Abstract
Obesity is accompanied by chronic, low-grade inflammation of adipose tissue, which promotes insulin resistance and type-2 diabetes. How does fat inflammation escape the powerful armamentarium of cells and molecules normally responsible for guarding against a run-away immune response? Regulatory CD4+ T cells expressing the transcription factor Foxp3 (termed Treg cells) are a lymphocyte lineage specialized in controlling immunologic reactivity. Treg cells with a unique phenotype were highly enriched in the abdominal fat of normal mice, but were strikingly and specifically reduced at this site in insulin-resistant models of obesity. In loss-of-function and gain-of-function experiments, Treg cells regulated the inflammatory state of adipose tissue and insulin resistance. Cytokines differentially synthesized by fat-resident regulatory and conventional T cells directly impacted on the synthesis of inflammatory mediators and glucose uptake by cultured adipocytes. These findings open the door to harnessing the anti-inflammatory properties of Treg cells to inhibit elements of the metabolic syndrome.
INTRODUCTION
Type-2 diabetes and other elements of the metabolic syndrome have increased at an alarming rate over the past several decades. There has been a parallel augmentation in obesity, now recognized to be a major contributor to the insulin resistance underlying this spectrum of metabolic abnormalities 1. Just how obesity promotes insulin resistance is not known, but results from clinical, epidemiological and molecular studies have converged to highlight the role of inflammation.
Prolonged nutrient overload results in a state of chronic, low-grade inflammation in adipose tissue 2 and systemically 3, in particular in visceral (abdominal) fat depots 4. Visceral fat produces a number of inflammatory cytokines and chemokines [leptin, tumor necrosis factor (TNF)-α, macrophage chemoattractant protein (MCP)-1, interleukin (IL)-6, etc], whose production can be elevated and expression dysregulated in the obese state, contributing importantly to insulin resistance 5.
At the cellular level, macrophages play a key role. With increasing obesity, they accumulate in visceral fat tissue, sometimes contributing as much as half of the cellularity 6, 7. The increase in number is accompanied by an evolution in phenotype from the anti-inflammatory (”alternatively” activated, M2) form to the pro-inflammatory (“classically activated”, M1) form 8, 9, which correlates with an increase in insulin resistance (e.g. 10). In the obese state, macrophages and adipocytes make several of the same regulators and mediators, including TNF-α, IL-6, matrix metalloproteinases (MMPs), peroxisome proliferator activated receptor (PPAR)-γ and fatty acid binding protein (FABP)-4 (reviewed in 1, 11). These findings, along with others, suggest that adipocytes and adipose tissue macrophages (ATMs) may contribute to insulin resistance in concert, both inhibiting and enhancing each other’s activities 11.
Like any inflammatory state, the chronic, low-grade inflammation associated with obesity should be subject to the control mechanisms that normally rein in over-exuberant immune responses. These mechanisms encompass a battery of cell-types, which can operate through cell-cell contact via a variety of receptors, or through a diversity of soluble mediators. Cells with a potentially regulatory phenotype have previously been associated with obesity [e.g. natural killer (NK)T cells] 12, and there have been prior reports of anti-inflammatory cytokines being detected in adipose tissue [e.g. IL-10 and transforming growth factor (TGF)-β] 8, 9. Yet, research on this topic has been very limited, and the influence – or loss of influence – of important control mechanisms remain unaddressed. For example, the role of arguably the most important regulatory cell population, CD4+Foxp3+ Treg cells, begs to be explored.
A small subset of T lymphocytes, normally constituting only 5–20% of the CD4+ compartment, Treg cells are thought to be one of the body’s most critical defenses against inappropriate immune responses – operating in contexts of autoimmunity, allergy, inflammation, infection and tumorigenesis 13, 14. Typically, they control the behavior of other T cell populations, but can also influence the activities of innate immune system cells 15–17. Treg cells are quite specifically characterized by high-level expression of the forkhead/winged-helix transcription factor, Foxp3. Deficiencies in this factor cause the lymphoproliferation and multi-organ autoimmunity found in scurfy mutant mice and human IPEX patients.
Here, we examined the Treg cells residing in murine adipose tissue, both their representation and phenotype, comparing visceral versus subcutaneous fat depots and lean versus obese mice. Their functional importance was evaluated in loss-of-function, gain-of-function and in vitro experiments. Lastly, an analogous population in human adipose tissue samples was sought. Our observations introduce an intriguing fat-resident T lymphocyte population whose products may have applications in treating the metabolic syndrome.
RESULTS
Adipose tissue Treg cells
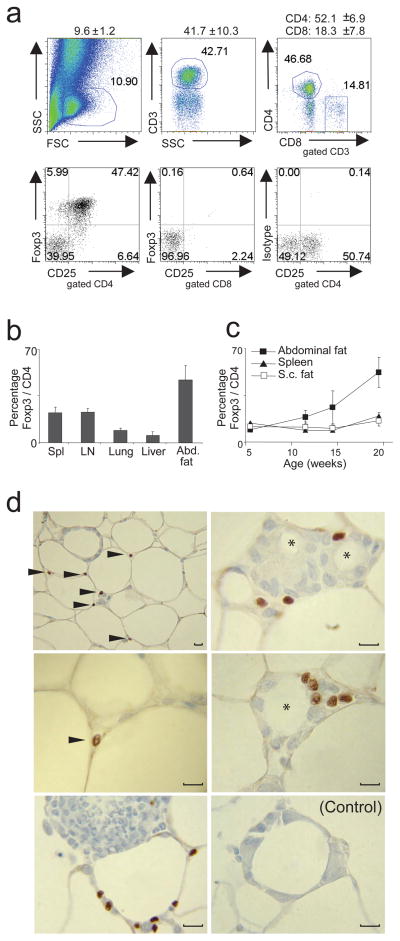
Adipose tissue is composed of multiple cell-types. Most prominent are adipocytes, but vascular endothelial cells, macrophages 6, 7 and lymphocytes 12, 18 are also found in the stromovascular fraction (SVF). According to multi-parameter flow cytometry, about 10% of SVF cells from the abdominal fat of ~30-week-old C57Bl/6 (B6) animals fell within the lymphocyte gate, close to half of which were of the CD3+ T lineage, split 3:1 between the CD4+ and CD8+ compartments, respectively (Fig. 1a, upper panels). Surprisingly, more than half of the CD4+ T cells expressed Foxp3 (Fig. 1a, lower panels), a much higher fraction than that normally found in lymphoid [e.g. spleen, lymph node (LN)] or non-lymphoid (lung, liver) tissues (Fig. 1b), including in subcutaneous fat (Fig. 1c). The two types of adipose tissue had similar low levels of Treg cells at birth, with a progressive accumulation over time in the visceral, though not the subcutaneous, depot (Fig. 1c). The dichotomy between visceral and subcutaneous fat is potentially important given the association of the former and not the latter with insulin resistance 4, 19.
Figure 1.
Abdominal (epidydimal) and subcutaneous (s.c.) fat pads as well as spleen, LN, lung and liver were isolated from retired-breeder B6 male mice, and the SVF fraction was stained for Foxp3, CD3, CD4, CD8 and CD25. (a) Upper panel: T cell distribution in SVF fraction from abdominal fat tissue. Numbers on top indicate the mean and standard deviation (SD) for cells in the lymphocyte gate after fixing and permeabilization, fraction of CD3+ T cells among lymphocyte-gated cells and distribution of CD4+ and CD8+ T cells. Lower panel: Percentage of Foxp3+CD25+ T cells in abdominal fat tissue gated on CD4+ or CD8+ T cells. Organs of 5 mice were pooled. Representative dot plots are shown. (b) Frequency of Foxp3+CD4+ T cells in different organs. Mean and SD from at least three independent experiments are shown, whereas organs from 4–5 mice per experiment were pooled. (c) Kinetic of Treg cell appearance in abdominal and s.c. fat tissue as well as in spleen. (d) Immunohistology of abdominal adipose tissue. Arrow-head indicates Foxp3 staining. Note: Foxp3 expression is restricted to the nucleus. * refers to dead-adipocyte residue surrounded by a crown-like structure formed by immune cells. Control staining with isotype antibody. Original magnification: left panel, upper picture 400x, all other 1000x.
Immunohistological examination revealed Foxp3+ cells in the spaces between adipocytes, mainly, but not only, in regions where several of them intersected (Fig. 1d, left panels). Fat tissue, especially from obese individuals, can host substantial numbers of macrophages, which accumulate in so-called “crown-like” structures, replete with dead-adipocyte residues 6, 7, 20. Treg cells were observed in such structures, in close proximity to macrophages and other leukocyte aggregates (Fig. 1d, right panels).
We estimate that 15,000–20,000 Foxp3+ cells reside in one gram of epididymal adipose tissue in an ~30-week-old B6 mouse. Given their known potency, this value very likely represents a biologically significant number – for example, transferring as few as 5,000–10,000 Treg cells can protect a mouse from autoimmune diabetes (and many of the cells do not even survive the transfer process) 21, 22.
A particular gene-expression profile
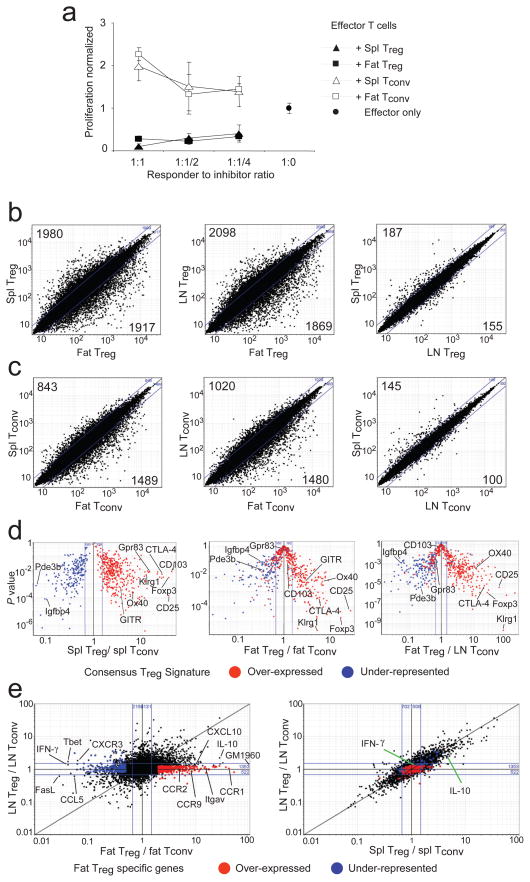
We wondered whether the CD25+Foxp3+ cells in abdominal adipose tissue were of typical Treg phenotype. They functioned as effectively as analogous cells isolated from the spleen when introduced into a standard in vitro suppression assay (Fig. 2a). [Fat T conventional (Tconv) cells also performed as expected, ie no suppressive activity and a normal proliferative response (Fig. 2a).] The lability and low recoverable numbers of fat Treg cells have so far prevented us from assaying their activities in in vivo suppressor assays. So we turned to the well-established transcriptional “Treg cell signature”, derived from the data of multiple groups 23–26, as an indicator of in vivo function.
Figure 2.
Functional comparison of Treg and Tconv cells from abdominal adipose tissue, LN and spleen. CD4+CD25+ Treg and Tconv cells were isolated from retired-breeder B6 mice. (a) A standard in vitro suppression assay was performed. Spleen-derived CD4+ effector T cells (responder cells) were incubated at various ratios with different T cell populations (spl: spleen, Fat: adipose tissue derived T cells). (b–e) Analysis with Affymetrix M430v2.0 chips. Normalized expression values for profiles directly comparing Treg cells (b) between: fat vs spleen (left panel), fat vs LN (center panel), LN vs spleen (right panel). Or for profiles directly comparing Tconv (c) between: fat vs spleen (left panel), fat vs LN (center panel), LN vs spleen (right panel). (b and c) Numbers are calculated on the basis of a cut-off of 2-fold from the individual comparisons. (d) “Volcano” plots of gene-expression data comparing P-value vs. fold-change for probes from the consensus Treg signature (23, 26). Plotted for: spleen Treg vs Tconv (left panel); fat Treg vs Tconv (center panel); fat Treg vs LN Tconv (right panel). (e) Fold-change to fold-change plots comparing Treg expression profiles between: fat Treg (x-axis) and LN Treg (y-axis) (left panel); spleen Treg (x-axis) and LN Treg (y-axis) (right panel). Genes uniquely up or down regulated in fat Treg cells are highlighted in red and blue.
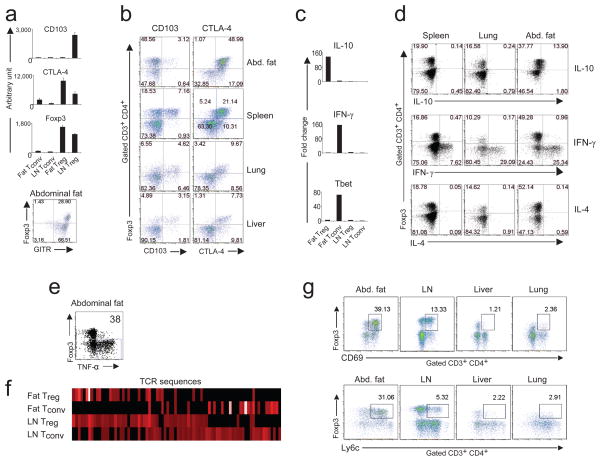
Clearly, the overall transcriptional profile of the Treg cell population from visceral fat differed from the patterns of its spleen and LN counterparts more than the latter two did from each other (Fig. 2b). This observation also held for the Tconv cell populations at these sites, though not as strikingly so (Fig. 2c). Focusing specifically on the documented Treg cell signature 23–26, we found the spleen data to show an excellent recapitulation of its major features: as anticipated, most genes known to be up-regulated in Treg cells (red) descended to the right on the p-value vs fold-change (FC) “volcano” plot, while most down-regulated loci (blue) dropped to the left (Fig. 2d, left panel). Fully 93% of the signature was present. In contrast, evidenced by their position at the volcano summit, many of the signature Treg cell genes were not significantly up- or down-regulated in the corresponding population from visceral fat, e.g. CD103 and Gpr83 (Fig. 2d, center and right panels). The data on CD103 (and others) were confirmed by flow cytometric analysis (Fig. 3b). These observations on the Treg cell signature were true whether the comparator was Tconv cells from the fat (Fig. 2d, center panel) or the LN (Fig. 2d, right panel), arguing that they reflect special features of adipose tissue Treg cells. Nonetheless, fat-resident CD4+Foxp3+ cells were clearly Treg cells, as much (63%) of the signature was intact, including over-expression of hallmark transcripts like those encoding CD25, GITR, CTLA-4, OX40 and KIrg1, in addition to Foxp3 itself. Confirmation of the elevated expression of several of these signature genes in fat Treg cells was obtained via RT-PCR and flow cytometric quantitation (Fig. 3a, and data not shown). The gene-expression differences observed in Treg cells isolated from the fat versus from the spleen and LN were not a simple reflection of different activation statuses, as a direct comparison between fat-derived and activated Treg cells showed clearly divergent transcription patterns (Suppl. Fig. 1)
Figure 3.
Phenotypic characterization of Treg cells from abdominal (epidydimal) fat tissue, spleen, lung and liver. (a and b) Cells were isolated from retired-breeder B6 mice, and the SVF fraction was stained for Foxp3, CD3, CD4, CD8, CD25, GITR, CD103 and CTLA-4. (a and c) Relative RNA expression of selected genes from Treg and Tconv cells from LN and fat. (d and e) Cytokine-expression profile from Treg and Tconv cells from spleen, lung and fat tissue. Profiles for IL-10, IFN-γ and IL-4. Representative dot plots of at least three independent experiments are shown. Organs from 4–6 mice were pooled per experiment. (f) TCR sequences of fat-derived Treg and Tconv cells. Abdominal fat and LN Treg and Tconv cells were isolated from old male animals from the Limited (LTD) mouse line. The frequency of the CDR3α sequences was analyzed on a single-cell base. Graphic display of the TCR sequences in a heat-map format from Treg and Tconv cells. (g) Cells were isolated from abdominal adipose tissue, LN, liver and lung from retired-breeder B6 mice, and the SVF fraction was stained for Foxp3, CD3, CD4, CD8, and for the activation marker CD69 and Ly6c. Representative dot plots are shown.
A large set of genes was over-expressed, many of them strikingly so, by the CD4+Foxp3+ T cells residing in abdominal adipose tissue, while not by the corresponding population at other sites examined (highlighted in red on Fig. 2e; listed in Suppl. Table 1). Chief amongst these were loci encoding molecules involved in leukocyte migration and extravasation: Gm1960 (an IL-10-inducible CXCR2 ligand 27), CCR1, CCR2, CCR9, CCL6, integrin alpha V, Alcam, CXCL2 and CXCL10 (Fig 2e; Suppl. Table 1; Suppl. Fig 2). On the other hand, some molecules of like function, eg CCL5 and CXCR3, were under-expressed in the visceral fat Treg cells (Fig. 2e, left panel). Also remarkable were the extremely high IL-10 transcript levels in CD4+Foxp3+ abdominal adipose cells (Fig. 2e, left vs right panels; Suppl. Fig. 2). A 136-fold augmentation of IL-10 transcripts in fat vs LN Treg cells was estimated from RT-PCR quantitation (Fig. 3c); the increase could also be detected by intracellular staining for IL-10 protein in the Treg cells of fat versus spleen and lung (Fig. 3d). Interestingly, pathway analysis (Suppl. Fig. 3a) suggested that the Treg cells not only produced large amounts of IL-10, but seemed also to be responding to it, as a number of genes downstream of the IL-10R were up-regulated in fat Treg cells compared with LN Treg cells.
Another set of genes was up-regulated specifically in CD4+Foxp3− T cells residing in adipose tissue vis a vis their LN counterparts, but not in spleen versus LN (indicated as blue in Fig. 2e; listed in Suppl. Table 1). Some of these loci also coded for molecules implicated in migration and extravasation, including CXCR3 and CCL5. Fat Tconv cells appeared to be highly polarized to a TH1 phenotype as they expressed high levels of Tbet and IFN-γ transcripts (Fig. 2e, left panel; Fig. 3c; and Suppl. Fig. 2), abundant intracellular interferon (IFN)-γ and TNF-α (Fig. 3, d and e), and little if any intracellular IL-4 (Fig. 3d). While the IL-10 effect could also be discerned with fat Tconv cells, it was not as striking (Suppl. Fig. 3b and c).
A specific T cell receptor repertoire
The T cell receptor (TCR) repertoire represents another parameter for assessing the degree of similarity of T cell populations: for example, it has been shown that Treg and Tconv cell populations have distinct repertoires, with only limited overlap 28–30. In addition, the TCR repertoire of Treg cells in the abdominal adipose tissue might give an indication of whether their abundance reflects an influx and/or retention of cells of a particular specificity or a local cytokine-induced conversion 31. To render the repertoire analysis more manageable and interpretable, we exploited the Limited (LTD) mouse line, wherein TCR diversity is restricted to the complementary-determining region (CDR)3α via the combination of a transgenic TCRα minilocus and the TCRα-knockout mutation 32. CDR3α sequences were determined from 98 individually sorted visceral fat CD4+CD25+ cells that also expressed Foxp3 RNA, and their distribution was compared with that of CDR3α sequences from fat Tconv cells or LN Treg and Tconv cells. (Note: We were unable to obtain enough Treg cells from subcutaneous fat to perform a parallel TCR sequence analysis on this depot.)
As expected, the “heat maps” generated from these sequences (Fig. 3f) revealed distinct TCR repertoires for the LN Treg and Tconv cell populations, with only limited overlap. Similarly, the fat Treg and Tconv cell populations also had different repertoires, rendering it very unlikely that the accumulation of Foxp3+ Treg cells in the abdominal adipose tissue resulted from local conversion of Tconv cells. Interestingly, the fat Treg cells had a very restricted distribution of sequences, representing a distinct subset of those normally found in their LN Treg cell counterparts (Fig. 3f and Suppl. Table 2). The CDR3α sequences characteristic of fat Treg cells were often independently generated by different nucleotide sequences: 50% of sequences found more then three times per individual mouse (3/6) showed such nucleotide variation (Suppl. Table 3). In contrast, none of the fat Tconv cells (0/10) did (Suppl. Table 4), suggesting the repeated selection of Treg cells with similar antigen receptors rather than the proliferation of a single clone. The sequences were reproducibly frequent in different mice, again pointing to TCR-driven selection (Suppl. Table 3). These data indicate that the specificity of the TCR may be instrumental in generating the high frequency of Treg cells in visceral fat, perhaps through local recognition of cognate antigen.
Indeed, fat Treg cells displayed unusually high levels of the early activation markers CD69 and Ly6c (Fig. 3g), although it remains possible that such increases instead, or also, reflect cytokine influences. Though TGF-β is readily detectable in adipose tissue 33, and is known to promote Treg cell differentiation/survival 34–36, its effects are an unlikely explanation for the high representation and activation state of Treg cells in fat because the typical changes in gene expression promoted by this growth factor were not observed in this population (Suppl. Fig. 4). For example, CD103 was not up-regulated (Fig. 2d, center and right panels; Fig. 3b). This observation also argues against TGF-β-mediated conversion of CD4+Foxp3− to CD4+Foxp3+ cells in visceral fat, as has been observed in a few systems 31.
Implication in metabolic control
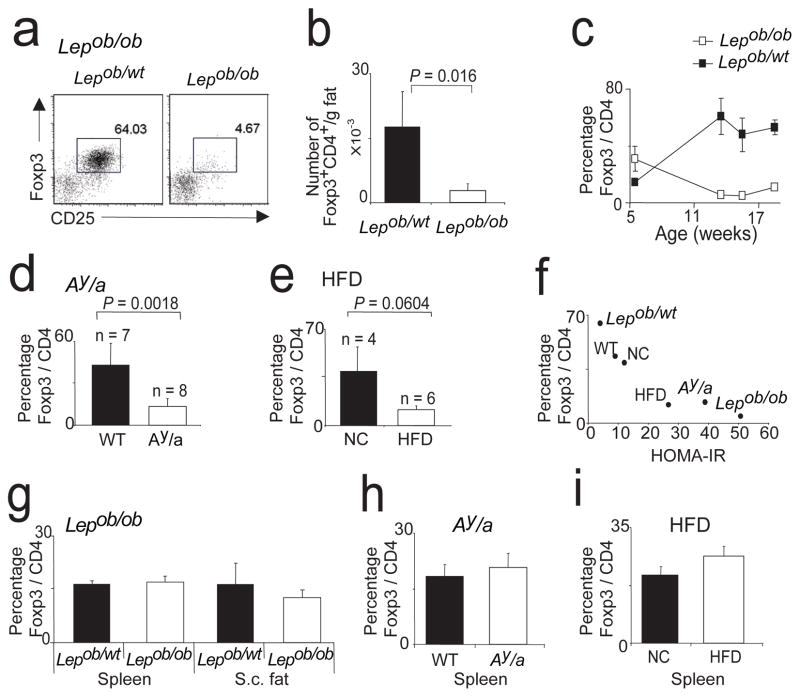
To learn how this peculiar population of Treg cells responds to excess adiposity, we examined it in three mouse models of obesity: leptin-deficient mice (Lepob/ob; commonly referred to as ob/ob) 37, mice heterozygote for the yellow spontaneous mutation (Ay/a) 38 and mice chronically fed a high-fat diet (HFD) 3, all on the B6 genetic background and all displaying insulin resistance (Suppl. Fig. 5). Strikingly, the Treg cell population in abdominal fat was drastically reduced in old ob/ob mice, whether the fraction of Treg cells in the CD4+ compartment or the number of Treg cells per gram of fat was quantitated (Fig. 4, a and b). While five-week-old leptin-deficient animals had somewhat higher (p=0.02) levels of CD4+Foxp3+ T cells in visceral fat (30%) than did wild-type age-matched littermates (10%), this subset progressively declined in the former case and rose in the latter (Fig. 4c) (p=0.0011). As anticipated, ob/ob fat Treg cells were relatively impoverished in IL-10 producers (Suppl. Fig. 6a). The normal representations of Treg cells in the spleen and subcutaneous fat of ob/ob mice (Fig. 4g) argue that the paucity of this subset in visceral fat was not just a reflection of the leptin defect; indeed, the absence of this adipokine was recently reported to foster the proliferation of Tregs 39. Numbers of conventional CD4+ T cells in the abdominal fat of ob/ob adults were only mildly reduced, and the percentage of these cells making IFN-γ remained very similar (Suppl. Fig. 6, b and c).
Figure 4.
Three mouse models of obesity: Lepob/ob, Ay/a and HFD. (a–c) Abdominal adipose tissue from Lepob/ob and heterozygote Lepob/wt mice was analyzed for Treg cell frequency. (a) Representative dot plots of 13-week-old mice. (b) Total number of Treg cells per one gram fat. (c) Changes of Treg representation over age. Mean and SD are shown. (d) Percentage of Treg cells in abdominal adipose tissue of 24-week-old Ay/a or littermate (WT) mice. (e) Percentage of fat Treg cells in mice fed for 29 weeks with HFD or NC. (f) Correlation of HOMAR-IR and fraction of Treg cells. (g–i) Observed changes of Treg cell proportion in adipose tissue of the three obesity models were not reflected in other organs. (g) Lepob/ob, (h) Ay/a, (i) HFD.
There were also reduced levels of CD4+Foxp3+ cells in abdominal fat, but not at other sites, in the Ay/a mice and in HFD-fed animals (Fig. 4, d and e; h and i). The reductions were not as striking as for ob/ob animals, consistent with less insulin resistance in the latter two models (Suppl. Fig. 5). Indeed, we saw a good correlation between insulin resistance and the fraction of Treg cells in abdominal fat (Fig. 4f).
The observed correlation between obesity and insulin resistance on the one hand and a dearth of CD4+Foxp3+ cells in abdominal adipose tissue on the other hand suggests that Treg cells might be implicated in the relationship between the inflammatory and metabolic parameters. To test this notion, we first attempted loss-of-function experiments. Given that it is not currently feasible to ablate Treg cells specifically in the fat, we employed mice expressing the diphtheria toxin (DT) receptor (R) under the control of the Foxp3 transcriptional regulatory elements, wherein administration of DT results in punctual systemic depletion of Treg cells. We recently developed a line of NOD BAC transgenic mice expressing a DTR-eGFP fusion protein under the dictates of Foxp3 transcriptional regulatory elements. Routinely, 85–90% of Treg cells are eliminated in the spleen and LNs two days after DT administration to these animals (unpublished data), similar to what has been described by the Rudensky group with their independent line 40. Cell death induced by DT is apoptotic, and therefore does not set off a pro-inflammatory immune response 41–44, prompting wide-spread use of this approach to probe diverse immunological issues through specific ablation of particular cell-types, including Treg cells 41, 45–47. Because Treg-cell-ablated mice develop multi-organ autoimmunity 2 weeks later 40, this strategy required us to look at early indicators of potential Treg cell function, namely alterations in adipose tissue mRNAs encoding inflammatory mediators or upstream changes in metabolic signaling pathways; previous data suggested that two weeks may be too early to see changes in more downstream metabolic parameters, including performance in glucose-tolerance tests (GTTs) 48.
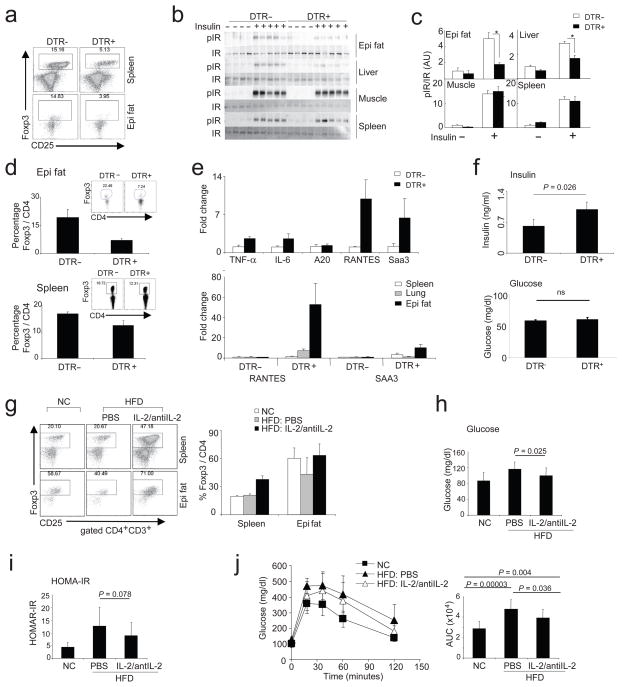
For one set of experiments, we treated 10-week-old male mice with DT every other day for four days, which reduced the Treg cell representation in abdominal fat to about 1/4 the normal (Fig. 5a, bottom panels), while the spleen and lung populations were at about 1/3 the usual (Fig. 5a, top panels and data not shown). The depletion of Treg cells was accompanied by substantial decreases in insulin-stimulated insulin-receptor (IR) tyrosine phosphorylation in epidydimal fat and liver, but not in muscle and spleen (Fig. 5, b and c). Parallel results were obtained on AKT phosphorylation (data not shown). At this early time-point, in vivo metabolic changes were marginal (not shown), so we conducted a second set of experiments in which mice were treated with DT for longer times. Mice injected with DT every other day for 9 days had a Treg cell fraction about 30% the usual size in the fat, while the spleen, lung and LN populations had bounced back to about 70% the normal (Fig. 5d). [We do not know why there is such a preferential impoverishment of Treg cells in adipose tissue with this protocol (which serves our purpose well) but it is likely to reflect slower repopulation kinetics.] Concomitantly, many of the genes encoding inflammatory mediators [e.g. TNF-α, IL-6, RANTES, Serum Amyloid A (SAA)-3] were induced in the visceral fat depot (Fig. 5e, upper panel), and much less so in the spleen and lung (Fig. 5e, lower panel). Insulin levels were significantly elevated in the Treg-cell-depleted mice, demonstrating insulin resistance (Fig. 5f, upper panel), although fasting blood-glucose levels at this early time-point were unchanged, consistent with adequate β-cell compensation (Fig. 5f, lower panel).
Figure 5.
Loss-of- and gain-of-function experiments. (a–f) Loss-of-function experiment by depleting Treg cells expressing DTR. 10-week-old male mice, either DTR-positive or -negative, were treated every other day for 4 days (a–c) or 9 days (d–f) with DT. (a) Percentage of Treg cells from spleen or the abdominal fat after 4 days of treatment. (b and c) Treg depletion affects insulin signaling in epididymal white adipose tissue (WAT) and liver. Immunoprecipitation and Western blotting of insulin-induced IR shows a decrease in IR phosphorylation (pIR) in epi WAT and liver without differences in muscle and spleen. (b) Blot data. (c) Quantification of pIR normalized by total IR. (N≥4, *p<0.004, T-test); (d) Percentage of Treg cells from the abdominal fat (upper panel) or spleen (lower panel) after 9 days of treatment, with a representative dot plot as an insert. (e) Upper panel: Expression of TNF-α, IL-6, A20, RANTES and SAA3 transcripts in abdominal adipose tissue. Three mice per group, one of two independent experiments is shown. Lower panel: comparison of RANTES and SAA3 transcripts in spleen, lung and abdominal fat (epi fat). (f) Fasting-insulin (upper panel) and -glucose levels (lower panel) after 9 days of treatment every other day. Six mice per group from two independent experiments were pooled. Significance was determined by the Mann-Whitney U test. (g–j) Gain-of-function experiment. In situ expansion of Treg cells via injection of a mAb specific for IL-2 coupled with recombinant IL-2. (g) Dot plots (left panel) and a summarizing bar graph (right panel) showing Treg cells from spleen and abdominal fat tissue (epi fat) from mice fed normal chow (NC) or with 15 weeks of HFD. Treated with IL-2/anti-IL2 complex or saline for 6 days and analyzed on day 14 (n=6 for each group). Blood glucose (h) HOMA-IR (i), and an i.p. GTT (j) of mice described in (g). (j, right panel), calculated area under the curve (AUC) from all mice tested by GTT (n=11 in each group), including the dataset described in (g). P-values were calculated with the T-test.
To provide additional evidence that the inflammation promoted by Treg cell depletion in the abdominal adipose tissue was not merely a response to cell death, we used mice 40 wherein most (95%) or only about half of the Treg cells were punctually ablated by DT-treating females carrying a DTR gene knocked into the Foxp3 locus on the X chromosome, in homozygous vs heterozygous state (Suppl. Fig. 7). In the latter case, random X-chromosome inactivation will protect about half of the Treg cells because the DTR-carrying allele resides within the inactivated chromosome. As expected, when most of the Treg cells were ablated, pro-inflammatory transcripts (eg RANTES, MCP-1) were strongly induced in the fat tissue. In contrast, when only half as many were killed, there was no such induction of pro-inflammatory transcripts, signifying that: 1) substantial DT-induced cell death was not in and of itself the stimulus, and 2) a demi-complement of Treg cells sufficed to protect the fat from inflammation.
As concerns gain-of-function approaches, the lability and low recoverable numbers of visceral fat Treg cells rendered unsuccessful our many attempts at standard adoptive transfer experiments; transfer of more limited numbers of fat Treg cells into lymphodeficient recipients also proved problematic because the resultant homeostatic proliferation altered the phenotype of the introduced population, perhaps most relevantly its profile of cell-surface homing receptors (data not shown). Therefore, as an alternative means to achieve gain-of-function, we turned to in situ expansion of Treg cells via injection of a particular recombinant IL-2:anti-IL-2 monoclonal antibody (mAb) complex demonstrated to selectively elicit Treg cells 49, 50. Daily injections of the complex for 6 days into mice pre-fed on HFD for fifteen weeks did substantially increase the fraction of Treg cells in the spleen and abdominal fat vis a vis PBS-injected controls (averages from multiple experiments 37+/− 4% vs. 21+/−2 % for spleen and 63+/−12% vs. 43+/−17% for abdominal fat) (Fig. 5g). [Note that the reduction in the Treg cell fraction in PBS-injected HFD-fed mice versus mice fed normal chow is less in Fig. 5 than in Fig. 4 because of the shorter time of HFD.] Since the complex-injected mice had been pre-challenged with an HFD, we could assess the influence of an elevated representation of Treg cells on various indicators of insulin resistance. Blood-glucose levels were significantly lower (Fig. 5h) [and, as expected, IL-10 transcript levels were significantly higher (Suppl. Fig. 6d)] in the HFD-fed mice with more Treg cells. While HOMA-IR (Fig. 5i) and glucose tolerance [measured via an intraperitoneal (ip) GTT] (Fig. 5j, left panel) all trended towards lower values in the Treg-cell-enriched HFD-fed animals, these differences fell short of statistical significance, probably due to the greater experimental variability inherent in these assays. In fact, given the very short experimental window that we had to work in to avoid expansion of effector T cells, small differences are not at all surprising. In order to enhance power, we injected a number of additional HFD-fed mice with IL-2:anti-IL-2 complexes vs. PBS under similar conditions, accumulating a total of 11 individuals for each group. The Treg cell fraction in the complex-injected HFD-fed mice ranged from 40–83% (Av= 68+/−13%), while that in the PBS-injected animals spanned 18–70% (Av= 45+/−13%). Both HFD-fed groups were glucose intolerant vis a vis control mice fed normal chow (NC); however, the complex-injected group, with the highest levels of Treg cells, showed a significant improvement compared with the PBS-injected group (Fig 5j, right panel). Similar results were obtained in the Ay/a system (Suppl. Fig. 8).
These findings indicate that Treg cells guard against excessive inflammation of the adipose tissue and its downstream systemic consequences, and strongly suggest that Treg cells residing in the fat are responsible. While it is not currently feasible to more definitively establish the latter point, given that no fat-Treg-specific reagent exists and that it has not been possible to successfully isolate and transfer fat Treg cells in the requisite quantities, a role for Treg cells, whatever their source, in metabolic homeostasis and its dysregulation in obesity is an unexpected finding.
In vitro effects on adipocytes
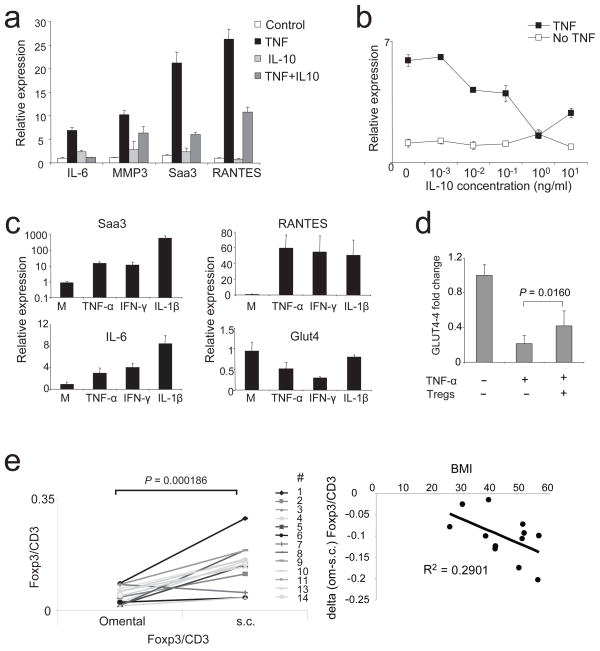
A likely mechanism by which T cells residing in adipose tissue impact on neighboring cells is through soluble mediators. Thus, we explored the influences of the major cytokines differentially produced by Treg and Tconv cells in fat vis a vis at other sites: according to our gene-expression profiling, IL-10 and IFN-γ, respectively. Fully differentiated, lipid-laden 3T3-L1 adipocytes were pretreated or not for 48h with IL-10, and were subsequently stimulated for 24h with TNF-α, an established method for in vitro induction of insulin resistance (Fig. 6, a and b). TNF-α induced changes in adipocyte expression of a number of transcripts encoding inflammatory mediators, for example IL-6, RANTES, SAA-3 and MMP3. Strikingly, IL-10 inhibited the TNF-α-induced expression of all of these mRNAs. TNF-α has also been shown to down-modulate insulin-dependent tyrosine phosphorylation of insulin receptor substrate (IRS)1 and to inhibit Glut4-mediated glucose uptake in 3T3-L1 adipocytes; these effects, too, were reversed by IL-10 8, indicating that this cytokine reverts insulin resistance by a mechanism directly impinging on adipose tissue cells (i.e. is cell autonomous). In striking contrast to the anti-inflammatory effects of this mediator made by visceral fat Treg cells, a major product of the Tconv cells at this site, IFN-γ, was pro-inflammatory in the same in vitro assay system: expression of SAA3, RANTES and IL-6 transcripts were all induced, and Glut4 mRNA was down-regulated (Fig. 6c).
Figure 6.
Cytokine effects on adipocytes and human correlates. (a and b) IL-10 can reverse TNF-α mediated inflammatory changes in differentiated adipocytes. (a) Expression of IL-6, MMP3, SAA3 and RANTES were measured with qPCR under unmanipulated culture conditions (control); adipocytes were treated with TNF-α (TNF); cells were treated IL-10 (IL-10) alone; or cells were treated with TNF-α and IL-10 (TNF+IL-10). (b) Relative expression of IL-6 in differentiated adipocytes, dose response curve of IL-10. TNF: TNF-α and different concentrations of IL-10. No TNF: only IL-10. Representative experiments are shown. (c) Expression of SAA3, RANTES, IL-6 and Glut4 in differentiated adipocytes, unmanipulated (M) or treated with TNF-α, IFN-γ and IL-1β. Representative experiments are shown. (d) Expression of Glut4 in differentiated adipocytes either unmanipulated or treated with TNF-α in presence or absence of spleen Treg cells. Mean and SD of 3 independent experiments are shown. P-value was calculated with T-test. (e) Paired human omental and s.c. adipose samples from mostly obese individuals (BMI range: 25.5–56.43, average: 44.85). Expression of FOXP3 and CD3 was measured by quantitative PCR. Plotted are the ratios of FOXP3 vs. CD3 for omental and s.c. adipose tissue (left panel). Right panel: the decrease in FOXP3/CD3 ratio in omental versus s.c. adipose tissue was plotted against the BMI for each individual donor from the left panel. Except the positive value of subject #7 (> 2 standard deviations from the mean) was not included. Each dot represents an individual donor.
Our many attempts to support these conclusions by performing co-cultures of fat Treg cells and 3T3-L1 pre-adipocytes have so far been unsuccessful due to rapid death of the Treg cells during the incubation period, likely due to pro-apoptotic factors present in the medium or produced by the adipocytes, eg TNF-α. However, spleen Treg cells were less fragile in these co-culture conditions, and we could demonstrate that they dampen levels of pro-inflammatory transcripts made by activated adipocytes in culture and, perhaps most relevant, inhibit their down-modulation of Glut-4 transcripts (Fig. 6d and data not shown). The greater fragility of fat vs spleen Treg cells may be explained by the former’s higher expression of TNF-Rs (data not shown).
And humans?
Finally, we sought to translate our findings to the human arena. We had access to a set of paired frozen omental and subcutaneous fat tissues from a number of individuals with an average body:mass index (BMI) of 44.85, thus (except for one case) falling within the obese (30–39.9) and morbidly obese (>40) range. Given that the samples were frozen, we could not perform flow cytometric analysis on or purification of lymphocyte populations, but could titer FOXP3 transcript levels by PCR (Fig. 6e, left panel). FOXP3 mRNA was readily detectable in both fat depots. Consistent with the observations on obese mice, we found higher levels of FOXP3 transcripts, presumably an indicator of Treg cells, in the subcutaneous adipose tissue. We did not have access to non-obese controls for these studies due to the rarity of performing bariatric surgery on normal individuals. However, we did find a correlation between BMI and the drop in Treg cells in omental vis-à-vis subcutaneous fat (Fig. 6e, right panel). These data suggest that our findings on mice may be translatable to humans, encouraging for more sophisticated analyses on purified T cell subsets from fresh adipose tissue.
DISCUSSION
Finding a peculiar population of regulatory T lymphocytes specifically in the abdominal adipose tissue of normal, but not obese, mice raises a number of questions. First, why and how do Treg cells accumulate at this site? One factor may be antigen stimulation, as suggested by the imprint of antigenic selection on the TCR repertoire of visceral fat Treg cells and by their unusually high state of activation. Depending on the location(s) of any such antigens, they could stimulate circulating Treg cells, provoking them to exit the lymph nodes and invade the fat, and/or re-stimulate Treg cells filtering through adipose tissue, thereby promoting their retention. As discussed above, neither the sequencing nor the gene-expression profiling data provided support for the notion that Tconv cells were induced to convert to Treg cells in the visceral fat. A second factor is almost certainly chemokines, given the unique pattern of chemokine/chemokine receptor gene expression by the Treg cells isolated from adipose tissue. Functional studies will be needed to unravel which of these molecules are indeed involved in cell attraction or access to fat, micro-localization once therein, or their recruitment of other leukocyte subsets. Thirdly, there may be a role for adipokines in nurturing Treg cell survival in adipose tissue. A recent study highlighted the negative effect of leptin on the proliferative capacity of Treg cells 39, which fits well with their opposite representations in normal and obese fat: many Treg cells and little leptin in the former; few Treg cells and much leptin in the latter. Given, in contrast, the parallel high levels of Treg cells and adiponectin in normal fat and low levels in obese fat, this adipokine is an interesting candidate as a positive factor, especially since it induces IL-10 synthesis, at least by macrophages 51, 52.
Next, what function(s) are Treg cells performing in normal abdominal adipose tissue? Recent reports have highlighted the interplay between adipocytes and a population of anti-inflammatory macrophages in this fat depot, suggesting a role for resident macrophages in promoting tissue repair and angiogenesis, and in maintaining insulin sensitivity 8, 9. Our results argue that the activities of coincident populations of Tconv and Treg cells need to be added to the mix. It may be relevant that the Tconv cells appear to be making an ongoing TH1 response to some stimulus and that their major product, IFN-γ, promotes synthesis of inflammatory mediators by adipocytes. The Treg cells may be keeping this response in check, as well as regulating the activities of their macrophage and adipocyte neighbors – indeed, their physical location in crown-like structures at adipocyte junctures would encourage interaction with both cell-types. IL-10 is one candidate for playing a role in Treg-cell-mediated regulatory activities, given its association with improved insulin sensitivity in a number of contexts in both rodents and humans 53–55, though this cytokine is also produced by the fat-resident anti-inflammatory macrophages 8.
Lastly, what provokes Treg cells to vacate abdominal fat in obesity, or perhaps more likely, to refrain from entering it? It may be a secondary effect. Increasing adiposity has been associated with an influx of macrophages 6, 7, in particular the inflammatory macrophage subset 8, 9, into the abdominal depot; as well as with increased local and systemic concentrations of inflammatory cytokines, such as TNF-α and IL-6. In addition, a reciprocal increase and decrease in the adipokines leptin and adiponectin, respectively, have been reported, fueling speculation of aggressive crosstalk between the two cell-types in the obese condition 11. One possible scenario, then, is that elements of this changing environment are unfavorable for Treg cell entry, expansion or survival, leading to a secondary decline of this regulatory population; in fact, leptin 39 and IL-6 56, 57 are already known to have such properties. However, it is also possible that loss of Treg cells from the abdominal fat is the primary effect. While their exaggerated numbers in lean adipose tissue had been sufficient to keep the chronic inflammation under control, reduced Treg cell numbers with increasing adiposity could open the gate to an invasion of inflammatory macrophages and thereby a more robust synthesis of inflammatory cytokines. Whether the influx of macrophages or the efflux (or death) of Treg cells proves upstream, both processes must be downstream of an initiating event, as yet undefined, though suggestions have included local hypoxia 58, increased adipocyte death 20 and adipocyte stress 59.
METHODS
Mice
Male B6 (at different ages and retired breeders 25–35 weeks old), mice homozygous for the obese spontaneous leptin mutation, (Lepob/ob; commonly referred to as ob/ob) and Lepob/wt mice, mice heterozygous for the yellow spontaneous mutation (Ay/a), Foxp3GFP/B6 reporter mice 23, and Limited (LTD) mice 32 were bred in our SPF facility at the Joslin Diabetes Center, or were purchased from the Jackson Laboratory (Bar Harbor, ME). Mice on HFD were fed with a rodent diet of 45 or 60 kcal% fat from Research Diet (New Brunswick, NJ; Cat# D12451). Others were bought from Jackson laboratory, as indicated. Mice expressing DTR under the control of Foxp3 (Foxp3DTR) were generated in our facility and will be described in detailed elsewhere. A construct expressing the DTR-eGFP cDNA was a gift from Dr Dan Littman. In brief, a BAC spanning from 150kb upstream to 70 kb downstream of Foxp3 transcription start site was isolated. The DTR-eGFP cDNA with a stop-codon was inserted between the first and second codon of the Foxp3 open reading frame. The recombinant Foxp3DTR BAC was injected into the NOD strain.
Isolation of T cells and cytokine stimulation assay
Abdominal (epidydimal) adipose tissue, subcutaneous adipose tissue, lung and liver were removed after flushing the organs through the portal vein and the heart ventricle, cut into small pieces (or passed through a sieve in the case of the liver) and digested for about 40 minutes with collagenase type II (adipose tissue, Sigma) or collagenase type IV (Sigma). Cell suspensions were then filtered through a sieve (or for the lung smashed through the sieve), and the SVF was harvested after spinning. Cells were stained with: anti-CD4, anti-CD8, anti-CD3, anti-CD25 and anti-B220; and for some experiments with anti-CD103, anti-GITR, anti-CD69 and anti-Ly6c mAb, and were fixed and permeabilized according to the manufacturer’s instructions (eBiosciences), followed by intracellular staining of Foxp3 (eBiosciences) and CTLA-4.
For intracellular cytokine staining, cells were stimulated with PMA (50ng/ml) (Sigma) and ionomycin (1nM) (Calbiochem) for 4 hours. Golgistop (BD) was added to the culture at the recommended amount during the last three hours. Cells were stained with: anti-CD4, anti-CD8, anti-CD3, anti-CD25 and anti-B220 antibody, and were fixed and permeabilized according to the manufacturer’s instructions (eBiosciences), followed by intracellular staining of Foxp3 (eBiosciences), IFN-γ, TNF-α, IL-10 or IL-4. Cells were then analyzed using a Moflo, Coulter Epics XL or LSRII instruments and FlowJo software.
Microarray analysis
Lymph node and abdominal fat TCR+CD4+ and CD25hi (Treg) or TCR+CD4+ and CD25− (Tconv) cells were sorted from retired male breeder B6 mice, and spleen Treg and Tconv cells were sorted from Foxp3GFP/B6 reporter mice 23. RNA was extracted with Trizol and amplified for two rounds using the MessageAmp aRNA kit (Ambion), followed by biotin labeling using the BioArray High Yield RNA Transcription Labeling Kit (Enzo Diagnostics), and purified using the RNeasy Mini Kit (Qiagen). The resulting cRNAs (three independent datasets for each sample type) were hybridized to M430 2.0 chips (Affymetrix) according to the manufacturer’s protocol. Initial reads were processed through Affymetrix software to obtain raw .cel files. Microarray data were background-corrected and normalized using the RMA algorithm implemented in the GenePattern software package 60, and replicates averaged (full datasets are deposited at NCBI under accession# GSE7852). A consensus Treg signature was compiled from four independent analyses 23, 26. The color-coding in the figures denoted genes 1.5 fold over- (red) or under- (blue) expressed in Treg cells in all four reference datasets. The fat Treg-specific gene set included loci specifically over- or under-expressed in fat Treg cells, and was generated by including genes 2-fold or more over- (red) or under- (blue) expressed in fat Treg vs. fat Tconv cells as well as more than 2-fold different between fat Treg and LN Tconv cells. To exclude the classical Treg-specific genes, LN Treg vs. LN Tconv had to be less then 1.25-fold for over- or more then 0.8 for under-represented genes.
In vivo depletion and expansion of Treg cells
For depletion: 10-week-old Foxp3DTR+ and Foxp3DTR− control mice were injected ip with DT (Sigma), 40ng/g body weight, every other day for 9 days or, in some experiments, for 4 days. Overnight fasting blood-glucose and-insulin levels were measured and abdominal adipose tissue, lung and spleen were removed for RNA extraction.
For expansion: mice fed 15 weeks on HFD were purchased from Jackson Laboratory (Bar Harbor ME), fed for 12 weeks with HFD in the Jackson facility [60 kcal % fat from Research Diet (New Brunswick, NJ; Cat# D12492)]. Complexes of the anti-IL-2 mAb JES6-5H4 (BD) and mouse IL-2 (PeproTech) were prepared and ip-injected as described 49. In brief, 5 μg of anti-IL2 and 0.5 μg mIL-2 per mouse were incubated for 20 minutes on ice followed by ip injection. Mice received daily injections for 6 or 9 days, and were analyzed on day 14. Control mice were injected with saline (PBS). In some experiments mice were fed with HFD for 8 weeks [60 kcal % fat from Research Diet (New Brunswick, NJ; Cat# D12492)] and were injected with the complex for 9 days. For glucose tolerance tests (GTT), glucose (2.0 g/kg) was administered by intraperitoneal injection after an overnight fast. Blood glucose was measured before, 15, 30, 60 and 120 minutes after glucose application.
Additional methodology are described in the Supplementary Methods.
Supplementary Material
Acknowledgments
We thank Dr Dan Littman for the DTR construct, Leilani Roser and Kimie Hattori for assistance with mice, Dr Sasha Rudensky for providing us with Foxp3DTR mice, Joyce LaVecchio and Giridesh Buruzala for flow-cytometry, and Dr Jonathan Hill, Jasmine Perez and Rachel Melamed for help with the microarray analysis. This work was supported by Young Chair funds to DM and CB; by the NIH (DK51729 and DK73547) and Adler Chair funds to SES; and by Joslin’s National Institutes of Diabetes and Digestive and Kidney Diseases-funded Diabetes and Endocrinology Research Center core facilities. Postdoctoral fellowship support for MF was from the German Research Foundation (Emmy-Noether Fellowship, FE 801/1-1) and the Charles A. King Trust Postdoctoral Fellowship, and for LH from the Ministry of Science of Spain. JW and DC were supported by predoctoral fellowships from the NIH (T32 DK7260) and the European School of Molecular Medicine (SEMM), respectfully.
References
- 1.Shoelson SE, Lee J, Goldfine AB. Inflammation and insulin resistance. J Clin Invest. 2006;116:1793–1801. doi: 10.1172/JCI29069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Hotamisligil GS, Shargill NS, Spiegelman BM. Adipose expression of tumor necrosis factor-alpha: direct role in obesity-linked insulin resistance. Science. 1993;259:87–91. doi: 10.1126/science.7678183. [DOI] [PubMed] [Google Scholar]
- 3.Cai D, et al. Local and systemic insulin resistance resulting from hepatic activation of IKK-beta and NF-kappaB. Nat Med. 2005;11:183–190. doi: 10.1038/nm1166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Bosello O, Zamboni M. Visceral obesity and metabolic syndrome. Obes Rev. 2000;1:47–56. doi: 10.1046/j.1467-789x.2000.00008.x. [DOI] [PubMed] [Google Scholar]
- 5.Fantuzzi G. Adipose tissue, adipokines, and inflammation. J Allergy Clin Immunol. 2005;115:911–919. doi: 10.1016/j.jaci.2005.02.023. [DOI] [PubMed] [Google Scholar]
- 6.Weisberg SP, et al. Obesity is associated with macrophage accumulation in adipose tissue. J Clin Invest. 2003;112:1796–1808. doi: 10.1172/JCI19246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Xu H, et al. Chronic inflammation in fat plays a crucial role in the development of obesity-related insulin resistance. J Clin Invest. 2003;112:1821–1830. doi: 10.1172/JCI19451. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Lumeng CN, Bodzin JL, Saltiel AR. Obesity induces a phenotypic switch in adipose tissue macrophage polarization. J Clin Invest. 2007;117:175–184. doi: 10.1172/JCI29881. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Lumeng CN, Deyoung SM, Bodzin JL, Saltiel AR. Increased inflammatory properties of adipose tissue macrophages recruited during diet-induced obesity. Diabetes. 2007;56:16–23. doi: 10.2337/db06-1076. [DOI] [PubMed] [Google Scholar]
- 10.Odegaard JI, et al. Macrophage-specific PPARgamma controls alternative activation and improves insulin resistance. Nature. 2007;447:1116–1120. doi: 10.1038/nature05894. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Suganami T, Nishida J, Ogawa Y. A paracrine loop between adipocytes and macrophages aggravates inflammatory changes: role of free fatty acids and tumor necrosis factor alpha. Arterioscler Thromb Vasc Biol. 2005;25:2062–2068. doi: 10.1161/01.ATV.0000183883.72263.13. [DOI] [PubMed] [Google Scholar]
- 12.Caspar-Bauguil S, et al. Adipose tissues as an ancestral immune organ: site-specific change in obesity. FEBS Lett. 2005;579:3487–3492. doi: 10.1016/j.febslet.2005.05.031. [DOI] [PubMed] [Google Scholar]
- 13.Zheng Y, Rudensky AY. Foxp3 in control of the regulatory T cell lineage. Nat Immunol. 2007;8:457–462. doi: 10.1038/ni1455. [DOI] [PubMed] [Google Scholar]
- 14.Sakaguchi S, Yamaguchi T, Nomura T, Ono M. Regulatory T cells and immune tolerance. Cell. 2008;133:775–787. doi: 10.1016/j.cell.2008.05.009. [DOI] [PubMed] [Google Scholar]
- 15.Maloy KJ, et al. CD4+CD25+ T(R) cells suppress innate immune pathology through cytokine-dependent mechanisms. J Exp Med. 2003;197:111–119. doi: 10.1084/jem.20021345. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Murphy TJ, Choileain NN, Zang Y, Mannick JA, Lederer JA. CD4+CD25+ regulatory T cells control innate immune reactivity after injury. J Immunol. 2005;174:2957–2963. doi: 10.4049/jimmunol.174.5.2957. [DOI] [PubMed] [Google Scholar]
- 17.Nguyen LT, Jacobs J, Mathis D, Benoist C. Where FoxP3-dependent regulatory T cells impinge on the development of inflammatory arthritis. Arthritis Rheum. 2007;56:509–520. doi: 10.1002/art.22272. [DOI] [PubMed] [Google Scholar]
- 18.Wu H, et al. T-cell accumulation and regulated on activation, normal T cell expressed and secreted upregulation in adipose tissue in obesity. Circulation. 2007;115:1029–1038. doi: 10.1161/CIRCULATIONAHA.106.638379. [DOI] [PubMed] [Google Scholar]
- 19.Tran TT, Yamamoto Y, Gesta S, Kahn CR. Beneficial effects of subcutaneous fat transplantation on metabolism. Cell Metab. 2008;7:410–420. doi: 10.1016/j.cmet.2008.04.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Cinti S, et al. Adipocyte death defines macrophage localization and function in adipose tissue of obese mice and humans. J Lipid Res. 2005;46:2347–2355. doi: 10.1194/jlr.M500294-JLR200. [DOI] [PubMed] [Google Scholar]
- 21.Herman AE, Freeman GJ, Mathis D, Benoist C. CD4+CD25+ T regulatory cells dependent on ICOS promote regulation of effector cells in the prediabetic lesion. J Exp Med. 2004;199:1479–1489. doi: 10.1084/jem.20040179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Chen Y, et al. Blockade of late stages of autoimmune diabetes by inhibition of the receptor for advanced glycation end products. J Immunol. 2004;173:1399–1405. doi: 10.4049/jimmunol.173.2.1399. [DOI] [PubMed] [Google Scholar]
- 23.Fontenot JD, et al. Regulatory T cell lineage specification by the forkhead transcription factor foxp3. Immunity. 2005;22:329–341. doi: 10.1016/j.immuni.2005.01.016. [DOI] [PubMed] [Google Scholar]
- 24.Huehn J, et al. Developmental stage, phenotype, and migration distinguish naive- and effector/memory-like CD4+ regulatory T cells. J Exp Med. 2004;199:303–313. doi: 10.1084/jem.20031562. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Herman AE, Freeman GJ, Mathis D, Benoist C. CD4+CD25+ T regulatory cells dependent on ICOS promote regulation of effector cells in the prediabetic lesion. J Exp Med. 2004;199:1479–1489. doi: 10.1084/jem.20040179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Hill J, et al. Foxp3-dependent and independent regulation of the Treg transcriptional signature. Immunity. 2007;25:693–695. doi: 10.1016/j.immuni.2007.09.010. [DOI] [PubMed] [Google Scholar]
- 27.Nolan KF, et al. IL-10-conditioned dendritic cells, decommissioned for recruitment of adaptive immunity, elicit innate inflammatory gene products in response to danger signals. J Immunol. 2004;172:2201–2209. doi: 10.4049/jimmunol.172.4.2201. [DOI] [PubMed] [Google Scholar]
- 28.Wong J, et al. Adaptation of TCR repertoires to self-peptides in regulatory and nonregulatory CD4+ T cells. J Immunol. 2007;178:7032–7041. doi: 10.4049/jimmunol.178.11.7032. [DOI] [PubMed] [Google Scholar]
- 29.Hsieh CS, Zheng Y, Liang Y, Fontenot JD, Rudensky AY. An intersection between the self-reactive regulatory and nonregulatory T cell receptor repertoires. Nat Immunol. 2006;7:401–410. doi: 10.1038/ni1318. [DOI] [PubMed] [Google Scholar]
- 30.Pacholczyk R, Ignatowicz H, Kraj P, Ignatowicz L. Origin and T cell receptor diversity of Foxp3+CD4+CD25+ T cells. Immunity. 2006;25:249–259. doi: 10.1016/j.immuni.2006.05.016. [DOI] [PubMed] [Google Scholar]
- 31.Kretschmer K, et al. Inducing and expanding regulatory T cell populations by foreign antigen. Nat Immunol. 2005;6:1219–1227. doi: 10.1038/ni1265. [DOI] [PubMed] [Google Scholar]
- 32.Correia-Neves M, Waltzinger C, Mathis D, Benoist C. The shaping of the T cell repertoire. Immunity. 2001;14:21–32. doi: 10.1016/s1074-7613(01)00086-3. [DOI] [PubMed] [Google Scholar]
- 33.Samad F, Yamamoto K, Pandey M, Loskutoff DJ. Elevated expression of transforming growth factor-beta in adipose tissue from obese mice. Mol Med. 1997;3:37–48. [PMC free article] [PubMed] [Google Scholar]
- 34.Chen W, et al. Conversion of peripheral CD4+CD25- naive T cells to CD4+CD25+ regulatory T cells by TGF-beta induction of transcription factor Foxp3. J Exp Med. 2003;198:1875–1886. doi: 10.1084/jem.20030152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Peng Y, Laouar Y, Li MO, Green EA, Flavell RA. TGF-beta regulates in vivo expansion of Foxp3-expressing CD4+CD25+ regulatory T cells responsible for protection against diabetes. Proc Natl Acad Sci U S A. 2004;101:4572–4577. doi: 10.1073/pnas.0400810101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Marie JC, Letterio JJ, Gavin M, Rudensky AY. TGF-beta1 maintains suppressor function and Foxp3 expression in CD4+CD25+ regulatory T cells. J Exp Med. 2005;201:1061–1067. doi: 10.1084/jem.20042276. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Pelleymounter MA, et al. Effects of the obese gene product on body weight regulation in ob/ob mice. Science. 1995;269:540–543. doi: 10.1126/science.7624776. [DOI] [PubMed] [Google Scholar]
- 38.Klebig ML, Wilkinson JE, Geisler JG, Woychik RP. Ectopic expression of the agouti gene in transgenic mice causes obesity, features of type II diabetes, and yellow fur. Proc Natl Acad Sci U S A. 1995;92:4728–4732. doi: 10.1073/pnas.92.11.4728. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.De RV, et al. A Key Role of Leptin in the Control of Regulatory T Cell Proliferation. Immunity. 2007;26:241–255. doi: 10.1016/j.immuni.2007.01.011. [DOI] [PubMed] [Google Scholar]
- 40.Kim JM, Rasmussen JP, Rudensky AY. Regulatory T cells prevent catastrophic autoimmunity throughout the lifespan of mice. Nat Immunol. 2007;8:191–197. doi: 10.1038/ni1428. [DOI] [PubMed] [Google Scholar]
- 41.Bennett CL, Clausen BE. DC ablation in mice: promises, pitfalls, and challenges. Trends Immunol. 2007;28:525–531. doi: 10.1016/j.it.2007.08.011. [DOI] [PubMed] [Google Scholar]
- 42.Thorburn J, Frankel AE, Thorburn A. Apoptosis by leukemia cell-targeted diphtheria toxin occurs via receptor-independent activation of Fas-associated death domain protein. Clin Cancer Res. 2003;9:861–865. [PubMed] [Google Scholar]
- 43.Miyake Y, et al. Protective role of macrophages in noninflammatory lung injury caused by selective ablation of alveolar epithelial type II Cells. J Immunol. 2007;178:5001–5009. doi: 10.4049/jimmunol.178.8.5001. [DOI] [PubMed] [Google Scholar]
- 44.Bennett CL, et al. Inducible ablation of mouse Langerhans cells diminishes but fails to abrogate contact hypersensitivity. J Cell Biol. 2005;169:569–576. doi: 10.1083/jcb.200501071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Duffield JS, et al. Conditional ablation of macrophages halts progression of crescentic glomerulonephritis. Am J Pathol. 2005;167:1207–1219. doi: 10.1016/S0002-9440(10)61209-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Duffield JS, et al. Selective depletion of macrophages reveals distinct, opposing roles during liver injury and repair. J Clin Invest. 2005;115:56–65. doi: 10.1172/JCI22675. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Walzer T, et al. Identification, activation, and selective in vivo ablation of mouse NK cells via NKp46. Proc Natl Acad Sci U S A. 2007;104:3384–3389. doi: 10.1073/pnas.0609692104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Yuan M, et al. Reversal of obesity- and diet-induced insulin resistance with salicylates or targeted disruption of Ikkbeta. Science. 2001;293:1673–1677. doi: 10.1126/science.1061620. [DOI] [PubMed] [Google Scholar]
- 49.Boyman O, Kovar M, Rubinstein MP, Surh CD, Sprent J. Selective stimulation of T cell subsets with antibody-cytokine immune complexes. Science. 2006;311:1924–1927. doi: 10.1126/science.1122927. [DOI] [PubMed] [Google Scholar]
- 50.Tang Q, et al. Central role of defective interleukin-2 production in the triggering of islet autoimmune destruction. Immunity. 2008;28:687–697. doi: 10.1016/j.immuni.2008.03.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Wolf AM, Wolf D, Rumpold H, Enrich B, Tilg H. Adiponectin induces the anti-inflammatory cytokines IL-10 and IL-1RA in human leukocytes. Biochem Biophys Res Commun. 2004;323:630–635. doi: 10.1016/j.bbrc.2004.08.145. [DOI] [PubMed] [Google Scholar]
- 52.Kumada M, et al. Adiponectin specifically increased tissue inhibitor of metalloproteinase-1 through interleukin-10 expression in human macrophages. Circulation. 2004;109:2046–2049. doi: 10.1161/01.CIR.0000127953.98131.ED. [DOI] [PubMed] [Google Scholar]
- 53.Kim HJ, et al. Differential effects of interleukin-6 and -10 on skeletal muscle and liver insulin action in vivo. Diabetes. 2004;53:1060–1067. doi: 10.2337/diabetes.53.4.1060. [DOI] [PubMed] [Google Scholar]
- 54.Bluher M, et al. Association of interleukin-6, C-reactive protein, interleukin-10 and adiponectin plasma concentrations with measures of obesity, insulin sensitivity and glucose metabolism. Exp Clin Endocrinol Diabetes. 2005;113:534–537. doi: 10.1055/s-2005-872851. [DOI] [PubMed] [Google Scholar]
- 55.Scarpelli D, et al. Variants of the interleukin-10 promoter gene are associated with obesity and insulin resistance but not type 2 diabetes in caucasian italian subjects. Diabetes. 2006;55:1529–1533. doi: 10.2337/db06-0047. [DOI] [PubMed] [Google Scholar]
- 56.Doganci A, et al. The IL-6R alpha chain controls lung CD4+CD25+ Treg development and function during allergic airway inflammation in vivo. J Clin Invest. 2005;115:313–325. doi: 10.1172/JCI22433. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Wan S, Xia C, Morel L. IL-6 produced by dendritic cells from lupus-prone mice inhibits CD4+CD25+ T cell regulatory functions. J Immunol. 2007;178:271–279. doi: 10.4049/jimmunol.178.1.271. [DOI] [PubMed] [Google Scholar]
- 58.Hosogai N, et al. Adipose tissue hypoxia in obesity and its impact on adipocytokine dysregulation. Diabetes. 2007;56:901–911. doi: 10.2337/db06-0911. [DOI] [PubMed] [Google Scholar]
- 59.Furukawa S, et al. Increased oxidative stress in obesity and its impact on metabolic syndrome. J Clin Invest. 2004;114:1752–1761. doi: 10.1172/JCI21625. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Reich M, et al. GenePattern 2.0. Nat Genet. 2006;38:500–501. doi: 10.1038/ng0506-500. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.