Non-technical summary
When muscles contract, a variety of signals interact and ultimately increase blood flow and oxygen delivery to the active muscle. However, when there is simultaneous activation of the sympathetic nervous system, noradrenaline (norepinephrine) is released which tries to cause vasoconstriction in the same blood vessels that are trying to relax (vasodilate). In young adults, we have shown that there is a unique ability of the contracting muscle to limit this vasoconstriction, and also that a circulating factor called ATP mimics the exercise response. In the present study, we demonstrate that older healthy adults have an impaired ability to limit the sympathetic vasoconstrictor signal during exercise; however, this ability is preserved when we administer ATP. Thus, if this impairment in the ability of contracting muscle to limit sympathetic vasoconstriction in older adults is related to ATP, we speculate that circulating levels of ATP may be impaired during exercise.
Abstract
Abstract
The ability to modulate sympathetic α-adrenergic vasoconstriction in contracting muscle is impaired with age. In young adults, adenosine triphosphate (ATP) has been shown to blunt sympathetic vasoconstrictor responsiveness similar to exercise. Therefore, we tested the hypothesis that modulation of postjunctional α-adrenergic vasoconstriction to exogenous ATP is impaired in ageing humans. We measured forearm blood flow (FBF; Doppler ultrasound) and calculated vascular conductance (FVC) to intra-arterial infusions of phenylephrine (α1-agonist) and dexmedetomidine (α2-agonist) during rhythmic handgrip exercise (15% MVC), a control non-exercise vasodilator condition (adenosine), and ATP infusion in seven older (64 ± 3 years) and seven young (22 ± 1 years) healthy adults. Forearm hyperaemia was matched across all vasodilatating conditions. During adenosine, forearm vasoconstrictor responses to direct α1-stimulation were lower in older compared with young adults (ΔFVC =−25 ± 3%vs.−41 ± 5%; P < 0.05), whereas the responses to α2-stimulation were not different (−35 ± 6%vs.−44 ± 8%; NS). During exercise, α1-mediated vasoconstriction was significantly blunted compared with adenosine in both young (−9 ± 2%vs.−41 ± 5%) and older adults (−15 ± 2%vs.−25 ± 3%); however, the magnitude of sympatholysis was reduced in older adults (32 ± 13 vs. 74 ± 8%; P < 0.05). Similarly, α2-mediated vasoconstriction during exercise was significantly blunted in both young (−15 ± 4%vs.−44 ± 8%) and older adults (−26 ± 3%vs.−35 ± 6%), however the magnitude of sympatholysis was reduced in older adults (19 ± 8%vs. 60 ± 10%; P < 0.05). During ATP, both α1- and α2-mediated vasoconstriction was nearly abolished in young and older adults (ΔFVC ∼−5%), and the magnitude of sympatholysis was similar in both age groups (∼85–90%). Our findings indicate that the ability to modulate postjunctional α-adrenergic vasoconstriction during exercise is impaired with age, whereas the sympatholytic effect of exogenous ATP is preserved. Thus, if impairments in vascular control during exercise in older adults involve vasoactive ATP, we speculate that circulating ATP is reduced with advancing age.
Introduction
Vascular regulation within contracting muscle exhibits a fine interplay between local vasodilator and sympathetic neural vasoconstrictor signals, and ultimately dictates blood flow and oxygen delivery to the active tissue. Moreover, the competition between these factors appears to increase with the intensity of exercise and the amount of muscle mass engaged (Saltin et al. 1998). Although it is clear that sympathetic vasoconstriction can restrain blood flow to active muscle, recent experimental evidence indicates that vasoconstrictor responses within contracting muscle are blunted compared to those within resting (quiescent) muscle; a phenomenon termed ‘functional sympatholysis’ (Remensnyder et al. 1962). Indeed, we and others have shown in young adults that muscle contractions have the ability to blunt sympathetically mediated vasoconstriction that is graded with the level of exercise intensity and is coupled with the metabolic demand of contracting skeletal muscle (Buckwalter et al. 2001; Tschakovsky et al. 2002; Dinenno & Joyner, 2003; Kirby et al. 2005). This integrative regulatory scheme is thought to appropriately regulate arterial blood pressure while optimizing blood flow and oxygen delivery to the metabolically active tissue (Marshall et al. 1961; Rowell, 1997; Joyner & Thomas, 2003).
Several substances have been proposed to be involved in the local modulation of sympathetic vasoconstriction in active muscle including adenosine (Nishigaki et al. 1991), nitric oxide (Thomas & Victor, 1998; Chavoshan et al. 2002), vasodilating prostaglandins (Faber et al. 1982), as well as the activation of ATP-sensitive potassium channels (KATP) (Pickkers et al. 2004). However, identifying the sympatholytic factor associated with contracting muscle in humans has proven quite difficult (Tschakovsky et al. 2002; Dinenno & Joyner, 2003, 2004; Rosenmeier et al. 2003). Recently, circulating ATP has been proposed to be a sympatholytic agent in the human circulation and is released from red blood cells (among other sources) when haemoglobin is deoxygenated (Jagger et al. 2001; Gonzalez-Alonso et al. 2002; Ellsworth et al. 2009), and this can be augmented by hypercapnia, acidosis and mechanical deformation (Bergfeld & Forrester, 1992; Ellsworth et al. 1995; Sprague et al. 1998). In this context, we and others have recently demonstrated that exogenous ATP has the ability to blunt α-adrenergic vasoconstriction in young adults (Rosenmeier et al. 2004; Kirby et al. 2008). Further, graded increases in arterial concentrations of ATP that evoke moderate limb hyperaemia result in graded inhibition of direct postjunctional α-adrenoceptor stimulation, such that low levels are not sympatholytic whereas progressive reductions in α-adrenoceptor-mediated vasoconstriction are observed with increasing [ATP] (Kirby et al. 2008). This is strikingly similar to the intensity-dependent sympatholytic nature of muscle contractions. Importantly, these unique responses appear specific to ATP and are not mediated by its degradation by-products (ADP, AMP or adenosine) (Rosenmeier et al. 2008).
With respect to human ageing, the control of vascular tone is altered whereby older adults have clear elevations in muscle sympathetic nervous system activity yet have diminished α-adrenoceptor responsiveness at rest (Seals & Esler, 2000; Seals & Dinenno, 2004; Dinenno & Joyner, 2006). In spite of this, there is general consensus in support of increased local vascular resistance with advancing age during exercise that is characterized either by augmented vasoconstrictor tone and/or blunted vasodilatation (Taylor et al. 1992; Lawrenson et al. 2003; Seals & Dinenno, 2004; Dinenno & Joyner, 2006; Richardson et al. 2006; Versari et al. 2009). Therefore, it was recently hypothesized that ageing is associated with an impaired modulation of α-adrenergic vasoconstriction in the vascular beds of contracting muscle (i.e. impaired functional sympatholysis). Data obtained during cycling exercise in older men (via cold pressor test; Koch et al. 2003), as well as during forearm exercise in older men (via direct α-adrenoceptor stimulation; Dinenno et al. 2005) and women (via lower body negative pressure; Fadel et al. 2004) indicate that modulation of sympathetic vasoconstriction in contracting muscle is impaired with age, and this involves both postjunctional α1- and α2-adrenoceptors (Dinenno et al. 2005). It is likely that this impairment contributes to reductions in blood flow and oxygen delivery in active muscle of older humans thereby potentially limiting oxygen uptake and exercise capacity, yet the mechanisms underlying this impairment have not been elucidated.
To date, the available information regarding how ATP interacts with sympathetic α-adrenergic vasoconstriction has only been obtained in young healthy humans. Therefore, in the present study we tested the hypothesis that the ability of exogenous ATP to modulate postjunctional α-adrenergic vasoconstriction is impaired in ageing humans. To do so, we utilized the exact study design as previously described in young subjects (Kirby et al. 2008) by measuring forearm haemodynamics (Doppler ultrasound) during intra-arterial infusions of phenylephrine (α1-agonist) and dexmedetomidine (α2-agonist) under conditions of moderate-intensity rhythmic handgrip exercise, a control non-exercise vasodilator condition (adenosine), and ATP infusion in healthy older adults.
Methods
Subjects
With Institutional Review Board approval and after written informed consent, a total of seven older healthy adults (5 men, 2 women; age range, 55–75 years, Table 1) participated in the present study. All were non-smokers, non-obese, normotensive, sedentary to moderately active, and not taking any medications. Subjects were instructed to not ingest caffeine or exercise for 24 h prior to the experimental day. Studies were performed after a 4 h fast with the subjects in the supine position. All studies were performed according to the Declaration of Helsinki.
Table 1.
Subject characteristics and baseline haemodynamics
| Variable | Young | Older |
|---|---|---|
| Male, female | 5, 2 | 5, 2 |
| Age (years) | 22 ± 1 | 64 ± 2* |
| BMI (kg·m−2) | 23 ± 1 | 25 ± 1 |
| Body fat (%) | 17 ± 2 | 19 ± 2 |
| Forearm volume (ml) | 946 ± 25 | 1104 ± 96 |
| MVC (kg) | 43 ± 2 | 42 ± 3 |
| Total cholesterol (mmol l−1) | — | 4.7 ± 0.2 |
| LDL cholesterol (mmol l−1) | — | 4.2 ± 0.2 |
| HDL cholesterol (mmol l−1) | — | 1.0 ± 0.1 |
| Triglycerides (mmol l−1) | — | 1.0 ± 0.1 |
| Heart rate (beats min−1) | 54 ± 2 | 58 ± 2 |
| Mean arterial pressure (mmHg) | 94 ± 1 | 103 ± 2* |
| Forearm blood flow (ml min−1) | 28 ± 3 | 27 ± 2 |
| Forearm vascular conductance (ml min−1 (100 mmHg)−1) | 29 ± 3 | 26 ± 2 |
MVC, maximum voluntary contraction; LDL, low-density lipoprotein; HDL, high-density lipoprotein. —, data not collected in young subjects
P < 0.05 vs. young adults.
Note: young data are adapted from Kirby et al. (2008).
Arterial catheterization
A 20-gauge, 7.6-cm catheter was placed in the brachial artery of the non-dominant arm under aseptic conditions after local anaesthesia (2% lidocaine) for local administration of study drugs. The catheter was connected to a 3-port connector as well as a pressure transducer for mean arterial pressure (MAP) measurement and continuously flushed at 3 ml h−1 with heparinized saline (Kirby et al. 2008). The two side ports were used for infusions of vasoactive drugs.
Body composition and forearm volume
Body composition was determined by dual-energy X-ray absorptiometry (DEXA; Hologic, Inc.; Bedford, MA, USA). Total forearm volume (FAV) was calculated from regional analysis of the experimental forearm (from the proximal to distal radioulnar joint) from whole-body DEXA scans with QDR series software for normalization of individual drug doses. Body mass index was calculated as bodyweight (kg) divided by height (meters) squared.
Forearm blood flow and vascular conductance
A 4 MHz pulsed Doppler probe (Model 500V, Multigon Industries, Mt Vernon, NY, USA) was used to measure brachial artery mean blood velocity (MBV) with the probe securely fixed to the skin over the brachial artery proximal to the catheter insertion site as previously described by our laboratory (Kirby et al. 2007, 2008). The probe insonation angle relative to the skin was 45 deg. A linear 12 MHz echo Doppler ultrasound probe (GE Vingmed Ultrasound Vivid7, Horten, Norway) was placed in a holder securely fixed to the skin immediately proximal to the velocity probe to measure brachial artery diameter in triplicate at end diastole between muscle contractions. Forearm blood flow was calculated as FBF = MBV (cm s−1) ×π (brachial artery diameter/2)2× 60, where the FBF is in ml min−1, the MBV is in cm s−1, the brachial diameter is in cm, and 60 is used to convert from ml s−1 to ml min−1. Forearm vascular conductance (FVC) was calculated as (FBF/MAP) × 100, and expressed as ml min−1 (100 mmHg−1). A fan was directed towards the forearm to minimize skin blood flow.
Rhythmic handgrip exercise
Maximum voluntary contraction (MVC) was determined for each subject as the average of at least three maximal squeezes of a handgrip dynamometer (Stoelting, Chicago, IL, USA) that were within 3% of each other. For the exercise trials, weights corresponding to 15% MVC were attached to a pulley system and lifted 4–5 cm over the pulley at a duty cycle of 1 s contraction–2 s relaxation (20 contractions per minute) using audio and visual signals to ensure the correct timing (Kirby et al. 2008). We (Dinenno et al. 2005; Carlson et al. 2008; Kirby et al. 2009) and others (Taylor et al. 1991; Momen et al. 2004; Kuipers et al. 2009) have previously demonstrated that certain aged healthy humans do not have significant differences in forearm MVC and thus exercise performed at the 15% MVC relative workload equates to the same average absolute workload. This is important in that the blood flow response to muscle contraction is proportional to the absolute work performed. We chose this moderate workload because it does not increase sympathetic nervous system activity, and because it significantly blunts (but does not abolish) superimposed sympathetic vasoconstriction in contracting muscle (Kirby et al. 2005, 2008).
Sympathetic α-adrenergic vasoconstrictor drugs
In male subjects, phenylephrine (a selective α1-agonist; Baxter, Irvine, CA, USA) was infused at 0.0625 μg (dl FAV)−1 min−1 and dexmedetomidine (a selective α2-agonist; Hospira, Lake Forest, IL, USA) was infused at 6.25 ng (dl FAV)−1 min−1. The doses of phenylephrine and dexmedetomidine were chosen based on our experience at rest and during handgrip exercise. In our prior study in young adults (Kirby et al. 2008), the doses of phenylephrine and dexmedetomidine were doubled for female participants because they typically have reduced vasoconstrictor responses to α-receptor stimulation compared with men (Kneale et al. 2000). Thus, this was also done in the two older women to allow for appropriate age group comparisons. All vasoconstrictor drug infusions were adjusted for the hyperaemic conditions as previously described (see below) (Kirby et al. 2008).
Given that exercise increases forearm blood flow, adenosine was infused to elevate resting forearm blood flow to similar levels observed during exercise. We have previously demonstrated that exercise blunts the vasoconstrictor responses to direct α1- and α2-adrenoceptor stimulation, whereas these vasoconstrictor responses are maintained when blood flow is elevated with adenosine and hence it was used to create a ‘high flow’ control state (Tschakovsky et al. 2002; Kirby et al. 2008). In an effort to normalise the concentration of each vasoconstricting drug in the blood perfusing the forearm, the infusions were adjusted on the basis of forearm blood flow and FAV (measured via regional analysis of whole-body DEXA scans). Various concentrations of each compound were available to keep the absolute infusion rates less than 3 ml min−1 in every trial.
Experimental protocols
General experimental protocol
Figure 1 is an example of a time-line for the specific trials. In the supine position, subjects performed either a bout of handgrip exercise, or received intra-arterial adenosine (Sicor, Irvine, CA, USA) or ATP (Sigma, USA); the total time for each trial was 8 min. After 2 min of baseline measurements, exercise or vasodilator infusion was initiated and steady-state FBF was reached within 3 min. Between 3 and 4 min of hyperaemia (minutes 5 and 6 of Fig. 1) the dose of the α1- or α2-agonist (vasoconstrictor) was calculated on the basis of FAV and blood flow. The vasoconstrictor infusion began at the 6 min mark and lasted for 2 min.
Figure 1. General experimental trial.
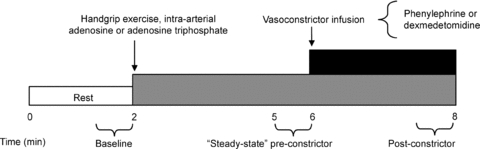
Each trial consisted of a 2 min baseline period. After this time period, subjects either began rhythmic handgrip exercise or received intra-arterial adenosine or adenosine triphosphate (ATP) to elevate resting forearm blood flow to levels observed during exercise. During minutes 5–6 (pre-constrictor), the doses of the α1- or α2-adrenoceptor agonists (phenylephrine or dexmedetomidine, respectively) were calculated on the basis of steady-state hyperaemic forearm blood flow and forearm volume. Subsequently, the α-agonist was infused for 2 min until minute 8. An average of forearm blood flow and mean arterial blood pressure during the final 30 s of α-agonist infusion was used to calculate the vasoconstrictor effect during all hyperaemic conditions.
Effects of exogenous ATP on postjunctional α-adrenergic vasoconstrictor responsiveness
We aimed to determine whether exogenous ATP blunts direct postjunctional α-adrenergic responsiveness in older healthy humans, and whether this is selective for α1- or α2-adrenoceptors. Therefore, in seven older subjects (5 men, 2 women), the vasoconstrictor responses to direct α1- and α2-adrenoceptor stimulation (via phenylephrine and dexmedetomidine, respectively) were assessed during control vasodilator infusion of adenosine, during moderate rhythmic handgrip exercise (15% MVC), and during infusion of ATP. In total, there were six experimental trials for each subject. In general, the goal was to match steady-state FBF during infusion of adenosine or ATP with that observed during exercise. To do so, adenosine (45 nmol (dl FAV)−1 min−1) and ATP (5 nmol (dl FAV)−1 min−1) were initially infused and doses were increased to elevate FBF accordingly. The final average doses of adenosine and ATP were 75 ± 21 and 10 ± 2 nmol (dl FAV)−1 min−1, respectively. The order of the adenosine, exercise and ATP trials were varied across subjects. Thus, for subjects that did not perform the exercise trial first, we had them perform 3–4 min of rhythmic handgrip exercise prior to any experimental trials with α-agonists to determine their individual steady-state FBF for this exercise intensity. In four of the subjects, vasoconstrictor responses to α1-adrenoceptor stimulation were determined under each hyperaemic condition, followed by the trials for α2-receptor stimulation. This order was reversed in the other three subjects, and all subjects rested for 15 min between each trial.
Previously published results from young adults
This experimental protocol was previously performed in young healthy adults (5 men, 2 women) to determine whether exogenous ATP blunts direct α1- or α2-mediated vasoconstriction, thus any reference to young adults refers to this published experiment (Kirby et al. 2008). The protocol, methods of data acquisition, as well as data analysis was exactly replicated in older adults for the present study, and this allows for the determination of how ageing impacts the ability of exogenous ATP and exercise to blunt direct postjunctional α-adrenergic vasoconstriction. In this prior study, the final average doses of adenosine and ATP were 73 ± 8 and 11 ± 2 nmol 100 ml−1 min−1, respectively. As vasoconstrictor responsiveness is not equal between age groups at rest in the forearm for a given receptor subtype (Dinenno et al. 2002, 2005), we calculated the magnitude of sympatholysis as a means of determining the ability of exercise or ATP to blunt α-adrenoceptor stimulation (Dinenno et al. 2005). The per cent magnitude of sympatholysis was calculated as ((%ΔFVCconstriction adenosine −%ΔFVCconstriction exercise)/%ΔFVCconstriction adenosine) × 100. Thus, if there is no attenuation of the vasoconstrictor response during exercise or ATP infusions compared with that observed during adenosine, the value would be zero. If the vasoconstrictor response was completely abolished, the value would be 100%.
Data acquisition and analysis
Data were collected and stored on computer at 250 Hz and analysed off-line with signal-processing software (WinDaq, DATAQ Instruments, Akron, OH, USA). Mean arterial pressure (MAP) was determined from the arterial pressure waveform. Baseline FBF, HR and MAP represent an average of the last minute of the resting time period, the steady-state hyperaemic values represent an average of minutes 3–4 (minutes 5–6 of Fig. 1; pre-vasoconstrictor) during exercise, adenosine or ATP and the effects of the α-agonists represent an average of the final 30 s of drug infusion (post-vasoconstrictor). The per cent reduction in FBF during vasoconstrictor administration was calculated as: (FBF post constrictor − FBF pre constrictor)/(FBF pre constrictor) × 100. We also calculated per cent reduction in FVC as our standard index to compare vasoconstrictor responses to the α-agonists across conditions, as this appears to be the most appropriate way to compare vasoconstrictor responsiveness under conditions where there might be differences in vascular tone (Lautt, 1989; O'Leary, 1991; Thomas et al. 1994). In an effort to be comprehensive, we have also presented absolute values of forearm haemodynamics for all conditions in tabular form.
Statistics
All values are reported as means ± SEM. Two-way repeated-measures analysis of variance (ANOVA) was used to determine differences in absolute haemodynamic values across conditions with trial (exercise, adenosine and ATP) and time-point (pre- and post-vasoconstrictor) as factors. Separate analyses were performed within older (present study) and young (previously published data; Kirby et al. 2008) for the phenylephrine and dexmedetomidine trials. Within each age group, differences in vasoconstrictor responses (%ΔFVC) between each trial (exercise, adenosine and ATP) were determined by one-way repeated-measures ANOVA. For all ANOVA tests, Student–Newman–Keuls post hoc pair-wise comparisons were made to identify significant differences. Specific hypothesis testing comparing age-group responses within each trial (exercise, adenosine and ATP) and subject characteristics were made using independent two-tailed Student's t tests. When analysing for magnitude of sympatholysis across age, one young subject from our previous study was identified as a significant outlier (Grubb's test, P < 0.05) and was thus excluded from the entire study analysis (total for young N = 7). Significance was set a priori at P < 0.05.
Results
Effects of exercise and exogneous ATP on postjunctional α-adrenergic vasoconstrictor responsiveness in older adults
Forearm haemodynamics, HR and MAP are presented in Table 2A and B. Intra-arterial infusion of both adenosine and ATP, as well as handgrip exercise, significantly increased FBF and FVC from baseline (P < 0.05). As desired by experimental design, steady-state (pre-vasoconstrictor) FBF responses to adenosine and ATP infusion were effectively matched to those observed during 15% MVC handgrip exercise within both phenylephrine (Fig. 2A) and dexmedetomidine conditions (Fig. 2B; P = 0.4–0.8). Infusion of phenylephrine (α1-agonist) significantly reduced FBF from steady-state hyperaemia during adenosine and exercise (P < 0.05), whereas FBF was unchanged during ATP (NS; Fig. 2A). Similarly, infusion of dexmedetomidine (α2-agonist) significantly reduced FBF from steady-state hyperaemia during adenosine and exercise (P < 0.05), whereas FBF was unchanged during ATP (NS; Fig. 2B).
Table 2.
Forearm and systemic haemodynamics in older adults
| Time | Condition | Forearm blood flow (ml min−1) | Mean arterial pressure (mmHg) | Forearm vascular conductance (ml min−1 (100 mmHg−1)) | Heart rate (beats min−1) |
|---|---|---|---|---|---|
| A. Phenylephrine trials | |||||
| Baseline | Adenosine | 26 ± 4 | 102 ± 4 | 25 ± 4 | 58 ± 3 |
| Exercise | 28 ± 5 | 105 ± 4 | 27 ± 5 | 59 ± 4 | |
| ATP | 27 ± 5 | 103 ± 3 | 26 ± 4 | 58 ± 3 | |
| Pre-phenylephrine | Adenosine | 130 ± 23 | 107 ± 3 | 123± 22 | 59 ± 3 |
| Exercise | 147 ± 16 | 106 ± 4 | 142 ± 17 | 62 ± 3 | |
| ATP | 132 ± 23 | 105 ± 4 | 132 ± 23 | 58 ± 3 | |
| Phenylephrine | Adenosine | 98 ± 17* | 108 ± 3 | 93 ± 16* | 60 ± 3 |
| Exercise | 126 ± 14* | 107 ± 4 | 120 ± 16* | 63 ± 4 | |
| ATP | 129 ± 21† | 104 ± 4 | 126 ± 21† | 57 ± 4 | |
| B. Dexmedetomidine trials | |||||
| Baseline | Adenosine | 25 ± 3 | 103 ± 4 | 25 ± 3 | 61 ± 3 |
| Exercise | 25 ± 4 | 104 ± 3 | 24 ± 4 | 61 ± 4 | |
| ATP | 28 ± 5 | 104 ± 4 | 27 ± 5 | 60 ± 3 | |
| Pre-dexmedetomidine | Adenosine | 132 ± 22 | 105 ± 4 | 127 ± 22 | 60 ± 3 |
| Exercise | 149 ± 16 | 105 ± 4 | 143 ± 15 | 64 ± 4 | |
| ATP | 136 ± 20 | 104 ± 3 | 133 ± 21 | 60 ± 3 | |
| Dexmedetomidine | Adenosine | 82 ± 10* | 107 ± 4 | 79 ± 11* | 61 ± 3 |
| Exercise | 110 ± 10* | 109 ± 4 | 103 ± 10* | 63 ± 4 | |
| ATP | 132 ± 22† | 105 ± 4 | 128 ± 23† | 60 ± 3 | |
All steady-state (pre-vasconstrictor) and constrictor FBF and FVC responses are significantly greater than respective baseline values (P < 0.05).
P < 0.05 vs. pre-vasoconstrictor, phenylephrine or dexmedetomidine (within condition)
P < 0.05 vs. adenosine (within condition).
Figure 2. Forearm blood flow at rest, during each hyperaemic condition and during infusion of α-agonists in older adults.
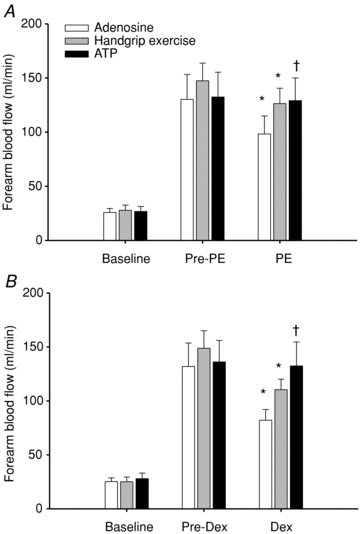
Steady-state hyperaemia was similar during rhythmic handgrip exercise, adenosine and ATP infusions for trials involving the α1-agonist phenylephrine (A; Pre-PE) and the α2-agonist dexmedetomidine (B; Pre-Dex). Forearm blood flow was reduced significantly with both α-agonists during adenosine and exercise, but the response was attenuated during exercise. In contrast, α-agonist infusion did not significantly reduce forearm blood flow during ATP. *P < 0.05 vs. steady state (Pre-vasoconstrictor; PE/Dex) within condition; †P < 0.05 vs. adenosine during α-agonist infusion.
The forearm vasoconstrictor responses to direct α1-adrenoceptor stimulation were blunted during steady-state exercise vs. adenosine (ΔFVC =−15 ± 2%vs.−25 ± 3%; P < 0.05), and were abolished during ATP infusion (−1 ± 4%; P = 0.8 vs. zero; Fig. 3A). Similarly, vasoconstrictor responses to α2-receptor stimulation were blunted during exercise vs. adenosine (−26 ± 4%vs.−35 ± 8%; P < 0.05), and were abolished during ATP infusion (−5 ± 5%; P = 0.3 vs. zero; Fig. 4A). MAP changed minimally within and between conditions (Table 2A and B), thus FBF responses were similar to FVC.
Figure 3. Forearm vascular responses to α1-adrenoceptor stimulation.
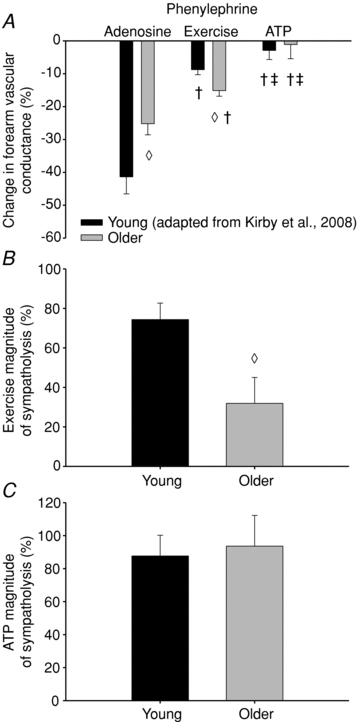
A, the vasoconstrictor responses to phenylephrine (α1-agonist) were significantly blunted in older compared with young during passive vasodilatation with adenosine, yet vasoconstriction was greater in older vs. young adults during exercise. In both groups, the vasoconstrictor responses during exercise were blunted compared with adenosine, and abolished during ATP infusion. B, calculating the ability of exercise to blunt α1-adrenoceptor stimulation demonstrates a significant impairment in older compared to young adults, yet (C) no age-associated impairment in the sympatholytic properties of ATP was observed. Forearm vascular conductance was calculated as (forearm blood flow/mean arterial pressure) × 100. The per cent magnitude of sympatholysis was calculated as ((%ΔFVCconstriction adenosine −%ΔFVCconstriction exercise)/%ΔFVCconstriction adenosine) × 100. †P < 0.05 vs. adenosine within age group; ‡P < 0.05 vs. exercise within age group; ◊P < 0.05 vs. young within condition. Note: young data are adapted from Kirby et al. (2008).
Figure 4. Forearm vascular responses to α2-adrenoceptor stimulation.
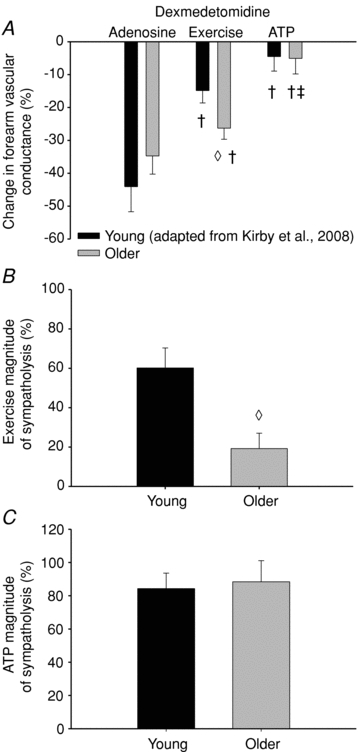
A, the vasoconstrictor responses to dexmedetomidine (α2-agonist) were significantly greater in older compared with young adults during exercise. In both groups, the vasoconstrictor responses during exercise were blunted, and abolished during ATP compared with adenosine infusions. B, calculating the ability of exercise to blunt α2-adrenoceptor stimulation demonstrates a significant impairment in older compared to young adults, yet (C) no age-associated impairment in the sympatholytic properties of ATP was observed. Forearm vascular conductance was calculated as (forearm blood flow/mean arterial pressure) × 100. The per cent magnitude of sympatholysis was calculated as ((%ΔFVCconstriction adenosine −%ΔFVCconstriction exercise)/%ΔFVCconstriction adenosine) × 100. †P < 0.05 vs. adenosine within age group; ‡P < 0.05 vs. exercise within age group; ◊P < 0.05 vs. young within condition. Note: young data are adapted from Kirby et al. (2008).
Magnitude of sympatholysis: impact of advancing age
A comparison between older adults included in the current study and younger subjects studied previously (Kirby et al. 2008) with respect to the vasoconstrictor responsiveness during handgrip exercise, and intra-arterial infusions of adenosine and ATP are presented in Table 3, and Figs 3 and 4. The vasoconstrictor responses to α1-adrenoceptor stimulation during adenosine were reduced in older compared with young adults (ΔFVC =−25 ± 3%vs.−41 ± 5%; P < 0.05), whereas the responses to α2-adrenoceptor stimulation were not different (ΔFVC =−35 ± 6%vs.−44 ± 8%; P = 0.34). In contrast, the vasoconstrictor responses to both α1- and α2-adrenoceptor stimulation during exercise was greater in older adults (ΔFVC =−15 ± 2%vs.–9 ± 2%, and −26 ± 3%vs.−15 ± 4%, respectively). ATP abolished the vasoconstrictor responses to both α1- and α2-adrenoceptor stimulation in young adults (ΔFVC =−3 ± 3% and −5 ± 4%, respectively), similar to older adults (ΔFVC =−1 ± 4% and −5 ± 5%, respectively). The ‘magnitude of sympatholysis’, or the ability to blunt α-adrenergic vasoconstriction, during exercise was significantly reduced in older adults compared to young adults for each receptor subtype (α1= 32 ± 13%vs. 74 ± 8%; α2= 19 ± 8%vs. 60 ± 10%; respectively, Figs 3B and 4B). However, exogenous ATP blunted the vasoconstriction to both α-receptor subtypes similarly in older and young adults (α1= 94 ± 19%vs. 88 ± 13%; α2= 88 ± 13%vs. 84 ± 9%; respectively, Figs 3C and 4C).
Table 3.
Forearm and systemic haemodynamics in young adults (adapted from Kirby et al. 2008)
| Time | Condition | Forearm vascular conductance (ml min−1 (mmHg−1)) | |
|---|---|---|---|
| Phenylephrine | Dexmedetomidine | ||
| Pre-constrictor drug | Adenosine | 159 ± 21 | 144 ± 29 |
| Exercise | 159 ± 14 | 159 ± 18 | |
| ATP | 146 ± 24 | 165 ± 25 | |
| Constrictor drug | Adenosine | 90 ± 11* | 78 ± 16* |
| Exercise | 145 ± 11† | 134 ± 14† | |
| ATP | 141 ± 24† | 160 ± 28† | |
All steady-state (pre-vasconstrictor) and constrictor FBF and FVC responses are significantly greater than respective baseline values (P < 0.05).
P < 0.05 vs. pre-vasoconstrictor, phenylephrine or dexmedetomidine (within condition)
P < 0.05 vs. adenosine (within condition).
Discussion
The primary findings from the present investigation are as follows. First, exogenous ATP required to match forearm hyperaemia during moderate handgrip exercise abolishes direct postjunctional α-adrenoceptor-mediated vasoconstriction in older adults, and this involves both α1- and α2-receptor subtypes. Second, the present data corroborate previous findings that α1-adrenoceptor responsiveness is blunted in older adults while α2-adrenoceptor sensitivity is relatively intact in the human forearm compared to young adults at rest and during passive vasodilatation (Dinenno et al. 2002, 2005). Further, we confirmed previous findings from our laboratory that older humans have enhanced vasoconstriction to direct postjunctional α-adrenoceptor stimulation during moderate-intensity forearm exercise and therefore demonstrate an impaired modulation of sympathetic vasoconstriction in contracting muscle (i.e. reduced magnitude of sympatholysis). However, in contrast to our hypothesis, the ability of exogenous ATP to blunt postjunctional α-adrenergic vasoconstriction is similar in young and older adults.
The rationale for the present study stems from the understanding that a competition exists between local vasodilator and sympathetic neural vasoconstrictor signals during exercise as a means to regulate blood flow and oxygen delivery to active skeletal muscle without compromising blood pressure (Rowell, 1997; Buckwalter & Clifford, 2001). As such, it is well recognized that vasodilatation and sympathetic activation occur simultaneously with the degree of exercise intensity and muscle mass recruited (Rowell, 1997; Saltin et al. 1998). Accumulating evidence indicates that muscle contractions can blunt sympathetic vasoconstriction in an intensity-dependent manner (Hansen et al. 1996; Tschakovsky et al. 2002; Kirby et al. 2005; Dinenno & Joyner, 2006). Teleologically, this phenomenon (termed ‘functional sympatholysis’; Remensnyder et al. 1962) probably allows for both optimal blood flow and blood pressure regulation during moderate- and heavy-intensity exercise in the upright human (Joyner & Thomas, 2003). Further, with respect to human ageing, the majority of evidence indicates that muscle blood flow is reduced and arterial pressure is augmented during large muscle mass exercise (Lawrenson et al. 2003; Proctor & Parker, 2006; Richardson et al. 2006), which could be consequent to impaired modulation of sympathetic α-adrenoceptor vasoconstriction in the vascular beds of contracting muscle (Koch et al. 2003; Fadel et al. 2004; Dinenno & Joyner, 2006).
Over the last decade, several investigators have designed experiments in animals and humans to determine what factor(s) are involved in the observed blunting of sympathetic vasoconstriction within active muscle (Hansen et al. 2000; Chavoshan et al. 2002; Dinenno & Joyner, 2003, 2004; Buckwalter et al. 2004; Keller et al. 2004; Pickkers et al. 2004). In humans, the collective data indicate that the traditional putative vasodilatating substances associated with exercise hyperaemia, such as adenosine, nitric oxide and prostaglandins, appear to have a minimal role in modulating α-adrenoceptor vasoconstriction during exercise (Tschakovsky et al. 2002; Dinenno & Joyner, 2004). Further, although it has been established that the mechanical effects of muscle contraction can evoke vasodilatation independent of muscle activation (Kirby et al. 2007), this physical mechanical influence on vascular tone does not limit sympathetically mediated vasoconstriction (Kirby et al. 2005). More recently, we and others have demonstrated that exogenous ATP can significantly blunt sympathetic α-adrenergic vasoconstriction in young adults (Rosenmeier et al. 2004; Kirby et al. 2008). In addition, given that both circulating ATP levels as well as the ability to blunt sympathetic vasoconstriction increase in an exercise intensity-dependent manner (Buckwalter et al. 2001; Gonzalez-Alonso et al. 2002), we had questioned whether this response was graded with the dose of ATP infusion, such that low doses of ATP are not sympatholytic yet higher doses of ATP produce progressive blunting of constriction (Kirby et al. 2008). This, in fact, does occur and gives credence to the possible role of endogenous ATP as a sympatholytic agent during exercise.
In humans, it is well recognized that control of the vasculature is altered with advancing age whereby sympathetic vasoconstrictor activity is elevated (Taylor et al. 1992; Ng et al. 1993; Proctor et al. 1998; Dinenno et al. 2002) and vasodilator mechanisms are diminished (Taddei et al. 2001; DeSouza et al. 2002; Newcomer et al. 2005) both at rest and during exercise (Dinenno & Joyner, 2006; Kirby et al. 2009). With specific relevance to the present study, Dinenno and colleagues demonstrated that aged men have impaired modulation of sympathetic α-adrenergic vasoconstriction in contracting muscle (Dinenno et al. 2005), supporting previous findings observed in older women (Fadel et al. 2004) and the leg of older men (Koch et al. 2003). Accordingly, in the present study we hypothesized that the sympatholytic properties of exogenous ATP are impaired in ageing humans when challenged with direct postjunctional α-adrenoceptor stimulation. In contrast to our hypothesis, we observed that the ability of exogenous ATP to blunt sympathetic vasoconstriction is intact in older adults, and the magnitude of this sympatholytic property of ATP is similar between young and older adults. However, despite these responses with exogenous ATP, older adults still exhibited an impaired ability to modulate direct α-adrenergic constriction during exercise.
As a means of quantifying the ability of either exercise or exogenous ATP to blunt α-adrenergic constriction relative to a control vasodilator condition (i.e. adenosine), we calculated a ‘magnitude of sympatholysis’ which represents the difference in vasoconstriction observed during control vasodilator infusion of adenosine with that observed during either exercise or ATP infusion. In the present study, we clearly demonstrate and support earlier findings that the magnitude of sympatholysis via exercise is significantly attenuated in older compared to young adults, independent of α-receptor subtype (Dinenno et al. 2005). However, in contrast to our hypothesis, our data show that the magnitude of sympatholysis via exogenous ATP was similar between young and older adults (∼90%). Although these findings may be somewhat surprising considering an impaired ability of exercise to blunt sympathetic constriction in ageing humans, we have recently demonstrated that older healthy adults have intact vasodilator responsiveness to graded doses of exogenous ATP despite clear endothelial dysfunction as shown by substantial reductions in vasodilatation during acetylcholine infusion (Kirby et al. 2010). Moreover, when comparing the average dose of ATP used in the present study (10 ± 2 nmol (dl FAV)−1 min−1) with our previous findings in young adults (11 ± 2 nmol (dl FAV)−1 min−1) (Kirby et al. 2008), there is no difference between age groups (P = 0.7). Collectively, these data indicate that the dual vasoactive signalling properties of ATP (vasodilatation and sympatholytic) are maintained with advancing age in humans. Thus, if ATP is mechanistically linked with impaired exercise sympatholysis in older adults, we speculate that the levels of circulating ATP or local release of ATP might be reduced with age.
In this context, it is of interest to consider whether the dose of ATP infused in both the young and older subjects is of physiological relevance. Using the ATP dose required to match exercise hyperaemia and the steady-state forearm blood flow values, we calculated the arterial [ATP] to be 932 ± 288 nmol l−1 in the older and 1073 ± 397 nmol l−1 in the young subjects (age-group difference not significant). These values are within the reported range during exercise in young adults (Gonzalez-Alonso et al. 2002), and thus we believe are indeed physiologically relevant. However, given that ATP can be rapidly degraded in the circulation, we still do not know the exact [ATP] in the circulation at the level of the resistance vessels. To our knowledge, no such studies aimed at characterizing circulating plasma [ATP] of healthy (or diseased) aged humans have yet been conducted; thus, it remains unknown whether the ATP draining the active muscle bed is impaired with age. Nevertheless, the present study clearly indicates that although older adults have a maintained ability to modulate α-adrenergic vasoconstriction during exogenous ATP infusion, this ability is significantly impaired during muscle contractions. This may suggest a lower quantity of sympatholytic agent(s) produced during exercise with advancing age.
Experimental considerations
First, it should be considered that we were unable to determine the role of endogenous ATP in modulating direct α-adrenergic stimulation during exercise as no pharmacological antagonist for the P2Y-receptor is yet available for human use. In addition, we did not measure circulating ATP levels in either age group, as this was not the main purpose of the present study. Nonetheless, it is clear that exogenous ATP has the ability to blunt sympathetic vasoconstriction in ageing humans despite an observed impairment of contracting muscle to modulate α-adrenoceptor responsiveness. Second, we acknowledge that the findings from the present study in older adults are compared to our previously published findings in young adults (Kirby et al. 2008). The experimental approach, methods of data collection and data analysis were identical between studies and thus our comparisons of the primary outcome variables are valid. In this context, this approach has been used in recent publications in The Journal (e.g. Schrage et al. 2007; Lundby et al. 2008).
Perspectives: impaired modulation of sympathetic vasoconstriction in contracting muscle of older healthy adults
The majority of evidence in humans indicates an impaired ability to modulate sympathetic vasoconstriction in contracting muscle in older adults (Koch et al. 2003; Fadel et al. 2004; Dinenno et al. 2005), and this impairment is evident at the level of the postjunctional α-adrenoceptors (Dinenno et al. 2005). The present data clearly indicate that exogenous ATP blunts α-adrenoceptor-mediated vasoconstriction in older adults; however, it is still possible that alterations in endogenous[ATP] during exercise of older adults in part explains age-related declines in exercise-mediated functional sympatholysis. Thus, if reduced circulating ATP does play a role in this age-associated impairment during exercise, it would appear to be due to either impaired local release of ATP from putative sources such as red blood cells (Ellsworth et al. 1995; Sprague et al. 1998) or the endothelium (Bodin & Burnstock, 1995; Hashimoto et al. 1995), or due to greater breakdown of ATP to its degradation products (ADP, AMP and adenosine) that do not possess sympatholytic capabilities (Yegutkin, 1997; Kirby et al. 2008; Rosenmeier et al. 2008). Although we have recently shown that ATP-mediated vasodilatation is not mediated via P1-receptors in older adults (i.e. not degraded to adenosine) (Kirby et al. 2010), this was performed under resting conditions and thus it remains unknown how acute exercise impacts ATP degradation in older humans. Alternatively, if this impaired modulation of sympathetic vasoconstriction during exercise in older adults is independent of circulating ATP, it could be due to some yet unidentified substance or mechanism (e.g. hyperpolarizing factor(s)) that is reduced with advancing age.
Conclusions
The ability of contracting muscle to modulate sympathetic vasoconstriction is impaired in ageing humans, and this impairment is observed for both α1- and α2-adrenoceptor subtypes. In young adults, circulating ATP is thought to play a role in regulating vascular tone during exercise via direct vasodilatation as well as modulation of sympathetic α-adrenergic vasoconstriction. We have clearly demonstrated that both the vasodilatory (Kirby et al. 2010) and sympatholytic properties of exogenous ATP are intact in older adults. These findings indicate that perhaps endogenous concentrations of ATP or other sympatholytic factors/signalling are reduced during exercise with advancing age, and may in part explain the impaired exercise sympatholysis in older adults. Collectively, the present findings emphasize the unique vasomotor properties of circulating ATP and draw further attention to the fact that impaired vasomotor control during exercise exists and may result in attenuated blood flow and oxygen delivery to contracting skeletal muscle in ageing humans.
Acknowledgments
We would like to thank Julia Davis for her assistance in these studies, as well as the subjects who volunteered to participate. This research was supported by National Institutes of Health awards AG022337, AG027150 and HL087952 (to F.A.D.).
Glossary
Abbreviations
- DEXA
dual-energy X-ray absorptiometry
- ECG
electrocardiogram
- FAV
forearm volume
- FBF
forearm blood flow
- FVC
forearm vascular conductance
- HR
heart rate
- MAP
mean arterial pressure
- MBV
mean blood velocity
- MVC
maximal voluntary contraction
- P-receptor
purinergic receptor
Author contributions
B.S.K. contributed to the conception and experimental design, data acquisition, data analysis, data interpretation and drafting of the manuscript. A.R.C. contributed to data acquisition and interpretation, and critical review of the manuscript. W.F.V. provided clinical support, invasive methodology, and contributed to data acquisition and interpretation, as well as critical review of the manuscript. F.A.D. contributed to the conception and experimental design, data acquisition and interpretation, and critical review of the manuscript. All authors approved the final version of the manuscript.
References
- Bergfeld GR, Forrester T. Release of ATP from human erythrocytes in response to a brief period of hypoxia and hypercapnia. Cardiovasc Res. 1992;26:40–47. doi: 10.1093/cvr/26.1.40. [DOI] [PubMed] [Google Scholar]
- Bodin P, Burnstock G. Synergistic effect of acute hypoxia on flow-induced release of ATP from cultured endothelial cells. Experientia. 1995;51:256–259. doi: 10.1007/BF01931108. [DOI] [PubMed] [Google Scholar]
- Buckwalter JB, Clifford PS. The paradox of sympathetic vasoconstriction in exercising skeletal muscle. Exerc Sport Sci Rev. 2001;29:159–163. doi: 10.1097/00003677-200110000-00005. [DOI] [PubMed] [Google Scholar]
- Buckwalter JB, Naik JS, Valic Z, Clifford PS. Exercise attenuates α-adrenergic-receptor responsiveness in skeletal muscle vasculature. J Appl Physiol. 2001;90:172–178. doi: 10.1152/jappl.2001.90.1.172. [DOI] [PubMed] [Google Scholar]
- Buckwalter JB, Taylor JC, Hamann JJ, Clifford PS. Role of nitric oxide in exercise sympatholysis. J Appl Physiol. 2004;97:417–423. doi: 10.1152/japplphysiol.01181.2003. [DOI] [PubMed] [Google Scholar]
- Carlson RE, Kirby BS, Voyles WF, Dinenno FA. Evidence for impaired skeletal muscle contraction-induced rapid vasodilation in aging humans. Am J Physiol Heart Circ Physiol. 2008;294:H1963–H1970. doi: 10.1152/ajpheart.01084.2007. [DOI] [PubMed] [Google Scholar]
- Chavoshan B, Sander M, Sybert TE, Hansen J, Victor RG, Thomas GD. Nitric oxide-dependent modulation of sympathetic neural control of oxygenation in exercising human skeletal muscle. J Physiol. 2002;540:377–386. doi: 10.1113/jphysiol.2001.013153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DeSouza CA, Clevenger CM, Greiner JJ, Smith DT, Hoetzer GL, Shapiro LF, Stauffer BL. Evidence for agonist-specific endothelial vasodilator dysfunction with ageing in healthy humans. J Physiol. 2002;542:255–262. doi: 10.1113/jphysiol.2002.019166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dinenno FA, Dietz NM, Joyner MJ. Aging and forearm postjunctional α-adrenergic vasoconstriction in healthy men. Circulation. 2002;106:1349–1354. doi: 10.1161/01.cir.0000028819.64790.be. [DOI] [PubMed] [Google Scholar]
- Dinenno FA, Joyner MJ. Blunted sympathetic vasoconstriction in contracting skeletal muscle of healthy humans: is nitric oxide obligatory? J Physiol. 2003;553:281–292. doi: 10.1113/jphysiol.2003.049940. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dinenno FA, Joyner MJ. Combined NO and PG inhibition augments α-adrenergic vasoconstriction in contracting human skeletal muscle. Am J Physiol Heart Circ Physiol. 2004;287:H2576–H2584. doi: 10.1152/ajpheart.00621.2004. [DOI] [PubMed] [Google Scholar]
- Dinenno FA, Joyner MJ. α-Adrenergic control of skeletal muscle circulation at rest and during exercise in aging humans. Microcirculation. 2006;13:329–341. doi: 10.1080/10739680600618843. [DOI] [PubMed] [Google Scholar]
- Dinenno FA, Masuki S, Joyner MJ. Impaired modulation of sympathetic α-adrenergic vasoconstriction in contracting forearm muscle of ageing men. J Physiol. 2005;567:311–321. doi: 10.1113/jphysiol.2005.087668. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ellsworth ML, Ellis CG, Goldman D, Stephenson AH, Dietrich HH, Sprague RS. Erythrocytes: oxygen sensors and modulators of vascular tone. Physiology (Bethesda) 2009;24:107–116. doi: 10.1152/physiol.00038.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ellsworth ML, Forrester T, Ellis CG, Dietrich HH. The erythrocyte as a regulator of vascular tone. Am J Physiol Heart Circ Physiol. 1995;269:H2155–H2161. doi: 10.1152/ajpheart.1995.269.6.H2155. [DOI] [PubMed] [Google Scholar]
- Faber JE, Harris PD, Joshua IG. Microvascular response to blockade of prostaglandin synthesis in rat skeletal muscle. Am J Physiol Heart Circ Physiol. 1982;243:H51–H60. doi: 10.1152/ajpheart.1982.243.1.H51. [DOI] [PubMed] [Google Scholar]
- Fadel PJ, Wang Z, Watanabe H, Arbique D, Vongpatanasin W, Thomas GD. Augmented sympathetic vasoconstriction in exercising forearms of postmenopausal women is reversed by oestrogen therapy. J Physiol. 2004;561:893–901. doi: 10.1113/jphysiol.2004.073619. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gonzalez-Alonso J, Olsen DB, Saltin B. Erythrocyte and the regulation of human skeletal muscle blood flow and oxygen delivery: role of circulating ATP. Circ Res. 2002;91:1046–1055. doi: 10.1161/01.res.0000044939.73286.e2. [DOI] [PubMed] [Google Scholar]
- Hansen J, Sander M, Hald CF, Victor RG, Thomas GD. Metabolic modulation of sympathetic vasoconstriction in human skeletal muscle: role of tissue hypoxia. J Physiol. 2000;527:387–396. doi: 10.1111/j.1469-7793.2000.00387.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hansen J, Thomas GD, Harris SA, Parsons WJ, Victor RG. Differential sympathetic neural control of oxygenation in resting and exercising human skeletal muscle. J Clin Invest. 1996;98:584–496. doi: 10.1172/JCI118826. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hashimoto M, Shinozuka K, Bjur RA, Westfall DP, Hattori K, Masumura S. The effects of age on the release of adenine nucleosides and nucleotides from rat caudal artery. J Physiol. 1995;489:841–848. doi: 10.1113/jphysiol.1995.sp021096. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jagger JE, Bateman RM, Ellsworth ML, Ellis CG. Role of erythrocyte in regulating local O2 delivery mediated by hemoglobin oxygenation. Am J Physiol Heart Circul Physiol. 2001;280:H2833–H2839. doi: 10.1152/ajpheart.2001.280.6.H2833. [DOI] [PubMed] [Google Scholar]
- Joyner MJ, Thomas GD. Having it both ways? Vasoconstriction in contracting muscles. J Physiol. 2003;550:333. doi: 10.1113/jphysiol.2003.044628. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Keller DM, Ogoh S, Greene S, Olivencia-Yurvati A, Raven PB. Inhibition of KATP channel activity augments baroreflex-mediated vasoconstriction in exercising human skeletal muscle. J Physiol. 2004;561:273–282. doi: 10.1113/jphysiol.2004.071993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kirby BS, Carlson RE, Markwald RR, Voyles WF, Dinenno FA. Mechanical influences on skeletal muscle vascular tone in humans: insight into contraction-induced rapid vasodilatation. J Physiol. 2007;583:861–874. doi: 10.1113/jphysiol.2007.131250. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kirby BS, Crecelius AR, Voyles WF, Dinenno FA. Vasodilatory responsiveness to adenosine triphosphate in ageing humans. J Physiol. 2010;588:4017–4027. doi: 10.1113/jphysiol.2010.197814. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kirby BS, Markwald RR, Smith EG, Dinenno FA. Mechanical effects of muscle contraction do not blunt sympathetic vasoconstriction in humans. Am J Physiol Heart Circ Physiol. 2005;289:H1610–H1617. doi: 10.1152/ajpheart.00391.2005. [DOI] [PubMed] [Google Scholar]
- Kirby BS, Voyles WF, Carlson RE, Dinenno FA. Graded sympatholytic effect of exogenous ATP on postjunctional α-adrenergic vasoconstriction in the human forearm: implications for vascular control in contracting muscle. J Physiol. 2008;586:4305–4316. doi: 10.1113/jphysiol.2008.154252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kirby BS, Voyles WF, Simpson CB, Carlson RE, Schrage WG, Dinenno FA. Endothelium-dependent vasodilatation and exercise hyperaemia in ageing humans: impact of acute ascorbic acid administration. J Physiol. 2009;587:1989–2003. doi: 10.1113/jphysiol.2008.167320. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kneale BJ, Chowienczyk PJ, Brett SE, Coltart DJ, Ritter JM. Gender differences in sensitivity to adrenergic agonists of forearm resistance vasculature. J Am Coll Cardiol. 2000;36:1233–1238. doi: 10.1016/s0735-1097(00)00849-4. [DOI] [PubMed] [Google Scholar]
- Koch DW, Leuenberger U, Proctor DN. Augmented leg vasoconstricion in dynamically exercising older men during acute sympathetic stimulation. J Physiol. 2003;551:337–344. doi: 10.1113/jphysiol.2003.042747. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kuipers NT, Sauder CL, Kearney ML, Ray CA. Interactive effect of aging and local muscle heating on renal vasoconstriction during isometric handgrip. Am J Physiol Renal Physiol. 2009;297:F327–F332. doi: 10.1152/ajprenal.00165.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lautt WW. Resistance or conductance for expression of arterial vascular tone. Microvasc Res. 1989;37:230–236. doi: 10.1016/0026-2862(89)90040-x. [DOI] [PubMed] [Google Scholar]
- Lawrenson L, Poole JG, Kim J, Brown C, Patel P, Richardson RS. Vascular and metabolic response to isolated small muscle mass exercise: effect of age. Am J Physiol Heart Circ Physiol. 2003;285:H1023–H1031. doi: 10.1152/ajpheart.00135.2003. [DOI] [PubMed] [Google Scholar]
- Lundby C, Boushel R, Robach P, Moller K, Saltin B, Calbet JA. During hypoxic exercise some vasoconstriction is needed to match O2 delivery with O2 demand at the microcirculatory level. J Physiol. 2008;586:123–130. doi: 10.1113/jphysiol.2007.146035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marshall RJ, Schirger A, Shepherd JT. Blood pressure during supine exercise in idiopathic orthostatic hypotension. Circulation. 1961;24:76–81. doi: 10.1161/01.cir.24.1.76. [DOI] [PubMed] [Google Scholar]
- Momen A, Leuenberger UA, Handly B, Sinoway LI. Effect of aging on renal blood flow velocity during static exercise. Am J Physiol Heart Circ Physiol. 2004;287:H735–H740. doi: 10.1152/ajpheart.00959.2003. [DOI] [PubMed] [Google Scholar]
- Newcomer SC, Leuenberger UA, Hogeman CS, Proctor DN. Heterogeneous vasodilator responses of human limbs: influence of age and habitual endurance training. Am J Physiol Heart Circ Physiol. 2005;289:H308–H315. doi: 10.1152/ajpheart.01151.2004. [DOI] [PubMed] [Google Scholar]
- Ng AV, Callister R, Johnson DG, Seals DR. Age and gender influence muscle sympathetic nerve activity at rest in healthy humans. Hypertension. 1993;21:498–503. doi: 10.1161/01.hyp.21.4.498. [DOI] [PubMed] [Google Scholar]
- Nishigaki K, Faber JE, Ohyanagi M. Interactions between α-adrenoceptors and adenosine receptors on microvascular smooth muscle. Am J Physiol Heart Circ Physiol. 1991;260:H1655–H1666. doi: 10.1152/ajpheart.1991.260.5.H1655. [DOI] [PubMed] [Google Scholar]
- O'Leary DS. Regional vascular resistance vs conductance: which index for baroreflex responses. Am J Physiol Heart Circ Physiol. 1991;260:H632–H637. doi: 10.1152/ajpheart.1991.260.2.H632. [DOI] [PubMed] [Google Scholar]
- Pickkers P, Jansen Van Rosendaal AJ, Van Der Hoeven JG, Smits P. Activation of the ATP-dependent potassium channel attenuates norepinephrine-induced vasoconstriction in the human forearm. Shock. 2004;22:320–325. doi: 10.1097/01.shk.0000142250.85264.10. [DOI] [PubMed] [Google Scholar]
- Proctor DN, Parker BA. Vasodilation and vascular control in contracting muscle of the aging human. Microcirculation. 2006;13:315–327. doi: 10.1080/10739680600618967. [DOI] [PubMed] [Google Scholar]
- Proctor DN, Shen PH, Dietz NM, Eickhoff TJ, Lawler LA, Ebersold EJ, Loeffler DL, Joyner MJ. Reduced leg blood flow during dynamic exercise in older endurance-trained men. J Appl Physiol. 1998;85:68–75. doi: 10.1152/jappl.1998.85.1.68. [DOI] [PubMed] [Google Scholar]
- Remensnyder JP, Mitchell JH, Sarnoff SJ. Functional sympatholysis during muscular activity. Circ Res. 1962;11:370–380. doi: 10.1161/01.res.11.3.370. [DOI] [PubMed] [Google Scholar]
- Richardson RS, Secher NH, Tschakovsky ME, Proctor DN, Wray DW. Metabolic and vascular limb differences affected by exercise, gender, age, and disease. Med Sci Sports Exerc. 2006;38:1792–1796. doi: 10.1249/01.mss.0000229568.17284.ab. [DOI] [PubMed] [Google Scholar]
- Rosenmeier JB, Dinenno FA, Fritzlar SJ, Joyner MJ. α1- and α2-adrenergic vasoconstriction is blunted in contracting human muscle. J Physiol. 2003;547:971–976. doi: 10.1113/jphysiol.2002.037937. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rosenmeier JB, Hansen J, Gonzalez-Alonso J. Circulating ATP-induced vasodilatation overrides sympathetic vasoconstrictor activity in human skeletal muscle. J Physiol. 2004;558:351–365. doi: 10.1113/jphysiol.2004.063107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rosenmeier JB, Yegutkin GG, Gonzalez-Alonso J. Activation of ATP/UTP-selective receptors increases blood flow and blunts sympathetic vasoconstriction in human skeletal muscle. J Physiol. 2008;586:4993–5002. doi: 10.1113/jphysiol.2008.155432. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rowell LB. Neural control of muscle blood flow: importance during dynamic exercise. Clin Exp Pharmacol Physiol. 1997;24:117–125. doi: 10.1111/j.1440-1681.1997.tb01793.x. [DOI] [PubMed] [Google Scholar]
- Saltin B, Radegran G, Koskolou MD, Roach RC. Skeletal muscle blood flow in humans and its regulation during exercise. Acta Physiol Scand. 1998;162:421–436. doi: 10.1046/j.1365-201X.1998.0293e.x. [DOI] [PubMed] [Google Scholar]
- Schrage WG, Eisenach JH, Joyner MJ. Ageing reduces nitric-oxide- and prostaglandin-mediated vasodilatation in exercising humans. J Physiol. 2007;579:227–236. doi: 10.1113/jphysiol.2006.124313. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seals DR, Dinenno FA. Collateral damage: cardiovascular consequences of chronic sympathetic activation with human aging. Am J Physiol Heart Circ Physiol. 2004;287:H1895–H1905. doi: 10.1152/ajpheart.00486.2004. [DOI] [PubMed] [Google Scholar]
- Seals DR, Esler MD. Human ageing and the sympathoadrenal system. J Physiol. 2000;528:407–417. doi: 10.1111/j.1469-7793.2000.00407.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sprague RS, Ellsworth ML, Stephenson AH, Kleinhenz ME, Lonigro AJ. Deformation-induced ATP release from red blood cells requires CFTR activity. Am J Physiol Heart Circ Physiol. 1998;275:H1726–H1732. doi: 10.1152/ajpheart.1998.275.5.H1726. [DOI] [PubMed] [Google Scholar]
- Taddei S, Virdis A, Ghiadoni L, Salvetti G, Bernini G, Magagna A, Salvetti A. Age-related reduction of NO availability and oxidative stress in humans. Hypertension. 2001;38:274–279. doi: 10.1161/01.hyp.38.2.274. [DOI] [PubMed] [Google Scholar]
- Taylor JA, Hand GA, Johnson DG, Seals DR. Sympathoadrenal-circulatory regulation during sustained isometric exercise in young and older men. Am J Physiol Renal Physiol. 1991;261:R1061–R1069. doi: 10.1152/ajpregu.1991.261.5.R1061. [DOI] [PubMed] [Google Scholar]
- Taylor JA, Hand GA, Johnson DG, Seals DR. Augmented forearm vasoconstriction during dynamic exercise in healthy older men. Circulation. 1992;86:1789–1799. doi: 10.1161/01.cir.86.6.1789. [DOI] [PubMed] [Google Scholar]
- Thomas GD, Hansen J, Victor RG. Inhibition of α-2 adrenergic vasoconstriction during contraction of glycolytic, not oxidative, rat hindlimb muscle. Am J Physiol Heart Circ Physiol. 1994;266:H920–H929. doi: 10.1152/ajpheart.1994.266.3.H920. [DOI] [PubMed] [Google Scholar]
- Thomas GD, Victor RG. Nitric oxide mediates contraction-induced attenuation of sympathetic vasoconstriction in rat skeletal muscle. J Physiol. 1998;506:817–826. doi: 10.1111/j.1469-7793.1998.817bv.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tschakovsky ME, Sujirattanawimol K, Ruble SB, Valic Z, Joyner MJ. Is sympathetic neural vasoconstriction blunted in the vascular bed of exercising human muscle? J Physiol. 2002;541:623–635. doi: 10.1113/jphysiol.2001.014431. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Versari D, Daghini E, Virdis A, Ghiadoni L, Taddei S. The ageing endothelium, cardiovascular risk and disease in man. Exp Physiol. 2009;94:317–321. doi: 10.1113/expphysiol.2008.043356. [DOI] [PubMed] [Google Scholar]
- Yegutkin GG. Kinetic analysis of enzymatic hydrolysis of ATP in human and rat blood serum. Biochemistry (Mosc) 1997;62:619–622. [PubMed] [Google Scholar]


