Abstract
Ixodes spp. tick-borne zoonotic diseases are present across the Holarctic in humans, domestic animals, and wildlife. Small mammals are reservoirs for the rickettsial pathogen Anaplasma phagocytophilum and tick vectors may include catholic-feeding bridge vectors as well as host-specialist or nidicolous ticks. Far western North American communities in which A. phagocytophilum is maintained are complex ecologically, with multiple reservoir host and tick species, multiple strains of the bacterial pathogen A. phagocytophilum and differences in dynamics of hosts and vectors across heterogeneous landscapes. We evaluated sites in northern California in order to identify primarily nidicolous ticks and the hosts they infest. A total of 667 ticks was found in 11 study sites, including 288 on flags and 379 attached to small mammals. Larvae were over-represented among attached ticks and adults on flags. The most abundant species was I. pacificus. Two-hundred fourteen nidicolous ticks were found, most abundantly I. angustus and I. spinipalpis. All adult I. ochotonae, I. auritulus, I. angustus, I. jellisoni, and I. woodi were female, while the male:female ratio of I. spinipalpis was 1.2:1 and 1:1 for I. pacificus. The greatest number of ticks was obtained from Tamias ochrogenys, Peromyscus spp., and Neotoma fuscipes. Of 234 small mammal individuals that were infested with Ixodes spp., only 81 (34.6%) were infested with I. pacificus. The remaining infested small mammals hosted nidicolous tick species. Eight ticks were PCR-positive, including 6 I. pacificus (one adult, one larva, and 6 nymphs), and 2 adult I. ochotonae and high PCR prevalences of 18% and 9% were detected in woodrats and chipmunks, respectively. Nymphal I. angustus ticks were active year-long with a possible increase in August while larval activity was only observed in December and spring months and adults only during spring and fall. Overall, we show high tick species richness and year-round high levels of infestation on rodents by several different nidicolous ticks in areas where A. phagocytophilum is enzootic, including on reported reservoir species.
Keywords: Anaplasma phagocytophilum, Granulocytic anaplasmosis, Ixodes angustus, Ixodes ochotonae, Ixodes pacificus
Introduction
Many tick-borne pathogens including Borrelia hermsii, Rickettsia rickettsii, and Babesia microti are zoonotic, with an enzootic cycle in wild small mammals as well as a zoonotic cycle in which a bridge vector transfers infection to humans and medically important domestic animals. This is true also for granulocytic anaplasmosis (GA) caused by Anaplasma phagocytophilum, which occurs across the Holarctic in humans, domestic animals, and wildlife (Madigan, 1993; Greig et al., 1996; Foley, 2000; Foley et al., 2001, 2004). Small mammals are reservoir hosts including white-footed mice (Peromyscus leucopus) in the eastern US (Telford et al., 1996) and woodrats (Neotoma fuscipes), squirrels (Sciurus spp.), and chipmunks (Tamias spp.) in the western US (Nicholson et al., 1999, Foley et al., 2002, Nieto and Foley, 2008, 2009).
The main tick vectors for GA are almost always reported to be catholic-feeding ticks in the Ixodes ricinus group including I. scapularis in the eastern US and I. pacificus in the western US (Foley et al., 2004). These three-host ticks feed primarily on large mammals as adults and utilize small mammals, reptiles, or birds in immature stages. Because transovarial transmission of A. phagocytophilum is not considered important (Munderloh and Kurtti, 1995), infection must be acquired by the tick during larval or nymphal stages before it can be transferred to humans or domestic animals by tick of subsequent stages. I. pacificus and I. scapularis are the most likely bridge vectors to humans, dogs, and horses in North America, but their importance as enzootic vectors may vary across regions. In several regions where the bridge vectors do not occur, studies have documented rodent-specialist ticks maintaining enzootic cycles of GA, including I. spinipalpis on the Mexican woodrat (N. mexicana) in Colorado and I. trianguliceps on small mammals in northwestern England (Zeidner et al., 2000; Bown et al., 2003). These ticks are nidicolous, i.e. living in small mammals. dens and burrows, where they gain access to their hosts. In far western North America, there are multiple reservoir host species, multiple strains of A. phagocytophilum (Foley et al., 2009b), and differences in dynamics of hosts and vectors across the diverse landscapes (Foley et al., 2009a). There also are numerous Ixodes spp. in the western US. In the present study, we evaluated multiple study sites in northern California and attempted to identify nidicolous ticks and the hosts they infest, any habitat and seasonal differences in their presence, and whether they were infected with A. phagocytophilum.
Materials and methods
Study sites, trapping, and sample collection
Small mammal trapping and tick collection were performed at 11 sites in northern California from February 2005 to July 2009 (Table 1). At each site, variable length transects were established by choosing among available deer trails or poorly used human trails or old roads. Flagging was performed over herbaceous and shrubby vegetation as well as duff and litter using a 1-m2 white cotton flag. In order to obtain small mammals and their attached ticks, extra-large (4×4.5×15 in.) Sherman (HB Sherman, Tallahassee, FL) and Tomahawk (Tomahawk Live Trap, Tomahawk, WI) live traps were set overnight at locations of observed active rodent usage or dens and baited with peanut butter and oats. Rodents were anesthetized with approximately 20 mg/kg ketamine and 3 mg/kg xylazine delivered SC, examined visually for ectoparasites and body condition and given a permanent individually numbered metal ear tag. Ticks were removed with forceps and preserved in 70% ethanol. Ixodes spp. were identified to species using keys (Furman and Loomis, 1984; Webb et al., 1990). Larvae were viewed under a compound microscope in a depression slide as well as with a dissecting microscope before identification was confirmed. All work with small mammals was performed under the oversight of the UC Davis Attending Veterinarian and the Institutional Animal Care and Use Committee.
Table 1.
Study sites evaluated for granulocytic anaplasmosis in nidicolous ticks and wild rodents across northern and central coastal California.
| Study Site | Region | Latitude and longitude (decimal degrees) | Elevation (m) | Distance to coast (km) |
|---|---|---|---|---|
| Big Basin State Park | Central coast range | 37°10.621; 122°12.328 | 368 | 21 |
| Hendy Woods State Park | Northern coast range | 39°04.25; 123°28.238 | 120 | 18 |
| Henry Cowell/Fall Creek State Park | Central coast range | 37°04.01; 122°06.40 | 150 | 10 |
| Humboldt Redwoods State Park | Northern coast range | 40°17.770; 123°59.178 | 610 | 26 |
| Hoopa Valley Indian Reservation | Northern coast range | 41°10.333; 123°56.520 | 109–1200 | 26 |
| King Range National Conservation Area | Northern coast range | 40°08.059; 124°07.404 | 61–610 | 3.9 |
| Quail Ridge/Cold Canyon Preserves | Northern interior coast range | 38°82.06; 122°23.47 | 300 | 72 |
| Samuel P. Taylor State Park | Northern coast range | 38°01.232; 122°40.774 | 134 | 11 |
| Soquel Demonstration Forest | Central coast range | 37°06.30; 121°83.19 | 450–600 | 8 |
| Sutter Buttes State Park | Central Valley butte | 39°12.805; 121°48.167 | 247 | 151 |
| Yosemite National Park | Central Sierra Nevada | 37°42–54; 119.15–51 | 1200–3000 | 217 |
Nucleic acid extraction, real-time TaqMan polymerase chain reaction (PCR), and DNA sequencing
Ticks and small mammal blood samples were assessed for infection by polymerase chain reaction (PCR). DNA was extracted from ticks by surface cleaning with 70% ethanol, followed by grinding in a mortar and pestle, and then boiling in TE buffer for 10 min. Tick extracts were diluted 1:100 in water for PCR. DNA was extracted from mammalian blood using a kit (Qiagen Blood kit, Valencia, CA) following the manufacturer.s instructions. Real-time quantitative PCR was performed targeting the multiple-copy msp2 gene of A. phagocytophilum as previously described (Drazenovich et al., 2006). Each 12-µl reaction contained 5 µl DNA, 1X TaqMan Universal Master Mix (Applied Biosystems), 2 nmol of each primer, and 400 pmol of probe. The thermocycling conditions consisted of 50°C for 2 min, 95°C for 10 min, and 40 cycles at 95°C for 15 s, followed by 60°C for 1 min. Samples were considered positive if they had a cycle threshold (CT) value <50 and characteristic amplification plots. For all reactions, 3 negative water controls were included during each run. In order to confirm that the animals were in fact infected with A. phagocytophilum, 2 rodent samples were randomly selected for DNA sequencing. A nested conventional PCR assay targeting the ribosomal RNA 23S-5S (rrl–rrs) intergenic spacer region was performed using newly designed external primers ITS2F, 5’-AGGATCTGACTCTAGTACGAG-3’ and ITS2R, 5’-CTCCCATGTCTTAAGACAAAG-3’, and internal primers ITS2iF, 5’-ATACCTCTGGTGTACCAGTTG-3’ and ITS2iR, 5’-TTAACTTCCGGGTTCGGAATG-3’ using the following thermocycler conditions: 94 °C for 2 min, followed by 35 cycles of 94°C for 30 s, 58°C for 30 s, and 72°C for 1 min, followed by a 5-min extension at 72°C. Amplified DNA was visualized on a 1% agarose gel stained with GelStar (Lonza, Rockland, ME) and then appropriately sized bands were excised and cleaned with a Qiagen gel extraction kit (Qiagen, Valencia, CA). Sequencing was performed in both forward and reverse directions on an ABI 3730 sequencer (Davis Sequencing, Davis, Ca). Sequences were aligned using the Clustal X program and evaluated for homology to previously reported sequencing by a BLAST search of the GenBank database (http://blast.ncbi.nlm.nih.gov/Blast.cgi).
Data analysis
Data were maintained in Excel (Microsoft, Redmond, WA) and analyzed with the statistical package “R” (R-Development Core Team, http://www.r-project.org). The cut-off for statistical significance was P=0.05. A chi-square contingency test was performed in order to evaluate whether the stage distribution of ticks varied between flagged ticks and those removed from small mammals.
Results
A total of 667 ticks was evaluated across 11 study sites, including 288 on flags and 379 attached to small mammals. Of the flagged ticks, 13.1% were larvae, 26.1% nymphs, and 62.5% adults, while the stage distribution among attached ticks was 46.8% larvae, 33.7% nymphs, and 19.5% adults, i.e. significantly more larvae among attached ticks (P=2.2×10−16). The most abundant species was I. pacificus (67.7% of all ticks collected) found in all study sites except Henry Cowell State Park and the nearby Fall Creek unit. Altogether, 214 nidicolous ticks were found: I. angustus and I. spinipalpis were abundant, while only one I. auritulus and one I. soricis were observed. Because tick collection effort was not equal across locations, statistical comparisons were not performed, but sites from which the greatest numbers of ticks were collected included Hendy Woods State Park, Samuel P. Taylor State Park, and Humboldt Redwoods State Park. Interestingly, all adults of the following species were female, including I. ochotonae, I. auritulus, I. angustus, I. jellisoni, and I. woodi. The male:female ratio of I. spinipalpis was 1.2:1 and 1:1 for I. pacificus.
A greater proportion of ticks 273 (44.4%) was obtained by flagging than from any individual host species (Table 2). Of the I. pacificus, 197 (44.0% of total I. pacificus) were attached to rodents, while 251 (56.0%) were obtained from flagging. Of the other species of ticks, 24 were detected on flags, including 3 I. angustus, one I. auritulus, one I. sculptus, and 19 I. spinipalpis, even though these are species which rarely quest openly. Finally, of 234 small mammal individuals that were infested with Ixodes spp., only 81 (34.6%) were infested with I. pacificus. The remaining infested small mammals hosted nidicolous tick species.
Table 2.
Number of Ixodes spp. obtained from flagging vegetation and trapping 14 species groups of rodents in 11 sites in northern California. In addition, one I. soricis was found on a Sorex townsendi.
| Number of host individuals captured |
I. angustus |
I. auritulus | I. jellisoni | I. ochotonae | I. pacificus | I. sculptus |
I. Spinipalpis |
I. woodi | |
|---|---|---|---|---|---|---|---|---|---|
| Flag | N/A | 3 | 1 | 0 | 0 | 249 | 1 | 19 | 0 |
| Sciurus carolinensis | 20 | 2 | 0 | 0 | 0 | 2 | 0 | 0 | 0 |
| Sciurus griseus | 31 | 0 | 0 | 0 | 0 | 4 | 0 | 3 | 0 |
| Tamiasciurus douglasii | 5 | 1 | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| Spermophilus beecheyi | 36 | 0 | 0 | 0 | 2 | 2 | 1 | 0 | 0 |
| Spermophilus beldingi | 151 | 0 | 0 | 0 | 0 | 0 | 1 | 0 | 0 |
| Glaucomys sabrinus | 13 | 4 | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| Neotoma fuscipes | 185 | 13 | 0 | 0 | 11 | 12 | 0 | 10 | 3 |
| Tamias ochrogenys | 206 | 35 | 0 | 0 | 7 | 128 | 0 | 4 | 0 |
| Tamias merriami | 9 | 0 | 0 | 0 | 1 | 0 | 0 | 0 | 0 |
| Tamias sonomae | 5 | 2 | 0 | 0 | 0 | 1 | 0 | 0 | 0 |
| Peromyscus spp. | 1134 | 40 | 0 | 3 | 11 | 34 | 0 | 3 | 0 |
| Myodes californicus | 18 | 0 | 0 | 0 | 0 | 1 | 0 | 0 | 0 |
| Microtus californicus | 19 | 0 | 0 | 0 | 0 | 1 | 0 | 0 | 0 |
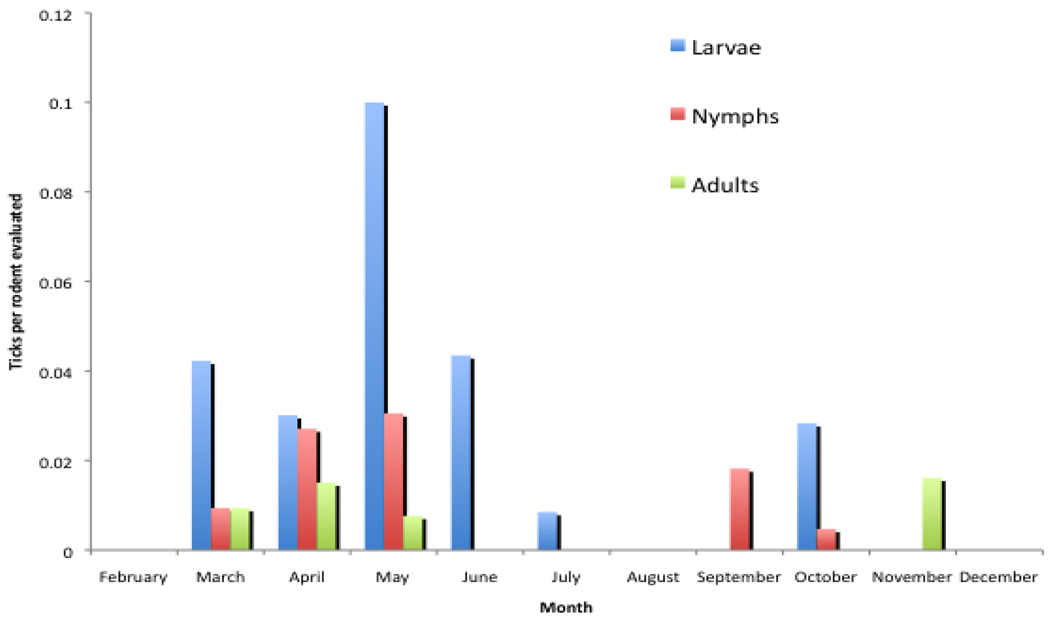
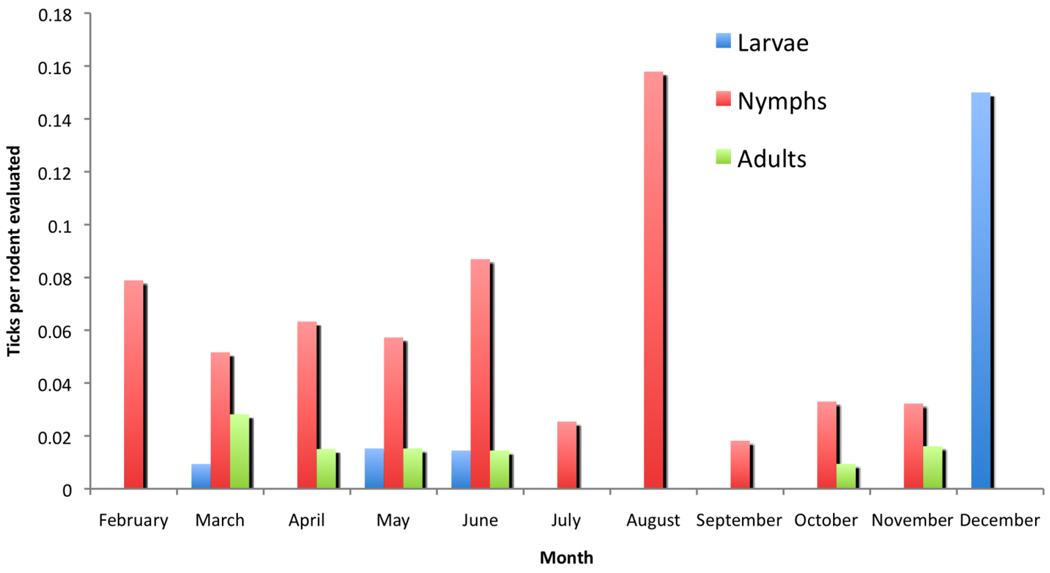
A total of 628 ticks (those with usable DNA) were tested for A. phagocytophilum by PCR, of which 8 were positive, including 6 I. pacificus (one adult, one larva, and 6 nymphs) and 2 adult I. ochotonae. All except one of the I. pacificus were from flags, while the remaining individual, the larva, was removed from a T. ochrogenys from Hendy Woods. The 2 I. ochotonae were removed from P. maniculatus also from Hendy Woods. The mean CT among PCR-positive ticks was 36.1 (range 33.7–39.0). Small mammals hosting ticks were also evaluated for infection by PCR (Table 3). PCR-positive individuals were identified among 18.0% of woodrats and 8.9% of chipmunks with an overall PCR prevalence in study animals of 4.7%. The mean CT for PCR-positive woodrats was 33.3 (range 32.0–36.0) and for chipmunks 36.8 (31.0–39.0). Two samples from woodrats were evaluated by DNA sequencing to confirm infection with A. phagocytophilum. Good-quality DNA sequences were obtained and compared to a database, which revealed 99% identity over 300 nucleotides with both the HZ and USG3 strains of A. phagocytophilum (GenBank accession numbers AF416766.1 and CP000235.1). Aside from these 2 accessions, identity to other species including A. marginale, Ehrlichia chaffeensis, and E. ruminantium did not exceed 86%, confirming the identity of the target as A. phagocytophilum. In order to evaluate the phenology of ticks, we evaluated numbers of I. pacificus and I. angustus ticks collected in each month (i.e. focusing on tick species with enough individuals to allow for this analysis). Flagging efforts for I. pacificus were not standardized precluding statistical analysis, but strong peaks for adults and nymphs were observed from December through April and March to July, respectively (data not shown). Thirty-two larvae were removed from flags in May. Because numbers of rodents caught varied over each sampling time, we presented the number of ticks observed divided by the number of rodents evaluated (Fig. 1). Patterns for rodent-feeding I. pacificus were related to those from flagged ticks, with a nymphal peak March through May and a smaller secondary peak in September and October. Very few adults were observed, in spring and November, while larvae were the most abundant ticks, occurring also in spring and October. These results were distinctly different from those of I. angustus (Fig. 2). In that species, nymphal ticks were active year-long with a possible increase in August. In contrast, larval activity was only observed in December and spring months, and the December results are strongly influenced by the small number of rodents (n=3) evaluated in that time period. Adult activity also was seasonal during spring and fall, but not in summer months.
Table 3.
Prevalence of Anaplasma phagocytophilum infection, based on PCR test results of blood samples in small mammals from 11 sites in northern California.
| Host | PCR-negative | PCR-positive | Total | Prevalence (%) |
|---|---|---|---|---|
| Ground squirrels | 5 | 0 | 5 | 0 |
| Voles | 2 | 0 | 2 | 0 |
| Woodrats | 30 | 4 | 42 | 11.8 |
| Flying squirrels | 1 | 0 | 1 | 0 |
| Deer mice | 89 | 0 | 89 | 0 |
| Tree squirrels | 8 | 0 | 8 | 0 |
| Shrews | 5 | 0 | 5 | 0 |
| Chipmunks | 61 | 6 | 81 | 8.9 |
| Grand total | 201 | 10 | 233 | 4.7 |
Fig. 1.
Monthly number of I. pacificus collected per rodents trapped in 11 sites in northern California.
Fig. 2.
Monthly number of I. angustus collected per rodents trapped in 11 sites in northern California.
Discussion
In this study, we show high tick species richness and year-round high levels of infestation on rodents by several different nidicolous ticks in areas where A. phagocytophilum is enzootic, including on reported reservoir species. I. pacificus, which is likely both an enzootic and bridge vector for A. phagocytophilum, was a common member of the tick fauna in most sites we evaluated and could be obtained both by flagging and by rodent trapping. Nidicolous tick species also found on small mammals in the areas we studied included I. angustus, I. ochotonae, I. spinipalpis, I. jellisoni, I. sculptus, and I. woodi and in fact, the majority of infested small mammals hosted ticks that were not I. pacificus.
In our data, I. angustus was a very abundant tick, particularly on chipmunks, woodrats, and deer mice. This species feeds on a variety of small mammals and occasionally on humans, and ranges in California throughout northern coastal mountains and the Sierra Nevada Mountain range including to relatively high elevations (Furman and Loomis, 1984). Interestingly, only female ticks appear to be recoverable from hosts, although males have been found in small mammal nests (Easton and Goulding, 1974). I. angustus may be naturally infected with B. burgdorferi (Banerjee et al., 1994) and is a competent vector for B. burgdorferi sensu stricto (Peavey et al., 2000). As in prior reports, I. angustus could be found throughout the year (Furman and Loomis, 1984) in contrast to the strong seasonality observed for both questing and host-attached I. pacificus. This implies that pathogen transmission could be continual in contrast to the more seasonal questing activity and resultant probable pulses of pathogen transmission that occur with I. pacificus (Eisen et al., 2002).
The next most commonly encountered ticks in the present study were I. spinipalpis (n=39) and I. ochotonae (n=32). I. spinipalpis (which now includes I. neotomae; Norris et al., 1997) is a common nidicolous tick of numerous host species including principally rodents, but also lagomorphs and rarely birds and humans (Furman and Loomis, 1984). It occurs across western North America often in chaparral and oak habitats and becomes more nidicolous in more mesic environments (Norris et al., 1997). As for I. angustus, nymphs can be collected year-round (Furman and Loomis, 1984), although sample size in the present study was insufficient to observe this. In our study, I. spinipalpis was found mostly on woodrats with fewer on deer mice and chipmunks. Prior research indicated that I. spinipalpis was an important vector of Borrelia sp. (likely B. bissettii) among woodrats in California (Brown and Lane, 1992, Brown et al., 2006). I. ochotonae is a relatively infrequently encountered tick, but with a broad geographical distribution across most of western North American (Furman and Loomis, 1984). Reported hosts include woodrats, chipmunks, pikas (Ochotona princeps), and grey foxes (Urocyon cinereoargenteus), and the specimens obtained in this study were removed from deer mice, woodrats, and chipmunks. I. ochotonae adults bear a strong resemblance to I. angustus, but were distinguished in this data set on the basis of having 2/2 denticles at the base of the hypostome. Two I. ochotonae were PCR-positive for A. phagocytophilum, but not only were the individual deer mice from which they were obtained not PCR-positive – no deer mice in this study were PCR-positive –, suggesting that these ticks were infected during earlier stages while feeding on other host species. Although this tick was uncommon in this and other studies in western North America, the PCR-positive ticks suggest that further study of this tick is warranted.
In general, nidicolous ticks are relatively host-specialist, non-‘questing’ ticks, relying on intimate associations with hosts in their nest sites to ensure a stable microenvironment and access to feeding resources, although this varies somewhat for some species such as I. spinipalpis in different environments (Furman and Loomis, 1984). This is a very different strategy than a catholic-feeding, questing tick such as I. pacificus which remains protected in leaf litter and duff, but seasonally emerges to seek mammalian, reptilian, or avian hosts. Because nidicolous ticks either are attached to mammalian hosts during feeding or spend non-feeding periods in host nest sites, standard methods of flagging litter and vegetation may overlook the majority of these ticks in an ecosystem. In the present study, most nidicolous ticks were removed directly from small mammals. However, we did obtain a low number of nidicolous ticks through flagging, notably I. spinipalpis, which reinforces the importance of individually examining all flagged ticks. Similarly, work in Colorado demonstrated I. spinipalpis questing more than 20 feet from a nearby woodrat house and no significant association of this tick on sentinel mice near or more distant from woodrat houses (Burkot et al., 2001). Eisen et al. (2006) also recovered host-seeking I. spinipalpis, I. angustus, and I. auritulus in dense woodlands in Mendocino County, California. We found I. angustus abundantly on Peromyscus spp. and to a lesser degree on voles (Microtus californicus), woodrats, house mice (Mus musculus), and rats (Rattus spp.). Less common ticks were I. pacificus on Peromyscus spp. and I. spinipalpis on woodrats and Peromyscus spp. One study compared ticks in small mammal and reptile communities from the Willamette Valley of Oregon and a coastal site with Sitka spruce (Picea sitchensis) and western hemlock (Tsuga heterophylla) (Easton and Goulding, 1974). In the Valley, abundant ticks were I. pacificus on deer mice, shrews, and alligator lizards (Elgaria multicarinatus). I. angustus was abundant at both sites: on the coast, notably on chipmunks, deer mice, flying squirrels (Glaucomys sabrinus), and shrews and in the Valley on shrews, woodrats, and deer mice. When rodent burrows were excavated, I. angustus was disproportionately found in voles. nests compared with nests of deer mice, woodrats, and shrews.
In the present study, pathogen testing revealed A. phagocytophilum DNA in I. pacificus and I. ochotonae. Overall, this is quite a low prevalence (1.3%) of positive ticks, but it is noteworthy that ticks in the present study were tested individually as opposed to some studies where ticks were pooled. Additionally, A. phagocytophilum loads in ticks tend to be relatively low, potentially reducing sensitivity of the assay (Foley and Nieto, 2007). Several PCR-positive ticks were adult, and it is not possible to determine what host species infected these ticks because they could have acquired infection while feeding earlier as larvae or nymphs. The finding of PCR-positive larvae could be consistent with transovarial transmission, although this is reported not to occur for A. phagocytophilum (Munderloh and Kurtti, 1995). It is more likely that the host on which these ticks fed was infected, but at very low levels that were below the detection ability of the PCR assay. In support of this, previous work has documented that tick-based xenodiagnosis can be more sensitive than direct testing of the rodent host (Levin and Ross, 2004).
Ecological complexity may increase persistence of some diseases in nature, while often also reducing the prevalence of certain pathogens, such as B. burgdorferi, in some ecosystems by a dilution effect. Very simple tick–A. phagocytophilum systems have been described comprising I. trianguliceps–small mammal hosts in England and I. spinipalpis–Mexican woodrats in Colorado (Zeidner et al., 2000). At the other extreme, California systems comprise at least 3 reservoir-competent hosts, potentially zooprophylactic lizards, possibly dozens of other tick host species of varying or unknown reservoir competence, multiple strains of the pathogen, and up to 10 different Ixodes species, each with different host preferences. Important future research will be to further evaluate the vector competence of nidicolous ticks for A. phagocytophilum including through experimental infection studies using natural small mammal hosts.
Acknowledgments
We thank Regina Dingler, Joy Worth, and Raymond Wong for help in the laboratory and in the field and the following for access and logistical support at the study sites: California State Parks, Pat Freeling, Scott Struckman, and Rene Pasquinelli at Hendy Woods State Park, Jay Harris at Humboldt Redwoods State Park, Jeff Maurer at Yosemite National Park, Richard Brown at Humboldt State University, and the Hoopa Indian Nation, Thomas Sutfin and Edgar Orre at Soquel Demonstration Forest, and the staff of Boggs Mountain Demonstration Forest, Big Basin State Park, Henry Cowell State Park, the UC Reserve System, Sutter Buttes State Park, and Samuel P. Taylor State Park. Funding was provided by the National Institutes of Health Allergy and Infectious Disease Evolution of Infectious Disease program #RO1 GM081714.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Bown K, Begon M, Bennett M, Woldehiwet Z, Ogden N. Seasonal dynamics of Anaplasma phagocytophila in a rodent-tick (Ixodes trianguliceps) system, United Kingdom. Emerging Infectious Diseases. 2003;9:63–70. doi: 10.3201/eid0901.020169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brown RN, Lane RS. Lyme disease in California: a novel enzootic transmission cycle of Borrelia burgdorferi. Science. 1992;256:1439–1442. doi: 10.1126/science.1604318. [DOI] [PubMed] [Google Scholar]
- Brown RN, Peot MA, Lane RS. Sylvatic maintenance of Borrelia burgdorferi (Spirochaetales) in Northern California: untangling the web of transmission. Journal of Medical Entomology. 2006;43:743–751. doi: 10.1603/0022-2585(2006)43[743:smobbs]2.0.co;2. [DOI] [PubMed] [Google Scholar]
- Burkot TR, Maupin GO, Schneider BS, Denatale C, Happ CM, Rutherford JS, Zeidner NS. Use of a sentinel host system to study the questing behavior of Ixodes spinipalpis and its role in the transmission of Borrelia bissettii, human granulocytic ehrlichiosis, and Babesia microti. American Journal of Tropical Medicine Hygiene. 2001;65:293–299. doi: 10.4269/ajtmh.2001.65.293. [DOI] [PubMed] [Google Scholar]
- Castro MB, Wright SA. Vertebrate hosts of Ixodes pacificus (Acari: Ixodidae) in California. Journal of Vector Ecology. 2007;32:140–149. doi: 10.3376/1081-1710(2007)32[140:vhoipa]2.0.co;2. [DOI] [PubMed] [Google Scholar]
- Drazenovich NL, Brown RN, Foley JE. Use of real-time quantitative PCR targeting the msp2 protein gene to identify cryptic Anaplasma phagocytophilum infections in wildlife and domestic animals. Vector Borne Zoonotic Disease. 2006;6:83–90. doi: 10.1089/vbz.2006.6.83. [DOI] [PubMed] [Google Scholar]
- Dumler JS, Choi KS, Garcia-Garcia JC, Barat NS, Scorpio DG, Garyu JW, Grab DJ, Bakken JS. Human granulocytic anaplasmosis and Anaplasma phagocytophilum. Emerging Infectious Diseases. 2005;11:1828–1834. doi: 10.3201/eid1112.050898. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Easton E, Goulding R. Ectoparasites in two diverse habitats in western Oregon: I. Ixodes (Acarina: Ixodidae) Journal of Medical Entomology. 1974;11:413–418. doi: 10.1093/jmedent/11.4.413. [DOI] [PubMed] [Google Scholar]
- Egenvall A, Hedhammar A, Bjoersdorff A. Clinical features and serology of 14 dogs affected by granulocytic ehrlichiosis in Sweden. Veterinary Record. 1997;140:222–226. doi: 10.1136/vr.140.9.222. [DOI] [PubMed] [Google Scholar]
- Eisen L, Eisen RJ, Lane RS. Seasonal activity patterns of Ixodes pacificus nymphs in relation to climatic conditions. Medical Veterinary Entomology. 2002;16:235–244. doi: 10.1046/j.1365-2915.2002.00372.x. [DOI] [PubMed] [Google Scholar]
- Eisen L, Eisen RJ, Lane RS. Geographical distribution patterns and habitat suitability models for presence of host-seeking ixodid ticks in dense woodlands of Mendocino County, California. Journal of Medical Entomology. 2006;43:415–427. doi: 10.1603/0022-2585(2006)043[0415:gdpahs]2.0.co;2. [DOI] [PubMed] [Google Scholar]
- Eisen RJ, Eisen L, Lane R. Habitat-related variation in infestation of lizards and rodents with Ixodes ticks in dense woodlands in Mendocino County, California. Experimental Applied Acarology. 2004;33:215–233. doi: 10.1023/b:appa.0000032954.71165.9e. [DOI] [PubMed] [Google Scholar]
- Foley J. Human ehrlichiosis: a review of clinical disease and epidemiology for the physician. Infectious Disease in Clinical Practice. 2000;9:93–98. [Google Scholar]
- Foley J, Nieto N. Anaplasma phagocytophilum subverts tick salivary gland proteins. Trends in Parasitology. 2007;23:3–5. doi: 10.1016/j.pt.2006.10.003. [DOI] [PubMed] [Google Scholar]
- Foley J, Clueit S, Brown RN. Differential exposure to Anaplasma phagocytophilum in rodent species in northern California. Vector Borne Zoonotic Disease. 2008a;8:49–55. doi: 10.1089/vbz.2007.0175. [DOI] [PubMed] [Google Scholar]
- Foley JE, Foley P, Madigan JE. The distribution of granulocytic ehrlichia seroreactive dogs in California. American Journal of Veterinary Research. 2001;62:1599–1605. doi: 10.2460/ajvr.2001.62.1599. [DOI] [PubMed] [Google Scholar]
- Foley JE, Kramer VL, Weber D. Experimental ehrlichiosis in dusky footed woodrats (Neotoma fuscipes) Journal of Wildlife Diseases. 2002;38:194–198. doi: 10.7589/0090-3558-38.1.194. [DOI] [PubMed] [Google Scholar]
- Foley JE, Nieto NC, Foley P. Emergence of tick-borne granulocytic anaplasmosis associated with habitat type and forest change in northern California. American Journal of Tropical Medicine Hygiene. 2009a;81:1132–1140. doi: 10.4269/ajtmh.2009.09-0372. [DOI] [PubMed] [Google Scholar]
- Foley JE, Nieto NC, Adjemian J, Dabritz H, Brown RN. Anaplasma phagocytophilum infection in small mammal hosts of Ixodes ticks, western United States. Emerging Infectious Diseases. 2008b;14:1147–1150. doi: 10.3201/eid1407.071599. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Foley JE, Foley P, Brown RN, Lane RS, Dumler JS, Madigan JE. Ecology of granulocytic ehrlichiosis and Lyme disease in the western United States. Journal of Vector Ecology. 2004;29:41–50. [PubMed] [Google Scholar]
- Foley JE, Nieto NC, Massung R, Barbet A, Madigan J, Brown RN. Distinct ecologically relevant strains of Anaplasma phagocytophilum. Emerging Infectious Diseases. 2009b;15:842–843. doi: 10.3201/eid1505.081502. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Furman DP, Loomis EC. The ticks of California (Acari: Ixodida) Berkeley, CA: University of California Press; 1984. [Google Scholar]
- Greig B, Asanovich KM, Armstrong PJ, Dumler JS. Geographic, clinical, serologic, and molecular evidence of granulocytic ehrlichiosis, a likely zoonotic disease, in Minnesota and Wisconsin dogs. Journal of Clinical Microbiology. 1996;34:44–48. doi: 10.1128/jcm.34.1.44-48.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Levin ML, Ross DE. Acquisition of different isolates of Anaplasma phagocytophilum by Ixodes scapularis from a model animal. Vector Borne Zoonotic Dis. 2004;4:53–59. doi: 10.1089/153036604773082997. [DOI] [PubMed] [Google Scholar]
- Lewis G, Huxsoll D, Ristic M, Johnson A. Experimentally induced infection of dogs, cats, and nonhuman primates with Ehrlichia equi, etiologic agent of equine ehrlichiosis. American Journal of Veterinary Research. 1975;36:85–88. [PubMed] [Google Scholar]
- Linsdale JM, Tevis LP. The dusky-footed wood rat; a record of observations made on the Hastings Natural History Reservation. Berkeley, CA: University of California Press; 1951. [Google Scholar]
- Madigan J. Equine ehrlichiosis. Veterinary Clinics of North America: Equine Practice. 1993;9:423–428. doi: 10.1016/s0749-0739(17)30408-x. [DOI] [PubMed] [Google Scholar]
- Munderloh U, Kurtti T. Cellular and molecular interrelationships between ticks and prokaryotic tick-borne pathogens. Annual Review of Entomology. 1995;40:221–243. doi: 10.1146/annurev.en.40.010195.001253. [DOI] [PubMed] [Google Scholar]
- Nicholson WL, Castro MB, Kramer VL, Sumner JW, Childs JE. Dusky-footed wood rats (Neotoma fuscipes) as reservoirs of granulocytic Ehrlichiae (Rickettsiales: Ehrlichieae) in northern California. Journal of Clinical Microbiology. 1999;37:3323–3327. doi: 10.1128/jcm.37.10.3323-3327.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nieto N, Foley J. Reservoir competence of the redwood chipmunk, Tamias ochrogenys, for Anaplasma phagocytophilum. Vector Borne Zoonotic Dis. 2009;9:573–577. doi: 10.1089/vbz.2008.0142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nieto N, Madigan JE, Foley J. The dusky-footed woodrat (Neotoma fuscipes) is susceptible to infection by Anaplasma phagocytophilum originating from woodrats, horses, and dogs. Journal of Wildlife Diseases. 2010;46:810–817. doi: 10.7589/0090-3558-46.3.810. [DOI] [PubMed] [Google Scholar]
- Nieto N, Foley P, Calder L, Dabritz H, Adjemian J, Conrad PA, Foley J. Ectoparasite diversity and exposure to vector-borne disease agents in wild rodents in central coastal California. Journal of Medical Entomology. 2007;44:328–335. doi: 10.1603/0022-2585(2007)44[328:edaetv]2.0.co;2. [DOI] [PubMed] [Google Scholar]
- Nieto NC, Foley JE. Evaluation of squirrels (Rodentia: Sciuridae) as ecologically significant hosts for Anaplasma phagocytophilum in California. Journal of Medical Entomology. 2008;45:763–769. doi: 10.1603/0022-2585(2008)45[763:eosrsa]2.0.co;2. [DOI] [PubMed] [Google Scholar]
- Norris DE, Klompen JS, Keirans JE, Lane RS, Piesman J, Black WCT. Taxonomic status of Ixodes neotomae and I. spinipalpis (Acari: Ixodidae) based on mitochondrial DNA evidence. Journal of Medical Entomolog. 1997;34:696–703. doi: 10.1093/jmedent/34.6.696. [DOI] [PubMed] [Google Scholar]
- Telford SR, Dawson JE, Katavolos P, Warner CK, Kolbert CP, Persing DH. Perpetuation of the agent of human granulocytic ehrlichiosis in a deer tick-rodent cycle. Proceedings of the National Academy of Sciences USA. 1996;93:6209–6214. doi: 10.1073/pnas.93.12.6209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Webb J, Bennett SN, Challet G. The larval ticks of the genus Ixodes Latreille (Acari: Ixodidae) of California. Bulletin of the Society of Vector Ecology. 1990;5:73–124. [Google Scholar]
- Zeidner NS, Burkot TR, Massung R, Nicholson WL, Dolan MC, Rutherford JS, Biggerstaff BJ, Maupin GO. Transmission of the agent of human granulocytic ehrlichiosis by Ixodes spinipalpis ticks: evidence of an enzootic cycle of dual infection with Borrelia burgdorferi in Northern Colorado. Journal of Infectious Diseases. 2000;182:616–619. doi: 10.1086/315715. [DOI] [PubMed] [Google Scholar]