Abstract
Objective:
The syndrome of cerebellar ataxia with bilateral vestibulopathy was delineated in 2004. Sensory neuropathy was mentioned in 3 of the 4 patients described. We aimed to characterize and estimate the frequency of neuropathy in this condition, and determine its typical MRI features.
Methods:
Retrospective review of 18 subjects (including 4 from the original description) who met the criteria for bilateral vestibulopathy with cerebellar ataxia.
Results:
The reported age at onset range was 39–71 years, and symptom duration was 3–38 years. The syndrome was identified in one sibling pair, suggesting that this may be a late-onset recessive disorder, although the other 16 cases were apparently sporadic. All 18 had sensory neuropathy with absent sensory nerve action potentials, although this was not apparent clinically in 2, and the presence of neuropathy was not a selection criterion. In 5, the loss of pinprick sensation was virtually global, mimicking a neuronopathy. However, findings in the other 11 with clinically manifest neuropathy suggested a length-dependent neuropathy. MRI scans showed cerebellar atrophy in 16, involving anterior and dorsal vermis, and hemispheric crus I, while 2 were normal. The inferior vermis and brainstem were spared.
Conclusions:
Sensory neuropathy is an integral component of this syndrome. It may result in severe sensory loss, which contributes significantly to the disability. The MRI changes are nonspecific, but, coupled with loss of sensory nerve action potentials, may aid diagnosis. We propose a new name for the condition: cerebellar ataxia with neuropathy and bilateral vestibular areflexia syndrome (CANVAS). Neurology® 2011;76:1903–1910
The syndrome of cerebellar ataxia with bilateral vestibular failure was probably initially reported by the Queen Square group, who described “cerebellar degeneration” in 7 of 53 patients with bilateral vestibular failure.1–3 Four of the 7 had “distal sensory impairment with absent ankle reflexes.” The typical neuro-otologic features of cerebellar ataxia with bilateral vestibulopathy (CABV) syndrome, with combined impairment of cerebellar and vestibular function, were first delineated in 2004.4 Visually enhanced vestibulo-ocular reflex (VVOR) (doll's eye reflex) impairment is the characteristic sign of CABV (see video and figure e-1 on the Neurology® Web site at www.neurology.org) as its failure reflects a compound deficit in the 3 compensatory reflexes involved in eye movement, namely the vestibulo-ocular reflex (VOR), smooth pursuit, and the optokinetic reflex (OKR). Although a sensory peripheral neuropathy was noted in 3 of 4 subjects, it was not classified as a core feature. That the association of cerebellar ataxia with bilateral vestibular failure was not mere coincidence was recently confirmed.5 These workers reported progressive ataxia in 54 of 255 patients with bilateral vestibular failure. An unspecified “peripheral polyneuropathy” accompanied the bilateral vestibulopathy in 17% of 54. Extensive overlap was recently reported between ataxia (with or without cerebellar atrophy) and bilateral vestibulopathy, peripheral neuropathy, or both in 117 patients exhibiting downbeating nystagmus.6 Another series reported the frequent occurrence of vestibular impairment in both axonal and demyelinating neuropathies, although there was no evidence of cerebellar dysfunction. The investigators postulated that there might be a continuum between these patients and those with cerebellar features as well.7
Our aim was to review patients with the CABV syndrome and to characterize the clinical and electrophysiologic features of the accompanying neuropathy, as well as the neuroradiologic features of the cerebellar degeneration.
METHODS
Standard protocol approvals, registrations, and patient consents.
Approval to conduct this study was obtained from the Alfred Hospital Ethics Committee. Written informed consent for research was obtained from all patients (or guardians of patients) participating in the study.
A retrospective case review was performed of patients diagnosed with the CABV syndrome by consultant neurologists at 4 neurology units in Australia and New Zealand. We confirmed that the diagnostic criteria proposed in 2004, of cerebellar ataxia with bilateral vestibular failure causing VOR and smooth pursuit impairment,4 were met in each case, although not all had had VVOR testing performed. Absence of neuropathic symptoms or signs was not an exclusion criterion. All the patients who were initially seen by each center were thoroughly investigated with screens for toxic, metabolic, and systemic disorders, including thyroid function tests, celiac serology, and vitamin B12 deficiency. We then reviewed the clinical and electrophysiologic features of the (invariably present) accompanying neuropathy, as well as MRI results. Clinical testing of vestibular function was undertaken by performing head impulse testing,8 and, in most, dynamic vs static visual acuity. Dynamic visual acuity testing involved assessment of visual acuity during passive head rotation at approximately 2 Hz.9 Normal degradation of acuity is a loss of 2 or less lines on the Snellen chart; impairment is present when this value is exceeded and often correlates with the symptom of movement-induced oscillopsia. Vestibular/proprioceptive interaction was assessed with Romberg test, and vestibular/smooth pursuit interaction with the VVOR.4
In addition to further clinical assessment of eye movements (documentation of nystagmus, visual smooth pursuit, and [in most] saccades to target), cerebellar functional assessment included evaluation for gait ataxia, cerebellar dysarthria, and dysmetria/dyssynergia/intention tremor on finger-nose and heel-knee-shin testing. Quantitative evaluation of vibration perception, where undertaken, employed a Rydel-Seiffer tuning fork, using published normative data.10 Impairment of pinprick perception was delineated qualitatively by exploring from the hypoesthetic to the normal areas.
Patients' routine brain MRI scans were assessed visually for regional cerebellar atrophy with the aid of The MRI Atlas of the Cerebellum.11 Nerve conduction studies (NCS) involved the application of standard methods in the measurement of the sensory and motor parameters assessed. Determination of abnormally slow nerve conduction velocities, indicative of demyelination, was carried out according to the 1991 recommendations of the Ad Hoc Subcommittee of the AAN AIDS Taskforce.12
Formal otoneurology testing involved a range of modalities including caloric, rotational chair testing, or both. Those who had rotational chair testing also underwent smooth pursuit analysis, VOR suppression (VORS), and measurement of spontaneous, gaze, and positional nystagmus. Rotational chair testing with recording of eye movement responses was performed using sinusoidal rotations at 0.01, 0.08, and 0.32 Hz and trapezoid (step) acceleration at 20 and 40 deg/s/s. Standard bithermal caloric testing was undertaken with measurement of maximal slow phase velocity of induced nystagmus, gaze, and positional nystagmus. The diagnostic criteria employed for bilateral vestibulopathy was as follows: less than 5 deg/s on all 4 caloric tests, less than 10 deg/s on rotational tests, positive clinical head impulse test, or all of the above.
In the first instance, neuropathy was defined by clinical presentation, that is, we looked for evidence of reduced pinprick, vibratory sense perception, and absent ankle jerks.
RESULTS
Eighteen patients (see table e-1 for demographic details) were identified in whom the diagnosis of CABV could be sustained. Sex distribution was equal (9:9). Mean age at reported onset was 58 (range 39–71) years, and patients were evaluated a mean of 13 (3–38) years after onset. Two subjects were sisters; family history was otherwise unrevealing.
Following an average of 13 (range 3–20) years of disease progression, 13 of 18 patients required a walking aid or were wheelchair-dependent, and a further one had repeated falls.
Clinical findings.
Thirteen patients presented with gait imbalance, 8 of whom noted this to be worse in the dark. Two patients reported dizziness on presentation, while only one patient gave an account of intrinsic falls. Another patient described oscillopsia, while 2 presented with lower limb dysesthesia (employing such descriptors as “lightening pain” and “tingling”).
All patients showed broken-up smooth visual pursuit and demonstrated additional evidence of cerebellar dysfunction, including gait ataxia, cerebellar-type dysarthria or appendicular ataxia, or combinations of these features (table 1). All but one of the subjects exhibited horizontal gaze-evoked nystagmus, with 5 also exhibiting downbeat nystagmus. All had clinical evidence of bilateral vestibular failure, with positive head impulse tests, and impaired dynamic visual acuity in all in whom it was sought (table 1). As expected, there was apparent preservation of VORS in the face of smooth pursuit impairment, reflecting the fact that there is no VOR to suppress.
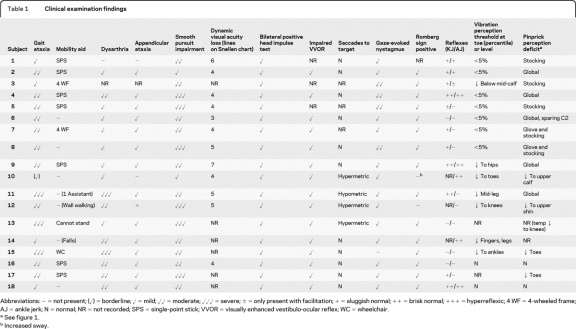
Table 1.
Clinical examination findings
Abbreviations: − = not present; (✓) = borderline; ✓ = mild; ✓✓ = moderate; ✓✓✓ = severe; ± = only present with facilitation; + = sluggish normal; ++ = brisk normal; +++ = hyperreflexic; 4 WF = 4-wheeled frame; AJ = ankle jerk; N = normal; NR = not recorded; SPS = single-point stick; VVOR = visually enhanced vestibulo-ocular reflex; WC = wheelchair.
See figure 1.
Increased sway.
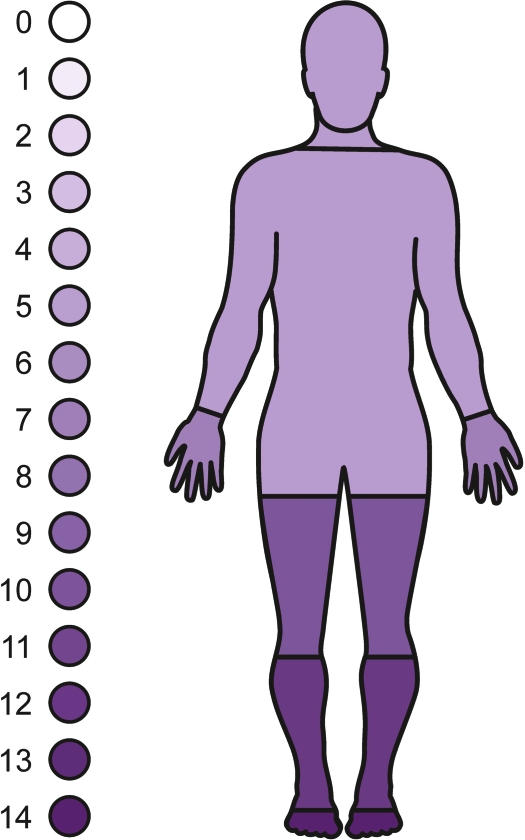
Clinical features of the neuropathy are shown in table 1. Ten of the 18 subjects had absent ankle jerks. Fourteen of 16 subjects displayed an impairment of vibration sensation, with a further one demonstrating joint position sense abnormalities. Seven of the subjects underwent quantitative testing of vibration perception threshold; all fell below the fifth percentile for age and gender. Fourteen of 16 had impairment of pinprick perception. Clinically, the peripheral neuropathy manifested as a length-dependent, pure, or predominantly sensory deficit. As illustrated in figure 1, the extent of the impairment of pin prick perception was variable, ranging from a “sock” or “glove and stocking distribution” to a pattern of almost total body involvement. Fiber type involvement, as judged by the clinical examination, varied from small-fiber predominance with almost complete loss of pinprick sensation accompanied by moderate distal reduction of vibration sense to predominant large-fiber length-dependent loss.
Figure 1. Homunculus demonstrating the pattern of sensory neuropathy.
The sensory modality represented is pinprick. Density of shading relates to the number of subjects demonstrating a sensory deficit in that anatomic region. It can be seen that some subjects have global loss, suggesting a neuronopathy, but that this probably represents extreme length-dependent loss.
Otoneurology investigation findings.
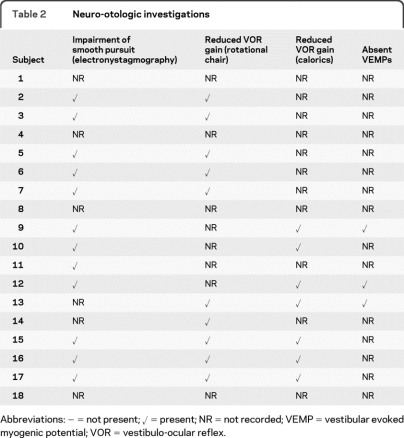
The results of neuro-otologic investigations are shown in table 2.
Table 2.
Neuro-otologic investigations
Abbreviations: − = not present; ✓ = present; NR = not recorded; VEMP = vestibular evoked myogenic potential; VOR = vestibulo-ocular reflex.
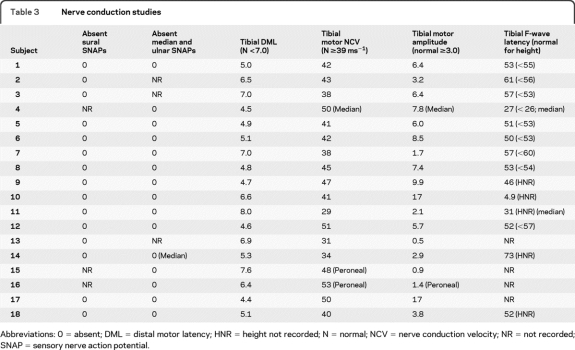
Nerve conduction studies.
The results of nerve conduction studies are shown in table 3. Sensory nerve action potentials (SNAPs) (upper limb, lower limb, or both) were unobtainable in all subjects. Reduced abductor hallucis (tibial motor) compound muscle action potential (CMAP) amplitudes were seen in 5, and reduced extensor digitorum brevis (peroneal motor) CMAPs in a further one. Three who underwent EMG showed evidence of mild chronic denervation in tibialis anterior. Mild prolongation of distal motor latency or mild slowing of motor nerve conduction were seen in 6, but CMAP amplitudes were reduced in all but one of these, who had only marginal slowing. None met current criteria for demyelination.12 Similarly, F-wave latencies were normal in 9 and only mildly prolonged in 5, with only one showing notable prolongation (tibial, 73 msec). In summary, the electrophysiology was consistent with an axonal sensory or sensorimotor neuropathy, with only minimal evidence for demyelination.
Table 3.
Nerve conduction studies
Abbreviations: 0 = absent; DML = distal motor latency; HNR = height not recorded; N = normal; NCV = nerve conduction velocity; NR = not recorded; SNAP = sensory nerve action potential.
Nerve biopsy.
A biopsy was taken from the sural nerve of 3 subjects (nos. 2, 14, and 16). That of subject 2 showed significant loss of both myelinated and nonmyelinated fibers with 34% segmental remyelination in a teased fiber presentation (see figure e-2), while those of subjects 14 and 16 revealed severe axonal neuropathies.
MRI.
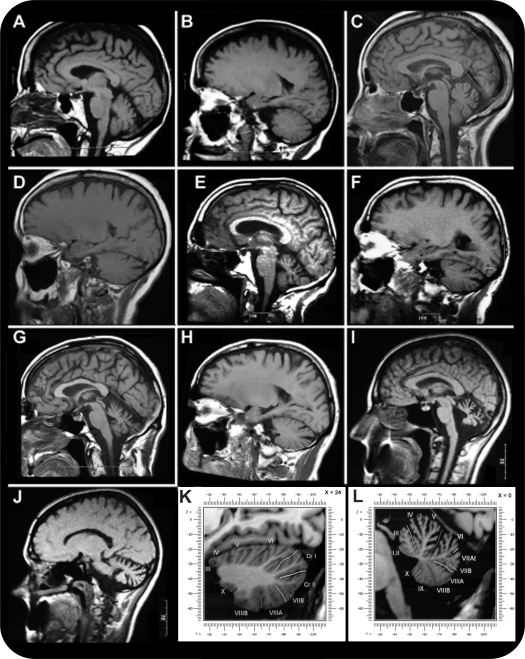
All subjects received MRI brain scans. Cerebellar atrophy was absent or negligible in 2; all others demonstrated a consistent pattern of anterior and dorsal vermis atrophy, the latter involving vermal lobules VI, VIIa, and VIIb. This was subjectively rated as mild in 11 and moderate in 5. Laterally, a pattern of hemispheric atrophy predominantly affecting crus I (corresponding to vermal lobule VII) was seen.11 None showed evidence of pontine atrophy or inferior olive pseudohypertrophy, and supratentorial structures were unremarkable (figure 2).
Figure 2. T1-weighted MRI illustrating the spectrum of cerebellar atrophy found in the cerebellar ataxia with neuropathy and bilateral vestibular areflexia syndrome (CANVAS).
(A) Sagittal view of very mild cerebellar atrophy; (B) parasagittal view of very mild cerebellar atrophy; (C) sagittal view of very mild cerebellar atrophy; (D) parasagittal view of very mild cerebellar atrophy; (E) sagittal view of mild cerebellar atrophy; (F) parasagittal view of mild cerebellar atrophy; (G) sagittal view of moderate cerebellar atrophy; (H) parasagittal view of moderate cerebellar atrophy; (I) sagittal view of non-CANVAS pattern cerebellar atrophy for comparison; (J) parasagittal view of non-CANVAS pattern cerebellar atrophy for comparison; (K) parasagittal view of the labeled cerebellar lobules, and crus I and II; (L) sagittal view of the labeled cerebellar lobules.
DISCUSSION
We provide further evidence for the existence of the syndrome of cerebellar ataxia and bilateral vestibulopathy as a distinct clinical entity,2–6 whose constellation of pathology does not occur simply as a matter of chance.5,6 This syndrome has previously been regarded as sporadic,4 but 2 of the patients described herein are sibling offspring of a nonconsanguineous union, raising the strong possibility of a late-onset recessive disorder. Confirmation will, however, have to await the reporting of other affected sibling pairs.
Although neuropathy was alluded to in the original description of the syndrome, we have found it to be a constant feature, and it may be severe enough to contribute to disability in its own right. We therefore propose a new name for this condition, which encompasses all essential clinical features: cerebellar ataxia with neuropathy and bilateral vestibular areflexia syndrome (CANVAS).
The pattern of the neuropathy is predominantly sensory, and clinically affects both small- and large-diameter fiber types. While total body involvement of smaller diameter fibers in some subjects might suggest a neuronopathy, most subjects demonstrated a length-dependent pattern of small-diameter fiber involvement. Hence, it is likely that total body involvement reflects an advanced length dependent process; however, a neuronopathy cannot be entirely excluded in some patients.
The nerve conduction study results are consistent with a predominantly sensory axonal neuropathy, with some also showing a minor to moderate degree of motor axonal loss. The results are not consistent with a primary demyelinating process, the sural nerve biopsy results in one subject notwithstanding.
The resultant disability is not inconsiderable. It should be noted that the finding of a positive Romberg sign may reflect functional impairment of either or both vestibular and proprioceptive function, a point which is particularly relevant to a condition which comprises defects in both of these systems. It has also been demonstrated that enhancement of the cervico-ocular reflex, one mechanism thought to be responsible for functional adaptation in patients with isolated bilateral vestibular failure, is absent in patients with additional cerebellar involvement, a factor which may also contribute to the prominent disability in this group.1,13
Additionally, we have found that a consistent pattern of cerebellar atrophy is present on MRI scanning. However, while likely to be invariably demonstrable in the more advanced stages of the disorder, it should be noted that this configuration of atrophy is not pathognomonic for CANVAS and can, for example, be seen in combination with pontine atrophy in patients with multiple system atrophy of cerebellar type. A similar pattern of crus I atrophy is also evident in a published case of SCA 12.14 Further, prospective, blinded neuroradiologic assessment would be required to establish the sensitivity and specificity of this pattern for clinically diagnosed CANVAS. The 2 MRI scans that demonstrated a radiologically normal cerebellar appearance were taken 4 and 5 years prior to the patients' assessment for this study, and as such, likely indicate that the MRI scan may be normal in the earlier stages of the disease. A similar conclusion was reached by others,6 and it seems probable to us that their separation of those patients with ataxia and vestibulopathy but without cerebellar atrophy on MRI from those with the same constellation of clinical findings but with cerebellar atrophy on MRI is artificial. A longitudinal study with repeated MRI scanning will be required to settle this point conclusively, however.
Whether apparently sporadic cerebellar ataxia plus vestibular failure but without sensory neuropathy, as described in some patients in 2 previous series,5,6 is the same condition as CANVAS is as yet unclear. Neuropathy might develop later in some patients, requiring careful longitudinal study for its exclusion, or it might be inapparent clinically, and escape attention unless NCS are done routinely, as in our patients 16 and 18. In at least one case, neuropathy preceded the other clinical features by a period of years. It is worth emphasizing that although all 18 of our patients had absent SNAPs, they were initially selected purely for the presence of cerebellar impairment with vestibular failure, regardless of the presence of neuropathy. This suggests, at least, that further series should include NCS routinely, and perhaps longitudinally, to determine the true frequency of neuropathy in CANVAS. On the basis of our series, NCS can at least be suggested as a useful adjunctive test in such patients. It is also clear from our series and from the original description of CABV that downbeating nystagmus is by no means a uniform feature of CANVAS,4 despite one previous series having originally selected the patients for their series on the basis of its presence.6
While the combination of late-onset recessive or sporadic progressive ataxia (usually with cerebellar atrophy), vestibular failure, and sensory neuropathy is distinctive, various aspects of the disorder may be mimicked by other conditions. Of the dominantly inherited ataxias, spinocerebellar ataxia type 3 (SCA 3; Machado-Joseph disease) may combine impairment of VOR gain,15–18 and neuropathy,19,20 with ataxia. Distinguishing features from CANVAS include a family history consistent with a dominant disorder, and a pattern of MRI involvement resulting in enlargement of the fourth ventricle (presumably partly as a result of prominent dentate nucleus involvement) with atrophy of vermal lobules I, II, IV, VIIIb, and IX, but without cerebellar hemispheric atrophy.19–21 The neuropathy in SCA 3 also typically affects motor as well as sensory neurons/fibers,20 in contrast to the severe sensory impairment with frequently normal motor studies in CANVAS.
Other SCAs typically (SCA 4, 25)22,23 or sometimes (SCAs 1, 8, 27)24–26 manifest sensory neuropathy, while a sensorimotor neuropathy was a feature of the only family described to date with SCA 18.27 Sensory loss is a typical although not universal feature of SCA 2, but the typically slow saccades and preserved VOR gain,15 obvious brainstem atrophy on MRI, and dominant inheritance help to exclude this disorder.
Friedreich ataxia (FRDA) is characterized by loss of sural SNAPs with preserved motor conduction velocities,28,29 and, being a common recessively inherited disorder, may appear to be sporadic. While a severe vestibulopathy has been found to be a component of FRDA,30 the disease typically has an onset below 25 years, although rare late-onset cases may present even after age 60.28 Furthermore, the MRI appearance is not reminiscent of CANVAS: the cerebellar cortex is relatively spared until late in the disease course, and the spinal cord is typically atrophic.28
Supplementary Material
ACKNOWLEDGMENT
The authors thank the following neurologists who referred subjects for this study: Peter J. Hand, John King, and Philip D. Thompson.
Supplemental data at www.neurology.org
- 4WF
- 4-wheeled frame
- CABV
- cerebellar ataxia with bilateral vestibulopathy
- CANVAS
- cerebellar ataxia with neuropathy and bilateral vestibular areflexia syndrome
- CMAP
- compound muscle action potential
- FRDA
- Friedreich ataxia
- NCS
- nerve conduction study
- NCV
- nerve conduction velocity
- OKR
- optokinetic reflex
- SCA
- spinocerebellar ataxia
- SNAP
- sensory nerve action potential
- SPS
- single point stick
- VEMP
- vestibular evoked myogenic potential
- VOR
- vestibulo-ocular reflex
- VORS
- vestibulo-ocular reflex suppression
- VVOR
- visually enhanced vestibulo-ocular reflex.
DISCLOSURE
Dr. Szmulewicz and Dr. Waterston report no disclosures. Dr. Halmagyi serves on the scientific board for the Brain Foundation of Australia; serves on the editorial boards of Acta Otolaryngologica, Otology, Neurotology, Audiology, Neuro-otology, and the Italian Journal of Otolaryngology; has served as a consultant for GN Otometrics; and receives research support from the National Health and Medical Research Council. Dr. Mossman reports no disclosures. Dr. Chancellor has received funding for travel from Biogen Idec and sanofi-aventis and serves on the editorial board for Practical Neurology. Dr. McLean reports no disclosures. Dr. Storey has received funding for travel (and speaker honoraria payable to his institution) from Pfizer Inc; serves as Neurology Co-Editor for Journal of Clinical Neuroscience; and receives research support from National Health and Medical Research Council (Australia), the NIH, Alfred Hospital Research Foundation, Wicking Foundation, and Bethlehem-Griffiths Foundation.
REFERENCES
- 1. Bronstein AM, Mossman S, Luxon LM. The neck-eye reflex in patients with reduced vestibular and optokinetic function. Brain 1991;114:1–11 [PubMed] [Google Scholar]
- 2. Rinne T, Bronstein AM, Rudge P, et al. Bilateral loss of vestibular function. Acta Otolaryngol 1995;(suppl 520):247–250 [DOI] [PubMed] [Google Scholar]
- 3. Rinne T, Bronstein AM, Rudge P, et al. Bilateral loss of vestibular function: clinical findings in 53 patients. J Neurol 1998;245:314–321 [DOI] [PubMed] [Google Scholar]
- 4. Migliaccio AA, Halmagyi GM, McGarvie LA, et al. Cerebellar ataxia with bilateral vestibulopathy: description of a syndrome and its characteristic clinical sign. Brain 2004;127:280–293 [DOI] [PubMed] [Google Scholar]
- 5. Zingler VC, Cnyrim C, Jahn K, et al. Causative factors and epidemiology of bilateral vestibulopathy in 255 patients. Ann Neurol 2007;61:524–532 [DOI] [PubMed] [Google Scholar]
- 6. Wagner JN, Glaser M, Brandt T, Strupp M. Downbeat nystagmus: aetiology and comorbidity in 117 patients. J Neurol Neurosurg Psychiatry 2008;79:672–677 [DOI] [PubMed] [Google Scholar]
- 7. Palla A, Schmid-Priscoveanu A, Studer A, Hess K, Straumann D. Deficient high-acceleration vestibular function in patients with polyneuropathy. Neurology 2009;72:2009–2013 [DOI] [PubMed] [Google Scholar]
- 8. Halmagyi GM, Cuthroys IS. A clinical sign of canal paresis. Arch Neurol 1988;45:737–739 [DOI] [PubMed] [Google Scholar]
- 9. Baloh RW, Kerber KA. Clinical Neurophysiology of the Vestibular System, 4th ed. New York: Oxford University Press; 2011:155 [Google Scholar]
- 10. Martina ISJ, van Koningsveld R, Schmitz PIM, et al. Measuring vibration threshold with a graduated tuning fork in normal aging and in patients with polyneuropathy. J Neurol Neurosurg Psychiatry 1998;65:743–747 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Schmahmann JD, Doyon J, Petrides M, Evans, et al. MRI Atlas of the Human Cerebellum. San Diego: Academic Press; 2000 [Google Scholar]
- 12. Ad Hoc Subcommittee of the American Academy of Neurology AIDS Task Force Research criteria for diagnosis of chronic inflammatory demyelinating polyneuropathy (CIDP): report from an Ad Hoc Subcommittee of the American Academy of Neurology AIDS Task Force. Neurology 1991;41:617–618 [PubMed] [Google Scholar]
- 13. Waterston JA, Barnes GR, Grealy MA, Luxon LM. Coordination of eye and head movements during smooth pursuit in patients with vestibular failure. J Neurol Neurosurg Psychiatry 1992;55:1125–1131 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Holmes SE, Hearn EO, Ross CA, Margolis RL. SCA12: an unusual mutation leads to an unusual spinocerebellar ataxia. Brain Res Bull 2001;56:397–403 [DOI] [PubMed] [Google Scholar]
- 15. Buttner N, Geschwind D, Jen JC, et al. Oculomotor phenotypes in autosomal dominant ataxias. Arch Neurol 1998;55:1353–1357 [DOI] [PubMed] [Google Scholar]
- 16. Burk K, Fetter M, Abele M, et al. Autosomal dominant cerebellar ataxia type I: oculomotor abnormalities in families with SCA1, SCA2 and SCA3. J Neurol 1999;246:789–797 [DOI] [PubMed] [Google Scholar]
- 17. Takegosh H, Murofushi T. Vestibular evoked myogenic potentials in patients with spinocerebellar degeneration. Acta Otolaryngol 2000;120:821–824 [DOI] [PubMed] [Google Scholar]
- 18. Gordon CR, Joffe V, Vainstein G, Gadoth N. Vestibulo-ocular areflexia in families with spinocerebellar ataxia type 3 (Machado-Joseph disease). J Neurol Neurosurg Psychiatry 2003;74:1403–1406 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Schols L, Amoiridis G, Buttner T, et al. Autosomal dominant cerebellar ataxia: phenotypic differences in genetically defined subtypes? Ann Neurol 1997;42:924–932 [DOI] [PubMed] [Google Scholar]
- 20. Klockgether T, Schols L, Abele M, et al. Age related axonal neuropathy in spinocerebellar ataxia type 3/Machado-Joseph disease (SCA3/MJD). J Neurol Neurosurg Psychiatry 1999;66:222–224 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Lukas C, Schols L, Bellenberg B, et al. Dissociation of grey and white matter reduction in spinocerebellar ataxia type 3 and 6: a voxel-based morphometry study. Neurosci Lett 2006;408:230–235 [DOI] [PubMed] [Google Scholar]
- 22. Flanigan K, Gardner K, Alderson K, et al. Autosomal dominant spinocerebellar ataxia with sensory axonal neuropathy (SCA4): clinical description and genetic localization to chromosome 16q22.1. Am J Hum Genet 1996;59:392–399 [PMC free article] [PubMed] [Google Scholar]
- 23. Stevanin G, Bouslam N, Thobois S, et al. Spinocerebellar ataxia with sensory neuropathy (SCA25) maps to chromosome 2p. Ann Neurol 2004;55:97–104 [DOI] [PubMed] [Google Scholar]
- 24. Subramony SH, Vig PJS. Clinical aspects of spinocerebellar ataxia 1. In: Wells RD, Warren ST. eds. Genetic Instabilities and Hereditary Neurological Diseases. San Diego: Academic Press; 1998:231–239 [Google Scholar]
- 25. Koob MD, Moseley ML, Schut LJ, et al. An untranslated CTG expansion causes a novel form of spinocerebellar ataxia (SCA8). Nat Genet 1999;21:379–384 [DOI] [PubMed] [Google Scholar]
- 26. Dalski A, Atici J, Kreuz FR, et al. Mutation analysis in the fibroblast growth factor 14 gene: frameshift mutation and polymorphisms in patients with inherited ataxias. Eur J Hum Genet 2005;13:118–120 [DOI] [PubMed] [Google Scholar]
- 27. Brkanac Z, Fernandez M, Matsushita M, et al. Autosomal dominant sensory/motor neuropathy with ataxia (SMNA): linkage to chromosome 7q22-q32. Am J Med Genet 2002;114:450–457 [DOI] [PubMed] [Google Scholar]
- 28. Pandolfo M. Friedreich ataxia. Semin Pediatr Neurol 2003;10:163–172 [DOI] [PubMed] [Google Scholar]
- 29. Alper G, Narayanan V. Friedreich's ataxia. Pediatr Neurol 2003;28:335–341 [DOI] [PubMed] [Google Scholar]
- 30. Fahey MC, Cremer PD, Aw ST, et al. Vestibular, saccadic and fixation abnormalities in genetically confirmed Friedreich ataxia. Brain 2008;131:1035–1045 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.