Abstract
Cytokines, such as interferons, erythropoietin, leptin and most interleukins, signal through type 1 cytokine receptors and activate the canonical JAK–STAT pathway. Aberrant cytokine signalling underlies numerous pathologies and adequate, temporary receptor activation is therefore under tight control. Negative-feedback mechanisms are very well studied, but cellular sensitivity also depends on the number of receptors exposed at the cell surface. This is determined by the equilibrium between receptor synthesis and transport to the plasma membrane, internalisation and recycling, degradation and ectodomain shedding, but the molecular basis of how cells establish steady state receptor levels is poorly understood. Here, we report that ring finger protein 41 (RNF41, also known as E3 ubiquitin-protein ligase Nrdp1) interacts with JAK2-associated cytokine receptor complexes and modulates their cell surface exposure and signalling. Moreover, ectopic expression of RNF41 affected turnover of leptin, leukaemia inhibitory factor and interleukin-6 receptor in a dual way: it blocked intracellular cathepsin-L-dependent receptor cleavage and concomitantly enhanced receptor shedding by metalloproteases of the ADAM family. Receptor degradation and shedding are thus interconnected phenomena with a single protein, RNF41, determining the balance.
Key words: Cathepsin L cleavage, Ectodomain shedding, MAPPIT, RNF41, Nrdp1, Type 1 cytokine receptor, ADAM
Introduction
Cytokines such as interferons, erythropoietin, leptin and most interleukins signal through type 1 cytokine receptors. Because aberrant cytokine signalling underlies many pathologies, including immunodeficiency, metabolic disorders and cancer, temporary cytokine receptor activation is tightly regulated. Negative-feedback mechanisms that operate to dampen signalling after receptor activation are very well studied. These include phosphatases (Xu and Qu, 2008), protein inhibitors of activated STAT (PIAS) proteins (Shuai and Liu, 2005) and the suppressor of cytokine signalling (SOCS) family (Lavens et al., 2006). Cellular sensitivity to cytokine signals, however, also depends on the number of receptors present at the time of stimulation. The molecular basis of how cells establish an appropriate number of receptors at their cell surface is far less well understood. Cell surface exposure of type 1 cytokine receptors is a highly dynamic process that, besides de novo synthesis, involves endocytosis followed by recycling or degradation. Internalised receptors, irrespective of ligand triggering, are targeted to early endosomal compartments, followed by sorting to recycling vesicles, signalling endosomes or multivesicular bodies (MVBs) and lysosomes for degradation (Jovic et al., 2010). Although ligand-induced endocytosis was originally thought to be solely a mechanism of receptor breakdown, receptors can remain active within endosomes and can couple to endosome-specific signalling pathways (Sorkin and von Zastrow, 2009). Protein ubiquitylation is a major mechanism that controls receptor internalisation, intracellular trafficking and proteasomal and lysosomal degradation (d'Azzo et al., 2005; Hicke and Dunn, 2003). Mono-ubiquitylation of cytokine receptors and the recruitment of ubiquitin-binding proteins serve as a regulated sorting mechanism for internalisation and sorting into the endocytic pathway. However, the specific ubiquitin ligases, ubiquitin-sorting signals and ubiquitin-binding proteins that control cytokine receptor trafficking and turnover remain poorly characterised and vary considerably between different cytokine receptor systems (Belouzard and Rouille, 2006; Irandoust et al., 2007; Strous and van Kerkhof, 2002).
The number of signalling-competent receptors at the cell surface is also determined by ectodomain shedding. Proteolytic cleavage of cell surface receptors by transmembrane metalloproteases of the ADAM (a disintegrin and metalloproteinase) family releases the receptor ectodomains and renders the cells desensitised to stimulation. ADAM10 and ADAM17 (or TNFα converting enzyme, TACE), the prototypical receptor sheddases, have been implicated in ectodomain cleavage of cytokine receptors of different families, including the tumor necrosis factor (TNF) and interleukin-1 (IL-1) receptor families and the type 1 cytokine receptors (Levine, 2008). The activity of ADAM17 is regulated by several mechanisms (for a review, see Huovila et al., 2005) including catalytic activation and cell surface exposure upon phosphorylation of its cytosolic domain (Diaz-Rodriguez et al., 2002; Xu and Derynck, 2010). In addition, because ADAM10 and ADAM17 activity is confined to cholesterol-rich membrane microdomains (lipid rafts) (Matthews et al., 2003; Tellier et al., 2006), substrates must be targeted to these rafts, but the molecular mechanisms regulating this routing remain unknown.
Ring finger protein 41 (RNF41), also referred to as E3 ubiquitin-protein ligase neuregulin receptor degradation protein-1 (Nrdp1) or fetal liver ring finger (FLRF) belongs to the family of single RING (really interesting new gene) finger-containing proteins. Members of this protein family function as E3 ubiquitin ligases. RNF41 serves as a scaffold by coordinating ubiquitin transfer from a ubiquitin-conjugating enzyme (E2) recruited by its N-terminal RING domain to a specific substrate that interacts with its C-terminal substrate binding domain. RNF41 has been implicated in the ubiquitylation and degradation of two other E3 ubiquitin ligases: BRUCE (Qiu et al., 2004), an inhibitor of apoptosis protein and parkin (Zhong et al., 2005), a protein involved in the onset of Parkinson's disease. More recently, RNF41 was found to control Toll-like receptor (TLR)-mediated responses through ubiquitylation of the central adaptor MyD88 and the kinase TBK1 (Wang et al., 2009). RNF41 also functions as a key regulator of steady-state cell surface levels of ErbB3 and ErbB4, two receptors that are closely related to the epidermal growth factor receptor (EGFR). RNF41 associates with these receptors independently of receptor stimulation (Diamonti et al., 2002; Qiu and Goldberg, 2002) and elicits ligand-independent ErbB3 ubiquitylation and degradation (Qiu and Goldberg, 2002). The biological significance of RNF41 in ErbB3 receptor signalling is underscored by the observation that perturbation of RNF41 activity results in enhanced ErbB3-activated cell growth and motility of human breast carcinoma cells. Moreover, loss of RNF41 expression correlates with in vivo overexpression and hypersignalling of ErbB3, as commonly found in primary human breast cancer tissue, and is related to tumor malignancy (Yen et al., 2006). Similarly to its impact on ErbB3 and ErbB4 receptor expression and function, RNF41 can modulate ligand-independent expression of the interleukin-3 (IL-3) and erythropoietin (Epo) cytokine receptors (Jing et al., 2008), but the underlying mechanism is unknown. Here, we demonstrate that RNF41 has a generic role in type 1 cytokine receptor signalling by controlling receptor degradation and shedding.
Results
RNF41 is an interaction partner of the leptin receptor complex in MAPPIT screening experiments
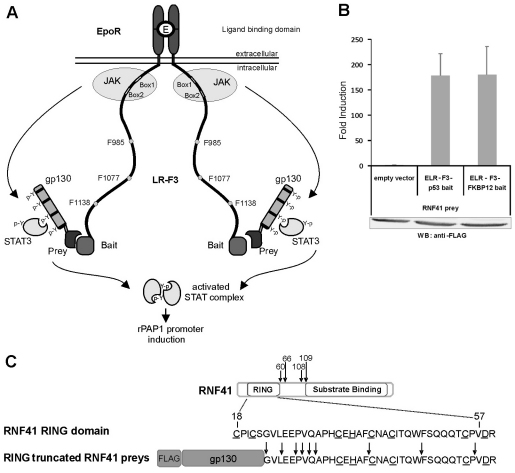
MAPPIT (mammalian protein-protein interaction trap) is a cytokine-receptor-based two-hybrid method that allows detection of protein interactions in mammalian cells (Eyckerman et al., 2001). In brief, a cytokine receptor, e.g. the leptin receptor (LR), is rendered inactive by mutating all cytosolic tyrosines (LR-F3): activation of the JAK–STAT signalling pathway thus depends on a bait–prey interaction, whereby the prey is fused to functional STAT recruitment sites (Fig. 1A). Using a FACS-based protocol, complex prey cDNA libraries can be screened for novel interaction partners of a selected bait (Lievens et al., 2004). It is intrinsic to this method that preys that bind to the receptor complex independently of the bait (e.g. to the LR cytoplasmic tail or JAK2) are identified as technical ‘false positives’. One such frequently identified protein in screening experiments corresponded to RNF41. Fig. 1B shows a standard MAPPIT experiment demonstrating the interaction of an RNF41 prey with the chimeric receptor complex, irrespective of the bait (p53 or FKBP12). Significantly, all 13 RNF41-encoding preys identified in MAPPIT screens featured a truncated N-terminal RING domain (Fig. 1C). An RNF41 protein lacking a functional RING domain acts as a dominant-negative inhibitor, potentiating ErbB3 signalling by interfering with receptor degradation (Diamonti et al., 2002; Qiu and Goldberg, 2002). Likewise, truncated RNF41 preys might stabilise the LR–bait complexes, readily explaining their efficient identification in MAPPIT screens.
Fig. 1.
Identification of RNF41 as a LR-complex-interacting protein. (A) Schematic outline of the MAPPIT technique using a chimeric EpoR-LR bait receptor (ELR-F3). (B) HEK293T cells transfected with an RNF41 prey arising from a MAPPIT screen and the indicated baits, were stimulated with Epo or left untreated. Luciferase data of triplicate measurements from a representative experiment are expressed as fold induction (stimulated/non stimulated) ± s.d. FLAG-tagged prey expression was verified by western blotting. (C) Human RNF41 structure and sequence of its RING domain. Arrows indicate in-frame fusion positions with gp130 in the RNF41 prey constructs identified in MAPPIT screens. The eight residues constituting a functional RING domain are underlined.
RNF41 modulates type 1 cytokine receptor cell surface expression and signalling
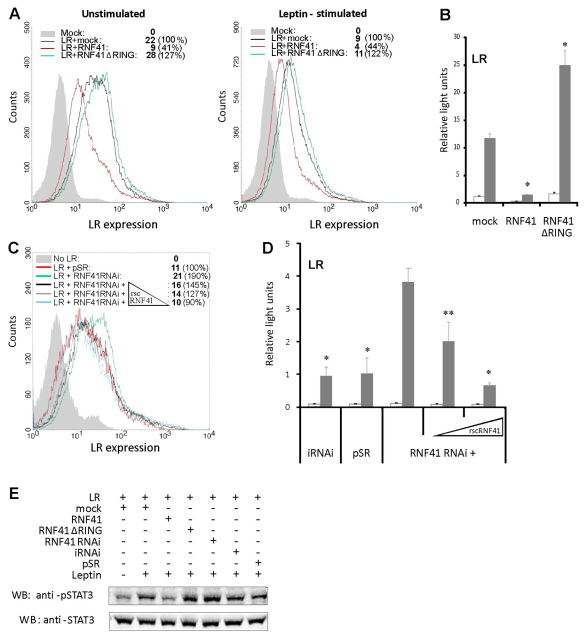
We next investigated whether modulation of RNF41 levels affected the expression of signalling competent LRs on the cell surface. Ectopic expression of full-length RNF41 or the dominant-negative RNF41 ΔRING clearly suppressed or enhanced LR cell surface expression (Fig. 2A) and signalling (Fig. 2B), respectively. Modulation of receptor expression was ligand independent (Fig. 2A). In addition, vector-based delivery of a short-hairpin oligonucleotide targeting endogenous RNF41 (supplementary material Fig. S1) enhanced LR surface expression and leptin signalling (Fig. 2C,D). These effects were specific to RNF41 because co-expression of a knockdown-resistant RNF41 construct (rscRNF41) (supplementary material Fig. S1) completely normalised LR surface expression and signalling (Fig. 2C,D). Modulation of RNF41 levels also affected STAT3 phosphorylation, as expected (Fig. 2E). Collectively, these results show that RNF41 regulates basal cell surface expression and signalling of the LR.
Fig. 2.
RNF41 attenuates LR surface expression and signalling. (A) Expression of RNF41 or RNF41 ΔRING suppresses or enhances LR surface expression, independently of leptin stimulation. FACS analysis of HEK293T cells transiently transfected with eGFP, combined with a mock vector (filled histogram) or an expression vector encoding the LR either alone (black) or together with RNF41 (red) or RNF41 ΔRING (green). Cells were either left untreated (left) or stimulated with 100 ng/ml leptin (right) 24 hours before analysis. Median fluorescence intensities of eGFP-positive, APC-labelled cells were used for quantification of LR surface expression and are presented in the inset of both panels for each transfection. (B) STAT3-responsive luciferase assay of HEK293T cells transfected with the LR and a mock, RNF41 or RNF41 ΔRING construct. Cells were either stimulated with leptin (grey) or left untreated (white). Absolute luciferase counts of triplicate measurements normalised for transfection efficiency are represented (mean ± s.d.) (n=3). *P<0.01 (Student's t-test) compared with mock-transfected cells. (C) FACS analysis of unstimulated eGFP-transfected HEK293T cells lacking LR expression (filled histogram) or cotransfected with the LR together with an empty knockdown vector (pSR, red) or expressing RNF41 RNAi (green), together with increasing amounts (0.3; 0.8 and 1.5 μg) of rscRNF41. Median fluorescence intensities of eGFP-positive, APC-labelled cells were used to quantify LR surface expression and are represented in the inset of the panel. (D) STAT3-responsive luciferase assay of HEK293T cells transfected with the LR and an irrelevant knockdown construct (iRNAi), an empty knockdown vector (pSR) or a knockdown vector targeting RNF41 (RNF41 RNAi), either without or with increasing amounts (0.1 and 0.5 μg) of a rescue RNF41 construct (rscRNF41). Cells were either stimulated with leptin (grey) or left untreated (white). Absolute luciferase counts of triplicate measurements normalised for transfection efficiency are represented (mean ± s.d.) (n=3). *P<0.01; **P<0.05 (Student's t-test) compared with RNF41 RNAi. (E) RNF41 modulates pSTAT3 levels. HEK293T cells expressing the LR and the indicated RNF41 or control constructs were either left untreated or were stimulated for 10 minutes with leptin following serum starvation. Endogenous pSTAT3 and STAT3 levels were visualised by western blotting.
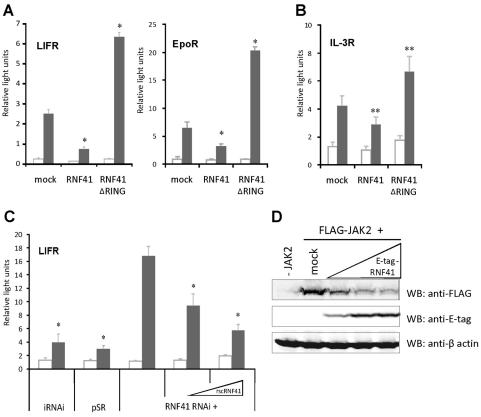
Similar effects were observed for other type 1 cytokine receptors: RNF41 expression attenuated signalling in HEK293T cells via the endogenous leukaemia inhibitory factor receptor (LIFR) and ectopically expressed EpoR (Fig. 3A) or via the endogenous IL-3R complex in hematopoietic Ba/F3 cells (Fig. 3B), demonstrating that this effect of RNF41 is not restricted to HEK293T cells. Conversely, RNF41 ΔRING acted as a dominant-negative inhibitor that potentiated signalling. Similarly to the LR, knockdown of endogenous RNF41 potentiated LIFR signalling, and expression of the rscRNF41 construct completely reversed this effect (Fig. 3C). The common denominator of all these receptor complexes is the receptor-associated JAK2 kinase, suggesting a direct impact of RNF41 on JAK2. Overexpression of RNF41 can suppress JAK2 levels (Fig. 3D), but we could not demonstrate efficient co-immunoprecipitation between RNF41 and JAK2, or RNF41-induced JAK2 ubiquitylation (data not shown).
Fig. 3.
RNF41 modulates signalling of several type 1 cytokine receptors. (A) Expression of RNF41 or RNF41 ΔRING suppresses or enhances STAT signalling of endogenously expressed hLIFR and ectopically expressed EpoR, respectively. STAT3- (left) or STAT5-dependent (right) luciferase assay of HEK293T cells stimulated with LIF (grey, left) or Epo (grey, right) or left untreated (white). (B) STAT5-dependent luciferase assay in Ba/F3 cells, expressing the endogenous IL-3R complex, transiently electroporated with a mock, RNF41 or RNF41 ΔRING construct. Cells were left untreated (white) or were stimulated overnight with IL-3 (grey). (C) RNF41 knockdown potentiates endogenous LIFR STAT signalling and this effect is dose-dependently reversed by expression of a rescue RNF41 construct (rscRNF41). STAT3-responsive luciferase assay of HEK293T cells transfected with an irrelevant knockdown construct (iRNAi), an empty knockdown vector (pSR) or a knockdown vector targeting RNF41 (RNF41 RNAi), either without or with increasing amounts (0.01 and 0.1 μg) of rscRNF41. Cells were either stimulated with LIF (grey) or left untreated (white). Absolute luciferase counts of triplicate measurements normalised for transfection efficiency are represented as mean ± s.d. (n=3). *P<0.01; **P<0.05 (Student's t-test) compared with mock-transfected sample (A,B) or iRNAi (C). (D) Etag–RNF41 expression (0.1; 0.5 and 1 μg) dose-dependently suppresses FLAG–JAK2 (1 μg) levels in HEK293T cells. β-actin was used as a control for loading.
Cathepsin L cleaves the leptin receptor
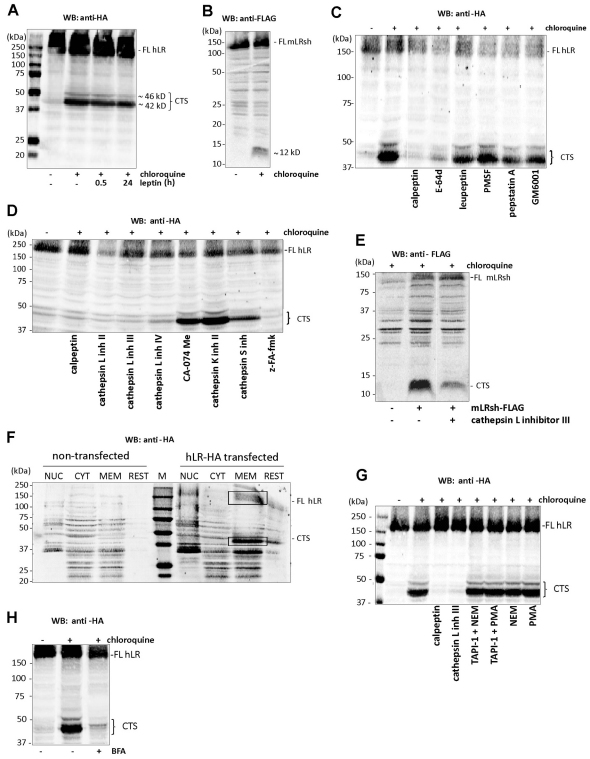
We next investigated the effect of RNF41 on ligand-independent LR turnover. Both the long (LRlo) and short (LRsh) isoforms of the LR are constitutively endocytosed and degraded in lysosomes (Belouzard et al., 2004). To monitor LR degradation, we incubated HEK293T cells expressing a human LRlo carrying a C-terminal HA-tag (hLR–HA) with chloroquine, an inhibitor of intralysosomal degradation. This led to the stabilisation of two C-terminal fragments (Fig. 4A). These degradation products of about 42 and 46 kDa, further referred to as the C-terminal LR stubs (LR-CTS), were generated independent of leptin stimulation. This cleavage is conserved between species and between different LR isoforms as a comparable 12 kDa LR-CTS was observed for the mouse LRsh fused to a C-terminal FLAG tag (mLRsh–FLAG) (Fig. 4B). We identified the protease involved in this cleavage using a series of pharmacological inhibitors. HEK293T cells expressing hLRlo–HA were first incubated with cell-permeable broad-spectrum protease blockers, each of which inhibited one of the four large protease classes (calpeptin and E-64d for cysteine proteases; leupeptin and PMSF for serine proteases; pepstatin A for aspartic proteases; GM6001 for metalloproteases). Only calpeptin and E-64d inhibited LR-CTS formation (Fig. 4C). Cathepsins are well-characterised cysteine proteases that are involved in protein degradation. The cathepsin B and L inhibitor z-FA-fmk and three different specific cathepsin L inhibitors completely blocked LR cleavage, whereas specific inhibitors of cathepsin B, cathepsin K or cathepsin S had no clear effect, demonstrating that cathepsin L causes cleavage of the LR (Fig. 4D). Similar results were obtained with mLRsh–FLAG (Fig. 4E). The size of the LR-CTS predicted cleavage close to the transmembrane region. Subcellular fragmentation revealed that the LR-CTS was associated with the membrane fraction (Fig. 4F), in line with juxtamembrane cleavage. Previous studies have shown that the hLR can undergo ectodomain shedding resulting in the generation of soluble LR. This shedding by a membrane-associated metalloprotease was enhanced by the phorbol ester PMA and N-ethylmaleimide (NEM) and could be blocked by the metalloprotease inhibitor TAPI-1 (Maamra et al., 2001). However, incubation of hLRlo–HA-expressing HEK293T cells with PMA or NEM, or co-incubating these shedding enhancers with TAPI-1 had no effect on the generation of LR-CTS, whereas calpeptin or a specific cathepsin L inhibitor completely blocked its formation (Fig. 4G). This indicates that, although processing must occur at very closeby positions in the LR ectodomain, shedding and LR-CTS cleavage are two independent mechanisms. Receptor trafficking is necessary to reach the correct subcellular location for cleavage, because incubation with Brefeldin A (BFA), an inhibitor of Arf1 activation that blocks transport from the ER to the Golgi, completely inhibited formation of the LR-CTS, supporting the notion that cleavage occurs in a post-ER subcellular compartment such as the MVBs or lysosomes (Fig. 4H).
Fig. 4.
The LR is cleaved by cathepsin L. Cell lysates of HEK293T cells transiently expressing the long isoform of the human LR with a C-terminal HA-tag (hLR–HA) or the short isoform of the mouse LR with a C-terminal FLAG-tag (mLRsh–FLAG) were analysed using western blotting (WB). (A) Cells expressing hLR–HA were left untreated (DMSO) or were incubated overnight with chloroquine, in the absence of stimulus or in combination with leptin for 0.5 or 24 hours. (B) Cells expressing mLRsh–FLAG were left untreated (DMSO) or were incubated overnight with chloroquine. (C,D) Cells expressing hLR–HA were left untreated (DMSO) or were incubated overnight with chloroquine alone or together with the indicated protease inhibitors. (E) Cells expressing mLRsh–FLAG were left untreated (DMSO) or were incubated overnight with chloroquine alone or together with cathepsin L inhibitor III. (F) The remaining C-terminal fragment is membrane anchored. Untransfected HEK293T cells or HEK293T cells transiently expressing hLR–HA were incubated overnight with chloroquine. Cell homogenates were separated in a nuclear (NUC), cytoplasmic (CYT), membrane (MEM) and rest fraction by differential centrifugation and analysed for the presence of the FL hLR (top square) or the CTS (bottom square) by western blotting (WB). M, molecular weight marker. (G) Activation or inhibition of ectodomain shedding has no effect on LR cleavage by cathepsin L. Cells expressing hLR–HA were left untreated (DMSO) or were incubated overnight with chloroquine alone or in combination with calpeptin, cathepsin L inhibitor III, NEM or PMA with or without TAPI-1. (H) Brefeldin A (BFA) incubation blocks LR-CTS formation. Cells expressing hLR–HA were left untreated (DMSO) or were incubated overnight with chloroquine alone or in combination with BFA. FL, full length; CTS, C-terminal stub.
RNF41 blocks LR-CTS cleavage and enhances leptin receptor ectodomain shedding
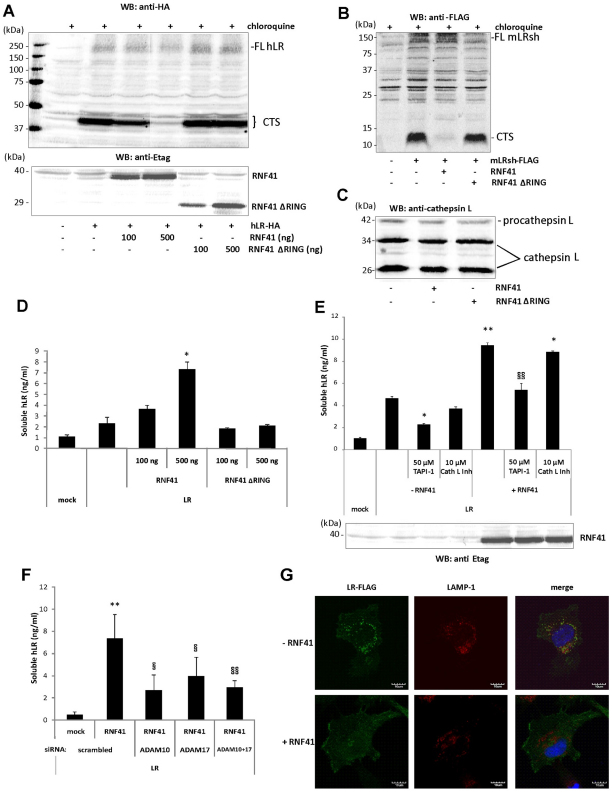
Importantly, RNF41 expression completely blocked the formation of the LR-CTS in hLR–HA-expressing HEK293T cells, without affecting the level of full-length LRs. No similar effect was seen for RNF41 ΔRING, implying a crucial role for the RING domain (Fig. 5A). Similar findings were obtained for mLRsh–FLAG (Fig. 5B). Direct inhibition of cathepsin L was ruled out because RNF41 or RNF41 ΔRING expression did not alter the levels of procathepsin L (42 kDa) or of its processed intermediate and active forms (34 kDa and 26 kDa, respectively) (Fig. 5C). We conclude that RNF41 inhibits lysosomal targeting of LRs.
Fig. 5.
RNF41 blocks formation of the LR CTS and concomitantly enhances LR shedding. (A) HEK293T cells were cotransfected with hLR–HA and increasing amounts of Etag–RNF41 or Etag–RNF41 ΔRING. After overnight chloroquine incubation, cell lysates were analysed using western blotting (WB). (B) HEK293T cells were cotransfected with mLRsh–FLAG and Etag–RNF41 or Etag–RNF41 ΔRING. After overnight chloroquine incubation, cell lysates were analysed using western blotting (WB). FL: full length; CTS: C-terminal stub. (C) Analysis of procathepsin L (42 kDa) and cathepsin L (34 and 26 kDa) expression after RNF41 (ΔRING) expression. (D) Cell media supernatants from the transfectants in Fig. 5A were analysed for soluble LR levels. (E) RNF41-enhanced LR shedding is reversed by TAPI-1. HEK293T cells transfected with hLR–HA with or without full length Etag–RNF41 were incubated overnight in starvation medium with TAPI-1 or cathepsin L inhibitor III and soluble LR levels in the cell media supernatants were quantified. (F) RNF41-enhanced LR shedding is reversed by silencing of ADAM10 and ADAM17. HEK293T cells, reverse transfected with siRNA targeting ADAM10 or ADAM17, were cotransfected the next day with hLR–HA with or without full-length Etag–RNF41 and soluble LR levels in the cell media supernatants were quantified. Values are means ± s.d. (n=3). *P<0.01 or **P<0.05 (Student's t-test) compared with –RNF41; §P<0.01 or §§P<0.05 (Student's t-test) compared with +RNF41. (G) RNF41 reduces colocalisation of LR–FLAG with LAMP-1. HeLa cells cotransfected with hLR–FLAG together with (bottom) or without (top) RNF41 were immunostained with anti-FLAG (green) and anti-LAMP-1 (red) antibodies following overnight chloroquine incubation. DAPI (blue) was used to visualise the nuclei. Images were acquired by confocal microscopy.
Cell culture supernatants were collected from the same transfected cells to analyse LR shedding. RNF41 expression significantly increased soluble LR levels from hLR–HA-expressing HEK293T cells, whereas expression of RNF41 ΔRING had no effect (Fig. 5D). As mentioned above, LR ectodomain shedding can be blocked by the metalloprotease inhibitor TAPI-1 (Maamra et al., 2001). Incubation of hLR–HA-expressing HEK293T cells with TAPI-1 reversed the enhancing effect of RNF41 on LR shedding (Fig. 5E). In contrast to TAPI-1, incubation with a specific cathepsin L inhibitor had no significant effect on basal or RNF41-induced soluble LR levels, confirming that LR ectodomain shedding by a metalloprotease and LR cleavage by cathepsin L are two distinct proteolytic events. TAPI-1 is known to inhibit several members of the ADAM family. Therefore, we tested the effect of silencing ADAM10 and ADAM17, two typical cytokine receptor sheddases, on RNF41-induced ectodomain shedding. Silencing of ADAM10 and ADAM17 gene expression reversed the enhancing effect of RNF41 on LR shedding (Fig. 5F; supplementary material Fig. S2). Importantly, because shedding and cleavage of the LR were studied simultaneously in the same cells, these observations provide evidence that RNF41 re-routes the LR from lysosomal degradation to subcellular microdomains where it is targeted by metalloproteases of the ADAM family, resulting in ectodomain release.
To visualise the intracellular LR redistribution predicted by such a scenario, we performed confocal microscopy on HeLa cells that expressed LR–FLAG. Following overnight incubation with chloroquine, RNF41 expression impaired colocalisation between the C-terminally FLAG-tagged LR and LAMP-1, which is a typical lysosomal marker (Fig. 5G). Together, these findings suggest an important impact of RNF41 on the intracellular routing of the LR.
RNF41 controls intracellular cleavage and ectodomain shedding of the LIFRα and IL-6Rα
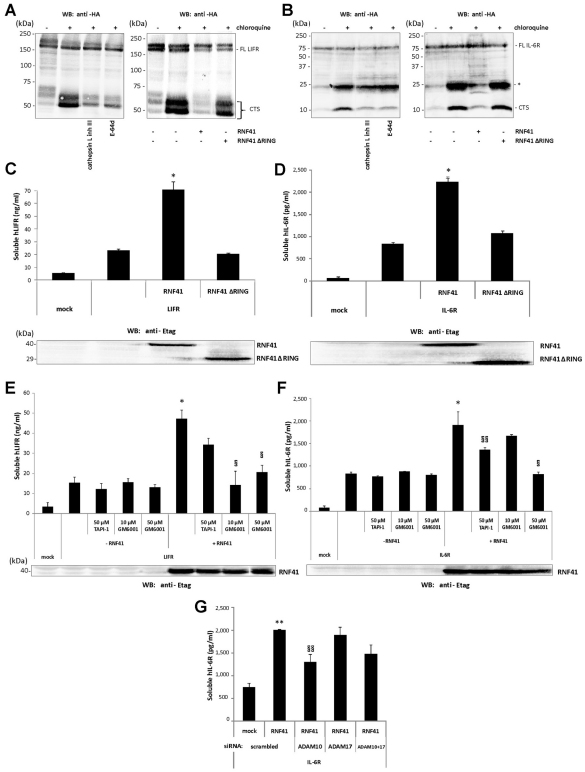
We next questioned whether RNF41 controlled degradation and shedding in a generic way, as we previously observed for JAK2-associated cytokine receptor exposure and signalling. As seen for the LR, chloroquine stabilised C-terminal stub fragments in HEK293T cells expressing LIFRα–HA or IL-6Rα–HA and pharmacological inhibition of cathepsin L or co-expression of RNF41 inhibited CTS formation without affecting levels of the full-length receptor (Fig. 6A,B). Incubation with chloroquine also stabilised another IL-6Rα C-terminal fragment (25 kDa) that was insensitive to cathepsin L inhibition. However, the formation of this fragment was also blocked by RNF41 expression, suggesting that the responsible protease resides at the same subcellular location. Soluble LIFRα and IL-6Rα can be generated by alternative splicing (Horiuchi et al., 1994; Tomida et al., 1994) or, in case of the IL-6Rα, also by ectodomain shedding (Mullberg et al., 1995). Again, as seen for the LR, RNF41 increased soluble LIFRα and IL-6Rα levels, whereas RNF41 ΔRING had no similar effect (Fig. 6C,D). This ectodomain shedding was caused by a sheddase activity because incubation with GM6001, a general metalloprotease inhibitor, reversed RNF41-induced ectodomain shedding of LIFRα and IL-6Rα (Fig. 6E,F). Induced ectodomain shedding of the IL-6Rα is mainly mediated by ADAM17, whereas constitutive shedding is mediated by ADAM10 (Matthews et al., 2003). In contrast to silencing of ADAM17, ADAM10 silencing reversed RNF41-induced IL-6Rα ectodomain shedding, which is in line with the constitutive nature of the effects of RNF41 on receptor shedding (Fig. 6G). TAPI-1, which inhibits shedding of LR (Maamra et al., 2001) and IL-6Rα (Mullberg et al., 1995), had only a partial effect on levels of soluble LIFRα, indicating that LIFRα shedding involves a different metalloprotease(s). LIFRα shedding has not been shown before as a mechanism for the generation of soluble LIFRα. Together, these results support the conclusion that RNF41 controls the routing and processing of many type 1 cytokine receptors in a similar way.
Fig. 6.
RNF41 blocks LIFRα and IL-6Rα cleavage by cathepsin L and enhances their shedding. (A,B) Chloroquine stabilises C-terminal LIFR and IL-6Rα fragments, which are blocked by cathepsin L inhibition (left panels) or RNF41 expression (right panels). HEK293T cells transiently transfected with C-terminal HA-tagged human LIFRα (LIFRα–HA) (A) or IL-6Rα (IL-6Rα–HA) (B) were left untreated (DMSO) or were incubated overnight solely with chloroquine or together with the indicated protease inhibitors (left panels); or were cotransfected with a full-length Etag–RNF41 or Etag–RNF41 ΔRING construct and left untreated or incubated with chloroquine (right). Cell lysates were analysed by western blotting (WB). FL, full length; *, intermediate IL-6Rα cleavage product; CTS, C-terminal stub. (C,D) RNF41 enhances LIFRα (C) and IL-6Rα (D) shedding. Cell media supernatants from the transfectants in the right panels of Fig. 6A,B were analysed for soluble LIFRα and IL-6Rα levels. E-tagged RNF41 or RNF41 ΔRING expression was verified by western blotting. (E,F) RNF41-enhanced LIFRα (E) and IL-6Rα (F) shedding is reversed by metalloprotease inhibitors. HEK293T cells transfected with hLIFRα–HA or hIL-6Rα–HA with or without full-length Etag–RNF41 were incubated overnight in starvation medium with TAPI-1 or GM6001 and soluble receptor levels in the cell medium supernatants were quantified. E-tagged RNF41 expression was verified by western blotting. (G) RNF41-enhanced IL-6R shedding is reversed by ADAM10 silencing. HEK293T cells, reverse transfected with siRNA targeting ADAM10 or ADAM17, were cotransfected the next day with hIL-6Rα–HA with or without full-length Etag–RNF41 and soluble IL-6Rα levels in the cell medium supernatants were quantified. Values are means ± s.d. (n=3). *P<0.01 or **P<0.05 (Student's t-test) compared with – RNF41; §P<0.01 or §§P<0.05 (Student's t-test) compared with +RNF41.
Discussion
The E3 ubiquitin ligase RNF41 is implicated in regulating the expression of a number of proteins with varying functions, including membrane receptors such as the EGFR family members ErbB3 and ErbB4 (Diamonti et al., 2002; Qiu and Goldberg, 2002) and the Epo and IL-3 cytokine receptors (Jing et al., 2008). As an alternative to the RNF41-mediated degradation of these receptors, we assign a role for RNF41 in restricting basal surface expression and signalling of three different type 1 cytokine receptors and provide evidence that RNF41 has a crucial role in ectodomain shedding. We initially identified RNF41 as a novel interaction partner of the leptin receptor in MAPPIT screens, whereby its N-terminal RING domain was invariably interrupted. Expression of such truncated RNF41 or silencing of its transcript enhanced LR cell surface expression and potentiated STAT signalling, whereas expression of full-length RNF41 had the opposite effect. In line with this, the recurrent identification of the truncated RNF41 preys isolated in the screens might be explained by a stabilised surface expression of the LR-derived MAPPIT bait receptor, generating pronounced MAPPIT signals. We confirmed this initial observation for different wild-type cytokine receptors, either transfected or endogenously expressed in different cell types. How RNF41 physically interacts with the type 1 cytokine receptor complexes is unclear at present. The common denominator of all receptor complexes analysed in this study is the receptor-associated JAK2 kinase, suggesting a direct impact of RNF41 on JAK2. JAK proteins have been shown to facilitate receptor folding, maturation and cell surface delivery by operating as a chaperone, constituting a quality control checkpoint in the ER or Golgi (Huang et al., 2001; Radtke et al., 2002). In addition, JAK kinases can promote receptor expression by decreasing basal internalisation rates (Kumar et al., 2008; Ragimbeau et al., 2003) or enhancing recycling (Royer et al., 2005). Furthermore, the human kinome tree shows that JAK kinases are most closely related to the EGFR family members, which are known targets of RNF41 (Manning et al., 2002). Although RNF41 can suppress levels of JAK2, we could not demonstrate efficient co-immunoprecipitation between RNF41 and JAK2, or RNF41-induced JAK2 ubiquitylation. This suggests that the JAK2–RNF41 interaction might be indirect and that other, so far unknown proteins bridge RNF41- and JAK2-associated cytokine receptor complexes. Additional studies involving protein–protein interaction analysis, subcellular localisation of RNF41 and JAK2 and the possible role of JAK2 in receptor routing are required to further clarify this issue.
The key finding of our study is that RNF41 controls the balance between type 1 cytokine receptor degradation and ectodomain shedding. For three different receptor systems (LR, LIFRα and IL-6Rα) we observed simultaneous inhibition of cathepsin-L-dependent cleavage and enhanced ectodomain shedding. In line with this, confocal imaging revealed a decrease in colocalisation of a C-terminally tagged LR with the lysosomal marker LAMP-1 upon RNF41 expression. Together, this implies an important role for RNF41 in cytokine receptor trafficking. Such a role for RNF41 in cargo trafficking is underscored by recent studies. RNF41 was shown to interact with Tsg101 (Vps23), a component of the endosomal sorting complex required for transport (ESCRT-I) that is involved in protein sorting to late endosomal membranes and MVBs (Markson et al., 2009; Williams and Urbé, 2007). Also, RNF41 can interact with the deubiquitylating enzyme USP8 (also called UBPy), which is required for maintenance of ESCRT-0 stability and endosomal sorting of ErbB3 and EGFR (Niendorf et al., 2007; Wu et al., 2004). Its role in preventing receptor degradation might also help to explain recent findings by Cao and co-workers, who reported that RNF41 could differentially modulate Toll-like receptor (TLR)-mediated responses. TLRs that reside at the plasma membrane engage the Mal/MyD88 adaptors leading to NF-κB activation, whereas activation of IRF3 is triggered by TRAM and TRIF adaptor-coupled endosomal TLRs. Next to ubiquitylation of crucial components in either pathway (Wang et al., 2009), RNF41 might promote TRAM- and TRIF-driven signalling by preventing targeting of the endosomal TLRs to the lysosomal degradation pathway.
In addition to alternative splicing, ectodomain shedding is a common mechanism for the generation of soluble receptors. We show here for the first time that LIFRα is susceptible to metalloprotease-dependent ectodomain shedding, as shown before for LR and IL-6Rα (Maamra et al., 2001; Mullberg et al., 1995). Silencing of both ADAM10 and ADAM17 (also called TACE) in the case of the LR, or ADAM10 in case of the IL-6Rα reverses the effects of RNF41 on receptor shedding. ADAM10 is responsible for the constitutive shedding of the IL-6Rα (Matthews et al., 2003), which is in line with the ligand independency of the RNF41 effects. The sheddase activity of ADAM10 and ADAM17, was shown to be sequestered in well-ordered membrane microdomains that are rich in cholesterol and sphingolipids, called lipid rafts (Matthews et al., 2003; Tellier et al., 2006). Furthermore, ADAM substrates are present in different proportions in lipid rafts, suggesting that the entry of these substrates in these particular membrane microdomains is specifically regulated (Tellier et al., 2006). However, the mechanisms or post-translational modifications modulating the entry of ADAM substrates to these lipid rafts are still unknown. Our findings imply that RNF41 sends receptors to such cellular domains for ectodomain shedding. Because rerouting by RNF41 depends on its RING domain and ubiquitin was shown to act as a sorting signal (Acconcia et al., 2009), (mono-)ubiquitylation of cargo proteins and/or proteins involved in the sorting machinery is probably involved. Future studies identifying the specific ubiquitylation targets of RNF41 (e.g. members of the ESCRT complex) will be required to unravel the precise molecular sorting mechanism. Enhanced shedding mediated by RNF41 expression might be directly correlated with decreased detection of surface receptors in our FACS assay and the attenuated signalling. However, additional effects of RNF41 on receptor stability and degradation cannot be ruled out at present and need further investigation.
In addition to its potentiating effect on metalloprotease-dependent ectodomain shedding, RNF41 also affects cleavage of the LR, LIFR and IL-6Rα. As shown before for the insulin receptor (IR) and insulin-like growth factor-1 receptor (IGF-1R) (Yang et al., 2007), we demonstrate using specific cathepsin inhibitors that both the LR (long and short isoform), LIFRα and IL-6Rα are cleaved by the cysteine protease cathepsin L, leading to the formation of a membrane-anchored C-terminal stub (CTS). Brefeldin A, an inhibitor of ER to Golgi transport, completely blocks formation of the LR-CTS, indicating that cleavage occurs in a post-ER subcellular compartment such as the MVBs or lysosomes. Interestingly, gene deletion or pharmacological inhibition of cathepsin L was shown to reduce body weight gain and glucose intolerance, partly because of increased levels of muscle IR (Yang et al., 2007). Cleavage by cathepsin L of cytokine receptors such as the LR, might contribute to this phenotype. Increased levels of cathepsin L in obese and diabetic patients suggest that this protease is a novel target for these metabolic disorders.
Future studies will have to determine how endogenous RNF41 expression is regulated. RNF41 expression was shown to be a TLR-inducible protein in RAW264.7 macrophages upon LPS stimulation (Wang et al., 2009). Moreover, auto-ubiquitylation and other post-transcriptional mechanisms are involved in suppressing RNF41 stability (Ingalla et al., 2010; Wu et al., 2004).
In conclusion, we have shown that a single protein, RNF41, can control the balance between JAK2-associated cytokine receptor degradation and ectodomain shedding. Our findings imply that RNF41 reroutes receptors from the lysosomal degradation pathway to cellular compartments for ectodomain shedding. As aberrant cytokine receptor expression and shedding has been correlated with the onset of various pathologies (Gooz, 2010; Murphy, 2008), a better understanding of the cellular control systems that safeguard correct exposure and shedding of receptors might contribute to developing therapies aimed at harnessing the associated diseases.
Materials and Methods
Constructs
The sequences encoding the human LR (hLR) and human LIFR (hLIFRα) were amplified and cloned in the pMET7 expression vector using 5′-AACTGCAGCACCATGATTTGTCAAAAATTCTGTG-3′ containing a PstI site and 5′-CTCTCTAGATTACGCATAATCCGGCACATCATACGGATACACAGTTAGGTCACACATCTTGTTTTC-3′ containing a XbaI site and a HA-coding sequence to generate pMET7-hLR-HA, and 5′-CGCGAATTCATGATGGATATTTACGTATGTTTG-3′ containing an EcoRI site and 5′-CCCTCTAGATTACGCATAATCCGGCACATCATACGGATAATCGTTTGGTTTGTTCTGAAAAAAG-3′ containing a XbaI site and a HA-coding sequence to generate pMET7-hLIFRα-HA. The pSVL-hIL-6Rα construct, encoding the human IL-6Rα, was from G. Müller-Newen (RWTH, Aachen, Germany) and a C-terminal HA-tag was inserted by site-directed mutagenesis. All bait receptors are generated based on the standard pSEL receptor construct, containing the extracellular part of the human EpoR and the transmembrane and intracellular parts of the mouse LR, that was reported elsewhere (Eyckerman et al., 2001). To generate the ELR-F3-FKBP12 bait, the FKBP12 sequence was amplified from a formerly constructed FKBP12 prey using a forward 5′-CGCGAGAGCTCAGGAGTGCAGGTGGAAACCATC-3′ and reverse 5′-CGCTGCGGCCGCTTATTCCAGTTTTAGAAGCTCC-3′ primer and then cloned in the standard pSEL bait receptor using SacI and NotI. The hRNF41 (AA109-317) prey was obtained by PCR amplification of cDNA prepared from a cell clone identified in a MAPPIT screen for proteins interacting with the LR-F3 receptor itself (unpublished data), using primers that recognise the sequences flanking the prey cDNA insert (Lievens et al., 2004). The pUT651 construct expressing β-galactosidase was obtained from Eurogentec. The pGL3-β-casein-luci reporter construct containing five repeats of the STAT5-responsive motif of the β-casein promoter was a gift from Ivo Touw (Erasmus MC, Rotterdam, The Netherlands). The generation of the following constructs was described previously: pMET7-mLRsh-FLAG (Eyckerman et al., 1999), the pXP2d2-rPAPI-luciferase reporter originating from the rPAPI (rat pancreatitis associated protein I) promoter, ELR-F3-p53 bait (Eyckerman et al., 2001), pMET7-mLRlo-FLAG (Zabeau et al., 2005), pSV-hEpoR (Montoye et al., 2005) and pMET7-FLAG-FKBP12 (Wauman et al., 2008). Full-length human RNF41 was amplified from HEK293T cDNA using the forward primer 5′-GCGGAATTCGCCATGGGGTATGATGTAACCCG-3′ containing an EcoRI site and the reverse primer 5′-CGCTCTAGATTAACGCGGTTCCAGCGGGTCCGGATACGGCACCGGCGCACCCTCGAGTATCTCTTCCACGCCATGCG-3′ containing an XbaI site. Insertion in an EcoRI and XbaI digested pMET7 vector generated the C-terminally E-tagged RNF41 construct. An N-terminally E-tagged RNF41 pMet7 construct was generated using the former construct as a template with the forward primer 5′-GAGCGGCCGCTGGGTATGATGTAAC-3′ containing a NotI site and the reverse primer 5′-CGCTCTAGATTATATCTCTTCCACGCCATGCG-3′ containing an XbaI site. The amplified sequence was cloned in a NotI-XbaI opened pMET7-Etag-mSOCS2 construct that was described previously (Piessevaux et al., 2006). An RNF41 rescue construct was designed by site-directed mutagenesis using the forward primer 5′-GAGCTGGAGAAGACTAGTGCCGAGCATAAGCACCAGCTGGCGGAG-3′ and the reverse primer 5′-CTCCGCCAGCTGGTGCTTATGCTCGGCACTAGTCTTCTCCAGCTC-3′. RNF41 ΔRING spanning amino acids 109–317 of human RNF41 were transferred from the hRNF41 (AA109–317) prey into a pMET7–FLAG vector via digestion with EcoRI and XbaI. A similar construct with an N-terminal E-tag was obtained by replacing the RNF41 sequence starting from amino acid 1 in the pMet7-Etag-RNF41 construct with the sequence starting from amino acid 109 in the pMet7-FLAG-RNF41 ΔRING construct, both flanked by NotI and BsaHI. For knockdown experiments the shRNA expression vector, pSUPER-retro (Oligoengine) was used for expression of shRNA. The RNF41-specific insert was a 19 nucleotide sequence corresponding to nucleotides 462–480 (5′-GACGTCAGCTGAACACAAA-3′) of RNF41, which was separated by a non-complementary spacer (TTCAAGAGA) from the reverse complement of the same 19 nucleotide sequence. As a control, we used either the iRNAi construct, which targets a sequence that bears no significant homology to any mammalian gene (5′-TTCTCCGAACGTGTCACGT-3′), or an empty pSUPER-retro vector (pSR). All constructs were verified by DNA sequence analysis.
Cell culture, transfection procedures and protease inhibitors
HEK293T and HeLa cells were cultured in an 8% CO2 humidified atmosphere at 37°C and grown in Dulbecco's modified Eagle's medium (DMEM) (Invitrogen) with 10% fetal calf serum (Perbio). For the cleavage and shedding assays, HEK293T cells were seeded in six-well plates (4×105 cells/well) and transfected overnight with 2 μg of receptor-encoding plasmid DNA (±2 μg RNF41-encoding plasmid DNA) using the calcium phosphate method. For confocal imaging, 2×105 cells/well were seeded on No. 1.5 glass coverslips (Zeiss), coated with poly-D-lysine (Sigma). The next day, they were transfected with JetPrime (Polyplus) according to the manufacturer's guidelines with 1 μg of receptor-encoding plasmid DNA ±1 μg of RNF41-encoding plasmid DNA. The pMET7-SVT construct was used to normalise for the amount of transfected DNA and load of the transcriptional and translational machinery. One day (or 8 hours for confocal imaging) after transfection, cells were left untreated (DMSO) or treated overnight with chloroquine (25 μM), phorbol 12-myristate 13-acetate (PMA) (1 μg/ml), N-ethylmaleimide (NEM) (5 nM), E-64d (10 μM), pepstatin A (10 μM), z-FA-fmk (10 μM) (Sigma), TAPI-1 (50 μM) (Peptides International), Brefeldin A (BFA) (5 μg/ml), calpeptin, phenylmethylsulfonyl fluoride (PMSF), leupeptin, GM6001, cathepsin L inhibitor II/III/IV, cathepsin K inhibitor II, cathepsin S inhibitor, CA-074 Me (all at 10 μM) (Calbiochem).
Ba/F3 cells were cultured in a 8% CO2 humidified atmosphere at 37°C and grown in RPMI 1640 medium (Invitrogen) supplemented with 10% heat-inactivated fetal calf serum (Perbio) and WEHI-3B conditioned medium (containing IL-3). Transfections were performed by electroporation (300 V, 1500 μF) (Clontech) with the RNF41-encoding plasmids (22 μg) and the pGL3-β-casein-luci reporter construct (8 μg).
Western blot analysis
HEK293T cells were washed twice with ice-cold PBS and lysed in modified RIPA buffer [200 mM NaCl, 50 mM Tris-HCl, pH 8.0, 0.05% SDS, 2 mM EDTA, 1% Nonidet P-40, 0.5% deoxycholic acid, 1 mM Na3VO4, 1 mM NaF, and Complete protease inhibitor cocktail (Roche)]. Lysates were cleared by centrifugation at 14,000 r.p.m. for 10 minutes at 4°C. The 5× loading buffer (156 mM Tris-HCl, pH 6.8, 2% SDS, 25% glycerol, 0.01% Bromophenol Blue sodium salt, 5% β-mercaptoethanol) was added to the cell lysates, which were then resolved by SDS-PAGE and transferred to nitrocellulose membranes (Amersham Biosciences). Blotting efficiency was checked by Ponceau S staining (Sigma). Blots were blocked in Odyssey blocking buffer (Li-Cor). β-actin, pSTAT3, STAT3, HA-tagged, FLAG-tagged and E-tagged proteins were revealed using a rabbit anti-β-actin (Sigma), rabbit anti-pSTAT3 (Y705), mouse anti-STAT3 (124H6) (Cell Signaling), monoclonal rat anti–HA (3F10) (Roche), mouse anti-FLAG-M2 (Sigma) and mouse anti-E-tag (Phadia) antibody, respectively, followed by an anti-rabbit, anti-rat or anti-mouse Alexa-Fluor-680-conjugated antibody (Molecular Probes), diluted in Odyssey blocking buffer (Li-Cor) with 0.1% Tween-20.
Subcellular fragmentation
hLR–HA-expressing HEK293T cells were incubated overnight with chloroquine and harvested into 500 μl cell lysis buffer [10 mM HEPES, 10 mM NaCl, 1 mM KH2PO4, 5 mM NaHCO3, 1 mM CaCl2, 0.5 mM MgCl2, 5 mM EDTA and Complete protease inhibitor cocktail (Roche)]. Cells were allowed to swell for 5 minutes and Dounce homogenised 50 times. After centrifugation (5 minutes, 4°C) at 7500 r.p.m., the pellet is the nuclei fraction (plus debris) and the supernatants is the cytosol plus membrane fraction. The supernatant was centrifuged (30 minutes, 4°C) at 25,000 r.p.m. Pure cytosol supernatants were collected and the membrane pellet was resuspended in 40 μl PBS. The nuclei fraction was resuspended in 1 ml Tris, Sucrose, EDTA (TSE) buffer (10 mM Tris-HCl, pH 7.5, 300 mM sucrose, 1 mM EDTA, 0.1% NP-40 and Complete protease inhibitor cocktail), Dounce homogenised 30 times and centrifuged (5 minutes, 4°C) at 5000 r.p.m. The supernatant (rest fraction) was collected and the pure nuclei pellet was resuspended in 40 μl TSE buffer.
ELISA for the detection of soluble cytokine receptors
In the final 24 hours before collection of the cell medium samples, cells were cultured in DMEM without fetal calf serum. The human sLeptinR and sIL-6Rα Quantikine® ELISA kit (R&D Systems) and human sLIF-R/gp190 ELISA kit (BioVendor) were used to determine soluble hLR, hIL-6Rα and hLIFR levels, respectively, following the manufacturer's instructions.
Luciferase reporter assays
For a typical luciferase experiment, HEK293T cells were transfected with the desired receptor (100 ng) and RNF41 (2 μg; 1 μg for iRNAi, pSR and RNF41 RNAi) constructs, together with a STAT3-dependent pXP2d2-rPAPI or STAT5-responsive pGL3-β-casein luciferase reporter plasmid (200 ng). Cells were additionally transfected with a β-gal reporter construct (200 ng) to correct for transfection efficiency. The pMET7-SVT construct was used to normalise for the amount of transfected DNA and load of the transcriptional and translational machinery. 24 hours after transfection, HEK293T cells were washed, transferred to a 96-well plate and left untreated or stimulated for at least 24 hours with mouse leptin (100 ng/ml), human Epo (5 ng/ml), human IL-3 (1 ng/ml) (R&D Systems) or human LIF (10 ng/ml) (Chemicon International). Luciferase activity from triplicate samples was measured by chemiluminescence in a TopCount luminometer (PerkinElmer) and expressed as fold induction (stimulated/non-stimulated relative light units) or as relative light units normalised for transfection efficiency. All luciferase data shown are based on at least three independent experiments (n=3).
FACS analysis
HEK293T cells were transiently transfected with a mock vector or an expression vector encoding the mLR either alone (0.5 μg) or together with the indicated RNF41 constructs (1.5 μg). In addition, all cells were transfected with an eGFP construct (0.1 μg) to allow gating for transfected cells during FACS analysis. The pMET7-SVT construct was used to normalise for the amount of transfected DNA and load of the transcriptional and translational machinery. 18 hours after transfection, HEK293T cells were left untreated or stimulated for another 24 hours with 100 ng/ml mouse leptin. Expression of the LR was monitored using a combination of two rat monoclonal antibodies directed against the extracellular domain of the mLR, which were produced in-house, and subsequent incubation with an anti-rat biotin (KPL) and streptavidin-APC (BD Biosciences Pharmingen) conjugated antibody. Fluorescence activated cell sorting (FACS) was performed on a FACSCalibur (Becton Dickinson).
Silencing of ADAM10 and ADAM17
ADAM10 siRNA (ADAM10 ON-TARGETplus SMARTpool, Dharmacon), ADAM17 siRNA (5′-AAGAAACAGAGUGCUAAUUUA-3′, Qiagen) and Stealth RNAi siRNA Negative Control Med GC (Invitrogen) were reverse transfected in HEK293T cells using Dharmafect (Dharmacon) 1 day before transfection of the plasmids expressing the appropriate receptor or RNF41. Silencing efficiency was evaluated using real-time PCR.
Confocal microscopy
24 hours after transfection, cells were rinsed with 1× PBS and fixed for 15 minutes at room temperature in 4% paraformaldehyde. After three washes with 1× PBS, cells were permeabilised with 0.1% Triton X-100 in 1× PBS for 10 minutes and blocked in 1% BSA in 1× PBS for another 10 minutes at room temperature. Samples were then incubated for 1 hour at room temperature with 1:500 rabbit anti-FLAG (F7425, Sigma) and 1:500 mouse anti-LAMP-1 (H4A3, Abcam) antibodies. After four washes in 1× PBS, cells were incubated for 1 hour at room temperature with goat anti-rabbit Alexa Fluor 488 and goat anti-mouse Alexa Fluor 594 secondary antibodies. Nuclei were stained with DAPI. Images were acquired using a 60× 1.35 NA objective on an Olympus IX-81 laser scanning confocal microscope and analysed using Fluoview 1000 software.
RNA isolation, cDNA synthesis and quantitative real-time PCR analysis
Total RNA was isolated from HEK293T cells using RNeasy spin columns and treated with RNase-free DNaseI (Qiagen). Concentration and purity of the extracted RNA were determined using the A260/A280 value measured on an ND1000 Spectrophotometer (NanoDrop Technologies). cDNA synthesis was carried out from 5 μg total RNA using SuperScript II Reverse transcriptase (Invitrogen) by the oligodT priming method. Relative mRNA transcript levels of endogenous RNF41 were assessed by real-time quantitative PCR using the LightCycler 480 Probes Master (Roche) in combination with the Universal ProbeLibrary probe #25 (Roche) and PCR primers (5′-TGCCCTATTTGCAGTGGAGT-3′ and 5′-CGTTGCAGAAAGCATGTTCA-3′) (Eurogentec) specific for RNF41 and designed using the Probe Library Assay Design Center (http://www.roche-applied-science.com/sis/rtpcr/upl/adc.jsp). For ADAM10, probe #70 and PCR primers 5′-TGTTGCTGAGAGTGTTAATTCTGC-3′ and 5′-TTAAAGGATTCCCATACTGACCTC-3′; and for ADAM17, probe #78 and PCR primers 5′-CCTTTCTGCGAGAGGGAAC-3′ and 5′-CACCTTGCAGGAGTTGTCAGT-3′ were used. The PCR cycle parameters were an initial denaturation of 95°C for 5 minutes and then 40 cycles of denaturation at 95°C for 10 seconds, annealing at 60°C for 15 seconds and extension at 72°C for 10 seconds. Each sample was run as a technical duplicate. GADPH (probe #60 and primers 5′-AGCCACATCGCTCAGACAC-3′ and 5′-GCCCAATACGACCAAATCC-3′) and HPRT (probe #73 and primers 5′-TGACCTTGATTTATTTTGCATACC-3′ and 5′-CGAGCAAGACGTTCAGTCCT-3′) served as internal controls and were used to normalise for differences in each sample. Relative RNF41 mRNA levels were quantified using LightCycler 480 Software 1.5.
Statistical analysis
Data are expressed as mean ± s.d. of at least three independent experiments. For statistical analyses of two groups, two-tailed Student's t-tests were performed. GraphPad Prism version 5.00 software was used for all statistical data analysis.
Supplementary Material
Acknowledgments
We thank Gerhard Müller-Newen for the pSVL-hIL-6Rα construct and for helpful discussion and Ivo Touw for the pGL3-β-casein-luci reporter construct. We also thank Frank Peelman for critical reading of the manuscript. This work was supported by grants from the FWO-V (G.0864.10), Ghent University (GOA 12051401) and IAP-6 (P6:28). J.W. and L.D.C. hold PhD grants from the FWO-V. J.W. designed and performed the degradation and shedding experiments; L.D.C. designed and performed the MAPPIT, FACS and signalling experiments; N.V. and S.L. performed the initial MAPPIT screens; J.T. reviewed the manuscript and supervised all work. J.W., L.D.C. and J.T. wrote the manuscript. The authors declare that they have no conflict of interest.
Footnotes
Supplementary material available online at http://jcs.biologists.org/cgi/content/full/124/6/921/DC1
References
- Acconcia F., Sigismund S., Polo S. (2009). Ubiquitin in trafficking: The network at work. Exp. Cell Res. 315, 1610-1618 [DOI] [PubMed] [Google Scholar]
- Belouzard S., Rouille Y. (2006). Ubiquitylation of leptin receptor OB-Ra regulates its clathrin-mediated endocytosis. EMBO J. 25, 932-942 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Belouzard S., Delcroix D., Rouillé Y. (2004). Low levels of expression of leptin receptor at the cell surface result from constitutive endocytosis and intracellular retention in the biosynthetic pathway. J. Biol. Chem. 279, 28499-28508 [DOI] [PubMed] [Google Scholar]
- d'Azzo A., Bongiovanni A., Nastasi T. (2005). E3 ubiquitin ligases as regulators of membrane protein trafficking and degradation. Traffic 6, 429-441 [DOI] [PubMed] [Google Scholar]
- Diamonti A. J., Guy P. M., Ivanof C., Wong K., Sweeney C., Carraway K. L. (2002). An RBCC protein implicated in maintenance of steady-state neuregulin receptor levels. Proc. Natl. Acad. Sci. USA 99, 2866-2871 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Diaz-Rodriguez E., Montero J. C., Esparis-Ogando A., Yuste L., Pandiella A. (2002). Extracellular signal-regulated kinase phosphorylates tumor necrosis factor alpha-converting enzyme at threonine 735, a potential role in regulated shedding. Mol. Biol. Cell 13, 2031-2044 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eyckerman S., Waelput W., Verhee A., Broekaert D., Vandekerckhove J., Tavernier J. (1999). Analysis of Tyr to Phe and fa/fa leptin receptor mutations in the PC12 cell line. Eur. Cytokine Netw. 10, 549-556 [PubMed] [Google Scholar]
- Eyckerman S., Verhee A., der Heyden J. V., Lemmens I., Ostade X. V., Vandekerckhove J., Tavernier J. (2001). Design and application of a cytokine-receptor-based interaction trap. Nat. Cell Biol. 3, 1114-1119 [DOI] [PubMed] [Google Scholar]
- Gooz M. (2010). ADAM-17: the enzyme that does it all. Crit. Rev. Biochem. Mol. Biol. 45, 146-169 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hicke L., Dunn R. (2003). Regulation of membrane protein transport by ubiquitin and ubiquitin-binding proteins. Annu. Rev. Cell Dev. Biol. 19, 141-172 [DOI] [PubMed] [Google Scholar]
- Horiuchi S., Koyanagiu Y., Zhouu Y., Miyamotou H., Tanakau Y., Waki M., Matsumoto A., Yamamotou M., Yamamotof N. (1994). Soluble interleukin-6 receptors released from T cell or granulocyte/macrophage cell lines and human peripheral blood mononuclear cells are generated through an alternative splicing mechanism. Eur. J. Immunol. 24, 1945-1948 [DOI] [PubMed] [Google Scholar]
- Huang L. J. S., Constantinescu S. N., Lodish H. F. (2001). The N-terminal domain of Janus kinase 2 is required for Golgi processing and cell surface expression of erythropoietin receptor. Mol. Cell 8, 1327-1338 [DOI] [PubMed] [Google Scholar]
- Huovila A. P. J., Turner A. J., Pelto-Huikko M., Karkkainen L., Ortiz R. M. (2005). Shedding light on ADAM metalloproteinases. Trends Biochem. Sci. 30, 413-422 [DOI] [PubMed] [Google Scholar]
- Ingalla E. Q., Miller J. K., Wald J. H., Workman H. C., Kaur R. P., Yen L., Fry W. H. D., Borowsky A. D., Young L. J. T., Sweeney C., et al. (2010). Post-transcriptional mechanisms contribute to the suppression of the ErbB3 negative regulator protein Nrdp1 in mammary tumors. J. Biol. Chem. 285, 28691-28697 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Irandoust M. I., Aarts L. H. J., Roovers O., Gits J., Erkeland S. J., Touw I. P. (2007). Suppressor of cytokine signaling 3 controls lysosomal routing of G-CSF receptor. EMBO J. 26, 1782-1793 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jing X., Infante J., Nachtman R. G., Jurecic R. (2008). E3 ligase FLRF (Rnf41) regulates differentiation of hematopoietic progenitors by governing steady-state levels of cytokine and retinoic acid receptors. Exp. Hematol. 36, 1110-1120 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jovic M., Sharma M., Rahajeng J., Caplan S. (2010). The early endosome: a busy sorting station for proteins at the crossroads. Histol. Histopathol. 25, 99-112 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kumar K. G. S., Varghese B., Banerjee A., Baker D. P., Constantinescu S. N., Pellegrini S., Fuchs S. Y. (2008). Basal ubiquitin-independent internalization of interferon alpha receptor is prevented by Tyk2-mediated masking of a linear endocytic motif. J. Biol. Chem. 283, 18566-18572 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lavens D., Piessevaux J., Tavernier J. (2006). Review: negative regtilation of leptin receptor signalling. Eur. Cytokine Netw. 17, 211-219 [PubMed] [Google Scholar]
- Levine S. J. (2008). Molecular mechanisms of soluble cytokine receptor generation. J. Biol. Chem. 283, 14177-14181 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lievens S., Van der Heyden J., Vertenten E., Plum J., Vandekerckhove J., Tavernier J. (2004). Design of a fluorescence-activated cell sorting-based Mammalian protein-protein interaction trap. Methods Mol. Biol. 263, 293-310 [DOI] [PubMed] [Google Scholar]
- Maamra M., Bidlingmaier M., Postel-Vinay M.-C., Wu Z., Strasburger C. J., Ross R. J. M. (2001). Generation of human soluble leptin receptor by proteolytic cleavage of membrane-anchored receptors. Endocrinology 142, 4389-4393 [DOI] [PubMed] [Google Scholar]
- Manning G., Whyte D. B., Martinez R., Hunter T., Sudarsanam S. (2002). The protein kinase complement of the human genome. Science 298, 1912-1934 [DOI] [PubMed] [Google Scholar]
- Markson G., Kiel C., Hyde R., Brown S., Charalabous P., Bremm A., Semple J., Woodsmith J., Duley S., Salehi-Ashtiani K., et al. (2009). Analysis of the human E2 ubiquitin conjugating enzyme protein interaction network. Genome Res. 19, 1905-1911 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matthews V., Schuster B., Schutze S., Bussmeyer I., Ludwig A., Hundhausen C., Sadowski T., Saftig P., Hartmann D., Kallen K. J., et al. (2003). Cellular cholesterol depletion triggers shedding of the human interleukin-6 receptor by ADAM10 and ADAM17 (TACE). J. Biol. Chem. 278, 38829-38839 [DOI] [PubMed] [Google Scholar]
- Montoye T., Lemmens I., Catteeuw D., Eyckerman S., Tavernier J. (2005). A systematic scan of interactions with tyrosine motifs in the erythropoietin receptor using a mammalian 2-hybrid approach. Blood 105, 4264-4271 [DOI] [PubMed] [Google Scholar]
- Mullberg J., Durie F. H., Ottenevans C., Alderson M. R., Rosejohn S., Cosman D., Black R. A., Mohler K. M. (1995). A metalloprotease inhibitor blocks shedding of the IL-6 receptor and the p60 TNF receptor. J. Immunol. 155, 5198-5205 [PubMed] [Google Scholar]
- Murphy G. (2008). The ADAMs: signalling scissors in the tumour microenvironment. Nat. Rev. Cancer 8, 929-941 [DOI] [PubMed] [Google Scholar]
- Niendorf S., Oksche A., Kisser A., Lohler J., Prinz M., Schorle H., Feller S., Lewitzky M., Horak I., Knobeloch K.-P. (2007). Essential role of ubiquitin-specific protease 8 for receptor tyrosine kinase stability and endocytic trafficking in vivo. Mol. Cell. Biol. 27, 5029-5039 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Piessevaux J., Lavens D., Montoye T., Wauman J., Catteeuw D., Vandekerckhove J., Belsham D., Peelman F., Tavernier J. (2006). Functional cross-modulation between SOCS proteins can stimulate cytokine signaling. J. Biol. Chem. 281, 32953-32966 [DOI] [PubMed] [Google Scholar]
- Qiu X.-B., Goldberg A. L. (2002). Nrdp1/FLRF is a ubiquitin ligase promoting ubiquitination and degradation of the epidermal growth factor receptor family member, ErbB3. Proc. Natl. Acad. Sci. USA 99, 14843-14848 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Qiu X. B., Markant S. L., Yuan J. Y., Goldberg A. L. (2004). Nrdp1-mediated degradation of the gigantic IAP, BRUCE, is a novel pathway for triggering apoptosis. EMBO J. 23, 800-810 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Radtke S., Hermanns H. M., Haan C., Schmitz-Van de Leur H., Gascan H., Heinrich P. C., Behrmann I. (2002). Novel role of Janus kinase 1 in the regulation of oncostatin M receptor surface expression. J. Biol. Chem. 277, 11297-11305 [DOI] [PubMed] [Google Scholar]
- Ragimbeau J., Dondi E., Alcover A., Eid P., Uze G., Pellegrini S. (2003). The tyrosine kinase Tyk2 controls IFNAR1 cell surface expression. EMBO J. 22, 537-547 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Royer Y., Staerk J., Costuleanu M., Courtoy P. J., Constantinescu S. N. (2005). Janus kinases affect thrombopoietin receptor cell surface localization and stability. J. Biol. Chem. 280, 27251-27261 [DOI] [PubMed] [Google Scholar]
- Shuai K., Liu B. (2005). Regulation of gene-activation pathways by pias proteins in the immune system. Nat. Rev. Immunol. 5, 593-605 [DOI] [PubMed] [Google Scholar]
- Sorkin A., von Zastrow M. (2009). Endocytosis and signalling: intertwining molecular networks. Nat. Rev. Mol. Cell Biol. 10, 609-622 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Strous G. J., van Kerkhof P. (2002). The ubiquitin-proteasome pathway and the regulation of growth hormone receptor availability. Mol. Cell. Endocrinol. 197, 143-151 [DOI] [PubMed] [Google Scholar]
- Tellier E., Canault M., Rebsomen L., Bonardo B., Juhan-Vague I., Nalbone G., Peiretti F. (2006). The shedding activity of ADAM17 is sequestered in lipid rafts. Exp. Cell Res. 312, 3969-3980 [DOI] [PubMed] [Google Scholar]
- Tomida M., Yamamoto-Yamaguchi Y., Hozumi M. (1994). Three different cDNAs encoding mouse D-Factor/LIF receptor. J. Biochem. 115, 557-562 [DOI] [PubMed] [Google Scholar]
- Wang C., Chen T., Zhang J., Yang M., Li N., Xu X., Cao X. (2009). The E3 ubiquitin ligase Nrdp1 ‘preferentially’ promotes TLR-mediated production of type I interferon. Nat. Immunol. 10, 744-752 [DOI] [PubMed] [Google Scholar]
- Wauman J., De Smet A. S., Catteeuw D., Belsham D., Tavernier J. (2008). Insulin receptor substrate 4 couples the leptin receptor to multiple signaling pathways. Mol. Endocrinol. 22, 965-977 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Williams R. L., Urbé S. (2007). The emerging shape of the ESCRT machinery. Nat. Rev. Mol. Cell Biol. 8, 355-368 [DOI] [PubMed] [Google Scholar]
- Wu X., Yen L., Irwin L., Sweeney C., Carraway K. L., III (2004). Stabilization of the E3 ubiquitin ligase Nrdp1 by the deubiquitinating enzyme USP8. Mol. Cell. Biol. 24, 7748-7757 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu D., Qu C. K. (2008). Protein tyrosine phosphatases in the JAK/STAT pathway. Front. Biosci. 13, 4925-4932 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu P., Derynck R. (2010). Direct activation of TACE-mediated ectodomain shedding by p38 MAp kinase regulates EGF receptor-dependent cell proliferation. Mol. Cell 37, 551-566 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang M., Zhang Y., Pan J., Sun J., Liu J., Libby P., Sukhova G. K., Doria A., Katunuma N., Peroni O. D., et al. (2007). Cathepsin L activity controls adipogenesis and glucose tolerance. Nat. Cell Biol. 9, 970-977 [DOI] [PubMed] [Google Scholar]
- Yen L., Cao Z. W., Wu X. L., Ingalla E. R. Q., Baron C., Young L. J. T., Gregg J. P., Cardiff R. D., Borowsky A. D., Sweeney C., et al. (2006). Loss of Nrdp1 enhances ErbB2/ErbB3-dependent breast tumor cell growth. Cancer Res. 66, 11279-11286 [DOI] [PubMed] [Google Scholar]
- Zabeau L., Defeau D., Iserentant H., Vandekerckhove J. l., Peelman F., Tavernier J. (2005). Leptin receptor activation depends on critical cysteine residues in its fibronectin type III subdomains. J. Biol. Chem. 280, 22632-22640 [DOI] [PubMed] [Google Scholar]
- Zhong L., Tan Y., Zhou A., Yu Q. M., Zhou J. H. (2005). RING finger ubiquitin-protein isopeptide ligase Nrdp1/FLRF regulates parkin stability and activity. J. Biol. Chem. 280, 9425-9430 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.