receptors (3,8-10,23). However, other studies have reported that there was no sex difference with other mu opioid agonists such as fentanyl (2) or that morphine antinociception was greater in female than in male rats (1). It is not yet clear whether a sex difference in opioid antinociception is a general phenomenon or if it depends on other conditions, including the particular opioid analgesics and types of nociceptive assays in different species.
Recently, a clinical study reported that female patients achieved greater pain relief than male patients by two opioid analgesics, nalbuphine and butorphanol, after dental surgery (19). Both genders received the same dose of analgesics IV. Whether the significantly less body weight of female subjects contributes to this gender difference is unknown (i.e., 59 kg in women vs. 76 kg in men). Nevertheless, the authors made the conclusion that kappa opioid analgesics produced greater analgesia in women than men, as they inferred that both analgesics were acting predominantly as kappa opioids.
This proposition requires further consideration in terms of its pharmacological basis. In particular, both nalbuphine and butorphanol have been characterized as partial opioid agonists with mixed mu/kappa opioid actions in animal studies (6,13,14,17,20,37). In vitro studies have also shown that both compounds display high affinity for mu and kappa opioid receptors (7,13) and they act as low to medium efficacy agonists in cell lines expressing mu or kappa receptors (15,34,40). In nonhuman primates, nalbuphine and butorphanol cause modest respiratory depression, which may indicate low efficacy at mu opioid receptors (6,20). In rodents, both compounds has been characterized as partial kappa agonists based on their diuretic effects or kappa antagonism studies (28,29,33). Both nalbuphine- and butorphanol-induced antinociception were also antagonized by a selective irreversible mu antagonist β-funaltrexamine (β-FNA), indicating the involvement of mu receptors in the actions of both compounds in mice (41). Furthermore, in healthy human subjects, nalbuphine produced a profile of subjective, psychomotor, and physiological effects similar to that of an equianalgesic dose of morphine (39). Taken together, these evidences strongly suggested that nalbuphine and butorphanol are not selective kappa opioids; instead, they are partial opioid agonists with mixed mu/kappa receptor components (6,14,20,28,29,41).
In order to investigate if there is a sex difference in kappa opioid antinociception, we chose to study the more selective kappa opioid agonists, U50,488 and CI-977, as well as nalbuphine and butorphanol. Two different antinociceptive assays, acid-induced writhing and warm water tail withdrawal, were utilized (i.e., visceral vs. somatic nociceptive reflex). The aim of this study was to examine if sex-related differences can be observed under these conditions in mice.
Methods
Subjects
Adult male and female (10–12 weeks old, 25–30 g) Swiss-Webster mice were housed 10–12 in the same gender per cage at approximately 22°C on a 12-h light/dark cycle. Food and water were available ad lib. Each animal was used only once. After the test session, they were sacrificed by an overdose of IP pentobarbital. Animals used in this study were maintained in accordance with the University Committee on the Use and Care of Animals in the University of Michigan, and the Guide for the Care and Use of Laboratory Animals (7th ed.) by the Institute of Laboratory Animal Resources (National Academic Press, Washington DC. revised 1996).
Antinociceptive Assays
Writhing Assay
The acetic acid-induced writhing assay was modified for use in this laboratory (11). Briefly, mice received an IP injection of 0.6% acetic acid (0.4 ml/animal) and were placed in individual Plexiglas boxes (18 × 28 × 13 cm) for observation. Five minutes after the acetic acid injection was given, there was a 5-min observation period, during which the number of writhes, typically a wave of the abdominal musculature followed by extension of the hind legs, was recorded. Vehicle or different doses of test drugs were administered SC 15 min prior to the administration of acetic acid.
Warm Water Tail Withdrawal Assay
Each mouse was placed into a cylindrical plastic mouse restrainer that left the tail fully exposed. The tail was then immersed in a container of 48°C water to approximately one third to one half of the exposed length of the tail. Latency of tail removal was measured by a hand-operated timer. The timer was stopped upon removal of the tail from the water. Mice were initially injected IP with sterile water and baseline latency was tested 25 min later. Then the agonist was administered IP by a cumulative dosing procedure with a 30-min interinjection interval. Subsequent tail withdrawal latencies were determined starting 25 min after each injection. This continued until the mouse failed to remove its tail from the water before the 20-s cutoff time was reached (maximum antinociception) or until toxic effects (e.g., convulsions) were exhibited.
Experimental Designs
In the writhing assay, U50,488 (1–10 mg/kg), CI-977 (0.01–0.1 mg/kg), nalbuphine (1–320 mg/kg), and butorphanol (0.032–0.32 mg/kg) were evaluated between male and female mice by a single dosing procedure. Each tested dose consisted of 8–10 mice of the same gender. The control number of writhes was determined as the mean number of writhes (n = 10) when a SC injection of vehicle (sterile water) was given 15 min before the acetic acid injection. As the value of the control was found to slightly vary between batches of mice, the control value was routinely redetermined for each new batch.
In the warm water tail withdrawal assay, higher doses of the agonists were required (U50,488: 10–100 mg/kg; CI-977: 0.1–3.2 mg/kg; nalbuphine: 32–320 mg/kg; butorphanol: 0.32–32 mg/kg). Each treatment group consisted of 9–10 mice of the same gender. The control value was the latency of tail withdrawal of each mouse after an IP injection of sterile water was given.
In the antagonist studies, either nor-binaltorphimine (nor-BNI), a selective kappa opioid antagonist, or β-FNA, a selective mu opioid antagonist, was administered SC to each mouse, 24 h before the agonist test. The agonist dosing procedure, antagonist doses, and pretreatment time were used based on previous studies that have shown the dose dependency and selective antagonism (4,11,41).
Data Analysis
Writhing Assay
For each tested dose of the agonists, the number of writhes for each mouse was expressed as percent of the control number of writhes. Then the percent control value for each dose was obtained. The ED50 values and 95% confidence limits (95% C.L.) in each dose–effect curve were determined by utilizing the statistical program PHARM/PCS (36). A significant difference was defined as a lack of overlap in their 95% C.L. of ED50 values between dose–effect curves.
Warm Water Tail Withdrawal Assay
For each treatment condition, individual tail withdrawal latencies were converted to the percent of maximum possible effect (MPE) by the following formula: %MPE = [(test latency − control latency)/(cutoff latency, 20 s − control latency) × 100. The ED50 and 95% C.L. values of each dose–effect curve were determined by the same statistical program (36). In the present study, nalbuphine and butorphanol did not produce full antinociceptive effects in this assay. Effects of both compounds and the antagonist study were analyzed with one-way ANOVA followed by the Newman-Keuls test (p < 0.05).
Drugs
U50,488 (Upjohn, Kalamazoo, MI), CI-977 (also known as enadoline) (Warner Lambert/Parke-Davis, Ann Arbor, MI), butorphanol tartrate (Bristol Myers Squibb, Wallingford, CT), nalbuphine hydrochloride (Mallinckrodt, St. Louis, MO), β-FNA (Dr. D. Zimmerman, Eli Lilly, Indianapolis, IN), and nor-BNI (Dr. H. Mosberg, Dept. of Medical Chemistry, University of Michigan, Ann Arbor, MI) were dissolved in sterile water. The agonists were administered SC (in the writhing assay) or IP (in the tail withdrawal assay) in volumes of 0.01 ml/g. Glacial acetic acid (Fisher Scientific Co., Fair Lawn, NJ) was diluted to a 0.6% solution with sterile water.
Results
Writhing Assay
The mean control values across different batches of male mice ranged from 11.6 ± 1.8 to 15.2 ± 1.9 writhes. The mean control values across different batches of female mice ranged from 12.6 ± 1.1 to 14.4 ± 1.3 writhes. Overall, there was no significant difference between genders regarding their writhing responses induced by IP injection of the acetic acid.
Figure 1 illustrates that all compounds used in this study (U50,488, CI-977, nalbuphine, and butorphanol) dose-dependently inhibited writhing responses. However, there was no sex difference found when comparing their ED50 values (Table 1). It is worth noting that nalbuphine is not as effective as other compounds to inhibit the writhing responses, as its dose–effect curve is very shallow across 2-log dose units.
Figure 1.
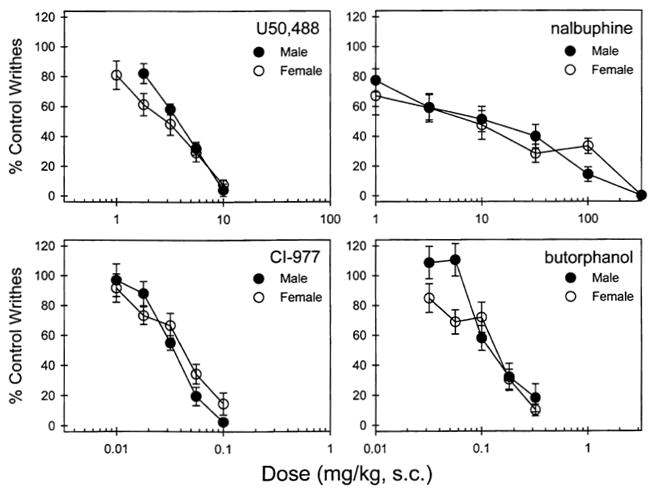
Antinociceptive effects of U50,488, CI-977, nalbuphine, and butorphanol measured by the writhing assay in a single dosing procedure. Abscissae (all panels): agonist dose in mg/kg. Ordinates (all panels): percent of writhing inhibition. Each value represents the mean ± SEM (n = 8–10).
Table 1.
Comparison of ED50 Values (mg/kg) of Kappa Opioids Between Genders
| ED50 (95% C.L.)* |
||
|---|---|---|
| Male Mice | Female Mice | |
| Writhing assay | ||
| U50,488 | 3.80 (3.35–4.31) | 2.93 (1.62–5.30) |
| CI-977 | 0.04 (0.03–0.06) | 0.05 (0.03–0.08) |
| Butorphanol | 0.10 (0.07–0.16) | 0.11 (0.06–0.18) |
| Nalbuphine | 12.7 (3.03–53.04) | 7.56 (3.17–18.1) |
| Tail withdrawal assay | ||
| U50,488 | 41.2 (29.7–57.0) | 27.09 (13.5–57.1) |
| CI-977 | 0.33 (0.10–1.10) | 0.33(0.21–0.52) |
| Butorphanol | NA | NA |
| Nalbuphine | NA | NA |
NA, not applicable.
95% C.L. is confidence limits (p < 0.05).
Warm Water Tail Withdrawal Assay
In this preparation (48°C water), the averaged tail withdrawal latencies of male mice from different groups ranged from 2.7 ± 0.2 to 5.4 ± 0.6 s. The averaged tail withdrawal latencies of female mice ranged from 2.8 ± 0.5 to 5.2 ± 0.6 s. There was no significant difference between genders regarding their baseline tail withdrawal latencies in 48°C water.
Figure 2 illustrates that selective kappa agonists, U50,488 and CI-977, dose-dependently produced thermal antinociception against 48°C water. There were no sex-related differences observed, as both compounds had similar potency (i.e., ED50 values) between both genders to produce antinociception (see Table I). Up to the dose of 320 mg/kg, nalbuphine did not significantly produce thermal antinociceptive effects. At the dose of 1000 mg/kg, some mice convulsed. In contrast, butorphanol only partially produced antinociception, as it reached the plateau when the dose was increased. Although we could not obtain ED50 values, there was a significant difference between genders at the doses of 3.2 and 10 mg/kg determined by the Newman-Keuls test (p < 0.05), in which male mice had more antinociceptive effect.
Figure 2.
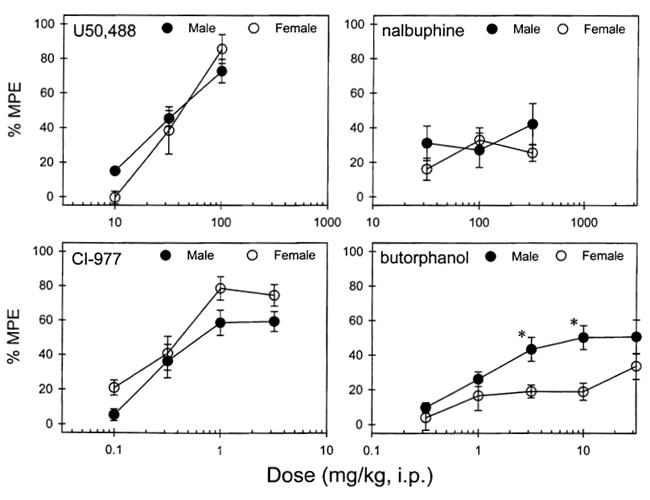
Antinociceptive effects of U50,488, CI-977. nalbuphine, and butorphanol measured by the 48°C water tail withdrawal assay in a cumulative dosing procedure. Abscissae (all panels): agonist dose in mg/kg. Ordinates (all panels): percent of maximum possible effect (%MPE). Each value represents the mean ± SEM (n = 9–10). *Significant difference (p < 0.05, male vs. female) determined by the Newman-Keuls test.
Because butorphanol produced greater antinociception in male mice than female mice (i.e., 50% MPE vs. 20% MPE), further antagonist studies were conducted in male mice to evaluate the selectivity of butorphanol effect in this preparation. It was difficult to conduct the antagonism study in female mice, as butorphanol only produced slight antinociception in the tail withdrawal assay. Figure 3 (top panel) shows that 24-h pretreatment with β-FNA, a selective mu antagonist, can block butorphanol-induced antinociception in a dose-dependent manner. In addition, 24-h pretreatment with nor-BNI, a selective kappa antagonist, also dose-dependently blocked antinociceptive effects of butorphanol (Fig. 3, middle panel). It needs to be noted that in the presence of nor-BNI (10 mg/kg), 320 mg/kg of butorphanol caused death and convulsions in some mice. Figure 3 (bottom panel) shows that 24-h pretreatment with low doses of both β-FNA (3.2 mg/kg) and nor-BNI (3.2 mg/kg) produced significant inhibition of butorphanol antinociception.
Figure 3.
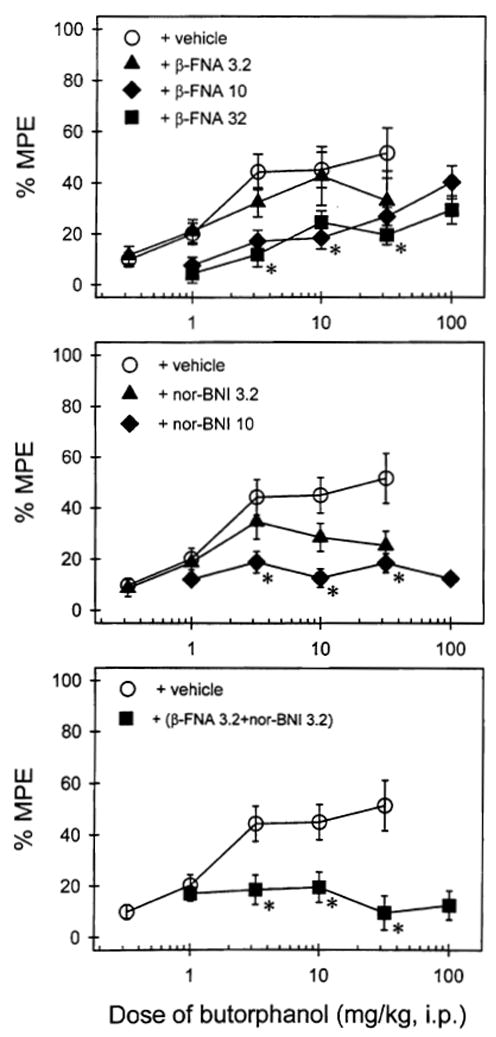
Antagonist effects of β-FNA (top panel) and nor-BNI (middle panel) against butorphanol in male mice measured by the 48°C water tail withdrawal assay. The bottom panel illustrated the effects of combination of the low dose 3.2 mg/kg of β-FNA and nor-BNI. Abscissae (all panels): agonist dose in mg/kg. Ordinates (all panels): percent of maximum possible effect (%MPE). Each value represents the mean ± SEM (n = 9–10). *Significant difference (p < 0.05) from the vehicle group determined by the Newman-Keuls test.
Discussion
The present study illustrated that there was no sex difference in response to selective kappa opioid agonists, U50,488 and CI-977, in terms of their antinociceptive effects in mice. Although butorphanol produced greater antinociception in male mice than in female mice, butorphanol was characterized as a mixed mu/kappa opioid agonist in this preparation.
Both U50,488 and CI-977 have been characterized as selective kappa opioid agonists in different species and behavioral assays including antinociception and diuresis (4,16,26,27,35). This study confirms other reports that both kappa ligands are full efficacy agonists under both in vivo and in vitro conditions (5,34,40), in which full kappa agonists are able to produce antinociception against more intensive noxious stimuli and to produce maximum stimulation measured by [35S] GTPγS binding in a cell line expressing kappa opioid receptors. Nevertheless, there was no sex difference in kappa opioid antinociception measured in either the writhing or tail withdrawal assays. This observation is similar to another study, which illustrated that U69,593 was equipotent between both genders in producing hot plate antinociception in rats (12). However, it was different from another finding (2), showing that bremazocine, but not U69,593, produced greater antinociception in the tail withdrawal assay, but not in the hot plate assay, in female rats. Although the authors suggested that sex differences were assay, dose, and time dependent, repeated measurements in the same subject may contribute to and confound the exact nature of gender difference. On the other hand, one study has shown that U50,488 produced greater antinociception in male deer mice (22). To date, there is no clear consensus regarding the sex difference in kappa opioid-induced antinociception (2,12,18,22). Further studies are required to clarify this phenomenon.
For example, several studies have emphasized that the sex difference may depend on different nociceptive assays and genotypes (25,30). Equivocal findings regarding opioid sex differences in the literature may be partially accounted for by the use of different strain populations (25). In addition, it is worth noting potential species differences in opioid pharmacodynamic profiles. In rats, kappa1 receptor populations comprise approximately less than 10% of the total amount of opioid receptor populations (31,32). In contrast, kappa1 receptors are much more abundant in cortex membranes in other species, including guinea pigs and monkeys (7,32,42). It will be valuable to determine the kappa receptor density between both genders among different species or strains. The limitation of the present study is lack of determination of time of peak effect for those opioid agonists and lack of control of the estrous cycle in female mice. Nevertheless, it has been shown that there was no sex difference in pharmacokinetic factors such as ligand concentrations in the brain or blood after systemic injection and the elimination half-life (10,12).
Nalbuphine produced antinociception in the writhing assay, but not in the tail withdrawal assay. This functional antinociception profile suggests that nalbuphine is a low-efficacy agonist, which is consistent with other reports indicating that nalbuphine acts as a partial agonist in vitro (15,34,40). Nalbuphine has been found to bind to both mu and kappa opioid receptors (7,13). Antinociceptive effects of nalbuphine in mice were antagonized by either a selective mu antagonist (β-FNA) or a selective kappa antagonist (nor-BNI) (33,41). Furthermore, the mu receptor component of nalbuphine actions has been demonstrated in the antinociception and drug discrimination assay in rhesus monkeys (20,21). In the same species, nalbuphine produced modest respiratory depression and it could be used to antagonize the marked respiratory depression induced by a full mu agonist, alfentanil (20). These animal studies clearly indicated that nalbuphine has low efficacy at mu opioid receptors in rodents and monkeys. More interestingly, in healthy human subjects, nalbuphine produced subjective effects, which is similar to that of morphine at the equianalgesic dose (10 mg, IV) (39). Nevertheless, the extent to which nalbuphine can relieve pain in humans through mu, kappa, or both opioid components remains to be determined.
Butorphanol displayed partial antinociceptive effects in the tail withdrawal assay, but produced full antinociception in the writhing assay in mice. This may indicate that butorphanol is an agonist with low to medium efficacy. In support of this supposition, in vitro studies have shown that butorphanol produces partial stimulation in [35S]GTPγS assay in cell lines expressing mu or kappa opioid receptors (15,34). Functional studies have also suggested that butorphanol has low efficacy at both mu and kappa opioid receptors in rodents and monkeys (6,14,17,28,29,41). In the present study, both β-FNA and nor-BNI dose-dependently blocked butorphanol antinociception in male mice. In particular, pretreatment with combination of small doses of β-FNA and nor-BNI significantly antagonized butorphanol, which indicated that butorphanol produced antinociception through both mu and kappa components under these conditions. It is important to note that butorphanol only produced greater antinociception in the tail withdrawal assay, but not in the writhing assay in male mice. Also, there was no sex difference in response to nalbuphine, which has also been characterized as a mixed mu/kappa opioid in mice (33,41). This observation may be partially due to different nociceptive assays and particular analgesics (2,30). Although both nalbuphine and butorphanol were partial opioid agonists with mixed mu/kappa opioid actions, both compounds may not have similar behavioral profiles. In human subjects, butorphanol produced a somewhat different profile of subjective effects and greater psychomotor impairment than morphine (38). It is possible that differential combined contribution of mu and kappa receptor components between nalbuphine and butorphanol results in different functional profiles.
The results of this study did not support a recent clinical study (19), suggesting that kappa opioids (e.g., nalbuphine and butorphanol) produced greater antinociception in women than in men. The major drawbacks of the clinical study are lack of kappa receptor selectivity of chosen analgesics and lack of control of systemic doses in patients with different body weights. The exact nature of kappa opioid antinociception between both genders could be complicated by a variety of factors. Even the gender differences in response to the noxious stimulus under baseline (i.e., vehicle/control) conditions are not consistent across studies. In many cases, including the present study, there was no significant sex difference in the baseline pain threshold (8,9,24). Sex-related differences in opioid antinociception is not yet a general phenomenon and its precise nature may depend on the particular analgesics, assays, strains, species, and measurement parameters such as the dose and time course (2,12,23,25,30). The present study illustrated that there was no sex difference in response to selective kappa opioid agonists, U50.488 and CI-977, and partial agonists with mixed mu/kappa opioid actions, nalbuphine and butorphanol, in writhing antinociception. Only butorphanol produced greater antinociception in tail withdrawal antinociception in male mice under these experimental conditions.
Acknowledgments
The authors wish to thank Jennifer Clemente for excellent technical assistance. This study was supported by USPHs Grant DA00254.
Footnotes
Preliminary results were present at the 60th annual meeting of College on Problems of Drug Dependence, Scottsdale, AZ, June 13–18, 1998.
References
- 1.Ali BH, Sharif SI, Elkadi A. Sex differences and the effect of gonadectomy on morphine-induced antinociception and dependence in rats and mice. Clin Exp Pharmacol Physiol. 1995;22:342–344. doi: 10.1111/j.1440-1681.1995.tb02012.x. [DOI] [PubMed] [Google Scholar]
- 2.Bartok RE, Craft RM. Sex differences in opioid antinociception. J Pharmacol Exp Ther. 1997;282:769–778. [PubMed] [Google Scholar]
- 3.Boyer J, Morgan M, Craft R. Microinjection of morphine into the rostral ventromedial medulla produces greater antinociception in male compared to female rats. Brain Res. 1998;796:315–318. doi: 10.1016/s0006-8993(98)00353-9. [DOI] [PubMed] [Google Scholar]
- 4.Broadbear JH, Negus SS, Butelman ER, de Costa BR, Woods JH. Differential effects of systemically administered nor-binaltorphimine (nor-BNI) on kappa-opioid agonists in the mouse writhing assay. Psychopharmacology (Berlin) 1994;115:311–319. doi: 10.1007/BF02245071. [DOI] [PubMed] [Google Scholar]
- 5.Butelman ER, Negus SS, Ai Y, de Costa BR, Woods JH. Kappa opioid antagonist effects of systemically administered nor-binaltorphimine in a thermal antinociception assay in rhesus monkeys. J Pharmacol Exp Ther. 1993;267:1269–1276. [PubMed] [Google Scholar]
- 6.Butelman ER, Winger G, Zemig G, Woods JH. Butorphanol: Characterization of agonist and antagonist effects in rhesus monkeys. J Pharmacol Exp Ther. 1995;272:845–853. [PubMed] [Google Scholar]
- 7.Butelman ER, KO MC, Sobczyk-kojiko K, Mosberg HI, Van Bemmel B, Zemig G, Woods JH. Kappa-opioid receptor binding populations in rhesus monkey brain: Relationship to an assay of thermal antinociception. J Pharmacol Exp Ther. 1998;285:595–601. [PubMed] [Google Scholar]
- 8.Candido J, Lutfy K, Billings B, Sierra V, Duttaroy A, Intunisi CE, Yobum BC. Effect of adrenal and sex hormones on opioid analgesia and opioid receptor regulation. Pharmacol Biochem Behav. 1992;42:685–692. doi: 10.1016/0091-3057(92)90015-8. [DOI] [PubMed] [Google Scholar]
- 9.Cicero TJ, Nock B, Meyer ER. Gender-related differences in the antinociceptive properties of morphine. J Pharmacol Exp Ther. 1996;279:767–773. [PubMed] [Google Scholar]
- 10.Cicero TJ, Nock B, Meyer ER. Sex-related differences in morphine’s antinociceptive activity: Relationship to serum and brain morphine concentrations. J Pharmacol Exp Ther. 1997;282:939–944. [PubMed] [Google Scholar]
- 11.Comer SD. Doctoral dissertation. The University of Michigan; Ann Arbor: 1992. BW373U86: Behavioral pharmacology of a putative non-peptide, systemically-active delta opioid agonist. [Google Scholar]
- 12.Craft RM, Kruzich PJ, Boyer JS, Harding JW, Hanesworth JM. Sex differences in discriminative stimulus and diuretic effects of the kappa opioid agonist U69,593 in the rat. Pharmacol Biochem Behav. 1998;61:395–403. doi: 10.1016/s0091-3057(98)00124-5. [DOI] [PubMed] [Google Scholar]
- 13.De Souza EB, Schmidt WK, Kuhar MJ. Nalbuphine: An autoradiographic opioid receptor binding profile in the central nervous system of an agonist/antagonist analgesic. J Pharmacol Exp Ther. 1988;244:391–402. [PubMed] [Google Scholar]
- 14.Dykstra LA. Butorphanol, levallorphan, nalbuphine and nalorphine as antagonists in the squirrel monkey. J Pharmacol Exp Ther. 1990;254:245–252. [PubMed] [Google Scholar]
- 15.Emmerson PJ, Clark MJ, Mansour A, Akil H, Woods JH, Medzihradsky F. Characterization of opioid agonist efficacy in a C6 glioma cell line expressing the mu opioid receptor. J Pharmacol Exp Ther. 1996;278:1121–1127. [PubMed] [Google Scholar]
- 16.France CP, Medzihradsky F, Woods JH. Comparison of kappa opioids in rhesus monkeys: Behavioral effects and receptor binding affinities. J Pharmacol Exp Ther. 1994;268:47–58. [PubMed] [Google Scholar]
- 17.Garner HR, Burke TF, Lawhom CD, Stoner JM, Wessinger WD. Butorphanol-mediated antinociception in mice: Partial agonist effects and mu receptor involvement. J Pharmacol Exp Ther. 1997;282:1253–1261. [PubMed] [Google Scholar]
- 18.Gear RW, Gordon NC, Heller PH, Paul S, Miaskowski C, Levine JD. Gender difference in analgesic response to the kappa-opioid pentazocine. Neurosci Lett. 1996;205:207–209. doi: 10.1016/0304-3940(96)12402-2. [DOI] [PubMed] [Google Scholar]
- 19.Gear RW, Miaskowski C, Gordon NC, Paul SM, Heller PH, Levine JD. Kappa-opioids produce significantly greater analgesia in women than in men. Nat Med. 1996;2:1248–1250. doi: 10.1038/nm1196-1248. [DOI] [PubMed] [Google Scholar]
- 20.Gerak LR, Butelman ER, Woods JH, France CP. Antinociceptive and respiratory effects of nalbuphine in rhesus monkeys. J Pharmacol Exp Ther. 1994;271:993–999. [PubMed] [Google Scholar]
- 21.Gerak LR, France CP. Discriminative stimulus effects of nalbuphine in rhesus monkeys. J Pharmacol Exp Ther. 1996;276:523–531. [PubMed] [Google Scholar]
- 22.Kavaliers M, Innes DG. Sex and day-night differences in opiate-induced responses of insular wild deer mice, Peromyscus maniculatus triangularis. Pharmacol Biochem Behav. 1987;27:477–482. doi: 10.1016/0091-3057(87)90351-0. [DOI] [PubMed] [Google Scholar]
- 23.Kepler KL, Kest B, Kiefel JM, Cooper ML, Bodnar RJ. Roles of gender, gonadectomy and estrous phase in the analgesic effects of intracerebroventricular morphine in rats. Pharmacol Biochem Behav. 1989;34:119–127. doi: 10.1016/0091-3057(89)90363-8. [DOI] [PubMed] [Google Scholar]
- 24.Kepler KL, Standifer KM, Paul D, Kest B, Pasternak GW, Bodnar RJ. Gender effects and central opioid analgesia. Pain. 1991;45:87–94. doi: 10.1016/0304-3959(91)90168-W. [DOI] [PubMed] [Google Scholar]
- 25.Kest B, Wilson SG, Mogil JS. Sex differences in supraspinal morphine analgesia are dependent on genotype. J Pharmacol Exp Ther. 1999;289:1370–1375. [PubMed] [Google Scholar]
- 26.KO MC, Butelman ER, Mosberg HI, Woods JH. Differential inhibition by opioid antagonists of CI-977-induced diuresis in rats. Conference proceedings, the 27th annual meeting of International Narcotics Research Conference (INRC); Long Beach, CA. 1996. [Google Scholar]
- 27.KO MC, Butelman ER, Traynor JR, Woods JH. Differentiation of kappa opioid agonist-induced antinociception by naltrexone apparent pA2 analysis in rhesus monkeys. J Pharmacol Exp Ther. 1998;285:518–526. [PMC free article] [PubMed] [Google Scholar]
- 28.Leander JD. Evidence that nalorphine, butorphanol and oxilorphan are partial agonists at a kappa-opioid receptor. Eur J Phannacol. 1983;86:467–470. doi: 10.1016/0014-2999(83)90198-x. [DOI] [PubMed] [Google Scholar]
- 29.Leander JD, Hart JC, Zerbe RL. Kappa agonist-induced diuresis: Evidence for stereoselectivity, strain differences, independence of hydration variables and a result of decreased plasma vasopressin levels. J Pharmacol Exp Ther. 1987;242:33–39. [PubMed] [Google Scholar]
- 30.Mogil JS, Kest B, Sadowski B, Belknap JK. Differential genetic mediation of sensitivity to morphine in genetic models of opiate antinociception: Influence of nociceptive assay. J Pharmacol Exp Ther. 1996;276:532–544. [PubMed] [Google Scholar]
- 31.Nock B, Giordano AY, Cicero TJ, O’Connor LH. Affinity of drugs and peptides for U-69,593-sensitive and -insensitive kappa opiate binding sites: The U-69,593-insensitive site appears to be the beta endorphine-specific epsilon receptor. J Pharmacol Exp Ther. 1990;254:412–419. [PubMed] [Google Scholar]
- 32.Nock B, Giordado AL, Moore BW, Cicero TJ. Properties of the putative epsilon opioid receptor: Identification in rat, guinea pig, cow, pig and chicken brain. J Pharmacol Exp Ther. 1993;264:349–359. [PubMed] [Google Scholar]
- 33.Pick CG, Paul D, Pastemak GW. Nalbuphine, a mixed kappa1 and kappa3 analgesic in mice. J Pharmacol Exp Ther. 1992;262:1044–1050. [PubMed] [Google Scholar]
- 34.Remmers AE, Clark MJ, Mansour A, Akil H, Woods JH, Medzihradsky F. Opioid efficacy in a C6 glioma cell line stably expressing the human kappa opioid receptor. J Pharmacol Exp Ther. 1999;288:827–833. [PubMed] [Google Scholar]
- 35.Takeniori AE, Schwartz MM, Portoghese PS. Suppression by nor-binaltorphimine of kappa opioid-mediated diuresis in rats. J Pharmacol Exp Ther. 1988;247:971–974. [PubMed] [Google Scholar]
- 36.Tallarida RJ, Murray RB. Manual of pharmacologic calculations with computer programs. New York: Springer-Verlag; 1987. [Google Scholar]
- 37.Vivian JA, van Bemmel B, Lewis JW, Woods JH. Behavioral effects of butorphanol after clocinnamox administration in rhesus monkeys. NIDA Res Monogr. 1997;178:226. [Google Scholar]
- 38.Zacny JP, Lichtor JL, Thapar P, Coalson DW, Flemming D, Thompson WK. Comparing the subjective, psychomotor and physiological effects of intravenous butorphanol and morphine in healthy volunteers. J Pharmacol Exp Ther. 1994;270:579–588. [PubMed] [Google Scholar]
- 39.Zacny JP, Conley K, Marks S. Comparing the subjective, psychomotor and physiological effects of intravenous nalbuphine and morphine in healthy volunteers. J Pharmacol Exp Ther. 1997;280:1159–1169. [PubMed] [Google Scholar]
- 40.Zhu J, Luo LY, Li JG, Chen C, Liu-Chen LY. Activation of the cloned human kappa opioid receptor by agonists enhances [ 35S] GTPgammaS binding to membranes: Determination of potencies and efficacies of ligands. J Pharmacol Exp Ther. 1997;282:676–684. [PubMed] [Google Scholar]
- 41.Zimmerman DM, Leander JD, Reel JK, Hynes MD. Use of beta-funaltrexamine to determine mu opioid receptor involvement in the analgesic activity of various opioid ligands. J Pharmacol Exp Ther. 1987;241:374–378. [PubMed] [Google Scholar]
- 42.Zukin RS, Eghbali M, Olive D, Untenvald EM, Tempel A. Characterization and visualization of rat and guinea pig brain k opioid receptors: Evidence for k1 and k2 opioid receptors. Proc Natl Acad Sci USA. 1988;85:4061–4065. doi: 10.1073/pnas.85.11.4061. [DOI] [PMC free article] [PubMed] [Google Scholar]


