Abstract
Purpose
In radiotherapy for prostate cancer, the rectum is the major dose-limiting structure. Physically separating the rectum from the prostate (e.g., by injecting a spacer) can reduce the rectal radiation dose. Despite pilot clinical studies, no careful analysis has been done of the risks, benefits, and dosimetric effects of this practice.
Methods and Materials
Using cadaveric specimens, 20 mL of a hydrogel was injected between the prostate and rectum using a transperineal approach. Imaging was performed before and after spacer placement, and the cadavers were subsequently dissected. Ten intensity-modulated radiotherapy plans were generated (five before and five after separation), allowing for characterization of the rectal dose reduction. To quantify the amount of prostate-rectum separation needed for effective rectal dose reduction, simulations were performed using nine clinically generated intensity-modulated radiotherapy plans.
Results
In the cadaveric studies, an average of 12.5 mm of prostate-rectum separation was generated with the 20-mL hydrogel injections (the seminal vesicles were also separated from the rectum). The average rectal volume receiving 70 Gy decreased from 19.9% to 4.5% (p < .05). In the simulation studies, a prostate-rectum separation of 10 mm was sufficient to reduce the mean rectal volume receiving 70 Gy by 83.1% (p < .05). No additional reduction in the average rectal volume receiving 70 Gy was noted after 15 mm of separation. In addition, spacer placement allowed for increased planning target volume margins without exceeding the rectal dose tolerance.
Conclusion
Prostate-rectum spacers can allow for reduced rectal toxicity rates, treatment intensification, and/or reduced dependence on complex planning and treatment delivery techniques.
Keywords: Prostate cancer, side effects, rectum, external beam radiotherapy
INTRODUCTION
Definitive external beam radiotherapy (RT) is a prevalent and effective therapy for men with low-, intermediate-, and high-risk localized prostate cancer. Acute and chronic side effects of treatment are generally well tolerated; however, the anterior rectal wall is the major dose-limiting structure. Although the use of intensity-modulated RT (IMRT) has reduced the frequency of acute and chronic rectal toxicity, side effects are still common. With dose-escalated (e.g., ≥78 Gy) IMRT, the rates of acute and chronic Grade 2 or greater rectal toxicity have ranged from 3% to 20% and 5% to 21%, respectively (1, 2). The risk of rectal toxicity depends on the volume of the rectum that receives a high radiation dose. In a large prospective series, the percentage of rectum receiving >70 Gy (V70) correlated with the occurrence of chronic rectal toxicity. For patients in whom the V70 was >26.2% vs. ≤26.2%, Grade 2 or greater chronic rectal toxicity occurred in 54% and 13%, respectively (3).
However, the region of the prostate most at risk of developing adenocarcinoma, the peripheral zone, is located immediately anterior to the rectum. Because of its location, this region typically has the smallest planning target volume (PTV) expansion. Commonly, a 10-mm expansion is applied in all directions, except posteriorly, where expansions of 5–7 mm (or less) are used (4). This relatively small expansion of a mobile pelvic organ necessitates daily image guidance and has led to some concerns about potential underdosing.
From basic radiation protection principles, it is well known that increasing the distance is a simple and effective way to reduce radiation exposure. The interest in recent years to physically separate the rectum from the prostate and thereby reduce the rectal radiation dose has been significant. The prostate-rectum anatomy is accommodating to this concept. Immediately posterior to the prostate is Denonvilliers fascia, a single, fused fascial layer composed of dense collagen, smooth muscle, and coarse elastic fibers (5). This layer is closely adherent to, and fused with, the prostatic capsule and seminal vesicles (6). In a series of 243 radical prostatectomy specimens, tumor progression was seen within Denonvilliers fascia in 19% of cases (6). Importantly, tumor invasion beyond Denonvilliers fascia was never seen. Therefore, during radical prostatectomy, dissection should be performed posterior to Denonvilliers fascia (6). The loose, areolar, adipose tissue of the mesorectum lies just behind Denonvilliers fascia, followed by the muscular layers of the rectal wall, and then the rectal mucosa. This loose, areolar tissue that separates the prostate and rectum is fairly easy to develop and separate. Injection of saline in this space before prostate cryotherapy is a recommended practice (7, 8). After the cryotherapy procedure, the saline is reabsorbed.
Just as the plane posterior to Denonvilliers fascia is developed during radical prostatectomy and cryotherapy, it is an ideal plane to expand before RT. To date, at least three approaches to expand this space have been applied in pilot clinical work. First, Noyes and Noyes (9) injected collagen between the prostate and rectum in 10 men before prostate IMRT. Prada et al. (10, 11) injected hyaluronic acid between the prostate and rectum in 27 patients before high-dose-rate brachytherapy and in 32 patients after low-dose-rate brachytherapy. A Phase I clinical trial is investigating a biodegradable balloon implanted between the prostate and rectum before RT (12).
Synthetic polyethylene-glycol (PEG)-based hydrogels might also be useful as prostate-rectum spacers. These hydrogels (which are >90% water by weight) are thin liquids when injected, but then polymerize in situ to form a soft hydrogel after the two precursor solutions mix. Although a variety of PEG hydrogels with various properties exist, we used one PEG hydrogel (DuraSeal, Confluent Surgical, Waltham, MA) as an example of this class of compounds (13). DuraSeal is Food and Drug Administration approved as an adjunct to surgical closure of the dura (to reduce the incidence of cerebrospinal fluid leakage). DuraSeal remains intact for 4–8 weeks after application, is degraded by hydrolysis of ester bonds, and is then excreted renally.
Despite significant interest from both academia and industry, no careful analysis of the dosimetric effects of prostate-rectum spacers has been done. It is unclear how much prostate-rectum separation is needed, what rectal dose reduction can realistically be achieved, and whether this benefit is applicable to all patients (i.e., those receiving prostate-only treatment vs. those receiving treatment to the prostate, seminal vesicles, and pelvic nodes). Also, the potential for harm from this practice should be considered. In the present study, using cadaveric specimens and RT plans from clinically treated patients, we have described and characterized the dosimetric effects of creating prostate-rectum separation. Although we believe that PEG hydrogel compounds have advantages for this application, our primary goal was to characterize the effects of prostate-rectum separation, independent of the particular substance or technique used.
METHODS AND MATERIALS
Preparation and imaging of cadaveric specimens
Using an approved protocol, two refrigerated, unfixed, unfrozen, cadaveric specimens were obtained within the first 3 postmortem days. Before intervention, the specimens underwent computed tomography (CT) simulation (Philips Brilliance Big Bore CT, 3-mm slices, 120 kVp, 300 mA, 60 cm field of view). Under endorectal ultrasound guidance (7.1 MHz, B-K Medical 2101 Falcon, 8658, 4–9-MHz endorectal probe), an 18-guage, 3.5-in. needle was advanced through the perineum and into the tissue plane between the prostate and rectum. The needle tip was placed posterior to the prostate at the mid-gland position and 20 mL of 50% diluted DuraSeal (Confluent Surgical) was injected without moving the needle (13). The polymer, when injected, has the viscosity of water but, within 5 s, polymerizes and forms a soft gel (undiluted DuraSeal polymerizes more quickly; the reason we diluted the substance for these studies). Repeat CT simulation and T2-weighted magnetic resonance imaging (MRI) were performed (Siemens Espree 1.5 T MRI, repetition time, 4.0 s; excitement time, 112 ms; field of view, 14 cm; 5.0-mm slices, 256×256 matrix). Imaging was followed by dissection and preparation of histologic sections to confirm hydrogel location (Fig. 1).
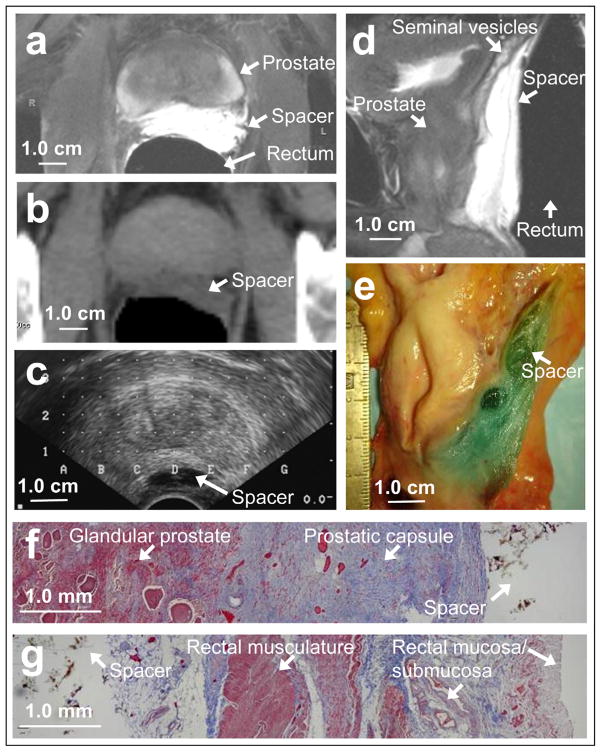
Fig. 1.
On (a) axial and (d) sagittal T2-weighted magnetic resonance imaging scans, hydrogel appears as T2-hyperintense region. On (b) computed tomography scan, hydrogel has water density, and on (c) ultrasound scan, it is hypoechoic. (e) On gross sagittal section (orientation corresponds to sagittal magnetic resonance imaging scan), blue hydrogel seen in areolar tissue separating prostate and seminal vesicles from rectum. (f) Masson’s trichrome histologic section showing glandular prostate (Left, red staining), prostatic capsule/Denonvilliers fascia (Middle, blue staining), and hydrogel (Right, nonstaining). (g) Posterior region of same section showing hydrogel and loose alveolar tissue (Left, blue/nonstaining), rectal musculature (Middle, red staining), and rectal mucosa/submucosa (Right).
Radiation planning using cadaveric data
On the CT volumes, both before and after spacer placement, the prostate, rectum, seminal vesicles, and bladder were contoured (Pinnacle3, Philips Radiation Oncology Systems, Milpitas, Ca). To avoid biases in plan evaluation, the volumes were consistently contoured on the pre- and postspacer CT volumes; all volumes differed by <1%. The mean volumes were as follows: rectum 46.5 cm3, prostate 17.2 cm3, bladder 261.0 cm3, and seminal vesicles 4.9 cm3. The clinical target volumes included either the prostate alone or the prostate and seminal vesicles. The PTV expansions were 10 mm in all directions, except for posterior, for which a 7-mm expansion was used (our current institutional practice).
Although we certainly were interested to achieve good-quality IMRT plans, our major objective was to provide a fair comparison of pre- and postspacer plans. As such, IMRT objectives and parameters were identical for all pre- and postspacer plans (i.e., the same number of beams, number of iterations, and objective functions were used). The IMRT parameters and objectives were as follows: 7 coplanar direct machine parameter optimization (DMPO), direct machine parameter optimization (DMPO), 25 iterations, PTV uniform dose 78 Gy (weight 50), PTV minimal dose 76 Gy (weight 50), rectal volume receiving ≥50 Gy of <20% (weight 1), bladder volume receiving ≥50 Gy of <20% (weight 1), PTV 2-cm ring volume receiving ≥50 Gy of <20% (weight 1).
A total of 10 IMRT plans were generated (5 using the prespacer CT scans and 5 using the postspacer CT scans). For four plans, the prostate alone was treated to 78 Gy. For four plans, the prostate and seminal vesicles were treated to 78 Gy. For the last two plans, the prostate and seminal vesicles were treated to 46 Gy, followed by a prostate cone down to 78 Gy. The target dose was prescribed to the 98% isodose. However, if the PTV receiving ≥78 Gy was <95%, the prescription isodose was lowered to achieve 95% dose coverage (but, the prescription isodose always remained between 97% and 98%). All plans had 107% hot spots of <3 cm3.
Radiation planning studies using clinical treatment plans
Nine recent, sequential prostate IMRT plans from our department were reviewed (all plans were generated by clinical dosimetrists, approved by physicians, and subsequently delivered to patients). The target volumes and doses are provided in Fig. 4. The PTV expansions were 10 mm except for the posterior, for which a 7-mm expansion was used.
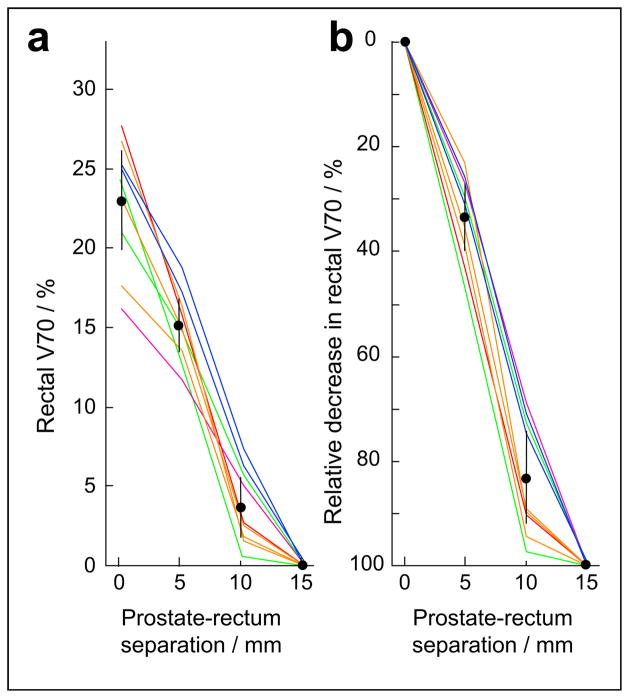
Fig. 4.
In nine, clinically generated and delivered intensity-modulated radiotherapy plans, 5, 10, and 15 mm of prostate-rectum separation were simulated and resulting rectal 70-Gy volumes calculated (a). An 83% relative reduction in mean rectal volume receiving ≥70 Gy achieved with 10 mm of separation (b). Bars indicate 95% confidence intervals. Intensity-modulated radiotherapy plans treated prostate to 78 Gy (purple), prostate plus seminal vesicles to 78 Gy (green), prostate plus seminal vesicles to 46 Gy with prostate cone-down to 78 Gy (red), prostate plus seminal vesicles plus lymph nodes to 46 Gy with prostate cone-down to 78 Gy (orange), and prostate plus seminal vesicles plus lymph nodes to 46 Gy with prostate plus seminal vesicle cone-down to 78 Gy (blue).
First, the rectal V70 was measured. Then, a new planning structure was made from the union of the prostate and seminal vesicles. This structure was expanded posteriorly by 5 mm and a new rectal structure (rectum minus 5 mm) was made by excluding the prostate plus seminal vesicles plus 5 mm from the rectum. The rectum minus 5 mm simulated the 5 mm of separation between the rectum and prostate/seminal vesicles. Similarly, a rectum minus 10 mm and rectum minus 15 mm were constructed by excluding 10 mm and 15 mm of space behind the prostate and seminal vesicles from the rectal volume, respectively. Without IMRT reoptimization, the V70 was measured for each of these new, modified rectal structures.
In vitro and in vivo studies of PEG-based hydrogel
To assay for in vitro radiation stability, hydrogel samples were formed using commercial DuraSeal kits. The precursor components were first diluted to 50% concentration using sterile water (to slow the polymerization time to approximately 5 s), the two components were injected together into 0.25-in. silicone tubing, cut into 1-cm segments, and stored in 20 mL of phosphate-buffered saline at 37 °C. One-half of the samples were irradiated to 150 Gy in a Shepherd Mark I Cesium Irradiator (J. L. Shepherd and Associates, San Fernando, CA).
The PEG hydrogel matrix is hypertonic and, therefore, particularly during the first 24 h, water is drawn in and swelling occurs (when the hydrogel is physically unconstrained). The integrity of the polymerized and cross-linked matrix resists this swelling force. Damage to the matrix (e.g., polymer cleavage due to ionizing radiation exposure) will weaken it, resulting in increased swelling. Therefore, the hydrogel percentage of swelling (during the 24 h after formation) provides a macroscopic index of the hydrogel integrity. Five irradiated and five unirradiated samples were weighed immediately after formation and again 24 h later.
Extracts from irradiated and unirradiated samples were also sent for high-performance liquid chromatography at our analytical pharmacology core laboratory using reverse-phase chromatography and gel-permeation chromatography on Days 2, 7, 21, and 35 after formation. The spectra from the irradiated and unirradiated samples were compared for new peaks, potentially indicating new radiation cleavage products.
Although PEG has an excellent biocompatibility profile (14), we also investigated whether irradiation of a PEG hydrogel implant in vivo might increase local tissue toxicity and reactivity. In five C57BL/6 mice, 0.2 mL of 50% dilute DuraSeal was injected subcutaneously in the bilateral hind flanks. Three days after injection, the left flank of each mouse was treated to 5 Gy (Shepherd Mark I Cesium Irradiator) for 5 consecutive days. After 2 weeks, the mice were killed, and the hindlimbs were harvested, fixed in 10% formalin for 48 h, decalcified for 24 h (Formacal-4), and sectioned through the implant site. A board-certified veterinary pathologist unaware of the experiment details reviewed each implant site and assigned a reaction score of 0–4 (0, no reaction; 1, nonirritant; 2, slight irritant; 3, moderate irritant; or 4, severe irritant) for each flank.
RESULTS
Implants well visualized on CT, ultrasonography, and MRI
On axial, T2-weighted MRI, the PEG hydrogel was clearly visible as a hyperintense region between the prostate and rectum (Fig. 1a). The mean separation was 12.5 mm. The sagittal MRI (Fig. 1d) showed that the separation was consistent along the length of the prostate and that separation also developed between the seminal vesicles and the rectum. On the axial CT slices (Fig. 1b), the hydrogel was seen as a water-density region between the prostate and rectum. On ultrasound imaging (Fig. 1c), the hydrogel was echolucent. Figure 1e shows a gross sagittal section corresponding to the sagittal MRI scan (dyed hydrogel appears blue). Histologic sections confirmed the location of the hydrogel posterior to the prostatic capsule/Denonvilliers fascia, within the areolar space anterior to the rectum (Fig. 1f,g).
Prostate-rectum separation reduced rectal V70 without compromising PTV coverage
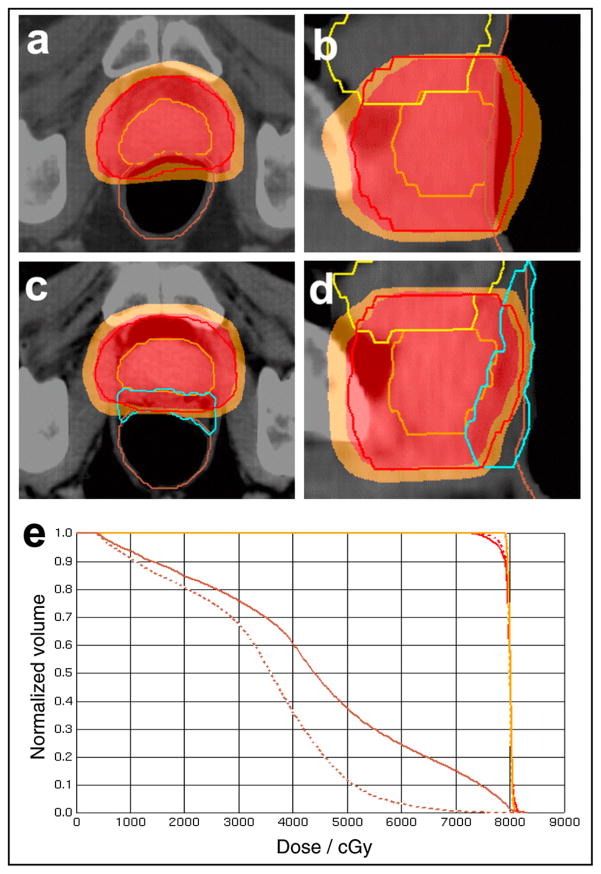
The dose distributions and dose–volume histogram from one cadaveric specimen IMRT plan are shown in Fig. 2 (prostate treated to 78 Gy). Before prostate-rectum separation (Fig. 2a,b), the 70-Gy volume (orange region) overlapped much of the anterior rectal wall. After separation, the anterior rectal wall was almost entirely excluded from the 70-Gy dose region (Fig. 2c,d). The rectal V70 decreased from 14.9% to 0.5% after prostate-rectum separation (Fig. 2e). Also, the dose homogeneity and PTV dose coverage improved (the PTV V78 increased from 95% to 97%). Most of the rectal dose reduction occurred within the range of 40–70 Gy, with relatively little change at <30 Gy.
Fig. 2.
Axial and sagittal sections of one cadaver intensity-modulated radiotherapy plan before (a,b) and after (c,d) spacer placement. Prostate (orange contour), prostate planning target volume (red contour), rectum (brown contour), hydrogel (blue contour), 78-Gy dose region (red overlay), and 70-Gy dose region (orange overlay) shown. After prostate-rectum separation (dashed dose–volume histogram), rectal dose reduced without compromising planning target volume coverage.
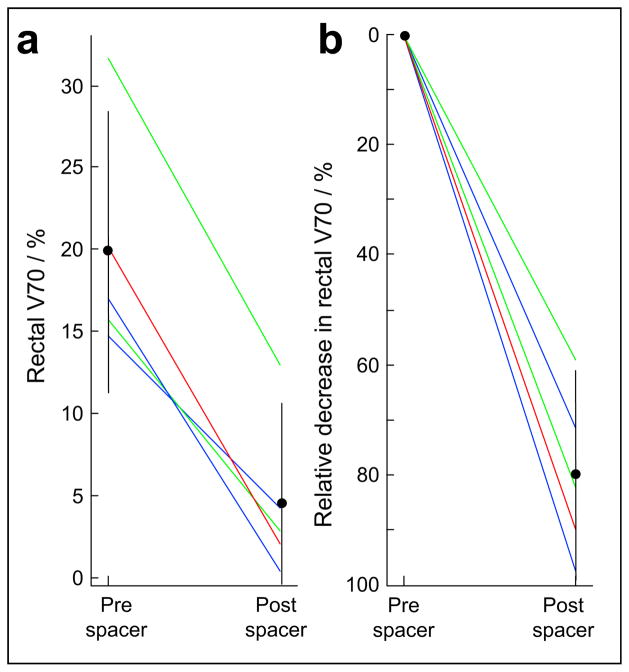
Figure 3 shows the composite data from all 10 IMRT plans generated using the cadaveric specimens (5 prespacer and 5 postspacer plans). The mean rectal V70 decreased from 19.9% before prostate-rectum separation to 4.5% after separation (p <.05). Expressed as a relative change, the prostate-rectum separation decreased the rectal V70 by 79.9% (95% confidence interval, 61.0–98.8%; p <.05). To produce comparable plans, IMRT planning parameters, objectives, and number of iterations were equal for all cases. The PTV receiving 78 Gy coverage met or exceeded 95%, and the volume with hot spots of 107% was <3 cm3.
Fig. 3.
Using cadaveric data, 10 intensity-modulated radiotherapy plans created to compare rectal radiation dose before and after spacer placement. Both (a) absolute and (b) relative rectal volume receiving ≥70 Gy (V70) significantly reduced (bars indicate 95% confidence intervals). Intensity-modulated radiotherapy plans treated prostate to 78 Gy (blue), prostate plus seminal vesicles to 78 Gy (green), and prostate plus seminal vesicles to 46 Gy with prostate cone-down to 78 Gy (red).
Separation of 10–15 mm sufficient to achieve 80% rectal dose reduction
From the cadaveric data, it was unclear how much separation would be needed to achieve an appreciable reduction in the rectal V70. In nine clinically generated and delivered IMRT plans, the rectum was “carved away” from the prostate and seminal vesicles by 5, 10, and 15 mm. Without IMRT reoptimization, the new rectal V70 were calculated. The mean rectal V70 decreased from 23.0% at baseline to 15.1%, 3.7%, and 0.0% with 5, 10, and 15 mm of separation, respectively (p <.05 for all separations compared with baseline; Fig. 4a). Expressed as a relative reduction, the mean rectal V70 decreased by 33.5%, 83.1%, and 100.0% with 5, 10, and 15 mm of separation, respectively (p <.05 for all separations; Fig. 4b). In the cadaveric studies (Fig. 3), an average of 12.5 mm of separation was generated, and the mean rectal V70 was reduced by 79.9%, which agrees well with these data. Thus, 10–15 mm of separation is sufficient to achieve the bulk of rectal V70 reduction.
Other effects of prostate-rectum separation
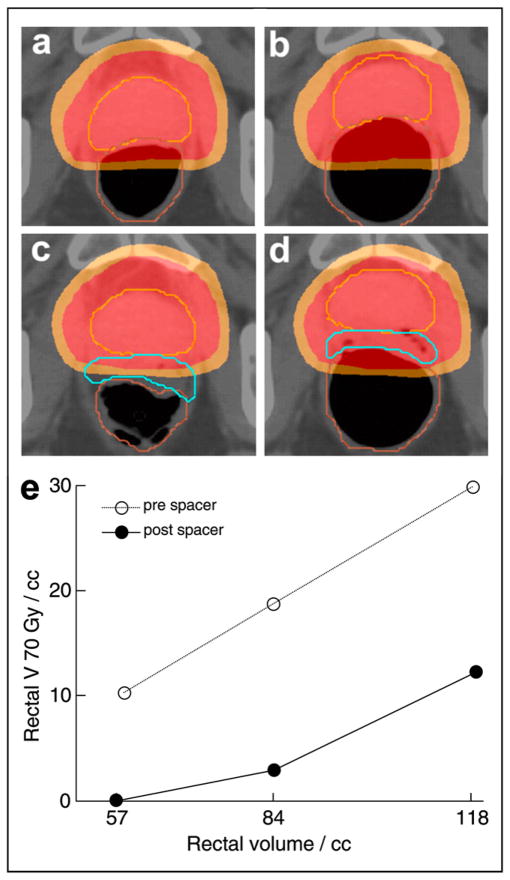
In addition to reducing the rectal V70, prostate-rectum spacing also made the treatment plans more tolerant to inadvertent changes in the rectal volume during treatment. An increase in the rectal volume from 57 cm3 to 84 cm3 and then to 118 cm3 was simulated in a cadaveric specimen using an endorectal balloon (before spacer placement; Fig. 5a,b,e). The rectal volume covered by the 70-Gy dose region increased from 10.3 to 18.7 to 29.9 cm3. However, after spacer placement, the same three rectal balloon volumes caused the rectal V70 to increase from 0.0 to 2.9 to 12.2 cm3 (Fig. 5c,d,e). Both the absolute rectal V70 and the change in the rectal V70 were decreased with prostate-rectum separation.
Fig. 5.
Using endorectal balloon, changes in rectal volume during treatment were simulated in cadaveric specimen. Before spacer placement, axial images showed increased rectal coverage by 78 Gy (red overlay) and 70 Gy (orange overlay) dose regions (a) before and (b) after rectal distension. However, after spacer placement, rectal distension caused minimal dose overlap on distension (c,d). Both absolute rectal volume receiving ≥70 Gy (V70) and rate of V70 increase were reduced after spacer placement (e).
In all plans generated and discussed, a 7-mm posterior PTV margin was used (as is our institutional practice). With increased prostate-rectum separation, however, a more generous posterior PTV expansion can be applied to decrease the possibility of a marginal miss. Using the same data and techniques discussed for Fig. 2, a 15-mm posterior PTV expansion was applied and an IMRT plan generated. Even with this large posterior PTV margin, the rectal V70 was 11.1% (percentage of PTV receiving ≥78 Gy was 96.2%).
Preclinical testing of PEG-based hydrogel
Given our interest in using PEG-based hydrogels for this application, in vitro and in vivo radiation stability testing of one PEG hydrogel (DuraSeal) was performed. Although this class of compounds has excellent biocompatibility and low tissue reactivity, their stability and reactivity in the radiation environment has not been previously established. To detect radiation-induced cleavage of the hydrogel matrix, unconstrained hydrogel swelling during the first 24 h after formation was measured (the native hydrogel is hypertonic and swells during this initial period). The polymer matrix resists swelling; therefore, increased swelling indicates damage to the polymer structure. Control, unirradiated hydrogel swelled an average of 45.2% (standard deviation, 3.0%). Irradiated hydrogel samples (treated to 150 Gy) swelled an average of 42.6% (standard deviation, 3.4%). No increased swelling was detected. To detect the formation of new, cleaved molecules after radiation exposure, reverse-phase and gel-permeability chromatography were performed on hydrogel extracts at 2, 7, 21, and 35 days after formation. No new spectral peaks were detected between the irradiated and unirradiated samples.
Five C57 Black 6 mice had 0.2-mL PEG hydrogel implants placed in the bilateral hindlimb flanks. The left flanks were irradiated to a dose of 5 Gy/d for 5 consecutive days, the mice were killed 14 days later, and hematoxylin-eosin slides of the implant sites were graded for local tissue reaction by a board-certified veterinary pathologist (reaction scale, 0, no reaction to 4, severe irritant). For both the irradiated flanks (n = 5) and the unirradiated flanks (n = 5), the mean reaction score was 1.8 (slight irritant). The mean difference in each mouse’s reaction score (irradiated flank minus unirradiated flank) was 0.0 (standard deviation, 1.4), indicating no increased tissue reaction from irradiation of the PEG hydrogel.
DISCUSSION
Separating the rectum from the prostate significantly reduces the link between the rectal and prostate radiation dose. Clinically, we can consider the potential utility of this from several perspectives. First, and most obviously, one could simply integrate this technique with the current paradigm of daily, image-guided, highly conformal, dose-escalated RT. As shown in the present study, extremely low rectal doses can be achieved and, therefore, a very low risk of rectal toxicity would be expected while maintaining current cure rates. Second, with reduced rectal dose and toxicity, increases in treatment intensity by additional dose escalation, hypofractionation, or combined chemoradiotherapy might be possible. However, when increasing the radiation dose to all or part of the prostate, other toxicities (owing to damage to the neurovascular bundles or urethra) could become limiting. Third, this technique could be used to reduce dependence on complex planning, treatment delivery and daily imaging techniques. As shown, low rectal V70 can be achieved despite generous posterior PTV margins. Also, as shown in one simplified example using a cadaveric specimen (Fig. 5), spacer placement can help to reduce the negative effects of rectal motion. Given the current and potential restrictions in health-care spending (in the United States and abroad), as well as the high incidence of prostate cancer, this perspective should not be dismissed.
The possible risks of this method should also be considered. In pilot clinical work, the acute and chronic tolerance of implants between the prostate and rectum have been excellent (9–11). Although 3 of 10 men in one study reported light rectal pressure acutely after collagen injection, we are unaware of any chronic symptoms caused by this procedure (9–11). Another concern is whether this technique could compromise local control rates by reducing the rectal radiation dose. A pathologic series of 243 radical prostatectomy specimens detected no local extension beyond Denonvilliers fascia (6). However, small pathologic series have also reported prostate cancer involvement of the rectal wall (15–17). In one series, prostate cancer involving the rectum was found in 30 cases at Johns Hopkins between 1987 and 2006 (18). All specimens showed high-grade prostate cancer (Gleason score 8–10) with 87% having a Gleason score of 9–10. Although the route of spread to the rectal wall in these cases was not known, the possibilities include direct extension, extension by the lymphatics, or seeding on biopsy. All men presented with either rectal bleeding, tenesmus, or obstruction, and 83% had a previous diagnosis of prostate cancer. Certainly, this separation technique should be contraindicated in any patient with imaging abnormalities posterior to the prostate. However, in men without findings on examination or imaging, the question remains whether a reduced rectal radiation dose could protect microscopic cancer that resides in the anterior rectal wall. Although this might be possible, it seems unlikely to affect the recurrence rates because the anterior rectal wall still receives ≥50 Gy (in all cases presented), and the baseline risk of involvement is small.
A few candidate compounds/devices are currently being examined for producing prostate-rectum separation. Our primary goal in the present study was not to promote one particular technique. However, to better understand this procedure, it is instructive to consider the relative merits and difficulties. Both collagen and hyaluronic acid have performed well in pilot clinical work (9–11). However, issues such as side effect rates, spacer persistence time, and cost could affect eventual clinical adoption. Collagen is available in both human and animal-derived forms. However, although human-derived collagen has excellent biocompatibility, it is very expensive and can be difficult to obtain (especially in the quantities necessary for widespread use of this technique) (19). Animal-derived collagen, although more widely available, is associated with immunologic reactions (20). Similarly, the high cost can be an issue for hyaluronic acid compounds (19). Although generally very well tolerated, occasional granulomatous reactions have been reported after hyaluronic acid injection (21–23). Both collagen and hyaluronic acid are associated with very long residence times between the prostate and rectum, with hyaluronic acid implants largely unchanged 1 year after placement (10). Although the stability of the implant might be a positive factor during the treatment period, a long residence time could increase the chronic toxicity rates. However, short residence times, such as the 4–8 weeks for DuraSeal, could also be problematic because changes could occur during treatment (other, related PEG compounds have longer residence times). In our experience, PEG compounds have the advantage of inexpensive manufacturing, excellent biocompatibility (PEG is routinely used in medications such as GoLYTELY, PEG-interferon-α, and PEG-filgrastim), and a shorter, controllable residence time. Although it is unclear which of these approaches (or another entirely) will prove optimal, multiple avenues of development do indicate a significant level of interest in this technique.
Acknowledgments
In vitro work was supported in part by National Institutes of Health Grants P30CA006973 and UL1RR025005.
We acknowledge Cory Brayton, D.V.M., for reviewing and scoring the mouse histology sections; Ming Zhao, Ph.D., Michelle A. Rudek, Pharm.D., Ph.D., Sarah Reinhardt, B.S., and Aleksandr Mnatsakanyan, M.D, for performing the chromatography analysis.
Footnotes
Conflict of interest: Johns Hopkins University, Department of Radiation Oncology and Molecular Radiation Sciences received an unrestricted gift from Augmenix. These funds were used, in part, to support this work. None of the authors have a financial interest in Augmenix or any other conflicts of interest.
References
- 1.Al-Mamgani A, Heemsbergen WD, Peeters ST, et al. Role of intensity-modulated radiotherapy in reducing toxicity in dose escalation for localized prostate cancer. Int J Radiat Oncol Biol Phys. 2009;73:685–691. doi: 10.1016/j.ijrobp.2008.04.063. [DOI] [PubMed] [Google Scholar]
- 2.Zelefsky MJ, Levin EJ, Hunt M, et al. Incidence of late rectal and urinary toxicities after three-dimensional conformal radiotherapy and intensity-modulated radiotherapy for localized prostate cancer. Int J Radiat Oncol Biol Phys. 2008;70:1124–1129. doi: 10.1016/j.ijrobp.2007.11.044. [DOI] [PubMed] [Google Scholar]
- 3.Huang EH, Pollack A, Levy L, et al. Late rectal toxicity: Dose–volume effects of conformal radiotherapy for prostate cancer. Int J Radiat Oncol Biol Phys. 2002;54:1314–1321. doi: 10.1016/s0360-3016(02)03742-2. [DOI] [PubMed] [Google Scholar]
- 4.Burman C, Chui CS, Kutcher G, et al. Planning, delivery, and quality assurance of intensity-modulated radiotherapy using dynamic multileaf collimator: A strategy for large-scale implementation for the treatment of carcinoma of the prostate. Int J Radiat Oncol Biol Phys. 1997;39:863–873. doi: 10.1016/s0360-3016(97)00458-6. [DOI] [PubMed] [Google Scholar]
- 5.Lindsey I, Guy RJ, Warren BF, et al. Anatomy of Denonvilliers fascia and pelvic nerves, impotence, and implications for the colorectal surgeon. Br J Surg. 2000;87:1288–1299. doi: 10.1046/j.1365-2168.2000.01542.x. [DOI] [PubMed] [Google Scholar]
- 6.Villers A, McNeal JE, Freiha FS, et al. Invasion of Denonvilliers fascia in radical prostatectomy specimens. J Urol. 1993;149:793–798. doi: 10.1016/s0022-5347(17)36209-2. [DOI] [PubMed] [Google Scholar]
- 7.Onik G. Image-guided prostate cryosurgery: State of the art. Cancer Control. 2001;8:522–531. doi: 10.1177/107327480100800607. [DOI] [PubMed] [Google Scholar]
- 8.Saliken JC, Donnelly BJ, Rewcastle JC. The evolution and state of modern technology for prostate cryosurgery. Urology. 2002;60:26–33. doi: 10.1016/s0090-4295(02)01681-3. [DOI] [PubMed] [Google Scholar]
- 9.Noyes WR, Noyes WR. Fillers and methods for displacing tissues to improve radiological outcomes. 2004/0094162 A1 US Patent.
- 10.Prada PJ, Gonzalez H, Menendez C, et al. Transperineal injection of hyaluronic acid in the anterior perirectal fat to decrease rectal toxicity from radiation delivered with low-dose-rate brachytherapy for prostate cancer patients. Brachytherapy. 2009;8:210–217. doi: 10.1016/j.brachy.2008.11.010. [DOI] [PubMed] [Google Scholar]
- 11.Prada PJ, Fernandez J, Martinez AA, et al. Transperineal injection of hyaluronic acid in anterior perirectal fat to decrease rectal toxicity from radiation delivered with intensity modulated brachytherapy or EBRT for prostate cancer patients. Int J Radiat Oncol Biol Phys. 2007;69:95–102. doi: 10.1016/j.ijrobp.2007.02.034. [DOI] [PubMed] [Google Scholar]
- 12.BioProtect: Evaluation of the safety and efficacy of the BioProtect balloon in prostate cancer subjects undergoing radiotherapy: NCT004621. [Accessed August 18, 2009];2008 :24. Available at: www.clinicaltrials.gov.
- 13.Confluent Surgical Inc. DuraSeal Dural Sealant System Instructions for Use. Waltham, MA: Confluent Surgical, Inc; 2007. [Google Scholar]
- 14.Food and Drug Administration. [Accessed August 18, 2009];DuraSeal Dural Sealant System: Summary of Safety and Effectiveness Data, 11/30/2004. Available at: www.fda.gov/MedicalDevices/ProductsandMedicalProcedures/DeviceApprovalsandClearances/Recently-ApprovedDevices/ucm078645.htm.
- 15.Bailey CM, Gilbert JM. Avoiding inappropriate surgery for secondary rectal cancer. Eur J Surg Oncol. 2002;28:220–224. doi: 10.1053/ejso.2001.1240. [DOI] [PubMed] [Google Scholar]
- 16.Bowrey DJ, Otter MI, Billings PJ. Rectal infiltration by prostatic adenocarcinoma: Report on six patients and review of the literature. Ann R Coll Surg Engl. 2003;85:382–385. doi: 10.1308/003588403322520726. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Murray SK, Breau RH, Guha AK, et al. Spread of prostate carcinoma to the perirectal lymph node basin: Analysis of 112 rectal resections over a 10-year span for primary rectal adenocarcinoma. Am J Surg Pathol. 2004;28:1154–1162. doi: 10.1097/01.pas.0000131543.80147.3d. [DOI] [PubMed] [Google Scholar]
- 18.Lane Z, Epstein JI, Ayub S, et al. Prostatic adenocarcinoma in colorectal biopsy: Clinical and pathologic features. Hum Pathol. 2008;39:543–549. doi: 10.1016/j.humpath.2007.08.011. [DOI] [PubMed] [Google Scholar]
- 19.2008 Drug Topics Red Book. Montvale, NJ: Blackwell; 2008. [Google Scholar]
- 20.Gorton E, Stanton S, Monga A, et al. Periurethral collagen injection: A long-term follow-up study. BJU Int. 1999;84:966–971. doi: 10.1046/j.1464-410x.1999.00321.x. [DOI] [PubMed] [Google Scholar]
- 21.Edwards PC, Fantasia JE, Iovino R. Foreign body reaction to hyaluronic acid (Restylane): An adverse outcome of lip augmentation. J Oral Maxillofac Surg. 2006;64:1296–1299. doi: 10.1016/j.joms.2006.04.028. [DOI] [PubMed] [Google Scholar]
- 22.Pinheiro MV, Bagatin E, Hassun KM, et al. Adverse effect of soft tissue augmentation with hyaluronic acid. J Cosmet Dermatol. 2005;4:184–186. doi: 10.1111/j.1473-2165.2005.00309.x. [DOI] [PubMed] [Google Scholar]
- 23.Ghislanzoni M, Bianchi F, Barbareschi M, et al. Cutaneous granulomatous reaction to injectable hyaluronic acid gel. Br J Dermatol. 2006;154:755–758. doi: 10.1111/j.1365-2133.2005.07074.x. [DOI] [PubMed] [Google Scholar]