Abstract
Recently, the side population (SP) phenotype has been introduced as a reliable marker to identify subpopulations of cells with stem/progenitor cell properties in various tissues. We and others have identified SP cells from post-mitotic tissues, including adult myocardium, in which they have been suggested to contribute to cellular regeneration following injury. SP cells are identified and characterized by a unique efflux of Hoechst 33342 dye. Abcg2 belongs to the ATP-binding cassette (ABC) transporter superfamily and constitutes the molecular basis for the dye efflux, hence the SP phenotype, in hematopoietic stem cells. While Abcg2 is also expressed in cardiac SP (cSP) cells, its role in regulating the SP phenotype and function of cSP cells is unknown. Herein, we demonstrate that regulation of the SP phenotype in cSP cells occurs in a dynamic, age-dependent fashion, with Abcg2 as the molecular determinant of the cSP phenotype in the neonatal heart and another ABC transporter, Mdr1, as the main contributor to the SP phenotype in the adult heart. Using loss- and gain-of-function experiments we find that Abcg2 tightly regulates cell fate and function. Adult cSP cells isolated from mice with genetic ablation of Abcg2, exhibit blunted proliferation capacity and augmented cell death. Conversely, overexpression of Abcg2 is sufficient to enhance cell proliferation, though with a limitation of cardiomyogenic differentiation. In summary, for the first time, we reveal a functional role for Abcg2 in modulating the proliferation, differentiation, and survival of adult cSP cells that goes beyond its distinct role in Hoechst dye efflux.
Keywords: Abcg2, Cardiomyocytes, Cell death, Contractility, Differentiation, Mdr1, Progenitor cells, Proliferation, SP cells, Stem cells
Introduction
Recently, the SP phenotype has been introduced as a reliable marker to identify subpopulations of cells with stem/progenitor cell properties in various tissues including the heart 1. On the molecular level, the SP phenotype is linked to the presence of ATP-binding cassette (ABC) transporters with the ability to efficiently efflux the DNA binding dye Hoechst 33342 2. This ABC transporter-dependent Hoechst efflux phenomenon confers the characteristic fluorescent-activated cell sorting (FACS) profile of SP cells as a Hoechst-low “side population” located to the periphery of the Hoechst-high main population 2.
Among the various members of the ABC transporter superfamily, Abcg2 (also referred to as breast cancer resistance protein 1 (Bcrp1)) and Mdr1 (also referred to as P-glycoprotein (p-gp) or Abcb1) have been shown to efficiently efflux Hoechst 33342 and thereby confer the SP phenotype 3. Although both transporters are highly expressed in bone marrow SP (BMSP) cells, studies performed in mice with targeted disruption of the Mdr1a and Mdr1b genes, the murine homologues of the human Abcb1/Mdr1 gene, demonstrated that Abcg2 is the sole molecular determinant of the SP phenotype in hematopoietic stem cells 4. Moreover, Abcg2 expression is conserved in SP cells from a wide range of tissues including blood, gonad, lung, skeletal muscle and the retina, suggesting an important role of Abcg2 in stem cells 4-7.
We and others have characterized SP cells isolated from adult myocardium 8-11. These cSP cells are phenotypically distinct from BMSP cells, in that they are not hematopoietic, but exhibit the potential to differentiate into functional cardiomyocytes 10. As in SP cells from the bone marrow, Abcg2 is expressed in SP cells from the heart 9. The contribution of Abcg2 to the cSP phenotype and its biological significance in cSP progenitor cells, however, remain unknown. In this study, we find that the contribution of Abcg2 to the SP phenotype in the heart exists in an age-dependent manner, with Abcg2 as the molecular determinant of the SP phenotype in the neonatal heart and Mdr1 as the basis for the cSP phenotype in the adult heart. In addition, we demonstrate that Abcg2 plays a crucial role in the maintenance of cSP cell progenitors by promoting cell proliferation and survival, while inhibiting lineage commitment. Intriguingly, for the first time, we reveal a functional role of Abcg2 in modulating proliferation, differentiation, and survival of cSP cells that goes beyond its distinct role of Hoechst dye efflux.
Methods
Animals
Male mice with genetic ablation of Abcg2 (Abcg2 -/-) or Mdr1a/b (Mdr1a/b -/-) were purchased from Taconic (cat# 002767-M and 001487-MM; Germantown, New York). Age-matched FVB mice were also purchased from Taconic to serve as wild type control (WT). Mice were studied at postnatal day 3 (p3), day 14 (p14), day 21 (p21) and between 8 and 12 weeks of age (adult). All animal studies strictly adhered to the guidelines of the Harvard Medical School, the Longwood Medical Area’s Institutional Animal Care and Use Committee (IACUC) and the National Society for Medical Research.
Cardiac and bone marrow SP cell preparation
Cardiomyocyte-depleted cardiac cell suspensions were prepared as previously described to obtain cSP cells 10 (For details please see the online supplemental materials and methods). Bone marrow SP cells were isolated form the tibia and femur as previously described 1.
Cell viability assay
Cell death was determined by an Annexin-V kit (Abcam) using FACS analysis and cell viability was determined by CellTiter-Glo® and CellTiter-Blue® (Promega) using a luminescence/fluorescence plate reader, respectively, according to the manufacturer’s instructions.
cSP expansion and lentiviral infection
Freshly isolated cSP cells were cultured in expansion medium (α-MEM culture medium supplemented with 20% FBS, 2 mM L-Glutamin and 1% penicillin/streptomycin). Vector pSPORT1 (ATCC vector # 10471063) was enzymatically digested with BamHI and the resulting Abcg2 cDNA was blunted and subsequently cloned into the HPV-422 lentivirus vector (kindly provided by Dr. P. Allen). The bicistronic vector HPV-422 encodes the Abcg2 and IRES-GFP under the promoter EF1a. WT cSP cells from passage 4-6 were infected with the Abcg2-IRES-GFP lentivirus and 48 hours post-infection GFP+ cSP cells were sorted and further expanded. Culture medium was replaced every 72 hours.
Proliferation assays
The proliferative capacity of cSP cells was determined in expanded passage 4-6 cSP cells using total cell number, expression of Ki67 and phospho-Histone H3, total protein and total DNA. For detailed protocols please see online supplemental materials and methods.
Cardiomyogenic differentiation capacity
The role of Abcg2 in regulating the ability of cSP cells to undergo cardiomyogenic differentiation was determined in our established co-culture system 10. cSP cells were transfected with GFP expressing lentivirus and co-cultures were stained for α-sarcomeric actinin (Sigma) (For details please see online supplemental materials and methods).
Statistical Analysis
Statistical differences between groups were evaluated using Student’s unpaired t test or analysis of variance (ANOVA), as appropriate. All data are presented as mean ± SEM. A p-value less than 0.05 was considered statistically significant.
Results
Abcg2 regulates the SP phenotype in cSP cells in an age-dependent manner
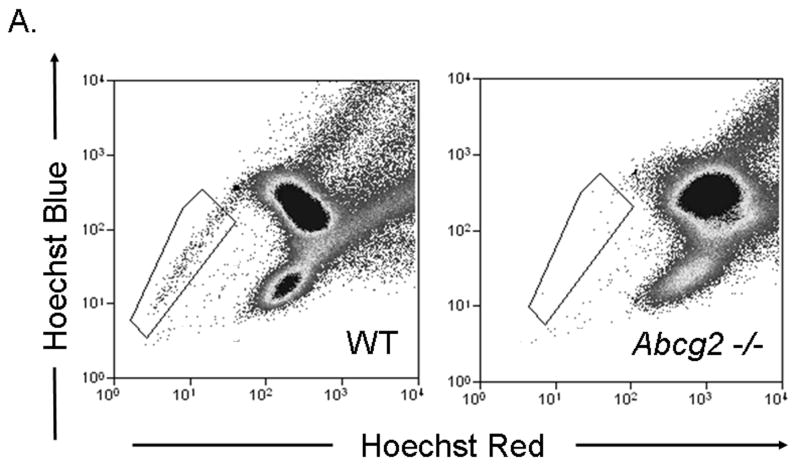
Given published data suggesting that Abcg2 is the sole molecular determinant of the SP cell phenotype in bone marrow cells, we first sought to determine whether Abcg2 also regulates the SP phenotype in cardiac cells. To establish the role of Abcg2 in mediating the SP cell phenotype, bone marrow and cardiac cell suspensions were isolated from 8-12 weeks old age-matched mice with genetic ablation of Abcg2 (Abcg2 -/-) and wild-type (WT) counterparts. FACS analysis of bone marrow cell suspensions from Abcg2 -/- mice demonstrated a complete lack of BMSP cells (WT: 0.20±0.05%; Abcg2 -/-: 0.02±0.02%) compared to WT mice, suggesting that Abcg2 is indeed required for conferring the SP phenotype in bone marrow cells (Figure 1A). In contrast, cSP cells from Abcg2 -/- hearts revealed a clearly detectable, though significantly reduced, SP population (WT: 0.8±0.1%; Abcg2 -/-: 0.46±0.1%) (Figure 1B).
Figure 1. The role of Abcg2 in mediating the SP cell phenotype in bone marrow and cardiac cells.
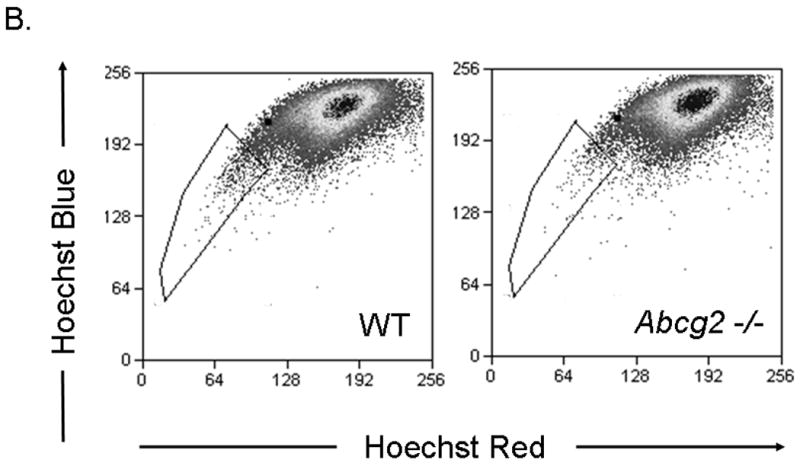
FACS analyses carried out on Hoechst-stained bone marrow and cardiac cells from wild type (WT) and Abcg2 -/- mice. Hoechst-extruding SP cells are located in the boxed area. (A) Compared to WT cells, bone marrow cells from Abcg2 -/- mice lack a detectable SP cell population, (B) whereas cardiac cells from Abcg2 -/- mice exhibit a clearly detectable SP cell population.
To confirm the correct FACS gating of SP cells (Figure 2A & B), we used two potent inhibitors of ABC transporters, verapamil and fumitremorgin C (FTC). Treatment of cells with verapamil or FTC completely abolished the SP cell band in both WT and Abcg2 -/- cardiac cell suspensions (Figure 2C - F). To further verify that Hoechst dye efflux in Abcg2 -/- hearts was mediated via ABC transport, cardiac cell suspensions were preincubated with 2-deoxyglucose to deplete ATP and thereby inactivate ATP-dependent transporter function. Depletion of ATP also resulted in a complete loss of SP cells in both WT and Abcg2 -/- cell suspensions, indicating that dye efflux in Abcg2 -/- cell suspensions is indeed mediated in an ATP-dependent manner (Figure 2G & H).
Figure 2. Hoechst dye efflux in Abcg2 -/- cardiac cells is mediated by ATP-dependent ABC transporter function.
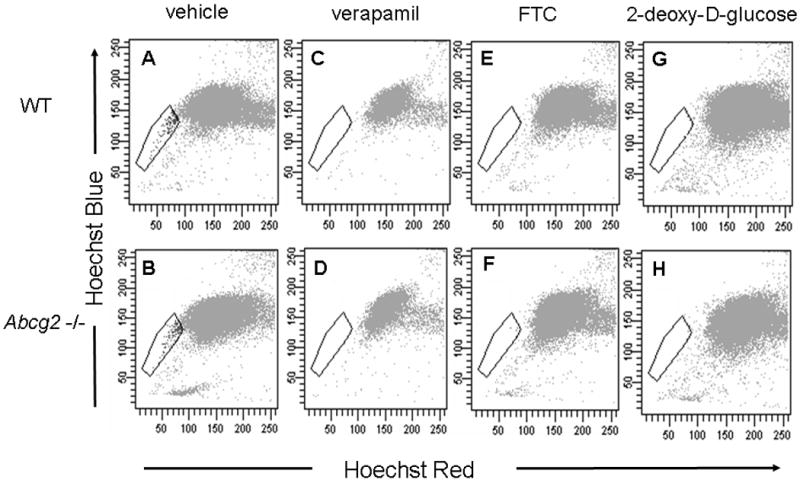
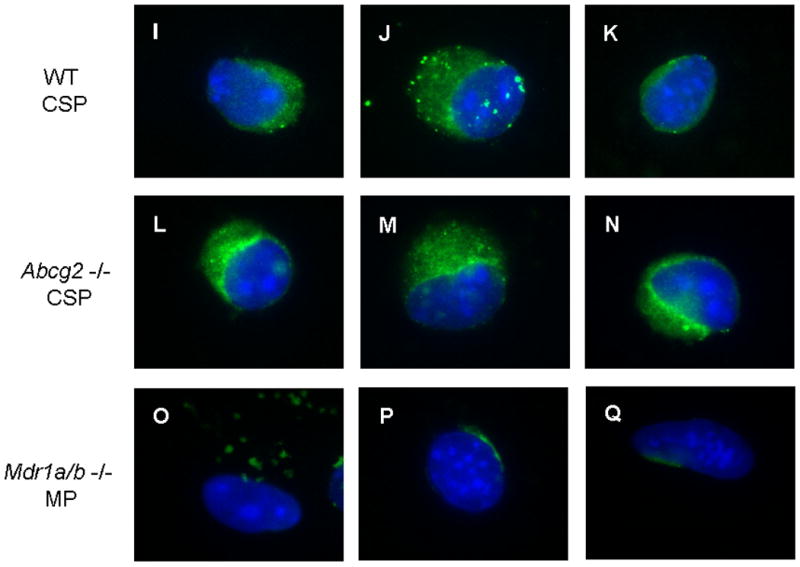
FACS analyses of Hoechst-stained cardiac cells from wild type (WT) and Abcg2 -/- mice pretreated with either vehicle (A, B), the ABC transporter inhibitors verapamil (C, D) or fumitremorgin C (FTC) (E, F), or after ATP depletion with 2-deoxy-D-glucose (G, H). Similar to wild type cardiac cells, inhibition of ABC transporter function as well as ATP depletion completely abolishes the SP phenotype in Abcg2 -/- cardiac cells, indicating that dye efflux in Abcg2 -/- cardiac cell suspensions is mediated via ABC transporter activity. Immunocytochemical analysis demonstrates the expression of Mdr1a/b in freshly isolated WT and Abcg2 -/- cSP cells (I-N). The lack of Mdr1a/b expression seen in Mdr1a/b -/- main population (MP) cells serves as negative control (O-Q).
We and others have previously shown that cSP cells express high levels of stem cell antigen 1 (Sca-1) and moderate levels of CD31 but lack the pan-hematopoietic marker CD45 10. To further immunophenotypically characterize Abcg2 -/- cSP cells, we compared the expression of cell surface markers, Sca-1, CD31 and CD45, in Abcg2 -/- and WT cSP cells using FACS analysis. As shown in Table 1, deficiency of Abcg2 did not alter the expression pattern of Sca-1, CD31 and CD45 as compared to WT cSP cells, further suggesting that lacking Abcg2 does not alter the expression pattern of major cell surface markers in adult cSP cells.
Table 1.
Cell Surface Marker Expression in WT and Abcg2 -/- Cardiac SP Cells
| Surface Marker | WT (%) | Abcg2 -/- (%) |
|---|---|---|
| Sca-1 | 90 ± 1 | 89 ± 2 |
| CD31 | 80 ± 2 | 79 ± 3 |
| CD45 | ≤ 1 | ≤ 1 |
In addition to Abcg2, another member of the ABC transporter superfamily, the P-glycoprotein Mdr1, has been demonstrated to exhibit the capacity to efflux the Hoechst 33342 dye 12. We therefore hypothesized that Mdr1 may be involved in the regulation of the cSP phenotype in the adult heart. Quantitative RT-PCR analysis (Online Figure I) and immunocytochemistry (Figure 2I-Q) in WT and Abcg2 -/- cSP cells confirmed the expression of Mdr1a and Mdr1b genes and proteins independent of the expression of Abcg2.
To determine the contribution of Mdr1 to the Hoechst 33342 efflux phenotype in the adult heart, cSP cells were isolated from hearts of adult mice with targeted disruption of Mdr1a/b genes. Strikingly, Mdr1a/b -/- hearts exhibited a severe depletion of cSP (Figure 3A). In contrast, bone marrow from Mdr1a/b -/- animals exhibited normal BMSP cell numbers (data not shown). To ensure the limited number of cSP cells observed in Mdr1a/b -/- hearts was not due to experimental variables, in particular Hoechst concentration, cell ratio and staining duration, we employed an internal control by mixing one part of cardiomyocyte depleted mononuclear cells ubiquitously expressing enhanced green fluorescent protein (GFP+ control cells) with three parts of non-GFP, Mdr1a/b -/- or WT cardiomyocyte depleted mononuclear cells (Figure 3B). Indeed, analysis of these cell mixtures revealed almost exclusively GFP+ cells among the cSP cells (> 98%) with no significant contribution from Mdr1a/b -/- cells to the SP band; thereby confirming the severely impaired Hoechst efflux capacity in Mdr1a/b -/- cSP cells (Figure 3B). In contrast, 1:3 cell mixtures of GFP+ control cells and WT cardiac cells revealed only ~25% GFP+ control cells within the SP cell population, confirming the dye efflux competence of WT cardiac cells (Figure 3B).
Figure 3. The ABC transporter Mdr1a/b mediates the Hoechst efflux phenotype of cSP cells in the adult heart.
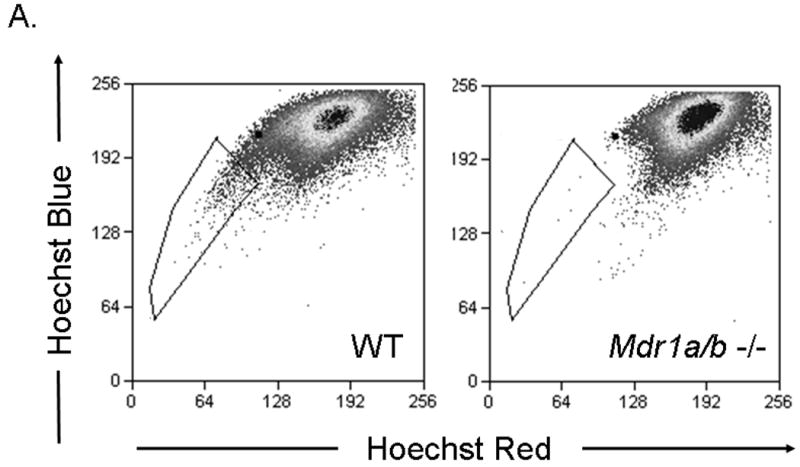
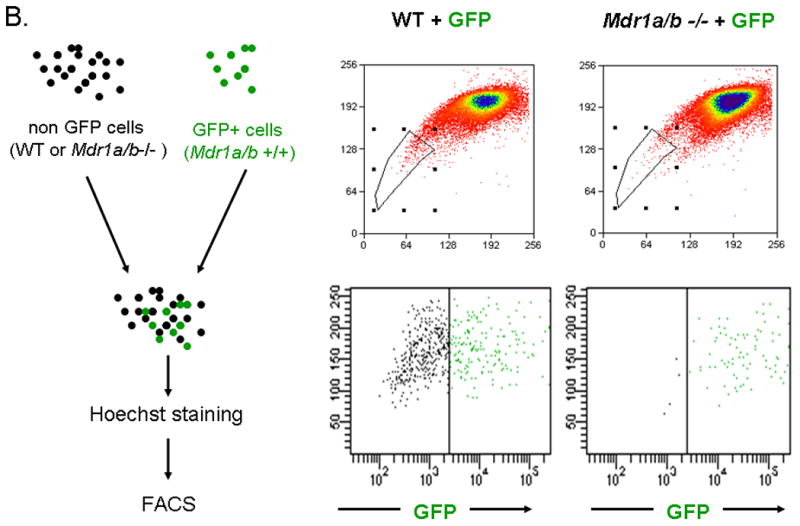
SP cell analyses of cardiac cells from WT and Mdr1a/b -/- mice. (A) Compared to WT cells, Mdr1a/b -/- cardiac cells almost completely lack cSP cells. (B) SP cell analyses of cell mixtures containing one part of WT cardiac cells expressing green fluorescent protein (GFP) and three parts of either WT or Mdr1a/b -/- cardiac cells not expressing GFP. In cell mixtures containing WT cells, GFP+ cells account for ~25% of total SP cells, thus reflecting the 1:3 ratio of the cell mixture. In cell mixtures containing GFP+ control- and Mdr1a/b -/- cells, however, SP cells are almost exclusively GFP positive implicating a dominant role of Mdr1a/b in the mediation of the cSP cell phenotype in adult hearts.
While Abcg2 is enriched in early neonatal cSP cells, its expression level is markedly decreased post-natally 9. To determine whether ABC-transporter expression in the heart is age-dependent, quantitative RT-PCR analysis for Abcg2 and Mdr1a/b was performed in neonatal and adult mouse hearts. These analyses confirmed profound down-regulation of Abcg2 gene expression in the adult cSP cells as compared to the neonatal cSP cells. In contrast, Mdr1a/b expression levels demonstrated a reverse pattern with low expression in the neonatal cSP cells and high expression in the adult cSP cells (Online Figure II). To determine whether Abcg2 and Mdr1a/b mediate the SP phenotype in cSP cells in an age-dependent manner, cSP cells were isolated from Abcg2 -/-, Mdr1a/b -/- and age-matched WT mice at postnatal day 3 (p3), 14 (p14), 21 (p21) and 8-12 weeks (adult) of age (Figure 4A-D). In contrast to adult hearts early postnatal (p3) Abcg2 -/- hearts demonstrated almost no detectable cSP cells, whereas a similar number of SP cells was observed in Mdr1a/b -/- and WT hearts (Figure 4A). The lack of Hoechst dye efflux was confirmed in cSP cells from Abcg2 -/- hearts at postnatal day 3 using GFP mixing studies, similar to those described above (data not shown). A gradual decrease in cSP cells from p3 (9.4±1.2%) to adulthood (0.8±0.1%) was noted in WT hearts. Abcg2 -/- hearts, however, demonstrated a gradual increase in cSP cells from day 3 post-natally (0.18±0.1%) to adult (0.46±0.1%), and thereafter maintained cSP cell levels into adulthood. The opposite profile was observed in Mdr1a/b -/- hearts, with cSP cell numbers dropping dramatically within the first three weeks of postnatal life and being barely detectable in adulthood. Taken together, these data demonstrate that the cSP cell phenotype is mediated by Abcg2 and Mdr1a/b in an age-dependent fashion.
Figure 4. Age-dependent regulation of the cSP phenotype by Abcg2 and Mdr1a/b.
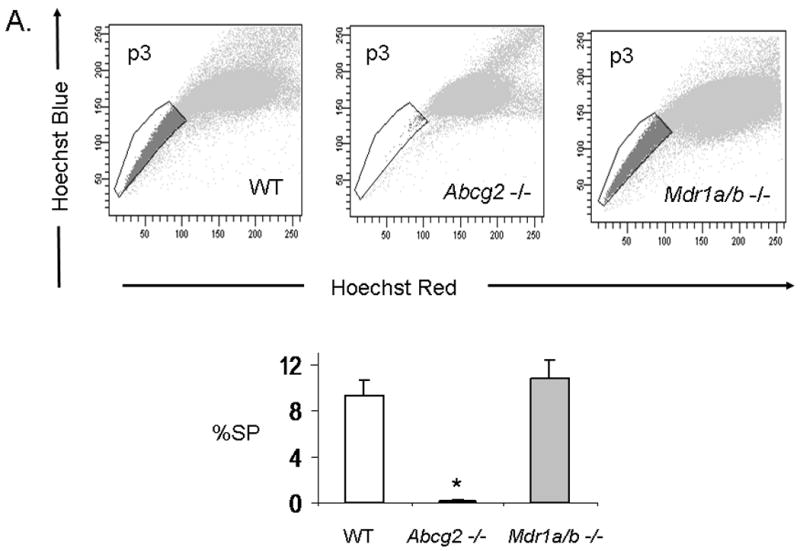
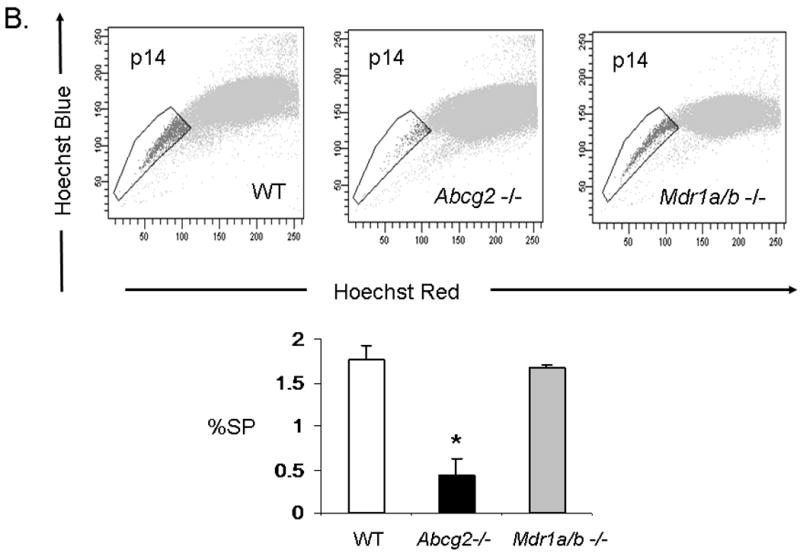
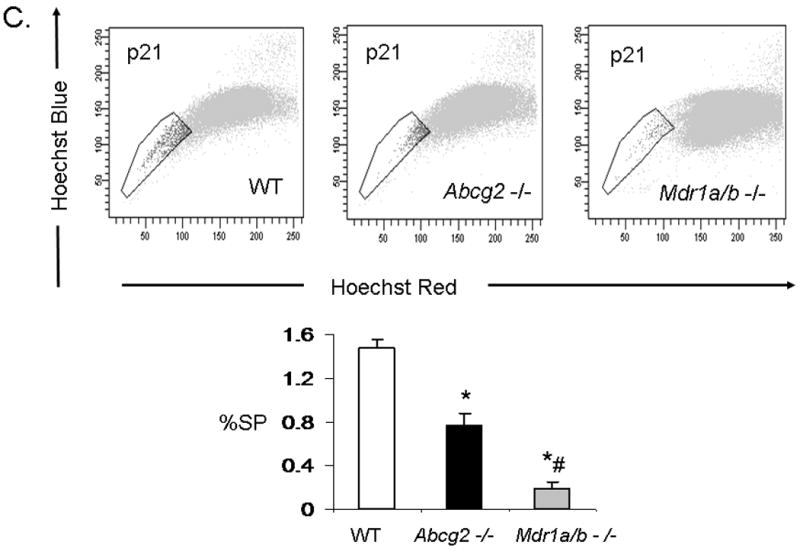
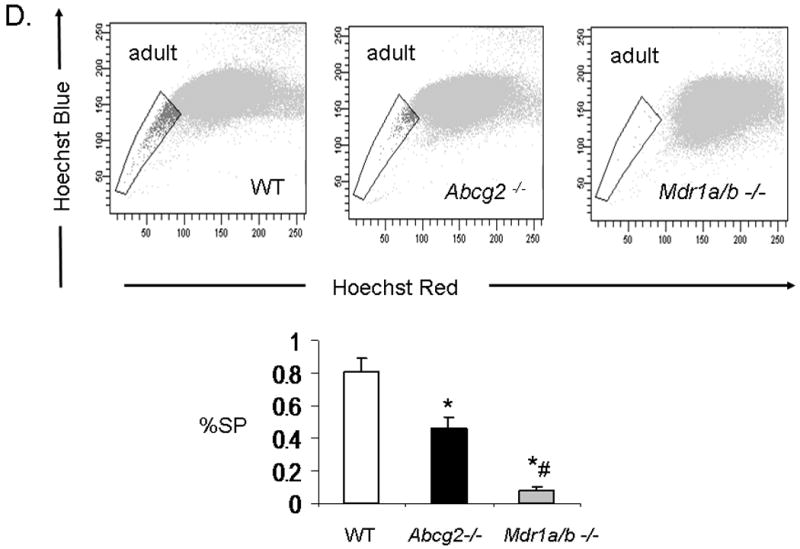
SP cell analyses of cardiac cells isolated from Abcg2 -/-, Mdr1a/b -/-and age-matched WT mice at postnatal day 3 (p3), 14 (p14), 21 (p21) and 8-12 weeks (adult) (A-D). A gradual decrease in cSP cells from early postnatal life through adulthood is evident in WT hearts. In contrast to WT hearts, early postnatal (p3) Abcg2 -/- hearts demonstrate almost no detectable cSP cells, whereas similar numbers of SP cells are observed between Mdr1a/b -/- and WT hearts (A). During postnatal development, Abcg2 -/- hearts demonstrate a steady increase in cSP cells from early postnatal into early adulthood (A–C) and maintain significant SP cell numbers throughout adulthood (D), while SP cell numbers of Mdr1a/b -/- hearts dramatically drop within the first 3 weeks of postnatal life and are barely detectable in adulthood (B–D).
Abcg2 regulates cSP cell proliferation
Expression of Abcg2 has been associated with cellular proliferation in cancer cell lines 13. Using gain- and loss-of-function approaches, we investigated the role of Abcg2 in the regulation of cSP cell proliferation. cSP cells isolated from adult age-matched Abcg2 -/- and WT mouse hearts were subjected to proliferation assays. As shown in Figure 5A, the proliferation capacity, as determined by total cell number, was markedly decreased in cSP cells lacking Abcg2. Consistent with the decrease in cell proliferation, the expression of the cell cycle markers Ki67 that identifies cells in G1, S, G2 and M phases (Figure 5B) and phospho-histone H3 (Figure 5C-D) that identifies cells in M phase were decreased by ~50% and ~70%, respectively, in Abcg2 -/- cSP cells when compared to WT cSP cells. Likewise, total protein and DNA content were significantly decreased in Abcg2 -/- cSP cells cultured in the expansion media, thus further supporting impaired cell proliferation seen in Abcg2 -/- cSP cells (Figure 5E-F). Conversely, the proliferation capacity was significantly enhanced in cSP cells following overexpression of Abcg2 via lentiviral-mediated gene transfer, with an increase in both total cell number (Figure 5G) and expression of Ki67 (data not shown) as compared to WT cSP cells. Taken together, these data demonstrate a functional role of Abcg2 in facilitating proliferation of cSP cells.
Figure 5. Functional role of Abcg2 in the regulation of cSP cell proliferation.
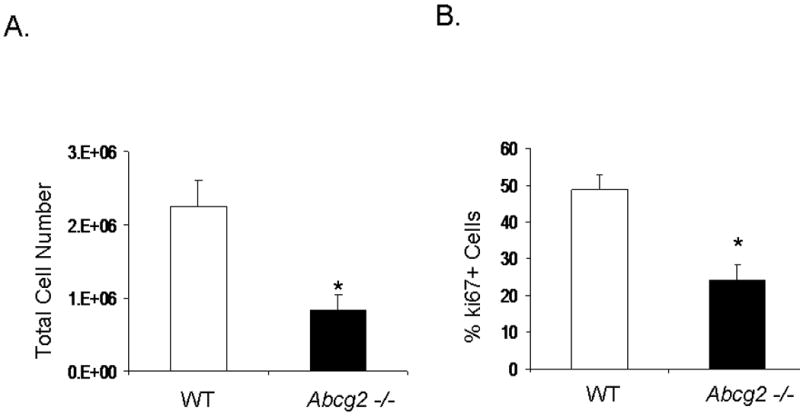
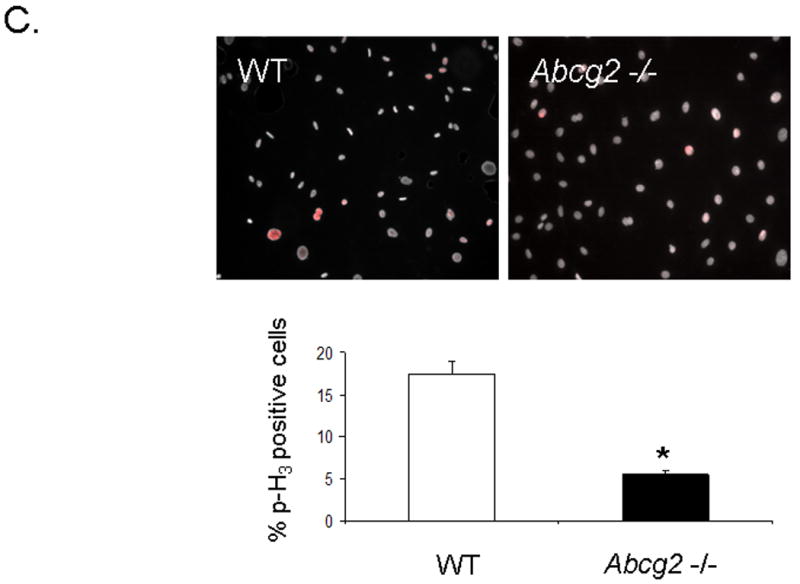
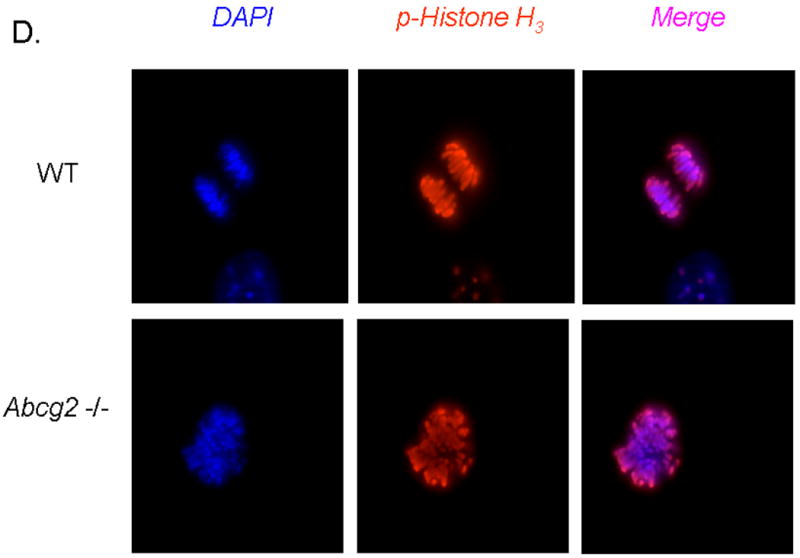
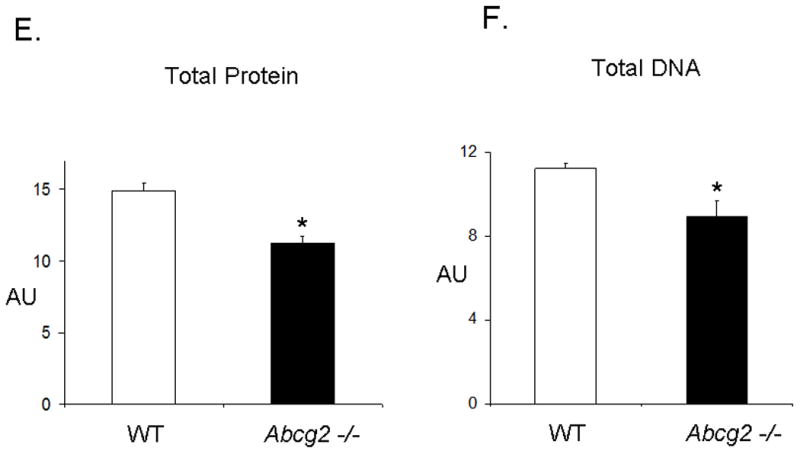
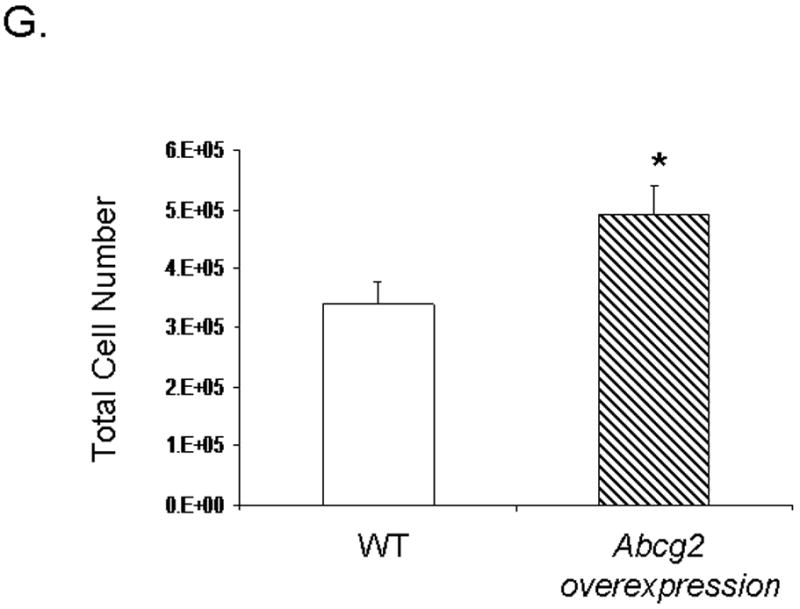
In vitro proliferation assay on cSP cells from adult age-matched WT and Abcg2 -/- mice, as well as cSP cells overexpressing Abcg2 via lentiviral mediated gene transfer. (A) Lack of Abcg2 significantly impairs the proliferation capacity of cSP cells as determined by total cell number (* p < 0.01) and (B) expression of the cell cycle marker Ki67 (*p < 0.01) and (C) phospho-histone-H3 using FACS analysis and immunofluorescence measurements. (D) Representative immunofluorescence staining of dividing WT and Abcg2 -/- cSP cells. Additionally, WT cSP cells exhibited increased total protein (E) and total DNA (F) in comparison with Abcg2 -/- cSP cells as determined by in cell western blot (p<0.01). Conversely, overexpression of Abcg2 significantly increases the proliferation capacity of cSP cells in terms of (G) total cell number (p < 0.05).
Regulation of cSP cell survival by Abcg2
Emerging evidence also suggests that Abcg2 may play a critical role in protecting primitive cells from cellular injury 14,15. To date, it is unclear whether Abcg2 also exerts cell protective effects on cSP cells. To determine whether Abcg2 is implicated in cSP cell survival, we first assessed apoptosis and necrosis in cSP cells under normal culture conditions using Annexin V and PI staining, respectively. As illustrated in Figure 6A-B, even under normal culture conditions, significantly elevated numbers of both apoptotic and necrotic cells were observed in cSP cells lacking Abcg2 as compared to WT cells. In addition, cell viability assays measuring cellular metabolic capacity by means of ATP quantitation (CellTiter-Glo®, Promega) or conversion of the redox dye resazurin to the fluorescent end product resorufin (CellTiter-Blue®, Promega) demonstrated significantly decreased metabolic capacity in Abcg2 -/- cSP cells (Figure 6C-D), thus further confirming impaired viability.
Figure 6. Abcg2 mediates survival in cSP cells.
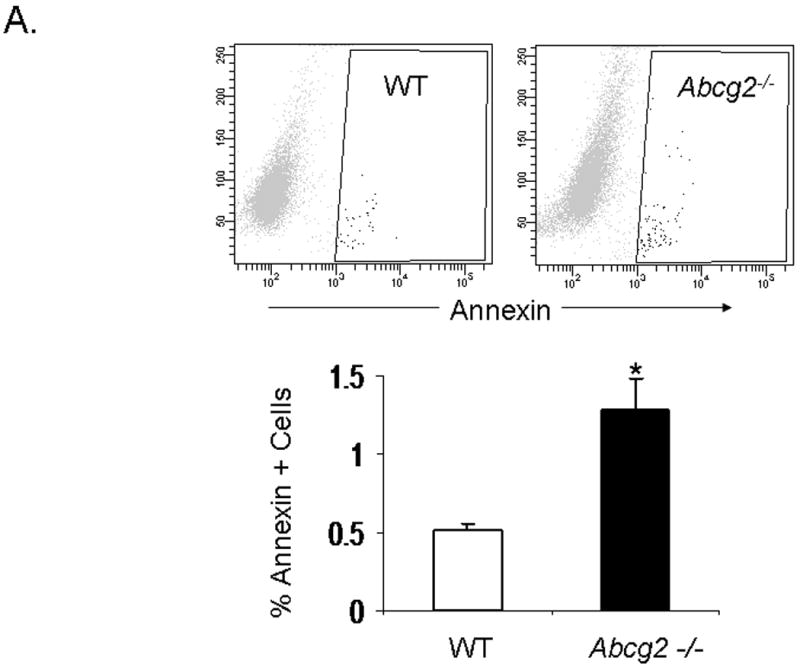
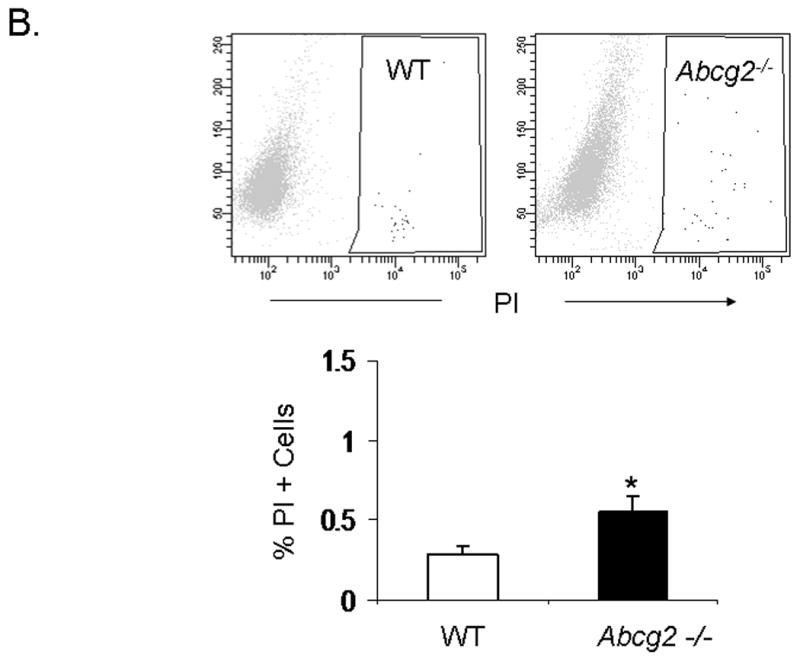
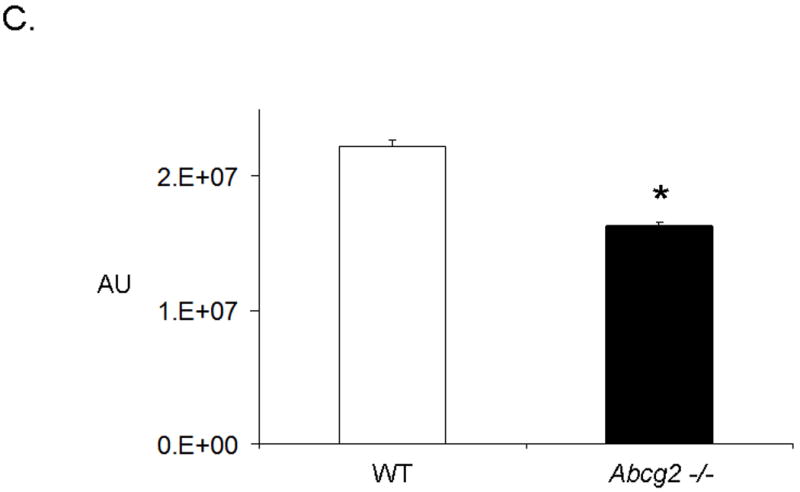
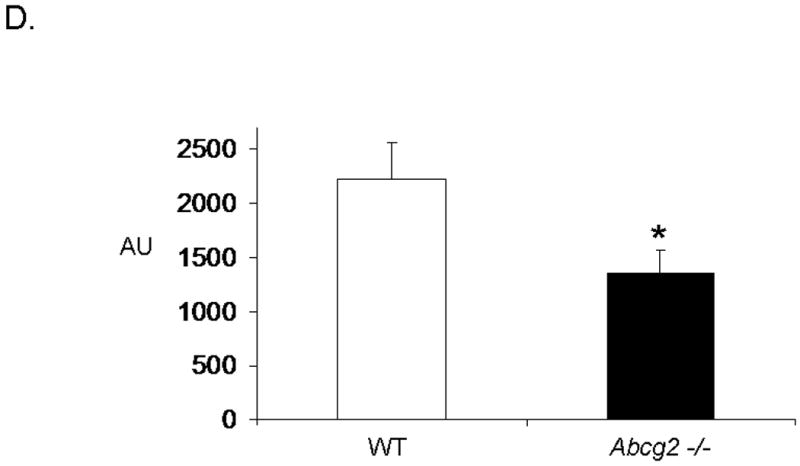
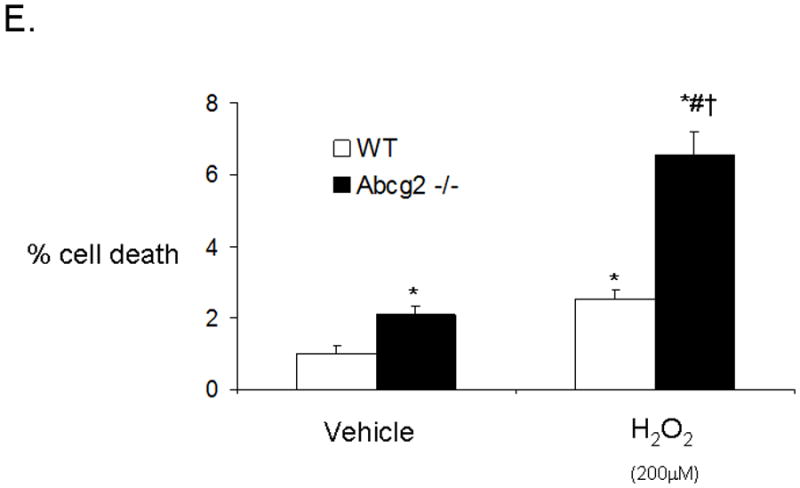
In vitro apoptosis / necrosis assay on cSP cells from adult age-matched WT and Abcg2 -/- mice. cSP cells lacking Abcg2 exhibit a significantly higher rate of apoptosis (p < 0.05) and necrosis (p < 0.1) under baseline culture conditions as determined by (A) Annexin V and (B) PI staining. The cell viability of WT and Abcg2 -/- cells is further determined by (C) CellTiter-Glo (p<0.05) and (D) CellTiter-Blue (p<0.05) viability assay. Abcg2 -/- cSP cells demonstrated reduced amount of ATP as indicated by the bioluminescence values (CellTiter-Glo) and metabolic capacity as determined by the ability of living cells to convert resazurin to fluorescent resorufin (CellTiter-Blue) as compared to the WT cSP cells. (E) Abcg2 -/- cSP cells exhibited higher levels of total cell death compared to the WT cSP cells in both vehicle and 200 μM H2O2-treated (p < 0.01, * vs WT vehicle, # vs. WT H2O2 treated, † vs. Abcg2 -/- H2O2 treated).
Because oxidative-stress is a common mediator of cell death in myocardial injury of various causes, we further investigated oxidative stress-induced cell death in WT and Abcg2 -/- cSP cells after exposing cSP cell cultures to 200μM H2O2. In this model, oxidative stress-induced cell death was significantly higher in cSP cells lacking Abcg2 as compared to WT cSP cells (Figure 6E). Taken together, our data indicate a protective role of Abcg2 in cSP cells under normal culture (21% O2) and H2O2 induced oxidative stress conditions.
Overexpression of Abcg2 impairs the ability of cSP cells to undergo cardiomyogenic differentiation
Regulation of proliferation and differentiation maintains progenitor cell homeostasis. We have found that Abcg2 is an essential regulator of cSP cell proliferation. We next sought to determine whether Abcg2 mediates cardiomyogenic differentiation of cSP cells. Utilizing a previously described co-culture system with adult rat cardiomyocytes, cardiomyogenic differentiation was assessed in WT and Abcg2 -/- cSP cells, as well as in Abcg2 overexpressing cSP cells (Figure 7A-I). In order to track the cell fate of cells in co-culture, cSP cells were infected with lentivirus expressing GFP. Genetic deficiency of Abcg2 did not limit the cardiomyogenic differentiation of cSP cells (Figure 7D-F). Overexpression of Abcg2 via lentiviral-mediated gene transfer significantly decreased cardiomyogenic differentiation of cSP cells (Figure 7G-I). Moreover, overexpression of Abcg2 maintained cSP cells in a proliferative state even under conditions promoting cardiomyogenic differentiation.
Figure 7. Abcg2 overexpression prevents cardiomyogenic differentiation of cSP cells.
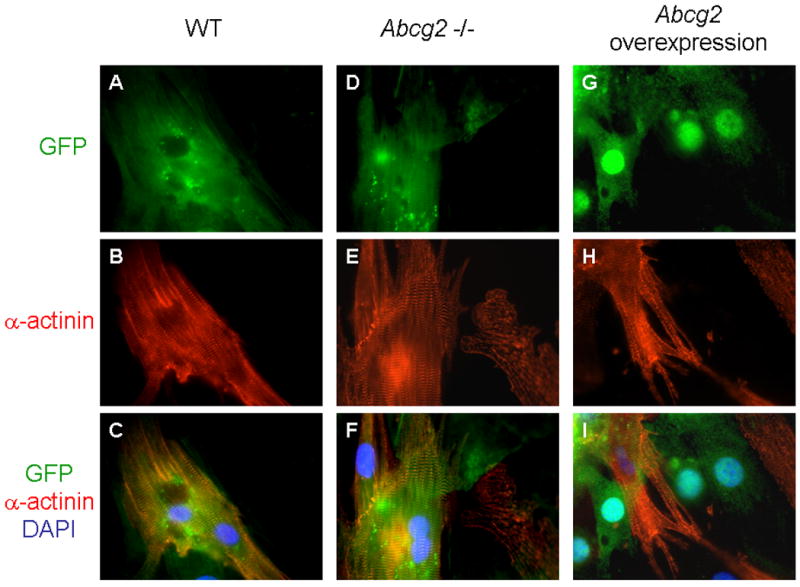
Fluorescence images of WT (A–C), Abcg2 -/-, (D–F) and Abcg2 overexpressing cSP cells (G– I) in co-culture with adult rat cardiomyocytes. Green fluorescence corresponds to GFP-expression that identifies respective cSP cells (upper row). Red fluorescence corresponds to the expression of α-actinin, a marker of cardiomyogenic differentiation (middle row). Merged images show areas of colocalization of GFP and α-actinin in yellow (bottom row). In this differentiation assay, lack of Abcg2 does not limit the cardiomyogenic differentiation of cSP cells as demonstrated by α-actinin-expression and appearance of clearly organized sarcomeric structures in differentiated Abcg2 -/- cSP cells (D–F) similar to as seen in WT cells (A–C). Abcg2 overexpression, however, prevents cSP cells from undergoing cardiomyogenic differentiation as demonstrated by lack of α-actinin-expression in Abcg2 overexpressing cSP cells co-cultured with cardiomyocytes (G–I).
Discussion
Since the first isolation of BMSP cells more than a decade ago 2, the SP phenotype has been widely used to identify stem/progenitor cells in various tissues 2,7,16-19. More recently, the ABC transporter Abcg2 has been identified as the sole molecular determinant of the SP phenotype in bone marrow cells 4. However, the role of ABC transporters in the regulation of SP phenotype and function of cSP cells remains unknown. Herein, we demonstrate not only a dynamic, age-dependent regulation of the cSP phenotype by Abcg2, but also a functional role of Abcg2 in modulating proliferation, differentiation, and survival of cSP cells that goes beyond its distinct role in Hoechst dye efflux.
Role of Abcg2 in conferring the SP phenotype in the heart
Although Abcg2 and Mdr1 are both expressed in hematopoietic stem cells identified by the SP phenotype, Abcg2 was shown to be the sole molecular determinant of the SP phenotype in bone marrow cells 4. These findings in the bone marrow led to the assumption that Abcg2 may also be responsible for the dye efflux observed in SP cells isolated from other tissues, particularly given the preferential expression pattern of Abcg2 in SP cells as compared to main population cells. Similar as in bone marrow, Abcg2 and Mdr1 were shown to be expressed in the heart, though their contribution to the cSP phenotype remained to be elucidated. Utilizing mouse models with targeted gene ablation of either Abcg2 or Mdr1, we demonstrate that the cSP cell phenotype is not governed by a single ABC transporter but rather is regulated in an age dependent manner by both Abcg2 and Mdr1. Our findings show that during early postnatal development, Abcg2 represents the main transporter responsible for dye efflux in cardiac cells and thus constitutes the molecular basis for the SP phenotype in the neonatal and early postnatal heart. This is in accordance with the role of Abcg2 in bone marrow-derived cells and may indicate that cSP cells of the early postnatal heart share phenotypic characteristics of bone marrow-derived SP cells. Whether cSP cells developmentally originate from blood borne cells, however, remains to be determined. It is important to point out that our current data provide no evidence to either support nor dispute such notion. Prior data from our laboratory using labeled bone marrow transplantation, however, suggest that extra-cardiac stem cells only contribute to the maintenance of resident cSP cell pools following injury, with little role in the maintenance of cSP cell numbers under normal physiologic conditions 20.
While our results agree with previous reports of persistent Abcg2 expression in cSP cells throughout adulthood 9, we find that the contribution of Abcg2 to the SP phenotype diminishes in the adult heart. Our results show that very limited cSP cells can be detected in early neonatal Abcg2 -/- hearts, with clearly detectable cSP cells in adult Abcg2 -/- hearts, albeit at a lower total number as compared to wild type hearts. These Abcg2 -/- cSP cells are sensitive to verapamil, FTC and 2-deoxyglucose treatment, thus suggesting that their Hoechst extruding ability is mediated through another ABC transporter. Analyses of mice completely lacking Mdr1 identified the P-glycoprotein as the essential ABC transporter for Hoechst efflux in adult cSP cells. It is important to note that the putative Abcg2 inhibitor FTC, that was previously shown to have no effect on Mdr1-mediated mitoxantrone efflux in mitoxantrone-resistance-selected human colon carcinoma cell lines 21, did inhibit Mdr1-mediated Hoechst efflux in cSP cells, suggesting that the specificity of FTC may depend on the cell type and the substrate.
Interestingly, this regulatory role of Mdr1 in the cSP phenotype is limited to cSP cells from mice older than 3 weeks of age with limited contribution of Mdr1 to the SP phenotype in early neonatal mouse hearts. While the origin of cardiac stem/progenitor cells and their relationship with bone marrow-derived stem cells remains speculative at this time, our data suggest that BMSP cells do not significantly contribute to the maintenance of cSP cells under physiologic conditions as it is evidenced by the lack of SP cells in adult Mdr1a/b -/- hearts whereas Mdr1a/b -/- bone marrow contains normal SP cells. Thus, the current data are consistent with our previous findings demonstrating that BMSP cells only contribute to the maintenance of cSP cells following cardiac injury such as myocardial infarction 20.
Our data dispute the perception that a single universal ABC transporter is responsible for the SP phenotype and suggest that developmental status and local microenvironment dictate the relative contribution of ABC transporters to the SP phenotype at the given tissue. In line with this observation is the recent demonstration of contribution of both Mdr1 and Abcg2 transporters to the SP phenotype in mammary glands 22.
Role of Abcg2 in regulating the function of cSP cells
Abcg2 has been found to be highly expressed in various proliferating stem/progenitor cells and tumor cell lines, though its role in the regulation of SP cell proliferation and differentiation remained unknown. Using gain- and loss-of function approaches, we demonstrate that overexpression of Abcg2 is sufficient to increase the proliferative capacity of cSP cells, whereas lack of Abcg2 expression markedly impairs their expandability in vitro. The marked decrease in cells being in M-phase of the cell cycle, as measured by phospho-histone H3 expression, suggests that the absence of Abcg2 may hamper cell cycle progression. This association of Abcg2 expression with cell proliferation is in line with the findings in cancer cells, where Abcg2 identified mainly fast cycling tumor progenitor cells 13. Our data provide convincing evidence demonstrating that Abcg2 may play a functional role in regulating the proliferation capacity of cSP cells, albeit the precise mechanisms are unknown. Further investigation, therefore, is warranted to dissect the molecular mechanisms by which Abcg2 facilitates the cell cycle progression in cardiac progenitor cells.
In addition to enhancing cell proliferation, we show that Abcg2 expression is necessary for protecting cSP cells from undergoing apoptosis and necrosis, specifically under conditions of increased oxidative stress. A similar pro-survival effect of Abcg2 was identified in trophoblast and hematopoietic stem cells 14,23. Moreover, Martin et al recently demonstrated that expression of Abcg2 induced low levels of oxidative stress in C2C12 myoblasts, which resulted in upregulation of cytoprotective and oxidative stress pathways 15. The cytoprotective effect of such Abcg2-mediated ROS preconditioning was also confirmed in mouse embryonic fibroblasts (MEFs) that displayed reduced oxidative-stress induced cell death, when transfected with Abcg2 15. Our data are in complete agreement with this concept by demonstrating increased tolerance of oxidative stress in Abcg2-competent WT cSP cells as compared to cSP cells lacking Abcg2.
Consistent with the notion that Abcg2 maintains progenitor cells in a proliferative stage and is downregulated during lineage specific differentiation, overexpression of Abcg2 prevented cSP cells from undergoing cardiomyogenic differentiation. Our data are supported by the recently published work in hematopoietic and retinal stem cells demonstrating highly regulated Abcg2 expression during stem cell differentiation with a sharp decline during lineage commitment 4,5. In contrast, overexpression of Abcg2 blocks the differentiation of hematopoietic and retinal stem cells, indicating a functional role of Abcg2 in the maintenance of the stem cell pool 4,5. In both cell types, overexpression of Abcg2 leads to increased cell expansion and adversely affects their lineage commitment.
We have shown that expression of Abcg2 not only promotes proliferation and survival of cSP cells but at the same time, inhibits cellular differentiation. Taken together our data suggest that Abcg2 is essential to the fate and function of cSP cells. As such, the tight regulation of Abcg2 expression may be critical for maintaining progenitor cells in either a pro-proliferative or pro-differentiation state. Moreover, such regulation may be especially essential following tissue injury, during which a rapid increase in cSP cell proliferation is observed in order to replenish tissue SP cell pools 20. Dysregulation of Abcg2 expression may also result in uncontrolled cell growth or cell death. To date, the exact mechanism whereby Abcg2 prevents stem cells from lineage commitment remains to be elucidated. Considering Abcg2’s primary function as a detoxifying transmembrane pump, however, it is tempting to speculate that active extrusion of key molecules of the differentiation-promoting pathway might be involved in this process.
In summary, our study highlights the importance of Abcg2 in regulating the function and homeostasis of cSP cells that goes beyond its traditional role as dye efflux transporter. Manipulation of Abcg2 expression and function may be of particular importance in promoting cardiac regeneration following injury by both endogenous and exogenously-delivered cSP cells. Given the role of Abcg2 in the proliferation of cancer cells, as well as cSP cells, there is great potential for cardiac toxicity with emerging chemotherapeutic agents specifically targeting Abcg2. Further investigation into the role of Abcg2 in cSP cells is of clinical importance to limit cancer drug-induced cardiac toxicity, and to promote cardiac regeneration.
Supplementary Material
Acknowledgments
The authors wish to thank Drs. Richard C. Mulligan and Alejandro B. Balazs for their help in the purification of SP cells and for useful discussions. Mr. Grigoriy Losyev at BWH Cardiovascular and Ms. Laura B. Prickett at MGH FACS Cores are acknowledged for assistance with cell sorting.
Sources of Funding This work was supported by NIH grants HL71775, HL86967, HL73756, and HL 88533 (RL). AO and GF were supported by American Heart Association Northeast Affiliate Pre-doctoral Fellowship and Sarnoff Cardiovascular Research Foundation Fellowship, respectively.
Footnotes
Disclosures: None.
References
- 1.Challen GA, Little MH. A side order of stem cells: the SP phenotype. Stem Cells. 2006;24:3–12. doi: 10.1634/stemcells.2005-0116. [DOI] [PubMed] [Google Scholar]
- 2.Goodell MA, Brose K, Paradis G, Conner AS, Mulligan RC. Isolation and functional properties of murine hematopoietic stem cells that are replicating in vivo. J Exp Med. 1996;183:1797–806. doi: 10.1084/jem.183.4.1797. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Bunting KD. ABC transporters as phenotypic markers and functional regulators of stem cells. Stem Cells. 2002;20:11–20. doi: 10.1002/stem.200011. [DOI] [PubMed] [Google Scholar]
- 4.Zhou S, Schuetz JD, Bunting KD, Colapietro AM, Sampath J, Morris JJ, Lagutina I, Grosveld GC, Osawa M, Nakauchi H, Sorrentino BP. The ABC transporter Bcrp1/ABCG2 is expressed in a wide variety of stem cells and is a molecular determinant of the side-population phenotype. Nat Med. 2001;7:1028–34. doi: 10.1038/nm0901-1028. [DOI] [PubMed] [Google Scholar]
- 5.Bhattacharya S, Das A, Mallya K, Ahmad I. Maintenance of retinal stem cells by Abcg2 is regulated by notch signaling. J Cell Sci. 2007;120:2652–62. doi: 10.1242/jcs.008417. [DOI] [PubMed] [Google Scholar]
- 6.Lassalle B, Bastos H, Louis JP, Riou L, Testart J, Dutrillaux B, Fouchet P, Allemand I. ‘Side Population’ cells in adult mouse testis express Bcrp1 gene and are enriched in spermatogonia and germinal stem cells. Development. 2004;131:479–87. doi: 10.1242/dev.00918. [DOI] [PubMed] [Google Scholar]
- 7.Summer R, Kotton DN, Sun X, Ma B, Fitzsimmons K, Fine A. Side population cells and Bcrp1 expression in lung. Am J Physiol Lung Cell Mol Physiol. 2003;285:L97–104. doi: 10.1152/ajplung.00009.2003. [DOI] [PubMed] [Google Scholar]
- 8.Hierlihy AM, Seale P, Lobe CG, Rudnicki MA, Megeney LA. The postnatal heart contains a myocardial stem cell population. FEBS Lett. 2002;530:239–43. doi: 10.1016/s0014-5793(02)03477-4. [DOI] [PubMed] [Google Scholar]
- 9.Martin CM, Meeson AP, Robertson SM, Hawke TJ, Richardson JA, Bates S, Goetsch SC, Gallardo TD, Garry DJ. Persistent expression of the ATP-binding cassette transporter, Abcg2, identifies cardiac SP cells in the developing and adult heart. Dev Biol. 2004;265:262–75. doi: 10.1016/j.ydbio.2003.09.028. [DOI] [PubMed] [Google Scholar]
- 10.Pfister O, Mouquet F, Jain M, Summer R, Helmes M, Fine A, Colucci WS, Liao R. CD31- but Not CD31+ cardiac side population cells exhibit functional cardiomyogenic differentiation. Circ Res. 2005;97:52–61. doi: 10.1161/01.RES.0000173297.53793.fa. [DOI] [PubMed] [Google Scholar]
- 11.Oyama T, Nagai T, Wada H, Naito AT, Matsuura K, Iwanaga K, Takahashi T, Goto M, Mikami Y, Yasuda N, Akazawa H, Uezumi A, Takeda S, Komuro I. Cardiac side population cells have a potential to migrate and differentiate into cardiomyocytes in vitro and in vivo. J Cell Biol. 2007;176:329–41. doi: 10.1083/jcb.200603014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Bunting KD, Zhou S, Lu T, Sorrentino BP. Enforced P-glycoprotein pump function in murine bone marrow cells results in expansion of side population stem cells in vitro and repopulating cells in vivo. Blood. 2000;96:902–9. [PubMed] [Google Scholar]
- 13.Patrawala L, Calhoun T, Schneider-Broussard R, Zhou J, Claypool K, Tang DG. Side population is enriched in tumorigenic, stem-like cancer cells, whereas ABCG2+ and ABCG2- cancer cells are similarly tumorigenic. Cancer Res. 2005;65:6207–19. doi: 10.1158/0008-5472.CAN-05-0592. [DOI] [PubMed] [Google Scholar]
- 14.Krishnamurthy P, Ross DD, Nakanishi T, Bailey-Dell K, Zhou S, Mercer KE, Sarkadi B, Sorrentino BP, Schuetz JD. The stem cell marker Bcrp/ABCG2 enhances hypoxic cell survival through interactions with heme. J Biol Chem. 2004;279:24218–25. doi: 10.1074/jbc.M313599200. [DOI] [PubMed] [Google Scholar]
- 15.Martin CM, Ferdous A, Gallardo T, Humphries C, Sadek H, Caprioli A, Garcia JA, Szweda LI, Garry MG, Garry DJ. Hypoxia-inducible factor-2alpha transactivates Abcg2 and promotes cytoprotection in cardiac side population cells. Circ Res. 2008;102:1075–81. doi: 10.1161/CIRCRESAHA.107.161729. [DOI] [PubMed] [Google Scholar]
- 16.Behbod F, Xian W, Shaw CA, Hilsenbeck SG, Tsimelzon A, Rosen JM. Transcriptional profiling of mammary gland side population cells. Stem Cells. 2006;24:1065–74. doi: 10.1634/stemcells.2005-0375. [DOI] [PubMed] [Google Scholar]
- 17.Hishikawa K, Marumo T, Miura S, Nakanishi A, Matsuzaki Y, Shibata K, Ichiyanagi T, Kohike H, Komori T, Takahashi I, Takase O, Imai N, Yoshikawa M, Inowa T, Hayashi M, Nakaki T, Nakauchi H, Okano H, Fujita T. Musculin/MyoR is expressed in kidney side population cells and can regulate their function. J Cell Biol. 2005;169:921–8. doi: 10.1083/jcb.200412167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Kotton DN, Fabian AJ, Mulligan RC. A novel stem-cell population in adult liver with potent hematopoietic-reconstitution activity. Blood. 2005;106:1574–80. doi: 10.1182/blood-2005-03-1017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Rivier F, Alkan O, Flint AF, Muskiewicz K, Allen PD, Leboulch P, Gussoni E. Role of bone marrow cell trafficking in replenishing skeletal muscle SP and MP cell populations. J Cell Sci. 2004;117:1979–88. doi: 10.1242/jcs.01051. [DOI] [PubMed] [Google Scholar]
- 20.Mouquet F, Pfister O, Jain M, Oikonomopoulos A, Ngoy S, Summer R, Fine A, Liao R. Restoration of cardiac progenitor cells after myocardial infarction by self-proliferation and selective homing of bone marrow-derived stem cells. Circ Res. 2005;97:1090–2. doi: 10.1161/01.RES.0000194330.66545.f5. [DOI] [PubMed] [Google Scholar]
- 21.Rabindran SK, He H, Singh M, Brown E, Collins KI, Annable T, Greenberger LM. Reversal of a novel multidrug resistance mechanism in human colon carcinoma cells by fumitremorgin C. Cancer Res. 1998;58:5850–8. [PubMed] [Google Scholar]
- 22.Jonker JW, Freeman J, Bolscher E, Musters S, Alvi AJ, Titley I, Schinkel AH, Dale TC. Contribution of the ABC transporters Bcrp1 and Mdr1a/1b to the side population phenotype in mammary gland and bone marrow of mice. Stem Cells. 2005;23:1059–65. doi: 10.1634/stemcells.2005-0150. [DOI] [PubMed] [Google Scholar]
- 23.Evseenko DA, Murthi P, Paxton JW, Reid G, Emerald BS, Mohankumar KM, Lobie PE, Brennecke SP, Kalionis B, Keelan JA. The ABC transporter BCRP/ABCG2 is a placental survival factor, and its expression is reduced in idiopathic human fetal growth restriction. Faseb J. 2007;21:3592–605. doi: 10.1096/fj.07-8688com. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.