Abstract
This paper evaluates an experiment in which individuals in rural Malawi were randomly assigned monetary incentives to learn their HIV results after being tested. Distance to the HIV results centers was also randomly assigned. Without any incentive, 34 percent of the participants learned their HIV results. However, even the smallest incentive doubled that share. Using the randomly assigned incentives and distance from results centers as instruments for the knowledge of HIV status, sexually active HIV-positive individuals who learned their results are three times more likely to purchase condoms two months later than sexually active HIV-positive individuals who did not learn their results; however, HIV-positive individuals who learned their results purchase only two additional condoms than those who did not. There is no significant effect of learning HIV-negative status on the purchase of condoms.
Over the past two decades, the HIV/AIDS epidemic has afflicted millions of individuals in Africa. In the absence of significantly expanded prevention and treatment programs, the epidemic is expected to worsen in many other parts of the world. One intervention often suggested to alleviate the spread of the disease is HIV testing, and some have gone so far as to say that voluntary counseling and testing (VCT) is the “missing weapon in the battle against AIDS.”1 Under the assumption that HIV testing is an effective prevention strategy, many international organizations and governments have called for increased investments in counseling and testing, requiring large amounts of monetary and human resources (Global Business Coalition 2005; Know HIV AIDS 2005). For example, in South Africa, government expenditures on counseling and testing increased from $2.4 million in 2000 to $17.3 million in 2004, and in Mozambique, 55 percent of all HIV/AIDS program expenditures in 2000 were for HIV counseling and testing (H. Gayle Martin 2003). Some governments have even suggested implementing universal testing programs, sending nurses door to door.2
Underlying the emphasis on HIV testing for prevention—and the large-scale expenditures on testing—are two rarely challenged assumptions. First, many believe that knowledge of HIV status has positive effects on sexual behavior that prevent the spread of the disease. In particular, it is assumed that those diagnosed HIV-negative will protect themselves from infection and those diagnosed HIV-positive will take precautions to protect others. Second, many believe that it is difficult to get people to learn their HIV status, due primarily to psychological or social barriers, thus justifying expenditures on destigmatization and advertising campaigns.
In this paper, I evaluate a field experiment in rural Malawi designed to address these assumptions. I find that barriers to obtaining HIV test results can be easily overcome by offering small cash incentives or by reducing the distance needed to travel for the results. I also find that while receiving an HIV-positive diagnosis has a significant effect on the subsequent purchase of condoms, the overall magnitude of the effect is small. The results in this paper suggest that, relative to other available prevention strategies or targeting high-risk populations, door-to-door HIV testing may not be the most effective HIV prevention strategy, as measured by condom purchases.
Previous studies have attempted to measure the demand for learning HIV status, as well as the subsequent behavioral effects. Most studies have relied on self-reported behavior by asking individuals if they want to know their HIV status (e.g., John H. Day et al. 2003; Joseph de Graft–Johnson et al. 2005; Susan M. Laver 2001; Stanley P Yoder and Pricilla Matinga 2004) or asking about reported sexual behavior (e.g., The Voluntary HIV-1 Counseling and Testing Efficacy Study Group 2000; M. Kamenga et al. 1994; M. Temmerman et al. 1990; and Lance S. Weinhardt et al. 1999). Self-selection is also a serious limitation to evaluating the effects of learning HIV results. Most, if not all, studies use a sample of individuals who self-select into knowing their HIV status.3
The design of this experiment avoids the usual complications of selection and reporting bias because it randomized individual incentives to learn HIV status, randomized the location of VCT centers where HIV results were available, measured actual post-test attendance at centers to obtain results, and measured actual condom purchases subsequent to learning HIV status. The experimental design of this study is important because factors influencing the decision to learn HIV results are generally correlated with behavioral outcomes, potentially biasing the estimates of the impact of learning HIV results on the demand for safe sex.
In this study, respondents in rural Malawi were offered a free door-to-door HIV test and were given randomly assigned vouchers between zero and three dollars, redeemable upon obtaining their results at a nearby VCT center. The demand for HIV test results among those who received no monetary incentive was moderate, at 34 percent. However, monetary incentives were highly effective in increasing result-seeking behavior: on average, respondents who received any cash-value voucher were twice as likely to go to the VCT center to obtain their HIV test results as were individuals receiving no incentive. Although the average incentive was worth about a day’s wage, even the smallest amount, approximately one-tenth of a day’s wage, resulted in large attendance gains. The location of each HIV results center was also randomly placed to evaluate the impact of distance on VCT attendance: living over 1.5 kilometers from the VCT center reduced attendance by 6 percent.
Several months later, follow-up interviews were conducted and respondents were given the opportunity to purchase condoms. Using the random allocation of incentives and distance as exogenous instruments for learning HIV status, I find that receiving an HIV-positive diagnosis significantly increased the likelihood of purchasing condoms among those with a sexual partner. However, the total number of condoms purchased by HIV-positive individuals who knew their test results was small. On average, those with a sexual partner who learned they were HIV-positive purchased two more condoms than those HIV-positives who did not learn their results. Learning HIV results had no impact on condom purchases among those who were HIV-negative or those who were not sexually active.
This paper measures the impact of learning HIV results on sexual activity and condom purchases two months later. While there may be other prevention strategies that individuals undertake after learning their results that are not measured in this paper, the findings presented here suggest that door-to-door testing may not be as cost-effective as other prevention programs in averting new infections. There may be a role for HIV testing, however, if it is targeted at high-risk groups. In addition, the findings in this paper suggest that offering small rewards to encourage people to learn their results may be very effective, for example, in giving HIV-positive individuals access to treatment or in distributing antiretroviral therapy to pregnant women to prevent HIV transmission to their baby.
I. Project Design
A. Background on Malawi and Description of the Data
The Malawi Diffusion and Ideational Change Project (MDICP) is conducted in Malawi, a land-locked country located in southern Africa (Figure 1). This collaborative project between the University of Pennsylvania and the Malawi College of Medicine is an ongoing study of men and women randomly selected from approximately 120 villages in the districts of Rumphi, Mchinji, and Balaka, located in the Northern, Central, and Southern regions respectively.4 Approximately 25 percent of all households in each village were randomly selected to participate in 1998, and ever-married women and their husbands from these households were interviewed in 1998, 2001, and 2004. During data collection in 2004, an additional sample of young adults (ages 15–24) residing in the original villages was added to the sample.
Figure 1.
Malawi and MDICP Study Sites
Between May and August of 2004, nurses from outside each area offered respondents free tests in their homes for HIV and three other sexually transmitted infections (STIs): gonorrhea, chlamydia, and trichomoniasis. At the time that tests were offered, respondents were given pretest counseling about HIV prevention strategies. Samples were taken through oral swabs to test for HIV and through urine samples (for men) or self-administered vaginal swabs (for women) to test for other STIs (Simona Bignami–Van Assche et al. 2004 and Francis Obare et al. 2008 provide the full testing protocol.). Across the three districts, 2,894 of the 3,185 respondents who were offered accepted an HIV test (91 percent). The main sample in this paper consists of those who both accepted an HIV test and had basic covariate data: 2,812 individuals (Table 1, panel A). Sample attrition and test refusals are discussed below.
Table 1.
Sample Size and Determinants of Participation
| Panel A: Sample size and attrition | ||
| Percent of 1998 adult sample interviewed in 2001 | 0.78 | |
| Percent of 1998 adult sample interviewed in 2004 | 0.72 | |
| Main sample (accepted HIV test with co-variate data) | 2,812 | |
| Follow-up sample (main sample interviewed at follow-up) | 1,524 | |
| Percent accepting a test for HIV | 0.91 | |
| Percent of main sample (Balaka and Rumphi) interviewed at 2004 follow-up survey | 0.75 | |
|
| ||
| Panel B: Determinants of accepting an HIV test | (1) | (2) |
| Male | −0.004 (0.009) | −0.002 (0.008) |
| Age | 0.002 (0.003) | 0.003 (0.002) |
| Age-squared | 0.000 (0.000) | 0.000 (0.000) |
| Married | −0.02 (0.017) | −0.030** (0.013) |
| Had a previous HIV test | 0.062*** (0.011) | 0.047*** (0.010) |
| Think treatment will become available | −0.012 (0.012) | |
| Think there is a likelihood of being HIV positive | −0.007 (0.010) | |
| Sample size | 3,141 | 2,682 |
| R2 | 0.01 | 0.01 |
|
| ||
| Panel C: Determinants of participation in the follow-up survey | (1) | |
| Got results | 0.240*** (0.022) | |
| Any incentive | 0.068 (0.044) | |
| Amount of incentive | −0.098* (0.056) | |
| Amount of incentive2 | 0.025 (0.017) | |
| Distance (km) | 0.013 (0.047) | |
| Distance2 | −0.007 (0.008) | |
| HIV positive | −0.171*** (0.044) | |
| Sample size | 2,021 | |
| R2 | 0.09 | |
Notes: Panel B represents one OLS regression of acceptance of an HIV test among those respondents offered a test. Panel C represents one OLS regression among respondents eligible for the follow-up survey (those tested for HIV in Balaka and Rumphi). Controls also include gender, age, and age-squared. Robust standard errors clustered by village with district fixed effects are in parentheses. “Any” is an indicator if the respondent received any nonzero monetary incentive and “amount” is the total value of the incentive.
Significantly different from zero at 99 percent confidence level.
Significantly different from zero at 95 percent confidence level.
Significantly different from zero at 90 percent confidence level.
The main sample is 46 percent male with an average age of 33 (Table 2, panel A). Seventy-one percent of the respondents were married at the time of the interview and 58 percent had ever attended school, with an average of almost four years. Ethnicity and religion vary across the three districts: the Chewas in Mchinji and the Tumbukas in Rumphi are primarily Christian; the Yaos in Balaka traditionally practice Islam. The majority of the respondents are subsistence farmers and 73 percent own land. The HIV prevalence rate was 6.3 percent (7.2 percent for females, 5.1 percent for males). Prevalence rates for other sexually transmitted diseases were even lower, with 3.2 percent men and women infected with gonorrhea, 0.3 percent men and women with chlamydia, and 2.4 percent women with trichomoniasis (Table 2, panel B). The prevalence of HIV in the MDICP sample is considerably lower than national prevalence rates. Another population-based study in Malawi found an overall HIV prevalence rate of 12.5 percent in 2004 (DHS 2004). Among the rural populations, however, the Malawi DHS found an HIV rate of 5.2 percent in the Northern region, 7.3 percent in the Central region, and 16.7 percent in the Southern region, which are comparable to the MDICP sample rates of 4.4 percent in the Northern region (Rumphi), 6.4 percent in the Central region (Mchinji), and 7.9 percent in the Southern region (Balaka). Longitudinal sample attrition from death and migration (discussed below) may also bias the estimates downward (Philip Anglewicz 2007), as well as the disproportionate number of young adults age 15–24 included in the sample (33.4 percent of respondents), who have a lower overall infection rate of 2.7 percent. See Section IC for further discussion.
Table 2.
Summary Statistics
| Full sample | Follow-up sample | |||
|---|---|---|---|---|
| Observations | 2,812 | 1,524 | ||
| Mean | SD | Mean | SD | |
| Panel A: Respondent characteristics | ||||
| Male | 0.46 | (0.50) | 0.46 | (0.50) |
| Age | 33.4 | (13.66) | 34.6 | (14.30) |
| Married | 0.71 | (0.45) | 0.72 | (0.45) |
| Years of education | 3.6 | (3.70) | 3.8 | (3.80) |
| Owns land | 0.73 | (0.44) | 0.74 | (0.44) |
|
| ||||
| Panel B: Health | ||||
| HIV positive | 0.063 | (0.24) | 0.048 | (0.21) |
| Gonorrhea positive | 0.032 | (0.18) | 0.034 | (0.18) |
| Chlamydia positive | 0.003 | (0.06) | 0.004 | (0.06) |
| Trichomoniasis positive | 0.024 | (0.15) | 0.014 | (0.12) |
| Ever had an HIV test (before 2004) | 0.181 | (0.385) | 0.217 | (0.412) |
| Thinks treatment will be available in five years | 0.341 | (0.474) | 0.372 | (0.484) |
| Reported having sex during 2004 | 0.761 | (0.43) | 0.759 | (0.43) |
| Reported using condoms during 2004 | 0.210 | (0.41) | 0.210 | (0.41) |
| Sexual acts in one month (if > 0) | 5.104 | (4.89) | 5.104 | (4.82) |
|
| ||||
| Panel C: Incentives, distance, and attendance at results centers | ||||
| Monetary incentive (dollars) | 1.01 | (0.90) | 1.05 | (0.91) |
| Distance to VCT center (km) | 2.02 | (1.27) | 2.20 | (1.34) |
| Attended VCT center | 0.69 | (0.46) | 0.72 | (0.45) |
| Attended VCT center (if incentive = 0) | 0.34 | (0.47) | 0.37 | (0.48) |
|
| ||||
| Panel D: Follow-up condom sales | ||||
| Purchased condoms at the follow-up | — | — | 0.24 | (0.43) |
| Number of condoms purchased (if > 0) | — | — | 3.66 | (2.18) |
| Reported purchasing condoms | — | — | 0.08 | (0.27) |
| Reported having sex after VCT | — | — | 0.62 | (0.49) |
| Reported having sex with more than one partner after VCT | — | — | 0.033 | (0.18) |
Notes: Full sample includes respondents who accepted a test for HIV in 2004 and have basic demographic data. Follow-up sample includes respondents in Balaka and Rumphi who tested and were reinterviewed in 2005. HIV prevalence rates do not include 14 respondents with indeterminate diagnoses. Other STI prevalence rates do not include 83 respondents with indeterminate diagnoses. Trichomoniasis was tested only among women. Respondents were asked about sexual acts per month only during the nurses’ survey in Balaka. The monetary incentive is a sum of an incentive for learning HIV results and an incentive for learning other STI results (in Mchinji and Balaka). Distance from assigned testing centers to respondents’ homes is a straight-line spherical distance measured in kilometers. Reported having sex with more than one partner does not include those who did not report having sex in the follow-up survey. Sexual acts per month was asked only among a subset of respondents between the baseline survey and time of the VCT.
B. Experimental Design
The first part of the experimental design involved offering monetary incentives to encourage respondents to obtain their test results at nearby centers. After taking the HIV test samples, nurses gave each respondent vouchers redeemable upon obtaining either HIV or STI results. Voucher amounts were randomized by letting each respondent draw a token out of a bag indicating a monetary amount. In Mchinji and Balaka each respondent received two vouchers, one for obtaining HIV results, and one for obtaining STI results. In Rumphi, respondents received only one voucher redeemable by returning for either HIV or STI results. For the analysis, I examine the impact of the total value of the incentive (the sum of the HIV and STI incentives) on obtaining results. In Mchinji and Balaka, all respondents learned both their HIV and STI results. Vouchers ranged between zero and three dollars, with an average total voucher amount (including zeros) of 1.01 dollars, worth approximately a day’s wage (Table 2, panel C).
The distribution of vouchers was carefully monitored to ensure that each nurse followed the rules of randomization.5 Figure 2 presents the distribution of the vouchers. Each voucher included the amount, a respondent ID, and the nurse’s signature; a carbon copy was made to prevent forgeries. Respondents who drew a zero token received no voucher; 22 percent received no incentive to return for either HIV or STI results. Drawing a “zero” may have had a demotivating effect on individuals wanting to attend the VCT, center which may have had an impact on attendance. Because all of the respondents participated in the “lottery” draw, it is impossible to estimate the potential effect of disappointment. However, this is likely to be minimal.
Figure 2. Distribution of Incentives to Return for HIV Results ($).
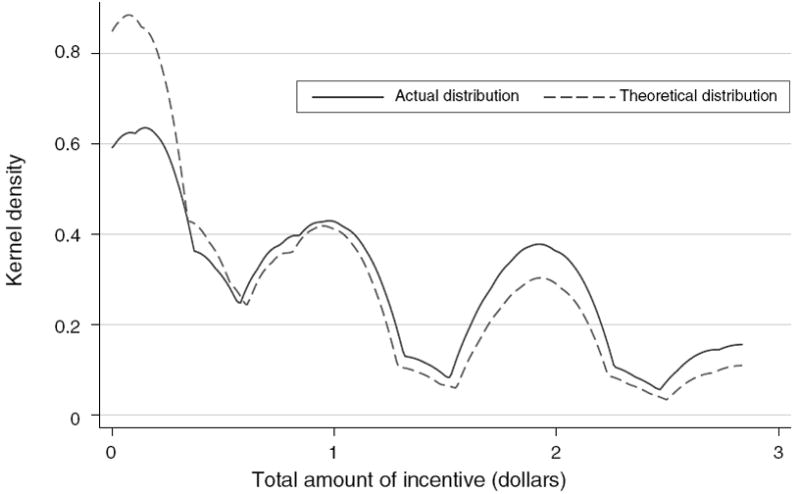
Notes: Sample includes 2,812 individuals who tested for HIV and have age data. Figure presents the actual kernel density of the distribution of monetary incentives given by nurses and the theoretical distribution of the incentives to be given.
Two to four months after sample collection, test results became available, and temporary test results (VCT) centers, consisting of small portable tents, were placed randomly throughout the districts. Based on their geospatial (GPS) coordinates, respondents’ households in villages were grouped into zones, and a location within each zone was randomly selected to place a tent. The average distance to a center was two kilometers and over 95 percent of those tested lived within five kilometers. Distance to the VCT center is calculated as a straight line and does not account for roads or paths. In most cases, tents were placed in the exact randomly selected location and paths were created for easy accessibility.6 Baseline characteristics are similar across groups receiving any incentive amount (including zero) and living within various VCT zones. Although there are some statistically significant differences among these groups, they are small in magnitude (Table 3).
Table 3.
Baseline Characteristics by Incentives and Distance
| Dependent variable | Male (1) | Age (2) | HIV positive (3) | Years of education (4) | Owns land (5) | Had sex in 2004 (6) | Used a condom in 2004 (7) |
|---|---|---|---|---|---|---|---|
| Any incentive | −0.051 (0.039) | 2.034*** (0.993) | 0.001 (0.020) | −0.25 (0.284) | 0.018 (0.033) | −0.027 (0.033) | 0.006 (0.029) |
| Amount of incentive | 0.06 (0.055) | −0.185 (1.331) | −0.018 (0.024) | 0.022 (0.356) | 0.005 (0.043) | −0.029 (0.048) | −0.061 (0.045) |
| Amount of incentive2 | −0.015 (0.018) | −0.103 (0.435) | 0.005 (0.008) | 0.038 (0.116) | 0.002 (0.014) | 0.008 (0.015) | 0.022 (0.016) |
| Distance (km) | −0.025 (0.022) | −0.211 (1.133) | 0.026 (0.016) | −0.089 (0.330) | −0.045 (0.033) | 0.012 (0.028) | 0.041 (0.028) |
| Distance2 | 0.006 (0.004) | 0.096 (0.240) | 0.004 (0.003) | −0.015 (0.064) | 0.011 (0.007) | -0.001 (0.005) | −0.006 (0.005) |
| Constant | 0.487*** (0.030) | 30.585*** (1.217) | 0.101*** (0.023) | 3.311*** (0.366) | 0.768*** (0.036) | 0.791*** (0.037) | 0.167*** (0.036) |
| Sample size | 2,812 | 2,812 | 2,812 | 2,530 | 2,594 | 2,343 | 2,374 |
| R2 | 0.00 | 0.01 | 0.01 | 0.17 | 0.07 | 0.01 | 0.01 |
Notes: Sample includes individuals who tested for HIV and have demographic data. Columns represent OLS coefficients; robust standard errors clustered by village (for 119 villages) with district fixed effects in parentheses. “Any incentive” is an indicator if the respondent received any nonzero monetary incentive. “HIV” is an indicator of being HIV positive. Also includes age, age-squared, and gender. Distance is measured as a straight-line spherical distance from a respondent’s home to randomly assigned VCT center from geospatial coordinates and is measured in kilometers.
Significantly different from zero at 99 percent confidence level.
Significantly different from zero at 95 percent confidence level.
Significantly different from zero at 90 percent confidence level.
Respondents were personally informed of the hours of operation and location of their assigned center (open Monday through Saturday from 8 a.m. to 7 p.m.) and centers were operational for approximately one week. Respondents were allowed to attend any of the VCT centers but were informed only of the location and hours of operation of their assigned center (fewer than 6 percent of respondents went to a center other than the one to which they were assigned). When they obtained their test results, respondents also received counseling. On average, nurses spent 30 minutes counseling each respondent about safe sexual practices, including abstinence and condom use, regardless of respondent’s HIV test results. Several studies have shown that education can have an impact on condom use and HIV prevention (see Jeff Stryker et al. 1995; K. J. Sikkema et al. 2000). In this study, however, it is impossible to distinguish a pure information effect (learning HIV status) from an educational effect (counseling). The counseling received at the VCT centers would then produce upper bounds of the effects of learning HIV test results alone. Couples were given their test results verbally and were informed of their results separately. Respondents could redeem their vouchers only after hearing their results. Those who were HIV-positive were referred to the nearest permanent clinic for further counseling. Those who were positive for other sexually transmitted diseases were also given free treatment at that time, which may have provided additional incentive to attend VCT centers, over and above the monetary incentive. However, STI prevalence rates were very low (see Table 2, panel B).
Approximately two months after results were available, respondents who tested for HIV in two districts, Balaka and Rumphi, were reinterviewed in their homes by interviewers who had no part in the testing and did not know the respondents’ HIV status. Both those who had obtained their results and those who had not were approached for this follow-up interview. During this interview, respondents were asked about their sexual behavior in the prior two months and their attitudes toward condom use. At the end of the interview, respondents were given approximately 30 cents as appreciation for participation and were offered the opportunity to purchase condoms at half the subsidized retail price: five cents for a package of three condoms or two cents for a single condom. Respondents were allowed to purchase condoms only from the 30 cents they had just been given in order to prevent condom purchases from being correlated with any monetary incentive received two months prior at the results center. The maximum number of condoms that could be purchased was 18.
C. Sample Attrition and Test Refusals
The sample used for the main analysis in this paper consists of 2,812 residents of rural Malawi who accepted an HIV test, who had provided basic demographic data during the 2004 survey (age, village location), and whose HIV test result was not indeterminant (14 individuals). Although the original sample in 1998 was randomly drawn, sample attrition across waves of data collection affects the degree to which this sample is representative. The primary reason for attrition across all waves of data is temporary and permanent migration. For example, in 2004, 21 percent of those interviewed in 2001 were away or had moved, which is comparable to the attrition rates due to out-migration of other longitudinal studies in Africa (Antony Chapoto and Thomas S. Jayne 2005; John Maluccio 2000; Simona Bignami–Van Assche, Reniers, and Weinreb 2003). No village ever refused to participate in data collection and less than 1 percent of those approached in 2004 refused to be interviewed. Despite the attrition across waves of the MDICP panel, baseline characteristics in 2004 are similar to those of a population-based survey that was also conducted in Malawi in 2004 (NSO Malawi 2005). In that survey, 72.8 percent of the women (age 15–49) living in rural Malawi were married or cohabitating with a partner (versus 71 percent in the MDICP 2004 data); 4.3 percent of women and 13.1 percent of men reported using a condom at last intercourse (versus 16.4 percent of the women and 27.3 percent of the men who reported using a condom in the previous year in the MDICP 2004 data); and 14 percent reported ever having an HIV test (versus 18 percent in the MDICP 2004 data). These comparisons provide support to the external validity of the findings of this study for other rural populations in Malawi, and perhaps other rural parts of Africa.
Test refusals may also be a source of bias: 9 percent of those approached refused to be tested for HIV. In comparison to other studies conducting HIV testing, such as the DHS Malawi (2004), this is a relatively low refusal rate. This may be attributable to the method of testing through saliva, or to the fact that respondents were not required to learn their results at the time of testing. Observable characteristics (such as gender, age, or marital status) are not significant predictors of accepting an HIV test. Respondents’ prior beliefs of infection status also do not predict refusing an HIV test (Table 1, panel B). See also Francis Obare (2006). Among the main sample who agreed to be tested, 18 percent reported having been tested previously for HIV, although only half of these individuals reported actually receiving their results. Those who reported having a previous test were 6 percentage points more likely to agree to be tested by the MDICP nurses (Table 1, panel B). Not all spouses of respondents were offered a test: men who divorced or whose spouse died and spouses of the newly sampled adolescents were ineligible for testing.
While attrition from the panel across years suggests that the sample has disproportionately fewer mobile individuals—perhaps leading to a downward bias in the HIV prevalence rate and posing a potential threat to external validity—the sample who accepted an HIV test is relevant from a policy perspective in that it represents those who would be present during an HIV testing campaign in rural areas.
The follow-up interviews were conducted among 75 percent of those in the main sample in Balaka and Rumphi. Learning HIV results and HIV status itself are both separately associated with attrition from the follow-up survey. Being HIV-positive increases the likelihood of attrition by 17 percentage points, which could be due to death or sickness (Table 1, panel C). Having obtained HIV results from the VCT center decreases the likelihood of attrition by 24 percentage points, which to a certain extent is mechanical since those who were available to attend the VCT center were also more likely to be available for the follow-up interview (if, for example, they had not temporarily migrated). Importantly, all exogenously assigned variables (receiving an incentive, the amount of the incentive, and the distance from home to the VCT center) have no significant direct effect on likelihood of attrition at the follow-up. Thus, while sample attrition and HIV test refusals may pose a potential threat to the external validity of the study, the lack of differential attrition associated with incentives or distance minimizes risk to the internal validity of the study (Table 1, panel C).
II. Learning HIV Results
A. Theoretical Considerations
To the extent that individuals use the knowledge of their HIV test results to alter their behavior, there could be positive effects from HIV testing on sexual behavior. Those diagnosed negative can practice safe sex to protect themselves from future infection; those diagnosed positive can seek treatment, and if altruistic, can prevent spreading the virus to children or sexual partners. Furthermore, knowing HIV status may allow individuals to more realistically and effectively plan for the future. However, while people with treatable diseases have strong motivations for testing and diagnosis, these incentives may be absent for people concerned about HIV because it is incurable. Also, in low-income countries access to antiretroviral therapies that would slow disease progress is often limited, further reducing the incentive to learn HIV results (Peter Glick 2005; Jo Stein 2005). Moreover, even when antiretroviral therapies are available, most patients must wait until they have severe symptoms before receiving treatment. The costs of testing and travel may also prevent individuals from learning their HIV status (Steven Forsythe et al. 2002; Laver 2001; Areleen A. Leibowitz and Stephanie L. Taylor 2007; M. Isabel Fernandez et al. 2005) although testing rates are usually low even when testing services are free or low-cost. For example, although HIV testing is free in Malawi, only 14 percent of respondents reported ever having been tested (Malawi DHS 2004). Even when individuals choose to be tested for HIV, many do not return for their results: in meta-studies of clinics across Africa, only approximately 65 percent of individuals who tested for HIV returned to learn their results (Michel Cartoux et al. 1998; Donatus Ekwueme et al. 2003).7
It is therefore commonly suggested that psychological costs are important, perhaps crucial, barriers to testing and learning results. The psychological costs associated with learning HIV results can be either internal, such as having worry or fear, or external, such as experiencing social stigma (HITS 2004; F. Mugusi et al. 2002; S. Ginwalla et al. 2002; Rachel Baggaley et al. 1998; Angela B. Hutchinson et al. 2004; Kathleen Ford et al. 2004; Djeneba Coulibaly et al. 1998; Seth C. Kalichman and Leickness C. Simbayi 2003; and Brent Wolff et al. 2005).8
Monetary incentives may operate through several mechanisms to motivate individuals to learn their HIV status through testing. Incentives may directly compensate for the costs of learning HIV results, including the monetary costs of time or travel, or psychological costs. Monetary incentives may also reduce actual or anticipated social stigma. For example, while others could interpret attending a VCT center as a signal of self-perceived risk of infection or of prior unsafe sexual behavior, monetary incentives may provide individuals with an excuse for going to the center, thereby reducing negative inferences made by others.
B. Impact of Incentives and Distance on Learning HIV Results
Across the three districts of the study area, 69 percent of all respondents attended a VCT center to obtain their HIV test results. Both incentives and distance to the center had large effects on seeking HIV results. Figure 3 presents the percent attending a results center as a function of receiving any incentive (panel A) and the total amount of the incentive (panel B). These figures illustrate the large impact of receiving an incentive on attendance. Error bars are also presented showing precisely estimated effects. Figure 4, panel A, presents the impact of distance of residence from the nearest results center on attendance, estimated by a nonparametric, locally weighted regression (Jianqing Fan 1992). Distance had a strong negative effect on attendance, especially pronounced among those living farther than 1.5 kilometers from the results center.
Figure 3. Percentage Returning for HIV Results.
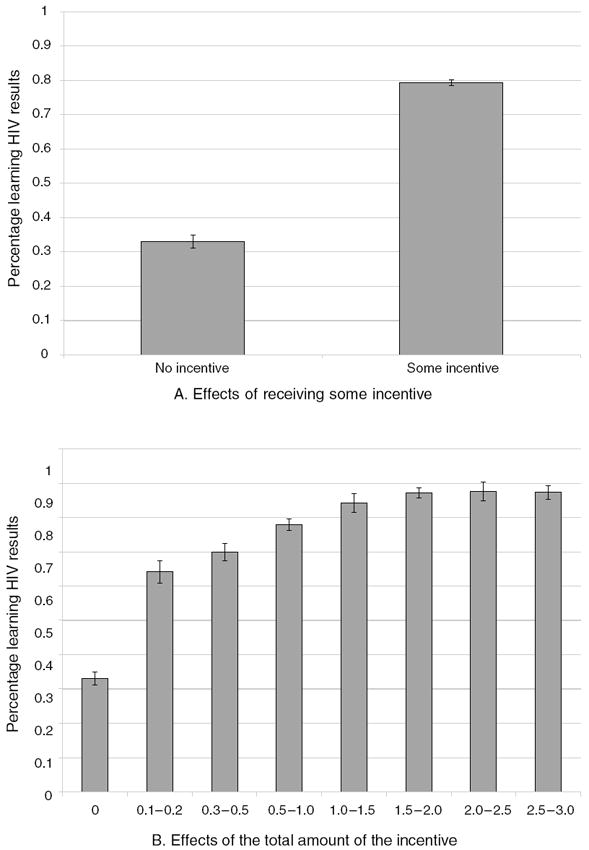
Notes: Sample includes 2,812 individuals who tested for HIV; 0.05 percent error bars are presented. Figures present the percentage of individuals attending HIV results centers.
Figure 4. Impact of Distance to VCT on Returning for HIV Results.
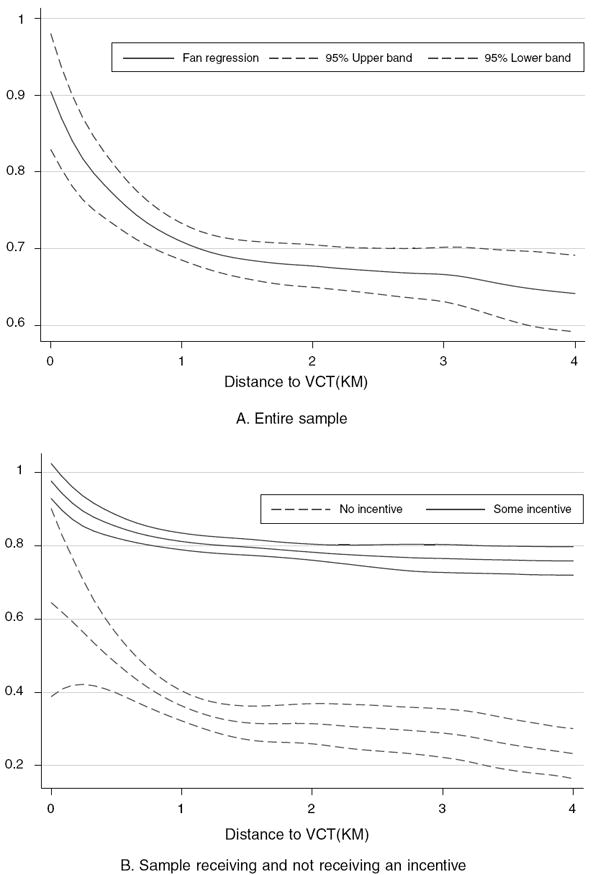
Notes: Nonparametric Fan regression where distance is measured as a straight-line spherical distance from a respondent’s home to randomly assigned VCT center from geospatial coordinates and is measured in kilometers. Sample includes 2,812 individuals who tested for HIV. Lines indicate percentage attending the results centers and upper and lower confidence intervals.
To measure the demand for learning HIV results in a regression framework, I estimate:
| (1) |
Attendance at the VCT center is indicated by GotResults = 1 for person i in village j. Any indicates if the respondent received any nonzero voucher and Amt is the dollar amount of the incentive. Including the binary indicator as well as the squared term allows for nonlinear effects of the incentive. Dist is the number of kilometers from the randomly placed VCT center assigned to person i. A vector of controls, X, includes covariates of gender, age, age-squared, HIV status, and district dummies, as well as a control for a simulated average distance in each VCT zone. Because the locations of the centers were chosen randomly, as opposed to randomly assigning the distance needed to travel, I draw 1,000 simulated random locations in each VCT zone and calculate the average distance of each tested respondent from each of the 1,000 simulated locations. I average this distance for each respondent and take the mean across all respondents living in each zone.9 Standard errors are clustered by village, for 119 villages.10 Although the dependent variable is binary, the linear specifications do not differ greatly from estimations from probits; both results are presented (see also Joshua D. Angrist 2001).
Seeking HIV results was highly responsive to incentives. The average VCT center attendance of those receiving any positive-valued voucher is more than twice that of those receiving no incentive, a difference of 43 percentage points (Table 4, column 1). Moreover, the probability of center attendance increased by an additional 9.1 percentage points with every additional dollar of incentive (Table 4, column 2), with the nonlinear effect of the incentive also important (Table 4, column 3). Distance and distance-squared also had a significant impact on the likelihood of obtaining HIV results (Table 4, column 4). Those living more than 1.5 kilometers from the center were 3.8 percentage points, or 6 percent, less likely to seek their HIV results than those living within 1.5 kilometers (Table 4, column 5). The effect of offering ten cents (as compared to offering no incentive) was greater than the effect of distance. The effects of incentives and distance are similar when using a nonlinear model (Table 4, columns 6–10).
Table 4.
Impact of Monetary Incentives and Distance on Learning HIV Test Results (Dependent variable: Attendance at HIV results centers)
| OLS
|
Marginal probit
|
|||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| (1) | (2) | (3) | (4) | (5) | (6) | (7) | (8) | (9) | (10) | |
| Any incentive | 0.431*** (0.023) | 0.309*** (0.026) | 0.218*** (0.029) | 0.220*** (0.029) | 0.219*** (0.029) | 0.438*** (0.022) | 0.279*** (0.030) | 0.175*** (0.033) | 0.181*** (0.033) | 0.178*** (0.033) |
| Amount of incentive | 0.091*** (0.012) | 0.274*** (0.036) | 0.274*** (0.035) | 0.273*** (0.036) | 0.115*** (0.016) | 0.311*** (0.042) | 0.309*** (0.042) | 0.309*** (0.043) | ||
| Amount of incentive2 | −0.063*** (0.011) | −0.063*** (0.011) | −0.063*** (0.011) | −0.070*** (0.014) | −0.069*** (0.014) | −0.069*** (0.014) | ||||
| HIV | −0.055* (0.031) | −0.052 (0.032) | −0.05 (0.032) | −0.058* (0.031) | −0.055* (0.031) | −0.062* (0.036) | −0.063* (0.038) | −0.06 (0.037) | −0.069* (0.037) | −0.066* (0.037) |
| Distance (km) | −0.076*** (0.027) | −0.094*** (0.034) | ||||||||
| Distance2 | 0.010** (0.005) | 0.013** (0.006) | ||||||||
| Over 1.5 km | −0.037 (0.023) | −0.044* (0.026) | ||||||||
| Male | −0.01 (0.019) | −0.013 (0.018) | −0.015 (0.018) | −0.015 (0.018) | −0.014 (0.018) | −0.011 (0.021) | −0.016 (0.021) | −0.018 (0.021) | −0.018 (0.021) | −0.018 (0.021) |
| Age | 0.005 (0.003) | 0.004 (0.003) | 0.004 (0.003) | 0.004 (0.003) | 0.004 (0.003) | 0.005 (0.004) | 0.005 (0.004) | 0.004 (0.004) | 0.005 (0.003) | 0.005 (0.003) |
| Simulated average distance | 0.005 (0.014) | −0.007 (0.012) | 0.005 (0.016) | −0.008 (0.014) | ||||||
| Rumphi | −0.137*** (0.029) | −0.155*** (0.029) | −0.161*** (0.029) | −0.151*** (0.028) | −0.156*** (0.029) | −0.163*** (0.037) | −0.187*** (0.037) | −0.195*** (0.037) | −0.184*** (0.036) | −0.189*** (0.037) |
| Balaka | −0.118*** (0.023) | −0.123*** (0.024) | −0.122*** (0.024) | −0.114*** (0.026) | −0.109*** (0.027) | −0.144*** (0.031) | −0.149*** (0.032) | −0.148*** (0.033) | −0.139*** (0.036) | −0.132*** (0.036) |
| Constant | 0.346*** (0.059) | 0.365*** (0.058) | 0.370*** (0.058) | 0.442*** (0.067) | 0.395*** (0.060) | −0.144*** (0.031) | −0.149*** (0.032) | −0.140*** (0.034) | −0.133*** (0.035) | |
| Sample size | 2,812 | 2,812 | 2,812 | 2,812 | 2,812 | 2,812 | 2,812 | 2,812 | 2,812 | 2,812 |
| R2 | 0.18 | 0.2 | 0.21 | 0.21 | 0.21 | 0.14 | 0.16 | 0.17 | 0.17 | 0.17 |
| Average attendance | 0.69 | 0.69 | 0.69 | 0.69 | 0.69 | 0.69 | 0.69 | 0.69 | 0.69 | 0.69 |
Notes: Sample includes individuals who tested for HIV and have demographic data. Columns 1–5 represent OLS coefficients and columns 6–10 represent marginal probit coefficients (dprobit); robust standard errors clustered by village (for 119 villages) with district fixed effects in parentheses. All specifications also include a term for age-squared. “Any incentive” is an indicator if the respondent received any nonzero monetary incentive. “HIV” is an indicator of being HIV positive. “Simulated average distance” is an average distance of respondents’ households to simulated randomized locations of HIV results centers. Distance is measured as a straight-line spherical distance from a respondent’s home to a randomly assigned VCT center from geospatial coordinates and is measured in kilometers.
Significantly different from zero at 99 percent confidence level.
Significantly different from zero at 95 percent confidence level.
Significantly different from zero at 90 percent confidence level.
VCT center attendance varied by HIV status. Overall, HIV-positive individuals were approximately 5.5 percentage points less likely to obtain their results (Table 4, column 1). In the district with the highest HIV prevalence rate, Balaka, those infected with HIV were 11 percentage points less likely to attend the center than those who were HIV-negative (standard error 0.043; Table 5, column 3). Overall, men were no more likely to attend than women. Age also had no impact on obtaining HIV results. Opportunity costs may also be important. For example, ever attending school reduced the probability of attending the VCT by 3.2 percentage points (standard error 0.019; regression not shown). VCT attendance varied by district and the coefficients on the district fixed-effects are significant (Table 4). Overall, 80 percent attended the result centers in Mchinji, 71 percent attended in Balaka, and 59 percent attended in Rumphi. These differences may be due, at least in part, to the timing of the test results availability, which coincided with planting season in Balaka and Rumphi and thus may have resulted in lower average attendance rates in these districts. Without more detailed time use data, however, the effects of the agricultural season cannot be distinguished from effects of characteristics that vary systematically by district.
Table 5.
Covariates and Interaction Effects with Incentives and Distance (Dependent variable: Attendance at HIV results centers)
| All respondents | Unmarried | Balaka | All respondents | |||
|---|---|---|---|---|---|---|
| (1) | (2) | (3) | (4) | (5) | (6) | |
| Any incentive | 0.229*** (0.030) | 0.144*** (0.065) | 0.393*** (0.037) | 0.198*** (0.043) | 0.220*** (0.029) | 0.224*** (0.030) |
| Amount of incentive | 0.257*** (0.038) | 0.345*** (0.071) | 0.276*** (0.049) | 0.273*** (0.036) | 0.272*** (0.036) | 0.273*** (0.036) |
| Amount of incentive2 | −0.055*** (0.012) | −0.089*** (0.024) | −0.056*** (0.016) | −0.063*** (0.011) | −0.062*** (0.011) | −0.062*** (0.011) |
| Male | −0.004 (0.018) | 0.03 (0.036) | 0.114 (0.077) | −0.014 (0.018) | −0.015 (0.018) | −0.014 (0.018) |
| HIV | −0.045 (0.031) | 0.034 (0.078) | −0.114** (0.042) | −0.056* (0.031) | −0.023 (0.038) | −0.004 (0.074) |
| Over 1.5 km | −0.039* (0.023) | −0.007 (0.033) | −0.065 (0.041) | −0.064 (0.043) | −0.032 (0.024) | −0.036 (0.023) |
| Had previous HIV test | −0.019 (0.024) | |||||
| Treatment for HIV will be available | 0.005 (0.018) | |||||
| Married | 0.051*** (0.018) | |||||
| Never had sex | −0.037 (0.077) | |||||
| Never had sex × any | 0.107 (0.079) | |||||
| Any incentive × male | −0.161** (0.066) | |||||
| Over 1.5 km × any | 0.037 (0.044) | |||||
| HIV × any | −0.067 (0.062) | |||||
| Over 1.5 km × HIV | −0.066 (0.083) | |||||
| Constant | 0.452*** (0.037) | 0.482*** (0.065) | 0.224*** (0.055) | 0.412*** (0.065) | 0.393*** (0.061) | 0.392*** (0.060) |
| Sample size | 2,635 | 810 | 1,037 | 2,812 | 2,812 | 2,812 |
| R2 | 0.21 | 0.22 | 0.20 | 0.21 | 0.21 | 0.21 |
| Average attendance | 0.69 | 0.66 | 0.71 | 0.69 | 0.69 | 0.69 |
Notes: Sample includes individuals who tested for HIV and have age data. Columns represent OLS coefficients; robust standard errors clustered by village (for 119 villages) with district fixed effects in parentheses. “Any” is an indicator if the respondent received any nonzero monetary incentive and “amount” is the total value of the incentive. “HIV” is an indicator of being HIV positive. Also includes a simulated average distance (average distance of respondents’ households to simulated randomized locations of HIV results centers), age, and age-squared. Distance is measured as a straight-line spherical distance from a respondent’s home to randomly assigned VCT center from geospatial coordinates and is measured in kilometers.
Significantly different from zero at 99 percent confidence level.
Significantly different from zero at 95 percent confidence level.
Significantly different from zero at 90 percent confidence level.
Theoretically, those who are most uncertain of their HIV status would expect to gain the most from learning their results since those most certain of their status would expect to receive no new information. However, those who reported having had a previous test for HIV were no less likely to attend a VCT center than their untested counterparts—although among those who had been tested, only 54 percent reported knowing their HIV results. The likelihood of attendance was not significantly affected by having learned HIV test results prior to the 2004 MDICP survey (not shown).
The likelihood of seeking HIV results at a center was also not affected by respondents’ belief in the near-term availability of treatment for HIV, nor a belief of their own infection probability. Respondents were asked: “Do you think treatment for HIV (ARVs) will become available to most people in your area in the next five years?” In all, 34 percent reported believing treatment would be available for HIV-positive persons (Table 2, panel B) but rate of attendance did not vary among those who believed treatment would be available and those who did not (Table 5, column 1). Individuals were also asked: “What is the chance that you are infected with HIV?” Possible answers were “no likelihood,” “some likelihood,” “high likelihood,” and “don’t know.” There were no significant differences in attendance between individuals having varying subjective probabilities of infection (not shown). One reason why attendance did not vary with prior beliefs may be that respondents’ priors may be unreliable. There is evidence that respondents had a tendency to overestimate their own likelihood of HIV infection (Philip Anglewicz and Hans-Peter Kohler 2006; Bignami–Van Assche et al. 2005; see also Table 10 and discussion below).
Table 10.
Average Belief of Likelihood of Infection before and after VCT (Dependent variable: Likely to be HIV infected)
| Panel A: HIV negative | |||||||
|---|---|---|---|---|---|---|---|
| Baseline survey | Follow-up survey
|
||||||
| (1) | Did not get results (2) | Got results (3) | Difference (4) | ||||
| 0.43 | (0.49) | 0.49 | (0.50) | 0.13 | (0.33) | −0.38*** | (0.03) |
|
| |||||||
| Panel B: HIV positive | |||||||
| Baseline survey | Follow-up survey
|
||||||
| (1) | Did not get results (2) | Got results (3) | Difference (4) | ||||
|
| |||||||
| 0.58 | (0.50) | 0.46 | (0.51) | 0.5 | (0.51) | 0.03 | (0.12) |
Notes: Sample includes respondents in Balaka and Rumphi who were tested for HIV and were reinterviewed in 2005. Robust standard errors clustered by village with district fixed effects are in parentheses. Controls also include gender, age, and age squared. “Likely to be infected” is equal to zero if the respondent reported no likelihood of HIV infection, and one otherwise. Sample includes 2,428 HIV-negatives and 165 HIV-positives in the baseline survey and 1,430 HIV-negatives and 72 HIV-positives in the follow-up survey.
Significantly different from zero at 99 percent confidence level.
Significantly different from zero at 95 percent confidence level.
Significantly different from zero at 90 percent confidence level.
Those who had never had sex would have little reason to attend the VCT center, except to redeem a monetary voucher. Although not statistically significant, the impact of receiving an incentive is much more elastic among unmarried respondents who report never having had sex, who were 11 percentage points more likely to attend a center (and redeem their voucher) than those who ever had sex, and who also received an incentive (Table 5, column 2). Alternatively, those who were married in 2004 were 5.1 percentage points more likely to return for their results than those who were not married (Table 5, column 1).
Receiving a voucher may have provided justification for some individuals to attend the center. For example, because of historical gender restrictions within Islam, women in Balaka may have more travel restrictions, preventing them from easily attending the results centers.11 In Balaka, men receiving no incentive were significantly more likely to attend the center than women receiving no incentive. However, men and women receiving an incentive are equally likely to attend, closing the gender gap of attendance in Balaka (Table 5, column 3). In Mchinji and Rumphi there were no differential impacts of the incentive on attendance between men and women.
Monetary incentives were also especially important for those living farther from the VCT center: for those living over 1.5 kilometers from the HIV results center, there was an additional impact of receiving an incentive, increasing attendance by 3.7 percentage points, although the difference is not statistically significant (Table 5, column 4). This effect can also be seen in Figure 4, panel B, which graphs the impact of distance on attendance among those receiving any incentive and those receiving no incentive. Despite the interaction between distance and incentives, evidence suggests an upper bound to the distance that individuals are willing to travel to learn their results, regardless of the incentives offered. Several VCT centers that were initially placed more than nine kilometers from sample households had no attendance for several days. These centers were re-sited at new random locations and respondents were informed of the new locations. These distant locations are excluded from the analysis. This suggests that in the absence of monetary incentives, distance and transaction costs may be the strongest contributing factors to low rates of obtaining results. On the other hand, it may be that increased distance also increased the likelihood that respondents did not know the location of the VCT sites, confounding the interpretation that travel costs alone reduce attendance.
HIV-positive individuals receiving an incentive were less likely to seek their test results than those who were HIV-negative (Table 5, column 5). There was also an effect of being HIV positive and living farther away from the VCT center: HIV-positives living over 1.5 kilometers from the center were approximately 6.6 percentage points less likely to attend than HIV-negatives living over 1.5 kilometers, perhaps because they were more likely to be sick, making travel more costly (Table 5, column 6). However, these differences were not statistically significant, likely due in part to the small number of HIV-positive individuals.
In sum, both monetary incentives and distance had large impacts on obtaining HIV results. Many have suggested that psychological barriers, such as fear or stigma, play an important role in deterring individuals from testing (or seeking test results). I find, however, that these barriers, if they exist at all, can be easily overcome by offering small cash rewards. Incentives may compensate individuals directly for their cost of obtaining results or serve as a public justification for attending a results center, indirectly reducing psychological costs. One woman’s remark, overheard by an interviewer, is especially illustrative:
Those who were lucky were picking [vouchers] with some figures and those were courageous to go and check their blood test results because they were also receiving their money there so like me where I got a zero. I did not even go and check the results because I knew that there was nothing for me there (Malawi Diffusion Ideational Change Project 2004).
These findings reemphasize the importance of economic costs such as travel or opportunity costs in decision making and the effects of monetary incentives to overcome these costs.
III. Impact of Learning HIV Status
A. Theoretical and Measurement Considerations
In a standard expected utility framework, individuals do not receive any additional utility from learning their HIV status. Testing is beneficial only to the extent that it provides new information that can be used for updating behavior.12 However, it is theoretically ambiguous how the knowledge of HIV status affects sexual behavior and, in particular, the demand for condoms. For those diagnosed HIV-positive, the direct benefits of using condoms fall because (if safe sex is costly) there is no longer any need of protection. However, HIV-positive individuals who are sufficiently altruistic may exhibit a higher demand for condoms after learning their status. On the other hand, if infected individuals behave selfishly, there may be a decrease in the demand for condoms (Stephane Mechoulan 2004). For HIV-negatives, it is similarly ambiguous: the benefit from using condoms—i.e., to remain uninfected—may increase after diagnosis; however, the lack of need to protect a sexual partner may cause condom use to fall.13 Thus, how learning HIV results affects sexual behavior is ultimately an empirical question.
Previous studies examining the effects of HIV testing on sexual behavior have not only been inconclusive, but also suffer from selection bias in terms of which individuals chose to learn their results.14 In this study, however, the randomly assigned VCT center distances and monetary incentives serve as exogenous instruments for knowledge of status. Nevertheless, several estimation challenges and limitations remain.
One challenge to measuring the demand for safe sex in response to HIV testing is that sexual behavior is difficult to measure and self-reports may be unreliable. Prior research has found that sexual behavior, such as number of sexual partners, is likely to be underreported and that contraceptive use is sometimes overreported (Landon Myer, C. Morroni, and B. G. Link 2004; Susan Allen et al. 2003). Because of the potential bias of self-reports, the primary outcome variable used to measure the demand for safe sex in this paper is the actual purchase of condoms from survey interviewers. To mitigate potential reporting biases during follow-up interviews, every effort was made to conduct interviews privately with an interviewer of the same sex as the respondent, who had no part in the HIV testing or in giving results.
It is important to keep in mind that condom purchases may not reflect the true demand for safe sex. If knowledge of HIV status increases abstinence, the demand for condoms could fall in response to obtaining test results. Also, the impact of learning HIV status on condom purchases is likely to differ between those with a sexual partner and those without. And because partnership may be endogenous to learning HIV results (Georges Reniers 2005), I differentiate between those who were and who were not sexually active at the time of the baseline survey (before testing). It is worth noting, however, that there were no significant changes in marriage or partnership attributable to learning HIV status between the time of the HIV results being available and the follow-up survey (not shown).
B. Summary Statistics of Sexual Activity and Condom Use
In the baseline 2004 survey, 76 percent of the follow-up sample reported having had sex during the previous year (Table 2, panel B). In the follow-up 2005 survey, 62 percent of the sample reported having had sex during the previous two months (Table 2, panel D). Of those who reported having sex, only 3.3 percent reported having sex with more than one partner, although the majority of these individuals were polygamous men. At the time of the follow-up survey, respondents were also asked if they had purchased condoms at any time between the opportunity to obtain test results and the follow-up interviews: only 8 percent of the sample reported purchasing condoms during those two months. In terms of the subsidized condoms that were offered at the end of the follow-up interview, 24 percent purchased at least one condom; of those who purchased any condoms, the average number purchased was 3.7 (Table 2, panel D). This rate of condom purchases is similar to the condom use reported in the 2004 main survey, where 21 percent of all respondents reported using a condom with a sexual partner during the prior year (Table 2, panel B). Only three individuals purchased the maximum possible number of condoms. Men were more likely to purchase than women: 31 percent of men purchased condoms while 18 percent of women purchased at least one condom. However, conditional on purchasing any condoms, men and women purchase the same number, on average (not shown). Among HIV-positives, men and women were equally likely to purchase condoms at the follow-up survey. Among all respondents, married and unmarried respondents were equally likely to purchase condoms. However, purchase of condoms did vary by reported sexual activity: condoms were purchased by 26 percent of respondents who in 2004 reported having had sex in the prior year as opposed to 21 percent of those who reported not having had sex.
C. Receiving HIV-Positive and Negative Diagnoses
Panel A of Figure 5 presents the percent purchasing condoms among those who knew and did not know their HIV status. For HIV-positive respondents, those who obtained their test results were more than twice as likely to purchase condoms as those who did not, while among HIV-negative individuals, condom purchase did not vary significantly by knowledge of HIV status (Figure 5, panel B).
Figure 5. Percentage Purchasing Condoms.
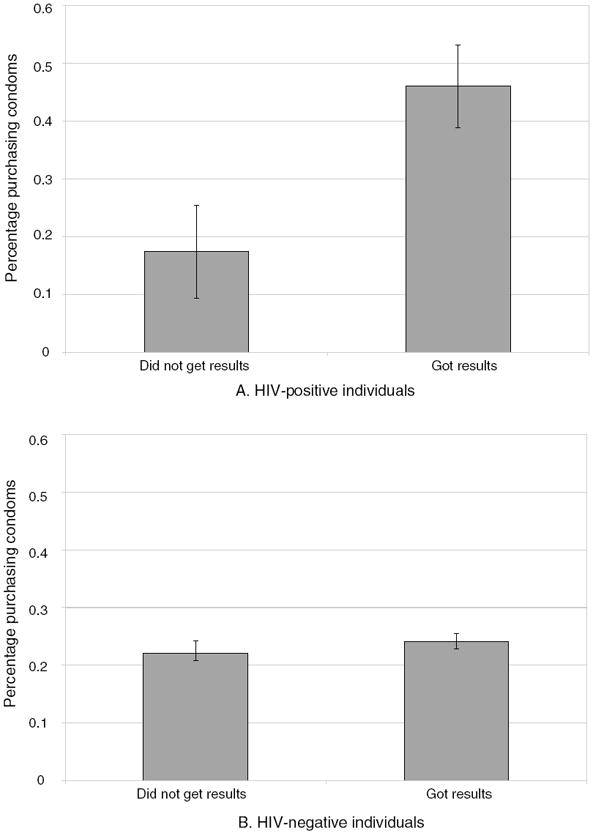
Notes: Sample includes 1,524 respondents in Balaka and Rumphi who were tested for HIV and reinterviewed in 2005 who reported having sex during 2004 (at the baseline). Figures present the percent purchasing condoms at a follow-up interview two months after testing.
I measure the effects of learning HIV results with the following regression:
| (2) |
Y indicates condom purchase at the time of the follow-up survey (as measured by whether the respondent purchased condoms or the total number of condoms purchased) or if the respondent reported having sex; and GotResults indicates knowledge of HIV status. The fact that individuals choose to learn their HIV status means that OLS estimates are likely to be biased, but estimating the effects of knowing HIV status with exogenous instruments provides unbiased estimates. In particular, I instrument GotResults with being offered any incentive, the amount of the incentive, the amount of the incentive squared, the distance from the HIV result center, and distance-squared. Table 6 presents first-stage OLS regressions; the F-statistics for this specification and sample of sexually active respondents are equal to 74.98 and 193.29, respectively. To account for differential effects among men and women, I also include interactions with gender:
| (3) |
Table 6.
First Stage
| Got results (1) | HIV × got results (2) | |
|---|---|---|
| Any incentive | 0.153* (0.081) | −0.001 (0.004) |
| Amount of incentive | 0.309*** (0.089) | 0.001 (0.002) |
| Amount of incentive2 | −0.067** (0.026) | 0.000 (0.001) |
| Distance (km) | −0.117* (0.062) | 0.003 (0.005) |
| Distance2 | 0.018 (0.011) | 0.000 (0.001) |
| Any × male | 0.047 (0.104) | −0.001 (0.006) |
| Amount × male | −0.058 (0.130) | −0.001 (0.002) |
| Amount2 × male | 0.023 (0.040) | 0.000 (0.001) |
| Distance × male | 0.199** (0.076) | −0.003 (0.011) |
| Distance2 × male | −0.033** (0.013) | 0.001 (0.002) |
| Any × male × HIV | −0.161 (0.442) | −0.185 (0.392) |
| Amount × male × HIV | −0.714 (0.638) | −0.778 (0.592) |
| Amount2 × male × HIV | 0.195 (0.177) | 0.23 (0.172) |
| Distance × male × HIV | 0.401** (0.193) | 0.385** (0.184) |
| Distance2 × male × HIV | −0.059 (0.036) | −0.054 (0.036) |
| Any × HIV | −0.067 (0.235) | 0.165 (0.213) |
| Amount × HIV | 0.750* (0.377) | 1.035*** (0.350) |
| Amount2 × HIV | −0.235** (0.115) | −0.301*** (0.109) |
| Distance × HIV | −0.171 (0.237) | −0.221 (0.224) |
| Distance2 × HIV | 0.034 (0.046) | 0.04 (0.045) |
| HIV | −0.231 (0.275) | 0.236 (0.259) |
| Male | −0.279** (0.112) | 0.004 (0.018) |
| Constant | 0.406*** (0.103) | 0.026* (0.015) |
| Observations | 1,008 | 1,008 |
| R2 | 0.22 | 0.76 |
| F–statistic | 74.98 | 193.29 |
Notes: Sample includes individuals who tested for HIV, have age data, and who had sex in 2004. Columns represent OLS coefficients; robust standard errors clustered by village (for 57 villages) with district fixed effects. “Any incentive” is an indicator if the respondent received any nonzero incentive. “HIV” indicates being HIV positive. Distance is measured as a straight-line spherical distance from a respondent’s home to randomly assigned VCT center from geospatial coordinates and is measured in kilometers. Also includes controls for age, age-squared, simulated average distance, and a district fixed effect.
Significantly different from zero at 99 percent confidence level.
Significantly different from zero at 95 percent confidence level.
Significantly different from zero at 90 percent confidence level.
In equation (2), GotResults×HIV represents the differential effect of receiving an HIV-positive diagnosis, and this is instrumented with the same set of instruments as in (3) above, interacted by HIV status. Because the monetary incentives and distances to VCT centers were both exogenously assigned to each individual, they are uncorrelated with the error term. In the analysis, although the measure of purchasing condoms is binary, estimates do not differ greatly from a nonlinear specification. Both specifications are presented.
Covariates, X, include age, age-squared, a dummy for male, simulated average distance to the VCT center, and district dummies. It is also important to point out that the IV estimates in (2) are local average treatment effects (LATE), which represent a weighted average per-unit treatment effect, where the weight is proportional to the number of people affected by the instruments, not necessarily equal to the average treatment effect (Guido W. Imbens and Angrist 1994; Angrist and Imbens 1995; Angrist et al. 1996). Also, while I account for some heterogeneous treatment effects by including interactions with gender, there may be other differential effects of the incentive and distance that are not included in the IV analysis (see Imbens and Angrist 1994; Heckman and Jeffrey Smith 1997; Heckman 2001; Heckman et al. 2006).15
I first examine the effects of receiving an HIV diagnosis on condom purchases and reported sexual activity among those who reported having sex at the time of the baseline survey: 63 percent of HIV-negative and 66 percent of HIV-positive individuals.16 Note that this reduces the sample of HIV-positives for the analysis and thus much of the focus of the analysis is among the HIV-negatives or pooled regressions. Row 1 of Table 7 shows no significant effects of learning HIV-negative results on any measure of condom purchase or recent sexual activity among those who had sex at baseline (Table 7, columns 2, 4, 6, and 8). However, the standard errors of the IV estimates are large, making it impossible to reject “no impact of learning HIV-negative status.”
Table 7.
Reactions to Learning HIV Results among Sexually Active at Baseline
| Dependent variables: | Bought condoms | Number of condoms bought | Reported buying condoms | Reported having sex at follow-up | ||||
|---|---|---|---|---|---|---|---|---|
| OLS (1) | IV (2) | OLS (3) | IV (4) | OLS (5) | IV (6) | OLS (7) | IV (8) | |
| Got results | −0.022 (0.025) | −0.069 (0.062) | −0.193 (0.148) | −0.303 (0.285) | −0.006 (0.018) | 0.017 (0.050) | 0.016 (0.032) | −0.004 (0.060) |
| Got results × HIV | 0.418*** (0.143) | 0.248 (0.169) | 1.778*** (0.564) | 1.689** (0.784) | 0.098 (0.084) | −0.027 (0.092) | −0.072 (0.121) | −0.079 (0.229) |
| HIV | −0.175** (0.085) | −0.073 (0.123) | −0.873*** (0.275) | −0.831 (0.375) | −0.029 (0.055) | 0.053 (0.074) | −0.043 (0.112) | −0.041 (0.156) |
| Male | 0.114*** (0.026) | 0.111*** (0.026) | 0.413*** (0.116) | 0.408*** (0.116) | 0.116*** (0.021) | 0.117*** (0.021) | −0.02 (0.028) | −0.021 (0.028) |
| Age | −0.007 (0.005) | −0.007 (0.005) | −0.03 (0.029) | −0.029 (0.028) | −0.004 (0.003) | −0.005 (0.003) | 0.027*** (0.005) | 0.027*** (0.005) |
| Age2 | 0.000 (0.000) | 0.000 (0.000) | 0.000 (0.000) | 0.000 (0.000) | 0.000 (0.000) | 0.000 (0.000) | −0.000*** (0.000) | −0.000*** (0.000) |
| Simulated average distance | −0.048** (0.022) | −0.048** (0.021) | −0.153** (0.065) | −0.154** (0.065) | −0.008 (0.012) | −0.008 (0.012) | 0.028** (0.013) | 0.028** (0.013) |
| Rumphi | −0.349*** (0.037) | −0.355*** (0.039) | −1.065*** (0.129) | −1.078*** (0.123) | −0.046** (0.023) | −0.044** (0.021) | −0.068** (0.029) | −0.070** (0.029) |
| Constant | 0.728*** (0.109) | 0.764*** (0.118) | 2.688*** (0.474) | 2.761*** (0.539) | 0.216*** (0.069) | 0.206** (0.076) | 0.207** (0.098) | 0.220** (0.101) |
| Sample Size | 1,008 | 1,008 | 1,008 | 1,008 | 1,008 | 1,008 | 1,008 | 1,008 |
| R2 | 0.19 | 0.18 | 0.10 | 0.10 | 0.06 | 0.06 | 0.04 | 0.02 |
| Mean | 0.26 | 0.26 | 0.95 | 0.95 | 0.09 | 0.09 | 0.73 | 0.73 |
| p-Statistic (got results + got results × HIV = 0) | 0.01 | 0.30 | 0.01 | 0.07 | 0.26 | 0.90 | 0.64 | 0.69 |
Notes: Sample includes HIV-positive and negative respondents in Balaka and Rumphi who were tested for HIV, were reinterviewed in 2005, and who had sex in 2004. Robust standard errors clustered by village (for 57 villages) with district fixed effects are in parentheses. Simulated average distance variable (an average distance of respondents’ households to simulated randomized locations of HIV results centers). Coefficients are either OLS or IV estimates where knowing HIV status is instrumented by having any positive-valued incentive, the total amount of the incentive, total amount squared, distance from the HIV results center, distance-squared, and all terms interacted with gender and HIV status. “Bought condoms” is an indicator if any condoms were purchased from interviewers at the follow-up survey; “number of condoms” is the total number of condoms purchased from interviewers; “reported buying condoms” and “reported having sex” were asked at the follow-up interview in 2005 and refer to the previous two months since the VCT was available.
Significantly different from zero at 99 percent confidence level.
Significantly different from zero at 95 percent confidence level.
Significantly different from zero at 90 percent confidence level.
Overall, while HIV-positive individuals were significantly less likely to purchase condoms from the survey interviewers or report purchasing condoms on their own, receiving an HIV-positive diagnosis resulted in an increase in condom purchases. Among those with a sexual partner at the time of the 2004 baseline survey, those obtaining HIV-positive results were 25 percentage points more likely to purchase condoms than HIV-positive persons who did not learn their results (although statistically insignificant in the IV specification of Table 7, column 2). This result is statistically significant when measuring the total number of condoms purchased as an outcome, where the average number of condoms purchased increased by 1.69 condoms after receiving an HIV-positive diagnosis (Table 7, column 4). The p-value of the total impact of receiving an HIV-positive diagnosis is 0.07, presented in Table 7.
Recall that the condoms sold during the follow-up study were subsidized in price and were offered for purchase at the respondent’s home–significantly lowering the cost of travel to obtain condoms at local stores. In regards to the nonsubsidized condoms, respondents were asked if they had purchased any condoms during the two months after that test results were available at the VCT centers. There is a positive, although statistically insignificant, relationship between receiving an HIV-positive test result and the reported purchase of nonsubsidized condoms during the two months after HIV test results were available in the OLS, but not in the IV (Table 7, columns 5 and 6). There is also a negative (also statistically insignificant) relationship between receiving HIV-positive results on the probability of having sex at follow-up (Table 7, column 8).
Theoretically, if individuals who were more likely to practice safe sex were also more likely to choose to learn their HIV status and also purchase more condoms at the follow-up, not accounting for selection bias would overestimate the true impact of learning HIV results on later sexual behavior. However, comparing the OLS estimates to the IV estimates of the impact of learning HIV results on condom purchases (Table 7) indicates no consistent pattern across each column, and the differences between the OLS and IV estimates are neither large nor statistically significant for either HIV-negative or HIV-positive individuals (not shown).
Several of the outcome variables in Table 7 are binary, possibly warranting a nonlinear estimation strategy. However, estimation of binary regression models with binary endogenous variables is difficult and there are often problems with convergence or concavity of the log likelihood surface (David A. Freedman and Jasjeet S. Sekhon 2008; see also Edward Vytlacil and Nese Yildiz 2007; Jacob Nielsen Arendt and Anders Holm 2006). One strategy suggested by Heckman (1978) is to estimate a bivariate probit model. In order to estimate the effects of learning HIV results on the likelihood of purchasing any condoms using this nonlinear strategy, it is necessary to divide the sample by HIV status and estimate the effect of obtaining HIV test results on positive and negative individuals separately. Because the sample is not pooled among HIV-positives and HIV-negatives, the nonlinear estimates presented in Table 8 are not directly comparable to those in Table 7. I therefore present linear OLS and IV estimates as well as the marginal probit and marginal biprobit (accounting for endogeneity) estimates on the impact of learning HIV results on condom purchases among HIV-positives and negatives.17
Table 8.
Probit Estimates: Reactions to Learning HIV Results
| Dependent variable: Bought any condoms | ||||
|---|---|---|---|---|
| OLS (1) | IV (2) | Probit (marginal effects) (3) | Bivariate probit (marginal effects) (4) | |
| Panel A: HIV positive | ||||
| Got results | 0.427*** (0.149) | 0.273 (0.200) | 0.437*** (0.138) | 0.370*** (0.141) |
| Male | 0.038 (0.115) | 0.024 (0.117) | 0.043 (0.129) | 0.026 (0.131) |
| Age | 0.001 (0.040) | −0.018 (0.043) | 0.004 (0.039) | −0.009 (0.038) |
| Age2 | 0.000 (0.001) | 0.000 (0.001) | 0.000 (0.000) | 0.000 (0.000) |
| Simulated average distance | 0.025 (0.074) | 0.026 (0.072) | 0.026 (0.074) | 0.025 (0.074) |
| Rumphi | −0.084 (0.179) | −0.08 (0.177) | −0.126 (0.195) | −0.117 (0.193) |
| Constant | 0.055 (0.823) | 0.511 (0.974) | ||
| Sample size | 52 | 52 | 52 | 52 |
| R2 | 0.21 | 0.18 | 0.18 | |
| Mean | 0.38 | 0.38 | 0.38 | 0.38 |
|
| ||||
| Panel B: HIV negative | ||||
| Got results | −0.023 (0.025) | −0.072 (0.061) | −0.033 (0.029) | −0.128 (0.088) |
| Male | 0.113*** (0.028) | 0.111*** (0.027) | 0.118*** (0.029) | 0.114*** (0.029) |
| Age | −0.007 (0.005) | −0.006 (0.005) | −0.005 (0.005) | −0.004 (0.005) |
| Age2 | 0.000 (0.000) | 0.000 (0.000) | 0.000 (0.000) | 0.000 (0.000) |
| Simulated average distance | −0.052** (0.022) | −0.053** (0.022) | −0.043** (0.018) | −0.044** (0.018) |
| Rumphi | −0.362*** (0.040) | −0.368*** (0.041) | −0.356*** (0.036) | −0.365*** (0.037) |
| Constant | 0.754*** (0.109) | 0.784*** (0.116) | ||
| Sample size | 956 | 956 | 956 | 956 |
| R2 | 0.19 | 0.19 | 0.18 | |
| Mean | 0.25 | 0.25 | 0.25 | 0.25 |
Notes: Sample includes HIV-positive and negative respondents in Balaka and Rumphi who were tested for HIV, were reinterviewed in 2005, and who had sex in 2004. Robust standard errors clustered by village (for 57 villages) with district fixed effects are in parentheses. Coefficients in column 1 are linear OLS estimates. Coefficients in column 2 are linear IV estimates where knowing HIV status is instrumented by having any nonzero incentive, the total amount of the incentive, total amount squared, distance from the HIV results center, distance-squared, and all terms interacted with gender. Column 3 presents marginal probit estimates, which do not control for endogenous selection into learning HIV results. Column 4 presents the marginal effects of a seemingly unrelated bivariate probit, controlling for endogenous selection into learning HIV results (see text).
Significantly different from zero at 99 percent confidence level.
Significantly different from zero at 95 percent confidence level.
Significantly different from zero at 90 percent confidence level.
The linear results in column 2, Table 8, do not differ substantively from the nonlinear biprobit specification in column 4, Table 8; however, the magnitudes of the coefficients and statistical significance do vary slightly. In particular, while the coefficient on GotResults does not differ greatly among the HIV-negatives (Table 8, panel B), among HIV-positives the coefficient in the nonlinear case is statistically significant at the 99 percent level (Table 8, panel A). The coefficient is also greater in the bivariate probit specification than in the linear model, suggesting an even larger effect of learning HIV-positive results on the likelihood of purchasing condoms. On the other hand, the difference between the two specifications may be due in part to the small sample size of HIV-positives. Given the difficulties with this estimation, including in particular the fact that it is necessary to separate the samples when doing the biprobit estimation, the linear IV specification in Table 7 remains the preferred estimation model.18
The results in Table 7 and Table 8 together suggest that overall, while there was no impact of receiving an HIV-negative diagnosis, receiving an HIV-positive diagnosis had positive effects on subsequent condom purchases. In the linear specification, the estimated impact of learning HIV-positive results on the likelihood of purchasing any condoms is large, although statistically insignificant at traditional confidence levels (Table 7, column 2). However, there is a statistically significant effect of learning HIV-positive results on the total number of condoms purchased (Table 7, column 4). In the nonlinear case, the coefficient on GotResults is statistically significant and large (Table 8, column 4): an HIV-positive individual who learned his HIV status was 37 percentage points more likely to purchase condoms than an HIV-positive individual who did not learn his results.
D. Other Considerations
It is possible that respondents who knew themselves to be HIV-positive were motivated to purchase condoms solely out of guilt, believing (incorrectly) that the interviewer knew their status. If this were the case, they may have purchased only one condom as a token, keeping the remaining money. However, only two of all the HIV-positives purchased a single condom; and omitting these individuals or coding their purchases as zero does not affect any of the results. Although impossible to rule out, this suggests that guilt or shame may not have been a large factor in the observed increase in condom sales among HIV-positives learning their results. Moreover, if HIV-positives purchased condoms solely out of guilt or social pressure, this would mean that the results of these analyses are upper bounds of the true impact of learning HIV status. Another consideration is that the outcome variable is condoms purchased rather than condoms used during sexual activity—which would also contribute to the results being an upward bias of the actual effect of learning HIV-positive results.19 It is also possible that respondents purchased condoms with the intention of reselling the condoms, rather than using them. To the extent that resale is uncorrelated with the randomly assigned incentives and distance, this would not threaten the internal validity of results.
There were no differential effects of learning HIV-negative status on condom purchases between those who were and were not sexually active at the baseline (Table 9). There were also no differential effects of learning HIV status (either positive or negative) on condom purchases among other observable respondent characteristics. For example, although men on average were more likely to purchase condoms than women, there were no differences in the impact of learning HIV status on purchases by gender. There were also no differences by age, or ever having attended school (not shown). Learning HIV-positive or negative results did not significantly affect having discussions with friends or spouses about condoms or AIDS. Respondents were also asked about their attitudes about condoms. These attitudes were strong determinants of purchasing condoms; for example, those who “agreed” that condoms were acceptable to use with a spouse were twice as likely to purchase condoms as those who “disagreed.” However, there were no effects of receiving either a positive or negative diagnosis on attitudes about condoms among either HIV-positive or negative individuals (not shown).
Table 9.
Learning HIV-Negative Results—Interactions with Prior Sexual Behavior
| Dependent variables: | Bought condoms
|
Number of condoms
|
Reported buying condoms
|
Reported having sex at follow-up
|
||||
|---|---|---|---|---|---|---|---|---|
| OLS (1) | IV (2) | OLS (3) | IV (4) | OLS (5) | IV (6) | OLS (7) | IV (8) | |
| Got results | 0.024 (0.047) | 0.007 (0.100) | 0.03 (0.189) | −0.141 (0.453) | −0.029 (0.028) | −0.041 (0.058) | 0.027 (0.062) | −0.073 (0.140) |
| Got results × had sex | −0.043 (0.054) | −0.061 (0.127) | −0.207 (0.250) | −0.13 (0.577) | 0.023 (0.033) | 0.058 (0.074) | −0.013 (0.066) | 0.068 (0.148) |
| Had sex | 0.068 (0.047) | 0.081 (0.086) | 0.355 (0.213) | 0.301 (0.409) | −0.001 (0.030) | −0.026 (0.052) | 0.247*** (0.063) | 0.189* (0.112) |
| Constant | 0.595*** (0.111) | 0.605*** (0.112) | 2.081*** (0.481) | 2.196*** (0.502) | 0.194*** (0.062) | 0.204** (0.077) | −0.206** (0.088) | −0.137 (0.129) |
| Sample size | 1,260 | 1,260 | 1,260 | 1,260 | 1,260 | 1,260 | 1,260 | 1,260 |
| R2 | 0.17 | 0.17 | 0.09 | 0.09 | 0.06 | 0.06 | 0.12 | 0.12 |
| Mean | 0.23 | 0.23 | 0.87 | 0.87 | 0.09 | 0.09 | 0.67 | 0.67 |
| P-statistic (got results + got results × had sex = 0) | 0.46 | 0.40 | 0.24 | 0.36 | 0.76 | 0.71 | 0.65 | 0.93 |
Notes: Sample includes HIV-negative respondents in Balaka and Rumphi who were tested for HIV and were reinterviewed in 2005. Robust standard errors clustered by village (for 57 villages) with district fixed effects are in parentheses. Controls also include gender, age, age-squared, and a simulated average distance variable (an average distance of respondents’ households to simulated randomized locations of HIV results centers). Coefficients are either OLS or IV estimates where knowing HIV status is instrumented by having any positive-valued incentive, the total amount of the incentive, total amount squared, distance from the HIV results center, distance-squared, all terms interacted with gender and HIV status. “Bought condoms” is an indicator if any condoms were purchased from interviewers at the follow-up survey; “number of condoms” is the total number of condoms purchased from interviewers; “reported buying condoms” and “reported having sex” were asked at the follow-up interview in 2005 and refer to the previous two months since the VCT was available.
Significantly different from zero at 99 percent confidence level.
Significantly different from zero at 95 percent confidence level.
Significantly different from zero at 90 percent confidence level.
Thus, it appears that individuals learning their HIV-positive status incur private costs to protect their sexual partners by purchasing condoms. It is important to note that the effects are greatest among those who are sexually active at the time condoms are made available to them to be purchased. There is no statistically significant effect on the likelihood of engaging in sexual intercourse after receiving an HIV-positive diagnosis. There was no significant effect of learning HIV-negative results on the purchase of condoms or on the likelihood of having sex two months after learning HIV-negative results.
IV. Robustness and Discussion
A. Credibility of Results
An important consideration is whether respondents believed the diagnoses they received at the VCT centers were credible. If not, there would be little reason that receiving an HIV diagnosis should affect condom purchases. A comparison of respondents’ belief of their likelihood of infection before and after learning results suggests that individuals updated their prior beliefs based on receiving HIV-negative test results. Before being tested, 43 percent of HIV-negative respondents thought there was a likelihood they were infected (Table 10, panel A, column 1). During the follow-up survey, respondents were again asked their beliefs about being infected. Among the HIV-negatives who had not received their test results, 50 percent believed that there was a likelihood of being infected. In contrast only 12 percent of the HIV-negatives who did learn their results thought that there was a likelihood of infection (Table 10, panel A, columns 2 and 3). Receiving an HIV-negative diagnosis significantly reduced the likelihood of believing there was a chance of being infected by 39 percentage points.
Among the HIV-positives, before being tested, 56 percent believed that there was some likelihood of infection (Table 10, panel B, column 1). At the follow-up survey, among the HIV positives who did not learn their HIV results, 43 percent reported some likelihood of being infected, as compared to 51 percent of those who had learned they were positive who reported some likelihood of being infected. While the difference in belief of infection is not statistically significant between HIV-positives who obtained their results and those who did not, this may be due, in part, to the small sample size of HIV positives (Table 10, panel B, column 4).
B. Prior Beliefs
Another argument as to why learning HIV status may have no measured effect on condom purchases (among the HIV-negatives) is that only individuals who are surprised by their HIV results should be expected to alter their sexual behavior in response to the information. Using data from unmarried respondents interviewed and tested in San Francisco during 1988 and 1989, Boozer and Philipson (2000) find asymmetric results: those who thought they were at risk and were diagnosed HIV-negative reported increased sexual contact by 20 percent; those who thought they were not at risk but were diagnosed HIV-positive reported decreasing sexual contact by 50 percent. Their findings give important theoretical insights into who benefits from the information provided by HIV testing and potential asymmetric behavior among those with differing prior beliefs of HIV status. Using the same measure of likelihood of infection as Boozer and Philipson, I find no significant relationship between prior beliefs and condom purchases.
Table 11 presents the impact of learning HIV results on condom purchases among those who had sex at the baseline, including an indicator of whether the respondent believed there was any likelihood of being infected, as well as an interaction of respondent prior beliefs with learning HIV status.20 Each specification is presented separately for HIV-positives and HIV-negatives. In the IV specification, knowing HIV test results (positive or negative) had no differential effects on condom purchases between those believing there was a likelihood of being infected and those believing there was no likelihood (Table 11, columns 2, 4). It is possible that respondents have uncertain prior beliefs about infection likelihood and measuring respondents’ confidence of their prior beliefs is difficult. Also, the respondents in the Boozer and Philipson study are quite different from those in this study and are likely to have different distributions of prior beliefs. Most important, this comparison is difficult to make without having a baseline level of condom purchases.
Table 11.
Interaction of Prior Belief of Infection and Condom Purchases (Dependent variable: Purchased any condoms)
| HIV-positive
|
HIV-negative
|
|||
|---|---|---|---|---|
| OLS (1) | IV (2) | OLS (3) | IV (4) | |
| Got results | 0.484** (0.197) | 0.535−0.36 | −0.048 (0.030) | −0.107 (0.083) |
| Got results × likely to be infected | −0.064 (0.237) | −0.421−0.399 | 0.054 (0.041) | 0.068 (0.104) |
| Likely to be infected | 0.072 (0.124) | 0.274−0.262 | −0.015 (0.037) | −0.027 (0.079) |
| Constant | 0.000 (0.844) | 0.389−1.059 | 0.763*** (0.109) | 0.799*** (0.121) |
| Sample size | 52 | 52 | 956 | 956 |
| R2 | 0.21 | 0.15 | 0.19 | 0.19 |
| p-statistic (got results + got results × likely to be infected = 0) | 0.03 | 0.69 | 0.87 | 0.62 |
Notes: Sample includes respondents in Balaka and Rumphi who were tested for HIV, were reinterviewed in 2005, and reported having sex at the baseline (2004). Robust standard errors clustered by village with district fixed effects are in parentheses. Controls also include gender, age, age-squared, and a simulated average distance variable (an average distance of respondents’ households to simulated randomized locations of HIV results centers). “Likely to be infected” indicates having any prior belief of HIV infection. Coefficients in columns 2 and 3 are IV estimates where knowing HIV status is instrumented by having any nonzero incentive, the total amount of the incentive, total amount squared, distance from the HIV results center, distance-squared, and all terms interacted with gender and having a likelihood of being infected.
Significantly different from zero at 99 percent confidence level.
Significantly different from zero at 95 percent confidence level.
Significantly different from zero at 90 percent confidence level.
C. Cost-Effectiveness
Sexually active HIV-positive individuals who learned their status were significantly more likely to purchase condoms than other respondents. However, the increase in the demand for condoms was seen almost entirely among this small proportion of the sample: there were virtually no effects among HIV-negatives or among those who were not sexually active. Further, learning HIV-positive results increased the number of condoms purchased by less than two additional condoms (Table 7, column 4) and there were no significant increases in reported purchases of nonsubsidized condoms among all respondents. If these results were to be generalized to other settings, the effectiveness of a similar door-to-door testing program in increasing condom purchases (and ultimately in preventing additional HIV infections) would be a function of the number of HIV-positives who were sexually active and who did not already have an infected sexual partner.
This is important to consider because the costs of testing are quite high. Table 12 presents the costs of testing, counseling/giving results, and selling condoms during 2004 fieldwork in Malawi, converted to US dollars (107 Malawi Kwacha per dollar). These costs do not include any research-related expenses (e.g., the incentives or survey costs) and the high and low estimates for any variable costs are also presented.21 The average total cost per respondent was $44.06 for testing, $5.51 for counseling/giving results, and $4.87 for selling condoms to respondents (costs comparable to other published expenses related to providing testing services are comparable).22
Table 12.
Program Cost per Respondent (Dollars)
| Average | Percent | Low | High | ||
|---|---|---|---|---|---|
| Testing | Transportation | 4.54 | 0.1 | 4.54 | 4.54 |
| Labor | 13.57 | 0.31 | 11.75 | 16.15 | |
| Training | 1.66 | 0.04 | 1.66 | 1.66 | |
| Laboratory costs | 22.45 | 0.51 | 6.76 | 22.45 | |
| Supplies | 1.84 | 0.04 | 1.06 | 2.87 | |
| Subtotal | 44.06 | 25.57 | 47.05 | ||
| Counseling and results | Transportation | 0.68 | 0.13 | 0.68 | 0.68 |
| Labor | 3.48 | 0.64 | 3.37 | 4.76 | |
| Training | 0.16 | 0.03 | 0.16 | 0.16 | |
| Supplies | 1.09 | 0.2 | 0.63 | 1.72 | |
| Subtotal | 5.51 | 4.53 | 6.64 | ||
| Selling condoms | Transportation | 0.9 | 0.18 | 0.9 | 0.9 |
| Labor | 3.33 | 0.68 | 3.23 | 3.48 | |
| Supplies | 0.64 | 0.13 | 0.18 | 1.06 | |
| Subtotal | 4.87 | 4.31 | 5.44 | ||
| Total cost | 55.34 | 34.41 | 59.14 |
Notes: Costs exclude all research-related expenses. See text for full details.
In a setting such as Malawi, with an overall HIV prevalence rate of approximately 10 percent, at least ten individuals would have to be tested using a door-to-door approach to inform one HIV-positive individual of her status. The cost for this could range between $80 (the lowest estimated cost published (Forsythe et al. 2002) and $537 (the highest estimated cost (MDICP)). Further, the results of this study indicate that this program would have no significant effect on nonsubsidized condom purchases or on the probability of having sex, although it might have an effect in uptake of heavily subsidized condom purchases (such as the door-to-door “sales”23) or on acceptance of freely distributed condoms.
Given the high ratio of costs to HIV-positive diagnosis in door-to-door testing in this setting, and the lack of significant effects of diagnosis on some condom purchase behavior and sexual activity, other interventions might be much more cost-effective for HIV prevention. For example, an evaluation of a randomized trial in Mwanza, Tanzania, estimated that treating non- HIV STIs had an incremental costs of $217.62 per HIV infection averted (Lucy Gilson et al. 1997) and another simulation of a similar intervention estimated a cost of $78.24 per infection averted (Oster 2005). Other less direct interventions may also be effective prevention strategies—for example, improving blood supply safety (estimated cost of $172 per infection averted; (Rex Winsbury 1995), preventing mother-to-child transmission ($298–$506 per infection averted; Elliot Marseille et al. 1999), or performing circumcisions (Bertran Auvert et al. 2005; Ronald H Gray et al. 2007).24
In sum, the significant effects claimed for broad-based HIV testing and counseling efforts, deemed the “single most influential driver for behavior change” (Global Business Coalition 2004), are not detected within these data. Neither the knowledge of HIV status nor the personal attention and education from the nurses to practice safe sex during the VCT counseling sessions appears to have had a significant impact on purchasing condoms or on the likelihood of having sex after two months.
V. Conclusion
This study is the first to analyze the impact on obtaining HIV results of randomized associated benefits (monetary incentives for attending a VCT center) and costs (travel distance to the VCT center). It also estimates the impact of learning HIV results on subsequent condom purchases. I find that a monetary incentive of less than a tenth of a day’s wage doubled the rate of result-seeking among respondents; I also find that distance to a VCT center from a respondent’s home had a strong negative impact on result-seeking. I find that HIV-positive individuals with a sexual partner who had obtained their test results exhibited a higher demand for condoms than those who had not, supporting the view that individuals aware of their HIV-positive status are willing to bear the costs of safe sex in order to protect sexual partners.
The finding that learning HIV-negative test results had little impact on the demand for condoms is difficult to interpret, since it implies indifference to using a highly effective prevention strategy to avert infection by a deadly virus. It should be noted, however, that these analyses are limited to examining the impact of learning HIV status on condom purchases. Future research will explore other preventive responses to learning HIV status.
This study contradicts widely held views that large psychological or stigma-related costs are barriers to learning HIV results. For example, one organization, discussing barriers faced by those traveling to HIV results centers in Zimbabwe, stated: “The cost of stigma is quite high, more so than the bus fare to town” (Emedie Gunduza 2002). However, the evidence from this experiment in Malawi indicates that such psychological barriers, if they exist, can easily and inexpensively be overcome. Cash incentives may directly compensate for the real costs (e.g., travel expenses, missed work) or psychological costs of obtaining HIV results, or they may indirectly reduce the stigma associated with HIV testing by providing individuals with a public excuse for attending the results center. This suggests that rather than expensive campaigns to reduce perceptions of testing stigma, simply offering incentives or reducing costs associated with testing may be effective strategies for increasing uptake. It is important to keep in mind, however, that these results may differ in settings with higher prevalence rates or different social circumstances.25
The fact that HIV-positive individuals are willing to incur private costs to protect sexual partners from infection suggests that offering free testing and incentives for obtaining test results and reduced-cost condoms might constitute a promising strategy to prevent new HIV infections. However, in this study, the magnitude of the effects of learning HIV status were relatively small and were observed only for a small fraction of sampled respondents. Further, testing had no impact on increasing the demand for condoms among HIV-negatives, or among those without a sexual partner, despite lengthy pre- and post-test counseling sessions with nurses, education about safe sex, and offers of low-priced condoms. These findings indicate that other prevention programs should be explored before investing in costly door-to-door testing programs. However, if governments or organizations choose to invest in testing programs in order to provide treatment, or if a testing program is already in place, offering modest monetary rewards may help to increase uptake and return rates. Given evidence of small positive behavioral effects on HIV-positives of learning their status, inexpensive boosts in participation may help to increase the effectiveness of such programs.26
It is important to revisit and challenge previous assumptions about HIV testing and sexual behavior. With rigorous empirical evidence and a better understanding of behavior in Africa, policies may be more accurately and effectively designed to reduce the spread of HIV.
Acknowledgments
I am grateful to Randall Akee, David Bishai, David Cutler, Eliza Hammel, Richard Holden, Erica Field, Edward Glaeser, Andrew Francis, Emir Kaminica, Larry Katz, Michael Kremer, David Laibson, Sendhil Mullainathan, Ben Olken, Emily Oster, Michelle Poulin, Mark Rosenzweig, Jesse Shapiro, Jeff Smith, and participants at seminars at Harvard University, University of Michigan, University of Toronto, University of Chicago, University of Virginia, Center for Global Development, and Washington University for helpful comments and discussion. I also thank three anonymous referees. I am very grateful to Jere Behrman, Hans-Peter Kohler, Susan Watkins, and the MDICP team for support, data, and assistance with fieldwork. The Malawi Diffusion and Ideational Change Project is supported by grants from the Rockefeller Foundation; NICHD (R01-HD4173, R01 HD372-276); NIA (AG1236-S3); the Center for AIDS Research; and the Center on the Demography of Aging at the University of Pennsylvania. Follow-up data were supported by the Warburg Foundation of the Economics of Poverty. I am grateful for financial support from the Harvard Population Center, Harvard Graduate Council, the Harvard Center for International Development, and the National Bureau of Economic Research Fellowship on Aging.
Footnotes
Richard Holbrooke and Richard Furman, “A Global Battle’s Missing Weapon.” New York Times, February 10, 2004.
John Donnelly, “Dire Situation, Drastic Measures: AIDS Testing Urged for All in Ravaged Nation.” Boston Globe, October 23, 2005.
One exception to this is a study that randomly phased in individuals being tested (The Voluntary HIV-1 Counseling and Testing Efficacy Study Group 2000). Their findings, that learning HIV results substantially reduced reported risky sexual behavior, has since been widely cited within the public health literature and used in subsequent simulations to conclude that counseling and testing is an effective strategy for preventing new infections (M. Sweat et al. 2000). However, that study was conducted among self-selected individuals who choose to have an HIV test at urban health clinics and relied on reported sexual behavior as their primary measure of behavioral change. A different study conducted by Michael Boozer and Tomas Philipson (2000) found effects of learning HIV status among gay men in San Francisco, accounting for their prior beliefs of infection; this study also suffers from many of the limitations above, including self-selection.
For further sampling details see http://www.malawi.pop.upenn.edu/Level%203/Malawi/level3_malawisampling.htm.
During a pilot of the questionnaire and HIV testing, nurses gave out higher incentive amounts than the distribution would suggest, possibly feeling sympathetic to poor villagers. Nurses were then instructed that continuation of employment was contingent upon following the instructions of randomization. However, it appears that fewer “zero incentives” were given out (Figure 2). On average, the cumulative distribution of actual incentives given out is $0.22 higher than the theoretical distribution (a Kolmogorov-Smirnov test rejects the null hypothesis of equality at the 99 percent level, not shown). For incentive amounts over one dollar, there is no significant difference between the actual amount and theoretical amount of incentive. It is possible that a few respondents were allowed to “redraw” the voucher amount if they had originally selected a zero. This would be most problematic if nurses favored individuals who were more likely to practice safe sex or purchase condoms after learning their HIV status. However, there is no systematic difference by observable respondent or nurse characteristics, and there is no change in the results of the analysis if the nurses whose distribution of incentives were significantly different from the theoretical distribution of incentives are omitted (not shown).
GPS locations are accurate between 10 and 15 meters. Calculating straight-line distance ignores natural boundaries such as roads or rivers and may underestimate the actual distance needed to travel. Approximately 7 percent of households have missing household GPS coordinates and, in this case, the distance to an assigned VCT center is replaced with the average village distance to the results center. Note that the permanent health clinics with VCT services nearest to each of the study sites were located between 20 and 50 kilometers away in 2004.
Although, when rapid testing is offered, close to 100 percent stay to learn their results. See also Nicole Angotti et al. 2007.
There is a growing body of literature in behavioral economics suggesting anxiety or fear may be important factors in decision making, especially in seeking health information (see Colin Camerer, George Loewenstein, and Matthew Rabin 2004; Richard G. Frank 2004; Rabin 1998; Steven Wu 2003). Andrew Caplin and John Leahy (2001) present a model of psychological expected utility and in an expansion of this work, Caplin and Dan Eliaz (2003) and Botond Kőszegi (2003) model anxiety to learn health status. See also Thomas Philipson and Richard Posner (1995). It is also possible that individuals overestimate the potential psychological effects of receiving an HIV diagnosis (Tim Wilson and Dan Gilbert 2003; Elaine Sieff, Robyn M. Dawes, and Loewenstein 1999).
Not controlling for this simulated average term would ignore the fact that each VCT zone is bounded and that, in expectation, more central households have shorter distances to travel from any randomized location (although including this term does not significantly change any of the estimates).
The VCT centers were placed throughout the study site such that many villages were assigned to the same center—this ranged from as few as 3 villages at one center (in Balaka) to as many as 14 villages attending another center (in Rumphi). Clustering standard errors by VCT center, rather than village, reduces the standard errors on the impact of incentives, but increases the standard error of the effect of distance on attendance. The results are not significantly different when using larger clusters. Also, because there were only 16 VCT centers, there may be omitted variables correlated with the center location that may be systematic, despite the randomized design. The results do not change significantly, however, if VCT center fixed effects are included (not shown).
In Balaka, 62 percent of the respondents are Muslim as opposed to less than 1 percent in Mchinji and Rumphi. Women in Balaka may also be less independent: in 2001, only 10 percent of Balaka women reported going to the health center without their husband’s permission, as opposed to 22 percent in the other districts.
For a formal model of testing see Michael Boozer and Philipson (2000).
Individuals may also update their priors, believing they have a lower likelihood of future infection, resulting in no change, or a decrease in safe sex, after learning HIV-negative results.
One review of 35 studies notes, “There is no question that HIV testing can and does motivate behavioral change in some individuals, but testing does not always lead to changes and does not have the same effect in all populations” (Donna Higgins et al. 1991; R. J. Wolitski et al. 1997). Several studies indicate little to no behavioral differences among those learning they are HIV-negative; there is some evidence that discordant couples may reduce risky sexual practices after testing (The Voluntary HIV-1 Counseling and Testing Efficacy Study Group 2000; Wolitski et al. 1997; Weinhardt 1999).
However, among HIV-positives and HIV-negatives, there are no large differences in prior belief of HIV status or in measures of wealth by incentive or distance among those who obtained their HIV results and those who did not, suggesting that heterogeneous treatment effects across incentives or distance may be minimal (not shown).
Approximately 17 percent of the HIV-negatives and 11 percent of the HIV-positives refused to answer if they had had sex in the past year and they are excluded from this analysis. The majority of those refusing to answer were unmarried adolescents.
The bivariate probit estimates for HIV-positive and HIV-negative individuals were calculated by iterating over maximization techniques (Newton-Raphson, Berndt-Hall-Hall-Hausman, Davidon-Fletcher-Powell, and Broyden- Fletcher-Goldfarb-Shanno algorithms). Given the low sample size of HIV-positives, convergence to a reasonable estimate of ρ is difficult. I therefore constrain ρ among HIV-positives to be equal to that among HIV-negatives (0.218).
See also Angrist (2001) who argues in favor of conventional 2SLS estimation of nonlinear models.
On the other hand, the effects on condom purchases could be understated to the extent to which individuals may have already purchased condoms in response to the HIV test. Given that only 8 percent reported purchasing condoms on their own, this downward bias is likely to be minimal.
Likelihood of HIV infection is coded as “one” if the respondent replied there was any chance of being infected or if she did not know. Likelihood of HIV infection is coded as “zero” if she reported no likelihood of infection (see also Table 10, column 1). Results do not change if those reporting “don’t know” are coded as “zero” or if they are omitted.
The HIV tests and related fees (e.g., equipment for sample collection, laboratory and processing fees) constituted the largest proportion of costs. Labor (e.g., salary, accommodations, and benefits) accounted for the second largest proportion of costs, followed by transportation costs that include vehicle rental and fuel to transport employees to the rural study sites, as well as the cost of transporting HIV samples to the laboratory. Training for collecting HIV samples lasted approximately one week and included the costs of paying salaries, instructors, and room rental fees; training for post-test counseling was a two-day seminar. Supplies included employee uniforms, freezers and cold packs for HIV samples, portable tents, and other necessary supplies and equipment.
One study of clients’ willingness to pay for VCT services in Kenya (Forsythe et al. 2002) estimated a total cost of $16.03 per client for three health centers during 1999. It is worth noting that this was the calculated “incremental” cost, which did not include donated items. The total cost was $47.34 per client before subtracting these other costs. This paper also suggested that with certain cost-savings steps, the costs could be reduced to as little as $8.00 per client. Another study of clinics in Kenya and Tanzania reported a cost of $26.65 and $28.93 per client, respectively. The biggest discrepancy between these studies and the MDICP costs are the costs of bringing the testing into local villages. The initial budget for the government of Lesotho to test each citizen going door-to-door was approximately $10.00 per person, which would reach a total cost of approximately $10 million. Actual costs of this program (begun in late 2005) are not yet available (Integrated Regional Information Networks 2004).
It should also be noted that purchases of subsidized condoms were only made after respondents were given 30 cents—without this gift, it is likely that condom sales would have been even lower.
Sweat et al. (2000) estimated that HIV testing in Kenya and Tanzania resulted in a cost per infection averted of 249 and 346 respectively although their results are limited by selection and reporting bias which may bias results towards more cost-effective analysis.
There may also be scope for offering subsidies or incentives for other positive health behavior, such as remaining HIV negative, adhering to antiretroviral therapy, getting circumcised, attending prenatal clinics, or being tested and treated for other sexually transmitted diseases (Oster 2005).
Simulations have shown that some condom use may be more effective than no condom use, especially when individuals are infected with a sexually transmitted disease (Michael Bracher, Gigi Santow, and Susan Cott Watkins 2004).
References
- ►.Allen Susan, Meinzen-Derr Jareen, Kautzman Michele, Zulu Isaac, Trask Stanley, Fideli Ulgen, Musonda Rosemary, Kasolo Francis, Gao Feng, Haworth Alan. Sexual Behavior of HIV Discordant Couples after HIV Counseling and Testing. AIDS. 2003;17(5):733–40. doi: 10.1097/00002030-200303280-00012. [DOI] [PubMed] [Google Scholar]
- Anglewicz Philip. PhD diss. University of Pennsylvania; 2007. Migration, Risk Perception and HIV Infection in Malawi. [Google Scholar]
- Anglewicz Philip, Kohler Hans-Peter. Overestimating HIV Infection: The Construction and Accuracy of Subjective Probabilities of HIV Infection in Rural Malawi. Paper presented at the Annual Meeting of the Population Association of America; Los Angeles. 2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Angotti Nicole, Bula Agatha, Lauren Gaydosh, Eitan Zeev Kimchi, Thornton Rebecca L, Yeatman Sara E. The Fear Factor as a Barrier to HIV Testing: Alternative Considerations from Rural Malawi. Paper presented at the American Sociological Association Annual Conference; New York, NY. 2007. [Google Scholar]
- ►.Angrist Joshua D. Estimations of Limited Dependent Variable Models with Dummy Endogenous Regressors: Simple Strategies for Empirical Practice. Journal of Business and Economic Statistics. 2001;19(1):2–16. [Google Scholar]
- ►.Angrist Joshua D, Imbens Guido W. Two-Stage Least Squares Estimation of Average Causal Effects in Models with Variable Treatment Intensity. Journal of the American Statistical Association. 1995;90(430):431–42. [Google Scholar]
- ►.Angrist Joshua D, Imbens Guido W, Rubin Donald B. Identification of Causal Effects Using Instrumental Variables. Journal of the American Statistical Association. 1996;91(434):444–55. [Google Scholar]
- Arendt Jacob Nielsen, Holm Anders. Probit Models with Binary Endogenous Regressors. Discussion Papers on Business and Economics 4/2006 2006 [Google Scholar]
- ►.Auvert Bertran, Taljaard Dirk, Lagarde Emmanuel, Sobngwi-Tambekou Joelle, Sitta Remi, Puren Adrian. Randomized, Controlled Intervention Trial of Male Circumcision for Reduction of HIV Infection Risk: The ANRS 1265 Trial. PLoS Medicine. 2005;2(11):298. doi: 10.1371/journal.pmed.0020298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baggaley Rachel, Kelly M, Weinreich S, Kayawe I, Phiri G, Mulongo W, Phiri M. HIV Counselling and Testing in Zambia: The Kara Counselling Experience. SAfAIDS News. 1998;6(2):2–8. [PubMed] [Google Scholar]
- Bignami-Van Assche Simona, Reniers Georges, Weinreb Alex. An Assessment of the KDICP and MDICP Data Quality: Interviewer Effects, Question Reliability and Sample Attrition. Demographic Research. 2003 Special Collection 1, Article 2. [Google Scholar]
- ►.Bignami-Van Assche Simona, Chao Li-Wei, Anglewicz Philip, Chilongozi David, Bula Agatha. The Validity of Self-reported Likelihood of HIV Infection among the General Population in Rural Malawi. Sexually Transmitted Infections. 2007;83(1):35–40. doi: 10.1136/sti.2006.020545. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bignami-Van Assche Simona, Smith Kirsten, Reniers Georges, Anglewicz Philip, Thornton Rebecca, Chao Li-Wei, Weinreb Alex, Watkins Susan Cotts, Hoffman Irving the MDICP STI Team. Protocol for Biomarker Testing in the 2004 Malawi Diffusion and Ideational Change Project. University of Pennsylvania Working Paper 7 2004 [Google Scholar]
- ►.Boozer Michael A, Philipson Tomas J. The Impact of Public Testing for Human Immunodeficiency Virus. Journal of Human Resources. 2000;35(3):419–46. [Google Scholar]
- ►.Bracher Michael, Santow Gigi, Watkins Susan Cotts. Assessing the Potential of Condom Use to Prevent the Spread of HIV: A Microsimulation Study. Studies in Family Planning. 2004;35(1):48–64. doi: 10.1111/j.1728-4465.2004.00005.x. [DOI] [PubMed] [Google Scholar]
- Camerer Colin F, Loewenstein George, Rabin Matthew., editors. Advances in Behavioral Economics. Princeton, NJ: Princeton University Press; 2004. [Google Scholar]
- ►.Caplin Andrew, Eliaz Kfir. Aids Policy and Psychology: A Mechanism-Design Approach. RAND Journal of Economics. 2003;34(4):631–46. [PubMed] [Google Scholar]
- ►.Caplin Andrew, Leahy John. Psychological Expected Utility Theory and Anticipatory Feelings. Quarterly Journal of Economics. 2001;116(1):55–79. [Google Scholar]
- ►.Cartoux Michel, Meda Nicolas, Van de Perre Philippe, Newell Marie Louise, de Vincenzi Isabelle, Dabis Francois. Acceptability of Voluntary HIV Testing by Pregnant Women in Developing Countries: an International Survey. AIDS. 1998;12(18):2489–93. doi: 10.1097/00002030-199818000-00019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chapoto Antony, Jayne Thomas S. Socioeconomic Characteristics of Individuals Afflicted by Aids-Related Prime-Age Mortality in Zambia. Paper presented at the International Food Policy Research Institute/Renewal Conference on HIV/AIDS and Food and Nutrition Security; Durban, South Africa. 2005. [Google Scholar]
- ►.Coates Thomas J, Grinstead Olga A, Gregorich SE, Heilbron DC, Wolf WP, Choi KH, Schachter J, et al. Efficacy of Voluntary HIV-1 Counseling and Testing in Individuals and Couples in Kenya, Tanzania, and Trinidad: A Randomised Trial. Lancet. 2000;356(9224):103–12. [PubMed] [Google Scholar]
- Coulibaly Djeneba, Msellati Philippe, Dedy Seri, Welffens-Ekra Christiane, Dabis Francois. Attitudes and Behavior of Pregnant Women towards HIV Screening in Abidjan (Ivory Coast) in 1995 and 1996. Santé. 1998;8(3):234–38. [PubMed] [Google Scholar]
- Day John H, Miyamura Kouichi, Grant Allison D, Leeuw A, Munsamy J, Baggaley R, Churchyard Gavin J. Attitudes to HIV Voluntary Counselling and Testing Among Mineworkers in South Africa: Will Availability of Antiretroviral Therapy Encourage Testing? AIDS Care: Psychological and Socio-Medical Aspects of AIDS/HIV. 2003;15(5):665–72. doi: 10.1080/0954012030001595140. [DOI] [PubMed] [Google Scholar]
- ►.de Graft-Johnson Joseph, Paz-Soldan Valerie, Kasote Antonio, Tsui Amy. HIV Voluntary Counseling and Testing Service Preferences in a Rural Malawi Population. AIDS and Behavior. 2005;9(4):475–84. doi: 10.1007/s10461-005-9018-x. [DOI] [PubMed] [Google Scholar]
- ►.Ekwueme Donatus U, Pinkerton Steven D, Holtgrave David R, Branson Bernard M. Cost Comparison of Three HIV Counseling and Testing Technologies. American Journal of Preventive Medicine. 2003;25(2):112–21. doi: 10.1016/s0749-3797(03)00115-6. [DOI] [PubMed] [Google Scholar]
- ►.Fan Jianqing. Design-Adaptive Nonparametric Regression. Journal of the American Statistical Association. 1992;87(420):998–1004. [Google Scholar]
- ►.Fernandez M Isabel, Collazo Jose B, Bowen G Stephen, Varga Leah M, Hernandez Nilda, Perrino Tatiana. Predictors of HIV Testing and Intention to Test Among Hispanic Farmworkers in South Florida. Journal of Rural Health. 2005;21(1):56–64. doi: 10.1111/j.1748-0361.2005.tb00062.x. [DOI] [PubMed] [Google Scholar]
- ►.Ford Kathleen, Wirawan Dewa Nyoman, Sumantera Gusti Made, Sawitri Anak Agung Sagung, Stahre Mandy. Voluntary HIV Testing, Disclosure, and Stigma Among Injection Drug Users in Bali, Indonesia. AIDS Education and Prevention. 2004;16(6):487–98. doi: 10.1521/aeap.16.6.487.53789. [DOI] [PubMed] [Google Scholar]
- Forsythe Steven, Arthur Gilly, Ngatia Gilbert, Mutemi Roselyn, Odhiambo Joseph, Gilks Charles. Using Economics to Reveal Why Kenyans Do (or Do Not) Get Tested for HIV. Meeting Abstract (E11511) presented at the XIV International AIDS Conference; Barcelona. 2002. [Google Scholar]
- Frank Richard G. Behavioral Economics and Health Economics. National Bureau of Economic Research Working Paper 10881 2004 [Google Scholar]
- Freedman David A, Sekhon Jasjeet S. Endogeneity in Probit Response Models. 2008 http://papers.ssrn.com/sol3/papers.cfm?abstract_id=1138489.
- ►.Gilson Lucy, Mkanje Rashid, Grosskurth Heiner, Mosha Frank, Picard John, Gavyole Awena, Todd James, et al. Cost-effectiveness of Improved Treatment Services for Sexually Transmitted Diseases in Preventing HIV-1 Infection in Mwanza Region, Tanzania. Lancet. 1997;350(9094):1805–09. doi: 10.1016/S0140-6736(97)08222-6. [DOI] [PubMed] [Google Scholar]
- ►.Ginwalla S, Grant A, Day J, Dlova T, Macintyre S, Baggaley Munsamy R, Churchyard GJ. Use of UNAIDS Tools to Evaluate HIV Voluntary Counseling and Testing Services for Mine-workers in South Africa. AIDS Care. 2002;14(5):707–26. doi: 10.1080/0954012021000005533. [DOI] [PubMed] [Google Scholar]
- ►.Glick Peter. Scaling Up HIV Voluntary Counseling and Testing in Africa: What Can Evaluation Studies Tell Us About Potential Prevention Impacts? Evaluation Review. 2005;29(4):331–57. doi: 10.1177/0193841X05276437. [DOI] [PubMed] [Google Scholar]
- Global Business Coalition. The Need to Know: Accelerating Access to Testing. 2004 http://www.gbcimpact.org/documents/media/publications/archive/Accelerating_access_to_testing_05_20_04.pdf.
- ►.Gneezy Uri, Rustichini Aldo. Pay Enough or Don’t Pay at All. Quarterly Journal of Economics. 2000;115(3):791–810. [Google Scholar]
- ►.Gray Ronald H, Kigozi Godfrey, Serwadda David, Makumbi Frederick, Watya Stephen, Nalugoda Fred, Kiwanuka Noah. Male Circumcision for HIV Prevention in Men in Rakai, Uganda: a Randomised Trial. Lancet. 2007;369(9562):657–66. doi: 10.1016/S0140-6736(07)60313-4. [DOI] [PubMed] [Google Scholar]
- Gunduza Emedie. The Hidden Cost of Voluntary Counseling and Testing for Women. Women and AIDS Support Network. 2002 December 31; http://www.kubatana.net/html/archive/hivaid/021231wasn1.asp?orgcode=wom003&year=2002&range_start=1.
- ►.Heckman James J. Dummy Endogenous Variables in a Simultaneous Equation System. Econometrica. 1978;46(4):931–59. [Google Scholar]
- ►.Heckman James J. Micro Data, Heterogeneity, and the Evaluation of Public Policy: Nobel Lecture. Journal of Political Economy. 2001;109(4):673–748. [Google Scholar]
- ►.Heckman James J, Smith Jeffrey. Making the Most out of Programme Evaluations and Social Experiments: Accounting for Heterogeneity in Programme Impacts. Review of Economic Studies. 1997;64(4):487–535. [Google Scholar]
- ►.Heckman James J, Urzua Sergio, Vytlacil Edward. Understanding Instrumental Variables in Models with Essential Heterogeneity. Review of Economics and Statistics. 2006;88(3):389–432. [Google Scholar]
- ►.Higgins Donna, Galavotti Christine, O’Reilly Kevin, Schnell Daniel, Moore M, Rugg D, Johnson R. Evidence for the Effects of HIV Antibody Counseling and Testing on Risk Behaviors. Journal of the American Medical Association. 1991;266(17):2419–29. [PubMed] [Google Scholar]
- ►.HITS-2000 Investigators. Failure to Return for HIV Test Results Among Persons at High Risk for HIV Infection: Results from a Multistate Interview Project. Journal of Acquired Immune Deficiency Syndrome. 2004;35(5):511–18. doi: 10.1097/00126334-200404150-00009. [DOI] [PubMed] [Google Scholar]
- ►.Hutchinson Angela B, Corbie-Smith Giselle, Thomas Stephen B, Mohanan Sveta, del Rio Carlos. Understanding the Patient’s Perspective on Rapid and Routine HIV Testing in an Inner-City Urgent Care Center. AIDS Education and Prevention. 2004;16(2):101–14. doi: 10.1521/aeap.16.2.101.29394. [DOI] [PubMed] [Google Scholar]
- ►.Imbens Guido W, Angrist Joshua D. Identification and Estimation of Local Average Treatment Effects. Econometrica. 1994;62(2):467–75. [Google Scholar]
- Integrated Regional Information Networks. LESOTHO: HIV/AIDS testing facilities still to be set up. 2004 http://www.irinnews.org/report.aspx?reportid=48985.
- ►.Kalichman Seth C, Simbayi Leickness C. HIV Testing Attitudes, AIDS Stigma, and Voluntary HIV Counselling and Testing in a Black Township in Cape Town, South Africa. Sexually Transmitted Infections. 2003;79(6):442–7. doi: 10.1136/sti.79.6.442. [DOI] [PMC free article] [PubMed] [Google Scholar]
- ►.Kamenga M, Ryder R, Jingu M, Mbuyi N, Mbu L, Behets Pattullo A, Malonza M, Kimani G, Muthee A, Otieno P, Odhiambo K, Moses S, Plummer F. Survey of Knowledge, Behaviour and Attitudes Relating to HIV Infection and AIDS Among Kenyan Secondary School Students. AIDS Care. 1994;6:173–81. doi: 10.1080/09540129408258628. [DOI] [PubMed] [Google Scholar]
- Know HIV AIDS. About Us. [6/2005];2005 http://www.knowhivaids.org/factstested.html.
- ►.Kőszegi Botond. Health Anxiety and Patient Behavior. Journal of Health Economics. 2003;22(6):1073–84. doi: 10.1016/j.jhealeco.2003.06.002. [DOI] [PubMed] [Google Scholar]
- Laver Susan M. Voluntary Testing and Counselling for HIV. ‘Are Adults in Rural Communities Ready to Test?’ A Descriptive Survey. Central African Journal of Medicine. 2001;47(4):92–7. [PubMed] [Google Scholar]
- ►.Leibowitz Arleen A, Taylor Stephanie L. Distance to Public Test Sites and HIV Testing. Medical Care Research and Review. 2007;64(5):568–84. doi: 10.1177/1077558707304634. [DOI] [PubMed] [Google Scholar]
- ►.Loewenstein George. Anticipation and the Valuation of Delayed Consumption. Economic Journal. 1987;97(387):666–84. [Google Scholar]
- Malawi Diffusion Ideational Change Project. Qualitative Field Journal Number 041115, Diston. 2004 November 15; [Google Scholar]
- Maluccio John. Attrition in the Kwazulu Natal Income Dynamics Study, 1993–1998. Food Consumption and Nutrition Division Discussion Paper 95 2000 [Google Scholar]
- ►.Marseille Elliot, Kahn James G, Mmiro Francis, Guay Laura, Musoke Philippa, Fowler Mary Glenn, Jackson J Brooks. Cost Effectiveness of Single-Dose Nevirapine Regimen for Mothers and Babies to Decrease Vertical HIV-1 Transmission in Sub-Saharan Africa. Lancet. 1999;354(9181):803–09. doi: 10.1016/S0140-6736(99)80009-9. [DOI] [PubMed] [Google Scholar]
- Martin H Gayle. A Comparative Analysis of the Financing of HIV/AIDS Programmes in Botswana, Lesotho, Mozambique, South Africa, Swaziland, and Zimbabwe. Cape Town: Human Sciences Research Council; 2003. [Google Scholar]
- Mechoulan Stephane. HIV Testing: A Trojan Horse? Topics in Economic Analysis and Policy. 2004;4(1):1–24. [Google Scholar]
- ►.Mugusi F, Josiah R, Moshi A, Chale S, Bakari M, Aris E, Magao P, et al. Dropouts in a Long-Term Follow-up Study Involving Voluntary Counseling and HIV Testing: Experience from a Cohort of Police Officers in Dar Es Salaam, Tanzania. Journal of Acquired Immune Deficiency Syndromes. 2002;30(1):119–23. doi: 10.1097/00126334-200205010-00016. [DOI] [PubMed] [Google Scholar]
- ►.Myer Landon, Morroni C, Link BG. Impact of Measurement Error in the Study of Sexually Transmitted Infections. Sexually Transmitted Infections. 2004;80(4):318–23. doi: 10.1136/sti.2003.006536. [DOI] [PMC free article] [PubMed] [Google Scholar]
- National Statistical Office (NSO) [Malawi], and ORC Macro. Malawi Demographic and Health Survey 2004. Calverton, MD: NSO and ORC Macro; 2005. [Google Scholar]
- Obare Francis. The Effect of Non-Response on Population-Based HIV Prevalence Estimates: The Case of Rural Malawi. Paper presented at the Annual Meeting of the Population Association of America; Los Angeles. 2006. [Google Scholar]
- Obare Francis, Fleming Peter, Anglewicz Philip, Thornton Rebecca L, Martinson Francis, Kapatuka Agatha, Poulin Michelle, Watkins Susan, Kohler Hans-Peter. Acceptance of Repeat Population-Based Voluntary Counseling and Testing for HIV in Rural Malawi. 2008 doi: 10.1136/sti.2008.030320. Unpublished. [DOI] [PMC free article] [PubMed] [Google Scholar]
- ►.Oster Emily. Sexually Transmitted Infections, Sexual Behavior, and the HIV/AIDS Epidemic. Quarterly Journal of Economics. 2005;120(2):467–515. [Google Scholar]
- ►.Philipson Thomas, Posner Richard. A Theoretical and Empirical Investigation of the Effects of Public Health Subsidies for STD Testing. Quarterly Journal of Economics. 1995;110(2):445–74. [Google Scholar]
- Rabin Matthew. Psychology and Economics. Journal of Economic Literature. 1998;36(1):11–46. [Google Scholar]
- Reniers Georges. Marital Strategies for Managing Exposure to HIV in Rural Malawi. Paper presented at the Annual Meeting of the Population Association of America; Philadelphia. 2005. [Google Scholar]
- ►.Sieff Elaine, Dawes Robyn M, Loewenstein George. Anticipated versus Actual Reaction to HIV Test Results. American Journal of Psychology. 1999;112(2):297–311. [PubMed] [Google Scholar]
- ►.Sikkema KJ, Kelly JA, Winett RA, Solomon LJ, Cargill VA, Roffman RA, McAuliffe TL, et al. Outcomes of a Randomized Community-Level HIV Prevention Intervention for Women Living in 18 Low-Income Housing Developments. American Journal of Public Health. 2000;90(1):57–63. doi: 10.2105/ajph.90.1.57. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stein Jo. The Impact of Antiretroviral (ARV) Provision on HIV/AIDS Prevention. AIDS Bulletin. 2005;14(1):58–71. [Google Scholar]
- ►.Stryker Jeff, Coates Thomas J, Decarlo Pamela, Haynes-Sanstad Katherine, Shriver Mike, Makadon Harvey J. Prevention of HIV Infection. Looking Back, Looking Ahead. Journal of the American Medical Association. 1995;273(14):1143–48. [PubMed] [Google Scholar]
- ►.Sweat Michael, Gregorich Steven, Sangiwa Gloria, Furlonge Colin, Balmer Donald, Kamenga Claudes, Grinstead Olga, Coates Thomas. Cost-effectiveness of Voluntary HIV-1 Counselling and Testing in Reducing Sexual Transmission of HIV-1 in Kenya and Tanzania. Lancet. 2000;356(9224):113–21. doi: 10.1016/S0140-6736(00)02447-8. [DOI] [PubMed] [Google Scholar]
- ►.Temmerman M, Moses S, Kiragu D, Fusallah S, Wamola IA, Piot P. Impact of Single Session Post-Partum Counseling of HIV Infected Women on their Subsequent Reproductive Behavior. AIDS Care. 1990;2(3):247–52. doi: 10.1080/09540129008257737. [DOI] [PubMed] [Google Scholar]
- ►.The Voluntary HIV-1 Counseling and Testing Efficacy Study Group. Efficacy of Voluntary HIV-1 Counselling and Testing in Individuals and Couples in Kenya, Tanzania, and Trinidad: A Randomised Trial. Lancet. 2000;356(9224):103–12. [PubMed] [Google Scholar]
- ►.Vytlacil Edward, Yildiz Nese. Dummy Endogenous Variables in Weakly Separable Models. Econometrica. 2007;75(3):757–79. [Google Scholar]
- ►.Weinhardt Lance S, Carey Michael P, Johnson Blair T, Bickham Nicole L. Effects of HIV Counseling and Testing on Sexual Risk Behavior: A Meta-Analytic Review of Published Research, 1985–1997. American Journal of Public Health. 1999;89(9):1397–1405. doi: 10.2105/ajph.89.9.1397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- ►.Wilson Tim, Gilbert Dan. Affective Forecasting. In: Zanna Mark P., editor. Advances in Experimental Social Psychology. Vol. 35. New York: Elsevier; 2003. pp. 345–411. [Google Scholar]
- Winsbury Rex., editor. Safe Blood in Developing Countries: The Lessons from Uganda. Luxembourg: Office for Official Publications of the European Communities; 1995. [Google Scholar]
- ►.Wolff Brent, Nyanzi Barbara, Katongole George, Ssesanga Deo, Ruberantwari Andy, Whitworth Jimmy. Evaluation of a Home-Based Voluntary Counselling and Testing Intervention in Rural Uganda. Health Policy Plan. 2005;20(2):109–16. doi: 10.1093/heapol/czi013. [DOI] [PubMed] [Google Scholar]
- Wolitski RJ, MacGowan RJ, Higgins DL, Jorgensen CM. The Effects of HIV Counseling and Testing on Risk-Related Practices and Help-Seeking Behavior. AIDS Education and Prevention. 1997;9(S):S52–67. [PubMed] [Google Scholar]
- ►.Wu Stephen. Sickness and Preventive Medical Behavior. Journal of Health Economics. 2003;22(4):675–89. doi: 10.1016/S0167-6296(03)00042-0. [DOI] [PubMed] [Google Scholar]
- Yoder P Stanley, Matinga Priscilla. Voluntary Counselling and Testing (VCT) for HIV in Malawi: Public Perspectives and Recent VCT Experiences. Calverton, MD: ORC Macro; 2004. [Google Scholar]


