Abstract
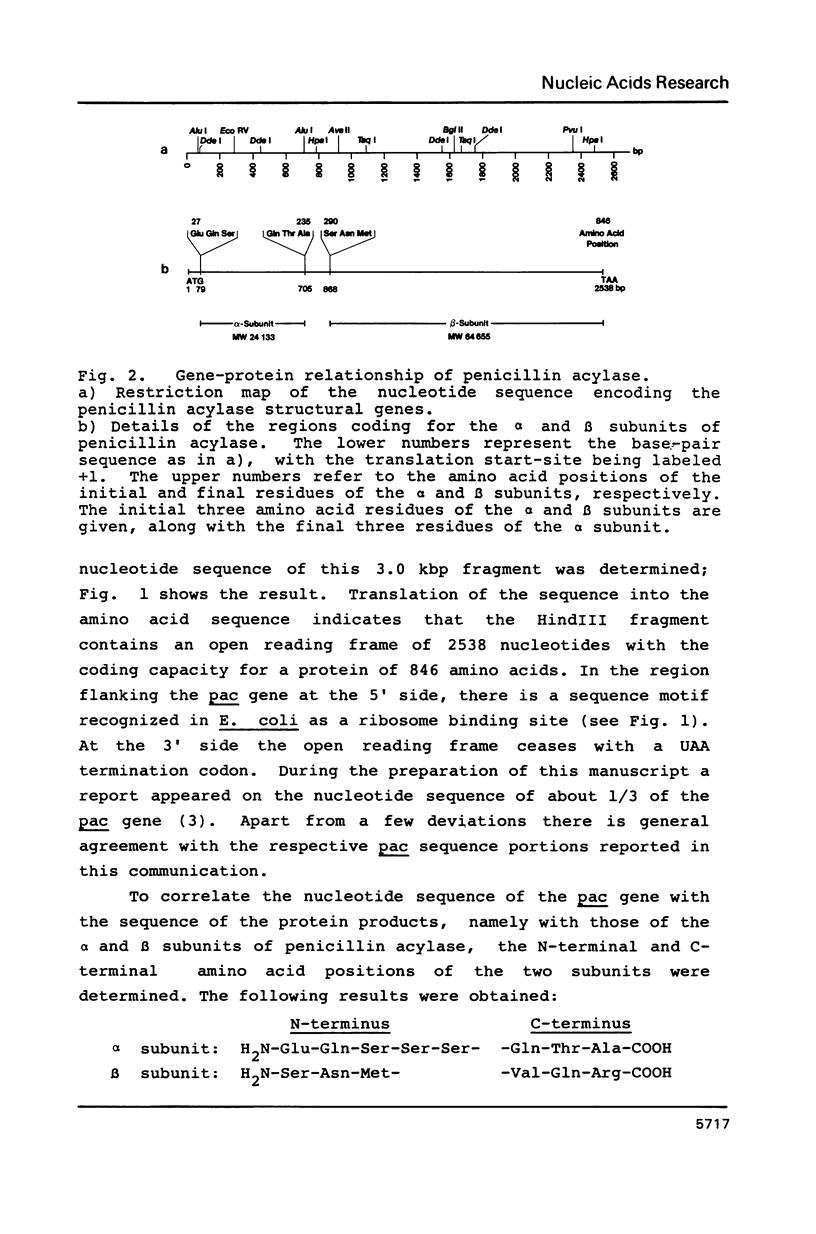
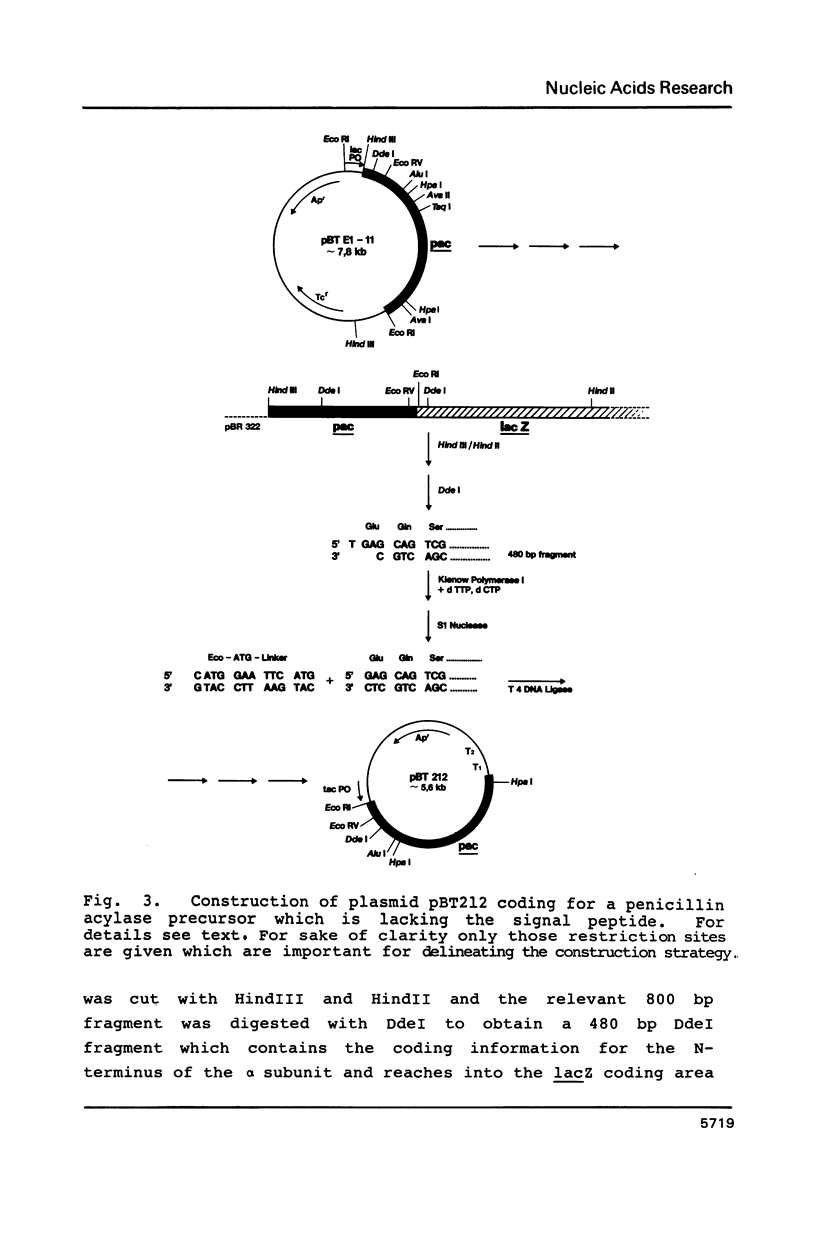
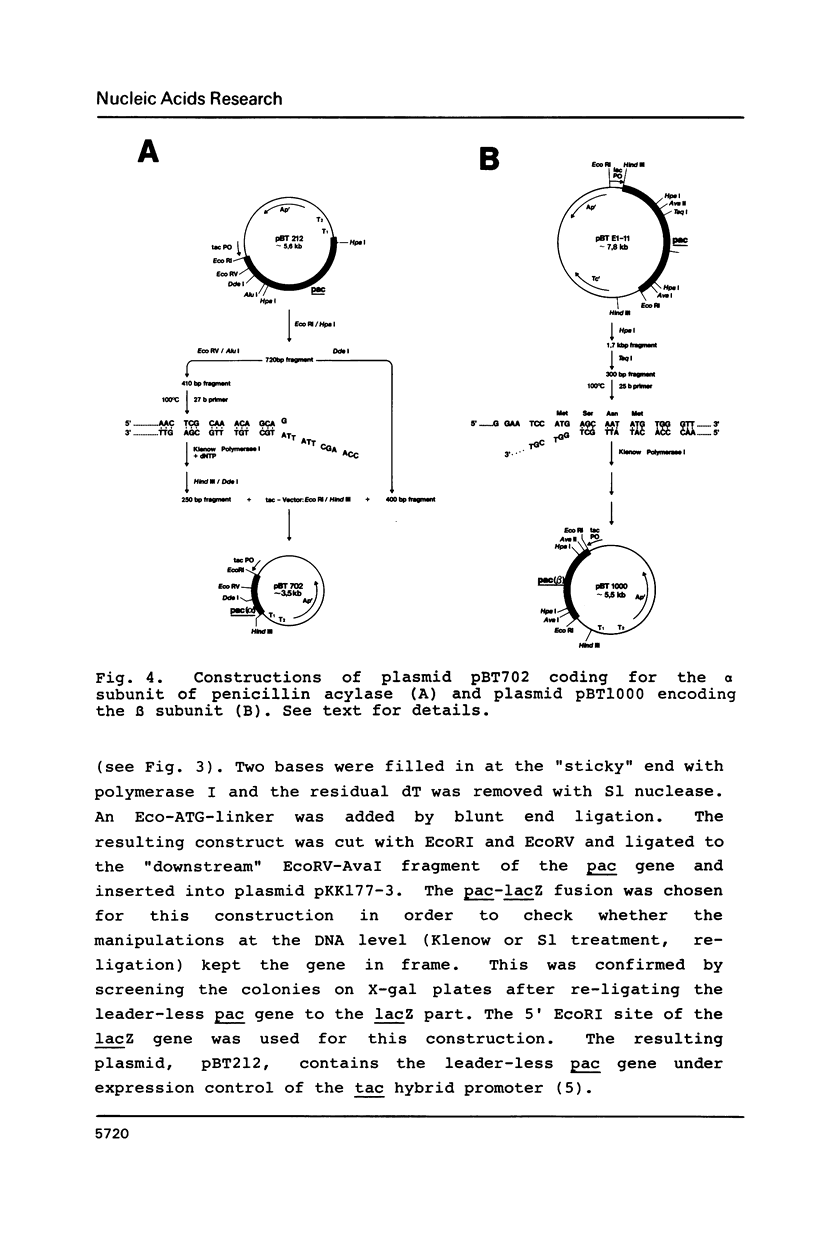
The nucleotide sequence of the gene (pac) coding for penicillin G acylase from E. coli ATCC 11105 was determined and correlated with the primary structure of the two constituent subunits of this enzyme. The pac gene open reading frame consists of four structural domains: Nucleotide positions 1-78 coding for a signal peptide, positions 79-705 coding for the alpha subunit, positions 706-867 coding for a spacer peptide, and positions 868-2538 coding for the beta subunit. Plasmids were constructed which direct the synthesis of a pac gene product lacking the signal peptide, and the synthesis of the alpha subunit or the beta subunit. The following results were obtained: The two dissimilar subunits are processing products of a single precursor polypeptide; the spacer peptide is removed during processing; the precursor polypeptide lacking the signal sequence is accumulated in the cytoplasm; it is not processed proteolytically in the cytoplasm and it does not display enzyme activity. Processing, therefore, requires translocation through the cytoplasmic membrane; processing follows a distinct sequential pathway in vitro.
Full text
PDF











Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Amann E., Brosius J., Ptashne M. Vectors bearing a hybrid trp-lac promoter useful for regulated expression of cloned genes in Escherichia coli. Gene. 1983 Nov;25(2-3):167–178. doi: 10.1016/0378-1119(83)90222-6. [DOI] [PubMed] [Google Scholar]
- Ames G. F., Prody C., Kustu S. Simple, rapid, and quantitative release of periplasmic proteins by chloroform. J Bacteriol. 1984 Dec;160(3):1181–1183. doi: 10.1128/jb.160.3.1181-1183.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Balasingham K., Warburton D., Dunnill P., Lilly M. D. The isolation and kinetics of penicillin amidase from Escherichia coli. Biochim Biophys Acta. 1972 Jul 13;276(1):250–256. doi: 10.1016/0005-2744(72)90027-7. [DOI] [PubMed] [Google Scholar]
- Crea R., Kraszewski A., Hirose T., Itakura K. Chemical synthesis of genes for human insulin. Proc Natl Acad Sci U S A. 1978 Dec;75(12):5765–5769. doi: 10.1073/pnas.75.12.5765. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frischauf A. M., Garoff H., Lehrach H. A subcloning strategy for DNA sequence analysis. Nucleic Acids Res. 1980 Dec 11;8(23):5541–5549. doi: 10.1093/nar/8.23.5541. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hayashi R., Moore S., Stein W. H. Carboxypeptidase from yeast. Large scale preparation and the application to COOH-terminal analysis of peptides and proteins. J Biol Chem. 1973 Apr 10;248(7):2296–2302. [PubMed] [Google Scholar]
- Howe J. G., Hershey J. W. A sensitive immunoblotting method for measuring protein synthesis initiation factor levels in lysates of Escherichia coli. J Biol Chem. 1981 Dec 25;256(24):12836–12839. [PubMed] [Google Scholar]
- Laemmli U. K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970 Aug 15;227(5259):680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- Maxam A. M., Gilbert W. Sequencing end-labeled DNA with base-specific chemical cleavages. Methods Enzymol. 1980;65(1):499–560. doi: 10.1016/s0076-6879(80)65059-9. [DOI] [PubMed] [Google Scholar]
- Oliver G., Valle F., Rosetti F., Gómez-Pedrozo M., Santamaría P., Gosset G., Bolivar F. A common precursor for the two subunits of the penicillin acylase from Escherichia coli ATCC11105. Gene. 1985;40(1):9–14. doi: 10.1016/0378-1119(85)90018-6. [DOI] [PubMed] [Google Scholar]
- Perlman D., Halvorson H. O. A putative signal peptidase recognition site and sequence in eukaryotic and prokaryotic signal peptides. J Mol Biol. 1983 Jun 25;167(2):391–409. doi: 10.1016/s0022-2836(83)80341-6. [DOI] [PubMed] [Google Scholar]
- Schmid G., Böck A. Immunological comparison of ribosomal proteins from archaebacteria. J Bacteriol. 1981 Aug;147(2):282–288. doi: 10.1128/jb.147.2.282-288.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yelverton E., Norton S., Obijeski J. F., Goeddel D. V. Rabies virus glycoprotein analogs: biosynthesis in Escherichia coli. Science. 1983 Feb 11;219(4585):614–620. doi: 10.1126/science.6297004. [DOI] [PubMed] [Google Scholar]





