Abstract
Mitogen-activated protein kinases (MAPK) integrate signals from numerous receptors and translate these signals into cell functions. MAPKs are critical for immune cell metabolism, migration, production of pro-inflammatory mediators, survival, and differentiation. We provide a concise review of the involvement of MAPK in important cells of the immune system. Certain cell functions e.g. production of pro-inflammatory mediators resolve quickly and may require a transient MAPK activation, other processes such as cell differentiation and long-term survival may require persistent MAPK signal. The persistent MAPK signal is frequently a consequence of positive feedback loops or double negative feedback loops which perpetuate the signal after removal of an external cell stimulus. This self-perpetuated activation of a signaling circuit is a manifestation of its bistability. Bistable systems can exist in “on” and “off” states and both states are stable. We have demonstrated the existence of self-perpetuated activation mechanism for ERK1/2 in bronchial epithelial cells. This sustained activation of ERK1/2 supports long-term survival of these cells and primes them for cytokine transcription. ERK1/2 bistability arises from repetitive stimulation of the cell. The repeated stimulation (e.g. repeated viral infection or repeated allergen exposure) seems to be a common theme in asthma and other chronic illnesses. We thus hypothesize that the self-perpetuated ERK1/2 signal plays an important role in the pathogenesis of asthma.
Introduction to MAPK
Mitogen-activated protein kinases belong to large family of proline-directed serine-threonine protein kinases that play a fundamental role in cellular functions. There are six distinct classes of MAPK—ERK1/2, ERK3/4, ERK5, ERK7/8, JNK1/2/3, and p38 (α/β/γ/δ) MAPK (reviewed in ref. 1-3). JNK1/2/3 and p38 (α/β/γ/δ) MAPK are collectively called stress-activated protein kinases (SAPK). ERK5 is also known as big MAPK (BMK1). Not much is known about ERK3/4 and ERK7/8. The activation of MAPK proceeds through a cascade of upstream molecules in an orderly fashion. The nature of upstream molecules depends upon the inciting trigger (receptor vs non-receptor), cell type and the subcellular location of activation. There is a low level activation of some of the MAPKs, especially that of ERK1/2, likely due to basal signaling and metabolic needs. Some of the MAPKs, e.g. ERK2 and p38α, have non-redundant role during embryonic development. Their null mutation is embryonic lethal (4).
MAPK signaling in the airways from asthmatic patients
The inhibition of MAPK activity via pharmacological or genetic approaches blocks allergic inflammation of airways. Surprisingly, the literature examining the activation of MAPK in asthmatic airways is limited. We thus studied MAPK signaling in the airway biopsy samples from allergic asthmatic patients and healthy controls (5). Asthmatic patients demonstrated increased immunostaining for phospho (p)-ERK1/2, p-p38α/β/γ (p-p38) and pJNK1/2/3 (pJNK) (5). pERK1/2 staining was observed especially in airway epithelium and smooth muscle cells. The phosphorylation of p38 was primarily observed in the basal layer of the columnar epithelium. It is likely that p38 drives basal metabolic processes for this particular cell type. There was significant correlation between clinical severity of asthma and intensity of immunostaining for pERK1/2 and p-p38, and, between pERK1/2 and the number of tissue eosinophils and neutrophils in the airways. p-JNK primarily stained airway smooth muscle cells. p-JNK staining was strongest in healthy control subjects.
The expression of two ERK1/2 inducible proteins JunB and sprouty-2 was also significantly increased in the airway tissue in asthmatic patients (5). JunB is a transcription factor and is a member of the AP-1 complex (6). Many transcriptional outcomes of ERK1/2 activation are mediated by the AP-1 complex (7, 8). JunB drives Th2 cell differentiation (6). Sprouty-2 is a cytosolic adapter protein, which regulates receptor-mediated ERK1/2 activation and plays an important role in bronchial branching during lung development (9). The expression pattern of Sprouty-2 mirrored that of p-ERK1/2 in the biopsy samples, i.e. predominant expression occurred in the airway epithelium (5). Another group studied phosphorylation of p38 in alveolar macrophages obtained from BAL samples of asthmatic patients (10). Lipopolysaccharide (LPS)-induced p38 activity correlated positively with the disease severity and negatively with the corticosteroid sensitivity. Results obtained on human samples are in agreement with data from mouse models of asthma which showed elevated phosphorylation of p38 in lung lysates after allergen challenge (11).
Effect of ERK1/2 and p38 inhibition on chemokine secretion by epithelial cells
The ERK1/2 and p38 MAPK pathways differentially regulate various functions of epithelial cells. Epithelial cells are an important source of chemokines. Both MAPKs regulate the production of RANTES and eotaxin-3 in primary epithelial cells (5). Only p38 but not ERK1/2 regulates IL-8 production. Neither pathway is essential for MCP-1 secretion. Based upon the sensitivity to the MEK1/2 inhibitor PD98059 and response to IL-13/TNFα dual stimulation we have identified three groups of genes in the BEAS-2B epithelial cell line (5). Group 1: Genes that are expressed basally and not modified by MEK1/2 inhibition or IL-13/TNFα stimulation. This group includes the EGF receptor, TSLP, PDGF-β, SCF, and beta actin. Group 2: Genes that are induced by IL-13/TNFα stimulation and further upregulated by MEK1/2 inhibition. They include fibronectin and endothelin-1. Group 3: Genes that are induced by IL-13/TNFα and inhibited by the MEK1/2 inhibitor. This group includes the chemokines eotaxin-3, RANTES, MCP-1, MMP-9, tenascin and MUC5AC. IL-13 induced goblet cell hyperplasia and mucin gene induction has been previously reported (12).
The role of ERK1/2 signaling in epithelial cells has been well documented in experimental models of asthma using mutant mice. IL-13 transgenic mimics many features of asthma (13). IL-13 signals through STAT6 and ERK1/2 pathways. Interestingly, most of the effects of IL-13 in this transgenic model were blocked by a MEK1/2 inhibitor suggesting that the ERK1/2 pathway plays a crucial role in IL-13 induced phenotype in this model (14). This notion was further confirmed in a recent study involving a knock-in of the Q576R gene variant of the IL-4 receptor. The Q576R polymorphism increases the risk of atopy and asthma in humans. The knock-in mutant mice have exaggerated asthma phenotype, which was mediated by ERK1/2 but not STAT6 or AKT pathways (15).
MAPK in endothelial cells
Endothelium controls leukocyte and plasma extravasation and its appropriate function is essential for inflammation. The p38 MAPK plays an important role in endothelial development and function. The endothelial p38 kinase is activated by growth factors (e.g. VEGF), pro-inflammatory cytokines (e.g. TNFα), coagulation factors (e.g. thrombin) and hypoxia (16-20). The p38 MAPK is essential for angiogenesis (21, 22). The p38 pathway is also important for endothelial expression of adhesion molecules (E-selectin, ICAM-1 and VCAM-1) and chemokines (19, 20, 23). The p38 MAPK promotes vasodilatation and increased blood flow through inflamed tissues via upregulation of the nitric oxide (NO) synthetase (24). The p38 kinase regulates endothelial permeability via effects on cytoskeleton and intercellular junctions (16, 17, 23, 25). The activation of p38 leads to increased stress fiber formation, cell contraction and formation of intercellular gaps. Described processes ultimately result in augmented plasma and leukocyte extravasation (20, 23). One of the downstream effectors of p38 is MK2 (26, 27). We have reported that MK2 is critical for endothelial permeability and for endothelial expression of VCAM-1 and other pro-inflammatory molecules (23). MK2 knockout mice do not develop Th2-type airway inflammation despite having an intact systemic Th2 response i.e. normal IL-4 and IgE production (23). The differential effect of MK2 on endothelium and lymphocytes is a reflection of the expression level of the kinase in these cell types (23). MK2 controls endothelium and airway inflammation through NFκB (23). NFκB controls many inflammation-related genes which can be divided into an early- and a late-induced group (28, 29). VCAM-1 is an example of late-induced genes, which requires sustained NFκB activity in the nucleus. We have shown that MK2 extends the duration of NFκB in the nucleus (23). In order to do so, MK2 reduces the amplitude of early NFκB activation and thus downmodulates the strength of a negative feedback in the NFκB pathway. MK2 suppresses the phosphorylation of NFκB by the kinase MSK1 (23). This phosphorylation augments NFκB activity (30). MSK1 is phosphorylated by p38 following its translocation to the nucleus (31, 32). This nuclear translocation of p38 is tightly controlled by MK2, which exports it from the nucleus soon after translocation (33). We have shown that through reduction of NFκB phosphorylation MK2 diminishes the transcription of the early gene - IκBα (23). IκBα protein creates a negative feedback by exporting NFκB from the nucleus. Overall, through effects on NFκB MK2 downmodulates the transcription of early genes (e.g. IκBα, IL-6, Rel) and promotes the transcription of late genes (e.g. VCAM-1, E-selectin, MCP-1) (23). The late gene transcripts encode adhesion molecules and chemokines which are critical for pro-inflammatory functions of endothelium and for development of airway inflammation (23).
MK2-mediated induction of VCAM-1 and MCP-1 in endothelium was subsequently shown to be critical for recruitment of macrophages into the blood vessel wall and atherogenesis in hypercholesterolemic mice (34). Another report demonstrated an important role for MK2 in stress fiber formation and contraction of the pulmonary endothelium in response to hypoxia (25). Thus, MK2 regulates pro-inflammatory functions of the endothelium in various disease conditions.
MAPK in T cells
The MAPK signaling pathways plays a pivotal role in the differentiation of CD4 and CD8 T cells during thymopoiesis. While ERK1/2 is critical for positive selection, p38 and JNK promote negative selection (35, 36). Targeted deletion of ERK2 in CD4 T cells causes a failure to differentiate along the CD4 lineage (37). The pharmacological inhibition of ERK1/2 favors CD8 development (38). The development of T cells in the thymus is an example of a process where opposite cell fates are linked to the activation of unique groups of MAPKs. MAPKs are also important for T cell function in the periphery. ERK1-/- T cells have slightly reduced capacity to proliferate, manifest reduced capacity to produce Th2 cytokines and increased propensity to differentiate into Th1 cells (39). ERK1-/- mice have increased severity of experimental autoimmune encephalitis (40, 41). ERK1/2 favors Th2 differentiation through multiple mechanisms (42, 43). In agreement with the foregoing a MEK1/2 inhibitor inhibits eosinophilic inflammation of the airway in a mouse model of asthma (44).
In contrast to ERK1/2, the JNK and p38 MAPKs favor Th1 differentiation. JNK1 (Mapk8)-/- mice produce increased levels of Th2 cytokines due to increased accumulation of JunB (45). JNK2 (Mapk9)-/- have impaired IFNγ production under Th1 conditions (46). Double knockout mice manifest heightened IL-2 production suggesting that physiologically JNK1 and JNK2 negatively regulate the IL-2 response (47). ERK5-/- is embryonic lethal due its non-redundant role in angiogenesis (48). Targeted null deletion of ERK5 has very little discernible effect on T cell development and function (49).
T cell resistance to the suppressive action of glucocorticoids is a frequent problem in asthma. This reduced sensitivity of T cells is attributed to the exposure to the inflammatory milieu. IL-2, IL-4, co-stimulation via CD28 and bacterial superantigens all play a role. These factors were shown to promote T cell resistance to glucocorticoids through activation of ERK1/2 and p38 MAPK (50-52).
MAPK in eosinophils
MAPK, especially p38 and ERK1/2 play an important role in eosinophil differentiation and activation (reviewed in ref. 53). ERK1/2 and p38 are activated by eosinophil growth factors e.g. IL-5 and by chemokines e.g. eotaxin (54-57). The stimulation with IL-5 and eotaxin results in different kinetics and amplitude of MAPK activation. This may account for differences in biological functions of IL-5 and eotaxin. The differentiation of eosinophils from the committed bone marrow stem cells in response to IL-5 requires the activation of p38 and, to a lesser extent, ERK1/2 (54). ERK1/2 is important for eosinophil priming, degranulation, locomotion, and cytokine and leukotriene production (58, 59). Increased ERK1/2 activation and mediator release have been noted in eosinophils recovered from the airways of asthmatic subjects (60). The results underscore the biological relevance of ERK1/2 activation in vivo. The p38 MAPK contributes to eosinophil degranulation, migration and cytokine production (54).
MAPK in mast cells
ERK1/2, p38, JNK and ERK5 are involved in mast cell activation. ERK1/2 is activated in response to stimulation of mast cells through c-kit or IgE receptors (61). ERK1/2 is important for mast cell differentiation/proliferation, survival and eicosanoid release (62-64). Pak1 is an upstream activator of both ERK1/2 and p38 pathways. Studies using PAK1 knockout mast cells suggest that ERK1/2 is responsible for mast cell proliferation whereas p38 is important for mast cell migration (63). ERK1/2 controls mast cell survival through phosphorylation of BIM (64). The regulation of mast cell mediator release and cytokine production is complex and seems to be receptor-dependent. MEKK2 activates JNK and ERK5. Mast cells from MEKK2-/- mice are unable to produce cytokines such as IL-4, IL-6, and TNFα upon stimulation through the IgE receptor or c-kit (65). Pharmacological inhibitor studies suggest that the release of eicosanoids and GM-CSF production depends upon ERK1/2 and possibly JNK (66). The p38 pathway has been reported to control IL-4 secretion (67). Studies with Fyn deficient mast cells showed an important role for JNK and p38 but not ERK1/2 in eicosanoid and cytokine secretion (68).
MAPK in dendritic cells
The p38 and ERK1/2 MAPK have opposing effects on the ability of dendritic cells (DC) to activate T cells. p38 is critical for upregulation of MHC II and co-stimulatory molecules, for production of IL-12 and for DC migration (69-71). We have shown that the activation of p38 in DCs plays a role in chronic asthma in the mouse model (72). In our model of chronic asthma mice are immunized and, then, repeatedly challenged via intranasal route with three allergens (dust-mite, ragweed and Aspergillus extracts) for 8 weeks. The airway inflammation, hyperreactivity to methacholine and remodeling persist at least for three weeks after the last allergen exposure. Triple allergen-stimulated DCs but not single allergen-treated DCs show strong p38 activation and p38 inhibitor-sensitive induction of MHC II and CD40 molecules. DCs isolated from mice with chronic asthma have enhanced ability to stimulate T cell proliferation. DC functions differentially depend on the strength and the duration of p38 signaling. DC migration seems to be induced by weak and transient p38 signals while strong and persistent signals favor cytokine production (71). The role of ERK1/2 in DC is complex. ERK1/2 antagonizes p38 and induces IL-10 production (71, 73, 74). ERK activation seems to be responsible for maintenance of DC in their immature state and for their tolerogenic effect. However, the role of DC-derived IL-10 in T cell differentiation is likely to be concentration and context dependent. Pulendran and colleagues (75, 76) have shown that ERK1/2, through the induction of c-Fos, inhibits IL-12 production by DC. An increase in IL-10 and decrease in IL-12 favors Th2 differentiation.
MAPK in macrophages
The p38 MAPK is an important regulator of macrophage function. p38α is a dominant variant of p38 in macrophages (77). Studies using pharmacological inhibitors or the dominant negative mutant of p38α indicated a role of the kinase in the expression of a wide range of pro-inflammatory factors e.g. TNFα IL-1β chemokines, inducible nitric oxide synthase (iNOS) or cyclooxygenase 2 (COX2) (77-81). A recent study on mice with conditional inactivation of p38α in macrophages has demonstrated that this kinase may have more limited role (82). p38α is important for production of select chemokines (CXCL1 and CXCL2) and IL-10. IL-10 is linked to anti-inflammatory functions of macrophages. Accordingly, dependent on the type and the duration of a disease p38 stimulates pro- or anti-inflammatory functions of macrophages (82). The macrophage-expressed p38α promotes the development of sodium dodecyl sulfate (SDS)-induced chronic skin inflammation but blocks UVB-induced acute skin inflammation (82). A separate study has shown that ERK1/2 synergizes with p38 in the production of IL-10 (83). They also mediate the induction of arginase I upon stimulation with IL-13 (84). Arginase I is a marker of alternatively activated macrophages and exerts anti-inflammatory activities through suppression of nitric oxide release. Alveolar macrophages have been shown to suppress allergic inflammation in airways in a mouse model of asthma (85, 86). Thus, p38 and ERK1/2 may exert both pro- and anti-inflammatory activities in asthma. The dual nature of p38α in regulation of inflammation may provide explanation for only transient effect of p38α inhibitors in rheumatoid arthritis in humans (87-89).
MAPK in B cells
ERK1/2 kinases are critical for signal transmission from pre-BCR and B cell development (90, 91). Deletion of both ERK1 and ERK2 blocks pre-BCR induced B cell proliferation and the transition of pro-B to pre-B cells. ERK1/2 are also activated downstream of BCR in mature B cells (92). ERK1/2 regulates B cell proliferation and survival (93, 94). In addition to the immunostimulatory signal, which promotes B cell proliferation and immunoglobulin secretion in response to foreign antigens, BCR can also induce an inhibitory signal which endorses B cell tolerance to self-antigens. The inhibitory mechanism involves chronic ERK1/2 activation (95). The self-antigen induced persistent ERK signal blocks the TLR-induced differentiation of B cells into plasma cells (95). This tolerance mechanism may be disrupted in lupus and other autoimmune diseases where CpG motifs in self-DNA are thought to trigger autoantibody production (95). B cell development and mature B cell proliferation is normal upon inactivation of JNK1, JNK2 or p38α genes (96). Normal phenotype is likely due to functional redundancy among stress-activated MAPK. Defects are observed upon simultaneous inactivation of JNK and p38 pathways. Expression of the kinase-dead JNK/p38 activator - MEKK1 in mice blocks the germinal center formation, thymus-dependent antigen-induced B cell proliferation and antibody production (97). p38 and ERK1/2 have opposing effects on the class switch recombination to IgE. While p38 promotes, ERK1/2 blocks this process (98, 99).
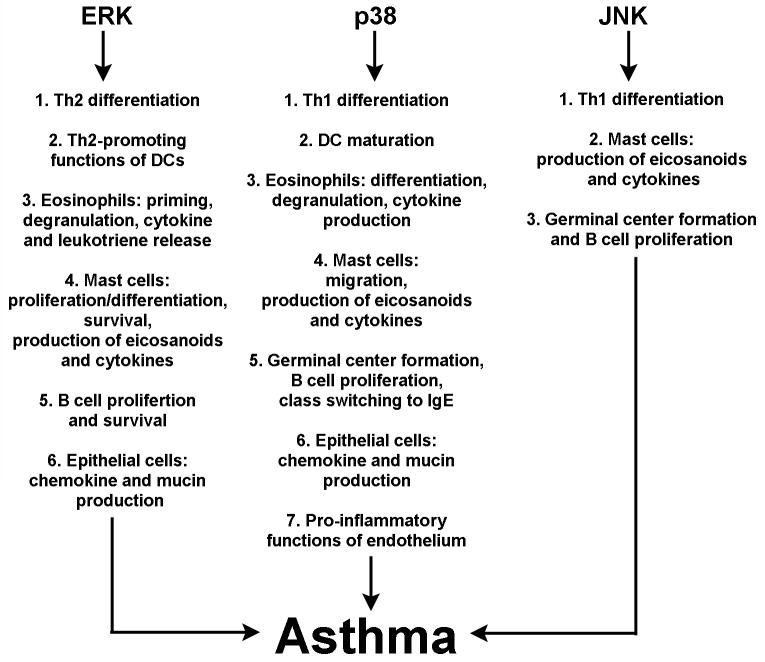
Figure 1 summarizes the role of various MAPK signaling pathways in the pathogenesis of asthma.
Figure 1.
The importance of ERK, p38 and JNK in asthma.
ERK1/2 and signaling bistability
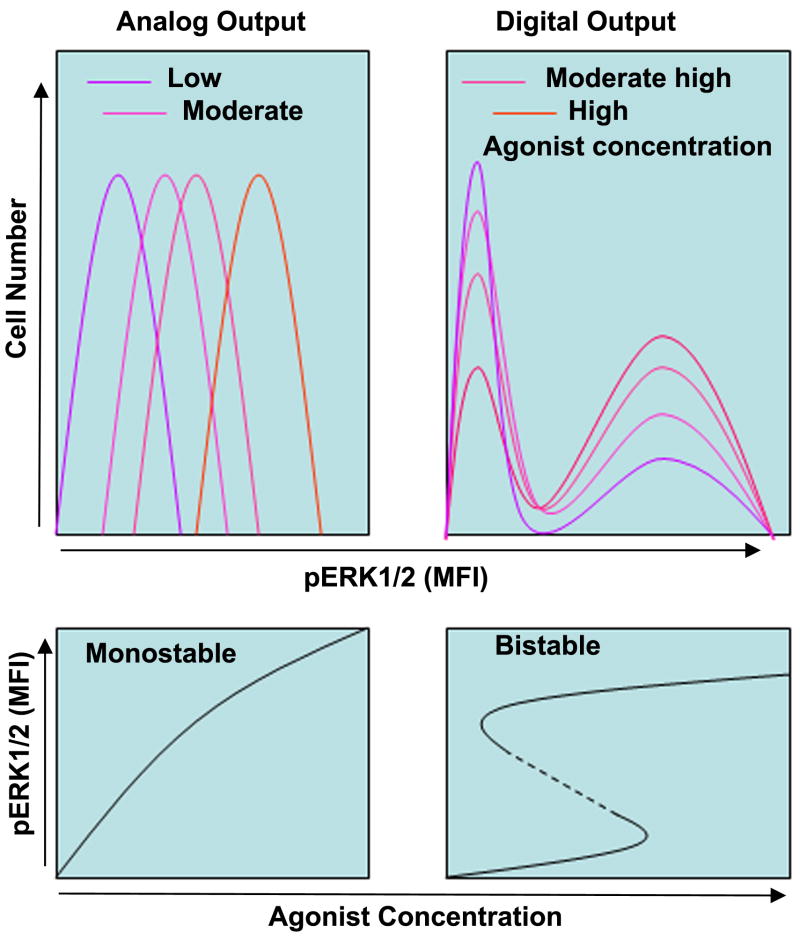
Biological systems respond to environmental cues with two distinct output modes. In an analog output mode the response is linear and proportionate to the strength of the agonist (Figure 2, left bottom panel). In a digital output mode the response is all or none at an individual cell level (Figure 2, right bottom panel). With the increasing strength of the agonist, the number of cells but not signaling intensity per cell increases (Figure 2, top right panel). One of the interesting properties of the ERK1/2 signaling module is its ability to switch back and forth between analog and digital output mode (100-102). Digital systems manifest hysteresis. In physics hysteresis is history dependence of physical systems (103). This frequently applies to magnetic materials -- as the external field with the signal from the microphone is turned off, the little magnetic domains in the tape do not return to their original configuration, thus storing memory (music). Systems that display hysteresis can toggle between two alternative stable steady states. This is known as system bistability. A bistable system exists in two states—an “on” state, and an “off” state. These two states are transitioned through an unstable intermediate state (101, 102). Early examples of biological bistable systems include the lambda phage lysis-lysogeny switch and the hysteretic lac repressor system (104). Lisman in 1985 first suggested that a bistable system could serve as a self-sustaining biochemical memory (105).
Figure 2.
Top panel: A diagrammatic presentation of analog and digital output of pERK1/2 with the increasing concentration of an agonist. Bottom panel, left: A monostable system showing a linear dose-response relationship. Right: A bistable system showing a non-linear hysteretic property with the increasing concentration of the agonist.
Bistability arises from a positive feedback loop or a mutually inhibitory, double-negative feedback loop (102). Ferrell and associates have mathematically shown that when the strength of positive feedback exceeds a certain limit (f=0.08), the system shows hysteresis and becomes bistable (106, 107). In confirmation they have shown that progesterone-induced activation of MAPK and Cdc2 induces a self-sustained activation mechanism in frog oocytes, which is dependent upon a positive feedback loop (107). Disruption of the positive feedback loop at the level of c-Mos (Raf-1) abrogates the signaling memory. ERK1/2 bistability is not unique to oocyte as it has also been studied in mammalian cells. Using a combination of computational and biological experiments Bhalla and Iyengar showed that a single stimulation of fibroblasts with PDGF led to ERK1/2 MAPK activation, whose duration was dependent upon a positive feedback loop through cytoplasmic phospholipase A2 (cPLA2) and protein kinase C (PKC) (108). The duration was shortened in the presence of cPLA2 and PKC inhibitors. The duration of ERK1/2 signal is also determined by the level of phosphatases that are present in the cells. ERK1/2 is regulated by MAPK specific phosphatases as well as non-specific phosphatases. The authors showed that at a low level expression of MKP1 ERK1/2 showed a switch-like “off’ and “on” response, which is typical of digital output from a bistable system. In contrast, at a high level expression of MKP1 the system showed a gradual time and dose-dependent response, typical of analog output from a monostable system. In T cells digital vs analog output is determined by the nature of the agonist used. Superantigen-loaded dendritic cell stimulation results in digital output whereas stimulation with SDF-1 elicits an analog output (109). KSR1, a scaffold protein, plays a crucial role in switching the output from analog to digital. Bistability is not unique for ERK1/2. Its presence has also been demonstrated in other MAPK family members (110) as well as upstream activators—Ras (111) and Sos (112). Since many biological processes have built-in negative and positive feedback loops, they all could potentially function as bistable systems.
As mentioned, bistability can function as a biochemical memory. For this reason, the ERK1/2 signaling pathway has been studied in the CNS for its potential role in memory. Indeed, ERK1/2 and one of its downstream effector CREB have been shown to play a critical role in neuronal plasticity, long-term potentiation (LTP) and memory formation (reviewed in ref. 113 & 114). Mice with functional ERK1/2 deficiency have impaired protein synthesis as well as selective defects in LTP and memory consolidation (115-117). The definition of memory is the retention of an acquired (learned) signal. In neuronal networks, the result of this signal is expression of an activation path whose physiological manifestation is a particular pattern of neural firing. System memory in organs outside the central nervous system (CNS) is rarely invoked and studied. There are memory T cells and B cells but their mechanism is different from that in the CNS. T and B cell memory is maintained by the persistence of antigen-specific cell clones and can be evoked by a specific antigen during a later encounter. Whether or not these cells utilize a signaling memory is unknown.
The CNS memory is classified into episodic memory and semantic memory (118). Episodic memory is the explicit memory of events and context, which includes time, space, and associated emotions. Semantic memory is knowledge independent of context. It is a form of declarative memory that requires repetition for consolidation (119). The repetition of the memory-forming event for consolidation is an important concept. Indeed, when one carefully examines the development of a chronic disease such as asthma, repetition of the initial inciting trigger seems to be a common finding. Many children develop wheezing during a viral infection. This usually resolves without sequelae. However, if the wheezing recurs on subsequent infections, this recurrence usually heralds the development of chronic asthma (120, 121). Once developed the affected children begin to experience wheezing and other asthma symptoms when exposed to diverse environmental stimuli without regard to the viral infection. This suggests that repetition of the inciting trigger leads to the formation of a system memory, which then drives the process in a self-perpetuated manner. We applied this logic of repeated stimulation inducing system memory in signaling studies. We asked if repeated stimulation would lead to the development of a self-perpetuated mechanism of activation of ERK1/2 (formation of bistability). We tested this hypothesis using airway epithelial cells.
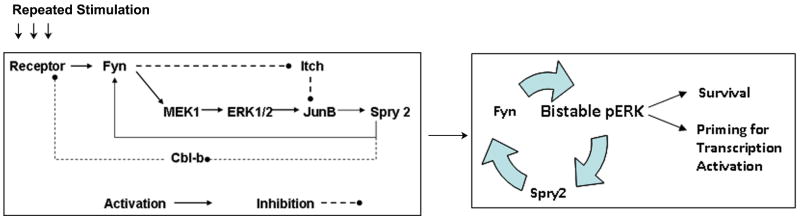
A single stimulation of epithelial cells with cytokines and growth factors (IL-4, IL-6, IL-13, eotaxin, and EGF) causes rapid ERK1/2 activation, which returns to baseline in 24 hr. Interestingly, repeated stimulation on 3 consecutive days leads to sustained activation of ERK1/2 but not JNK, p38 or STAT6 (122). The ERK1/2 activation lasts for 3-7 days and depends upon a positive feedback mechanism involving spry 2 and Fyn. Repeated stimulation leads to increasing expression of the adapter protein Spry 2, which directly activates Fyn kinase. The activation of Fyn creates a positive feedback loop by further activating ERK1/2 (Figure 3) (122). It also creates a double negative feedback loop by phosphorylating and inhibiting the E3 ubiquitin ligase Itch (123). Itch physiologically ubiquitinates JunB, a downstream ERK-inducible transcription factor (124). By inhibiting Itch Fyn stabilizes JunB and further augments spry 2 production. Spry 2 is known to inhibit another E3 ubiquitin ligase Cbl, which is responsible for receptor degradation (125-127). By inhibiting Cbl spry 2 prolongs receptor signaling. This is another example of a double negative feedback loop. Thus, there are multiple positive and double negative feedback loops that can perpetuate ERK1/2 signaling in the cell. Overexpression of spry 2 induces and its genetic deletion abrogates ERK1/2 bistability. The sustained pERK1/2 is excluded from the nucleus and partially compartmentalized to the Rab5+ endosomal compartment. Consequently, many genes which are induced during a single stimulation are silent upon repeated stimulation. The epigenetic regulation may also be involved as demonstrated in the repeated stimulation model of macrophages using LPS (128). We have shown that there is only a small group of genes which remains active in repeatedly stimulated epithelial cells and this group includes regulators of MAPK signaling (122). We hypothesize that these MAPK regulators contribute to sustained ERK1/2 signal.
Figure 3.
A schematic presentation of positive and double negative feedback loops leading to ERK1/2 bistability
What are the benefits of sustained but compartmentalized ERK1/2 activation in epithelial cells? We have shown that cells with this sustained endosomal pERK1/2 manifest resistance against growth factor withdrawal-induced cell death (122). The survival pathway likely involves endosomal proteins as cells with reduced amounts of Rab5 demonstrate increased apoptosis. Cells with sustained endosomal pERK1/2 are also primed for heightened cytokine production. Prolonged cell survival and cellular priming are two important features of asthma. We examined the biological relevance for ERK1/2 bistability in asthma (122). Epithelial cells from human asthma and from the mouse model of chronic asthma manifest increased pERK1/2. A significant part of this pERK1/2 is associated with Rab5+ endosomes. The increase in pERK1/2 is associated with a simultaneous increase in spry 2 expression in these tissues. The results from this study suggest that the ERK1/2 pathway develops bistability upon repeated stimulation. We speculate that ERK1/2 bistability serves as a signaling memory for epithelial priming of the immune system in chronic asthma.
Conclusions and future perspective
One of the key molecules that played a critical role in ERK1/2 bistability was the adapter protein spry 2. Depending upon the structure (presence of binding sites for agonists and antagonists), concentration and subcellular localization the adapter/scaffold proteins can generate a linear (analog) or a non-linear (digital) response from a system (109, 111, and 112). Engineered modification of adapters and their application in simple biological systems such as yeasts have validated this point (129, 130). Interestingly, this principle applies not only to cytosolic adapters but also transcription factors and repressors. For example, it has been shown that the transcriptional output is analog with increasing concentrations and DNA binding of the transcription factor NFκB (131). On the other hand, if the DNA binding sites show cooperativity, which is an adapter/scaffold-like behavior, then the output is digital. Adapters/scaffold proteins that bind both agonists and antagonists have the potential to drive a system from analog to digital and generate system memory. Interestingly, most signaling pathways employ adapter proteins along their activation pathway. Adapter proteins are seldom pathway-specific. So, the use of non-specific adapter proteins allows versatility (analog vs. digital), robustness and memory generation, all of which serve to make the system adaptive—the principal tenet of evolution.
Acknowledgments
This work was supported by NIH grants RO1 AI68088, AI059719 and PPG HL36577.
References
- 1.Roux PP, Blenis J. ERK and p38 MAPK-activated protein kinases: a family of protein kinases with diverse biological functions. Microbiol Mol Biol Rev. 2004;68:320–44. doi: 10.1128/MMBR.68.2.320-344.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Rincón M, Davis RJ. Regulation of the immune response by stress-activated protein kinases. Immunol Rev. 2009;228:212–24. doi: 10.1111/j.1600-065X.2008.00744.x. [DOI] [PubMed] [Google Scholar]
- 3.Brown MD, Sacks DB. Protein scaffolds in MAP kinase signalling. Cell Signal. 2009;21(4):462–9. doi: 10.1016/j.cellsig.2008.11.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Yao Y, Li W, Wu J, Germann UA, Su MS, Kuida K, Boucher DM. Extracellular signal-regulated kinase 2 is necessary for mesoderm differentiation. Proc Natl Acad Sci U S A. 2003;100:12759–64. doi: 10.1073/pnas.2134254100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Liu W, Liang Q, Balzar S, Wenzel S, Gorska M, Alam R. Cell-specific activation profile of extracellular signal-regulated kinase 1/2, Jun N-terminal kinase, and p38 mitogen-activated protein kinases in asthmatic airways. J Allergy Clin Immunol. 2008;121:893–902.e2. doi: 10.1016/j.jaci.2008.02.004. [DOI] [PubMed] [Google Scholar]
- 6.Li B, Tournier C, Davis RJ, Flavell RA. Regulation of IL-4 expression by the transcription factor JunB during T helper cell differentiation. EMBO J. 1999;18:420–32. doi: 10.1093/emboj/18.2.420. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Nakakuki T, Birtwistle MR, Saeki Y, Yumoto N, Ide K, Nagashima T, Brusch L, Ogunnaike BA, Okada-Hatakeyama M, Kholodenko BN. Ligand-specific c-Fos expression emerges from the spatiotemporal control of ErbB network dynamics. Cell. 2010;141:884–96. doi: 10.1016/j.cell.2010.03.054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Eferl R, Wagner EF. AP-1: a double-edged sword in tumorigenesis. Nat Rev Cancer. 2003;3:859–68. doi: 10.1038/nrc1209. [DOI] [PubMed] [Google Scholar]
- 9.Tefft JD, Lee M, Smith S, Leinwand M, Zhao J, Bringas P, Jr, Crowe DL, Warburton D. Conserved function of mSpry-2, a murine homolog of Drosophila sprouty, which negatively modulates respiratory organogenesis. Curr Biol. 1999;9:219–22. doi: 10.1016/s0960-9822(99)80094-3. [DOI] [PubMed] [Google Scholar]
- 10.Bhavsar P, Khorasani N, Hew M, Johnson M, Chung KF. Effect of p38 MAPK inhibition on corticosteroid suppression of cytokine release in severe asthma. Eur Respir J. 2010;35:750–6. doi: 10.1183/09031936.00071309. [DOI] [PubMed] [Google Scholar]
- 11.Nath P, Leung SY, Williams A, Noble A, Chakravarty SD, Luedtke GR, Medicherla S, Higgins LS, Protter A, Chung KF. Importance of p38 mitogen-activated protein kinase pathway in allergic airway remodelling and bronchial hyperresponsiveness. Eur J Pharmacol. 2006;544:160–7. doi: 10.1016/j.ejphar.2006.06.031. [DOI] [PubMed] [Google Scholar]
- 12.Atherton HC, Jones G, Danahay H. IL-13-induced changes in the goblet cell density of human bronchial epithelial cell cultures: MAP kinase and phosphatidylinositol 3-kinase regulation. Am J Physiol Lung Cell Mol Physiol. 2003;285:L730–9. doi: 10.1152/ajplung.00089.2003. [DOI] [PubMed] [Google Scholar]
- 13.Zhu Z, Homer RJ, Wang Z, Chen Q, Geba GP, Wang J, Zhang Y, Elias JA. Pulmonary expression of interleukin-13 causes inflammation, mucus hypersecretion, subepithelial fibrosis, physiologic abnormalities, and eotaxin production. J Clin Invest. 1999;103:779–88. doi: 10.1172/JCI5909. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Lee PJ, Zhang X, Shan P, Ma B, Lee CG, Homer RJ, Zhu Z, Rincon M, Mossman BT, Elias JA. ERK1/2 mitogen-activated protein kinase selectively mediates IL-13-induced lung inflammation and remodeling in vivo. J Clin Invest. 2006;116:163–73. doi: 10.1172/JCI25711. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Tachdjian R, Mathias C, Al Khatib S, Bryce PJ, Kim HS, Blaeser F, O'Connor BD, Rzymkiewicz D, Chen A, Holtzman MJ, Hershey GK, Garn H, Harb H, Renz H, Oettgen HC, Chatila TA. Pathogenicity of a disease-associated human IL-4 receptor allele in experimental asthma. J Exp Med. 2009;206:2191–204. doi: 10.1084/jem.20091480. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Rousseau S, Houle F, Landry J, Huot J. p38 MAP kinase activation by vascular endothelial growth factor mediates actin reorganization and cell migration in human endothelial cells. Oncogene. 1997;15:2169–77. doi: 10.1038/sj.onc.1201380. [DOI] [PubMed] [Google Scholar]
- 17.Park JH, Okayama N, Gute D, Krsmanovic A, Battarbee H, Alexander JS. Hypoxia/aglycemia increases endothelial permeability: role of second messengers and cytoskeleton. Am J Physiol. 1999;277:C1066–74. doi: 10.1152/ajpcell.1999.277.6.C1066. [DOI] [PubMed] [Google Scholar]
- 18.Huang H, Rose JL, Hoyt DG. p38 Mitogen-activated protein kinase mediates synergistic induction of inducible nitric-oxide synthase by lipopolysaccharide and interferon-gamma through signal transducer and activator of transcription 1 Ser727 phosphorylation in murine aortic endothelial cells. Mol Pharmacol. 2004;66:302–11. doi: 10.1124/mol.66.2.302. [DOI] [PubMed] [Google Scholar]
- 19.Borbiev T, Birukova A, Liu F, Nurmukhambetova S, Gerthoffer WT, Garcia JG, Verin AD. p38 MAP kinase-dependent regulation of endothelial cell permeability. Am J Physiol Lung Cell Mol Physiol. 2004;287:L911–8. doi: 10.1152/ajplung.00372.2003. [DOI] [PubMed] [Google Scholar]
- 20.Roussel L, Houle F, Chan C, Yao Y, Bérubé J, Olivenstein R, Martin JG, Huot J, Hamid Q, Ferri L, Rousseau S. IL-17 promotes p38 MAPK-dependent endothelial activation enhancing neutrophil recruitment to sites of inflammation. J Immunol. 2010;184:4531–7. doi: 10.4049/jimmunol.0903162. [DOI] [PubMed] [Google Scholar]
- 21.Mudgett JS, Ding J, Guh-Siesel L, Chartrain NA, Yang L, Gopal S, Shen MM. Essential role for p38alpha mitogen-activated protein kinase in placental angiogenesis. Proc Natl Acad Sci U S A. 2000;97:10454–9. doi: 10.1073/pnas.180316397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Yang J, Boerm M, McCarty M, Bucana C, Fidler IJ, Zhuang Y, Su B. Mekk3 is essential for early embryonic cardiovascular development. Nat Genet. 2000;24:309–13. doi: 10.1038/73550. [DOI] [PubMed] [Google Scholar]
- 23.Gorska MM, Liang Q, Stafford SJ, Goplen N, Dharajiya N, Guo L, Sur S, Gaestel M, Alam R. MK2 controls the level of negative feedback in the NF-kappaB pathway and is essential for vascular permeability and airway inflammation. J Exp Med. 2007;204:1637–52. doi: 10.1084/jem.20062621. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Schett G, Zwerina J, Firestein G. The p38 mitogen-activated protein kinase(MAPK) pathway in rheumatoid arthritis. Ann Rheum Dis. 2008;67:909–16. doi: 10.1136/ard.2007.074278. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Kayyali US, Pennella CM, Trujillo C, Villa O, Gaestel M, Hassoun PM. Cytoskeletal changes in hypoxic pulmonary endothelial cells are dependent on MAPK-activated protein kinase MK2. J Biol Chem. 2002;277:42596–602. doi: 10.1074/jbc.M205863200. [DOI] [PubMed] [Google Scholar]
- 26.Ben-Levy R, Leighton IA, Doza YN, Attwood P, Morrice N, Marshall CJ, Cohen P. Identification of novel phosphorylation sites required for activation of MAPKAP kinase-2. EMBO J. 1995;14:5920–30. doi: 10.1002/j.1460-2075.1995.tb00280.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Kotlyarov A, Neininger A, Schubert C, Eckert R, Birchmeier C, Volk HD, Gaestel M. MAPKAP kinase 2 is essential for LPS-induced TNF-alpha biosynthesis. Nat Cell Biol. 1999;1:94–7. doi: 10.1038/10061. [DOI] [PubMed] [Google Scholar]
- 28.Hoffmann A, Levchenko A, Scott ML, Baltimore D. The IkappaB-NF-kappaB signaling module: temporal control and selective gene activation. Science. 2002;298:1241–5. doi: 10.1126/science.1071914. [DOI] [PubMed] [Google Scholar]
- 29.Tian B, Nowak DE, Brasier AR. A TNF-induced gene expression program under oscillatory NF-kappaB control. BMC Genomics. 2005;6:137. doi: 10.1186/1471-2164-6-137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Vermeulen L, De Wilde G, Van Damme P, Vanden Berghe W, Haegeman G. Transcriptional activation of the NF-kappaB p65 subunit by mitogen- and stress-activated protein kinase-1 (MSK1) EMBO J. 2003;22:1313–1324. doi: 10.1093/emboj/cdg139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.McCoy CE, Campbell DG, Deak M, Bloomberg GB, Arthur JS. MSK1 activity is controlled by multiple phosphorylation sites. Biochem J. 2005;387:507–17. doi: 10.1042/BJ20041501. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Deak M, Clifton AD, Lucocq LM, Alessi DR. Mitogen- and stress-activated protein kinase-1 (MSK1) is directly activated by MAPK and SAPK2/p38, and may mediate activation of CREB. EMBO J. 1998;17:4426–41. doi: 10.1093/emboj/17.15.4426. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Ben-Levy R, Hooper S, Wilson R, Paterson HF, Marshall CJ. Nuclear export of the stress-activated protein kinase p38 mediated by its substrate MAPKAP kinase-2. Curr Biol. 1998;8:1049–57. doi: 10.1016/s0960-9822(98)70442-7. [DOI] [PubMed] [Google Scholar]
- 34.Jagavelu K, Tietge UJ, Gaestel M, Drexler H, Schieffer B, Bavendiek U. Systemic deficiency of the MAP kinase-activated protein kinase 2 reduces atherosclerosis in hypercholesterolemic mice. Circ Res. 2007;101:1104–12. doi: 10.1161/CIRCRESAHA.107.156075. [DOI] [PubMed] [Google Scholar]
- 35.Sugawara T, Moriguchi T, Nishida E, Takahama Y. Differential roles of ERK and p38 MAP kinase pathways in positive and negative selection of T lymphocytes. Immunity. 1998;9:565–74. doi: 10.1016/s1074-7613(00)80639-1. [DOI] [PubMed] [Google Scholar]
- 36.Werlen G, Hausmann B, Palmer E. A motif in the alphabeta T-cell receptor controls positive selection by modulating ERK activity. Nature. 2000;406:422–6. doi: 10.1038/35019094. [DOI] [PubMed] [Google Scholar]
- 37.Fischer AM, Katayama CD, Pagès G, Pouysségur J, Hedrick SM. The role of erk1 and erk2 in multiple stages of T cell development. Immunity. 2005;23:431–43. doi: 10.1016/j.immuni.2005.08.013. [DOI] [PubMed] [Google Scholar]
- 38.Sharp LL, Schwarz DA, Bott CM, Marshall CJ, Hedrick SM. The influence of the MAPK pathway on T cell lineage commitment. Immunity. 1997;7:609–18. doi: 10.1016/s1074-7613(00)80382-9. [DOI] [PubMed] [Google Scholar]
- 39.Pagès G, Guérin S, Grall D, Bonino F, Smith A, Anjuere F, Auberger P, Pouysségur J. Defective thymocyte maturation in p44 MAP kinase (Erk 1) knockout mice. Science. 1999;286:1374–7. doi: 10.1126/science.286.5443.1374. [DOI] [PubMed] [Google Scholar]
- 40.Nekrasova T, Shive C, Gao Y, Kawamura K, Guardia R, Landreth G, Forsthuber TG. ERK1-deficient mice show normal T cell effector function and are highly susceptible to experimental autoimmune encephalomyelitis. J Immunol. 2005;175:2374–80. doi: 10.4049/jimmunol.175.4.2374. [DOI] [PubMed] [Google Scholar]
- 41.Agrawal A, Dillon S, Denning TL, Pulendran B. ERK1-/- mice exhibit Th1 cell polarization and increased susceptibility to experimental autoimmune encephalomyelitis. J Immunol. 2006;176:5788–96. doi: 10.4049/jimmunol.176.10.5788. [DOI] [PubMed] [Google Scholar]
- 42.Nakayama T, Yamashita M. The TCR-mediated signaling pathways that control the direction of helper T cell differentiation. Semin Immunol. 2010 May 18; doi: 10.1016/j.smim.2010.04.010. Epub ahead of print. [DOI] [PubMed] [Google Scholar]
- 43.Yamashita M, Kimura M, Kubo M, Shimizu C, Tada T, Perlmutter RM, Nakayama T. T cell antigen receptor-mediated activation of the Ras/mitogen-activated protein kinase pathway controls interleukin 4 receptor function and type-2 helper T cell differentiation. Proc Natl Acad Sci USA. 1999;96:1024–9. doi: 10.1073/pnas.96.3.1024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Duan W, Chan JH, Wong CH, Leung BP, Wong WS. Anti-inflammatory effects of mitogen-activated protein kinase kinase inhibitor U0126 in an asthma mouse model. J Immunol. 2004;172:7053–9. doi: 10.4049/jimmunol.172.11.7053. [DOI] [PubMed] [Google Scholar]
- 45.Dong C, Yang DD, Wysk M, Whitmarsh AJ, Davis RJ, Flavell RA. Defective T cell differentiation in the absence of Jnk1. Science. 1998;282:2092–5. doi: 10.1126/science.282.5396.2092. [DOI] [PubMed] [Google Scholar]
- 46.Yang DD, Conze D, Whitmarsh AJ, Barrett T, Davis RJ, Rincón M, Flavell RA. Differentiation of CD4+ T cells to Th1 cells requires MAP kinase JNK2. Immunity. 1998;9:575–85. doi: 10.1016/s1074-7613(00)80640-8. [DOI] [PubMed] [Google Scholar]
- 47.Dong C, Yang DD, Tournier C, Whitmarsh AJ, Xu J, Davis RJ, Flavell RA. JNK is required for effector T-cell function but not for T-cell activation. Nature. 2000;405:91–4. doi: 10.1038/35011091. [DOI] [PubMed] [Google Scholar]
- 48.Sohn SJ, Sarvis BK, Cado D, Winoto A. ERK5 MAPK regulates embryonic angiogenesis and acts as a hypoxia-sensitive repressor of vascular endothelial growth factor expression. J Biol Chem. 2002;277:43344–51. doi: 10.1074/jbc.M207573200. [DOI] [PubMed] [Google Scholar]
- 49.Ananieva O, Macdonald A, Wang X, McCoy CE, McIlrath J, Tournier C, Arthur JS. ERK5 regulation in naïve T-cell activation and survival. Eur J Immunol. 2008;38:2534–47. doi: 10.1002/eji.200737867. [DOI] [PubMed] [Google Scholar]
- 50.Tsitoura DC, Rothman PB. Enhancement of MEK/ERK signaling promotes glucocorticoid resistance in CD4+ T cells. J Clin Invest. 2004;113:619–27. doi: 10.1172/JCI18975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Li LB, Goleva E, Hall CF, Ou LS, Leung DY. Superantigen-inducedcorticosteroid resistance of human T cells occurs through activation of the mitogen-activated protein kinase kinase/extracellular signal-regulated kinase(MEK-ERK) pathway. J Allergy Clin Immunol. 2004;114:1059–69. doi: 10.1016/j.jaci.2004.08.005. [DOI] [PubMed] [Google Scholar]
- 52.Goleva E, Li LB, Leung DY. IFN-gamma reverses IL-2- and IL-4-mediated T-cell steroid resistance. Am J Respir Cell Mol Biol. 2009;40:223–30. doi: 10.1165/rcmb.2007-0327OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Adachi T, Alam R. The mechanism of IL-5 signal transduction. Am J Physiol. 1998;275(3 Pt 1):C623–33. doi: 10.1152/ajpcell.1998.275.3.C623. [DOI] [PubMed] [Google Scholar]
- 54.Adachi T, Choudhury BK, Stafford S, Sur S, Alam R. The differential role of extracellular signal-regulated kinases and p38 mitogen-activated protein kinase in eosinophil functions. J Immunol. 2000;165:2198–204. doi: 10.4049/jimmunol.165.4.2198. [DOI] [PubMed] [Google Scholar]
- 55.Pazdrak K, Schreiber D, Forsythe P, Justement L, Alam R. The intracellular signal transduction mechanism of interleukin 5 in eosinophils: the involvement of lyn tyrosine kinase and the Ras-Raf-1-MEK-microtubule-associated protein kinase pathway. J Exp Med. 1995;181:1827–34. doi: 10.1084/jem.181.5.1827. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Kampen GT, Stafford S, Adachi T, Jinquan T, Quan S, Grant JA, Skov PS, Poulsen LK, Alam R. Eotaxin induces degranulation and chemotaxis of eosinophils through the activation of ERK2 and p38 mitogen-activated protein kinases. Blood. 2000;95:1911–7. [PubMed] [Google Scholar]
- 57.Goplen N, Gorska MM, Stafford SJ, Rozario S, Guo L, Liang Q, Alam R. A phosphosite screen identifies autocrine TGF-beta-driven activation of protein kinase R as a survival-limiting factor for eosinophils. J Immunol. 2008;180:4256–64. doi: 10.4049/jimmunol.180.6.4256. [DOI] [PubMed] [Google Scholar]
- 58.Pazdrak K, Olszewska-Pazdrak B, Stafford S, Garofalo RP, Alam R. Lyn, Jak2, and Raf-1 kinases are critical for the antiapoptotic effect of interleukin 5, whereas only Raf-1 kinase is essential for eosinophil activation and degranulation. J Exp Med. 1998;188:421–9. doi: 10.1084/jem.188.3.421. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Bates ME, Green VL, Bertics PJ. ERK1 and ERK2 activation by chemotactic factors in human eosinophils is interleukin 5-dependent and contributes to leukotriene C(4) biosynthesis. J Biol Chem. 2000;275:10968–75. doi: 10.1074/jbc.275.15.10968. [DOI] [PubMed] [Google Scholar]
- 60.Bates ME, Sedgwick JB, Zhu Y, Liu LY, Heuser RG, Jarjour NN, Kita H, Bertics PJ. Human airway eosinophils respond to chemoattractants with greater eosinophil-derived neurotoxin release, adherence to fibronectin, and activation of the Ras-ERK pathway when compared with blood eosinophils. J Immunol. 2010;184:7125–33. doi: 10.4049/jimmunol.0900634. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Tsai M, Chen RH, Tam SY, Blenis J, Galli SJ. Activation of MAP kinases, pp90rsk and pp70-S6 kinases in mouse mast cells by signaling through the c-kit receptor tyrosine kinase or Fc epsilon RI: rapamycin inhibits activation of pp70-S6 kinase and proliferation in mouse mast cells. Eur J Immunol. 1993;23:3286–91. doi: 10.1002/eji.1830231234. [DOI] [PubMed] [Google Scholar]
- 62.Hirasawa N, Santini F, Beaven MA. Activation of the mitogen-activated protein kinase/cytosolic phospholipase A2 pathway in a rat mast cell line. Indications of different pathways for release of arachidonic acid and secretory granules. J Immunol. 1995;154:5391–402. [PubMed] [Google Scholar]
- 63.McDaniel AS, Allen JD, Park SJ, Jaffer ZM, Michels EG, Burgin SJ, Chen S, Bessler WK, Hofmann C, Ingram DA, Chernoff J, Clapp DW. Pak1 regulates multiple c-Kit mediated Ras-MAPK gain-in-function phenotypes in Nf1+/- mast cells. Blood. 2008;112:4646–54. doi: 10.1182/blood-2008-04-155085. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Möller C, Alfredsson J, Engström M, Wootz H, Xiang Z, Lennartsson J, Jönsson JI, Nilsson G. Stem cell factor promotes mast cell survival via inactivation of FOXO3a-mediated transcriptional induction and MEK-regulated phosphorylation of the proapoptotic protein Bim. Blood. 2005;106:1330–6. doi: 10.1182/blood-2004-12-4792. [DOI] [PubMed] [Google Scholar]
- 65.Garrington TP, Ishizuka T, Papst PJ, Chayama K, Webb S, Yujiri T, Sun W, Sather S, Russell DM, Gibson SB, Keller G, Gelfand EW, Johnson GL. MEKK2 gene disruption causes loss of cytokine production in response to IgE and c-Kit ligand stimulation of ES cell-derived mast cells. EMBO J. 2000;19:5387–95. doi: 10.1093/emboj/19.20.5387. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Kimata M, Inagaki N, Kato T, Miura T, Serizawa I, Nagai H. Roles of mitogen-activated protein kinase pathways for mediator release from human cultured mast cells. Biochem Pharmacol. 2000;60:589–94. doi: 10.1016/s0006-2952(00)00354-3. [DOI] [PubMed] [Google Scholar]
- 67.Frossi B, Rivera J, Hirsch E, Pucillo C. Selective activation of Fyn/PI3K and p38 MAPK regulates IL-4 production in BMMC under nontoxic stress condition. J Immunol. 2007;178:2549–55. doi: 10.4049/jimmunol.178.4.2549. [DOI] [PubMed] [Google Scholar]
- 68.Gomez G, Gonzalez-Espinosa C, Odom S, Baez G, Cid ME, Ryan JJ, Rivera J. Impaired FcepsilonRI-dependent gene expression and defective eicosanoid and cytokine production as a consequence of Fyn deficiency in mast cells. J Immunol. 2005;175:7602–10. doi: 10.4049/jimmunol.175.11.7602. [DOI] [PubMed] [Google Scholar]
- 69.Arrighi JF, Rebsamen M, Rousset F, Kindler V, Hauser C. A critical role for p38 mitogen-activated protein kinase in the maturation of human blood-derived dendritic cells induced by lipopolysaccharide, TNF-alpha, and contact sensitizers. J Immunol. 2001;166:3837–45. doi: 10.4049/jimmunol.166.6.3837. [DOI] [PubMed] [Google Scholar]
- 70.Puig-Kröger A, Relloso M, Fernández-Capetillo O, Zubiaga A, Silva A, Bernabéu C, Corbí AL. Extracellular signal-regulated protein kinase signaling pathway negatively regulates the phenotypic and functional maturation of monocyte-derived human dendritic cells. Blood. 2001;98:2175–82. doi: 10.1182/blood.v98.7.2175. [DOI] [PubMed] [Google Scholar]
- 71.Luft T, Maraskovsky E, Schnurr M, Knebel K, Kirsch M, Görner M, Skoda R, Ho AD, Nawroth P, Bierhaus A. Tuning the volume of the immune response: strength and persistence of stimulation determine migration and cytokine secretion of dendritic cells. Blood. 2004;104:1066–74. doi: 10.1182/blood-2003-12-4146. [DOI] [PubMed] [Google Scholar]
- 72.Goplen N, Karim MZ, Liang Q, Gorska MM, Rozario S, Guo L, Alam R. Combined sensitization of mice to extracts of dust mite, ragweed, and Aspergillus species breaks through tolerance and establishes chronic features of asthma. J Allergy Clin Immunol. 2009;123:925–32.e11. doi: 10.1016/j.jaci.2009.02.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Xia CQ, Peng R, Beato F, Clare-Salzler MJ. Dexamethasone induces IL-10-producing monocyte-derived dendritic cells with durable immaturity. Scand J Immunol. 2005;62:45–54. doi: 10.1111/j.1365-3083.2005.01640.x. [DOI] [PubMed] [Google Scholar]
- 74.Qian C, Jiang X, An H, Yu Y, Guo Z, Liu S, Xu H, Cao X. TLR agonists promote ERK-mediated preferential IL-10 production of regulatory dendritic cells(diffDCs), leading to NK-cell activation. Blood. 2006;108:2307–15. doi: 10.1182/blood-2006-03-005595. [DOI] [PubMed] [Google Scholar]
- 75.Dillon S, Agrawal A, Van Dyke T, Landreth G, McCauley L, Koh A, Maliszewski C, Akira S, Pulendran B. A Toll-like receptor 2 ligand stimulates Th2 responses in vivo, via induction of extracellular signal-regulated kinase mitogen-activated protein kinase and c-Fos in dendritic cells. J Immunol. 2004;172:4733–43. doi: 10.4049/jimmunol.172.8.4733. [DOI] [PubMed] [Google Scholar]
- 76.Dillon S, Agrawal S, Banerjee K, Letterio J, Denning TL, Oswald-Richter K, Kasprowicz DJ, Kellar K, Pare J, van Dyke T, Ziegler S, Unutmaz D, Pulendran B. Yeast zymosan, a stimulus for TLR2 and dectin-1, induces regulatory antigen-presenting cells and immunological tolerance. J Clin Invest. 2006;116:916–28. doi: 10.1172/JCI27203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Hale KK, Trollinger D, Rihanek M, Manthey CL. Differential expression and activation of p38 mitogen-activated protein kinase alpha, beta, gamma, and delta in inflammatory cell lineages. J Immunol. 1999;162:4246–52. [PubMed] [Google Scholar]
- 78.Baldassare JJ, Bi Y, Bellone CJ. The role of p38 mitogen-activated protein kinase in IL-1 beta transcription. J Immunol. 1999;162:5367–73. [PubMed] [Google Scholar]
- 79.Ajizian SJ, English BK, Meals EA. Specific inhibitors of p38 and extracellular signal-regulated kinase mitogen-activated protein kinase pathways block inducible nitric oxide synthase and tumor necrosis factor accumulation in murine macrophages stimulated with lipopolysaccharide and interferon-gamma. J Infect Dis. 1999;179:939–44. doi: 10.1086/314659. [DOI] [PubMed] [Google Scholar]
- 80.Chen C, Chen YH, Lin WW. Involvement of p38 mitogen-activated protein kinase in lipopolysaccharide-induced iNOS and COX-2 expression in J774 macrophages. Immunology. 1999;97:124–9. doi: 10.1046/j.1365-2567.1999.00747.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Yeo SJ, Gravis D, Yoon JG, Yi AK. Myeloid differentiation factor 88-dependent transcriptional regulation of cyclooxygenase-2 expression by CpG DNA: role of NF-kappaB and p38. J Biol Chem. 2003;278:22563–73. doi: 10.1074/jbc.M302076200. [DOI] [PubMed] [Google Scholar]
- 82.Kim C, Sano Y, Todorova K, Carlson BA, Arpa L, Celada A, Lawrence T, Otsu K, Brissette JL, Arthur JS, Park JM. The kinase p38 alpha serves cell type-specific inflammatory functions in skin injury and coordinates pro- and anti-inflammatory gene expression. Nat Immunol. 2008;9:1019–27. doi: 10.1038/ni.1640. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Lucas M, Zhang X, Prasanna V, Mosser DM. ERK activation following macrophage FcgammaR ligation leads to chromatin modifications at the IL-10 locus. J Immunol. 2005;175:469–77. doi: 10.4049/jimmunol.175.1.469. [DOI] [PubMed] [Google Scholar]
- 84.Chang CI, Zoghi B, Liao JC, Kuo L. The involvement of tyrosine kinases, cyclic AMP/protein kinase A, and p38 mitogen-activated protein kinase in IL-13-mediated arginase I induction in macrophages: its implications in IL-13-inhibited nitric oxide production. J Immunol. 2000;165:2134–41. doi: 10.4049/jimmunol.165.4.2134. [DOI] [PubMed] [Google Scholar]
- 85.Thepen T, Van Rooijen N, Kraal G. Alveolar macrophage elimination in vivo is associated with an increase in pulmonary immune response in mice. J Exp Med. 1989;170:499–509. doi: 10.1084/jem.170.2.499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Tang C, Inman MD, van Rooijen N, Yang P, Shen H, Matsumoto K, O'Byrne PM. Th type 1-stimulating activity of lung macrophages inhibits Th2-mediated allergic airway inflammation by an IFN-gamma-dependent mechanism. J Immunol. 2001;166:1471–81. doi: 10.4049/jimmunol.166.3.1471. [DOI] [PubMed] [Google Scholar]
- 87.Page TH, Brown A, Timms EM, Foxwell BM, Ray KP. p38 inhibitors suppress cytokine production in RA synovial membranes: Does variable inhibition of IL-6 production limit effectiveness in vivo? Arthritis Rheum. 2010 Jun 29; doi: 10.1002/art.27631. Epub ahead of print. [DOI] [PubMed] [Google Scholar]
- 88.Espelin CW, Goldsipe A, Sorger PK, Lauffenburger DA, de Graaf D, Hendriks BS. Elevated GM-CSF and IL-1beta levels compromise the ability of p38 MAPK inhibitors to modulate TNFalpha levels in the human monocytic/macrophage U937 cell line. Mol Biosyst. 2010 Jul 9; doi: 10.1039/c002848g. Epub ahead of print. [DOI] [PubMed] [Google Scholar]
- 89.Hammaker D, Firestein GS. “Go upstream, young man”: lessons learned from the p38 saga. Ann Rheum Dis. 2010;69 1:i77–82. doi: 10.1136/ard.2009.119479. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Yasuda T, Sanjo H, Pagès G, Kawano Y, Karasuyama H, Pouysségur J, Ogata M, Kurosaki T. Erk kinases link pre-B cell receptor signaling to transcriptional events required for early B cell expansion. Immunity. 2008;28:499–508. doi: 10.1016/j.immuni.2008.02.015. [DOI] [PubMed] [Google Scholar]
- 91.Kurosaki T, Shinohara H, Baba Y. B cell signaling and fate decision. Annu Rev Immunol. 2010;28:21–55. doi: 10.1146/annurev.immunol.021908.132541. [DOI] [PubMed] [Google Scholar]
- 92.Hashimoto A, Okada H, Jiang A, Kurosaki M, Greenberg S, Clark EA, Kurosaki T. Involvement of guanosine triphosphatases and phospholipase C-gamma2 in extracellular signal-regulated kinase, c-Jun NH2-terminal kinase, and p38 mitogen-activated protein kinase activation by the B cell antigen receptor. J Exp Med. 1998;188:1287–95. doi: 10.1084/jem.188.7.1287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Richards JD, Davé SH, Chou CH, Mamchak AA, DeFranco AL. Inhibition of the MEK/ERK signaling pathway blocks a subset of B cell responses to antigen. J Immunol. 2001;166:3855–64. doi: 10.4049/jimmunol.166.6.3855. [DOI] [PubMed] [Google Scholar]
- 94.Sanjo H, Hikida M, Aiba Y, Mori Y, Hatano N, Ogata M, Kurosaki T. Extracellular signal-regulated protein kinase 2 is required for efficient generation of B cells bearing antigen-specific immunoglobulin G. Mol Cell Biol. 2007;27:1236–46. doi: 10.1128/MCB.01530-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Rui L, Vinuesa CG, Blasioli J, Goodnow CC. Resistance to CpG DNA-induced autoimmunity through tolerogenic B cell antigen receptor ERK signaling. Nat Immunol. 2003;4:594–600. doi: 10.1038/ni924. [DOI] [PubMed] [Google Scholar]
- 96.Rincón M, Davis RJ. Regulation of the immune response by stress-activated protein kinases. Immunol Rev. 2009;228:212–24. doi: 10.1111/j.1600-065X.2008.00744.x. [DOI] [PubMed] [Google Scholar]
- 97.Gallagher E, Enzler T, Matsuzawa A, Anzelon-Mills A, Otero D, Holzer R, Janssen E, Gao M, Karin M. Kinase MEKK1 is required for CD40-dependent activation of the kinases Jnk and p38, germinal center formation, B cell proliferation and antibody production. Nat Immunol. 2007;8:57–63. doi: 10.1038/ni1421. [DOI] [PubMed] [Google Scholar]
- 98.Yamada T, Zhu D, Zhang K, Saxon A. Inhibition of interleukin-4-induced class switch recombination by a human immunoglobulin Fc gamma-Fc epsilon chimeric protein. J Biol Chem. 2003;278:32818–24. doi: 10.1074/jbc.M304590200. [DOI] [PubMed] [Google Scholar]
- 99.Zhang K, Zhang L, Zhu D, Bae D, Nel A, Saxon A. CD40-mediated p38 mitogen-activated protein kinase activation is required for immunoglobulin class switch recombination to IgE. J Allergy Clin Immunol. 2002;110:421–8. doi: 10.1067/mai.2002.126382. [DOI] [PubMed] [Google Scholar]
- 100.Altan-Bonnet G, Germain RN. Modeling T cell antigen discrimination based on feedback control of digital ERK responses. PLoS Biol. 2005;3:e356. doi: 10.1371/journal.pbio.0030356. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Legewie S, Schoeberl B, Blüthgen N, Herzel H. Competing docking interactions can bring about bistability in the MAPK cascade. Biophys J. 2007;93:2279–88. doi: 10.1529/biophysj.107.109132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Markevich NI, Hoek JB, Kholodenko BN. Signaling switches and bistability arising from multisite phosphorylation in protein kinase cascades. J Cell Biol. 2004;164:353–9. doi: 10.1083/jcb.200308060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Sethna J. What is hysteresis? http://www.lassp.cornell.edu/sethna/hysteresis/WhatIsHysteresis.html.
- 104.Yildirim N, Santillan M, Horike D, Mackey MC. Dynamics and bistability in a reduced model of the lac operon. Chaos. 2004;14:279–92. doi: 10.1063/1.1689451. [DOI] [PubMed] [Google Scholar]
- 105.Lisman JE. A mechanism for memory storage insensitive to molecular turnover: a bistable autophosphorylating kinase. Proc Natl Acad Sci USA. 1985;82:3055–7. doi: 10.1073/pnas.82.9.3055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Ferrell JE, Xiong W. Bistability in cell signaling: How to make continuous processes discontinuous, and reversible processes irreversible. Chaos. 2001;11:227–236. doi: 10.1063/1.1349894. [DOI] [PubMed] [Google Scholar]
- 107.Xiong W, Ferrell JE., Jr A positive-feedback-based bistable ‘memory module’ that governs a cell fate decision. Nature. 2003;426:460–5. doi: 10.1038/nature02089. [DOI] [PubMed] [Google Scholar]
- 108.Bhalla US, Ram PT, Iyengar R. MAP kinase phosphatase as a locus of flexibility in a mitogen-activated protein kinase signaling network. Science. 2002;297:1018–23. doi: 10.1126/science.1068873. [DOI] [PubMed] [Google Scholar]
- 109.Lin J, Harding A, Giurisato E, Shaw AS. KSR1 modulates the sensitivity of mitogen-activated protein kinase pathway activation in T cells without altering fundamental system outputs. Mol Cell Biol. 2009;29:2082–91. doi: 10.1128/MCB.01634-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Bagowski CP, Ferrell JE., Jr Bistability in the JNK cascade. Curr Biol. 2001;11:1176–82. doi: 10.1016/s0960-9822(01)00330-x. [DOI] [PubMed] [Google Scholar]
- 111.Das J, Ho M, Zikherman J, Govern C, Yang M, Weiss A, Chakraborty AK, Roose JP. Digital signaling and hysteresis characterize ras activation in lymphoid cells. Cell. 2009;136:337–51. doi: 10.1016/j.cell.2008.11.051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Inder K, Harding A, Plowman SJ, Philips MR, Parton RG, Hancock JF. Activation of the MAPK module from different spatial locations generates distinct system outputs. Mol Biol Cell. 2008;19:4776–84. doi: 10.1091/mbc.E08-04-0407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Kelleher RJ, 3rd, Govindarajan A, Tonegawa S. Translational regulatory mechanisms in persistent forms of synaptic plasticity. Neuron. 2004;44:59–73. doi: 10.1016/j.neuron.2004.09.013. [DOI] [PubMed] [Google Scholar]
- 114.Giovannini MG. The role of the extracellular signal-regulated kinase pathway in memory encoding. Rev Neurosci. 2006;17:619–34. doi: 10.1515/revneuro.2006.17.6.619. [DOI] [PubMed] [Google Scholar]
- 115.Kelleher RJ, 3rd, Govindarajan A, Jung HY, Kang H, Tonegawa S. Translational control by MAPK signaling in long-term synaptic plasticity and memory. Cell. 2004;116:467–79. doi: 10.1016/s0092-8674(04)00115-1. [DOI] [PubMed] [Google Scholar]
- 116.Davis S, Vanhoutte P, Pages C, Caboche J, Laroche S. The MAPK/ERK cascade argets both Elk-1 and cAMP response element-binding protein to control long-term potentiation-dependent gene expression in the dentate gyrus in vivo. J Neurosci. 2000;20:4563–72. doi: 10.1523/JNEUROSCI.20-12-04563.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Satoh Y, Endo S, Ikeda T, Yamada K, Ito M, Kuroki M, Hiramoto T, Imamura O, Kobayashi Y, Watanabe Y, Itohara S, Takishima K. Extracellular signal-regulated kinase 2 (ERK2) knockdown mice show deficits in long-term memory; ERK2 has a specific function in learning and memory. J Neurosci. 2007;27:10765–76. doi: 10.1523/JNEUROSCI.0117-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Miyashita Y. Cognitive memory: cellular and network machineries and their top-down control. Science. 2004;306:435–40. doi: 10.1126/science.1101864. [DOI] [PubMed] [Google Scholar]
- 119.Ofen-Noy N, Dudai Y, Karni A. Skill learning in mirror reading: how repetition determines acquisition. Brain Res Cogn Brain Res. 2003;17:507–21. doi: 10.1016/s0926-6410(03)00166-6. [DOI] [PubMed] [Google Scholar]
- 120.Gern JE. Viral and bacterial infections in the development and progression of asthma. J Allergy Clin Immunol. 2000;105:S497–502. doi: 10.1016/s0091-6749(00)90050-2. [DOI] [PubMed] [Google Scholar]
- 121.Martinez FD. Viruses and atopic sensitization in the first years of life. Am J Respir Crit Care Med. 2000;162:S95–9. doi: 10.1164/ajrccm.162.supplement_2.ras-8. [DOI] [PubMed] [Google Scholar]
- 122.Liu W, Tundwal K, Liang Q, Goplen N, Rozario S, Quayum N, Gorska M, Wenzel S, Balzar S, Alam R. Establishment of extracellular signal-regulated kinase 1/2 bistability and sustained activation through Sprouty 2 and its relevance for epithelial function. Mol Cell Biol. 2010;30:1783–99. doi: 10.1128/MCB.01003-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Yang C, Zhou W, Jeon MS, Demydenko D, Harada Y, Zhou H, Liu YC. Negative regulation of the E3 ubiquitin ligase itch via Fyn-mediated tyrosine phosphorylation. Mol Cell. 2006;21:135–41. doi: 10.1016/j.molcel.2005.11.014. [DOI] [PubMed] [Google Scholar]
- 124.Gao M, Labuda T, Xia Y, Gallagher E, Fang D, Liu YC, Karin M. Jun turnover is controlled through JNK-dependent phosphorylation of the E3 ligase Itch. Science. 2004;306:271–5. doi: 10.1126/science.1099414. [DOI] [PubMed] [Google Scholar]
- 125.Egan JE, Hall AB, Yatsula BA, Bar-Sagi D. The bimodal regulation of epidermal growth factor signaling by human Sprouty proteins. Proc Natl Acad Sci USA. 2002;99:6041–6. doi: 10.1073/pnas.052090899. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Wong ES, Fong CW, Lim J, Yusoff P, Low BC, Langdon WY, Guy GR. Sprouty2 attenuates epidermal growth factor receptor ubiquitylation and endocytosis, and consequently enhances Ras/ERK signalling. EMBO J. 2002;21:4796–808. doi: 10.1093/emboj/cdf493. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Rubin C, Litvak V, Medvedovsky H, Zwang Y, Lev S, Yarden Y. Sprouty fine-tunes EGF signaling through interlinked positive and negative feedback loops. Curr Biol. 2003;13:297–307. doi: 10.1016/s0960-9822(03)00053-8. [DOI] [PubMed] [Google Scholar]
- 128.Foster SL, Hargreaves DC, Medzhitov R. Gene-specific control of inflammation by TLR-induced chromatin modifications. Nature. 2007;447:972–8. doi: 10.1038/nature05836. [DOI] [PubMed] [Google Scholar]
- 129.Bashor CJ, Helman NC, Yan S, Lim WA. Using engineered scaffold interactions to reshape MAP kinase pathway signaling dynamics Science. 2008;319:1539–43. doi: 10.1126/science.1151153. [DOI] [PubMed] [Google Scholar]
- 130.Bhattacharyya RP, Reményi A, Good MC, Bashor CJ, Falick AM, Lim WA. The Ste5 scaffold allosterically modulates signaling output of the yeast mating pathway. Science. 2006;311:822–6. doi: 10.1126/science.1120941. [DOI] [PubMed] [Google Scholar]
- 131.Giorgetti L, Siggers T, Tiana G, Caprara G, Notarbartolo S, Corona T, Pasparakis M, Milani P, Bulyk ML, Natoli G. Noncooperative interactions between transcription factors and clustered DNA binding sites enable graded transcriptional responses to environmental inputs. Mol Cell. 2010;37:418–28. doi: 10.1016/j.molcel.2010.01.016. [DOI] [PubMed] [Google Scholar]