Non-technical summary
It is well known that cardiovascular disease is more frequent in postmenopausal than in premenopausal women. Moreover, it has been shown that dehydroepiandrosterone (DHEA), a steroid hormone secreted by adrenal glands, reduces during ageing. Its reduced plasma level has been related to increased prevalence of obesity, insulin resistance and cardiovascular disease. We show that DHEA treatment in ovariectomized rats, an experimental model of menopause, reduces blood pressure and improves vascular function. Furthermore, DHEA reduced reactive oxygen species (ROS), correcting the reduced protein expression of Cu/Zn-SOD, an antioxidant protein, and increased protein expression of NADPH oxidase, a pro-oxidant protein. This work shows the potential effect of DHEA upon correction of endothelial dysfunction observed on oestrogen deprivation.
Abstract
Abstract
Cardiovascular disease is less frequent in premenopausal women than in age-matched men or postmenopausal women. Moreover, the marked age-related decline in serum dehydroepiandrosterone (DHEA) level has been associated to cardiovascular disease. The aim of this study was to evaluate the effects of DHEA treatment on vascular function in ovariectomized rats. At 8 weeks of age, female Wistar rats were ovariectomized (OVX) or sham (SHAM) operated and 8 weeks after surgery both groups were treated with vehicle or DHEA (10 mg kg−1 week−1) for 3 weeks. Aortic rings were used to evaluate the vasoconstrictor response to phenylephrine (PHE) and the relaxation responses to acetylcholine (ACh) and sodium nitroprusside (SNP). Tissue reactive oxygen species (ROS) production and SOD, NADPH oxidase and eNOS protein expression were analysed. PHE-induced contraction was increased in aortic rings from OVX compared to SHAM, associated with a reduction in NO bioavailability. Furthermore, the relaxation induced by ACh was reduced in arteries from OVX, while SNP relaxation did not change. The incubation of aortic rings with SOD or apocynin restored the enhanced PHE-contraction and the impaired ACh-relaxation only in OVX. DHEA treatment corrected the increased PHE contraction and the impaired ACh-induced relaxation observed in OVX by an increment in NO bioavailability and decrease in ROS production. Besides, DHEA treatment restores the reduced Cu/Zn-SOD protein expression and eNOS phosphorylation and the increased NADPH oxidase protein expression in the aorta of OVX rats. The present results suggest an important action of DHEA, improving endothelial function in OVX rats by acting as an antioxidant and enhancing the NO bioavailability.
Introduction
It is known that oestrogen has profound effects on the cardiovascular system (White, 2002). Indeed, cardiovascular disease is much less frequent in premenopausal women than in age-matched men or postmenopausal women (Burt et al. 1995).
Endothelial dysfunction is a common factor in the development of many cardiovascular diseases. In line with this, endothelial function was shown to be impaired in ovariectomized women (Virdis et al. 2000) and hypertensive female rats (Wassmann et al. 2001; Widder et al. 2003). Indeed, it has been observed that ovariectomy reduces vascular response to acetylcholine in Sprague–Dawley rats (Squadrito et al. 2000; Lam et al. 2006), female hypertensive rats (Wassmann et al. 2001; Widder et al. 2003), and Wistar rats (Paredes-Cabejal et al. 1995). Ovariectomy also increases vascular response to phenylephrine in Sprague–Dawley rats (Lam et al. 2006), and Wistar rats (Paredes-Cabejal et al. 1995). This impairment in endothelial function was associated with an increment in superoxide anion production (Wassmann et al. 2001; Lam et al. 2006) as well as a reduction in eNOS protein expression (Widder et al. 2003). Moreover, ovariectomy promoted an increment in blood pressure of normotensive women (Mercuro et al. 2004), as well as in female Wistar rats (Mendonça et al. 2007), and in hypertensive rats (Ito et al. 2006).
Oestrogen replacement therapy (ORT) in ovariectomized women and female rats prevents the deleterious effect of ovariectomy by reducing blood pressure and increasing antioxidant defence and eNOS protein expression. However, large controlled clinical trials have not shown benefits of ORT, or have even shown deleterious effects, such as an increment of venous thromboembolic disease, stroke and breast cancer incidence (Prentice et al. 2009). Therefore, new therapeutic strategies to prevent oestrogen deprivation-induced deleterious effects on the cardiovascular system will be valuable.
Dehydroepiandrosterone (DHEA) has been considered a potential alternative hormone for ORT, due to its apparent safe, antioxidant and metabolic effects (Labrie et al. 2005). DHEA and its sulfated form (DHEAS) are the most abundant cholesterol-derived hormones in humans and their plasma concentrations progressively decline with age. An inverse relation between plasma DHEA concentration and obesity, diabetes and incidence of cardiovascular disease has been observed (Barrett-Connor et al. 1986; Nestler et al. 1988; Schriock et al. 1988). Moreover, DHEA treatment protects heart of diabetic rats against oxidative stress (Aragno et al. 2008); it furthermore protects endothelial cells against apoptosis (Liu et al. 2007), and stimulates ser1177 eNOS phosphorylation (Formoso et al. 2006).
It is well known that ovariectomized female rats present endothelial dysfunction and high blood pressure levels (Wassmann et al. 2001; Widder et al. 2003). Therefore, DHEA evokes beneficial mechanisms that could reduce endothelial dysfunction and reduce the cardiovascular risk present after surgical menopause in rats. Thus, the present work hypothesizes that DHEA could be an alternative hormonal therapy, and provide benefits to the vascular system of postmenopausal women. To evaluate this hypothesis, the aim of the present study was to assess the effects of DHEA treatment on endothelium-dependent relaxation, focusing on the mechanisms involved, mainly related to NO and oxidative stress, in an experimental menopause models in rats.
Methods
Animal care and protocol
All experimental procedures were approved by and conducted in accordance with the guidelines of the Animal Care and Use Committee of the Biomedical Institute of Sao Paulo University, according to the guidelines of the Brazilian Societies of Experimental Biology. Seven-week-old female Wistar rats were obtained from colonies maintained at the Institute of Biomedical Sciences of the University of São Paulo. At 8 weeks of age, the rats were anaesthetized with a ketamine–xylazine–acepromazine mixture (64.9, 3.2 and 0.78 mg kg−1, respectively, i.p.) and were ovariectomized (OVX) or sham-operated (SHAM). After 8 weeks of surgery, OVX and SHAM rats were treated with vehicle (soybean oil) or DHEA (10 mg kg−1 week−1, s.c.) for 3 weeks. The DHEA administration protocol was based on studies published by us and others (Campbell et al. 2004; Medina et al. 2006; Jacob et al. 2009). During all the procedures rats were housed in a constant room temperature environment, with 12:12 h light–dark cycle, with free access to standard rat chow and tap water. Rats were randomly divided into four groups: (1) SHAM, (2) SHAM+DHEA, (3) OVX, and (4) OVX+DHEA.
Biometrics characteristics
At the end of the treatment period, the body weight gain, retroperitoneal fat pad and uterus weight were determined.
Arterial blood pressure measurement
Rats were anaesthetized with a ketamine–xylazine– acepromazine mixture (64.9, 3.2 and 0.78 mg kg−1, respectively, i.p.) and allowed to breathe room air spontaneously. The right carotid artery was cannulated with a polyethylene catheter (PE-50 with heparinized saline) that was exteriorized in the mid-scapular region. After 24 h, arterial pressure and heart rate were measured in conscious animals by a pressure transducer (model TRA 021, PanLab, Barcelona, Spain) and recorded by using an interface and software for computer data acquisition (Power Lab 4/25; ADInstruments, Sydney, Australia). Heart rate was determined from the interbeat intervals.
Vascular reactivity study
Vascular function was evaluated in aortic rings from all groups studied. We used the aortic rings as a model to study vascular function because in this artery the main endothelium-dependent vasodilator factor is NO. Thus, since the aim of the present study is to assess the role of NO and reactive oxygen species on the DHEA treatment in OVX rats, the aortic rings are the best model to answer our questions without misinterpretation. Segments of the thoracic aorta (4 mm in length), free of connective tissue, were mounted in an isolated tissue chamber containing Krebs–Henseleit solution (containing (in mm): 118 NaCl, 4.7 KCl, 25 NaHCO3, 2.5 CaCl2.2H2O, 1.2 KH2PO4, 1.2 MgSO4.7H2O, 11 glucose, and 0.01 EDTA), gassed with 95% O2–5% CO2, and maintained resting tension of 1 g at 37°C at pH 7.4 as previously published by our group (Davel et al. 2008). In order to analyse the influence of the endothelium on vascular responses, the endothelial layer was mechanically removed in certain experiments by rubbing the lumen with a needle. Isometric tension was recorded by using an isometric force transducer (Letica TRI 210, Barcelona, Spain) connected to an acquisition system (MP100, Biopac Systems, Goleta, CA, USA).
After 30 min equilibration, concentration–response curves to the α1-adrenoceptor agonist phenylephrine (PHE, 10−10–10−4 mol l−1), to the endothelium-dependent vasodilator acetylcholine (ACh, 10−11–10−5 mol l−1) and to the nitric oxide donor sodium nitroprusside (SNP, 10−11–10−5 mol l−1) were obtained. At the end of the experiment, the presence or absence of functional endothelium was verified in all aortic rings by observing whether relaxation occurred on exposure to ACh (10−5 mol l−1) or not. The endothelium was considered intact if the aortic ring relaxed more than 80% to ACh, while endothelial denudation was confirmed by less than 10% relaxation. At the end of experiments, aortic rings were allowed to dry for at least 24 h and then were weighed. Vasoconstrictor responses to PHE are expressed as gram (g) of tension per milligram (mg) of tissue. Vasodilator concentration–response curves were obtained in the aortic rings pre-contracted with PHE (∼10−6 mol l−1), which is approximately 80% of maximal contraction. Relaxation induced by ACh and SNP was expressed as the percentage of the tonus obtained with PHE.
The role of NO and superoxide anion in the response to PHE and ACh was evaluated by incubating some aortic rings with the non-selective NOS inhibitor l-NG-nitro-l-arginine methyl ester (l-NAME; 100 μmol l−1), the superoxide anion scavenger superoxide dismutase (SOD; 150 U ml−1) or the inhibitor of NADPH oxidase, apocynin (100 μmol l−1), added 30 min before the construction of the concentration–response curves to PHE or ACh.
For each concentration–response curve to PHE and ACh, the maximal response (Rmax) and the agonist concentration log resulting in 50% of the Rmax (log EC50) were calculated using non-linear regression analysis (GraphPad Prism software, USA).
Reactive oxygen species measurement in aorta
Thoracic aortas were carefully dissected out and cleaned of connective tissue. Aortas were then divided into cylindrical segments 4 mm in length and were first immersed in an embedding medium (tissue freezing medium) and then frozen and kept at −80°C until superoxide anion was measured. The oxidative fluorescent dye hydroethidine was used to evaluate the in situ production of reactive oxygen species (ROS) as previously described (Hernanz et al. 2004). Transverse aortic sections (10 μm) were obtained in a cryostat from previously frozen aorta and collected on glass slides and left to reach equilibrium for 30 min at 37°C in phosphate-buffered saline (PBS). Fresh buffer containing hydroethidine (5 μmol l−1) was topically applied to each tissue section and coverslipped. Slides were incubated in a light-protected, humidified chamber at 37°C for 30 min. Control sections received the same volume of PBS. Images were obtained with a Nikon E1000 microscope equipped for epifluorescence (excitation at 488 nm; emission at 610 nm). Fluorescence was detected with a 585 nm long-pass filter. The fluorescence intensity was quantified using ImageJ software (NIH) and was expressed as arbitrary units.
Western blot analysis
For analysis of eNOS, ser1177 phosphorylated eNOS, SOD and NADPH oxidase protein expression, aortas were dissected out, cleaned of connective tissue, frozen in liquid nitrogen and kept at −70°C until the day of analysis. They were homogenized in RIPA lysis buffer containing protease inhibitor cocktail (1:5000 dilution), sodium fluoride (100 mm), sodium pyrophosphate (10 mm), sodium orthovanadate (100 mm), and PMSF (10 mm). Proteins from homogenized aorta (75 μg of protein extracts) were electrophoretically separated by 7.5% or 12% SDS-PAGE and then transferred to polyvinylidene difluoride membranes overnight at 4°C using a Mini Trans-Blot Cell system (Bio-Rad) containing 25 mm Tris, 190 mm glycine, 20% methanol and 0.05% SDS.
After blockade of non-specific sites with 5% non-fat dry milk, membranes were incubated overnight at 4°C with the following primary antibodies: anti-eNOS (1:1000, BD Transduction Laboratories, Lexington, KY, USA), anti-phospho eNOSser1177 (1:1000, Cell Signaling Technology, Inc., Danvers, MA, USA), anti-Cu/Zn SOD (1:2000, Sigma-Aldrich, Germany), anti-Mn SOD (1:2000, Sigma-Aldrich), anti-gp91phox (1:1000, Upstate Biotechnology, Inc., Lake Placid, NY, USA) and anti-p22phox (1:500, Santa Cruz Biotechnology, Inc., Santa Cruz, CA, USA). After washing (10 mm Tris, 100 mm NaCl, and 0.1% Tween 20), membranes were incubated with horseradish peroxidase-conjugated anti-rabbit IgG antibody (1:1500, Bio-Rad Laboratories, Hercules, CA, USA) for gp91phox, p22phox, Mn-SOD and phopho eNOS, and with anti-mouse IgG (1:1500, Bio-Rad) for Cu/Zn-SOD and eNOS. The same membranes were used to determine α-actin protein expression using a mouse monoclonal antibody (1:1500, Sigma-Aldrich). Membranes were thoroughly washed, and immune complexes were detected using an enhanced luminol chemiluminescence system (ECLPlus, Amersham GE Healthcare, UK) and subjected to MF-Chemibis-Bio-Imageing System (BioAmerica Inc., Miami, FL, USA). Signals on the immunoblot were quantified with Scion Image software.
Total expression of the proteins is given as the ratio between the optical density for the specific protein and the signal for α-actin protein expression.
Drugs
Dehydroepiandrosterone, phenylephrine hydrochloride, acetylcholine hydrochloride, sodium nitroprusside, SOD (bovine erythrocyte), l-NAME and apocynin were purchased from Sigma-Aldrich (USA); stock solutions (10 mmol l−1) were prepared in distilled water, except for DHEA, which was suspended in soybean oil (10 mg ml−1).
Statistical analysis
Results were expressed as means ± SEM, and n represents the numbers of animals used in each experiment. All results were analysed by two-way ANOVA followed by the Bonferroni post hoc test. A value of P < 0.05 was considered significant.
Results
Biometrics and haemodynamic parameters
OVX rats displayed elevated body weight gain (SHAM: 47.8 ± 3.0 vs. OVX: 83.3 ± 4.3 g; n = 10, P < 0.01) and retroperitoneal fat pad (SHAM: 0.5 ± 0.04 vs. OVX: 1.1 ± 0.09 mg (100 g BW)−1; n = 10, P < 0.01) (Table 1). However, they presented reduced uterus weight (SHAM: 613 ± 103 vs. OVX: 176 ± 14 mg; n = 10, P < 0.01) as compared to SHAM rats (Table 1). DHEA treatment did not modify these parameters; body weight gain (SHAM+DHEA: 49.2 ± 2.6 and OVX+DHEA: 79.3 ± 4.5 g; n = 10), retroperitoneal fat pad (SHAM+DHEA: 0.6 ± 0.06 and OVX+DHEA: 1.1 ± 0.10 mg (100 g BW)−1; n = 10) and uterus weight (SHAM+DHEA: 594 ± 50 and OVX+DHEA: 178 ± 24 mg; n = 10), compared to respective groups treated with vehicle (Table 1).
Table 1.
Body weight gain, retroperitoneal fat pad and uterus weight from ovariectomized (OVX) or sham-operated (SHAM) rats treated with vehicle or DHEA for 3 weeks
| SHAM | SHAM + DHEA | OVX | OVX + DHEA | |
|---|---|---|---|---|
| Body weight gain (g) | 47.8 ± 3.0 | 49.2 ± 2.6 | 83.3 ± 4.3* | 79.3 ± 4.5* |
| Retroperitoneal fat pad (mg (100 g BW)−1) | 0.5 ± 0.04 | 0.6 ± 0.06 | 1.1 ± 0.09* | 1.1 ± 0.10* |
| Uterus weight (mg) | 613 ± 103 | 594 ± 50 | 176 ± 14* | 178 ± 24* |
The values are expressed as means ± SEM. Two-way ANOVA
P < 0.01 in comparison to SHAM and SHAM+DHEA.
Table 2 shows that OVX rats presented higher systolic and diastolic blood pressure as compared to SHAM rats, while heart rate was not different in different groups. DHEA treatment corrected the systolic and diastolic blood pressure in OVX rats (Table 2). However, it did not change the blood pressure levels in SHAM rats as well as heart rate in both groups (Table 2).
Table 2.
Changes in systolic (SBP) and diastolic blood pressure (DBP) and in heart rate from ovariectomized (OVX) or sham-operated (SHAM) rats treated with vehicle or DHEA for 3 weeks
| SHAM | SHAM + DHEA | OVX | OVX + DHEA | |
|---|---|---|---|---|
| SBP (mmHg) | 129.2 ± 2.5 | 130.5 ± 2.6 | 139.6 ± 1.8* | 128.4 ± 2.7‡ |
| DBP (mmHg) | 91.5 ± 1.0 | 90.2 ± 2.6 | 100.2 ± 2.3* | 94.0 ± 1.6‡ |
| Heart rate (bpm) | 356.8 ± 9.1 | 365.2 ± 14.6 | 384.0 ± 11.9 | 354.5 ± 10.0 |
The values are expressed as mean ± SEM. Two-way ANOVA
P < 0.05 in comparison to SHAM
P < 0.05 in comparison to OVX.
Vascular reactivity
The contraction induced by PHE was increased in aorta from OVX rats compared with SHAM (Fig. 1A) (Rmax: SHAM 0.84 ± 0.03 vs. OVX 1.27 ± 0.07 g of tension per mg of tissue; n = 11, P < 0.01). On the other hand, the endothelium-dependent relaxation induced by ACh was reduced in aorta from OVX rats (Fig. 1B) (Rmax: SHAM 91.6 ± 2.7 vs. OVX 74.1 ± 2.7% of relaxation; n = 9, P < 0.01), while there was no change in the relaxation response to the NO donor SNP (Fig. 1C). DHEA treatment in OVX rats corrected both the increased PHE-induced contraction (Fig. 1A) (Rmax: OVX+DHEA 0.90 ± 0.04 g of tension per mg of tissue; n = 11) and the impaired ACh-induced relaxation (Fig. 1B) (Rmax: OVX+DHEA 90.3 ± 2.4% of relaxation; n = 9); while DHEA treatment in SHAM rats did not change either PHE-induced contraction (Fig. 1A) (Rmax: SHAM+DHEA 0.86 ± 0.06 g of tension per mg of tissue; n = 11) or ACh-induced relaxation (Fig. 1B) (Rmax: SHAM+DHEA 95.7 ± 3.7% of relaxation; n = 9). There were no changes in EC50 values among groups for each drug studied (data not shown).
Figure 1. Vascular reactivity to phenylephrine, acetylcholine and sodium nitroprusside.
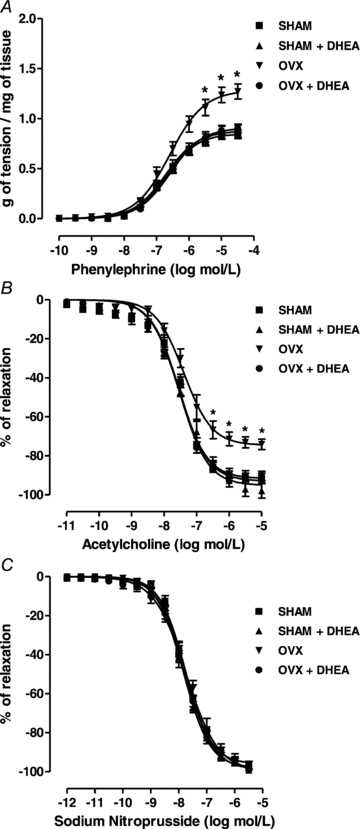
Concentration–response curve to phenylephrine (A), acetylcholine (B) and sodium nitroprusside (C) in endothelium-intact aortic rings from SHAM, OVX and DHEA-treated rats. Results are expressed as means ± SEM. Two-way ANOVA: *P < 0.01 in comparison to SHAM (n = 9–13).
Effects of DHEA treatment on the endothelial and NO modulation of phenylephrine-induced contraction in OVX rats
Endothelium and NO modulation of PHE-induced contraction were evaluated by endothelium denudation or l-NAME incubation, respectively. An increase in Rmax to PHE was observed in all groups of animals after endothelium removal (Table 3) or l-NAME incubation (Table 3). It is important to note that in the absence of endothelial cells or in the presence of nitric oxide inhibitor (l-NAME) there were no differences among groups. EC50 values were similar in all conditions (data not shown).
Table 3.
Effect of endothelium denudation (E−), NG-nitro-l-arginine methyl ester (l-NAME), SOD and apocynin (Apo) on the maximal response (Rmax) to phenylephrine
| SHAM | SHAM + DHEA | OVX | OVX + DHEA | |
|---|---|---|---|---|
| E+ | 0.84 ± 0.03 | 0.86 ± 0.06 | 1.27 ± 0.07* | 0.90 ± 0.04† |
| E− | 2.69 ± 0.17‡ | 2.71 ± 0.25‡ | 2.54 ± 0.10‡ | 2.66 ± 0.16‡ |
| l-NAME | 2.53 ± 0.13‡ | 2.37 ± 0.07‡ | 2.33 ± 0.11‡ | 2.42 ± 0.10‡ |
| SOD | 0.84 ± 0.05 | 0.80 ± 0.02 | 0.90 ± 0.02‡ | 0.84 ± 0.04 |
| Apo | 0.85 ± 0.09 | 0.74 ± 0.09 | 0.88 ± 0.11‡ | 0.82 ± 0.07 |
The values are expressed as mean ± SEM (g of tension per mg of tissue). Two-way ANOVA:
P < 0.01 in comparison to SHAM
P < 0.01 in comparison to OVX
P < 0.01 in comparison to E+ rings (endothelium intact).
Effects of DHEA treatment on the role of superoxide anion in the phenylephrine-induced contraction in OVX rats
To evaluate the possible role of superoxide anion modulation, derived from NADPH oxidase, on PHE-induced contraction, SOD and apocynin were incubated in some aortic rings 30 min before the concentration–response curves to PHE. Both SOD and apocynin significantly reduced the Rmax to PHE in aorta from OVX rats, whereas they did not have any effects in aorta from SHAM and DHEA-treated rats (Table 3). There were no differences among groups in EC50 in any situation (data not shown).
Effects of DHEA treatment on the role of superoxide anion in the acetylcholine-induced relaxation in OVX rats
The involvement of superoxide anion in ACh-induced relaxation was also evaluated. Both SOD and apocynin significantly enhanced the Rmax to ACh in aortic rings from OVX rats, whereas they did not have any effect in aorta from SHAM and DHEA-treated rats (Table 4). In addition, there were no differences among groups in EC50 to ACh in the presence of SOD or apocynin (data not shown).
Table 4.
Effect of SOD and apocynin (Apo) on the maximal response (Rmax) to acetylcholine
| SHAM | SHAM + DHEA | OVX | OVX + DHEA | |
|---|---|---|---|---|
| E+ | 91.6 ± 2.7 | 95.7 ± 3.7 | 74.1 ± 2.7* | 90.3 ± 2.4† |
| SOD | 93.4 ± 2.5 | 95.7 ± 1.6 | 96.5 ± 2.2‡ | 94.9 ± 1.6 |
| Apo | 92.5 ± 3.9 | 94.4 ± 1.6 | 93.4 ± 1.7‡ | 90.5 ± 2.3 |
The values are expressed as mean ± SEM (% of relaxation). Two-way ANOVA:
P < 0.01 in comparison to SHAM
P < 0.01 in comparison to OVX
P < 0.01 in comparison to E+ rings (endothelium intact).
Reactive oxygen species production
In order to evaluate tissue reactive oxygen species (ROS) production, dihydroethidium (DHE) staining was performed in aorta. As observed in Fig. 2 dihydroethidium-emitted fluorescence intensity was higher in aorta from OVX rats than in SHAM, indicating increased ROS production (Fig. 2). Treatment with DHEA for 3 weeks did not change ROS production in aorta from SHAM rats, although it corrected the enhanced ROS production in aorta from OVX rats (Fig. 2).
Figure 2. Reactive oxygen species in aorta.
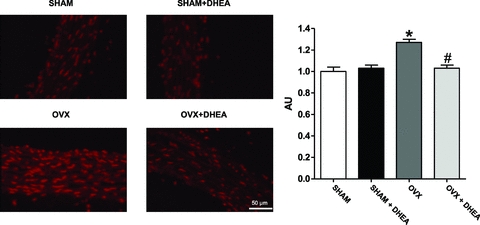
Representative fluorescence photomicrographs of microscopic sections of thoracic aorta from SHAM, OVX and DHEA-treated rats. Vessels were labelled with the oxidative dye hydroethidine, which produces a red fluorescence when oxidized to ethidium bromide by superoxide anion. Quantitative analysis for fluorescence photomicrographs of microscopic sections of thoracic aorta from SHAM, OVX and DHEA-treated rats. Results are expressed as means ± SEM. Two-way ANOVA: *P < 0.01 in comparison to SHAM and #P < 0.01 in comparison to OVX (n = 7).
Western blot analysis of endothelial nitric oxide synthase, superoxide dismutase and subunits of NADPH oxidase
Ovariectomy or DHEA treatment did not change endothelial nitric oxide synthase (eNOS) protein expression in aorta (Fig. 3A). However, in aorta from OVX rats there was observed a reduction on eNOS phosphorylation compared to SHAM (Fig. 3B). Furthermore, DHEA treatment in OVX rats corrected this reduction (Fig. 3B).
Figure 3. eNOS protein expression and phosphorylation in aorta.
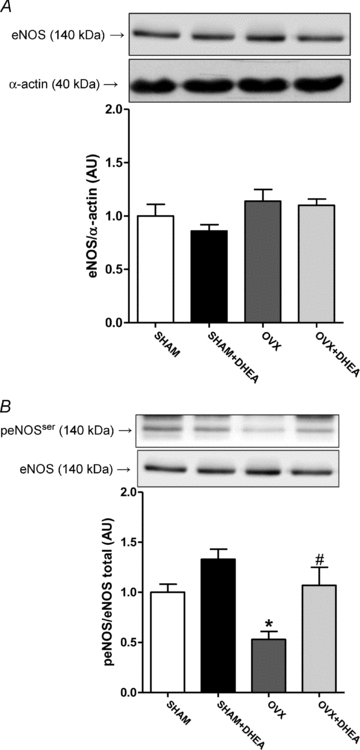
Representative Western blots (top) and quantitative analysis (bottom) for total eNOS protein expression (A) and ser1177 eNOS phosphorylation (B) in thoracic aorta from SHAM, OVX and DHEA-treated rats. Results are expressed as means ± SEM. Two-way ANOVA: *P < 0.01 in comparison to SHAM and #P < 0.01 in comparison to OVX (n = 9).
In addition, Cu/Zn-SOD protein expression was reduced in aorta from OVX rats and corrected by DHEA treatment (Fig. 4A), while there was no change in Mn-SOD protein expression among groups (Fig. 4B). Besides reduction of Cu/Zn-SOD protein expression, the aorta from OVX rats displayed an increased protein expression of gp91phox and p22phox (Fig. 5) NADPH oxidase subunits. Moreover, DHEA treatment of OVX rats corrected the increased of both NADPH oxidase subunits in aorta (Fig. 5).
Figure 4. Cu/Zn-SOD and Mn-SOD protein expression in aorta.
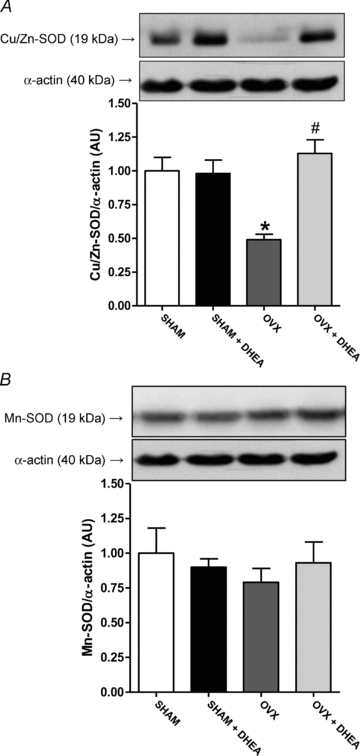
Representative Western blots (top) and quantitative analysis (bottom) for Cu/Zn-SOD (A) and Mn-SOD (B) protein expression in thoracic aorta from SHAM, OVX and DHEA-treated rats. Results are expressed as means ± SEM. Two-way ANOVA: *P < 0.01 in comparison to SHAM and #P < 0.01 in comparison to OVX (n = 8).
Figure 5. NADPH oxidase subunits protein expression in aorta.
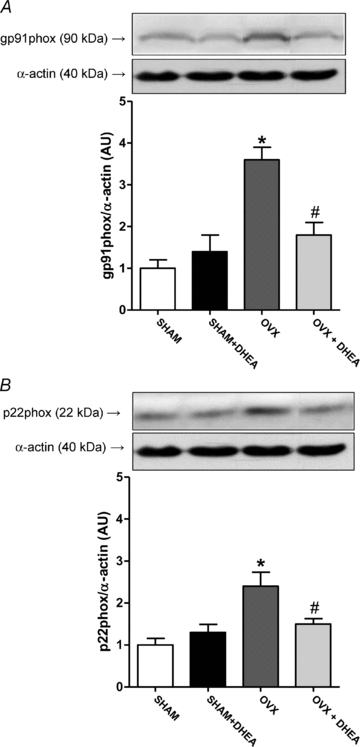
Representative Western blots (top) and quantitative analysis (bottom) for gp91phox (A) and p22phox (B) protein expression in thoracic aorta from SHAM, OVX and DHEA-treated rats. Results are expressed as means ± SEM. Two-way ANOVA: *P < 0.01 in comparison to SHAM and #P < 0.01 in comparison to OVX (n = 8).
Discussion
It has been shown that oestrogens have protective effects on the vascular system (White, 2002), based on epidemiological, clinical and molecular findings. In line with this affirmation, the loss of oestrogens may have profound effects on the cardiovascular system, with increase in blood pressure and endothelial dysfunction, both in women (Virdis et al. 2000; Mercuro et al. 2004) and in experimental models of menopause (Wassmann et al. 2001; Ito et al. 2006). In the present study we demonstrated that DHEA treatment reduces blood pressure and protects against oxidative stress-induced endothelial dysfunction in ovariectomized rats.
The favourable biological effects of oestrogen in the cardiovascular system have been recognized, in spite of the fact that several randomized clinical trials have failed to observe a benefit of hormone replacement therapy on the incidence of cardiovascular events in postmenopausal women (Hulley et al. 1998; Rossouw et al. 2002; Prentice et al. 2009). On the other hand, oestrogen replacement therapy corrected, in ovariectomized women, endothelial dysfunction (Virdis et al. 2000) and increased blood pressure (Mercuro et al. 2004). It is well known that ovariectomized rats display endothelial dysfunction, showing reduction in vascular response to ACh and increase in vascular response to PHE (Wassmann et al. 2001; Wong et al. 2006). Furthermore, Mendonça et al. (2007) found increased blood pressure after 11 weeks of ovariectomy in female Wistar rats. Interestingly, oestrogen seems to be important not only for females, since aromatize knockout male mice displayed reduced vascular response to ACh (Kimura et al. 2003). In line with those observations, our study shows an enhancement in blood pressure, increased vascular contraction to PHE and reduced endothelium-dependent relaxation to ACh following ovariectomy in rats.
Supporting the hypothesis of the present study, 3 weeks of DHEA treatment reduced the systolic and diastolic blood pressure, and corrected vascular responses to PHE and ACh in ovariectomized rats. The plasma DHEA level reduces during the ageing process and this has been related to the prevalence of body weight gain, obesity and insulin resistance, and mortality by cardiovascular disease (Barrett-Connor et al. 1986; Nestler et al. 1988; Schriock et al. 1988). Both aged men and aged women submitted to DHEA treatment for 6 months displayed visceral and subcutaneous fat reduction, besides increased insulin sensitivity (Villareal & Holloszy, 2004). Moreover, in a double-blind study, DHEA-treated postmenopausal women for 12 weeks showed an enhancement of endothelial function (Williams et al. 2004). Considering that the adrenal steroid DHEA is the unique sexual steroid hormone source in postmenopausal women (Labrie, 2010) and the risks associated with ORT (Prentice et al. 2009), the data presented here concerning the mechanism of DHEA action suggest that DHEA could be used as a successful alternative hormone replacement therapy for postmenopausal women.
Indeed, some studies using animal models demonstrated that DHEA treatment induces several beneficial effects. It may reduce cardiac fibrosis in diabetic rats (Aragno et al. 2008), delay atherosclerotic plaque formation in rabbits (Hayashi et al. 2000), reduce weight gain and fat deposition in rats (Han et al. 1998), increase insulin sensitivity (Campbell et al. 2004) and insulin secretion in rats (Medina et al. 2006), and reduce blood pressure in ovariectomized rats (Bhuiyan & Fukunaga, 2010). In addition, DHEA increases in vitro endothelial proliferation (Williams et al. 2004), and protects endothelial cells against apoptosis (Liu et al. 2007), both effects independently of oestrogen receptors. Besides, the present study has for the first time shown that DHEA treatment in vivo corrects both contractile and relaxation responses to PHE and ACh in OVX rats. Therefore, DHEA has several effects on the cardiovascular system, which could be direct or indirect, which reduce cardiovascular risk factors such as body weight gain or insulin resistance.
The present results suggest that the correction of increase in PHE-induced contraction and decrease in ACh-induced relaxation observed in aortas from ovariectomized rats by DHEA treatment in part are related to an increment in eNOS phosphorylation, improving NO production, since the eNOS phosphorylation on ser-1177 enhances the NO production by eNOS (Dimmeler et al. 1999). In line with the present results obtained in aorta, Bhuiyan & Fukunaga (2010) showed a decrease in eNOS phosphorylation in kidney from ovariectomized Wistar rats. Moreover, Widder et al. (2003) demonstrated that ovariectomy is related to a reduction of eNOS protein expression in aorta from SHR rats, which was not observed in our study using aorta from Wistar rats. Recently, consistent with our results, Bhuiyan & Fukunaga (2010) also showed that DHEA enhances eNOS phosphorylation in kidney from ovariectomized rats, leading to blood pressure reduction. In addition, there are some in vitro studies which have demonstrated that DHEA directly stimulates eNOS phosphorylation in endothelial cells (Liu & Dillon, 2004; Formoso et al. 2006). Furthermore, our results exclude a possible role of DHEA treatment on impaired ability of vascular smooth muscle to relax in response to exogenous NO donor, since the endothelium-independent relaxation induced by SNP was similar in all groups and was not changed by DHEA treatment.
Besides the reduction of NO production by eNOS, the increased superoxide anion in vascular cells provokes a decline in NO bioavailability, leading also to endothelial dysfunction (Cai & Harrison, 2000). The main source of superoxide anion in the vascular system is the enzyme NADPH oxidase (Cai & Harrison, 2000). Similar to other studies (Wassmann et al. 2001; Tsuda et al. 2005), here we observed that beyond decrease in eNOS phosphorylation, oxidative stress is also involved in the reduced NO bioavailability in aorta from OVX rats. Therefore, endothelial dysfunction in OVX rats is due to both reduced eNOS phosphorylation and oxidative stress. In fact, PHE and ACh response of aortic rings from OVX rats were also corrected by scavenging superoxide anion and inhibiting NADPH oxidase. Reinforcing our results, aorta from OVX rats displayed an increase in ROS production. Oxidative stress-induced endothelial dysfunction by oestrogen withdrawal has already been shown by several studies. Virdis et al. (2000) observed that endothelial dysfunction resulting from acute oestrogen deprivation in women was caused by oxidative stress. In SHR OVX rats reduction in relaxation response to carbachol in aortic rings associated with increased superoxide anion production was demonstrated (Wassmann et al. 2001). Moreover, oestrogen protects female OVX mice against increase in ROS production in aorta, and angiotensin II-induced intracellular ROS production in vascular smooth muscle (Strehlow et al. 2003).
Several studies have shown a potential antioxidant effect of DHEA. The present study also demonstrates a role of DHEA in reducing superoxide anion content in aorta from OVX rats. This effect may represent the mechanism by which DHEA treatment corrects the vascular response to PHE and ACh in aorta from OVX rats. Yorek et al. (2002) observed that DHEA reduced plasma thiobarbituric acid-reactive substances (TBARS) and superoxide anion production in arterioles from diabetic rats, and Aragno et al. (2006) also demonstrated reduction in ROS content in hearts from diabetic rats treated with DHEA. It seems that DHEA has an antioxidant effect that is not only in the cardiovascular system, since it reduced oxidative stress-induced skeletal muscle damage in diabetic rats (Aragno et al. 2004). Furthermore, oxidative stress parameters in plasma and in peripheral blood mononuclear cells in diabetic subjects were significantly decreased by DHEA treatment (Brignardello et al. 2007). Besides, in vitro studies have shown direct effects of DHEA on oxidative stress parameters. DHEA reduced ROS production and NF-κB activation in endothelial cells treated with TNF-α (Gutiérrez et al. 2007), and protected endothelial cells against apoptosis (Liu et al. 2007) and stimulated their proliferation (Williams et al. 2004).
As discussed above and demonstrated in the present study, menopause and ovariectomy induce oxidative stress, mainly in the cardiovascular system. One reason for this outcome could be the modulation of the activity or expression of antioxidant enzymes. Tsuda et al. (2005) demonstrated that aorta from female mice displayed less NADPH oxidase activity and expression than aorta from male mice, while ovariectomy showed an increase in NADPH oxidase activity and expression in females. This outcome has an important role in endothelial dysfunction observed in OVX rats, since NADPH oxidase is the main source of superoxide anion associated with endothelial dysfunction (Cai & Harrison, 2000). In addition, antioxidant systems are also depressed by oestrogen withdrawal, which contributes to oxidative stress-induced endothelial dysfunction in OVX rats. Oestrogen deficiency decreased SOD mRNA expression in aorta from OVX mice, which was related to an increase in the presence of the superoxide anion, and oestrogen replacement therapy prevented this downregulation (Strehlow et al. 2003).
In our study we also observed a modulation of SOD and NADPH oxidase protein expression following ovariectomy, displaying reduced Cu/Zn-SOD protein expression and increased gp91phox and p22phox (subunits of NADPH oxidase) protein expression, which would explain the oxidative stress-induced endothelial dysfunction. Besides, we observed a correction of Cu/Zn-SOD downregulation and gp91phox and p22phox increment by DHEA treatment, which could be the main result of DHEA treatment, leading to reduced ROS production and consequently enhanced vascular function in OVX rats. Besides the vascular antioxidant effect by modulating expression of enzymes involved in oxidant/antioxidant activity, the present work shows that DHEA treatment also enhances SOD activity in aorta from aged rats (Wu et al. 2007), prevents oxidative injury in obstructive jaundice in rats by increasing SOD activity in liver (Celebi et al. 2004), and also raises SOD activity in liver from diabetic rats (Aragno et al. 1999). However, the present study is the first to demonstrate that DHEA treatment can modulate Cu/Zn-SOD, gp91phox and p22phox (NADPH oxidase) protein expression.
In summary, this study confirms the pivotal role of oxidative stress-induced endothelial dysfunction in OVX rats. Furthermore, it indicates the potential role of DHEA therapy, instead of oestrogen replacement therapy, as an antioxidant, improving endothelial function, reducing systolic and diastolic blood pressure, and modulating eNOS phosphorylation, and Cu/Zn-SOD, gp91phox and p22phox protein expression in aorta from OVX rats.
Acknowledgments
We are grateful to Dr Ronald D. P. K. Ranvaud for helpful suggestions on the manuscript. This work was funded by research grants from FAPESP and CNPq, Brazil.
Glossary
Abbreviations
- ACh
acetylcholine
- DHEA
dehydroepiandrosterone
- NO
nitric oxide
- ORT
oestrogen replacement therapy
- OVX
ovariectomized rats
- PHE
phenylephrine
- ROS
reactive oxygen species
- SNP
sodium nitroprusside
Author contributions
J.P.G.C., E.H.A. and C.R.O.C. contributed to the conception and design of the experiments. J.P.G.C. performed and analysed most of the experiments in this study, with technical assistance from E.H.A., A.P.D., C.R.F. and L.V.R. All authors analysed and interpreted data. J.P.G.C., L.V.R. and C.R.O.C. wrote the article. Each of the authors approved the final version of the manuscript. There were no conflicts of interest.
References
- Aragno M, Mastrocola R, Alloatti G, Vercellinatto I, Bardini P, Geuna S, Catalano MG, Danni O, Boccuzzi G. Oxidative stress triggers cardiac fibrosis in the heart of diabetic rats. Endocrinology. 2008;149:380–388. doi: 10.1210/en.2007-0877. [DOI] [PubMed] [Google Scholar]
- Aragno M, Mastrocola R, Catalano MG, Brignardello E, Danni O, Boccuzzi G. Oxidative stress impairs skeletal muscle repair in diabetic rats. Diabetes. 2004;53:1082–1088. doi: 10.2337/diabetes.53.4.1082. [DOI] [PubMed] [Google Scholar]
- Aragno M, Mastrocola R, Medana C, Graziella M, Vercellinatto I, Danni O, Boccuzzi G. Oxidative stress-dependent impairment of cardiac-specific transcription factors in experimental diabetes. Endocrinology. 2006;147:5967–5974. doi: 10.1210/en.2006-0728. [DOI] [PubMed] [Google Scholar]
- Aragno M, Tamagno E, Gatto V, Brignardello E, Parola S, Danni O, Boccuzzi G. Dehydroepiandrosterone protects tissues of streptozotocin-treated rats against oxidative stress. Free Radic Biol Med. 1999;26:1467–1474. doi: 10.1016/s0891-5849(99)00012-x. [DOI] [PubMed] [Google Scholar]
- Barrett-Connor E, Khaw KT, Yen S. A prospective study of dehydroepiandrosterone sulfate, mortality, and cardiovascular disease. N Engl J Med. 1986;315:1519–1524. doi: 10.1056/NEJM198612113152405. [DOI] [PubMed] [Google Scholar]
- Bhuiyan S, Fukunaga K. Stimulation of Sigma-1 receptor by dehydroepiandrosterone ameliorates hypertension-induced kidney hypertrophy in ovariectomized rats. Exp Biol Med. 2010;235:356–364. doi: 10.1258/ebm.2009.009177. [DOI] [PubMed] [Google Scholar]
- Brignardello E, Runzo C, Aragno M, Catalano MG, Cassader M, Perin PC, Boccuzzi G. Dehydroepiandrosterone administration counteracts oxidative imbalance and advanced glycation end product formation in type 2 diabetic patients. Diabetes Care. 2007;30:2922–2927. doi: 10.2337/dc07-1110. [DOI] [PubMed] [Google Scholar]
- Burt VL, Whelton P, Roccella EJ, Brown C, Cutler JA, Higgins M, Horan MJ, Labarthe D. Prevalence of hypertension in the US adult population. Results from the Third National Health and Nutrition Examination Survey, 1988–1991. Hypertension. 1995;25:305–313. doi: 10.1161/01.hyp.25.3.305. [DOI] [PubMed] [Google Scholar]
- Cai H, Harrison DG. Endothelial dysfunction in cardiovascular diseases: the role of oxidant stress. Circ Res. 2000;87:840–844. doi: 10.1161/01.res.87.10.840. [DOI] [PubMed] [Google Scholar]
- Campbell CS, Caperuto LC, Hirata AE, Araújo EP, Velloso LA, Saad MJ, Carvalho CRO. The phosphatidylinositol/Akt/atypical PKC pathway is involved in the improved insulin sensitivity by DHEA in muscle and liver of rats in vivo. Life Sci. 2004;76:57–70. doi: 10.1016/j.lfs.2004.06.017. [DOI] [PubMed] [Google Scholar]
- Celebi F, Yilmaz I, Aksoy H, Gümüş M, Taysi S, Oren D. Dehydroepiandrosterone prevents oxidative injury in obstructive jaundice in rats. J Int Med Res. 2004;32:400–405. doi: 10.1177/147323000403200408. [DOI] [PubMed] [Google Scholar]
- Davel AP, Fukuda LE, De Sá LL, Munhoz CD, Scavone C, Sanz-Rosa D, Cachofeiro V, Lahera V, Rossoni LV. Effects of isoproterenol treatment for 7 days on inflammatory mediators in the rat aorta. Am J Physiol Heart Circ Physiol. 2008;295:H211–H219. doi: 10.1152/ajpheart.00581.2007. [DOI] [PubMed] [Google Scholar]
- Dimmeler S, Fleming I, Fisslthaler B, Hermann C, Busse R, Zeiher AM. Activation of nitric oxide synthase in endothelial cells by Akt-dependent phosphorylation. Nature. 1999;399:601–605. doi: 10.1038/21224. [DOI] [PubMed] [Google Scholar]
- Formoso G, Chen H, Kim J, Montagnani M, Consoli A, Quon MJ. Dehydroepiandrosterone mimics acute actions of insulin to stimulate production of both nitric oxide and endothelin 1 via distinct phosphatidylinositol 3-kinase-and mitogen-activated protein kinase-dependent pathways in vascular endothelium. Mol Endocrinol. 2006;20:1153–1163. doi: 10.1210/me.2005-0266. [DOI] [PubMed] [Google Scholar]
- Gutiérrez G, Mendoza C, Zapata E, Montiel A, Reyes E, Montaño LF, Lópes-Marure R. Dehydroepiandrosterone inhibits the TNF-α-induced inflammatory response in human umbilical vein endothelial cells. Atherosclerosis. 2007;190:90–99. doi: 10.1016/j.atherosclerosis.2006.02.031. [DOI] [PubMed] [Google Scholar]
- Han DH, Hansen PA, Chen MM, Holloszy JO. DHEA treatment reduces fat accumulation and protects against insulin resistance in male rats. J Gerontol A Biol Sci Med Sci. 1998;53:B19–B24. doi: 10.1093/gerona/53a.1.b19. [DOI] [PubMed] [Google Scholar]
- Hayashi T, Esaki T, Muto E, Kano H, Asai Y, Thakur NK, Sumi D, Jayachandran M, Iguchi A. Dehydroepiandrosterone retards atherosclerosis formation through its conversion to estrogen: the possible role of nitric oxide. Arterioscler Thromb Vasc Biol. 2000;20:782–792. doi: 10.1161/01.atv.20.3.782. [DOI] [PubMed] [Google Scholar]
- Hernanz R, Briones AM, Alonso MJ, Vila E, Salaices M. Hypertension alters role of iNOS, COX-2, and oxidative stress in bradykinin relaxation impairment after LPS in rat cerebral arteries. Am J Physiol Heart Circ Physiol. 2004;287:H225–H234. doi: 10.1152/ajpheart.00548.2003. [DOI] [PubMed] [Google Scholar]
- Hulley S, Grady D, Bush T, Furberg C, Herrington D, Riggs B, Vittinghoff E. Randomized trial of estrogen plus progestin for secondary prevention of coronary heart disease in postmenopausal women. Heart and Estrogen/progestin Replacement Study (HERS) Research Group. JAMA. 1998;280:605–613. doi: 10.1001/jama.280.7.605. [DOI] [PubMed] [Google Scholar]
- Ito K, Hirooka Y, Kimura Y, Sagara Y, Sunagawa K. Ovariectomy augments hypertension through rho-kinase activation in the brain stem in female spontaneously hypertensive rats. Hypertension. 2006;48:651–657. doi: 10.1161/01.HYP.0000238125.21656.9e. [DOI] [PubMed] [Google Scholar]
- Jacob MH, Janner DR, Jahn MP, Kucharski LC, Belló-Klein A, Ribeiro MFM. DHEA effects on myocardial Akt signaling modulation and oxidative stress changes in aged rats. Steroids. 2009;74:1045–1050. doi: 10.1016/j.steroids.2009.08.005. [DOI] [PubMed] [Google Scholar]
- Kimura M, Sudhir K, Jones M, Simpson E, Jefferis AM, Chin-Dusting JP. Impaired acetylcholine-induced release of nitric oxide in the aorta of male aromatase-knockout mice: regulation of nitric oxide production by endogenous sex hormones in males. Circ Res. 2003;93:1267–1271. doi: 10.1161/01.RES.0000103172.98986.25. [DOI] [PubMed] [Google Scholar]
- Labrie F. DHEA, important source of sex steroids in men and even more in women. Prog Brain Res. 2010;182:97–148. doi: 10.1016/S0079-6123(10)82004-7. [DOI] [PubMed] [Google Scholar]
- Labrie F, Luu-The V, Bélanger A, Lin S-X, Simard J, Pelletier G, Labrie C. Is dehydroepiandrosterone a hormone? J Endocrinol. 2005;187:169–196. doi: 10.1677/joe.1.06264. [DOI] [PubMed] [Google Scholar]
- Lam KK, Lee YM, Hsiao G, Chen SY, Yen MH. Estrogen therapy replenishes vascular tetrahydrobiopterin and reduces oxidative stress in ovariectomized rats. Menopause. 2006;13:294–302. doi: 10.1097/01.gme.0000182806.99137.5e. [DOI] [PubMed] [Google Scholar]
- Liu D, Dillon JS. Dehydroepiandrosterone stimulates nitric oxide release in vascular endothelial cells: evidence for a cell surface receptor. Steroids. 2004;69:279–289. doi: 10.1016/j.steroids.2004.02.004. [DOI] [PubMed] [Google Scholar]
- Liu D, Si H, Reynolds KA, Zhen W, Jia Z, Dillon JS. Dehydroepiandrosterone protects vascular endothelial cells against apoptosis through a Gαi protein-dependent activation of phosphatidylinositol 3-kinase/Akt and regulation of antiapoptotic Bcl-2 expression. Endocrinology. 2007;148:3068–3076. doi: 10.1210/en.2006-1378. [DOI] [PubMed] [Google Scholar]
- Medina MC, Souza LC, Caperuto LC, Anhê GF, Amanso AM, Teixeira VP, Bordin S, Carpinelli AR, Britto LR, Barbieri RL, Borella MI, Carvalho CRO. Dehydroepiandrosterone increases β-cell mass and improves the glucose-induced insulin secretion by pancreatic islets from aged rats. FEBS Lett. 2006;580:285–290. doi: 10.1016/j.febslet.2005.12.014. [DOI] [PubMed] [Google Scholar]
- Mendonça LS, Fernandes-Santos C, Mandarim-de-Lacerda CA. Cardiac and aortic structural alterations due to surgically-induced menopause associated with renovascular hypertension in rats. Int J Exp Pathol. 2007;88:301–309. doi: 10.1111/j.1365-2613.2007.00546.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mercuro G, Zoncu S, Saiu F, Mascia M, Melis GB, Rosano GMC. Menopause induced by oophorectomy reveals a role of ovarian estrogen on the maintenance of pressure homeostasis. Maturitas. 2004;47:131–138. doi: 10.1016/s0378-5122(03)00252-4. [DOI] [PubMed] [Google Scholar]
- Nestler JE, Barlascini CO, Clore JN, Blackard WG. Dehydroepiandrosterone reduces serum low density lipoprotein levels and body fat but does not alter insulin sensitivity in normal men. J Clin Endocrinol Metabol. 1988;66:57–61. doi: 10.1210/jcem-66-1-57. [DOI] [PubMed] [Google Scholar]
- Paredes-Carbajal MC, Juárez-Oropeza MA, Ortíz-Mendoza CM, Mascher D. Effects of acute and chronic estrogenic treatment on vasomotor responses of aortic rings from ovariectomized rats. Life Sci. 1995;57:473–486. doi: 10.1016/0024-3205(95)00281-a. [DOI] [PubMed] [Google Scholar]
- Prentice RL, Manson JE, Langer RD, Anderson GL, Pettinger M, Jackson RD, Johnson KC, Kuller LH, Lane DS, Wactawski-Wende J, Brzyski R, Allison M, Ockene J, Sarto G, Rossouw JE. Benefits and risks of postmenopausal hormone therapy when it is initiated soon after menopause. Am J Epidemiol. 2009;170:12–23. doi: 10.1093/aje/kwp115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rossouw JE, Anderson GL, Prentice RL, LaCroix AZ, Kooperberg C, Stefanick ML, Jackson RD, Beresford SA, Howard BV, Johnson KC, Kotchen JM, Ockene J. Risks and benefits of estrogen plus progestin in healthy postmenopausal women: principal results from the Women's Health Initiative randomized controlled trial. JAMA. 2002;288:321–333. doi: 10.1001/jama.288.3.321. [DOI] [PubMed] [Google Scholar]
- Schriock ED, Buffington CK, Hubert GD, Kurtz BR, Kitabchi AE, Buster JE, Givens JR. Divergent correlations of circulating dehydroepiandrosterone sulfate and testosterone with insulin levels and insulin receptor binding. J Clin Endocrinol Metabol. 1988;66:1329–1331. doi: 10.1210/jcem-66-6-1329. [DOI] [PubMed] [Google Scholar]
- Squadrito F, Altavilla D, Squadrito G, Saitta A, Cucinotta D, Minutoli L, Deodato B, Ferlito M, Campo GM, Bova A, Caputi AP. Genistein supplementation and estrogen replacement therapy improve endothelial dysfunction induced by ovariectomy in rats. Cardiovasc Res. 2000;45:454–462. doi: 10.1016/s0008-6363(99)00359-4. [DOI] [PubMed] [Google Scholar]
- Strehlow K, Rotter S, Wassmann S, Adam O, Grohé C, Laufs K, Böhm M, Nickenig G. Modulation of antioxidant enzyme expression and function by estrogen. Circ Res. 2003;93:170–177. doi: 10.1161/01.RES.0000082334.17947.11. [DOI] [PubMed] [Google Scholar]
- Tsuda M, Iwai M, Li JM, Li HS, Min LJ, Ide A, Okumura M, Suzuki J, Mogi M, Suzuki H, Horiuchi M. Inhibitory effects of AT1 receptor blocker, olmesartan, and estrogen on atherosclerosis via anti-oxidative stress. Hypertension. 2005;45:545–551. doi: 10.1161/01.HYP.0000157409.88971.fc. [DOI] [PubMed] [Google Scholar]
- Villareal DT, Holloszy JO. Effect of DHEA on abdominal fat and insulin action in elderly women and men: a randomized controlled trial. JAMA. 2004;292:2243–2248. doi: 10.1001/jama.292.18.2243. [DOI] [PubMed] [Google Scholar]
- Virdis A, Ghiadoni L, Pinto S, Lombardo M, Petraglia F, Gennazzani A, Buralli S, Taddei S, Salvetti A. Mechanisms responsible for endothelial dysfunction associated with acute estrogen deprivation in normotensive women. Circulation. 2000;101:2258–2263. doi: 10.1161/01.cir.101.19.2258. [DOI] [PubMed] [Google Scholar]
- Wassmann S, Bäumer AT, Strehlow K, Eickels MV, Grohé C, Ahlbory K, Rösen R, Böhm M, Nickenig G. Endothelial dysfunction and oxidative stress during estrogen deficiency in spontaneously hypertensive rats. Circulation. 2001;103:435–441. doi: 10.1161/01.cir.103.3.435. [DOI] [PubMed] [Google Scholar]
- White RE. Estrogen and vascular function. Vascul Pharmacol. 2002;38:73–80. doi: 10.1016/s0306-3623(02)00129-5. [DOI] [PubMed] [Google Scholar]
- Widder J, Pelzer T, Poser-Klein CV, Hu K, Jazbutyte V, Fritzemeier K, Hegele-Hartung C, Neyses L, Bauersachs J. Improvement of endothelial dysfunction by selective estrogen receptor-α stimulation in ovariectomized SHR. Hypertension. 2003;42:991–996. doi: 10.1161/01.HYP.0000098661.37637.89. [DOI] [PubMed] [Google Scholar]
- Williams MR, Dawood T, Ling S, Dai A, Lew R, Myles K, Funder JW, Sudhir K, Komesaroff PA. Dehydroepiandrosterone increases endothelial cell proliferation in vitro and improves endothelial function in vivo by mechanisms independent of androgen and estrogen receptors. J Clin Endocrinol Metab. 2004;89:4708–4715. doi: 10.1210/jc.2003-031560. [DOI] [PubMed] [Google Scholar]
- Wong CM, Yao X, Au CL, Tsang SY, Fung KP, Laher I, Vanhoutte PM, Huang Y. Raloxifene prevents endothelial dysfunction in aging ovariectomized female rats. Vascul Pharmacol. 2006;44:290–298. doi: 10.1016/j.vph.2005.12.005. [DOI] [PubMed] [Google Scholar]
- Wu S, Ruan Y, Yin M, Lai W. Research on the age-related changes in the nitric oxide pathway in the arteries of rats and the intervention effect of dehydroepiandrosterone. Gerontology. 2007;53:234–237. doi: 10.1159/000100961. [DOI] [PubMed] [Google Scholar]
- Yorek MA, Coppey LJ, Gellett JS, Davidson EP, Bing X, Lund DD, Dillon JS. Effect of treatment of diabetic rats with dehydroepiandrosterone on vascular and neural function. Am J Physiol Endocrinol Metab. 2002;283:E1067–E1075. doi: 10.1152/ajpendo.00173.2002. [DOI] [PubMed] [Google Scholar]


