Abstract
Objective:
To examine the potential utility of conventional MRI signs in differentiating pseudoprogression (PsP) from early progression (EP).
Methods:
This retrospective study reviewed initial postradiotherapy MRI scans of 321 patients with glioblastoma undergoing chemotherapy and radiotherapy. A total of 93 patients were found to have new or increased enhancing mass lesions, raising the possibility of PsP. Final diagnosis of PsP or EP was established upon review of surgical specimens from a second resection or by clinical and radiologic follow-up. A total of 11 MRI signs potentially helpful in the differentiation between PsP and EP were examined on the initial post-RT MRI and were correlated with the final diagnosis through χ2 or Fisher exact test.
Results:
Sixty-three (67.7%) of the 93 patients had EP, of which 22 (34.9%) were diagnosed by pathology. Thirty patients (32.3%) had PsP; 6 (16.7% of the 30) were diagnosed by pathology. Subependymal enhancement was predictive for EP (p = 0.001) with 38.1% sensitivity, 93.3% specificity, and 41.8% negative predictive value. The other 10 signs had no predictive value (p = 0.06–1.0).
Conclusions:
Conventional MRI signs have limited utility in diagnosing PsP in patients with recently treated glioblastomas and worsening enhancing lesions. We did not find a sign with a high negative predictive value for PsP that would have been the most useful for the clinical physician. When present, subependymal spread of the enhancing lesion is a useful MRI marker in identifying EP rather than PsP. Neurology® 2011;76:1918–1924
Radiation therapy (RT) plus concurrent and adjuvant chemotherapy with temozolomide (TMZ) is the standard of care for patients with newly diagnosed glioblastoma.1 Soon after completion of RT, patients may demonstrate pseudoprogression (PsP), defined as the transient worsening of enhancing abnormalities or mass effect on MRI. PsP may occur in 14%–31% of patients with treated malignant glioma,2–5 and up to 58% of patients with methylated O6-methylguanine-methyltransferase enzyme (MGMT) promoter status.6 PsP is thought to be due to potentiated radiation-induced tissue injury with associated inflammatory reaction and necrosis. The worsening lesions, which reflect treatment effects rather than treatment failure, subsequently stabilize or improve and are not correlated with poorer outcomes.7
The increased or new enhancing lesions of PsP and early progression (EP) may both fulfill criteria for worsening disease when applying standard response criteria.8 There is therefore a need for improved imaging biomarkers to distinguish EP from PsP in order to optimize patient treatments and the study design of clinical trials. Identifying conventional MRI signs to determine PsP may spare these patients from unnecessary surgery or inappropriate and possibly more toxic chemotherapy, both of which may become necessary later in the course of the disease. The purpose of this article is to examine the potential utility of conventional MRI signs in differentiating PsP from EP.
METHODS
Standard protocol approvals, registrations, and patient consents.
This retrospective study was granted a Waiver of Informed Consent by the Memorial Sloan-Kettering Cancer Center Institutional Review Board. With the approval of the hospital Privacy Board and compliant with Health Insurance Portability and Accountability Act regulations, we retrospectively identified 321 consecutive patients with glioblastoma who were treated between January 2003 and November 2009 from the hospital database.
Patients.
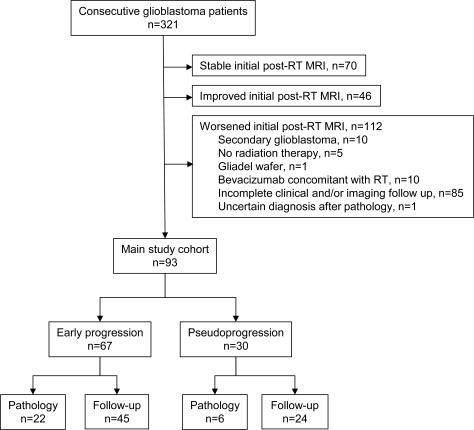
All patients had a pathologic diagnosis of glioblastoma according to revised World Health Organization criteria after biopsy, subtotal resection, or gross total resection. Selection of the main study cohort is summarized in figure 1. Only newly diagnosed patients with primary glioblastomas who underwent initial combination RT and chemotherapy treatment were included in this study. A total of 112 patients were excluded due to transformed low-grade or anaplastic glioma (n = 10), no RT (n = 5), Gliadel wafer implantation (n = 1), bevacizumab concomitant with RT (n = 10), incomplete clinical or imaging follow-up (n = 85), or uncertain diagnosis after pathology (n = 1). Upon review of initial post-RT MRI findings, a total of 93 patients with either EP or PsP were identified, as per the following inclusion criteria: 1) successfully completed RT with concurrent chemotherapy; 2) developed worsening (new or increased) enhancing mass lesions on the initial post-RT MRI (usually 2–4 weeks after completion of RT) as compared to the pre-RT MRI (usually <48 hours before beginning RT); and 3) diagnosis of the worsening lesions by either pathology after repeat resection, or, when pathology was not available, by clinical and imaging follow-up assessed every 1–2 months. These patients constituted the main study cohort and were reviewed as further described below. The remaining 116 patients who did not display worsening disease (stable, n = 70, or improved, n = 46) on the initial post-RT MRI did not undergo additional analysis, aside from collection of mortality/survival data.
Figure 1. STARD diagram.
RT = radiation therapy.
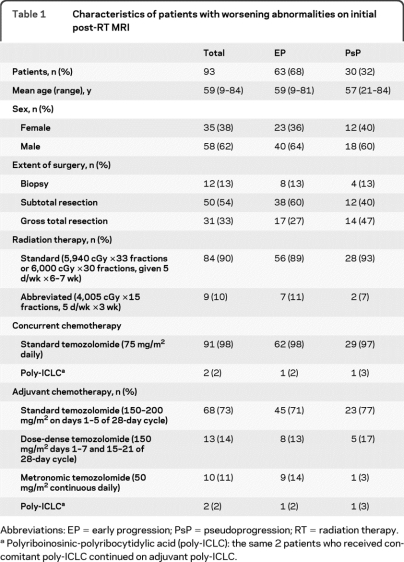
All 93 patients in the main study cohort underwent partial brain external beam RT using conventional or intensity modulated planning. As summarized in table 1, most patients (90%) received a standard course of RT with 5,940 or 6,000 cGy given over 6–7 weeks. Some poorly functioning or elderly patients (10%) who were considered by the radiation oncologist as unlikely to complete the standard course received an abbreviated course of RT to 4,005 cGy given over 3 weeks, an acceptable alternative.9
Table 1.
Characteristics of patients with worsening abnormalities on initial post-RT MRI
Abbreviations: EP = early progression; PsP = pseudoprogression; RT = radiation therapy.
Polyriboinosinic-polyribocytidylic acid (poly-ICLC): the same 2 patients who received concomitant poly-ICLC continued on adjuvant poly-ICLC.
Almost all patients (97.8%) received TMZ at standard doses (75 mg/m2) daily concomitant with RT. For adjuvant therapy after completion of RT, most patients (75.2%) received standard TMZ (150–200 mg/m2 × 5/28 days), while others were included in a phase 2 trial10 randomizing patients to receive either low-dose metronomic TMZ (14.0%) at 50 mg/m2/day or dose-dense TMZ (10.8%) at 150 mg/m2/day 1 week on and 1 week off. Only 2 patients (2.2%) did not receive TMZ; they both received polyriboinosinic-polyribocytidylic (poly-ICLC) concomitant with RT followed by adjuvant poly-ICLC.
When available, MGMT methylation status was obtained through chart review. The methylation of this DNA repair enzyme promoter was determined by methylation-specific PCR analysis. Decisions to perform MGMT assays were based on enrollment into a clinical trial requiring MGMT analysis for some patients, or as part of an emerging standard of practice for all patients with glioblastoma seen at Memorial Sloan-Kettering Cancer Center toward the end of the study.
Diagnosis of PsP and EP.
The worsening lesions on the initial post-RT MRI was determined to represent either EP or PsP based on pathologic analysis after repeat biopsy or resection when available. PsP was characterized when necrotizing treatment effects were present with no to minimal identifiable tumor. The presence of residual or recurrent tumor established EP.
If repeat pathology was not available, the clinical diagnosis of EP or PsP was made by consensus of 2 neuro-oncologists (with 3 and 10 years of experience) after complete chart and imaging review. The diagnosis of PsP was made if no change in treatment was required for a minimum of 6 months from the end of RT. This definition allows for the continued mild increase of the worsening enhancing lesions, as compared to the usual decrease or stabilization, as long as no treatment change occurred during this time period. The diagnosis of EP was made if imaging or clinical worsening prompted a change in treatment. Diagnosis was made while blinded to the patient's MGMT status.
MRI parameters.
MRI was obtained using 1.5 T (Signa Excite) and 3 T magnets (Discovery 750, GE Healthcare, Waukesha, WI). All studies were acquired using 5-mm slice thickness and no interslice gap. For 1.5 T, axial fast spin-echo T2-weighted (repetition time [TR]/echo time [TE] = 4,000/100 msec, matrix 256 × 256); axial fluid-attenuated inversion recovery (FLAIR; TR/TE/inversion time [TI] = 10,000/160/2,200 msec, matrix 256 × 256); sagittal and axial T1-weighted; and contrast coronal, sagittal, and axial T1-weighted images (TR/TE = 500/10 msec, matrix 256 × 256) were obtained. For 3 T, axial fast spin-echo T2-weighted (TR/TE = 4,000/100 msec, matrix 512 × 512); axial FLAIR (TR/TE/TI = 9,000/125/2,250 msec, matrix 512 × 512); sagittal and axial T1-weighted/FLAIR; and contrast coronal, sagittal, and axial T1-weighted/FLAIR images (TR/TE/TI = 2,500/6/860 msec, matrix 512 × 512) were obtained. Gadopentetate dimeglumine (Magnevist, HealthCare Pharmaceuticals Inc. Wayne, NJ) was injected though a peripheral angiocatheter (18–21 gauge) at a standard dose (0.2 mL/kg body weight, maximum 20 mL). The same dose of contrast was administered for both 1.5 T and 3 T scans. The mean ± SD of the first, second, and third MRI scans obtained after completing RT were 20 ± 9 days, 90 ± 30 days, and 150 ± 38 days, respectively.
Conventional MRI signs.
Two neuroradiologists (1 with 5 years experience and the other with 10 years experience and holding a Certificate of Added Qualification in Neuroradiology) blinded to the final diagnosis of EP or PsP described by consensus the worsening lesions on the initial post-RT MRI according to these signs: 1) new enhancement; 2) marginal enhancement around the surgical cavity; 3) nodular enhancement; 4) callosal enhancement; 5) subependymal enhancement; 6) spreading wavefront of enhancement; 7) cystic or necrotic change; 8) increased peritumoral T2 abnormality; and 9) diffusion restriction. Diffusion restriction was visually determined as hyperintense signal on diffusion-weighted images (DWI) and corresponding hypointense signal on the apparent diffusion coefficient (ADC) maps, while avoiding areas of hemorrhage and calcification. Two additional parameters were described by comparing the initial post-RT MRI to subsequent post-RT MRIs performed over the next 2–5 months to evaluate evolution of the enhancing lesions prior to any change in treatment; 10) decreasing enhancement intensity; and 11) increasing cystic or necrotic change.
Statistical analysis.
Univariate analysis using χ2 or Fisher exact test was performed to determine the relative utility of the conventional MRI signs in predicting PsP vs EP. Univariate analyses were also performed to determine potential correlations with the degree of resection (gross total vs subtotal vs biopsy), dose of RT (dichotomized at 5,940 cGy), and different schedules for adjuvant TMZ administration. A Fisher exact test was used to test the potential correlation between diagnosis and MGMT status. Statistical significance was set at p = 0.05.
Overall survival (OS) among the different groups was calculated from the date of RT completion to the date of death or last follow-up using the Kaplan-Meier method. OS curves were compared using the log-rank test.
RESULTS
The characteristics and treatments received by the 93 main analysis patients are summarized in table 1. Sixty-three (67.7%) of the 93 patients were determined to have EP, of which 22 (34.9% of the 63 patients) were diagnosed by repeat pathology. Thirty patients (32.3%) were determined to have PsP, of which 6 (16.7% of the 30 patients) were diagnosed by repeat pathology. Of the other 24 patients diagnosed with PsP, follow-up showed that the enhancing disease had improved in 8, stabilized in 9, slightly worsened (by <25%) in 5, and worsened (by >25%) then stabilized in 2. The diagnosis of EP or PsP in all cases was made prior to any change in treatment.
Conventional MRI signs.
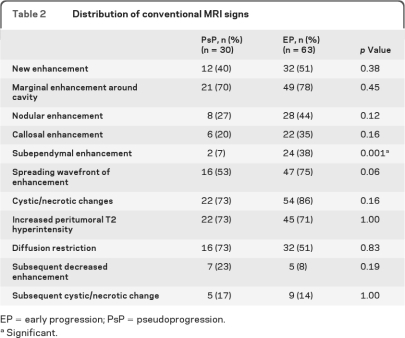
The conventional MRI results are summarized in table 2. Only subependymal enhancement was found to be predictive for EP (p = 0.001), with 38.1% sensitivity, 93.3% specificity, 92.3% positive predictive value, and 41.8% negative predictive value. A representative case is shown in figure 2. The other 10 signs had no predictive value (p = 0.06 to 1.0). Among the patients who displayed new enhancement, all lesions occurred at or immediately adjacent to the primary tumor site; no patient developed remote enhancement (i.e., >3 cm away).
Table 2.
Distribution of conventional MRI signs
EP = early progression; PsP = pseudoprogression.
Significant.
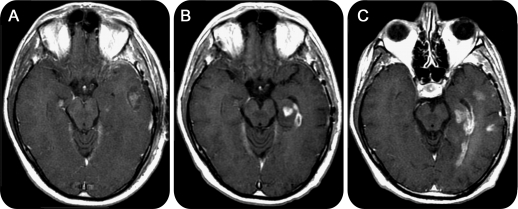
Figure 2. Subependymal enhancement in a patient with early progression.
Contrast T1-weighted images. Pre–radiation therapy (RT) and 1 day after resection (A), the patient shows a left pterional craniotomy with a blood/fluid-filled surgical cavity in the temporal lobe and minute enhancement in the mesial temporal lobe. One month post-RT (B), there is increased enhancement in the mesial temporal lobe and new enhancement along the subependymal margin of the temporal horn. Five months post-RT (C), the subependymal enhancement has extended posteriorly to the occipital horn, and there are 2 new sites of enhancement in the lateral temporal lobe. The patient continued to show clinical deterioration, and treatment was switched from adjuvant temozolomide to bevacizumab for tumor progression.
Clinical variables.
Information on MGMT promoter methylation status was available in 22 patients. Among those, MGMT promoter methylation was detected in 5 patients, all of them in the EP group. Unmethylated MGMT promoter was found in 11 patients with EP and 6 patients with PsP. There was no association between EP/PsP status and MGMT status (p = 0.27), nor with the degree of resection (p = 0.14), dose of RT (p = 0.49), or different schedules of adjuvant TMZ (p = 0.27).
Overall survival.
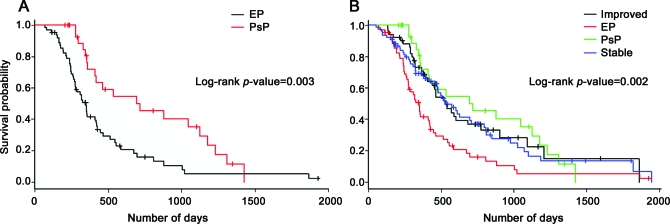
Among the patients with worsening initial post-RT MRI (n = 93), the median OS was 318 days (range, 70–1,926 days) for the EP group and 440 days (206–1,422 days) for the PsP group. Survival was longer for the PsP group as compared to the EP group (p = 0.003). Median OS was 459 days (52–1,943 days) for patients with stable initial post-RT MRI, and 463 days (132–1,861 days) for patients with improved initial post-RT MRI. OS of the stable and improved groups was similar to that of the PsP group (p = 0.75) and different from the EP group (p = 0.0002). Kaplan-Meier curves are shown in figure 3. No differences in survival according to MGMT promoter methylation status could be detected (p = 0.94; n = 22), although analysis was limited by the low number of patients with methylated MGMT promoter.
Figure 3. Kaplan-Meier survival curves.
(A) Overall survival of the pseudoprogression (PsP) group was longer than of the early progression (EP) group. (B) Overall survival of the EP group was shorter than the PsP, stable, and improved groups. The latter 3 groups had similar overall survival.
DISCUSSION
Reliable imaging biomarkers are necessary to efficiently conduct clinical trials that compare the efficacy of new therapies. Nearly 75% of oncologic clinical trials rely on surrogate imaging endpoints rather than patient survival,11 with most clinical trials for malignant glioma treatment using modified Macdonald criteria to determine treatment response.8 A major limitation of the Macdonald criteria, which were developed 2 decades ago, is the reliance on changes in size of the enhancing tumor. PsP may be indistinguishable from EP using these response criteria. Recognizing this difficulty in diagnosis, many phase II trials for recurrent malignant gliomas exclude patients with worsening enhancing lesions within 3 months after RT.4,5,12 Although advanced imaging techniques such as MR perfusion, MR spectroscopy, and PET may have increased accuracy and sensitivity, these technologies are not as well-studied or ubiquitously available as conventional MRI. As a result, even the most recent attempts to establish new criteria by the Response Assessment by Neuro-Oncology13 group rely on conventional MRI characteristics (contrast enhancement and T2/FLAIR signal abnormality). Determining conventional MRI signs that would best determine PsP would assist the management of these patients and potentially impact clinical decision-making processes.
In this study, we report the largest cohort of patients systematically reviewed for MRI findings that could differentiate PsP from EP. We found that subependymal enhancement predicts the development of EP rather than PsP in worsening enhancing lesions that occur soon after completion of combination chemoradiation therapy. Although subependymal enhancement has high specificity for EP (93.3%), its low sensitivity (38.1%) and low negative predictive value (41.8%) suggest that it may have only limited utility in the majority of patients with suspected PsP.
Subependymal spread of tumor is a known pattern of glioma failure, although it is less common than local progression.14,15 Infiltration of the margins of the ventricles may occur by direct spread of tumor cells in the subependymal space or by deposits transferred by the CSF.16 One study of 51 multifocal gliomas (of which 31 were glioblastoma) reported subependymal spread to be the second most common route for disseminated disease at 24%.17 Less frequent rates of subependymal or spinal spread have been described by other groups,14 ranging from 0% to 14%.
Conventional MRI signs to distinguish radiation necrosis from tumor recurrence have been investigated in patients with malignant gliomas. One study of 27 patients did not find individual signs to be useful predictors for tumor recurrence, although combining 2 signs with involvement of the corpus callosum and multiple enhancing lesions was useful (p = 0.02), as were combining 3 signs with involvement of the corpus callosum, multiple enhancing lesions, and crossing of the midline (p = 0.04) or subependymal spread (p = 0.01).18 The lack of significance for individual signs such as subependymal spread (p = 0.26),18 in contrast to the findings in our study (p = 0.001), may reflect the smaller number of patients or differing central distributions of lesions in that series. In addition, those patients all had new enhancing lesions that occurred more than 6 months after proton beam RT, which were more consistent with radiation necrosis than with PsP. The 2 entities are similar but not synonymous, with PsP showing earlier onset after completion of RT at 1–3 months that reflects an intermediate stage between subacute radiation reaction and later radiation necrosis at 6–18 months or more.2
Treatment-related necrosis can also occur in the periventricular region and mimic subependymal spread of tumor.19 This is thought to reflect the relatively poor vascularity of the periventricular region, which is supplied by long medullary arteries without collateral supply that are vulnerable to radiation-induced vasculopathy.19 The low incidence of subependymal enhancement in PsP (6.7%) found in our study, however, suggests that this complication may be less frequent than direct spread to the subependymal region by centrally located tumors. Treatment-related necrosis becomes more common with increasing total doses, high fraction doses, hyperfractionation, and concurrent chemotherapy.2,20 The majority of our patients were treated with standard RT plans, with relatively equal small proportions of the EP and PsP groups instead receiving abbreviated RT plans that are acceptable alternatives.9 Although we did not detect a correlation with the RT dose, further examination of the potential relationship between RT dose and fields with subependymal enhancement may be useful.
Survival has been reported as longer in patients with methylated MGMT promoter status who receive temozolomide.6,21 A study attempting to correlate MGMT status and PsP found that methylated MGMT promoter status is a strong predictor of PsP, occurring in 21/23 (91.3%) of methylated vs 11/27 (40.7%) of unmethylated patients (p = 0.0002).6 Less than a quarter (23.7%) of our patients had known MGMT promoter status, with methylated MGMT promoter detected in 5 patients with EP, including 2 patients with pathologic confirmation of their EP status, and none of the patients with PsP. The low number of patients precludes further analysis, although this finding does highlight the unreliability of using MGMT status to predict PsP or EP in an individual patient. We are prospectively collecting molecular and genetic data in contemporary patients, and plan a separate project to specifically examine the potential relationship between MRI and MGMT status.
One potential limitation of our study relates to the lack of a widely accepted definition of PsP. Specific clinical, imaging, and pathologic criteria for the diagnosis of PsP were established for this study, including patients who did not require additional treatment for a minimum of 6 months. Although this may have underestimated the true incidence of PsP, the primary intent of the study was to determine clinically useful conventional MRI signs that could guide treatment decisions by confidently identifying the patients who would not have required a change in treatment. We recognize that this definition also potentially biased the survival analysis, since the condition of a 6-month interval was not imposed upon the EP group.
Another limitation is that most but not all patients received standard RT and TMZ chemotherapy as established by Stupp et al.1 Since PsP or early treatment-related necrosis has been described with other RT and chemotherapy regimens,19,22 the heterogeneity of TMZ vs non-TMZ treatment and differing adjuvant TMZ schedules should have little effect on the analysis. In addition, the proportion of patients determined to have PsP (32.3%) is similar to previously published rates.2–5 The majority (75.2%) of patients in this study received adjuvant TMZ according to the standard 5/28 day cycle. We did not detect a correlation between PsP and TMZ schedule (p = 0.27). It is possible that a more effective adjuvant dosing schedule could cause EP to remit and mimic PsP, although differential rates of PsP have not been described with dose-dense vs metronomic TMZ treatment.10
Conventional MRI signs have limited utility in the diagnosis of PsP in patients with recently treated glioblastomas and worsening enhancing lesions. The distinction is important for making treatment decisions, patient counseling, and establishing prognosis. We did not find a sign with a high negative predictive value for PsP, which would have provided the most useful information for treating clinicians. When present, direct subependymal spread of the enhancing lesion is a useful MRI marker in identifying EP rather than PsP. Additional research into advanced imaging modalities or biomarkers such as MRI perfusion, diffusion tensor, spectroscopy, and PET/CT is necessary.
ACKNOWLEDGMENT
The authors thank Judith A. Lampron for editorial advice.
Footnotes
- ADC
- apparent diffusion coefficient
- DWI
- diffusion-weighted image
- EP
- early progression
- FLAIR
- fluid-attenuated inversion recovery
- MGMT
- O6-methylguanine-methyltransferase enzyme
- OS
- overall survival
- poly-ICLC
- polyriboinosinic-polyribocytidylic
- PsP
- pseudoprogression
- RT
- radiation therapy
- TE
- echo time
- TI
- inversion time
- TMZ
- temozolomide
- TR
- repetition time
AUTHOR CONTRIBUTIONS
Statistical analysis was conducted by Dr. Zhigang Zhang and Weiji Shi. Dr. Robert J. Young takes full responsibility for the data, analyses and interpretation, and the conduct of the research, and has full access to all of the data with the right to publish all data separate and apart from any sponsor.
DISCLOSURE
Dr. Young, Dr. Gupta, Dr. Shah, Dr. Graber, Dr. Zhang, and W. Shi report no disclosures. Dr. Holodny has served on a scientific advisory board for Bayer Schering Pharma and serves on the editorial advisory board of the American Journal of Neuroradiology. Dr. Omuro served as a Section Editor for Current Opinion in Neurology and receives research support from Genentech, Inc., Schering-Plough Corp/Merck Serono, Exelixis Inc., the NIH/NCI, the BCured Foundation, and the Collaborative Ependymoma Research Network (CERN).
REFERENCES
- 1. Stupp R, Mason WP, van den Bent MJ, et al. Radiotherapy plus concomitant and adjuvant temozolomide for glioblastoma. N Engl J Med 2005;352:987–996 [DOI] [PubMed] [Google Scholar]
- 2. Brandsma D, Stalpers L, Taal W, Sminia P, van den Bent MJ. Clinical features, mechanisms, and management of pseudoprogression in malignant gliomas. Lancet Oncol 2008;9:453–461 [DOI] [PubMed] [Google Scholar]
- 3. Chamberlain MC, Glantz MJ, Chalmers L, Van Horn A, Sloan AE. Early necrosis following concurrent Temodar and radiotherapy in patients with glioblastoma. J Neurooncol 2007;82:81–83 [DOI] [PubMed] [Google Scholar]
- 4. de Wit MC, de Bruin HG, Eijkenboom W, Sillevis Smitt PA, van den Bent MJ. Immediate post-radiotherapy changes in malignant glioma can mimic tumor progression. Neurology 2004;63:535–537 [DOI] [PubMed] [Google Scholar]
- 5. Taal W, Brandsma D, de Bruin HG, et al. Incidence of early pseudo-progression in a cohort of malignant glioma patients treated with chemoirradiation with temozolomide. Cancer 2008;113:405–410 [DOI] [PubMed] [Google Scholar]
- 6. Brandes AA, Franceschi E, Tosoni A, et al. MGMT promoter methylation status can predict the incidence and outcome of pseudoprogression after concomitant radiochemotherapy in newly diagnosed glioblastoma patients. J Clin Oncol 2008;26:2192–2197 [DOI] [PubMed] [Google Scholar]
- 7. Brandsma D, Stalpers L, Taal W, Sminia P, van den Bent MJ. Clinical features, mechanisms, and management of pseudoprogression in malignant gliomas. Lancet Oncol 2008;9:453–461 [DOI] [PubMed] [Google Scholar]
- 8. Macdonald DR, Cascino TL, Schold SC, Jr, Cairncross JG. Response criteria for phase II studies of supratentorial malignant glioma. J Clin Oncol 1990;8:1277–1280 [DOI] [PubMed] [Google Scholar]
- 9. Roa W, Brasher PM, Bauman G, et al. Abbreviated course of radiation therapy in older patients with glioblastoma multiforme: a prospective randomized clinical trial. J Clin Oncol 2004;22:1583–1588 [DOI] [PubMed] [Google Scholar]
- 10. Clarke JL, Iwamoto FM, Sul J, et al. Randomized phase II trial of chemoradiotherapy followed by either dose-dense or metronomic temozolomide for newly diagnosed glioblastoma. J Clin Oncol 2009;27:3861–3867 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Johnson JR, Williams G, Pazdur R. End points and United States Food and Drug Administration approval of oncology drugs. J Clin Oncol 2003;21:1404–1411 [DOI] [PubMed] [Google Scholar]
- 12. Chaskis C, Neyns B, Michotte A, De Ridder M, Everaert H. Pseudoprogression after radiotherapy with concurrent temozolomide for high-grade glioma: clinical observations and working recommendations. Surg Neurol 2009;72:423–428 [DOI] [PubMed] [Google Scholar]
- 13. Wen PY, Macdonald DR, Reardon DA, et al. Updated response assessment criteria for high-grade gliomas: response assessment in neuro-oncology working group. J Clin Oncol 2010;28:1963–1972 [DOI] [PubMed] [Google Scholar]
- 14. Sneed PK, Gutin PH, Larson DA, et al. Patterns of recurrence of glioblastoma multiforme after external irradiation followed by implant boost. Int J Radiat Oncol Biol Phys 1994;29:719–727 [DOI] [PubMed] [Google Scholar]
- 15. Garden AS, Maor MH, Yung WK, et al. Outcome and patterns of failure following limited-volume irradiation for malignant astrocytomas. Radiother Oncol 1991;20:99–110 [DOI] [PubMed] [Google Scholar]
- 16. McGeachie RE, Gold LHA, Latchaw RE. Periventricular spread of tumor demonstrated by computed tomography. Radiology 1977;125:407–410 [DOI] [PubMed] [Google Scholar]
- 17. Kyritsis AP, Levin VA, Alfred Yung WK, Leeds NE. Imaging patterns of multifocal gliomas. Eur J Radiol 1993;16:163–170 [DOI] [PubMed] [Google Scholar]
- 18. Mullins ME, Barest GD, Schaefer PW, Hochberg FH, Gonzalez RG, Lev MH. Radiation necrosis versus glioma recurrence: conventional MR imaging clues to diagnosis. AJNR Am J Neuroradiol 2005;26:1967–1972 [PMC free article] [PubMed] [Google Scholar]
- 19. Kumar AJ, Leeds NE, Fuller GN, et al. Malignant gliomas: MR imaging spectrum of radiation therapy- and chemotherapy-induced necrosis of the brain after treatment. Radiology 2000;217:377–384 [DOI] [PubMed] [Google Scholar]
- 20. Ruben JD, Dally M, Bailey M, Smith R, McLean CA, Fedele P. Cerebral radiation necrosis: incidence, outcomes, and risk factors with emphasis on radiation parameters and chemotherapy. Int J Radiat Oncol Biol Phys 2006;65:499–508 [DOI] [PubMed] [Google Scholar]
- 21. Hegi ME, Diserens AC, Gorlia T, et al. MGMT gene silencing and benefit from temozolomide in glioblastoma. N Engl J Med 2005;352:997–1003 [DOI] [PubMed] [Google Scholar]
- 22. Watne K, Hager B, Heier M, Hirschberg H. Reversible oedema and necrosis after irradiation of the brain: diagnostic procedures and clinical manifestations. Acta Oncol 1990;29:891–895 [DOI] [PubMed] [Google Scholar]