Abstract
The use of mice for the evaluation and study of cardiovascular pathophysiology is growing rapidly, primarily due to the relative ease for developing genetically engineered mouse models. Arterial pressure monitoring is central to the evaluation of the phenotypic changes associated with cardiovascular pathology and interventions in these transgenic and knockout models. There are four major techniques for measuring arterial pressure in the mouse: tail cuff system, implanted fluid filled catheters, Millar catheters and implanted telemetry systems. Here we provide protocols for their use and discuss the advantages and limitations for each of these techniques .
Keywords: Arterial pressure monitoring, mice, methods
INTRODUCTION
Since the mouse is most suited to the development of different transgenic and knockout models, potential biological and molecular targets are further explored in these genetically altered mice. It is essential to characterize and evaluate the phenotypic changes that are present within these different models. One key variable that must be monitored and considered for cardiovascular pathophysiology research is arterial pressure. The accurate determination of arterial pressure is important not only for pathological conditions such as hypertension or determining the effects of pharmacological intervention, but is also important for the assessment of the condition of the animal at the time of the experiment. Excessively elevated or depressed arterial pressure may affect the experimental design and values. Indeed, it is not possible to interpret left ventricular (LV) function and ejection fraction (EF) in the absence of after load, which is most commonly assessed by systolic arterial pressure. The major techniques for measuring arterial pressure in the mouse are tail cuff, implanted fluid filled catheters, Millar solid state catheters and implanted telemetry systems, all of which are described here. Since the implantation of fluid filled catheters, Millar solid state catheters and telemetry systems require anesthesia and surgery, the authors first compare conscious versus sedated arterial pressure monitoring.
Comparing conscious vs. sedated arterial pressure monitoring
Anesthesia affects the heart, blood vessels and reflex control of the circulation (Vatner and Braunwald, 1975; Vatner et al., 2002). Accordingly, values obtained under anesthesia for arterial pressure are depressed (Table 1). Because of the hemodynamic effects of the anesthetic agents, studies in conscious animals are preferred when possible (Kurtz et al., 2005; Seidel et al., 2005). However, it is difficult to train mice to remain still in the conscious state, and movement and excitement all affect arterial pressure. Even though training will help minimize variability, it will not eliminate the normal physiologic response to stress. This is particularly important for experiments requiring a stable baseline of pressure for a prolonged period of time. Furthermore, catheter migration and bleeding is less of an issue in anesthetized animals compared to those studied in the conscious state. For studies in tranquilized mice many choices of injectable or inhalant anesthetics are available. The mixture ketamine/xylazine decreases cardiac contractility and output with the advantage of rapid anesthesia induction and recovery, providing an adequate duration of anesthesia (approximately 30 min) to allow for the completion of most surgical procedures, while Avertin lowers cardiac function at a more modest level without affecting cardiac output (Hart et al., 2001; Kiatchoosakun et al., 2001), thus is ideal for hemodynamic monitoring under anesthesia; however, with the relatively short anesthetic duration (10-15 minutes) it is more suited for surgery of relatively shorter duration. Isoflurane results in the lowest number of complications after anesthesia (Pena and Wolska, 2005; Szczesny et al., 2004); however, it may lead to an initial tachycardia with gradual decrease to a heart rate similar to that seen with Avertin (Roth et al., 2002). The major advantage of isoflurane anesthesia is that it allows for titratable continuous anesthesia with a relatively quick recovery time. However, the use of isoflurane requires an anesthetic chamber in a well ventilated operating room with continuous monitoring of oxygen saturation. The hemodynamic effects of some of the common anesthetic agents used in mice are listed in Table 1.
Table 1.
Mean arterial pressure (MAP) and heart rate (HR) in conscious and anesthetized mice
| Type of Anesthesia |
None (Conscious) |
Avertin | pentobarbital | K+X | isoflurane |
|---|---|---|---|---|---|
| Strain | C57/B6SJL | C57BL/6 | Webster 4 | C57/B6JSL | Swiss, C57BL/6 |
| MAP(mmHg) | 103±1 | 75±2 | 89±2 | 64±3 | 79±3 |
| HR(bpm) | 588±14 | 431±15 | 514±17 | 297±15 | 544±31 |
| Dose | none | 300mg/kg ip | 60mg/kg ip | 65+13mg/kg, ip | 2% inhalation |
| Data resource | Uechi et al. 1998 | Odashima et al. 2007 | Ma et al. 2002 | Vatner et al. 2000 | Janssen et al. 2004 |
All values are mean±SE. The heart rate and arterial pressure in conscious mice were much higher than those seen in anesthetized mice. (K=ketamine, X=xylazine; HR=heart rate; IP=intraperitoneal injection, IM=intramuscular injection, SC=subcutaneous injection).
BASIC PROTOCOL 1
TAIL CUFF SYSTEM
Tail cuff systems are routinely used for the monitoring of arterial pressure in rats (Krege et al., 1995; van Nimwegen et al., 1973) and over time this method has been adapted for the monitoring of arterial pressure in mice (Feng et al., 2009). In brief, the system involves a period of training for the mice with repeated arterial pressure measurements taken via the placement of a computer controlled arterial pressure cuff around the tail of the mouse. The tail cuff system uses a sphygmomanometer coupled to a method for measuring blood flow in the tail artery, e.g. photoplethysmography or piezoplethysmography. Photoplethysmography utilizes a light source (incandescent or LED) to sense and record the pulse wave signal while the piezoplethysmography uses piezoelectric crystals to sense the pulse. In general, the tail cuff is inflated to occlude the blood flow and the disappearance of arterial pressure during inflation or the first appearance of the pressure wave during deflation is taken as the systolic pressure. Since flow cannot be quantified by either of these methods, in both cases diastolic pressure is determined by mathematical calculation. Recently, a new technique has been developed where tail volume as a measure of blood flow into the tail is monitored and used to determine the diastolic pressure as the pressure upon which the blood flow into and out of the tail are equalized. This technique provides the most accurate of the three methods for tail cuff arterial pressure measurements (Feng et al., 2008).
Advantages and disadvantages of the tail cuff system
The advantages of this system are that it provides a less costly, simple, non-invasive method for measurements of arterial pressure in conscious mice and also can be used for regular monitoring of large groups of mice. A disadvantage is that the mice almost always continue to experience some degree of anxiety and stress during balloon inflation on the tail, as evidenced by elevated heart rate (Lorenz, 2002). However, arterial pressure seems to be affected to a lesser extent by this stress (Janssen et al., 2004; Krege et al., 1995; Ma et al., 2002; Odashima et al., 2007; Whitesall et al., 2004), most likely due to the powerful arterial baroreflexes. The degree of correlation between the arterial pressure obtained via tail cuff versus invasive methods is operator dependent (Krege et al., 1995; Whitesall et al., 2004), but in our hands there is a good correlation (Figure 1). One additional problem is that tail cuff arterial pressure does not always reflect central arterial pressure (Lorenz, 2002). Due to the inherent properties of peripheral arteries, environmental temperature, increased sympathetic tone, hypotension or vasoactive substances could all lead to vasoconstriction of the tail artery and affect the accuracy of the measurements. Tail pressure is sensitive to temperature, blood volume status and vascular tone, therefore, control of volume status and temperature is essential. Thus, all these factors should be considered when using tail cuff for arterial pressure measurements. Finally, there is a decrease in sensitivity with a narrowing of the pulse pressure (Doevendans et al., 1998), and due to the time required for the inflation and deflation of the tail cuff continuous monitoring of arterial pressure is not possible. This method is best suited for single point, periodic monitoring of arterial pressure in mice where the overall arterial pressure trend may be more important than the instantaneous arterial pressure.
Figure 1.
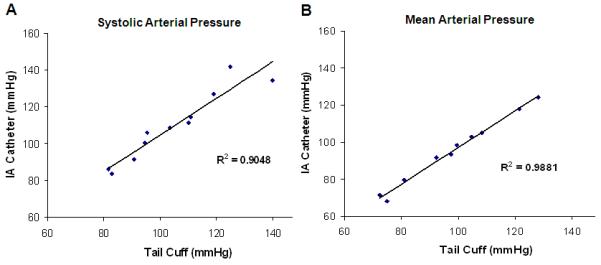
A comparison of arterial pressure obtained by tail cuff and intra-arterial catheter (IA) using the same male C57BL/6J mouse. A good correlation between systolic, R2=0.9048 (A) and mean arterial pressure, R2=0.9881 (B) is observed with the two methods.
Materials
Visitech BP-2000 series II arterial pressure analysis system™, four channel mouse platform, control unit, arterial pressure analysis software.
- Train the mice continuously for five days prior to the scheduled experiment. During training, the animals are subjected to the entire recording process and are placed in the restrainer before performing the following steps (2- 7c).IMPORTANT NOTE: Take care not to place mice of different gender near one another during training and recording period, as proximity to mice of the opposite sex could lead to excitation in some of the animals.
Adjust the heating mechanism on the platform by clicking on the “adjust temperature” option, under the “configuration” menu. Check the temperature of the platform to ensure a stable temperature of 38-40°C depending on the level of anesthesia and condition of the animal.
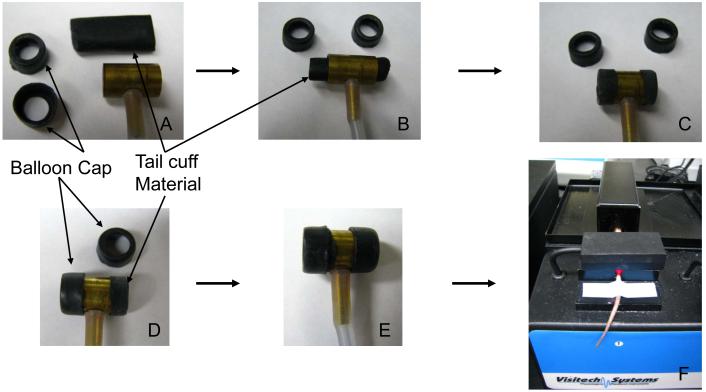
- Prepare the tail cuff by inspecting, cleaning with alcohol, and calibrating the tail cuff apparatus.The tail cuff material may degrade and become brittle over time. To replace the balloon, as per manufacturer’s instructions, first remove the old balloon, cut 4 pieces of 1-1/3” mouse tail cuff material (Visitech, BP-CE-M-38-100), thread the balloon through the tail-cuff and fold back the ends to wrap around the end of tail-cuff. Use the tail-cuff balloon cap to hold the balloon in place, to ensure proper inflation of the balloon (Figure 2).
Connect the pressurizing tubing to the platform, and check the balloons for leakage with “pressure leak wizard”, found under the “configuration” menu.
- Load the animals onto the platform for acclimation while the system is being calibrated.
- Place the mouse inside one of the magnetic restrainers.
- Pass the tail through the cuff and pulse sensor. Make sure the pulse sensor holds the tail within the groove. Taping the distal part of the tail onto the platform will help avoid excessive movements.
- Calibrate the system. Connect the platform to the sphygmomanometer, then select “Calibrate Pressure” from the “Configuration” menu, and follow the on screen directions.Ideally, four pressure inputs should be performed for this calibration setup. The system will ask to verify the pressure calibration by re-pressurizing again.
- Obtain arterial pressure measurements as follows:
- Re-connect the pressurizing tubing to the platform.
- Using the “specimen registration” option from the “Analysis” menu, enter the experimental groups and animal IDs used for data acquisition and analysis.
- Start recording. A user entered number of measurements (1-30) will be used to allow acclimation of the animal to the balloon inflation and deflation. These acclimation measurements are done automatically by the tail cuff apparatus, and are performed during each of the training sessions as well as prior to the experimental arterial pressure recording.IMPORTANT NOTE: The recording system should be set up in a quiet room with minimal through traffic to ensure the reproducibility of the obtained arterial pressure measurements.
- Additional user determined number of measurements will be recorded and analyzed. This number should not be less than 20.
- After the data acquisition is finished, to view the results look under the “report” option within the “Data” menu.According to the individual needs, “measurement set statistics” or “individual measurements” is selected and exported to an Excel or Text file for further analysis.
Figure 2.
Illustration of the tail cuff system (A). To replace the tail cuff balloon the tail cuff material is threaded through the tail cuff device (B), the ends are then everted over the ends (C), and the balloon caps are then placed on to each of the two ends (D,E). The mice are restrained individually during the recording process while the tail cuff system is connected to a computer for data acquisition (F).
Five days of training prior to the actual experimental recording is recommended by the manufacturer to obtain the most reliable data, as stress from balloon inflation and being in the restrainer can all lead to excitation of the mice and result in falsely elevated heart rate and arterial pressure. With an increasing number of training sessions the mice are able to adapt quicker to the procedures and provide more reproducible results. Investigators should record the arterial pressures from each of the daily training sessions and perform the actual experimental recording on day 6. By comparing the arterial pressures from each of the training days the investigators can then determine when the heart rate and arterial pressure measurements have reached a stable level. Usually, the results from the last 3 days of recording are relatively consistent and all of these can be used in the final data analysis. If excessively high variation in blood pressure, heart rate or movement is noted additional training sessions will be needed. The actual number of training sessions required for acclimations will very depending on the strain, gender and age of the mice.
BASIC PROTOCOL 2
FLUID FILLED CATHETER SYSTEM
The use of indwelling fluid filled catheters implanted into the aorta through either the femoral or carotid artery results in direct and continuous measurements of arterial pressure. The catheter is connected to a strain gauge manometer for transduction of the pressure signal to an electrical signal where it is observed and stored using analog or digital recorders. When these catheters are exteriorized and secured they can be tethered to a swivel device on the cage to allow free movement of the animals. In this way the tethered system is used for monitoring of arterial pressure in a conscious animal (Figure 3).
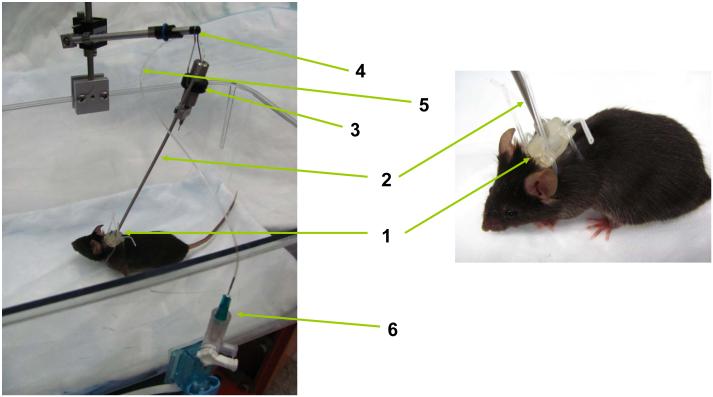
Figure 3.
Schematic diagram of the tether system. After the arterial line has been placed in the mouse the line is inserted into the catheter harness (1), threaded up the spring casing (2) for the catheter, and then attached to the swivel device (3) on the swivel arm (4). The catheter tubing (5) exits from the spring casing and is attached to the pressure transducer (6) set at the level of the heart.
Advantages and disadvantages of the fluid filled catheters
The advantage of this system is that it provides direct and continuous measurements of arterial pressure. In addition, it allows not only for monitoring of arterial pressure, but also for vascular access, thus allowing more experimental flexibility (Mattson, 1998). One example is the delivery of phenylephrine or sodium nitroprusside in a conscious mouse leading to a corresponding increase or decrease in arterial pressure (Figure 4).
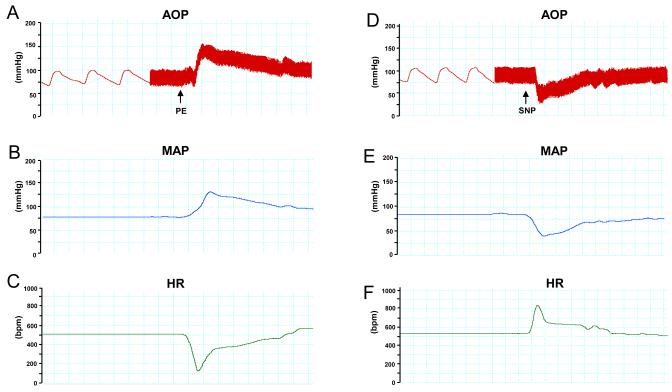
Figure 4.
Arterial pressure recording with an implanted fluid filled catheter showing a sample of blood pressure change in response to vasoactive compounds in a conscious C57BL/6J mouse. (A) An increase in both the aortic pressure (AOP), (B) mean arterial pressure (MAP) and (C) a decrease in heart rate (HR) are noted with the administration of phenylephrine (PE). (D) A decrease in AOP and (E) MAP with (F) an increased HR are noted with the administration of sodium nitroprusside (SNP).
The disadvantages, aside from morbidity and mortality associated with surgery, under such a system are that the catheters require a heparin lock and daily flushing to prevent clotting of the catheter. This system requires single housing of the animal and is not suited for continuous prolonged recording, as catheter patency diminishes and signal dampening occurs with time. Although flushing of the catheter will restore signal intensity due to the low total blood volume of a mouse, frequent flushing may result in volume overload. Finally, when the heart rate of the mouse is elevated, the arterial signal may be dampened (Mattson, 1998).
Materials
- Catheter: PE-05 tubing (0.28 mm ID × 0.61mm OD) for the carotid artery
- PE-08 tubing (0.20 mm ID × 0.36 mm OD) for the femoral artery
Suture: 7-0 Silk for vessel ligation, 6-0 nylon for skin closure
Anesthesia of choice (see Table 1 and step 2)
Heparinized-saline solution
Customized needle-wire plug
Pressure transducer
Signal amplifier
Recording system (e.g., Power Lab (AdInstruments), Dataquest (Data Sciences International), NOTOCORD-hem™ (Notocord), EMKA IOX (EMKA Technologies))
Prepare and insert catheters
Calibrate the fluid filled catheters. The general range for the calibration of the catheters in mice is from 0 to 200 mmHg.
- Anesthetize, shave and place mouse in supine position.Depending on experimental design and the experience of each individual laboratory the choice of anesthesia will vary, please refer to Table 1 for reference on the hemodynamic effects of each of the anesthetic agents. Different anesthetic agents may be used for the day of the surgery and the day of the experiment. In our laboratory we generally use a mixture of ketamine and xylazine for catheter implantation. This mixture has a shorter induction time and a more rapid recovery time when compared against pentobarbital; however, any anesthetic agent that the individual surgeon is comfortable with could be used. As for recording, the conscious condition is preferred. If needed, Avertin anesthesia is used as the agent of choice for hemodynamic monitoring under sedation because of its lower decreased suppressive effects on cardiac function, heart rate and temperature when compared against pentobarbital or ketamine and xylazine mixture.
Make a 1-2 cm midline neck incision from just below the mandible to the thoracic inlet. Under a dissecting microscope, the right carotid artery is exposed and carefully separated from other neighboring structures including the vagus nerve.
- Once the carotid artery has been isolated, place a silk suture (7-0 or 6-0) distally (closer to the head) for the complete ligation of the vessel. Place a second silk suture proximally (closer to the heart) to allow temporary obstruction of blood flow. Finally, place a third silk suture loosely between the first two ligatures and make a small incision (arteriotomy) distal to the middle ligature (i.e., between the first and the third suture) (Figure 5).OPTIONAL: Due to the smaller diameter of the femoral artery as compared to the carotid artery, a smaller caliber catheter (PE-08 tubing) is needed for the femoral artery. Due to the anatomical relationship between the femoral artery and the abdominal aorta it is technically more difficult to advance the femoral catheter beyond the bifurcation point into the abdominal aorta. Once the femoral catheter is successfully inserted into the abdominal aorta the caliber of the catheter remains a point of consideration. With the smaller catheter used for femoral artery there is a decrease in signal wave form quality and intensity of pressure. Furthermore, because of the location and associated potential movement of the legs, the femoral catheter is more prone to signal lost during movement. For similar reasons the insertion of the femoral artery catheter requires a more secure fixation of the surrounding musculature compared to the insertion of carotid artery catheters. Although not preferred, either the left or the right femoral artery can still be used in place of the carotid. Using the three suture technique in a similar fashion as the one described above for the carotid artery the femoral vessels are exposed and the artery isolated for cannulation through a 1-2 cm inguinal skin incision.
Insert the tip of a catheter that had been pre-filled with 10-30% heparinized saline into the carotid artery via the arteriotomy in the direction of the heart and secure it in place by tying the mid (i.e., the third) suture once that catheter has been advanced past the ligature.
- Release the proximal ligature and re-ligate after the catheter has been advanced for about 11-12 mm into the ascending aorta or 18-20 mm into the left ventricular chamber.When advancing or withdrawing the catheter, hold the catheter between the thumb and index finger; use a gentle twisting motion in conjunction with the slow advancement of the catheter to help avoid vascular injury. Pay special attention to the resulting waveform to ensure the proper placement of the catheter.If using the femoral artery: Upon isolation of a section of the femoral artery, apply a couple drops of 2% lidocaine to the area to help dilate the vessel. Insert the catheter and advance it past the bifurcation point into the abdominal aorta. If resistance is felt, do not force the catheter; reposition the mouse to line up the operated leg with the axis of the body. Using a twisting motion move the catheter forward to advance the catheter beyond the bifurcation.
- Verify cannula patency by blood return via withdraw then flush with a little more heparinized-saline and cap the distal end of the catheter with a customized needle-wire plug.With the low total blood volume of a mouse (80 ml/kg, body weight), caution should be used when flushing the catheter to avoid excessive volume loading.
- Place the mouse in the right lateral recumbent position (i.e., right side down), and subcutaneously tunnel and externalize the catheter through a mid-scapular skin incision on the back.If using the femoral artery secure the catheter in place through the application of sutures, again tunnel subcutaneously and externalize in the midscapular region on the back.
- Close the neck incision with 6-0 nylon sutures and fix the external portion of the catheters in the back to the underlying muscle.For both the carotid and femoral catheters once exteriorized in the midscapular region, protect the catheters from being chewed by the animal by housing the catheter within a plastic cap transfixed to the midscapular region of the dorsal skin.
Unless immediate post-anesthesia measurements are performed, allow the animals to recover inside a pre-warmed (31°C) rodent cage after the surgery.
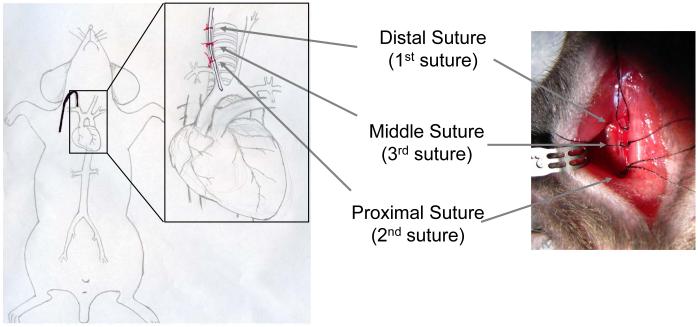
Figure 5.
Illustration of the suture placements for the three suture technique used during carotid artery catheter insertion. The sutures are placed around the right carotid artery. The numbers indicate the sequence of suture placement.
Record arterial pressure
11. Connect the catheter to a solid state pressure transducer placed at the level of the mouse heart.
12. Connect the pressure transducer to a signal amplifier that is connected to the recording system of choice.
Additional steps for tether
The exteriorized catheter can be attached to the tether device for conscious recording of freely mobile animals.
- 13. First thread the catheter through the harness and spring casing of the tether.The spring casing is connected to a swivel device which is connected to the swivel arm attached to the side of the cage.
14. Once the catheter exits from the spring, attach the casing to a section of PE5 tubing which is in turn attached to the pressure transducer placed at the level of the mouse heart.
15. Attach the pressure transducer to a signal amplifier connected to the recording system (Figure 3).
BASIC PROTOCOL 3
MILLAR SOLID STATE MICRO-PRESSURE TRANSDUCER TIPPED CATHETER
Solid state Millar catheters provide the frequency response needed for assessing alterations in the phasic arterial pressure waveform. In brief, the Millar solid state catheter contains a micro-pressure transducer mounted at its distal end allowing the conversion of the pressure wave into an electrical signal. The procedure used for implantation of a Millar catheter is similar to those used for a fluid filled catheter.
Advantages and disadvantages of the Millar solid state catheters
The most important advantages of the Millar solid state catheter system is its frequency response, making it the most sensitive and precise method for the monitoring of arterial pressure and for analysis of changes in the phasic arterial pressure waveform. In addition, unlike the fluid filled catheters, Millar solid state catheters do not require flushing and do not have the problem of decreasing signal intensity with increasing catheter length. One main disadvantage is the cost, and others include surgical morbidity and mortality. Furthermore, due to its extreme sensitivity, the recordings are affected by ambient conditions such as temperature and blood viscosity or electrolyte change (Lorenz, 2002), so multiple parameters must be closely monitored and controlled. Additionally, we have noted electrical interference from other surgical equipment during hemodynamic measurements when utilizing this system. Finally, at present, Millar solid state catheters are not convenient for conscious monitoring of arterial pressure in mice, although a mouse telemetry system utilizing the Millar solid state catheter is in development (AdInstruments, Inc.). Therefore, the Millar solid state catheters are best suited for acute studies under anesthesia where the monitoring of arterial pressure needs to be coupled to left ventricular pressure measurements.
Materials
Micromanometer catheter: 1.4F (Millar Instruments)
Suture: Silk 7-0 or 6-0 for vessel ligation
- Implant the Millar solid state catheter following the procedure described in detail for the implantation of fluid filled catheters (see Basic Protocol 2). Either the carotid or the femoral artery can be employed as vascular access.In smaller mice catheter advancement into either the carotid or the femoral artery can be difficult. Application of an acoustic gel around the catheter entry site will provide lubrication.IMPORTANT NOTE: Throughout the implantation process, when the Millar solid state catheter is not inside the animal, the tip of the catheter needs to be kept in warm saline (37°C) at all times.
Subject the Millar solid state catheter to a cleaning process as described in the instructional manual following the experiment. Keep the tip of the catheter protected at all times.
IMPORTANT: turn on the recording instruments prior to connecting the catheter for calibration (warm-up time; 20-30 min) since the Millar catheter is sensitive to sudden electrical surge.
- Keep the vessel(s) around the catheter wet before and during implantation.If a shift in baseline or abnormal wave form, such as a spike noted on the top of the pressure wave, is observed try repositioning the body of the animal or the catheter tip, as the shift may be the result of catheter position and the change in waveform may be the result of the catheter sensor coming into contact with the vessel or ventricular wall.
BASIC PROTOCOL 4
IMPLANTED TELEMETRY SYSTEM
Radiotelemetry units have been accepted as the gold standard for the monitoring of arterial pressure in intact, conscious mice. These devices contain a short segment of catheter attached to a signal transmitter. The catheters function to sense pressure waves in a similar manner as the fluid filled catheter. The catheter is inserted into either the carotid artery with subcutaneous placement of the transmitter or into the abdominal aorta with intraperitoneal placement of the transmitter. Prior to insertion into the animal the catheters are pre-filled with a gel material (AD instruments) to improve signal conduction and help prevent clotting of the catheter. The unit functions in a similar manner as a fluid filled catheter, the difference being the electrical signal is transmitted wirelessly to a receiving device. The units are activated via a magnet and the transmitted signals are then be received by a receiver placed on the outside of the animal cage and the data recorded by a connected computer.
Advantages and disadvantages of the implanted telemetry device
The major advantages of implanted telemetry systems include remote monitoring of arterial pressure in conscious mice living in their natural environment while providing sensitive, accurate, long term data (Figure 6) (Desjardins et al., 2008; Kramer et al., 2000). On the other hand, disadvantages include the cost and the surgical skill needed for implantation and consequently surgically related morbidity and mortality. Furthermore, a 5-7 day recovery period is often needed after implantation (Lorenz, 2002). In the event of an acute study where intravenous (iv) drug delivery is needed, the insertion of an iv catheter is still required. When the gel filled sensing catheters are implanted into the abdominal aorta, cases of vascular occlusion leading to decreased blood flow to the hindquarters has been reported (Lorenz, 2002). This is avoided by using the carotid artery where ischemia is much less of an issue. With the use of newer techniques and models, these devices are implanted into mice as small as 17g with a reported success rate of 90% (Carlson and Wyss, 2000). Telemetry systems are best suited for long term continuous monitoring of mice either within its natural living environment or with exercise.
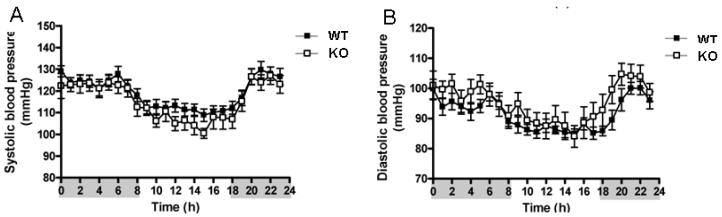
Figure 6.
An example of telemetry blood pressure recording over a 24 hour time period. The circadian variation in arterial pressure and heart rate are clearly observed in this recording. (A) Arterial systolic pressure (SBP) and (B) arterial diastolic pressure (DBP) variations are seen in both the wild type (WT, C57BL/6) and caveolin-1 knockout (KO) mice. The shaded and non-shaded areas on the X-axis represent the dark and light cycles, respectively (Desjardin et al., 2008). Figure reproduced and modified with permission.
Materials
Telemetry transmitter device (PA-C20 or PA-C10) (Data Sciences International, MN, Figure 7)
Central Recorder/monitor
Platforms for transmission
Suture: 7-0 Silk for vessel ligation, 6-0 nylon for skin closure, 5-0 nylon for telemetry device fixation.
Anesthesia of choice (see Table 1 and step 1)
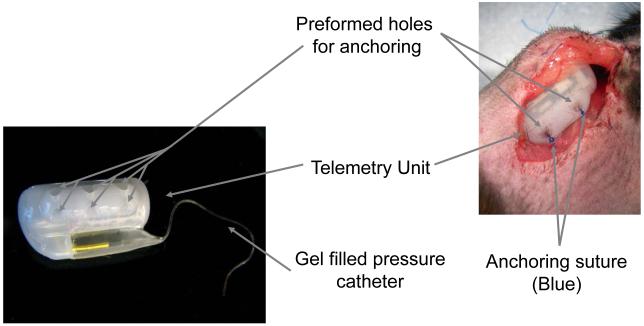
Figure 7.
Illustration of the telemetry unit with the anchoring sutures in place. In general, 2-3 anchoring sutures are used to fix the telemetry unit to the chest wall of the mice.
- Anesthetize, shave and place mouse in the supine position.Depending on experimental design and the experience of each individual laboratory the choice of anesthesia will vary, please refer to Table 1 for reference on the hemodynamic effects of each of the anesthetic agents. Different anesthetic agents may be used for the day of the surgery and the day of the experiment. In our laboratory we generally use a mixture of ketamine and xylazine for surgery; however, any anesthetic agent that the surgeon is comfortable with could be used for telemetry implantation. Recording is generally done under the conscious condition; however, if anesthesia is needed Avertin is used for hemodynamic monitoring under sedation.
Make a 2-cm mid-cervical incision in the neck of the mouse and create a subcutaneous space by undermining the skin on the right chest to create a pocket large enough to accommodate the device.
Insert the catheter tip via an arteriotomy in the left carotid artery and advance it into the transverse aorta for about 13 mm following the three-suture placement technique described in Basic Protocol 2 step 4 for fluid catheter implantation.
Perform extra fixation of the catheter onto muscle and soft tissue of the mouse to prevent the dislodgement of the catheter tip from the arterial lumen which could lead to inaccurate readings.
- Insert the transmitter device into the pocket in the right chest with the side containing the predrilled suture holes facing up and transfix the device to the overlying skin using a few 5-0 nylon sutures to prevent migration (Figure 7).Due to the potential irritation to the mouse associated with the implanted telemetry device, the body of the device should be secured to prevent migration of the device and keep the mouse from scratching and biting the site of surgery or device.
Recording
6. After inserting the telemetry unit, allow the mice to recover for at least 5-7 days.
- 7. Once the animals have recovered from the insertion of the telemetry unit, house them individually in a regular mouse cage placed on top of the telemetry receiver plate (Figure 8).
- If the recording of multiple animals is needed, each plate should be placed at least 17 inches from each other.When fully charged, the battery will allow for 1.5 months of continuous recording. If the arterial pressure waveform becomes dampened or the signal is lost the device should be removed.
- If telemetric recording is desired during exercise, the receiver plate should be placed above the treadmill.
- The device can be turned on/off with magnetic activation and can be used in combination with drug administration should the experimental needs arise.
- After the device is removed from the animal, it should be sent back to the manufacturer for cleaning, re-conditioning (insertion of new battery, catheter and re-calibration) and sterilization.
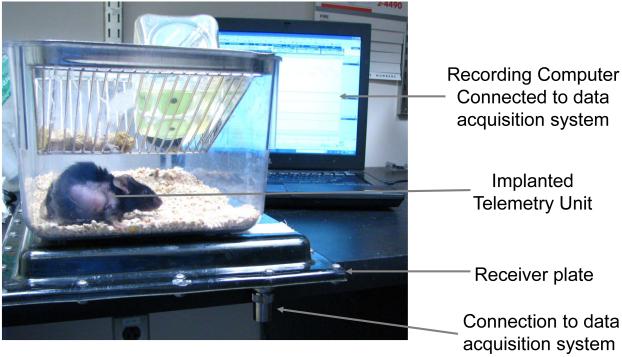
Figure 8.
Illustration of a telemetric recording of mouse arterial pressure. The mice are housed individually during the recording process with the recording receiver placed below each cage and connected to a computer for data acquisition.
COMMENTARY
Background Information
In cardiovascular research and the development of new pharmacological agents, arterial pressure monitoring is of particular importance for evaluating cardiovascular function and response, such as change in arterial pressure in response to vasoactive drugs and change in vessel stiffness in aged and hypertensive animal models. Mice are an important animal model and widely used in laboratory and clinical research. Arterial pressure monitoring in mice is challenging due to the size of the animal and the rapid heart rate. This article reviews the four major methods: tail cuff, intravascular fluid filled catheter (including the tether system), solid state Millar catheter, and telemetry for arterial pressure monitoring in mice.
Tail cuff system
Utilizes a sphygmomanometer coupled to blood flow measurement in the tail artery via photoplethysmography or piezoplethysmography. In general, the tail cuff is inflated to occlude the blood flow and the disappearance of arterial pressure during inflation or the first appearance of the pressure wave during deflation is taken as the systolic pressure and the diastolic pressure is determined by mathematical calculation. Since flow can not be quantified by either of these methods, diastolic pressure is determined by mathematical calculation. However, using a method that uses tail volume as a measure of blood flow into the tail, diastolic pressure is determined as the pressure upon which the blood flow into and out of the tail are equalized.
Fluid filled catheter system
Uses implanted indwelling strain gauge manometer connected fluid filled catheters in either the aorta, the femoral or carotid artery for direct and continuous measurements of arterial pressure. The pressure signal is then converted to an electrical signal where it is observed and stored using analog or digital recorders.
Millar solid state micro-pressure transducer tipped catheter
Consists of a micro-pressure transducer mounted at the distal end of a wire catheter converting the measured pressure wave into an electrical signal that are then recorded in a similar manner as the signal obtained via the fluid filled catheter.
Implanted telemetry systems
Implanted telemetry systems are accepted as the gold standard for monitoring of arterial pressure in intact, conscious mice. These devices consist of a short segment of catheter attached to a signal transmitter where the catheters are inserted into either the carotid artery or the abdominal aorta, after which the pressure wave signals are then converted to radio waves to be received by a signal receiver. For detailed background for each of the individual methods please refer to Basic Protocols 1 to 4.
Advantages and disadvantages
The tail cuff system is the simplest method for measuring arterial pressure within multiple animals, there is no need for anesthesia or invasive procedures. However, due to the stress associated balloon inflation and deflation and the peripheral arterial vasoconstriction, the obtained arterial pressure measurement may be influenced. The fluid filled catheters are widely used for the measurements of central arterial pressure prior to the development of solid state Millar catheters. This method is used for continuous monitoring of central arterial pressure in both conscious and anesthetized animals. Based on the skill of the surgeon, the less the blood loss during catheter insertion and the better the catheter patency, the more physiologic the results. The most often encountered problem is catheter patency, when the catheter is partially clogged, the pressure waveform may be dampened and the pulse pressure will be decreased. However, aside from the surgically associated morbidity and mortality, this system requires a heparin lock and daily flushing of the catheter to prevent clotting. Furthermore, due to the need for continuous monitoring of catheter patency and signal quality, this system is not suited for continuous prolonged recording. Compared to the fluid filled catheter, the Millar solid state catheter provides increased sensitivity and responsiveness. The cost of the Millar catheter requires a financial commitment and the recording may be affected by physiologic as well as ambient conditions, so much care must be taken to minimize these influences. Finally, the telemetry system allows for the recording of arterial pressure in conscious animals within their normal living environment providing the most physiologic of the arterial pressure readings. However, since it is an implanted intravascular catheter, careful calibration and sterilizing of the device prior to implantation is of particular importance. For details of the advantages and disadvantages of each individual method please refer to Basic Protocols 1 to 4.
Critical parameters
Invasive vs. Non-invasive
Due to the stress, morbidity and mortality associated with invasive methods of arterial pressure monitoring, non-invasive methods are preferred whenever possible. However, the tail cuff arterial pressure monitoring system is not stress free and does have some level of stress associated with the use of a restrainer. The non-invasive tail cuff arterial pressure monitoring method allows for rapid, periodic monitoring of arterial pressure of experimental animals, thus allowing for the monitoring of arterial pressure change over time. However, it is important to remember that the tail cuff system provides information only on peripheral arterial pressure as compared to the central arterial pressure obtained by other invasive techniques, such as fluid filled catheter, telemetry and Millar catheter. If high infidelity of data or an unexpected result is noticed either due to the physical condition of the animal or lack of acclimation of the animal to the tail cuff device resulting in missing data points during the recording, the investigator should consider invasive methods for monitoring of arterial pressure. It is generally recommended that indirect methods of arterial pressure monitoring (e.g. tail cuff) should not be used to measure blood pressure variability, diastolic blood pressure or determination of pulse pressure in conscious mice. In addition, this technique is not suited for studies in non-stressed, unrestrained mice. It is also not well suited for drug studies or the detection of mild or intermittent hypertension (Kurtz, 2005b).
Direct vs. Indirect
Comparing direct invasive arterial pressure monitoring techniques to those of indirect arterial pressure measurements in the peripheral artery may result in different results due to the condition of the animal, a low peripheral temperature, or difference in regional effects of vasoconstrictive compounds (e.g. phenylephrine). In drug dose response experiments, a direct measurement is preferred over indirect methods. Direct methods allow for continuous monitoring of arterial pressure change, whereas tail cuff provides only intermittent arterial pressure reading such that if the duration of the response is short the drug response could be missed completely. Furthermore, the observed central arterial pressure effect could be very different from that of the peripheral arterial pressure, thus depending on the area of interest, the desire for peripheral versus central pressure monitoring, continuous versus intermittent arterial pressure monitoring, should all be considered in the experimental design.
Need for Telemetry
The implanted telemetry system is ideal for long term continuous monitoring of conscious arterial pressure and is the gold standard for this purpose. For chronic studies, such as monitoring chronic vasoactive drug infusion, circadian arterial pressure change, heart failure studies, comparison of phenotypic arterial blood pressure difference between genetically modified animals, telemetry is preferred over other methods. Although the manipulation of the carotid artery during catheter insertion may injure the ipsilateral carotid barorefelx center, this is well compensated by the baroreflex center on the contralateral carotid and the aortic arch.
Need for Hi-fidelity Waveform
Solid state Millar catheter system provides the highest fidelity of the arterial pressure waveform with the most rapid response time of all the methods. In mice under stimulation, heart rate could be as high as 700 beats per minute (bpm), while under resting conditions mice generally have a heart rate of 500-600 bpm. Therefore, to achieve a sufficient frequency-response rate for mouse LV blood pressure monitoring with dP/dt, a system capable of sampling at a rate of 1000-2000 Hz may be needed (Lorenz and Robbins, 1997). In order to achieve this goal the Millar catheter is the most suitable method. Thus, for anesthetized drug response experiments the Millar solid state catheters provide the most accurate measurement among all other techniques. The experimental needs and design of a particular study will dictate the optimal method required for monitoring arterial pressure for that setting. The material discussed in this article will aid in selecting the technique that is best suited for any distinct application.
Troubleshooting
Tail cuff system
Even after multiple training sessions, mice may still be agitated during the pressurizing process of the tail cuff. This will in turn affect the accuracy of the pressure readings. Taping the distal part of the tail on the platform may help avoid excessive movement from the animal.
Although the restrainer is made of metal, there are predrilled holes through which ambient light will leak in and the animals will be able to sense nearby movements. Using a black cover on the outside of the platform and the restrainer will help reduce light. In addition, a quiet room and a calm recording operator are indispensable to reduce noise level and to decrease the agitation experienced by the animal.
Fluid filled catheter system
Due to the rapid blood flow at the entrance of the ascending aorta, at times it is difficult to advance the catheter into the left ventricle. Moreover, forcefully advancing the catheter may result in aortic valve damage. While advancing the catheter, placing the catheter between the thumb and index fingers, using a gentle twisting motion in conjunction with the slow advancement of the catheter, the catheter is placed into the left ventricle. The entrance of the catheter into the left ventricle is often felt as a sudden disappearance of resistance, and to-and-fro movement of blood flow inside the catheter will be visibly intensified.
During femoral artery catheter implantation it may be difficult to advance the catheter beyond the aortic bifurcation of the iliac artery. If resistance is felt, reposition the mouse to line up the operated leg with the longitudinal axis of the body and twist the catheter forward, this body position will help to advance the catheter beyond the bifurcation point.
Millar solid state micro-pressure transducer tipped catheter
After the Millar catheter is implanted into the left ventricle sometimes spike artifacts are observed on the top of the pressure wave as seen on the monitor or paper strip chart, this is at times associated with arrhythmia. One possible explanation for this observation is left ventricular irritation secondary to contact between the catheter sensor and the ventricular wall. This is remedied by repositioning the catheter tip via a change in direction or depth. Alternatively, the contact between the catheter and LV wall may be secondary to pharmacological stimulation resulting in severely contracting ventricles. In this case, the spike artifact may persist until the drug effect has worn off. Occasionally, an upward or downward drift of the waveform may be observed and this could lead to inaccurate pressure readings. This could be secondary to the sudden re-positioning of the animal or catheter. Therefore, by repositioning the body of the animal or the catheter tip, stabilization of the waveforms may be observed.
Due to tight fitting of the catheter in the vessel, catheter advancement in either the carotid or the femoral artery can be difficult. Application of an acoustic gel around the catheter entry site may serve as helpful lubricant. Application of 2% lidocaine is almost always necessary in femoral arterial catheter insertion.
In mice with transverse aortic banding, the right carotid artery is usually bulging and dilated making catheter insertion and advancement easier; however, care should be taken to avoid having the catheter pushed out due to the high pressure (hypertensive) present in the carotid artery.
Implanted telemetry system
Due to the chronic nature of the implanted telemetry system the mice may chew on the surgical sites due to associated irritation. Therefore, the body of the device should be secured properly in the pocket to prevent migration of the device.
Movement may lead to the migration of the implanted catheter, resulting in the dislodgement of the catheter tip from the arterial lumen, thus leading to massive lethal bleed. Extra fixation of the catheter onto the musculature and soft tissue of the mouse is necessary to help decrease the possibility of such an event.
The battery in the telemetry device lasts for 1.5 months with continuous use. However, if a damped arterial pressure waveform or a lost of signal is noticed the device may have lost its efficacy. At this point, the device should be removed and cleaned, then sent back to the manufacturer for re-conditioning.
Anticipated results
The anticipated result for the tail cuff system is a digital read out of a series of numbers representing each individual reading and the average arterial pressures, while the fluid filled catheters, Millar solid state catheters and the telemetry system will provide arterial pressure wave forms in addition to the digital read out of the measured arterial pressures. Multiple variables are known to affect the measured arterial pressure including the experimental and physiologic conditions, the level of sedation, volume status, age and gender, as well as many environmental factors. As a general reference, the average conscious arterial pressure found in some common strains of mice used for cardiovascular research can be found in Table 2. In general, hypertension can be defined as blood pressure greater than two standard deviations above the mean.
Table 2.
Mean arterial pressure (MAP) and heart rate (HR) in three strains of conscious mice
| Strain | C57/B6SJL | 129Sv/J | FVB |
|---|---|---|---|
| MAP (mmHg) | 103±1 | 102±8 | 99±2 |
| HR (bpm) | 588±14 | 522±11 | 617±26 |
| Data source | Uechi et al., 1998 | Gross et al., 2003 | Lin et al., 2010 |
All values are mean±SE. (MAP=mean arterial pressure; HR=heart rate)
Time considerations
The time required for tail cuff system of arterial pressure monitoring depends upon the number of acclimation and actual readings set by the investigator, but in general expect 1-2 hours. As for the other methods, the actual time required for the completion of the method will depend on the skill of the surgeon. The time required for the completion of each of these methods is found below:
Tail cuff system
Depends on the number of acclimations and actual readings desired for each animal. On average expect 1-2 hours to complete the process, allow 5 minutes for preparation and 5 minutes for calibration of the device. This process is repeated for multiple days to reduce variation on different days.
Fluid filled catheters
On average, depending upon the skill of the surgeon, without tether, expect 30-40 minutes to complete the process. If tether is needed, add an additional 5-10 minutes for tether placement. Prior to recording the time required for calibration and preparation is approximately 5 minutes each.
Millar solid state catheters
On average, depending upon the skill of the surgeon, expect 25-35 minutes to complete the process. Similar to that seen in fluid filled catheter and tail cuff systems, the time required for calibration and preparation prior to recording is approximately 5 minutes each.
Telemetry system
On average depending upon the skill of the surgeon, expect 30-40 minutes to complete the process. The recording process can be initiated with approximately 5 minutes of preparation time.
Acknowledgements
Supported in part by grants HL033107, HL069020, AG027211, HL101420, HL093481, HL059139, HL095888, DK083826, and HL102472.
Literature cited
- Carlson SH, Wyss JM. Long-term telemetric recording of arterial pressure and heart rate in mice fed basal and high NaCl diets. Hypertension. 2000;35:E1–5. doi: 10.1161/01.hyp.35.2.e1. [DOI] [PubMed] [Google Scholar]
- Desjardins F, Lobysheva I, Pelat M, Gallez B, Feron O, Dessy C, Balligand JL. Control of blood pressure variability in caveolin-1-deficient mice: role of nitric oxide identified in vivo through spectral analysis. Cardiovasc Res. 2008;79:527–536. doi: 10.1093/cvr/cvn080. [DOI] [PubMed] [Google Scholar]
- Doevendans PA, Daemen MJ, de Muinck ED, Smits JF. Cardiovascular phenotyping in mice. Cardiovasc Res. 1998;39:34–49. doi: 10.1016/s0008-6363(98)00073-x. [DOI] [PubMed] [Google Scholar]
- Feng M, Deerhake ME, Keating R, Thaisz J, Xu L, Tsaih SW, Smith R, Ishige T, Sugiyama F, Churchill GA, et al. Genetic analysis of blood pressure in 8 mouse intercross populations. Hypertension. 2009;54:802–809. doi: 10.1161/HYPERTENSIONAHA.109.134569. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Feng M, Whitesall S, Zhang Y, Beibel M, D’Alecy L, DiPetrillo K. Validation of volume-pressure recording tail-cuff blood pressure measurements. Am J Hypertens. 2008;21:1288–1291. doi: 10.1038/ajh.2008.301. [DOI] [PubMed] [Google Scholar]
- Gross V, Luft FC. Exercising restraint in measuring blood pressure in conscious mice. Hypertension. 2003;41:879–881. doi: 10.1161/01.HYP.0000060866.69947.D1. [DOI] [PubMed] [Google Scholar]
- Hart CY, Burnett JC, Jr., Redfield MM. Effects of avertin versus xylazine-ketamine anesthesia on cardiac function in normal mice. Am J Physiol Heart Circ Physiol. 2001;281:H1938–1945. doi: 10.1152/ajpheart.2001.281.5.H1938. [DOI] [PubMed] [Google Scholar]
- Janssen BJ, De Celle T, Debets JJ, Brouns AE, Callahan MF, Smith TL. Effects of anesthetics on systemic hemodynamics in mice. Am J Physiol Heart Circ Physiol. 2004;287:H1618–1624. doi: 10.1152/ajpheart.01192.2003. [DOI] [PubMed] [Google Scholar]
- Kiatchoosakun S, Kirkpatrick D, Hoit BD. Effects of tribromoethanol anesthesia on echocardiographic assessment of left ventricular function in mice. Comp Med. 2001;51:26–29. [PubMed] [Google Scholar]
- Kramer K, Voss HP, Grimbergen JA, Mills PA, Huetteman D, Zwiers L, Brockway B. Telemetric monitoring of blood pressure in freely moving mice: a preliminary study. Lab Anim. 2000;34:272–280. doi: 10.1258/002367700780384663. [DOI] [PubMed] [Google Scholar]
- Krege JH, Hodgin JB, Hagaman JR, Smithies O. A noninvasive computerized tail-cuff system for measuring blood pressure in mice. Hypertension. 1995;25:1111–1115. doi: 10.1161/01.hyp.25.5.1111. [DOI] [PubMed] [Google Scholar]
- Kurtz ME, Kurtz JC, Given CW, Given B. A randomized, controlled trial of a patient/caregiver symptom control intervention: effects on depressive symptomatology of caregivers of cancer patients. J Pain Symptom Manage. 2005;30:112–122. doi: 10.1016/j.jpainsymman.2005.02.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kurtz TW, Griffin KA, Bidani AK, Davisson RL, Hall JE. Recommendations for blood pressure measurement in humans and experimental animals. Part 2:Blood pressure measurements in experimental animals. Hypertension. 2005;45:299–310. doi: 10.1161/01.HYP.0000150857.39919.cb. [DOI] [PubMed] [Google Scholar]
- Lin M, Harden SW, Li L, Wurster RD, Chen Z. Impairment of barorefelx control of heart rate in conscious transgenic mice of type 1 diabetes (OVE26) Auton Neurosci. 2010;152:67–74. doi: 10.1016/j.autneu.2009.09.020. [DOI] [PubMed] [Google Scholar]
- Lorenz JN. A practical guide to evaluating cardiovascular, renal, and pulmonary function in mice. Am J Physiol Regul Integr Comp Physiol. 2002;282:R1565–1582. doi: 10.1152/ajpregu.00759.2001. [DOI] [PubMed] [Google Scholar]
- Lorenz JN, Robbins J. Measurement of intraventricular pressure and cardiac performance in the intact closed-chest anesthetized mouse. Am J Physiol. 1997;272:H1137–1146. doi: 10.1152/ajpheart.1997.272.3.H1137. [DOI] [PubMed] [Google Scholar]
- Ma X, Abboud FM, Chapleau MW. Analysis of afferent, central, and efferent components of the baroreceptor reflex in mice. Am J Physiol Regul Integr Comp Physiol. 2002;283:R1033–1040. doi: 10.1152/ajpregu.00768.2001. [DOI] [PubMed] [Google Scholar]
- Mattson DL. Long-term measurement of arterial blood pressure in conscious mice. Am J Physiol. 1998;274:R564–570. doi: 10.1152/ajpregu.1998.274.2.R564. [DOI] [PubMed] [Google Scholar]
- Odashima M, Usui S, Takagi H, Hong C, Liu J, Yokota M, Sadoshima J. Inhibition of endogenous Mst1 prevents apoptosis and cardiac dysfunction without affecting cardiac hypertrophy after myocardial infarction. Circ Res. 2007;100:1344–1352. doi: 10.1161/01.RES.0000265846.23485.7a. [DOI] [PubMed] [Google Scholar]
- Pena JR, Wolska BM. Differential effects of isoflurane and ketamine/inactin anesthesia on cAMP and cardiac function in FVB/N mice during basal state and beta-adrenergic stimulation. Basic Res Cardiol. 2005;100:147–153. doi: 10.1007/s00395-004-0503-6. [DOI] [PubMed] [Google Scholar]
- Roth DM, Swaney JS, Dalton ND, Gilpin EA, Ross J., Jr. Impact of anesthesia on cardiac function during echocardiography in mice. Am J Physiol Heart Circ Physiol. 2002;282:H2134–2140. doi: 10.1152/ajpheart.00845.2001. [DOI] [PubMed] [Google Scholar]
- Seidel G, Kurtz V, Krause H, Dierks ML. Patient and consumer information centres according to 65b Social Security Code V--means for high-quality information exchange and counselling. Z Arztl Fortbild Qualitatssich. 2005;99:397–403. [PubMed] [Google Scholar]
- Szczesny G, Veihelmann A, Massberg S, Nolte D, Messmer K. Long-term anaesthesia using inhalatory isoflurane in different strains of mice-the haemodynamic effects. Lab Anim. 2004;38:64–69. doi: 10.1258/00236770460734416. [DOI] [PubMed] [Google Scholar]
- Uechi M, Asai K, Osaka M, Smith A, Sato N, Wagner TE, Ishikawa Y, Hayakawa H, Vatner DE, Shannon RP, Homcy CJ, Vatner SF. Depressed heart rate variability and arterial baroreflex in conscious transgenic mice with overexpression of cardiac Gsα. Circ Res. 1998;82:416–423. doi: 10.1161/01.res.82.4.416. [DOI] [PubMed] [Google Scholar]
- van Nimwegen C, van Eijnsbergen B, Boter J, Mullink JW. A simple device for indirect measurement of blood pressure in mice. Lab Anim. 1973;7:73–84. doi: 10.1258/002367773781005905. [DOI] [PubMed] [Google Scholar]
- Vatner DE, Yan GP, Geng YJ, Asai K, Yun JS, Wagner TE, Ishikawa Y, Bishop SP, Homcy CJ, Vatner SF. Determinants of the cardiomyopathic phenotype in chimeric mice overexpressing cardiac Gsα. Circ Res. 2000;86:802–806. doi: 10.1161/01.res.86.7.802. [DOI] [PubMed] [Google Scholar]
- Vatner SF, Braunwald E. Cardiovascular control mechanisms in the conscious state. N Engl J Med. 1975;293:970–976. doi: 10.1056/NEJM197511062931906. [DOI] [PubMed] [Google Scholar]
- Vatner SF, T. G, Asai K, Shannon RP. Cardiovascular physiology in mice: conscious measurements and effects of anesthesia. In: Hoit BDWR, editor. Cardiovascular Physiology in the Genetically Engineered Mouse. Kluwer Academic Publishers; 2002. pp. 257–275. [Google Scholar]
- Whitesall SE, Hoff JB, Vollmer AP, D’Alecy LG. Comparison of simultaneous measurement of mouse systolic arterial blood pressure by radiotelemetry and tail-cuff methods. Am J Physiol Heart Circ Physiol. 2004;286:H2408–2415. doi: 10.1152/ajpheart.01089.2003. [DOI] [PubMed] [Google Scholar]