Abstract
Sudden unexpected death in epilepsy (SUDEP) is a devastating complication of epilepsy and is not rare. The NIH and National Institute of Neurological Disorders and Stroke sponsored a 3-day multidisciplinary workshop to advance research into SUDEP and its prevention. Parallel sessions were held: one with a focus on the science of SUDEP, and the other with a focus on issues related to the education of health care practitioners and people with epilepsy. This report summarizes the discussions and recommendations of the workshop, including lessons learned from investigations of sudden infant death syndrome (SIDS), sudden cardiac death, autonomic and respiratory physiology, medical devices, genetics, and animal models. Recommendations include educating all people with epilepsy about SUDEP as part of their general education on the potential harm of seizures, except in extenuating circumstances. Increasing awareness of SUDEP may facilitate improved seizure control, possibly decreasing SUDEP incidence. There have been significant advances in our understanding of the clinical and physiologic features of SIDS, sudden cardiac death, and SUDEP in both people and animals. Research should continue to focus on the cardiac, autonomic, respiratory, and genetic factors that likely contribute to the risk of SUDEP. Multicenter collaborative research should be encouraged, especially investigations with direct implications for the prevention of SUDEP. An ongoing SUDEP Coalition has been established to facilitate this effort. With the expansion of clinical, genetic, and basic science research, there is reasonable hope of advancing our understanding of SUDEP and ultimately our ability to prevent it. Neurology® 2011;76:1932–1938
Throughout the world, approximately 0.5%–1% of the population has epilepsy. One-third of people with epilepsy have persistent seizures despite appropriate treatment. Each year, slightly less than one of every thousand people with epilepsy dies of sudden, unexpected, unexplained death. In those with refractory epilepsy, this occurs in 1 in 150 people each year. The risk is particularly high in those with uncontrolled tonic-clonic seizures.
In 2007, the American Epilepsy Society and the Epilepsy Foundation formed a task force to address the research and educational issues concerning the phenomenon of sudden unexpected (unexplained) death in epilepsy (SUDEP). Among the published recommendations of the task force was that a multidisciplinary workshop on SUDEP be convened.1 The goal of the workshop was to bring together a multidisciplinary group of professionals and lay advocates with diverse expertise to further our understanding of SUDEP and our ability to prevent it. The depth and breadth of the participants included epileptologists and other neurologists, patient and professional educators, advocates from the bereaved community, and experts in guideline development as well as experts from related fields, such as sudden cardiac death (cardiology), neurocardiology, the autonomic nervous system, sudden infant death syndrome (SIDS), genetics, animal models of sudden death, respiratory physiology, and pathology (medical examiners/coroners).
In November 2008, a unique, 3-day multidisciplinary SUDEP workshop was convened by the NIH and the National Institute of Neurological Disorders and Stroke (NINDS) in Bethesda, MD. The workshop consisted of parallel sessions, one with a focus on the science of SUDEP, and the other with a focus on issues related to the education of health care practitioners and people with epilepsy. This report summarizes the discussions and recommendations from the workshop. For brevity, only a few select references are included.
This report is a workshop summary, not a guideline, practice parameter, or evidence-based review. While some of the suggestions involve clinical practice, these recommendations only represent the views of the majority of the workshop participants at this time. Further study is required to develop future evidence-based guidelines.
The definition of SUDEP used in this document is based on that of Nashef and Brown2: “Sudden, unexpected, witnessed or unwitnessed, nontraumatic and nondrowning death in a patient with epilepsy, with or without evidence of a seizure and excluding documented status epilepticus.”
Definite SUDEP requires a postmortem examination showing no definite cause of death (such as high levels of illicit drugs or acute myocardial infarction).
Probable SUDEP is used when postmortem examination is not performed, but the definition is otherwise fulfilled.
Possible SUDEP is applied to less clear cases that might have been SUDEP, but where there is inadequate information to be certain or competing possible causes of death.
For this workshop and summary, the term near-SUDEP is used to describe cases in which death was likely if resuscitation or other intervention had not been applied.
DEVELOPING A RESEARCH AGENDA TO UNDERSTAND AND PREVENT SUDEP
Lessons learned from SIDS.
The prone sleep position is associated with autonomic dysfunction, decreased arousability, heat trapping, rebreathing of exhaled gases, and higher risk of SIDS. Many patients with SUDEP are found prone. Exposure to cigarette smoke, especially in utero, is a risk factor for SIDS. In both animal and human infant studies, smoke exposure decreases ventilatory responses to hypoxia and decreases arousal from sleep. There appears to be a higher risk of SIDS with recent infection, autonomic dysfunction, and decreased sighs, gasps, spontaneous arousals, and arousability. Brainstem serotonin dysfunction appears to play a significant role in SIDS, possibly explaining up to half of cases.
As many as 10% of SIDS cases appear to be associated with genetic variations known to be associated with cardiac arrhythmias and sudden death, or linked to regulation of CNS serotonin levels. In addition to genes associated with cardiac ion channelopathies (both sodium and potassium), genetic variants have been reported in genes relating to serotonin transport, autonomic nervous system and brainstem development, cytokines, and energy production (mitochondrial function).
These factors that have been associated with SIDS have either not been investigated in SUDEP (cigarette smoke exposure, infection, arousability, brainstem physiology) or require additional investigation.
Lessons learned from sudden cardiac death, neurocardiology, and studies of the autonomic nervous system.
In general, increases in sympathetic activity and decreases in parasympathetic activity are markers for increased risk of cardiac arrhythmia. Heart rate variability and baroreflex sensitivity are reasonable measures of autonomic system dysfunction that might predispose to sudden cardiac death. Baroreflex sensitivity may be more relevant to SUDEP, as it is a measure of an acute, reactive vagal response rather then chronic vagal tone as measured by heart rate variability.
Inflammation, fever, and high C-reactive protein appear to be associated with an increased risk of sudden cardiac death.
Genetic factors are clearly important in cardiac arrhythmias. Many of the known cardiac arrhythmia genes associated with either long or short QT syndromes, Brugada syndrome, or catecholaminergic polymorphic ventricular arrhythmia are dually expressed in heart and brain. Examples include KCNQ1, KCNH2, RyR2, and some sodium and calcium channel genes. SCN1A, a sodium channel gene known to be associated with generalized epilepsy with febrile seizures plus and severe myoclonic epilepsy of infancy (Dravet syndrome), is also expressed in the heart. SUDEP may be particularly common in Dravet syndrome.
There are several mechanisms of sudden cardiac death or myocardial injury that may be relevant to patients with epilepsy, especially during seizures. This includes a hyperadrenergic state, which is associated with coagulative myocytolysis (also known as contraction band necrosis), and can occur with subarachnoid hemorrhage and “scared-to-death” syndrome (also known as “voodoo death” or “broken heart” syndrome). This can be associated with apical ballooning or Takotsubo cardiomyopathy. Other acute autonomic changes that may occur in “voodoo death,” including hyperparasympathetic activity and possibly acute adrenal failure, may play a role as well. With some seizures in animals, brain discharges become directly linked to the activity of small intracardiac autonomic nerves (the “lock-step” phenomenon), which predisposes to arrhythmias in animal models. Intracardiac release of catecholamines is an important cause of cardiac injury. Cumulative injury may occur over time via this mechanism. Seizures can by associated with hyperactive parasympathetic activity, including asystole, and possibly hyperactive baroreflex activity. Cardiac ischemia due to coronary atherosclerosis can be exacerbated by seizures, which are a form of “stress test”; this cause of death is not typically considered SUDEP, but is still a potentially preventable cause of sudden seizure-related death in epilepsy patients. Other possible mechanisms include arrhythmias/channelopathies, conduction or autonomic effects of antiepileptic drugs or their withdrawal, and combinations of the above, with or without pulmonary mechanisms, especially hypoxia.
Lessons learned from respiratory physiology.
Serotonin plays a role in respiratory drive and the response to hypercapnia. In sheep and mouse models of SUDEP, seizures are associated with death due to respiratory arrest. In one strain of mice (DBA), pharmacologically increasing serotonin or its action can prevent death by preventing seizure-related respiratory arrest, and blocking serotonin activity increases seizure-related death. In other studies, increasing serotonin may decrease sleep apnea and death after stroke. In at least one animal model, administration of oxygen prevented seizure-related sudden death.
Possible respiratory mechanisms contributing to SUDEP include central and obstructive apnea, pulmonary edema (especially neurogenic edema, as seen in a sheep model of SUDEP), ictal hypoxia, aspiration (not typically considered SUDEP, but another potential cause of sudden nontraumatic, nondrowning, seizure-related death), and laryngospasm. Central apnea may be most important, either related to serotonin as above, other substances released during seizures such as adenosine or opiates, or to “cerebral shutdown” of all brain activity after a seizure. This “shutdown” may be due to ictal or postictal dysfunction of monoamine neurons, including serotonergic neurons. Inactivity of monoamine neurons could lead to simultaneous central apnea and decreased arousal, both thought to occur in SUDEP. Ictal hypoxia is frequently observed with seizures, including complex partial seizures without generalization. Hypoxia can be present without obvious respiratory distress or dysfunction. It typically involves a component of central apnea, but may also involve ventilation-perfusion mismatch or pulmonary edema. Life-threatening laryngospasm has been reported in relation to seizures, perhaps induced by aspiration. This may leave no obvious findings on autopsy, and therefore is likely to be classified as SUDEP.
Recommendations to advance research in SUDEP.
For details on recommendations to advance research in SUDEP, see table 1 and online report (appendix e-1 on the Neurology® Web site at www.neurology.org). We need to continue to develop animal models of SUDEP, including genetic ones. Sudden death, typically seizure-related, is known to occur in animal models of epilepsy. These deaths should be viewed as a research opportunity to learn more about their relevance to SUDEP. Ideally, investigators should simultaneously monitor cardiac, respiratory (including for central and obstructive components), cortical (EEG), brainstem, and autonomic function in these models.
Table 1.
Avenues of scientific research related to SUDEP and its preventiona
Abbreviations: AED = antiepileptic drug; SIDS = sudden infant death syndrome; SSRI = selective serotonin reuptake inhibitor; SUDEP = sudden unexpected death in epilepsy.
See text, plus full report (appendix e-1 on the Neurology® Web site at www.neurology.org) for details.
Clinical investigations should include the role of a screening EKG in all people with epilepsy, other methods to identify those at risk of arrhythmias, and the role of anti-arrhythmic medication or devices. Peri-ictal cardiac injury, autonomic dysfunction, and their prevention require further study. The role of respiratory drive, arousability, sleep position, serotonin, adenosine, sleep apnea, hypoxia, pulmonary edema, postictal EEG flattening, nocturnal supervision, tactile stimulation, and pulmonary edema all warrant further study.
Further genetic investigation is needed to search for SUDEP-related genes, especially genes coding for the numerous channels that are dually expressed in heart and brain.
Improved medical devices are needed, including reliable and convenient home oxygen and pulse monitors and seizure detectors. Implanted devices that can monitor respiratory, cardiac, and cerebral activity could define pathophysiology, identify high-risk patients, and be linked to specific treatment modalities such as cardiac defibrillators and pacemakers, alerting stimuli, and diaphragmatic pacing.
Collaboration with coroners and medical examiners will be crucial to improve recognition, documentation, and investigation of SUDEP. Protocols for investigating deaths should be standardized.
Creating a research consortium and SUDEP registry may be the most effective approach. A multicenter study of high-risk patients could be performed (e.g., those with refractory convulsions, especially in sleep), enrolling subjects and studying the following while in the epilepsy monitoring unit:
12-lead EKG
Blood/DNA for banking. Blood for DNA can be banked via blood-spot cards that are easy to store and can be kept at room temperature
Baseline echocardiogram; cardiac monitoring, including ictal and postictal
Autonomic evaluation, including heart rate variability, baroreceptor sensitivity, and response to Valsalva
Respiratory evaluation, including oxygen saturation in the interictal, ictal, and postictal states; nasal airflow, chest and abdominal wall movement; sighs/yawns/arousability measures (as in SIDS studies); possibly full polysomnography
Standardized history including family history of sudden death, in utero and postnatal smoke exposure, sleep habits/environment, alcohol and drug use
Check C-reactive protein, postictal troponin, and postictal brain natriuretic peptide
Consider including a volunteer high-risk subgroup in whom a device would be implanted to obtain long-term recordings of the O2 level, EKG, EEG, and respiratory effort
Annual phone follow-up and questionnaire, including information about medications, illicit drugs, alcohol, compliance, sleep habits, SUDEP awareness
In the event of near-SUDEP or SUDEP, provide readily accessible information to first responders, emergency room staff, or medical examiner office on how to contact the study center (i.e., prior educational programs, medical alert ID or bracelet)
If probable or possible SUDEP occurs, perform a standardized autopsy, preferably with select tissues analyzed at a central or regional site. Of note, one should not rely on formalin-fixed, paraffin-embedded tissue for genetic studies but rather blood-spot cards, blood in EDTA, or frozen tissue. In addition, detailed cardiopulmonary examination; specialized brainstem neuropathology, including serotonin evaluation; further genetic studies on tissue
Create a SUDEP registry and central tissue bank. It was estimated that there are about 2,000 SUDEP deaths per year in the United States, and perhaps 400–500 per year in the United Kingdom. Include cases with and without known seizures at the time of death, and possibly include prolonged seizures/status epilepticus if no obvious cause of death
EDUCATING PEOPLE WITH EPILEPSY AND THEIR FAMILIES ABOUT SUDEP
Ethics: The right to know vs the right not to know.
To address the issue of specific benefits and harms of discussing SUDEP with people with epilepsy and their families, primary bioethics principles were considered: respect for autonomy, nonmaleficence, beneficence, and justice.3
The potential benefits of health care providers discussing SUDEP with people with epilepsy and their families include the following:
Helps health care providers and people with epilepsy share in treatment goals
Helps to establish a “truth-telling” relationship
Avoids a false sense of security and resulting complacency regarding epilepsy and its treatment
Allows for expression of the natural anxiety regarding epilepsy and encourages it to be dealt with in a constructive fashion
Allows people with epilepsy to organize their lives with reasonable expectations
Allows people with epilepsy and their families to help reduce possible risk factors for SUDEP, e.g., by ensuring medical compliance and minimizing behavior that can exacerbate seizures
Significantly reduces the fear of SUDEP in low-risk populations, especially in those who fear dying or fear the death of their loved one due to seizures, but have been afraid to inquire
If SUDEP does occur, the family's pain, grief, and blame may be lessened by having been fully informed, knowing the patient was fully informed, and knowing how to get information and grief counseling, including discussing with other affected individuals' families
The potential risks of health care providers discussing SUDEP with people with epilepsy and their families include the following:
Precipitating anxiety, depression, or posttraumatic stress disorder in individuals with a predisposed psychological makeup
In certain cultures, the discussion could be interpreted as predisposing the individual to the event
Misunderstanding of “low risk” as “no risk”
When and how to provide information to people with epilepsy and their families.
At the time of the workshop, 2 published studies addressed the issue of practitioners' communication with their patients about SUDEP. Lewis et al.4 conducted a survey of members of the UK Clinical Nurse Epilepsy Specialists association and other nurses with an interest in epilepsy. They found that 50% discuss SUDEP with most or all patients; improved adherence to treatment was reported in 62% of cases.
Morton et al.5 conducted a survey of UK neurologists to determine compliance with the National Institute of Clinical Excellence guidelines, which recommend that SUDEP be discussed with people with epilepsy. A total of 26% of physicians surveyed reported that they discussed SUDEP with the majority of their patients, 61% with some, 7.5% with none, and 5% with all. Doctors who discussed SUDEP with most or all patients were significantly less likely to report negative reactions from their patients, and noted most patients received the information with equanimity or positively.
There is no existing literature to guide the health care provider in assessing the readiness of a person with epilepsy or their family to learn about SUDEP, the timing and content of these discussions, or the appropriate cultural and social considerations.
Recommendations.
Along with the following, see table 2 for a list of recommendations.
Table 2.
Avenues of research related to SUDEP education and awareness
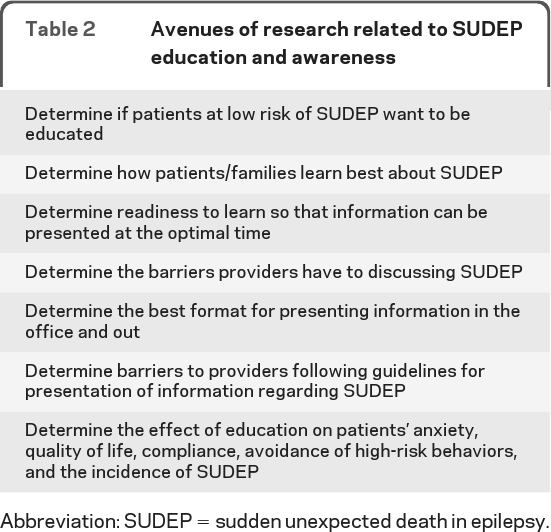
Abbreviation: SUDEP = sudden unexpected death in epilepsy.
Except for patients with cultural or psychological circumstances which preclude safe discussion, it was the consensus of the discussants that the benefits of disclosing the risk of SUDEP to patients outweigh the harms. This is particularly (but not only) true in patients with generalized tonic-clonic seizures. Further research is needed in this area.
The increased risk of sudden death, including SUDEP, associated with epilepsy should be disclosed as part of the overall education and counseling to patients about their condition and prognosis of living with epilepsy.
A brief clinical tool should be designed and validated to assess readiness to learn about SUDEP. This could be used to determine how and when people with epilepsy and their families should receive information about SUDEP.
Focus groups should be held for people recently diagnosed with epilepsy, people with medically intractable epilepsy, and families who have been affected by SUDEP to determine when and how much information should be presented.
Research studies should be performed to determine the best methods of educating people with epilepsy and their families, the effects of discussing SUDEP on the patient and family, and the role of SUDEP disclosure on the reduction of identified risk factors and the incidence of SUDEP.
Learning materials should include consistent, appropriate, widely available information for the public to ensure that SUDEP education is accurate and communicated appropriately.
EDUCATING HEALTH CARE PROVIDERS ABOUT SUDEP
There is a paucity of data regarding health care provider knowledge of SUDEP and attitudes toward disclosure of risk.
Recommendations
Develop a survey aimed at identifying health professionals' knowledge of SUDEP, expectations of patients' and families' reactions to information on SUDEP, comfort with and timing of discussion of SUDEP with patients and families, and perception of the utility of educational tools.
Develop and disseminate tailored but consistent information regarding SUDEP to professionals.
Develop evidence-based guidelines that provide recommendations for why, when, and how SUDEP should be discussed with people affected by epilepsy. Health care providers should be part of the guideline development. The intended and unintended consequences of guidelines should be considered based upon the experience of countries in which guidelines are in place. Specific consideration should be given to meeting the needs of the broad spectrum of individuals affected by epilepsy (as determined by future research) as well as the social and legal implications of the proposed guidelines.
PREVENTION OF SUDEP WITH CURRENT KNOWLEDGE AND DATA
Based on current knowledge, the most effective means of SUDEP prevention is to reduce the frequency of seizures, especially but not only generalized tonic-clonic seizures, through optimized epilepsy care, including maximizing compliance with medications, avoiding seizure triggers such as sleep deprivation and heavy ethanol use, and consideration of epilepsy surgery in appropriate candidates in a timely fashion, as recommended in the prior AES/EF SUDEP Task Force report1; unnecessary polytherapy should be avoided as well. To this end, the measures discussed above regarding patient, family, and care provider education were endorsed. The recommendation to develop a research agenda in SUDEP will aid with prevention in the long term. Preliminary evidence suggests that nocturnal supervision or monitoring devices may be protective for SUDEP, but this requires further study.
Additional recommendations to aid in SUDEP prevention include the following:
Educate patients about research promotion and participation.
Increase awareness of what constitutes good seizure management, both in care providers and patients.
Increase awareness of SUDEP in the public domain, including through the use of lay media.
Establish collaborations among support groups, funding sources, health care professionals, and others, both nationally and internationally, to advance the public discussion of SUDEP.
Consider developing a SUDEP practice guideline via the American Academy of Neurology.
For future clinical studies, consider using social science study designs to compare SUDEP rates before and after interventions in various regions, and following trends over time. Consider randomizing communities to “aggressive educational campaign” or standard care and following SUDEP rates.
Concentrate research on modifiable risk factors, both at the animal and human level.
FUTURE DIRECTIONS
Create an ongoing SUDEP workgroup. This has already begun. The SUDEP Coalition has been formed jointly by the American Epilepsy Society, The Epilepsy Foundation, Citizens United for Research in Epilepsy, and NINDS. The goal is to promote and organize SUDEP research and other SUDEP-related activities, including the creation of a SUDEP registry and tissue banks. See www.aesnet.org/SUDEP for further information.
Encourage funding sources to solicit and fund proposals on SUDEP.
Supplementary Material
APPENDIX
Participants. Scientific session cochairs: Elson So, Jeff Noebels. Subsection moderators: Clinical factors: Elizabeth J. Donner, Anne Berg. Cardiorespiratory and autonomic mechanisms: Lawrence Hirsch, Martin Samuels. Genetics: Alica Goldman, Michael Ackerman. Case identification: Nancy Temkin, Paul Schraeder. Prevention: Nina Graves, Lina Nashef. Education session cochairs: Jeffrey Buchhalter and Tess Sierzant. Subsection moderators: Ethics: Nancy Collins, Jane Hanna. Educating patients and families: Joan Austin, Fran London. Educating professionals: Andres Kanner, Jacci Bainbridge. Guidelines: Cynthia Harden, Susan Duncan. Prevention: Rosemary Panelli, David Thurman. Other participants are listed in appendix e-1 on the Neurology® Web site at www.neurology.org.
Supplemental data at www.neurology.org
- NINDS
- National Institute of Neurological Disorders and Stroke
- SIDS
- sudden infant death syndrome
- SUDEP
- sudden unexpected death in epilepsy
DISCLOSURE
Dr. Hirsch has received speaker honoraria from UCB, GlaxoSmithKline, Pfizer Inc, and Lundbeck Inc.; serves on the editorial board of the Journal of Clinical Neurophysiology and as a contributing editor for Epilepsy Currents; receives publishing royalties for Atlas of EEG in Critical Care (Wiley-Blackwell, 2010) and UpToDate, Inc.; serves as a consultant for Lundbeck Inc. and Ikano Therapeutics Inc.; serves/has served on speakers' bureaus for GlaxoSmithKline, UCB, Pfizer Inc, and Lundbeck Inc.; and receives research support from Eisai Inc., Pfizer Inc, UCB, Lundbeck Inc., Upsher-Smith, the American Epilepsy Society, and the Epilepsy Foundation. Dr. Donner serves on the editorial board of the Journal of Child Neurology; has received speaker honoraria from Nutrica, Inc. and the American Academy of Neurology; and has received research support from the Canadian Institutes of Health Research and C.U.R.E. Dr. So serves on the editorial board of Epilepsia, Epilepsy Research, and Journal of Clinical Neurophysiology. M. Jacobs reports no disclosures. Dr. Nashef is a trustee for Epilepsy Research UK and serves on the scientific advisory committee of Epilepsy Bereaved; receives publishing royalties for Oxford Specialist Handbooks in Neurology: Epilepsy (Oxford University Press, 2009); and has attended medical conferences as a guest of pharmaceutical companies and supervised staff funded by pharmaceutical companies carrying out audits (GlaxoSmithKline, UCB, Pfizer Inc, and Eisai Inc.). Dr. Noebels served on the editorial board of the Journal of Neuroscience and receives research support from the NIH/NINDS and the Blue Bird Circle Foundation for Pediatric Research. Dr. Buchhalter serves on scientific advisory boards for the NIH and the Charlie Foundation; serves on the editorial advisory board of Clinical Neurology News; and receives research support from Lundbeck Inc, Pfizer Inc, and the NIH/NINDS.
REFERENCES
- 1. So EL, Bainbridge J, Buchhalter JR, et al. Report of the American Epilepsy Society and the Epilepsy Foundation joint task force on sudden unexplained death in epilepsy. Epilepsia 2009;50:917–922 [DOI] [PubMed] [Google Scholar]
- 2. Nashef L, Brown S. Epilepsy and sudden death. Lancet 1996;348:1324–1325 [DOI] [PubMed] [Google Scholar]
- 3. Jonsen AR, Siegler M, Winslade WJ. Clinical Ethics: A Practical Approach to Ethical Decisions in Clinical Medicine, 6th ed. New York: McGraw-Hill; 2006 [Google Scholar]
- 4. Lewis S, Higgins S, Goodwin M. Informing patients about sudden unexpected death in epilepsy: a survey of specialist nurses. Br J Neurosci Nursing 2008;41:30–34 [Google Scholar]
- 5. Morton B, Richardson A, Duncan S. Sudden unexpected death in epilepsy (SUDEP): don't ask, don't tell? J Neurol Neurosurg Psychiatry 2006;77:199–202 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.



