Abstract
Objective:
To evaluate associations between vascular risk factors and changes in burden of infarcts, ventricular size (VS), sulcal widening (SW), and white matter hyperintensities (WMH) in an initially middle-aged, biracial cohort from the Atherosclerosis Risk in Communities (ARIC) study.
Methods:
Initial brain magnetic resonance (MR) scans and evaluations for vascular risk factors were performed in 1,812 ARIC participants in 1994–1995. In 2004–2006, 1,130 ARIC participants underwent repeat MR scans. MR scans were rated using a validated 9-point scale for VS, SW, and WMH. Infarcts were recorded. Multiple logistic regression analysis was used to assess associations between vascular risk factors and change between MR scans of one or more grades in VS, SW, WMH, or appearance of new infarcts, controlling for age, sex, and race.
Results:
At baseline, the 1,112 participants with usable scans (385 black women, 200 black men, 304 white women, 223 white men) had a mean age of 61.7 ± 4.3 years. In adjusted models, diabetes at baseline was associated with incident infarcts (odds ratio [OR] 1.95, 95% confidence interval [CI] 1.29–2.95) and worsening SW (OR 2.10, 95% CI 1.36–3.24). Hypertension at baseline was associated with incident infarcts (OR 1.73, 95% CI 1.23–2.42). In subjects with the highest tertile of fasting blood sugar and systolic blood pressure at baseline, the risk of incident infarcts was 3.68 times higher (95% CI 1.89–7.19) than those in the lowest tertile for both.
Conclusion:
Both atrophic and ischemic imaging changes were driven by altered glycemic and blood pressure control beginning in midlife. Neurology® 2011;76:1879–1885
Midlife vascular risk factors are well-known to be associated with late-life cognitive impairment and dementia,1,2 but the mechanisms by which midlife vascular risk factors cause brain injury are not well-understood. Both ischemic and degenerative pathways are likely involved, but speculation abounds about their relative contributions. There are few autopsy studies in persons in midlife, so what disease-related processes are occurring in midlife must be inferred from late-life neuropathologic studies,3,4 or from brain imaging. Brain imaging in middle-aged populations has begun to address the issue, but most work is cross-sectional. Cross-sectional studies have shown associations with vascular risk factors and imaging features.5–8 There are only a few longitudinal studies9–12 of white matter hyperintensities (WMH) and infarcts, but only one study13 that we are aware of has assessed the impact of vascular risk factors on brain atrophy.
We carried out MRI in an initially middle-aged cohort from the Atherosclerosis Risk in Communities (ARIC) study and have reported on the cross-sectional associations of cognitive and risk factor correlates with magnetic resonance (MR) findings.5,14 Diabetes and hypertension were the 2 most important vascular risk factors in those analyses. MR scans were repeated in over half of the original participants roughly 10 years later. The goal of the present analysis is to describe the imaging changes and their relationships to the presence of diabetes and hypertension, as well as other risk factors. We sought to learn whether vascular risk factors were associated with ischemic and atrophic brain imaging changes.
METHODS
Design and patients.
At inception in 1987–1989, the ARIC study recruited 15,792 women and men, aged 45–64, from probability samples in 4 US communities: Forsyth County, NC; Jackson, MS (black subjects only); selected suburbs of Minneapolis, MN; and Washington County, MD. Details of the ARIC study sampling and study design have been published.15 The current analyses involve a subset of the original ARIC cohort who participated in the ARIC MRI Study (2004–2006). These individuals were recruited for a follow-up brain MR scan and cognitive testing from the subset of the ARIC cohort (n = 1,812) who had an initial MR scan at the third ARIC examination.
During the first 2 years (1993 and 1994) of the third ARIC examination, 2,891 participants aged 55 and older from the ARIC study sites in Forsyth County and Jackson were invited for cerebral MRI and cognitive assessment. Cognitive assessments and vascular risk factor assessments were conducted at this visit, and the vascular risk factors recorded at this third ARIC visit constitute the baseline for the present analyses. For reasons of participant safety, the following exclusion criteria were used for selection of participants in 1993–1994: prior surgery for an aneurysm in the brain; metal fragments in the eyes, brain, or spinal cord; valvular prosthesis, cardiac pacemaker, cochlear implant, spinal cord stimulator, or other internal electrical device; and occupations associated with exposure to metal fragments. Of those screened, 2% of women and 6% of men were ineligible. A total of 1,945 participants successfully underwent cerebral MRI, 1,812 of whom had scans of sufficient quality to perform ratings. The cognitive and vascular risk factor relationships to the imaging obtained in 1993–1994 have been previously described.5,14
Between 2004 and 2006, 10 years after the initial MR scans, all participants who had undergone MR scanning at the third ARIC visit were invited to participate in the ARIC MRI study and undergo a repeat MR and cognitive assessment. There were 1,112 (African American 585, white 527) subjects who had both an initial scan and a follow-up scan of sufficient quality for analysis, as well as assessment of vascular risk factors at the time of the initial MR scan 10 years earlier, who form the study group for this report. There were 808 subjects who did not undergo scanning because of death (n = 268), refusal (n = 452), requests for no further contact with study (n = 20), neurologic disorders (n = 13), surgery/radiation to skull/brain (n = 9), or ineligible (n = 46). The flow of subjects is also shown in figure e-1 on the Neurology® Web site at www.neurology.org.
Standard protocol approvals and patient consents.
All subjects provided written informed consent to participate, based on local standards at Wake Forest University School of Medicine or the University of Mississippi Medical Center.
Risk factors.
Risk factor measurements were obtained in 1993–1994 at the time of the third ARIC visit, with the exception of strokes, which were captured on an ongoing basis. These have been described previously,5,16 so only those measurements for risk factors pertinent to the current report are included here.
Prevalent diabetes mellitus was defined as a fasting glucose of >126 mg/dL, nonfasting glucose of >200 mg/dL, a self-reported history of diabetes, or treatment for diabetes in the past 2 weeks. Serum glucose was assessed by the hexokinase method.
Hypertension was defined as systolic blood pressure >140 mm Hg, diastolic blood pressure >90 mm Hg, or use of antihypertensive medications in the past 2 weeks.
Prevalent stroke prior to first MR was defined as a stroke validated by an ARIC physician through review of medical records17,18 occurring prior to the first MR scan.
Incident stroke was similarly defined as stroke occurring after baseline validated by an ARIC clinician through review of medical records occurring after baseline. The identification and validation of incident strokes is complete to December 31, 2004. Approximately 90% of incident strokes were characterized as ischemic (embolic or thrombotic strokes), the remainder as hemorrhagic.
APOE genotype determinations were available on most subjects. Genotyping of the APOE polymorphisms was performed using the TaqMan assay (Applied Biosystems, Foster City, CA). The current analyses were done using a dichotomous variable to represent the presence or absence of an ϵ4 allele.
Imaging measures.
At the time that the original scans were obtained, quantitative volumetric brain imaging was not available, and consequently, our analyses used visual ratings of white matter hyperintensities, ventricular volume, and sulcal width. While visual ratings lack the precision of volumetric analyses, there is an excellent correspondence between the 2 methods.19 See figure e-2 for templates for the imaging ratings.
All scans were performed at 1.5 T and included axial 5-mm contiguous T1, T2, and proton density–weighted images. The baseline brain MRs in 1993–1994 were obtained on GE or Picker scanners, and were interpreted at the ARIC MRI Reading Center at Johns Hopkins Medical Institutions using a scoring protocol developed and validated by the Cardiovascular Health Study.20,21 Results using the baseline scans have been reported.5,14,22
The follow-up MR scans were performed in 2004–2006 (all on GE scanners), as part of the ARIC MRI study. Although there were interval hardware and software upgrades, these studies were again obtained on 1.5 T scanners and the parameters used for scanning were chosen to match as closely as possible the signal-to-noise, resolution, and contrast weighting used for the earlier scans. These scans were scored at the University of Washington by neuroradiologists who were trained by one of the readers involved in the baseline study. The current study neuroradiologists were tested on a sample of the earlier scans to verify highly similar scoring. The criterion was ≥85% agreement within one grade for the WMH, sulci, and ventricle grading and ≥85% agreement for the presence or absence of infarcts compared to the original adjudicated scoring. This level of concordance was chosen to be similar to the interreader agreement from the baseline scoring. Thus, it was assured that both the scan technique and the scoring of the follow-up scans were as similar as possible to the baseline scans.
The ratings for the baseline and follow-up scans were obtained independently. We did not perform side-to-side comparisons.
Infarcts were defined based on signal characteristics on T1, T2, and proton density images: bright on T2 and proton density and dark on T1 images. Infarcts were counted only if they were >3 mm in maximum diameter. All scans were subjected to double-reads for infarcts scoring, and scans with discrepancies in numbers of infarcts between readers were read a third time for adjudication. A reliability exercise using 104 randomly selected cases was carried out, and interrater agreement was 89%.19
Proton density images were used to estimate the extent of white matter hyperintensities (WMH). Periventricular and subcortical WMH were combined for these analyses. The burden of WMH was rated on a 0–9 scale as previously described for ARIC22 and Cardiovascular Health Study.6 An analysis of the visually rated WMH burden had an excellent linear correlation with quantitatively estimated WMH (R2 = 0.75).19 Interrater reliability for WMH showed a weighted κ of 0.76.
Axial T1-weighted images were used for assessment of ventricular size (VS) and sulcal width (SW) using a 0 to 9 scale.20,21 An analysis of the visual ratings compared to quantitative estimates of ventricular volume showed an excellent correlation (R2 = 0.79) in a subsample of scans from 2004 to 2006.19 The reliability coefficients for 26 pairs of readings by 2 independent neuroradiologists for VS = 0.87, SW = 0.63, and WMH = 0.94.
Analytic procedures.
All outcomes were dichotomized for WMH, VS, and SW. The outcomes were worsening of one or more grades vs no change; for incident infarcts, present vs absent. Multiple logistic regression (SAS Institute, Cary, NC) was used to estimate outcome odds ratios (OR) and 95% confidence intervals (CI) for each vascular risk factor individually, controlling for age, sex, and race. For continuous risk factors, the unit of difference was 1 SD. Cases with missing data were excluded from analyses. Based on our prior studies,5,16 prevalent stroke, incident stroke, diabetes, and hypertension were the risk factors of primary interest. All other risk factors were considered exploratory. With 4 imaging features and 3 risk factors, our analyses involve multiple applications of statistical testing. We used a p value of 0.05, however, because the association of a risk factor with one imaging feature is likely to be correlated with the other imaging features.
Differences in associations among the imaging features should be viewed with the caveat that the metrics for each are different. For example, one incident infarct is not equivalent to an increase in one grade of SW, VS, or WMH.
RESULTS
Demographics and baseline vascular risk factor status of the 1,112 subjects with usable scans are shown in table 1. There were notable differences between racial and gender groups in the prevalence of diabetes, hypertension, and stroke. Compared to current participants, those who died, were ineligible, or refused to participate in the follow-up scan were older, had a much higher stroke rate, had a higher rate of diabetes and hypertension, and had worse imaging at the baseline scan (table e-1).16
Table 1.
Characteristics of the ARIC MRI study population at baseline MR examination
Abbreviations: ARIC = Atherosclerosis Risk in Communities; MR = magnetic resonance.
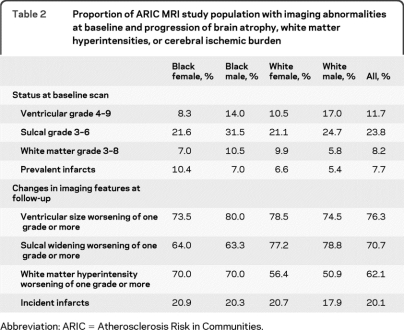
Over a median interval of 10.6 years between scans, most subjects experienced worsening of VS, SW, and WMH, but only 20% experienced new infarcts (table 2). Older age was strongly associated with worsening of imaging features (table e-2). For example, the number of subjects with new infarcts was 16.1% in the 55–59 year age group, but 25.4% in the 65- to 72-year-old subjects. Infarct incidence was weakly correlated with changes in WMH (Pearson correlation 0.17) but virtually uncorrelated with changes in VS or SW (Pearson correlation coefficients of 0.06 and 0.04, respectively). Black subjects had more infarcts at baseline and more increase in WMH (table 2) but ethnic differences were generally small for the other MR features.
Table 2.
Proportion of ARIC MRI study population with imaging abnormalities at baseline and progression of brain atrophy, white matter hyperintensities, or cerebral ischemic burden
Abbreviation: ARIC = Atherosclerosis Risk in Communities.
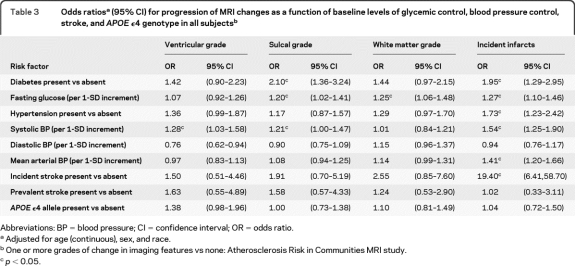
Measures of glycemic control and blood pressure were associated with progression of all 4 imaging features (table 3). Other risk factors showed associations with one or more imaging feature (table e-3), but glycemic control and hypertension alone were the only risk factors that were associated with both ischemic changes and atrophic changes. APOE ϵ4 genotype was not associated with any changes in imaging features. There was, as expected, a strong association between incident infarcts and incident stroke.
Table 3.
Odds ratiosa (95% CI) for progression of MRI changes as a function of baseline levels of glycemic control, blood pressure control, stroke, and APOE ϵ4 genotype in all subjectsb
Abbreviations: BP = blood pressure; CI = confidence interval; OR = odds ratio.
Adjusted for age (continuous), sex, and race.
One or more grades of change in imaging features vs none: Atherosclerosis Risk in Communities MRI study.
p < 0.05.
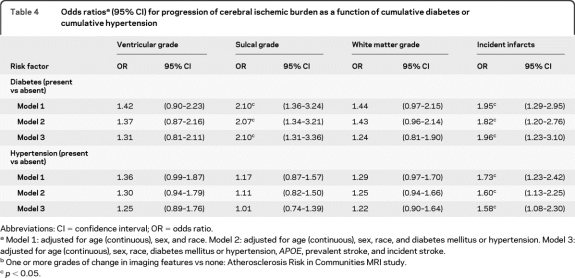
In all 3 multivariable models (table 4), both diabetes and hypertension at baseline were strongly and independently associated with incident infarcts. Diabetes alone was associated with worsening SW in all 3 models.
Table 4.
Odds ratiosa (95% CI) for progression of cerebral ischemic burden as a function of cumulative diabetes or cumulative hypertension
Abbreviations: CI = confidence interval; OR = odds ratio.
Model 1: adjusted for age (continuous), sex, and race. Model 2: adjusted for age (continuous), sex, race, and diabetes mellitus or hypertension. Model 3: adjusted for age (continuous), sex, race, diabetes mellitus or hypertension, APOE, prevalent stroke, and incident stroke.
One or more grades of change in imaging features vs none: Atherosclerosis Risk in Communities MRI study.
p < 0.05.
At baseline, 539 (50.3%) subjects were free of both hypertension and diabetes (“low vascular risk”) and 99 (9.2%) had both (“high vascular risk”). Incident infarcts were seen in 32.6% of the high vascular risk group compared to 15.1% in the low vascular risk group. The corresponding figures for change of one grade or more in the other MRI features were 84.7% vs 73.2% for VS progression, 76.5% vs 55.5% for WMH progression, and 80.0% vs 69.6% for SW progression. The combined effect of hypertension and diabetes was further illustrated by an analysis by tertiles of fasting blood sugar, systolic blood pressure, and risk for incident infarcts. Those in the highest tertile for both fasting blood sugar and systolic blood pressure had 3.68 higher risk (95% CI 1.89–7.19) of new infarcts compared to subjects in the lowest tertile for both conditions (figure 1). Analyses with VS, SW, and WMH progression showed no consistent pattern for the combined factors and ORs were low (data not shown).
Figure 1. Odds ratios of new infarcts by combinations of fasting blood glucose levels and systolic blood pressure in all subjects.
Tertiles of baseline systolic blood pressure are depicted on the x-axis, tertiles of baseline fasting glucose are depicted on the y-axis, and odds ratios on the z-axis. The reference group is the lowest tertile for both systolic blood pressure and fasting blood glucose. Gray bars indicate odds ratios that did not include 1. The odds ratio of the group with the highest tertile of systolic blood pressure and highest fasting blood glucose level was 3.68 (95% confidence interval 1.89–7.19).
We examined the risk factors in table 4 separately for men, women, and black and white subjects and found differences in the pattern of associations achieving p values of <0.05 between gender and racial groups. However, in models that included all subjects, none of the interaction terms for race or gender were significant.
DISCUSSION
There are several important findings from this study of serial imaging of an initially middle-aged cohort. First, over two-thirds of the ARIC MRI study participants experienced a detectable worsening of VS, SW, and WMH over the 10-year interval. Approximately 20% experienced new infarcts, the vast majority of which were lacunar. Second, altered glycemic control and hypertension were associated with incident infarcts and to a less consistent degree, worsening of VS, SW, or WMH. Third, altered glycemic control and elevated blood pressure were independent of one another, and for infarcts, showed additive effects. Fourth, despite the substantial differences at baseline across racial and gender groups, there were no race- or sex-specific interactions between changes in brain imaging and vascular risk factors, APOE ϵ4 genotype, or stroke history. Our observations from this longitudinal study offer convincing evidence for a causal relationship between alterations in glycemic control and blood pressure and subsequent brain ischemic and atrophic changes.
Strengths of the ARIC MRI study include the large sample size, its biracial composition, extensive risk factor assessment at baseline, and the 10-year interval between scans. There are several weaknesses, however. Loss of subjects over the 10 years of follow-up to death and worsening disability is an unavoidable bias in any prospective study. Considering that those persons who had follow-up scans were healthier in all respects including lower burdens of vascular risk factors, and less pathology on imaging,16 our findings probably understate the links between diabetes and hypertension. Unfortunately, when the first scans were obtained, volumetric MRI was not available. We were unable to use volumetric techniques for the serial comparisons. Our rating system for imaging features has been validated, but it is clearly less precise than newer quantitative techniques. Despite our large sample size, there were few APOE ϵ4 homozygotes (n = 33), precluding analysis of homozygotes vs heterozygotes.
The associations of altered glycemic control and hypertension with incident infarcts were the most consistent across the different markers of each (table 3) that we evaluated. Even if the risk factors could have other mechanisms, an ischemic one is not trivial. Our findings are consistent with neuropathologic studies of diabetic23–25 and hypertensive3,26 subjects that have shown that these 2 risk factors are both associated with infarcts.
Our observations on WMH also support a role for vascular processes in the longitudinal evolution of imaging changes with altered glycemic control and hypertension. WMH are strongly associated with cerebrovascular disease by imaging12 and pathology.27 Both cross-sectional5,6,8,22 and longitudinal imaging including ones previously reported from this same ARIC cohort28 and other studies10–12 have shown associations between WMH and vascular risk factors, most notably hypertension. Yet, in both cross-sectional and longitudinal studies, the associations have been modest.
The lack of significant association of incident or prevalent stroke with brain atrophy measures in the present study suggests that atrophic processes and macroscopic ischemic processes are not tightly linked. Associations were generally positive, however, and the number of incident strokes (n = 28) may be inadequate for reliable estimates of association. Lacunar infarcts are not likely to be the proximal cause of brain volume loss, but they might be associated with more widespread cerebral microvascular disease, which in turn is believed to be the core substrate of brain dysfunction causing dementia.29–31
However, epidemiologic investigations,2,32 theoretical considerations,33 and neuropathologic studies have raised the prospect that diabetes mellitus4,34 or hypertension3 might facilitate neurodegenerative mechanisms of the Alzheimer type. Prior cross-sectional studies of vascular risk factors and brain atrophy have generally shown associations with vascular risk factors.5,7,13,35–38 Our observations of the association of worsening SW and prevalent diabetes suggests that some aspect of altered glycemic control leads to synaptic loss, neuronal death, and brain volume loss but does not clarify the underlying mechanisms.
Carriers of the APOE ϵ4 allele are at greater risk for the appearance at a younger age of Alzheimer pathology,39 but are not at greater risk for cerebrovascular disease. We were not able to demonstrate an association in the cross-sectional analyses previously,5 nor currently in longitudinal analyses. Broken down by race, an association between APOE ϵ4 genotype and VS was seen in white but not in black subjects. APOE ϵ4 genotype has a more attenuated relationship to AD in black subjects,40 which perhaps accounts for the lack of association in the group as a whole.
Our observations imply that control of blood sugar and blood pressure in midlife should reduce the likelihood of ischemic and atrophic changes in the brain in subsequent decades. Future clinical trials in midlife aimed at these and other risk factors could use imaging as a marker for relevant brain disease.
Supplementary Material
ACKNOWLEDGMENT
The authors thank the staff and participants of the ARIC study for their important contributions.
- ARIC
- Atherosclerosis Risk in Communities
- CI
- confidence interval
- MR
- magnetic resonance
- OR
- odds ratio
- SW
- sulcal widening
- VS
- ventricular size
- WMH
- white matter hyperintensity
DISCLOSURE
Dr. Knopman serves as Deputy Editor of Neurology®; serves on a data safety monitoring board for Eli Lilly and Company; is an investigator in clinical trials sponsored by Elan Corporation, Baxter International Inc., and Forest Laboratories, Inc.; and receives research support from the NIH. Dr. Penman receives research support from the NIH/NHLBI. Dr. Catellier reports no disclosures. Dr. Coker receives research support from the NIH/NHLBI. Dr. Shibata reports no disclosures. Dr. Sharrett receives research support from the NIH/NHLBI. Dr. Mosley reports no disclosures.
REFERENCES
- 1. Kivipelto M, Ngandu T, Fratiglioni L, et al. Obesity and vascular risk factors at midlife and the risk of dementia and Alzheimer disease. Arch Neurol 2005;62:1556–1560 [DOI] [PubMed] [Google Scholar]
- 2. Whitmer RA, Sidney S, Selby J, Johnston SC, Yaffe K. Midlife cardiovascular risk factors and risk of dementia in late life. Neurology 2005;64:277–281 [DOI] [PubMed] [Google Scholar]
- 3. Petrovitch H, White LR, Izmirilian G, et al. Midlife blood pressure and neuritic plaques, neurofibrillary tangles, and brain weight at death: the HAAS (Honolulu-Asia Aging Study). Neurobiol Aging 2000;21:57–62 [DOI] [PubMed] [Google Scholar]
- 4. Matsuzaki T, Sasaki K, Tanizaki Y, et al. Insulin resistance is associated with the pathology of Alzheimer disease: the Hisayama study. Neurology 2010;75:764–770 [DOI] [PubMed] [Google Scholar]
- 5. Knopman DS, Mosley TH, Catellier DJ, Sharrett AR. Cardiovascular risk factors and cerebral atrophy in a middle-aged cohort. Neurology 2005;65:876–881 [DOI] [PubMed] [Google Scholar]
- 6. Longstreth WT, Jr, Manolio TA, Arnold A, et al. Clinical correlates of white matter findings on cranial magnetic resonance imaging of 3301 elderly people: the Cardiovascular Health Study. Stroke 1996;27:1274–1282 [DOI] [PubMed] [Google Scholar]
- 7. Longstreth WT, Jr, Arnold AM, Manolio TA, et al. Clinical correlates of ventricular and sulcal size on cranial magnetic resonance imaging of 3,301 elderly people: the Cardiovascular Health Study: Collaborative Research Group. Neuroepidemiology 2000;19:30–42 [DOI] [PubMed] [Google Scholar]
- 8. de Leeuw FE, de Groot JC, Oudkerk M, et al. A follow-up study of blood pressure and cerebral white matter lesions. Ann Neurol 1999;46:827–833 [DOI] [PubMed] [Google Scholar]
- 9. Vermeer SE, Den Heijer T, Koudstaal PJ, et al. Incidence and risk factors of silent brain infarcts in the population-based Rotterdam Scan Study. Stroke 2003;34:392–396 [DOI] [PubMed] [Google Scholar]
- 10. Longstreth WT, Jr, Arnold AM, Beauchamp NJ, Jr, et al. Incidence, manifestations, and predictors of worsening white matter on serial cranial magnetic resonance imaging in the elderly: the Cardiovascular Health Study. Stroke 2005;36:56–61 [DOI] [PubMed] [Google Scholar]
- 11. van Dijk EJ, Prins ND, Vrooman HA, et al. Progression of cerebral small vessel disease in relation to risk factors and cognitive consequences: Rotterdam Scan Study. Stroke 2008;39:2712–2719 [DOI] [PubMed] [Google Scholar]
- 12. Gouw AA, van der Flier WM, Fazekas F, et al. Progression of white matter hyperintensities and incidence of new lacunes over a 3-year period: the Leukoaraiosis and Disability Study. Stroke 2008;39:1414–1420 [DOI] [PubMed] [Google Scholar]
- 13. Enzinger C, Fazekas F, Matthews PM, et al. Risk factors for progression of brain atrophy in aging: six-year follow-up of normal subjects. Neurology 2005;64:1704–1711 [DOI] [PubMed] [Google Scholar]
- 14. Mosley TH, Jr, Knopman DS, Catellier DJ, et al. Cerebral MRI findings and cognitive functioning: the Atherosclerosis Risk in Communities Study. Neurology 2005;64:2056–2062 [DOI] [PubMed] [Google Scholar]
- 15. ARIC Investigators The Atherosclerosis Risk in Communities (ARIC) Study: design and objectives. Am J Epidemiol 1989;129:687–702 [PubMed] [Google Scholar]
- 16. Knopman DS, Mosley TH, Catellier DJ, Coker LH. Fourteen-year longitudinal study of vascular risk factors, APOE genotype, and cognition: the ARIC MRI Study. Alzheimers Dement 2009;5:207–214 [DOI] [PubMed] [Google Scholar]
- 17. Rosamond WD, Folsom AR, Chambless LE, et al. Stroke incidence and survival among middle-aged adults: 9-year follow- up of the Atherosclerosis Risk in Communities (ARIC) cohort. Stroke 1999;30:736–743 [DOI] [PubMed] [Google Scholar]
- 18. Toole JF, Lefkowitz DS, Chambless LE, et al. Self-reported transient ischemic attack and stroke symptoms: methods and baseline prevalence: The ARIC Study, 1987–1989. Am J Epidemiol 1996;144:849–856 [DOI] [PubMed] [Google Scholar]
- 19. Shibata DK, Mosley TH, Catellier DJ, et al. Comparison of volumetric segmentation to visual scoring for assessment of white matter ischemic disease. Am Soc Neuroradiol 2007;(Proc):145–146 [Google Scholar]
- 20. Bryan RN, Manolio TA, Schertz LD, et al. A method for using MR to evaluate the effects of cardiovascular disease on the brain: the Cardiovascular Health Study. AJNR Am J Neuroradiol 1994;15:1625–1633 [PMC free article] [PubMed] [Google Scholar]
- 21. Yue NC, Arnold AM, Longstreth WT, Jr, et al. Sulcal, ventricular, and white matter changes at MR imaging in the aging brain: data from the Cardiovascular Health Study. Radiology 1997;202:33–39 [DOI] [PubMed] [Google Scholar]
- 22. Liao D, Cooper L, Cai J, et al. The prevalence and severity of white matter lesions, their relationship with age, ethnicity, gender, and cardiovascular disease risk factors: the ARIC study. Neuroepidemiology 1997;16:149–162 [DOI] [PubMed] [Google Scholar]
- 23. Sonnen JA, Larson EB, Brickell K, et al. Different patterns of cerebral injury in dementia with or without diabetes. Arch Neurol 2009;66:315–322 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Beeri MS, Silverman JM, Davis KL, et al. Type 2 diabetes is negatively associated with Alzheimer's disease neuropathology. J Gerontol A Biol Sci Med Sci 2005;60:471–475 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Ahtiluoto S, Polvikoski T, Peltonen M, et al. Diabetes, Alzheimer disease, and vascular dementia: a population-based neuropathologic study. Neurology 2010;75:1195–1202 [DOI] [PubMed] [Google Scholar]
- 26. Hoffman LB, Schmeidler J, Lesser GT, et al. Less Alzheimer disease neuropathology in medicated hypertensive than nonhypertensive persons. Neurology 2009;72:1720–1726 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Young VG, Halliday GM, Kril JJ. Neuropathologic correlates of white matter hyperintensities. Neurology 2008;71:804–811 [DOI] [PubMed] [Google Scholar]
- 28. Gottesman RF, Coresh J, Catellier DJ, et al. Blood pressure and white matter disease progression in a biethnic cohort: the Atherosclerosis Risk in Communities (ARIC) Study. Stroke 2010;41:3–8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Longstreth WT, Jr, Sonnen JA, Koepsell TD, et al. Associations between microinfarcts and other macroscopic vascular findings on neuropathologic examination in 2 databases. Alzheimer Dis Assoc Disord 2009;23:291–294 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Gold G, Giannakopoulos P, Herrmann FR, Bouras C, Kovari E. Identification of Alzheimer and vascular lesion thresholds for mixed dementia. Brain 2007:130:2830–2836 [DOI] [PubMed] [Google Scholar]
- 31. White L, Petrovitch H, Hardman J, et al. Cerebrovascular pathology and dementia in autopsied Honolulu-Asia Aging Study participants. Ann NY Acad Sci 2002;977:9–23 [DOI] [PubMed] [Google Scholar]
- 32. Luchsinger JA, Mayeux R. Cardiovascular risk factors and Alzheimer's disease. Curr Atheroscler Rep 2004;6:261–266 [DOI] [PubMed] [Google Scholar]
- 33. Roher AE, Kuo YM, Esh C, et al. Cortical and leptomeningeal cerebrovascular amyloid and white matter pathology in Alzheimer's disease. Mol Med 2003;9:112–122 [PMC free article] [PubMed] [Google Scholar]
- 34. Ronnemaa E, Zethelius B, Sundelof J, et al. Impaired insulin secretion increases the risk of Alzheimer disease. Neurology 2008;71:1065–1071 [DOI] [PubMed] [Google Scholar]
- 35. Schmidt R, Launer LJ, Nilsson LG, et al. Magnetic resonance imaging of the brain in diabetes: The Cardiovascular Determinants of Dementia (CASCADE) Study. Diabetes 2004;53:687–692 [DOI] [PubMed] [Google Scholar]
- 36. Jefferson AL, Massaro JM, Wolf PA, et al. Inflammatory biomarkers are associated with total brain volume: the Framingham Heart Study. Neurology 2007;68:1032–1038 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Seshadri S, Wolf PA, Beiser A, et al. Stroke risk profile, brain volume, and cognitive function: the Framingham Offspring Study. Neurology 2004;63:1591–1599 [DOI] [PubMed] [Google Scholar]
- 38. den Heijer T, Launer LJ, Prins ND, et al. Association between blood pressure, white matter lesions, and atrophy of the medial temporal lobe. Neurology 2005;64:263–267 [DOI] [PubMed] [Google Scholar]
- 39. Kok E, Haikonen S, Luoto T, et al. Apolipoprotein E-dependent accumulation of Alzheimer disease-related lesions begins in middle age. Ann Neurol 2009;65:650–657 [DOI] [PubMed] [Google Scholar]
- 40. Green RC, Cupples LA, Go R, et al. Risk of dementia among white and African American relatives of patients with Alzheimer disease. JAMA 2002;287:329–336 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.