Abstract
Objective
To determine whether there is a relationship between depressive symptoms and cortisol assessed at first morning awakening, 6PM, and 9PM in a population-based sample of midlife women. If this relationship is not linear, we aim to test whether this relationship is nonlinear, only present in those with more severe depressive symptoms, better accounted for by diurnal slope, or only apparent under uncontaminated conditions.
Methods
We investigated the cross-sectional association between cortisol and depressive symptoms, assessed by the Center for Epidemiological Studies Depression Scale (CES-D) in 408 midlife women (45.7% African-Americans, 54.3% white; mean age = 50.4) participating in the Chicago site of the Study of Women’s Health Across the Nation (SWAN).
Results
Diurnal cortisol slope is significantly flatter for women with higher CES-D scores than for less depressed women (p<.05 for the interaction). This relationship remains significant even after adjusting for age, smoking status, race, education, income, menopausal status, hormone replacement therapy (HRT), body mass index (BMI), medications, and wake time as well as possibly contaminating factors including physical activity, smoking, eating, or caffeine or alcohol consumption prior to saliva collection. Results using depression assessed categorically (CES-D cutoff ≥ 16) were similar to those using continuous depression in both unadjusted and adjusted analyses (p=.005 for the interaction of CES-D by time).
Conclusions
In this population-based sample of midlife women, greater depressive symptoms were associated with a significantly flatter diurnal cortisol slope than those with fewer symptoms, even after adjusting for covariates and possibly contaminating behaviors.
Keywords: diurnal cortisol, depressive symptoms, midlife women, women’s health
INTRODUCTION
Hypercortisolism is a well-established correlate of severe depressive states (1-4). Earlier studies noted that patients with severe depressive illness exhibited hypercortisolism, particularly in the late evening or early morning, that was resistant to dexamethasone suppression (5-7). Significant associations have been demonstrated in samples that compare only those with severe depression to controls (8) as well as those that look only at depressed patients (9,10).
However, the literature examining the relationship between depressive symptoms and cortisol is inconsistent, with some studies demonstrating no significant association at all (11-16). The characterization of hypothalamic-pituitary-adrenal (HPA) axis dysfunction is complicated and affected by a multitude of variables, thus providing an explanation for this observed inconsistency. Moreover, studies that include people with underlying mental health disorders (8,17) tend to find this effect more often than those involving subjects without these issues (12,18).
There are a number of reasons this inconsistency could exist. Alternatively, the relationship between depressive symptoms and cortisol may not be linear. Later life depression has been shown to be associated with both hypo- and hypercortisolemia without the presence of a main effect for depression (11,19).
There are distinct ways to evaluate cortisol levels, including at various time points throughout the day (17), as a response to awakening (12,13,18), expressly at morning or evening times (20), or as the diurnal slope (14). Due to the variation in cortisol levels over the course of the day, assessing it based on its change over time, or slope, may be a more comprehensive approach. Diurnal cortisol fluctuations are being increasingly used as a marker for depressive symptoms (21-23) with recent evidence suggesting altered diurnal secretion of cortisol as a possible endophenotype of depression and indicator of depression susceptibility (24).
Studies evaluating the relationship between depression and cortisol use a wide range of cortisol sampling techniques with inconsistent findings. These techniques include urinary (15,25), salivary (12,16,17) and serum (8,20) samples measured at different times. These varying collection techniques have not yielded a clear consensus about which is most valid. Additionally, collection technique varies between home collection (16) versus the laboratory setting (20) where variables can be more tightly controlled. In the home setting, extraneous factors such as smoking, caffeine, alcohol, food intake, and exercise, if they closely precede salivary sampling, could affect the relationship.
Finally, variations in characteristics of the sample, including such characteristics as physical activity, smoking, age, income, education, race, menopausal status, use of hormone replacement therapy, body mass index (BMI), sleep, and medications affect cortisol levels. Failure to account for these variables as covariates in analyses could affect the consistency of observed associations (26,27).
The purpose of this study was to determine whether there is a relationship between depressive symptoms and cortisol assessed at first morning awakening, 6PM, and 9PM in a population-based sample of midlife women. If this relationship is not linear, we aim to test whether this relationship is nonlinear, only present in those with more severe depressive symptoms, better accounted for by diurnal cortisol slope, or only apparent under uncontaminated conditions.
METHODS
Participants
Participants were 408 women (45.7% African-American; 54.3% White) from the Chicago site of the Study of Women’s Health Across the Nation (SWAN) who were participating in an ancillary study of the impact of menopause on accumulation of visceral adipose tissue (SWAN Fat Patterning Study). SWAN is an ongoing longitudinal study conducted at seven clinical sites of women transitioning through menopause. Eligibility was determined and initial data on socioeconomic, demographic, lifestyle, and health factors were collected via telephone and in-person screening surveys at all sites from November 1995 through October 1997. By design, the Chicago site recruited only non-Hispanic white and black women. A unique feature of the Chicago SWAN site is that it is a population-based design, which drew on a complete community census to recruit a sample of black and white women with a 72% participation rate. These women were recruited in a way that featured comparability on socioeconomic status (SES) within the black and white women, thus minimizing any confound between ethnicity and SES. Details of SWAN recruitment have been reported previously (28,29).
Women were enrolled in the SWAN Fat Patterning Study between August 2002 and December 2005 coincident with their annual SWAN follow-up visits. To be eligible for this ancillary study, women had to have no history of diabetes, chronic liver or renal disease, anorexia nervosa (self-report), hysterectomy and/or bilateral oophorectomy, or alcohol or drug abuse. They were also not currently pregnant or planning to become pregnant. Equipment limitations precluded participation of women with breast implants, hip replacements, or weighing ≥299 pounds. Seventy-seven percent of eligible Chicago SWAN participants enrolled in the Fat Patterning Study. Since the menopausal transition was under investigation for the Fat Patterning Study and many of the initial participants were already post-menopausal when the ancillary study began, additional subjects who had yet to complete the menopausal transition were recruited. Thus, 138 women (65% of eligible) who were screened for the original SWAN recruitment but were too young to participate in 1996 were recruited to the Fat Patterning Study. These newly recruited women were younger but did not differ in depressive symptoms, BMI, education, or age-adjusted total fat or visceral adipose tissue from previously recruited women. The final cohort included 435 women (199 African-Americans; 236 Whites). Missing data on depressive symptoms (n = 22) and all cortisol levels (n = 5), left 408 women (196 African-Americans, 212 Whites) for the current analyses.
Protocol
At entry into SWAN and annually thereafter, all participants completed a standard protocol, with self- and interviewer-administered questionnaires about psychosocial and lifestyle factors, health status, medical history and medication use, menstrual status and symptoms. Full details of the SWAN protocol have been reported previously (29). Covariates of interest for the present analyses were measured during the annual SWAN assessment coincident with recruitment to the Fat Patterning Study. The 138 women recruited specifically for the Fat Patterning Study completed the full SWAN protocol, including all measurements obtained for the annual SWAN visit.
The Rush University Medical Center Institutional Review Boards approved all aspects of the study and all women provided their written informed consent.
Study Measures
Depressive Symptoms
Depressive symptoms were measured continuously according to the Center for Epidemiologic Studies-Depression (CES-D) Scale (30). Subjects were considered to have significant depressive symptoms if they had a CES-D score of 16 or higher and they were considered to have severe depressive symptoms if they had a score of 24 or higher (31). The CES-D value from the closest preceding SWAN core visit to the baseline fat patterning cortisol sample was used. 92% of the CES-D values were collected no more than 4 months prior to cortisol sampling, and none were collected more than one year prior.
Cortisol Measurements
Participants were instructed to collect home salivary cortisol samples after their SWAN baseline fat patterning visit. They were instructed to collect saliva at 6PM and 9PM that evening and again the following day immediately upon awakening.
All samples were sent to the Kirschbaum laboratory in Germany for quantification. Free cortisol was assayed from saliva centrifuged to obtain a clear specimen of low viscosity. Cortisol levels were then quantified by radioimmunoassay with time-resolved flurometric detection (32). Inter- and intra-assay coefficients of variation were <10% and <8%, respectively.
Covariate Measurements
Covariates were obtained from the SWAN core visit and included age, BMI, education, income, race, menopausal status, use of hormone replacement therapy, medications, and smoking. Education was categorized as having had post-high school training or not. Income was categorized as less than $20,000, $20,000-$50,000, and greater than $50,000. Menopausal status was divided into four categories for consideration: 1) Premenopausal - regular bleeding, 2) Early perimenopausal - irregular bleeding with bleeding in the last 3 months, 3) Late perimenopausal – bled in the last 3-12 months, and 4) Postmenopausal – no bleeding in the last 12 months.
Subjects maintained a “saliva diary” that included the actual time cortisol samples were taken as well as whether and what time subjects were physically active, drank caffeine or alcohol, smoked, or ate. These variables were analyzed individually with the other covariates as well as packaged together as “contaminating factors” for analysis purposes in order to assess their ability to directly affect the actual cortisol sampling. Wake time was also obtained via the diary but was only used as a covariate, not contaminating factor. All 15 covariates above were used for the adjusted models.
Analyses
Graphical and descriptive statistics confirmed that the cortisol concentrations at all three time points did not follow a normal distribution and were heavily skewed with a long right tail; transformation by natural logarithm normalized the three distributions. All further analyses used these transformed cortisol levels. Univariate statistics were computed for continuous variables and evaluated for consistency with a normal distribution. Means (SD) were used to describe normally distributed variables such as age and body mass index. Median and interquartile range (difference between 75th and the 25th percentiles) were used to characterize variables with highly skewed distributions. Frequencies were determined for categorical variables.
We used t-tests and chi square tests (p<.05) to evaluate differences between women with depressive symptoms and those without for population descriptors and potential covariates. To investigate the hypothesis that cortisol levels at any one time point (morning, 6pm, 9pm) vary with increasing depressive symptoms, we used linear regression models of the (log transformed) cortisol levels with continuous depression (CES-D) as the only predictor at each separate time point. To test for a non-linear relationship, quadratic and cubic terms of the continuous depression value were added to the regression models of cortisol at the three collection points. Linear models were adjusted using backward variable selection for age, education, physical activity, smoking status, race, menopausal status, hormone replacement therapy, steroid and antidepressent use. Variables significant with a liberal p-value of <.15 were retained. After that set of covariates was identified, use of cigarettes, caffeine, alcohol, exercise and food intake before the cortisol collection that day were adjusted for as a single indicator variable. To investigate the hypothesis that cortisol levels for women with higher levels of depressive symptoms were greater than those with lower levels, we compared the log transformed cortisol levels at each collection time with a t-test. In addition to the well-established cut-point of 16, we also used a score of 24 as an cut-off for more severe depressive symptoms.
To investigate the difference in diurnal slope associated with different levels of depressive symptoms, we treated the morning collection value as if it was collected on the same day as evening cortisol measures. Time of collection was taken as hours from awakening to collection of cortisol levels. We used a mixed effects regression model with a random intercept and random time variables. This type of model allows for a woman-specific intercept and slope and the assessment of an overall trend. We included a time by CES-D score interaction to investigate the difference in the diurnal slope. We adjusted this model for hours from mean wake time to account for difference in sleep patterns. We used stepwise backward selection to identify significant predictors of the diurnal cortisol slope from a list of variables identified in the literature (education, physical activity, smoking status, race, menopausal status, hormone replacement therapy, steroid and antidepressent/anxiolytic use). Variables significant with a liberal p-value of <.15 were retained. Secondary analyses used the dichotomous CES-D as a predictor. These results were very similar to the continuous depression scores. We therefore only present a graphic summary.
RESULTS
Table 1 presents the baseline characteristics of the 408 women participating in the study overall and comparing CES-D ≥ 16 and CES-D < 16. There were significantly more smokers (p<.001), African-Americans (p=.009), and lower income people (p=.005) in the higher CES-D group. No other differences were detected between the two groups.
Table 1.
Baseline Characteristics of the Cohort
| All N = 408 |
CESD ≥ 16 N = 56 |
CESD < 16 N = 352 |
p-value* | |
|---|---|---|---|---|
| Age, mean (Std) | 50.4 (3.8) | 50.4(3.6) | 50.4 (3.8) | .94 |
| Smoker, N(%) | 82 (20.1) | 22 (39.3) | 60 (17.0) | <.001 |
| African American, N(%) | 196 (45.7) | 34 (60.7) | 148 (42.0) | .009 |
| Education <= HS, N(%) | 44 (11.1) | 7 (13.2) | 37 (10.8) | .61 |
| Income, N(%) | .005 | |||
| < $20,000 | 28 (7.0) | 8 (15.1) | 20 (5.8) | |
| $20,000-$50,000 | 64 (16.1) | 13 (24.5) | 51 (14.8) | |
| >$50,000 | 305 (76.8) | 32 (60.3) | 273 (79.4) | |
| Menopausal Status, N(%) | .96 | |||
| Surgical | 22 (5.4) | 3 (5.4) | 19 (5.4) | |
| Post | 147(36.0) | 19 (33.9) | 128 (36.4) | |
| Late Peri | 33 (8.1) | 3 (5.4) | 30(8.5) | |
| Early Peri | 136(33.3) | 21(37.5) | 115(32.7) | |
| Pre | 48 (11.8) | 7(12.5) | 41(11.7) | |
| undetermined | 22 (5.4) | 3(5.4) | 19(5.4) | |
| Uses hormones, N(%) | 45 (11.0) | 8 (14.3) | 37 (10.5) | .40 |
| Corticosteroid Use, N (%) | 11 (2.7) | 0 (0.0) | 11 (3.1) | .18 |
| Antidepressant Use, N(%) | 44 (10.8) | 12 (21.4) | 32 (9.1) | .006 |
| BMI, mean (std) | 29.2 (6.4) | 29.9 (6.7) | 29.0 (6.3) | .32 |
| Ate before 6 pm, N(%) | 240 (58.8) | 30 (53.6) | 210 (59.7) | .39 |
| Ate before 9 pm, N(%) | 341 (83.6) | 47 (83.9) | 294 (83.5) | .94 |
| Exercised before 6 pm, N(%) | 28 (6.9) | 3 (5.4) | 25 (7.1) | .63 |
| Exercised before 9 pm, N(%) | 5 (1.2) | 1 (1.8) | 4 (1.1) | .68 |
| Caffeine before 6 pm, N(%) | 100 (25.7) | 20 (37) | 80 (23.9) | .04 |
| Alcohol before 6 pm, N(%) | 70 (17.9) | 9 (16.4) | 61 (18.1) | .76 |
| Smoking after 5 pm, N(%) | 76 (19.4) | 21 (38.2) | 55 (16.3) | <.001 |
| Wake time | 06:53 AM | 07:08 AM | 06:50 AM | .16 |
t-tests was used for normally distributed variables and chi square tests for frequencies
Table 2 demonstrates that women with higher levels of depressive symptoms had significantly higher mean levels of cortisol at 6PM (β = .014, p<.05) in the unadjusted linear regression. Depressive symptoms were not found to be predictive of morning or 9PM cortisol levels. This model using depressive symptoms measured continuously was rerun for the 6PM cortisol level using the aforementioned covariates in a backwards stepwise linear regression model. The relationship between cortisol and depression failed to hold after adjustment for smoking and education (p=.13 for CES-D, p<.05 for smoking). The relationship between cortisol concentrations at the three time points and depressive symptoms, using CES-D as a categorical variable (CES-D ≥ 16 vs CES-D < 16), was examined using a t-test for the unadjusted comparisons and linear regression models for the adjusted models and revealed the same pattern of results.
Table 2.
Multivariate Correlates of Cortisol at Three Separate Times (Morning, 6PM, and 9PM)
| Morning | 6 pm | 9 pm | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Unadjusted | Adjusted | Unadjusted | Adjusted | Unadjusted | Adjusted | |||||||
| β est. | p-value | β est. | p-value | β est. | p-value | β est. | p-value | β est. | p-value | β est. | p-value | |
| CESDa | -.010 | .156 | -.006 | .44 | .014 | .018 | .009 | .13 | .004 | .518 | -.002 | .67 |
| Income < $20,000 | -.044 | .82 | - | - | - | - | ||||||
| Income $20,000 - $50,000 | -.293 | .03 | - | - | - | - | ||||||
| African American | -.138 | .18 | - | - | .419 | <.001 | ||||||
| Smoker | - | - | .228 | .04 | .346 | .005 | ||||||
| Education <= HS | - | - | .240 | .08 | - | - | ||||||
| Age | - | - | - | - | .028 | .058 | ||||||
| Alcohol consumed prior to cortisol sampling that day | -.194 | .14 | ||||||||||
| Post Menopausal | -.150 | .18 | ||||||||||
Estimates are beta coefficients from linear models
CES-D measured continuously
Quadratic terms were added to the linear models in order to assess a curvilinear relationship between CES-D and cortisol levels at any time point but no curvilinear relationship was detected.
No significant difference at p=.05 level was found between those who have severe depressive symptoms (CES-D≥24, N=19) and those who have less severe depressive symptoms (CES-D<24). Cortisol levels at the 6PM collection time were marginally lower in the more depressed group (p<.10).
Diurnal Cortisol Slope
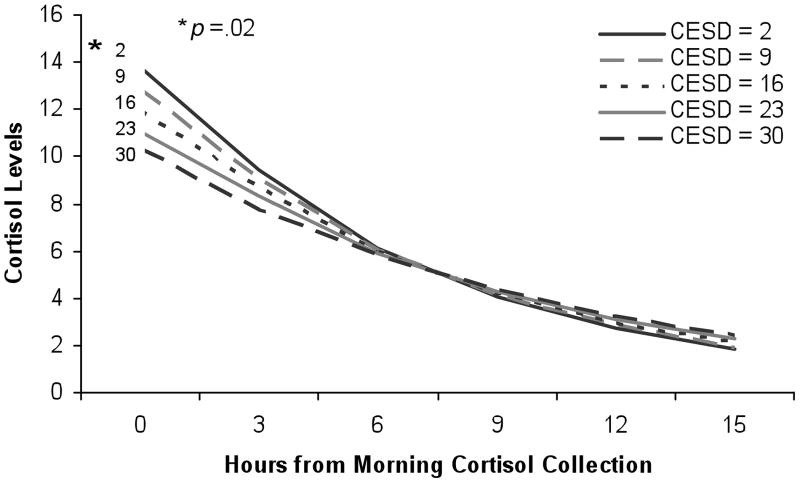
Table 3 shows the results of the mixed effects regression models to test the hypothesis that the pattern of cortisol over time differs by depressive symptoms as a continuous variable. The basic model on the left shows that in the unadjusted analysis, there is a significant overall decrease in cortisol from morning to night (β= −0.138, p<.001). With every increase in CES-D by 1 point, the slope flattens, as indicated by the interaction between CES-D and time (β= 0.001, p=0.02). Figure 1 presents this interaction graphically. Results were very similar in adjusted analyses. Results were also very similar in analyses using the dichotomous CES-D score, as Figure 2 illustrates.
Table 3.
Multivariate Correlates of Cortisol Slope
| Variable | Unadjusted | Adjusted** | ||
|---|---|---|---|---|
| β est. | p-value | β est. | p-value | |
| Intercept | 2.644 | <.001 | 2.610 | <.001 |
| CES-Da | -0.010 | 0.13 | -0.013 | 0.055 |
| Time | -0.138 | <.001 | -0.139 | <.001 |
| Time* (CES-D) | 0.001 | 0.02 | 0.001 | 0.02 |
| Wake time from 7 AM | - | - | 0.018 | 0.42 |
| Smoker | - | - | 0.135 | 0.11 |
| African American | - | - | 0.109 | 0.10 |
| Contaminated | - | - | -0.035 | 0.59 |
Estimates are beta coefficients from mixed models
CES-D measured continuously
Adjusted for age, smoking status, race, education, income, menopausal status, HRT, BMI, wake time, medications as well as physical activity, smoking, eating, or caffeine or alcohol consumption prior to saliva collection as indicated by “saliva diary”
Figure 1.
Graded association of depression and the diurnal slope of cortisol after adjustment for all covariates. The interaction of CES-D by time is significant (p<.05) using the continuous version of the CES-D.
Figure 2.
Average diurnal slope of cortisol for depressed and non-depressed women after adjustment for all covariates. The slope is significantly steeper (p=.005 for the interaction of CES-D by time) for women with CES-D<16 than for more depressed women.
Contamination Factors
The significant continuous (Figure 1) and categorical (Figure 2) relationships between CES-D and diurnal cortisol slope remained significant with the addition of contaminating factors to the model. There was no interaction of contamination and CES-D score (p=.60).
DISCUSSION
Due to the inconsistency in the literature regarding the relationship between cortisol and depressive symptoms (11-16), we aimed to understand this association better by studying a large, population-based sample of midlife women. We found that the diurnal slope of salivary cortisol was strongly and independently associated with depressive symptoms in these midlife women. This is a novel finding given that this is a large, generalizable, non-clinical, and community-based epidemiologic sample.
The results of the current study are significant for several reasons. First, they are biologically plausible. Support for this association has been provided by clinical studies aimed at developing hypotheses about the etiology and pathogenesis of depression (33). Of particular relevance is the hypothesis that depression involves changes in the set point of the HPA system, resulting in altered regulation of cortisol secretory activity via changes in corticotropin-releasing hormone (34). The suprachiasmatic nucleus (SCN) is one possible mediator of this diurnal cortisol-depression relationship. The SCN is the hypothalamic clock responsible for the rhythmic changes of the stress system, and it appears to have decreased activity in depression (33). It also has CRH fibers (33), demonstrating an innate hard wiring for a link between depression, corticosteroids, and diurnal variance. Complex systemic dysregulation involving increased CRH and impaired corticosteroid receptor signaling (34) may be clinically manifest as impaired diurnal cortisol variation with depression, as we see with our cohort.
Our study also provides a naturalistic, population-based investigation of cortisol response as opposed to more tightly controlled experimental samples (6,19) or clinical populations (8,17,25). As noted by Breslau, the suspected association between a disease and a characteristic may be most pronounced in, and often comes from, clinical samples, however, the validity of this association frequently comes from its replication in epidemiological studies (35).
Since cortisol is sensitive to, and affected by, a multitude of extraneous influences (36), measuring cortisol reliably in a population-based sample is especially challenging. Our findings indicate that the association with depressive symptoms is not altered by specific behaviors at the time of saliva sampling that may contaminate the cortisol specimen. It is important to identify reliable, rigorous, low cost, and minimal burden physiologic markers of psychosocial stressors for use in large-scale epidemiologic studies (37). We have shown that with only three cortisol samples collected at home over the course of a day we can obtain a slope that demonstrates a valid association with depressive symptoms.
Diurnal cortisol is a reliable marker for depressive symptoms even under the influence of the myriad of variables inevitably present in a population-based sample, including differences in sociodemographic factors as well as health and lifestyle behaviors. This supports the independence of the relationship from other known predictors.
Interestingly, the flattened diurnal cortisol slope we found possesses different characteristics than some cortisol rhythm disturbances previously demonstrated. Early timing of the nadir of ACTH-cortisol secretion has been demonstrated in major depressive disorder (38) and steeper, more rhythmic cortisol slopes driven by elevated morning cortisol has been shown in depressed women with metastatic breast cancer (23). Our findings, however, illustrate that the dominant effect characterizing flattened cortisol slope seems to be a lower morning cortisol level, which is related in a dose-dependent way to depressive symptoms and does not result in an overall hypercortisolemic state. CES-D scores in this relatively healthy population may reflect less obvious symptomatology than clinical depression, and this blunted or reduced morning rise may be a more subtle, initial sign. In this study of community participants, it appears that a blunting of the diurnal cortisol rhythm is a more sensitive indicator of depression than elevated salivary cortisol levels. Due to the complex and likely bidirectional interaction of cortisol and depressive symptoms, it is difficult to comment on its clinical significance in particular, however, its physiological significance is noteworthy given the disparate findings from previous research.
There may be variation in phenotypes underlying the mechanisms linking psychological processes to health and disease. It has been demonstrated that age, gender, and severity of psychological distress may have varying physiologic manifestations (9). Certain medical comorbidities may also affect this association as flatter diurnal cortisol slope has been found in depressed people with memory problems (21) as well as depressed people with coronary artery disease (22). Understanding these various phenotypes is crucial to targeting populations at risk for adverse health consequences of psychological distress.
Finally, our study population is of clinical importance as midlife women in the late stage of the menopausal transition are particularly vulnerable to depressed mood (39), with suggestive data that estrogen deficiency (40) as well as an increasingly testosterone dominated milieu (41) may contribute to this increased susceptibility. Combined with the loss of protective cardiovascular sex hormones during this time period (42) as well as already lower bone mineral density in premenopausal women with depression (43), this particular population is especially susceptible to adverse physiological effects of depression.
There are several limitations to this study. We had a relatively small number of depressed subjects in this population-based sample. However, given that the diurnal cortisol slope has a strong association with depressive symptoms even with this small number, it does not appear to have affected results. While we have awakening times and information on overall sleep problems, we do not have specific information about sleep from the night preceding morning cortisol measurements. It would be interesting to see whether sleep patterns the night before demonstrate an association with cortisol. Nor do we have overnight cortisol levels that, despite its impracticality, would be interesting to have and see whether depressive symptoms affected very early morning cortisol response. Finally, our CES-D and cortisol assessments were separated in time by as many as a few months, which may not be the traditional nature of how this relationship is usually examined. If this in fact introduced error, the direction of bias would be in the null, however, this is not what we found, lending further strength to the relationship we observed. It is unclear whether a flattened diurnal cortisol slope represents a persistent state characteristic of depression or whether this may be more consistent with a trait characteristic. Further studies investigating the temporal relationship between these variables are needed.
Future research should focus on clarifying its clinical significance and determining whether alteration in diurnal cortisol slope may be associated with adverse health consequences observed in depressed individuals (44). Everson-Rose et al recently showed that increased visceral fat may be one pathway by which depression contributes to excess risk for cardiovascular disease and diabetes (45); it would be meaningful to examine whether diurnal cortisol slope is a mediator in this relationship.
In conclusion, midlife women from a population-based sample with higher CES-D scores have a significantly flatter diurnal cortisol slope than those with lower scores, even after adjusting for covariates and possibly contaminating behaviors. Further research is needed to examine the health implications of this association.
Acknowledgments
The Study of Women’s Health Across the Nation (SWAN) has grant support from the National Institutes of Health (NIH), DHHS, through the National Institute on Aging (NIA), the National Institute of Nursing Research (NINR) and the NIH Office of Research on Women’s Health (ORWH) (Grants NR004061; AG012505, AG012535, AG012531, AG012539, AG012546, AG012553, AG012554, AG012495). The SWAN Fat Patterning Study is supported by the National Heart, Lung and Blood Institute (Grant HL067128) and the Charles J. and Margaret Roberts Trust. The content of this manuscript is solely the responsibility of the authors and does not necessarily represent the official views of the NHLBI, NIMH, NIA, NINR, ORWH, or NIH.
Abbreviations
- HPA
hypothalamic-pituitary-adrenocortical
- BMI
body mass index
- SWAN
Study of Women’s Health Across the Nation
- SES
socioeconomic status
- CES-D
Center for Epidemiologic Studies-Depression
- SCN
Suprachiasmatic Nucleus
- CRH
Corticotropin-Releasing Hormone
References
- 1.Pariante CM, Miller AH. Glucocorticoid receptors in major depression: relevance to pathophysiology and treatment. Biol Psychiatry. 2001;49:391–404. doi: 10.1016/s0006-3223(00)01088-x. [DOI] [PubMed] [Google Scholar]
- 2.Gold PW, Goodwin FK, Chrousos GP. Clinical and biochemical manifestations of depression: relation to the neurobiology of stress (second of two parts) N Engl J Med. 1988;319(7):413–420. doi: 10.1056/NEJM198808183190706. [DOI] [PubMed] [Google Scholar]
- 3.Irwin MR, Miller AH. Depressive disorders and immunity: 20 years of progress and discovery. Brain Behav Immun. 2007;21:374–383. doi: 10.1016/j.bbi.2007.01.010. [DOI] [PubMed] [Google Scholar]
- 4.Barden N, Reul JMHM, Holsboer F. Do antidepressants stabilize mood through actions on the hypothalamic-pituitary-adrenocortical system? Trends Neurosci. 1995;18(1):6–11. doi: 10.1016/0166-2236(95)93942-q. [DOI] [PubMed] [Google Scholar]
- 5.Sachar EJ. Neuroendocrine dysfunction in depressive illness. Annu Rev Med. 1976;27:389–396. doi: 10.1146/annurev.me.27.020176.002133. [DOI] [PubMed] [Google Scholar]
- 6.Carroll BJ, Curtis GC, Mendels J. Neuroendocrine regulation in depression. II. Discrimination of depressed from nondepressed patients. Arch Gen Psychiatry. 1976;33:1051–1058. doi: 10.1001/archpsyc.1976.01770090041003. [DOI] [PubMed] [Google Scholar]
- 7.Kocsis JH, Brockner N, Butler T, Fanelli C, Stokes PE. Dexamethasone suppression in major depression. Biol Psychiatry. 1984;19:1255–1259. [PubMed] [Google Scholar]
- 8.Deuschle M, Schweiger U, Weber B, Gotthardt U, Korner A, Schmider J, Standhardt H, Lammers C, Heuser I. Diurnal activity and pulsatility of the hypothalamus-pituitary-adrenal system in male depressed patients and healthy controls. J Clin Endocrinol Metab. 1997;82:234–238. doi: 10.1210/jcem.82.1.3689. [DOI] [PubMed] [Google Scholar]
- 9.Grant MM, Friedman ES, Haskett RF, Riso LP, Thase ME. Urinary free cortisol levels among depressed men and women: differential relationships to age and symptom severity? Arch Womens Ment Health. 2007;10:73–78. doi: 10.1007/s00737-007-0171-2. [DOI] [PubMed] [Google Scholar]
- 10.Vreeburg SA, Hoogendijk WJG, van Pelt J, DeRijk RH, Verhagen JCM, van Dyck R, Smit JH, Zitman FG, Penninx BWJH. Major depressive disorder and hypothalamic-pituitary-adrenal axis activity. Arch Gen Psychiatry. 2009;66(6):617–626. doi: 10.1001/archgenpsychiatry.2009.50. [DOI] [PubMed] [Google Scholar]
- 11.Penninx BWJH, Beekman ATF, Bandinelli S, Corsi AM, Bremmer M, Hoogendijk WJ, Guralnik JM, Ferrucci L. Late-life depressive symptoms are associated with both hyperactivity and hypoactivity of the hypothalamo-pituitary-adrenal axis. Am J Geriatr Psychiatry. 2007;15:522–529. doi: 10.1097/JGP.0b013e318033ed80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Whitehead DL, Perkins-Porras L, Strike PC, Magid K, Steptoe A. Cortisol awakening response is elevated in acute coronary syndrome patients with Type-D personality. J Psychosom Res. 2007;62:419–425. doi: 10.1016/j.jpsychores.2006.11.005. [DOI] [PubMed] [Google Scholar]
- 13.Conrad A, Wilhelm FH, Roth WT, Spiegel D, Taylor CB. Circadian affective, cardiopulmonary, and cortisol variability in depressed and nondepressed individuals at risk for cardiovascular disease. J Psychiatr Res. 2008;42:769–777. doi: 10.1016/j.jpsychires.2007.08.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Gallagher-Thompson D, Shurgot GR, Rider K, Gray HL, McKibbin CL, Kraemer HC, Sephton SE, Thompson LW. Ethnicity, stress, and cortisol function in Hispanic and non-Hispanic white women: a preliminary study of family dementia caregivers and noncaregivers. Am J Geriatr Psychiatry. 2006;14:334–342. doi: 10.1097/01.JGP.0000206485.73618.87. [DOI] [PubMed] [Google Scholar]
- 15.Vogelzangs N, Suthers K, Ferucci L, Simonsick EM, Ble A, Schrager M, Bandinelli S, Lauretani F, Gianelli SV, Penninx BW. Hypercortisolemic depression is associated with the metabolic syndrome in late-life. Psychoneuroendocrinology. 2007;32:151–159. doi: 10.1016/j.psyneuen.2006.11.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.van Eck M, Berkhof H, Nicolson N, Sulon J. The effects of perceived stress, traits, mood states, and stressful daily events on salivary cortisol. Psychosom Med. 1996;58:447–458. doi: 10.1097/00006842-199609000-00007. [DOI] [PubMed] [Google Scholar]
- 17.Inslicht SS, Marmar CR, Neylan TC, Metzler TJ, Hart SL, Otte C, McCaslin SE, Larkin GL, Hyman KB, Baum A. Increased cortisol in women with intimate partner violence-related posttraumatic stress disorder. Psychoneuroendocrinology. 2006;31:825–838. doi: 10.1016/j.psyneuen.2006.03.007. [DOI] [PubMed] [Google Scholar]
- 18.O’Donnell K, Badrick E, Kumari M, Steptoe Psychological coping styles and cortisol over the day in healthy older adults. Psychoneuroendocrinology. 2008;33:601–611. doi: 10.1016/j.psyneuen.2008.01.015. [DOI] [PubMed] [Google Scholar]
- 19.Bremmer MA, Deeg DJH, Beekman ATF, Penninx BWJH, Lips P, Hoogendijk WJG. Major depression in late life is associated with both hypo- and hypercortisolemia. Biol Psychiatry. 2007;62:479–486. doi: 10.1016/j.biopsych.2006.11.033. [DOI] [PubMed] [Google Scholar]
- 20.Strickland PL, Deakin JFW, Percival C, Dixon J, Gater RA, Goldberg DP. Biosocial origins of depression in the community. Br J Psychiatry. 2002;180:168–173. doi: 10.1192/bjp.180.2.168. [DOI] [PubMed] [Google Scholar]
- 21.Fiocco AJ, Wan N, Weekes N, Pim H, Lupien SJ. Diurnal cycle of salivary cortisol in older adult men and women with subjective complaints of memory deficits and/or depressive symptoms: relation to cognitive functioning. Stress. 2006;9(3):143–52. doi: 10.1080/10253890600965674. [DOI] [PubMed] [Google Scholar]
- 22.Bhattacharyya MR, Molloy GJ, Steptoe A. Depression is associated with flatter cortisol rhythms in patients with coronary artery disease. J Psychosom Res. 2008;65(2):107–13. doi: 10.1016/j.jpsychores.2008.03.012. [DOI] [PubMed] [Google Scholar]
- 23.Sephton SE, Dhabjar FS, Keuroghlian AS, Giese-Davis J, McEwen BS, Ionana AC, Spiegel D. Depression, cortisol, and suppressed cell-mediated immunity in metastatic breast cancer. Brain Behav Immun. 2009 doi: 10.1016/j.bbi.2009.07.007. [DOI] [PubMed] [Google Scholar]
- 24.Wichers MC, Myin-Germeys I, Jacobs N, Kenis G, Derom C, Vlietnick R, Delespaul P, Mengelers R, Peeters F, Nicolson N, Van Os J. Susceptibility to depression expressed as alterations in cortisol day curve: a cross-twin, cross-trait study. Psychosom Med. 2008;70:314–18. doi: 10.1097/PSY.0b013e31816b1eee. [DOI] [PubMed] [Google Scholar]
- 25.Otte C, Marmar CR, Pipkin SS, Moos R, Browner WS, Whooley MA. Depression and 24-hour urinary cortisol in medical outpatients with coronary heart disease: The heart and soul study. Biol Psychiatry. 2004;56:241–247. doi: 10.1016/j.biopsych.2004.06.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Kudielka BM, Hellhammer DH, Wust S. Why do we respond so differently? Reviewing determinants of human salivary cortisol responses to challenge. Psychoneuroendocrinology. 2009;34(1):2–18. doi: 10.1016/j.psyneuen.2008.10.004. [DOI] [PubMed] [Google Scholar]
- 27.Hanse AM, Garde AH, Persson R. Sources of biological and methodological variation in salivary cortisol and their impact on measurement among healthy adults: a review. Scand J Clin Lab Invest. 2008;68(6):448–458. doi: 10.1080/00365510701819127. [DOI] [PubMed] [Google Scholar]
- 28.Sowers MF, Crawford S, Sternfeld B, Morganstein D, Gold EB, Greendale GA, Evans D, Neer R, Matthews K, Sherman S, Lo A, Weiss G, Kelsey J. SWAN: a multicenter, multiethnic, community-based cohort study of women and the menopausal transition. In: Lobo RA, Kelsey J, Marcus R, editors. Menopause: Biology and Pathobiology. San Diego: Academic Press; 2000. [Google Scholar]
- 29.Bromberger JT, Meyer PM, Kravitz HM, Sommer B, Cordal A, Powell L, Ganz PA, Sutton-Tyrrell K. Psychologic distress and natural menopause: a multiethnic community study. Am J Public Health. 2001;91:1435–42. doi: 10.2105/ajph.91.9.1435. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Radloff LS. The CES-D scale. A self-report depression scale for research in the general population. Appl Psychol Meas. 1977;1:385–401. [Google Scholar]
- 31.Cohen LS, Soares CN, Vitonis AF, Otto MW, Harlow BL. Risk for new onset of depression during the menopausal transition. Arch Gen Psychiatry. 2006;63:385–390. doi: 10.1001/archpsyc.63.4.385. [DOI] [PubMed] [Google Scholar]
- 32.Drossendoerfer RA, Kirschbaum C, Rohde W, Stahl F, Strasburger CJ. Synthesis of a cortisol-biotin conjugate and evaluation as tracer in an immunoassay for salivary cortisol measurement. J Steroid Biochem Molec Biol. 1992;43:683–92. doi: 10.1016/0960-0760(92)90294-s. [DOI] [PubMed] [Google Scholar]
- 33.Bao AM, Meynen G, Swaab DF. The stress system in depression and neurodegeneration: focus on the human hypothalamus. Brain Res Rev. 2008;57:531–553. doi: 10.1016/j.brainresrev.2007.04.005. [DOI] [PubMed] [Google Scholar]
- 34.Holsboer F. The corticosteroid receptor hypothesis of depression. Neuropsychopharmacology. 2000;23(5):477–501. doi: 10.1016/S0893-133X(00)00159-7. [DOI] [PubMed] [Google Scholar]
- 35.Breslau N. Neurobiological research on sleep and stress hormones in epidemiological samples. Ann N Y Acad Sci. 2006;1071:221–230. doi: 10.1196/annals.1364.017. [DOI] [PubMed] [Google Scholar]
- 36.Burke HM, Davis MC, Otte C, Mohr DC. Depression and cortisol responses to psychological stress: a meta-analysis. Psychoneuroendocrinology. 2005;30:846–56. doi: 10.1016/j.psyneuen.2005.02.010. [DOI] [PubMed] [Google Scholar]
- 37.Powell LH, Lovallo WR, Matthews KA, Meyer P, Midgley AR, Baum A, Stone AA, Underwood L, McCann JJ, Herro KJ, Ory MG. Physiologic markers of chronic stress in premenopausal, middle-aged women. Psychosom Med. 2002;64:502–509. doi: 10.1097/00006842-200205000-00015. [DOI] [PubMed] [Google Scholar]
- 38.Linkowski P, Mendlewicz J, Leclerq R, Brasseur M, Hubain P, Golstein J, Copinschi G, Van Cauter E. The 24-hour profile of adrenocorticotropin and cortisol in major depressive illness. J Clin Endocrinol Metab. 1985;61(3):429–438. doi: 10.1210/jcem-61-3-429. [DOI] [PubMed] [Google Scholar]
- 39.Woods NF, Smith-DiJulio K, Percival DB, Tao EY, Mariella A, Mitchell ES. Depressed mood during the menopausal transition and early postmenopause: observations form the Seattle Midlife Women’s Health Study. Menopause. 2008;15:223–232. doi: 10.1097/gme.0b013e3181450fc2. [DOI] [PubMed] [Google Scholar]
- 40.Birkhauser M. Depression, menopause and estrogens: is there a correlation? Maturitas. 2002;41(Supp 1):S3–S8. doi: 10.1016/s0378-5122(02)00009-9. [DOI] [PubMed] [Google Scholar]
- 41.Janssen I, Powell LH, Crawford S, Lasley B, Sutton-Tyrrell K. Menopause and the metabolic syndrome: The Study of Women’s Health Across the Nation. Arch Intern Med. 2008;164(14):1568–75. doi: 10.1001/archinte.168.14.1568. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Kannel WB, Hjortland MC, McNamara PM, Gordon T. Menopause and risk of cardiovascular disease: the Framingham study. Ann Intern Med. 1976;85:447–452. doi: 10.7326/0003-4819-85-4-447. [DOI] [PubMed] [Google Scholar]
- 43.Eskandara F, Martinez PE, Torvik S, Phillips TM, Sternberg E, Mistry S, Ronsaville D, Wesley R, Toomey C, Sebring NG, Reynolds JC, Blackman MR, Calis KA, Gold PW, Cizza G. Low bone mass in premenopausal women with depression. Arch Intern Med. 2007;167(21):2329–2336. doi: 10.1001/archinte.167.21.2329. [DOI] [PubMed] [Google Scholar]
- 44.Vaccarino V, McClure C, Johnson BD, Sheps DS, Bittner V, Rutledge T, Shaw LJ, Sopko G, Olson MB, Krantz DS, Parashar S, Marroquin OC, Bairey Merz N. Depression, the metabolic syndrome and cardiovascular risk. Psychosom Med. 2008;70(40-48) doi: 10.1097/PSY.0b013e31815c1b85. [DOI] [PubMed] [Google Scholar]
- 45.Everson-Rose SA, Lewis TT, Karavolos K, Dugan SA, Wesley D, Powell LH. Depressive symptoms and increased visceral fat in middle-aged women. Psychosom Med. 2009;71:410–416. doi: 10.1097/PSY.0b013e3181a20c9c. [DOI] [PMC free article] [PubMed] [Google Scholar]