Abstract
Reactive oxygen species (ROS), particularly superoxide (O2·−), have been identified as key signaling intermediates in ANG II-induced neuronal activation and sympathoexcitation associated with cardiovascular diseases, such as hypertension and heart failure. Studies of the central nervous system have identified NADPH oxidase as a primary source of O2·− in ANG II-stimulated neurons; however, additional sources of O2·−, including mitochondria, have been mostly overlooked. Here, we tested the hypothesis that ANG II increases mitochondria-produced O2·− in neurons and that increased scavenging of mitochondria-produced O2·− attenuates ANG II-dependent intraneuronal signaling. Stimulation of catecholaminergic (CATH.a) neurons with ANG II (100 nM) increased mitochondria-localized O2·− levels, as measured by MitoSOX Red fluorescence. This response was significantly attenuated in neurons overexpressing the mitochondria-targeted O2·−-scavenging enzyme Mn-SOD. To examine the biological significance of the ANG II-mediated increase in mitochondria-produced O2·−, we used the whole cell configuration of the patch-clamp technique to record the well-characterized ANG II-induced inhibition of voltage-gated K+ current (IKv) in neurons. Adenovirus-mediated Mn-SOD overexpression or pretreatment with the cell-permeable antioxidant tempol (1 mM) significantly attenuated ANG II-induced inhibition of IKv. In contrast, pretreatment with extracellular SOD protein (400 U/ml) had no effect. Mn-SOD overexpression also inhibited ANG II-induced activation of Ca2+/calmodulin kinase II, a redox-sensitive protein known to modulate IKv. These data indicate that ANG II increases mitochondrial O2·−, which mediates, at least in part, ANG II-induced activation of Ca2+/calmodulin kinase II and inhibition of IKv in neurons.
Keywords: manganese superoxide dismutase, SOD2, calcium/calmodulin kinase II, CATH.a neurons, MitoSOX Red
the pathogenesis of various cardiovascular diseases, including hypertension and heart failure, involves increased ANG II-dependent signaling in the central nervous system (CNS) (32, 41). Elevated levels of ANG II in the CNS enhance neuronal activation by modulating ion currents, particularly voltage-gated Ca2+ and K+ current (IKv), across neuronal cell membranes (27). Increased excitatory neuronal firing in autonomic control regions of the brain, including the subfornical organ (SFO) (9), paraventricular nucleus (1), and rostral ventrolateral medulla (RVLM) (11), ultimately drives the deleterious sympathoexcitation commonly associated with cardiovascular disorders. Recently, numerous studies have identified NADPH oxidase-derived reactive oxygen species (ROS), particularly superoxide (O2·−), as key intermediates in brain angiotensinergic signaling (15, 36). In contrast, few studies have focused on mitochondria as a source of O2·− in ANG II-stimulated neurons.
The involvement of mitochondria-produced O2·− in modulating central ANG II-induced cardiovascular responses was first reported in a study showing that overexpression of Mn-SOD, the mitochondria-targeted isoform of the O2·−-scavenging enzyme, in the SFO of mice markedly attenuates acute, central ANG II-induced cardiovascular responses (38). A similar study reported that Mn-SOD overexpression in the RVLM inhibits the ANG II pressor response following RVLM administration. RVLM levels of Mn-SOD mRNA, protein, and activity are drastically reduced in spontaneously hypertensive rats (SHR) compared with normotensive Wistar-Kyoto (WKY) rats. Furthermore, overexpression of Mn-SOD in the RVLM of SHR significantly attenuates mean arterial pressure (4). In addition, decreased Mn-SOD levels in the RVLM are observed in rabbits with chronic heart failure (12). Together, these data indicate that mitochondria-produced O2·− is involved in the pathogenesis of cardiovascular diseases, including hypertension and heart failure, associated with increased sympathetic activation and brain angiotensinergic signaling. However, a direct link between ANG II and increased levels of mitochondrial O2·− in neurons, as well as the mechanism(s) by which mitochondria-produced O2·− modulates ANG II-dependent intraneuronal signaling and neuronal activation, has yet to be elucidated.
ANG II increases neuronal activity or firing rate by modulating the frequency of action potentials, which ultimately depends on the activity of membrane ion channels. Previous studies by Raizada, Sumners, and colleagues clearly identified ANG II-induced inhibition of IKv, particularly the delayed rectifier K+ current, as a key mechanism by which ANG II evokes changes in neuronal activity (28–30, 35). Using primary neurons isolated from rat hypothalamus and brain stem or a catecholaminergic neuronal cell line (CATH.a neurons), they demonstrated that an ANG II-mediated increase in neuronal activity involves the activation of PKC and Ca2+/calmodulin kinase II (CaMKII) (28, 30). Additionally, they previously demonstrated that the ANG II-induced inhibition of IKv and the increase in neuronal firing rate are mediated, at least in part, by NADPH oxidase-derived ROS (29).
Considering that, in most cells, mitochondria are a primary source of O2·− (31), along with the evidence that scavenging mitochondria-produced O2·− via Mn-SOD overexpression inhibits central ANG II-induced cardiovascular responses (38), we hypothesized that ANG II-dependent intraneuronal signaling is mediated by mitochondria-produced O2·−. To address this hypothesis, we measured mitochondria-localized O2·− levels in acute ANG II-stimulated CATH.a neurons and investigated ANG II-induced changes in IKv and CaMKII activation in CATH.a neurons overexpressing Mn-SOD.
MATERIALS AND METHODS
CATH.a neuronal cell culture.
Catecholaminergic CATH.a neuronal cells (stock no. CRL-11179, American Type Culture Collection, Manassas, VA) were cultured in RPMI 1640 medium supplemented with 8% normal horse serum, 4% fetal bovine serum, and 1% penicillin-streptomycin, as recommended by the supplier of the cell line. Before experimentation, CATH.a neurons were differentiated for 6–8 days by addition of N6,2′-O-dibutyryladenosine 3′,5′-cyclic monophosphate sodium salt (1 mM) to the culture medium, as previously described (7). CATH.a neurons are commonly used to examine ANG II-dependent signaling mechanisms, as these cells express the ANG II type 1 (AT1) and type 2 (AT2) receptors (28).
Adenoviral vectors.
Replication-deficient recombinant adenovirus encoding human Mn-SOD (AdMnSOD), constructed as previously described (39), was obtained from Viraquest (North Liberty, IA). Control adenovirus containing only the cytomegalovirus promoter (AdEmpty) was also provided by Viraquest. Adenoviral titers were 1–2 × 1012 viral particles/ml and 3–5 × 1010 plaque-forming units/ml. Pilot studies revealed that 50 multiplicity of infection of adenovirus was sufficient to induce gene expression without inducing neuronal toxicity (data not shown); thus, for studies reported here, CATH.a neurons were infected with 50 multiplicity of infection of adenovirus 4 days before experimentation.
Measurement of mitochondria-localized O2·−.
Levels of mitochondria-localized O2·− were measured using the mitochondria-targeted, O2·−-sensitive fluorogenic probe MitoSOX Red (Invitrogen/Molecular Probes, Carlsbad, CA), as we previously described (39). Briefly, noninfected control, AdEmpty-infected, or AdMnSOD-infected CATH.a neurons were loaded with MitoSOX Red (1 μM) for 20 min, and baseline MitoSOX Red fluorescence was captured using a confocal laser scanning microscope (Zeiss 510 Meta). After 20 min of acute ANG II (100 nM) stimulation, MitoSOX Red fluorescence in the same cells was recaptured. Fluorescence intensity was quantified by establishing each neuron as a region of interest using Zeiss LSM 510 analysis software. Pre-ANG II (baseline) and post-ANG II MitoSOX Red fluorescence intensities were measured. Then the percent increase in fluorescence from baseline in each cell was determined by dividing the post-ANG II fluorescence intensity value by the baseline value of that particular cell. As previously characterized and described by Robinson and colleagues (24, 25), we detected MitoSOX Red fluorescence using a 405-nm excitation wavelength, which selectively detects the O2·−-specific 2-hydroxyethidium product of oxidized MitoSOX Red. To confirm mitochondrial localization of MitoSOX Red, neurons were also loaded with 50 nM MitoTracker Green (Invitrogen/Molecular Probes) for 30 min.
Isolation of mitochondria from CATH.a neurons.
To confirm mitochondrial localization of Mn-SOD after adenovirus-mediated gene transfer, mitochondria were isolated as described previously with minor modifications (23). CATH.a neurons were resuspended in ice-cold buffer A (225 mM mannitol, 65 mM sucrose, 1 mM EGTA, and 10 mM HEPES, pH 7.2) and processed with a glass Dounce homogenizer. Homogenates were centrifuged at 500 g for 6 min at 4°C, and supernatants were collected and saved. Pellets were resuspended in buffer A, homogenized, and centrifuged again at 500 g for 6 min at 4°C. This process was repeated 3 times. Finally, all supernatants were pooled and centrifuged at 10,000 g for 10 min at 4°C to obtain mitochondria-enriched pellets. Mitochondria pellets were resuspended in ice-cold buffer B (225 mM mannitol, 65 mM sucrose, and 10 mM HEPES, pH 7.2), centrifuged, and washed five times. The final mitochondria-enriched pellet was resuspended in buffer B (∼30% vol/vol).
Mn-SOD activity assay.
Mn-SOD activity in whole cell lysates and isolated mitochondria was measured using a semiquantitative native in-gel SOD activity assay, as previously described (2). After separation of 60 μg of whole cell lysate or mitochondria protein on a nondenaturing native gel using electrophoresis, the gel was stained with 2.4 mM nitro blue tetrazolium, 28 μM riboflavin, and 28 mM N,N,N,N-tetramethylethylenediamine for 20 min in darkness. Mn-SOD activity was visualized by illumination of the gel under a fluorescent light until achromatic bands appeared.
Western blot analysis.
Adenovirus-mediated overexpression of Mn-SOD in whole cell lysates and isolated mitochondria was confirmed using standard Western blot analysis. At 4 days after viral infection, 5 μg of whole cell lysate or mitochondria protein were separated by SDS-PAGE and transferred to a nitrocellulose membrane. Membranes were incubated with anti-Mn-SOD polyclonal IgG (Upstate Biotech/Millipore, Billerica, MA), and bands were visualized using the Pierce enhanced chemiluminescence detection system (Thermo Scientific, Rockford, IL) after incubation with appropriate horseradish peroxidase-conjugated secondary antibodies. Purity of mitochondrial preparations was monitored using antibodies directed against lactate dehydrogenase (1:1,000 dilution; Abcam, Cambridge, MA), a cytosol marker, and cytochrome c oxidase subunit IV (1:1,000 dilution; Abcam), a mitochondria marker.
ANG II-induced activation of CaMKII was determined by Western blot analysis of phosphorylated CaMKII. For investigation of the time course of CaMKII activation, noninfected control neurons were incubated with ANG II (100 nM) for 0, 1, 2, or 5 min. In a separate set of experiments, CATH.a neurons infected with AdEmpty or AdMnSOD were stimulated with ANG II. Cells were harvested in lysis buffer containing protease and phosphatase inhibitors (Roche, Indianapolis, IN), and whole cell lysates were separated by SDS-PAGE. Membranes were incubated with anti-phosphorylated (Thr286) CaMKII (Affinity BioReagents). Densitometric analysis of band intensity was determined using Image J analysis software. Actin was used to control for protein loading and to normalize signal intensities.
Immunofluorescence.
At 4 days after viral infection, CATH.a neurons were incubated with the mitochondria marker MitoTracker Red (250 nM; Invitrogen/Molecular Probes) for 30 min in HBSS and then washed with 0.1 M phosphate buffer (PB) before initiation of immunofluorescence staining for Mn-SOD. Neurons were fixed with 4% paraformaldehyde for 20 min at room temperature and incubated with blocking solution (10% normal horse serum in 0.1 M PB) for 1 h at room temperature and then with antibody directed against human Mn-SOD (1:500 dilution; The Binding Site, Birmingham, UK) in 0.1 M PB containing 2% normal horse serum and 0.3% Triton X-100 at 4°C overnight. On the following day, neurons were washed and incubated with FITC-conjugated secondary antibody (1:200 dilution; The Binding Site) at room temperature for 2 h. After washout of secondary antibody, fluorescent images were captured using a confocal laser scanning microscope (Zeiss LSM 510 Meta).
Electrophysiology.
K+ currents (IKv) were recorded from CATH.a neurons using a patch-clamp amplifier (model PC-505B, Warner Instrument, Hamden, CT) and the whole cell configuration of the patch-clamp technique. The patch pipette had resistances of 4–6 MΩ when filled with (in mM) 130 KCl, 2 MgCl2, 0.25 CaCl2, 5 EGTA, 1 Mg-ATP, 0.1 Tris-GTP, 10 HEPES, and 8 glucose, pH 7.2. The extracellular solution consisted of (in mM) 137 NaCl, 5.4 KCl, 1.35 CaCl2, 2 MgCl2, 0.3 NaH2PO4, 10 HEPES, and 10 sucrose, pH 7.4. Na+ and Ca2+ channels were blocked by TTX (0.5 μM) and CdCl2 (0.3 mM), respectively. Current traces were sampled at 10 kHz and filtered at 5 kHz. Holding potential was −80 mV. Current-voltage relations were elicited by test potentials of −80 to +80 mV in 10-mV increments over 400-ms duration (5 s between steps). Following this protocol, we were able to measure peak K+ current (Ipeak), which includes a combination of the transient outward K+ current and the rising phase of the steady-state current (Iss) or delayed rectifier current. Since the contribution of transient outward K+ current is negligible at the end of a 400-ms pulse, current at that point (Iss) was measured to assess delayed rectifier current. Current density of Ipeak and Iss was calculated by dividing the respective current by membrane capacitance (Cm). Cm was determined by integrating the capacity current evoked by a voltage step of 5 mV and dividing the resulting charge by the voltage step. Cm for the CATH.a neurons used in this study ranged from 15 to 35 pF and was not significantly different between experimental groups.
The data points of current density were plotted against the corresponding test potential. pClamp 8.1 (Axon Instruments) was used for data acquisition and analysis. Recordings were performed at 22°C. The effect of acute ANG II (100 nM) on current density was tested by superfusing neurons with the peptide for 5 min and repeating the voltage pulse regimen. ANG II inhibited IKv in 87% of control CATH.a neurons tested in this study; therefore, to confirm that a given neuron was responsive to ANG II, each cell was treated with ANG II (100 nM), which was washed out before superfusion with experimental agents (see below). If ANG II did not inhibit IKv, that cell was excluded from further analysis. To determine the contribution of AT1 vs. AT2 receptors, a subset of neurons were superfused with the AT1 receptor antagonist losartan (1 μM) or the AT2 receptor antagonist PD-123319 (1 μM) before and during ANG II superfusion. To investigate the involvement of extracellular vs. intracellular ROS, additional groups of neurons were superfused with extracellular SOD (ecSOD) protein (400 U/ml) or the cell-permeable antioxidant tempol (1 mM). Notably, for these experiments, the same neuron was subjected to the patch-clamp protocol after superfusion with vehicle, ecSOD, tempol, ecSOD + ANG II, or tempol + ANG II. Finally, subsets of neurons were infected with AdMnSOD or AdEmpty for specific examination of the role of mitochondria-produced O2·−.
Statistical analysis.
Values are means ± SE. Data were analyzed by Student's t-test for two-group comparisons or by ANOVA followed by Newman-Keuls correction for multiple comparisons. Differences were considered significant at P < 0.05.
RESULTS
ANG II increases mitochondria-localized O2·− levels in CATH.a neurons.
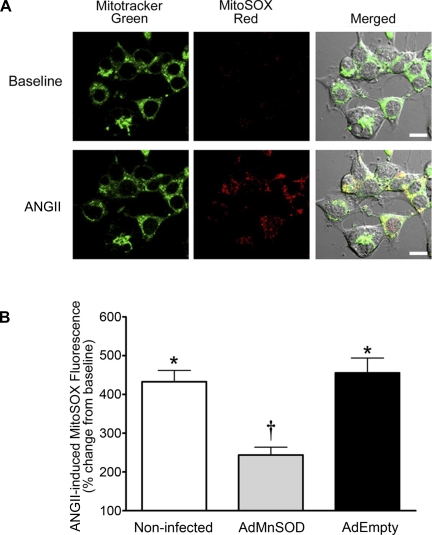
Adenovirus-mediated overexpression of Mn-SOD in SFO (38) or RVLM (20) neurons attenuates the pressor response of ANG II administered into these cardiovascular control brain regions. Considering that Mn-SOD is efficiently targeted to mitochondria and is the SOD isoform that specifically scavenges mitochondria-localized O2·−, we speculated that ANG II increases mitochondria-produced O2·− in central neurons. Using CATH.a neurons, a central catecholaminergic neuronal cell line, and the mitochondria-targeted O2·−-sensitive fluorogenic probe MitoSOX Red, we observed that acute ANG II stimulation significantly increases O2·− levels in neuron mitochondria. As demonstrated in the representative confocal microcopy images and summary data (Fig. 1; n = 58 neurons on 5 coverslips), ANG II significantly increased MitoSOX Red fluorescence (P < 0.05 vs. baseline) in noninfected control CATH.a neurons, thus indicating an increase in mitochondria-produced O2·−. Corroborating the fidelity of the assay and showing that MitoSOX Red reacts with mitochondria-localized O2·−, MitoSOX Red fluorescence colocalized with MitoTracker Green, yielding yellow fluorescence (merged images in Fig. 1A). Additionally, the ANG II-induced increase in MitoSOX Red fluorescence was markedly attenuated in neurons infected with AdMnSOD (Fig. 1B; n = 49 neurons on 5 coverslips, P < 0.05 vs. control neurons), whereas AdEmpty-treated neurons (n = 44 neurons on 4 coverslips) exhibited a significant increase in MitoSOX Red fluorescence similar to that of noninfected neurons following ANG II stimulation (Fig. 1B). These data clearly show that ANG II increases mitochondria-produced O2·− in neurons and that overexpression of Mn-SOD significantly reduces these elevated levels of O2·−.
Fig. 1.
ANG II increases mitochondria-localized O2·− levels. A: representative confocal microscopy images showing fluorescence of MitoTracker Green and MitoSOX Red in CATH.a neurons before (baseline) and after ANG II (100 nM) stimulation. Colocalization of MitoTracker Green and MitoSOX Red (yellow fluorescence) in neurons is shown in merged image. Scale bars, 10 μm. B: summary data of ANG II-induced MitoSOX Red fluorescence intensity in control (noninfected, n = 58 neurons on 5 coverslips), AdMnSOD-infected (n = 49 neurons on 5 coverslips), and AdEmpty-infected (n = 44 neurons on 4 coverslips) CATH.a neurons. Post-ANG II MitoSOX Red fluorescence in each neuron was divided by the baseline (pre-ANG II) fluorescence in that particular cell to obtain ANG II-induced percent change from baseline (y-axis). *P < 0.05 vs. baseline (pre-ANG II). †P < 0.05 vs. noninfected and AdEmpty.
AdMnSOD infection increases Mn-SOD expression and activity in mitochondria.
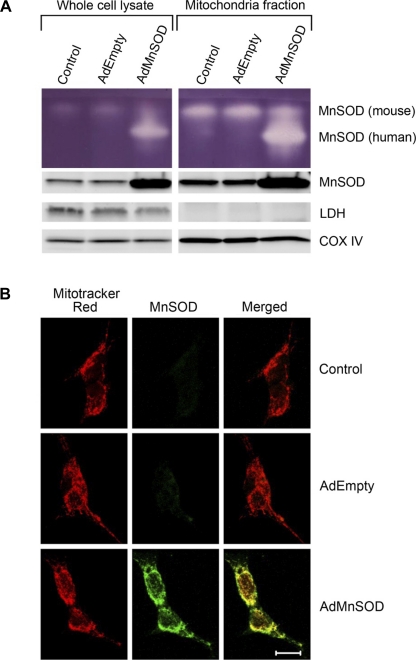
To confirm adenovirus-mediated overexpression of active Mn-SOD in mitochondria, we measured Mn-SOD activity and protein levels in whole cell lysates and isolated mitochondria from control, AdEmpty-treated, and AdMnSOD-infected CATH.a neurons. Mn-SOD activity (Fig. 2A, top) and protein levels (Fig. 2A, bottom) were markedly increased in whole cell lysates and mitochondria-enriched fractions 4 days after infection of CATH.a neurons with AdMnSOD compared with neurons infected with control virus AdEmpty and noninfected control cells. The enhanced endogenous (mouse) and adenovirus-encoded (human) Mn-SOD activity in the mitochondrial fraction compared with whole cell lysates is consistent with obtaining enriched fractions of mitochondria. Additionally, the lack of lactate dehydrogenase (LDH, a cytosolic marker) expression and the increased expression of cytochrome c oxidase subunit IV (oxIV, a mitochondria marker) in mitochondria preparations compared with whole cell lysates further demonstrate that pure mitochondria were isolated.
Fig. 2.
Adenovirus-mediated overexpression of active Mn-SOD in mitochondria. A: representative in-gel SOD activity assay (top) and Western blot (bottom) showing Mn-SOD activity and protein expression, respectively, in whole cell lysates and mitochondrial fractions from noninfected (control), AdEmpty-infected, and AdMnSOD-infected CATH.a neurons. LDH, lactate dehydrogenase; Cox IV, cytochrome c oxidase subunit IV. B: representative confocal microscopy images showing MitoTracker Red fluorescence, human Mn-SOD immunoreactivity (green fluorescence), and colocalization of Mn-SOD in mitochondria (yellow fluorescence) from noninfected control, AdEmpty-infected, and AdMnSOD-infected CATH.a neurons. Scale bar, 10 μm.
To provide additional evidence that AdMnSOD infection increases Mn-SOD expression in mitochondria, we used confocal microscopy to examine the colocalization of Mn-SOD and MitoTracker Red, a fluorescent mitochondria marker, in control, AdEmpty-treated, and AdMnSOD-infected cells. Representative fluorescent images (Fig. 2B) illustrate colocalization (yellow fluorescence in merged image) of Mn-SOD and MitoTracker Red in AdMnSOD-treated cells. We utilized an Mn-SOD primary antibody directed against human Mn-SOD to specifically detect adenovirus-encoded Mn-SOD expression; thus control and AdEmpty-infected CATH.a neurons did not exhibit Mn-SOD immunoreactivity (Fig. 2B). In contrast, we utilized an Mn-SOD antibody that recognizes human and mouse Mn-SOD for the Western blot analysis (Fig. 2A).
ANG II inhibits K+ current in CATH.a neurons via AT1 receptor activation.
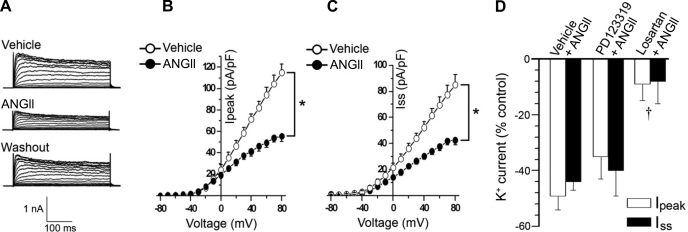
To examine the biological significance of the ANG II-induced increase in neuron mitochondria O2·− levels and to begin investigating the mechanism(s) by which Mn-SOD overexpression inhibits the central ANG II-induced pressor response (20, 38), we sought to measure ANG II-dependent changes in the electrophysiological properties, particularly IKv, of neurons overexpressing Mn-SOD. We initiated this series of experiments by first recording acute ANG II-induced changes in IKv in control CATH.a neurons. Compared with vehicle-treated CATH.a neurons, ANG II inhibited IKv, which was restored after washout of ANG II (Fig. 3A). In addition to inhibiting the delayed K+ current (Iss) by 45 ± 4% (Fig. 3C; P < 0.05 vs. vehicle), ANG II also significantly attenuated the transient current (Ipeak) by 49 ± 7% compared with neurons superfused with vehicle (Fig. 3B; P < 0.05 vs. vehicle, n = 14 neurons). These results are similar to those reported previously by others (7, 28, 29, 35). To determine the contribution of AT1 vs. AT2 receptors in mediating the ANG II-induced inhibition of IKv, a subset of neurons were pretreated with losartan (n = 6) or PD-123319 (n = 8), respectively. Summary data presented in Fig. 3D show that losartan significantly attenuated ANG II-induced inhibition of Ipeak and Iss, whereas ANG II induced a similar inhibition of IKv in PD-123319-treated neurons compared with vehicle-treated cells. Together, these results confirm previous data indicating that acute ANG II signaling results in inhibition of neuronal IKv through an AT1 receptor-dependent mechanism.
Fig. 3.
ANG II inhibits K+ current in CATH.a neurons. A: representative traces of evoked K+ currents in CATH.a neurons superfused with vehicle or ANG II (100 nM) and after washout of ANG II. Voltage-gated K+ current (IKv) was evoked by depolarizing voltage pulses from −80 to +80 mV in 10-mV increments over 400 ms. B and C: summary data of peak (Ipeak) and steady-state (Iss) current density-voltage relationships, in CATH.a neurons (n = 14) superfused with vehicle or ANG II. D: summary data showing ANG II-induced inhibition of Ipeak and Iss in CATH.a neurons pretreated with vehicle (n = 14), PD-123319 (1 μM, n = 8), and losartan (1 μM, n = 6). *P < 0.05 vehicle vs. ANG II. †P < 0.05 vs. vehicle and PD-123319.
Intracellular, but not extracellular, O2·− mediates ANG II-induced inhibition of IKv.
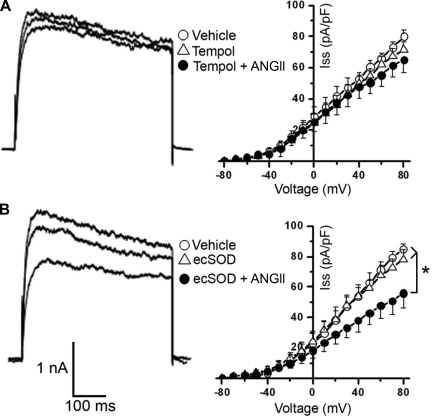
Next, we sought to determine whether intracellular and/or extracellular O2·− mediates the ANG II inhibition of IKv. ANG II stimulation of CATH.a neurons pretreated with tempol, a cell-permeable SOD mimetic commonly used to scavenge O2·−, failed to provoke the characteristic IKv inhibition, as demonstrated in the representative IKv traces (Fig. 4A, left) and the summary data showing the current density-voltage relationship for Iss (Fig. 4A, right; n = 10 neurons). However, ANG II did significantly reduce Iss in CATH.a neurons preincubated with ecSOD protein (n = 10 neurons, P < 0.05 for vehicle vs. ecSOD + ANG II; Fig. 4B). The ANG II-induced inhibition of Ipeak (49 ± 7%) was also significantly attenuated by tempol (13 ± 4%), but not by ecSOD (33 ± 7%). Superfusion of tempol or ecSOD alone did not significantly alter IKv in CATH.a neurons (Fig. 4). Together, these data indicate that intracellular, but not extracellular, O2·− is involved in the ANG II intraneuronal signaling pathway leading to IKv inhibition.
Fig. 4.
Intracellular O2·− mediates ANG II-induced inhibition of K+ current. A: representative traces of IKv induced by 1 episodic voltage step (−80 to +80 mV) during superfusion of vehicle, cell-permeable SOD mimetic tempol (1 mM), or tempol + ANG II (100 nM) and summary data (n = 10 neurons) of Iss current-voltage relationships. B: representative traces of IKv induced by 1 episodic voltage step (−80 to +80 mV) during superfusion of vehicle, extracellular SOD (ecSOD) protein (400 U/ml), or ecSOD + ANG II and summary data (n = 10 neurons) of Iss current-voltage relationships. *P < 0.05 vehicle and ecSOD alone vs. ecSOD + ANG II.
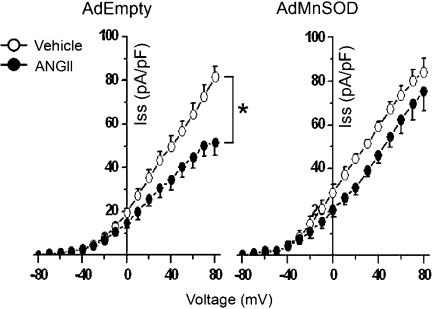
Mn-SOD overexpression attenuates ANG II-induced inhibition of IKv.
Although tempol is commonly used as an SOD mimetic, as it can scavenge O2·−, it can work as an antioxidant through an array of diverse mechanisms. For example, tempol can promote heme-mediated catalase-like activity, terminate radical chain reactions, and trap carbon-centered radicals (21). Because of these nonspecific actions of tempol and to directly investigate the role of mitochondria-produced O2·−, we utilized adenovirus-mediated gene transfer of Mn-SOD and examined ANG II-induced changes in IKv. Overexpression of Mn-SOD virtually abolished ANG II-induced inhibition of IKv (11 ± 2% inhibition of Ipeak and 9 ± 3% inhibition of Iss, n = 7 neurons) compared with control neurons infected with AdEmpty (34 ± 5% inhibition of Ipeak and 36 ± 4% inhibition of Iss, P < 0.05 vs. AdMnSOD-infected neurons, n = 8 neurons; Fig. 5). Taken together with data presented in Figs. 1–4, these results indicate that, through an AT1 receptor-dependent mechanism, ANG II-induced inhibition of IKv in CATH.a neurons is mediated by an increase in mitochondria-localized O2·− levels.
Fig. 5.
Mn-SOD overexpression attenuates ANG II-induced inhibition of K+ current: summary data of Iss current-voltage relationships from CATH.a neurons (n = 7–8 neurons/group) infected with 50 multiplicity of infection of AdEmpty or AdMnSOD during superfusion of vehicle or ANG II (100 nM). *P < 0.05 vehicle vs. ANG II.
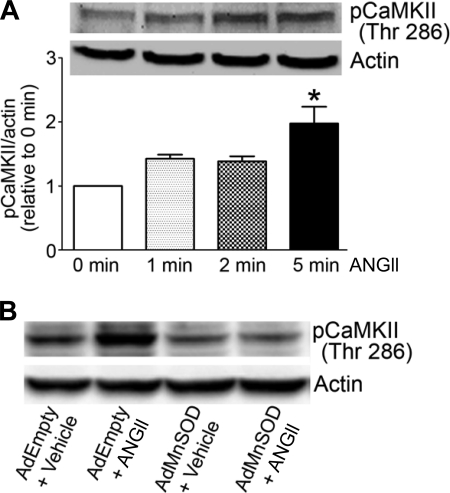
Mn-SOD overexpression inhibits ANG II-induced activation of CaMKII.
To begin exploring a possible mechanism by which mitochondria-produced O2·− mediates ANG II-induced inhibition of IKv in neurons, we tested the hypothesis that Mn-SOD overexpression modulates ANG II-induced activation of CaMKII. CaMKII is a redox-sensitive protein (14) known to be involved in mediating ANG II-induced neuronal firing and inhibition of IKv in primary and CATH.a neurons (28, 30). In control CATH.a neurons, ANG II rapidly increases the phosphorylation of CaMKII with a significant increase observed after 5 min of ANG II stimulation (Fig. 6A; P < 0.05 vs. 0 min ANG II). However, ANG II-induced phosphorylation of CaMKII was attenuated in AdMnSOD-infected neurons compared with control, AdEmpty-infected neurons (Fig. 6B), thus suggesting that mitochondrial O2·− is involved in the ANG II signaling pathway leading to CaMKII activation. Together with previous reports clearly demonstrating that CaMKII activation is required for the ANG II-induced inhibition of IKv and neuronal activation (28, 30, 35), these data provide evidence for a mechanism by which mitochondria-produced O2·− mediates these acute ANG II-induced electrophysiological responses.
Fig. 6.
Overexpression of Mn-SOD inhibits ANG II-induced activation of Ca2+/calmodulin kinase II (CaMKII). A: representative Western blot analysis showing levels of phosphorylated (Thr286) CaMKII (pCaMKII) in CATH.a neurons without ANG II and after ANG II (100 nM) stimulation for 1, 2, and 5 min. Actin was used as a protein loading control. Levels of pCaMKII were quantified using densitometric analysis, normalized to actin, and expressed relative to basal (0 min ANG II) levels (n = 5 separate experiments). *P < 0.05 vs. 0 min ANG II. B: representative Western blot analysis showing levels of pCaMKII in AdEmpty- and AdMnSOD-infected neurons stimulated with vehicle or ANG II (100 nM).
DISCUSSION
The present study provides direct evidence that ANG II increases mitochondria-produced O2·− in neurons, which in turn, mediates acute ANG II-dependent intraneuronal signaling. More specifically, we have demonstrated that increased scavenging of mitochondria-produced O2·− by overexpression of Mn-SOD, the mitochondria-localized isoform of SOD, significantly attenuates ANG II-induced CaMKII activation and inhibition of IKv in CATH.a neurons. These data provide new insight into the contribution of mitochondria-produced O2·− in the intraneuronal signaling pathway of ANG II.
Over the past decade, numerous studies have demonstrated that dysregulation of brain angiotensinergic signaling and increased levels of ROS in central neurons are involved in the pathogenesis and sequelae of hypertension and chronic heart failure. In particular, increased sympathoexcitation, which mediates, at least in part, progression of these cardiovascular disorders, has been linked to elevated ROS in the CNS (3, 10, 16, 17, 19, 26, 34). Many of these previous studies used adenovirus expressing SOD, PEGylated-SOD, and/or SOD mimetics to increase SOD levels in specific ANG II-sensitive, cardiovascular-regulating nuclei in the brain and, in doing so, specifically identified O2·− as a critical ROS in brain angiotensinergic signaling (3, 12, 16, 17, 26, 34). Additional studies utilizing various inhibitors of NADPH oxidase convincingly demonstrated that NADPH oxidase is an important source of ANG II-induced O2·− production in the CNS (19, 33, 37). However, until most recently, additional sources of O2·−, including mitochondria, have received little attention.
Home to the electron transport chain where 1–4% of molecular oxygen consumed is incompletely reduced to O2·−, mitochondria are a primary intracellular source for O2·− in most cells (31). Zimmerman et al. (38) first suggested a role for mitochondria-produced O2·− in mediating brain angiotensinergic signaling by demonstrating that Mn-SOD overexpression in mouse brain, particularly the SFO, markedly attenuates central ANG II-induced pressor, bradycardic, and dipsogenic responses. More recently, Nozoe and colleagues (20) reported that Mn-SOD overexpression in the RVLM via adenovirus-mediated gene transfer significantly inhibits the pressor response induced by RVLM-administered ANG II. Mn-SOD expression and activity are decreased in the RVLM in SHR compared with normotensive WKY rats, and overexpression of the mitochondria-targeted SOD significantly reduces arterial pressure in SHR (4). Similarly, in rabbits with pacing-induced heart failure, Mn-SOD expression is significantly reduced in the RVLM (12). Together, these studies suggest that elevated levels of mitochondria-localized O2·− contribute to sympathetic neuronal activation in cardiovascular diseases associated with increased ANG II signaling in the brain. Our data presented here provide evidence for an intraneuronal signaling mechanism by which acute ANG II stimulation increases mitochondria-produced O2·−, which ultimately contributes to neuronal activation.
To directly test the hypothesis that ANG II intraneuronal signaling involves elevated levels of mitochondria-produced O2·−, we took advantage of the fact that Mn-SOD is the SOD isoform known to exclusively reside in mitochondria. Endogenous Mn-SOD is a nuclear encoded gene, but with a mitochondrial targeting sequence in the NH2 terminus of the polypeptide, it is efficiently and specifically targeted to mitochondria. In our study, we used adenoviral-mediated gene transfer to increase Mn-SOD protein and activity in CATH.a neuron mitochondria. With such a huge increase in Mn-SOD expression (Fig. 2), it should be considered that some of the adenoviral-expressed Mn-SOD protein may not enter the mitochondria. Although this is a potential limitation of the present study, previous reports clearly show that Mn-SOD protein is loaded with manganese, to make it active, only as the protein is unfolded and transported into mitochondria (6, 18). Therefore, endogenous or adenoviral-expressed Mn-SOD in the cytoplasm is inactive. As such, our data showing that adenovirus-mediated overexpression of Mn-SOD virtually abolishes ANG II-dependent neuronal responses and that ANG II directly increases levels of O2·− in mitochondria indicate that mitochondria-produced O2·− is involved in ANG II intraneuronal signaling.
Raizada and Sumners and colleagues clearly identified CaMKII and PKC as signaling proteins that mediate IKv inhibition and neuronal activation in isolated primary neurons and CATH.a neurons after ANG II stimulation (22, 28, 30, 35). In the present study, we focused on CaMKII because of recent reports indicating that activation of CaMKII is redox sensitive. In particular, Erickson et al. (8) reported that, in cardiomyocytes, CaMKII is activated by direct ANG II-induced oxidation. In addition, Hongpaisan et al. (13) reported that, in neurons, mitochondria-produced O2·− increases CaMKII activation by inhibiting protein phosphatases. Our results showing that Mn-SOD overexpression inhibits ANG II-induced phosphorylation of CaMKII in CATH.a neurons corroborate these previous findings. Taken together, these studies provide evidence that mitochondria-produced O2·− activates CaMKII, which, as previously reported (28, 30, 35), plays a role in ANG II-induced inhibition of neuronal IKv.
In addition to activating CaMKII in neurons, our results show that mitochondria-produced O2·− inhibits IKv following ANG II stimulation. Previous studies using the cell-permeable SOD mimetic tempol or overexpression of the primarily cytoplasm-localized SOD, Cu/Zn-SOD, or NADPH oxidase inhibitors have demonstrated that intracellular O2·− contributes to ANG II-induced neuronal activation by modulating K+ and Ca2+ currents across the plasma membrane of neurons (29, 33, 40). In the present study, we confirmed that ANG II-induced inhibition of IKv is dependent on intracellular O2·−, as tempol attenuated this response, whereas ecSOD protein did not. We also extended these findings by investigating the specific role of mitochondria-produced O2·− in modulating IKv. We found that overexpressing Mn-SOD virtually abolished the ANG II-induced inhibition of IKv, indicating that O2·− produced in mitochondria mediates this acute ANG II response.
Although we clearly demonstrate that ANG II increases mitochondria-localized O2·− and that increased scavenging of mitochondria-produced O2·− via Mn-SOD overexpression inhibits ANG II signaling in neurons, the mechanism(s) by which ANG II increases O2·− in mitochondria remains to be fully elucidated. Recently, Chan and colleagues (5) proposed a feedforward ROS-induced ROS mechanism in RVLM neurons whereby NADPH oxidase-derived ROS damage mitochondrial electron transport chain complexes, which, in turn, results in an increase in mitochondria-produced ROS. Supporting this hypothesis, they report that NADPH oxidase inhibition via p22phox antisense attenuates an ANG II-induced increase in mitochondria-produced ROS. In addition, restoring electron transport capacity in the RVLM by direct administration of coenzyme Q10, a mitochondria electron transporter and antioxidant, significantly attenuated mean arterial pressure and sympathetic tone in rats centrally infused with ANG II (5). Together, these studies suggest a signaling mechanism and identify a source (i.e., damaged electron transport chain complexes) for the elevated levels of mitochondria-localized O2·− in ANG II-stimulated neurons, as we report here.
Because the present data were obtained from the catecholaminergic CATH.a neuronal cell line, additional studies using an in vivo model of ANG II-dependent neurogenic hypertension are needed to confirm that, in central neurons, mitochondria-produced O2·− is involved in the signaling pathway of ANG II. Nevertheless, previous reports (28, 30, 35) clearly demonstrate that the known ANG II intraneuronal signaling mechanisms in primary neurons isolated from the brain are mimicked in CATH.a neurons. In particular, Sumners, Raizada, and colleagues showed that, through an AT1 receptor-dependent mechanism, ANG II inhibits IKv similarly in CATH.a neurons and primary neurons isolated from the hypothalamus and brain stem (28, 30, 35). In addition, they demonstrated that CaMKII inhibition attenuates ANG II-induced reduction of IKv in CATH.a and primary neurons (28, 30, 35). Together, these previous studies convincingly identified CATH.a neurons as a useful neuronal cell model to study ANG II intraneuronal signaling. Nevertheless, as with all cell culture-based studies, future studies should include in vivo experiments designed to explore the role of mitochondria-produced O2·− in brain angiotensinergic signaling.
In summary, dysregulation of brain ANG II signaling is involved in the pathogenesis of cardiovascular diseases associated with sympathoexcitation, including heart failure and hypertension. To better understand the central actions of ANG II in mediating neuronal activation and sympathoexcitation, it is essential to precisely identify the intraneuronal signaling mechanisms of ANG II. In ANG II-stimulated neurons, NADPH oxidase has been identified as a source of O2·−, a signaling intermediate involved in controlling membrane ion currents and neuronal activation. Our present study also identifies mitochondria as a critical source of the O2·− in response to ANG II. In support of this conclusion, overexpression of the mitochondria-targeted O2·−-scavenging enzyme Mn-SOD attenuates ANG II-induced activation of CaMKII, and inhibition of IKv in neurons. We propose that new antioxidant-based therapies for cardiovascular diseases associated with ANG II-mediated sympathoexcitation may need to be developed to target mitochondria.
GRANTS
This study was supported by National Institutes of Health Grants P20 RR-017675 (M. C. Zimmerman) and P01 HL-062222 (H. D. Sshultz) and by American Heart Association Scientist Development Grant 0930204N (M. C. Zimmerman).
DISCLOSURES
No conflicts of interest are declared by the author(s).
ACKNOWLEDGMENTS
We acknowledge the expert technical assistance of Jocelyn Jones. We thank Janice A. Taylor and James R. Talaska (Confocal Laser Scanning Microscope Core Facility at the University of Nebraska Medical Center) for assistance with confocal microscopy and the Nebraska Research Initiative and the Eppley Cancer Center for support of the Core Facility.
REFERENCES
- 1. Bains JS, Potyok A, Ferguson AV. Angiotensin II actions in paraventricular nucleus: functional evidence for neurotransmitter role in efferents originating in subfornical organ. Brain Res 599: 223–229, 1992 [DOI] [PubMed] [Google Scholar]
- 2. Beauchamp C, Fridovich I. Superoxide dismutase: improved assays and an assay applicable to acrylamide gels. Anal Biochem 44: 276–287, 1971 [DOI] [PubMed] [Google Scholar]
- 3. Campese VM, Shaohua Y, Huiquin Z. Oxidative stress mediates angiotensin II-dependent stimulation of sympathetic nerve activity. Hypertension 46: 533–539, 2005 [DOI] [PubMed] [Google Scholar]
- 4. Chan SH, Tai MH, Li CY, Chan JY. Reduction in molecular synthesis or enzyme activity of superoxide dismutases and catalase contributes to oxidative stress and neurogenic hypertension in spontaneously hypertensive rats. Free Radic Biol Med 40: 2028–2039, 2006 [DOI] [PubMed] [Google Scholar]
- 5. Chan SHH, Wu KLH, Chang AYW, Tai MH, Chan JYH. Oxidative impairment of mitochondrial electron transport chain complexes in RVLM contributes to neurogenic hypertension. Hypertension 53: 217–222, 2009 [DOI] [PubMed] [Google Scholar]
- 6. Culotta VC, Yang M, O'Halloran TV. Activation of superoxide dismutases: putting the metal to the pedal. Biochim Biophys Acta 1763: 747–758, 2006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Du JQ, Sun CW, Tang JS. Effect of angiotensin II type 1 receptor on delayed rectifier potassium current in catecholaminergic CATH.a cells. Acta Pharmacol Sin 25: 1145–1150, 2004 [PubMed] [Google Scholar]
- 8. Erickson JR, Joiner ML, Guan X, Kutschke W, Yang J, Oddis CV, Bartlett RK, Lowe JS, O'Donnell SE, Aykin-Burns N, Zimmerman MC, Zimmerman K, Ham AJ, Weiss RM, Spitz DR, Shea MA, Colbran RJ, Mohler PJ, Anderson ME. A dynamic pathway for calcium-independent activation of CaMKII by methionine oxidation. Cell 133: 462–474, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Ferguson AV, Bains JS. Actions of angiotensin in the subfornical organ and area postrema: implications for long term control of autonomic output. Clin Exp Pharmacol Physiol 24: 96–101, 1997 [DOI] [PubMed] [Google Scholar]
- 10. Gao L, Wang W, Li YL, Schultz HD, Liu D, Cornish KG, Zucker IH. Superoxide mediates sympathoexcitation in heart failure: roles of angiotensin II and NAD(P)H oxidase. Circ Res 95: 937–944, 2004 [DOI] [PubMed] [Google Scholar]
- 11. Gao L, Wang W, Li YL, Schultz HD, Liu D, Cornish KG, Zucker IH. Sympathoexcitation by central ANG II: roles for AT1 receptor upregulation and NAD(P)H oxidase in RVLM. Am J Physiol Heart Circ Physiol 288: H2271–H2279, 2005 [DOI] [PubMed] [Google Scholar]
- 12. Gao L, Wang W, Liu D, Zucker IH. Exercise training normalizes sympathetic outflow by central antioxidant mechanisms in rabbits with pacing-induced chronic heart failure. Circulation 115: 3095–3102, 2007 [DOI] [PubMed] [Google Scholar]
- 13. Hongpaisan J, Winters CA, Andrews SB. Strong calcium entry activates mitochondrial superoxide generation, upregulating kinase signaling in hippocampal neurons. J Neurosci 24: 10878–10887, 2004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Howe CJ, Lahair MM, McCubrey JA, Franklin RA. Redox regulation of the calcium/calmodulin-dependent protein kinases. J Biol Chem 279: 44573–44581, 2004 [DOI] [PubMed] [Google Scholar]
- 15. Infanger DW, Sharma RV, Davisson RL. NADPH oxidases of the brain: distribution, regulation, and function. Antioxid Redox Signal 8: 1583–1596, 2006 [DOI] [PubMed] [Google Scholar]
- 16. Lindley TE, Doobay MF, Sharma RV, Davisson RL. Superoxide is involved in the central nervous system activation and sympathoexcitation of myocardial infarction-induced heart failure. Circ Res 94: 402–409, 2004 [DOI] [PubMed] [Google Scholar]
- 17. Lu N, Helwig BG, Fels RJ, Parimi S, Kenney MJ. Central Tempol alters basal sympathetic nerve discharge and attenuates sympathetic excitation to central ANG II. Am J Physiol Heart Circ Physiol 287: H2626–H2633, 2004 [DOI] [PubMed] [Google Scholar]
- 18. Luk E, Yang M, Jensen LT, Bourbonnais Y, Culotta VC. Manganese activation of superoxide dismutase 2 in the mitochondria of Saccharomyces cerevisiae. J Biol Chem 280: 22715–22720, 2005 [DOI] [PubMed] [Google Scholar]
- 19. Nagae A, Fujita M, Kawarazaki H, Matsui H, Ando K, Fujita T. Sympathoexcitation by oxidative stress in the brain mediates arterial pressure elevation in obesity-induced hypertension. Circulation 119: 978–986, 2009 [DOI] [PubMed] [Google Scholar]
- 20. Nozoe M, Hirooka Y, Koga Y, Araki S, Konno S, Kishi T, Ide T, Sunagawa K. Mitochondria-derived reactive oxygen species mediate sympathoexcitation induced by angiotensin II in the rostral ventrolateral medulla. J Hypertens 26: 2176–2184, 2008 [DOI] [PubMed] [Google Scholar]
- 21. Offer T, Russo A, Samuni A. The pro-oxidative activity of SOD and nitroxide SOD mimics. FASEB J 14: 1215–1223, 2000 [DOI] [PubMed] [Google Scholar]
- 22. Pan SJ, Zhu M, Raizada MK, Sumners C, Gelband CH. ANG II-mediated inhibition of neuronal delayed rectifier K+ current: role of protein kinase C-α. Am J Physiol Cell Physiol 281: C17–C23, 2001 [DOI] [PubMed] [Google Scholar]
- 23. Rehncrona S, Mela L, Siesjo BK. Recovery of brain mitochondrial function in the rat after complete and incomplete cerebral ischemia. Stroke 10: 437–446, 1979 [DOI] [PubMed] [Google Scholar]
- 24. Robinson KM, Janes MS, Beckman JS. The selective detection of mitochondrial superoxide by live cell imaging. Nat Protoc 3: 941–947, 2008 [DOI] [PubMed] [Google Scholar]
- 25. Robinson KM, Janes MS, Pehar M, Monette JS, Ross MF, Hagen TM, Murphy MP, Beckman JS. Selective fluorescent imaging of superoxide in vivo using ethidium-based probes. Proc Natl Acad Sci USA 103: 15038–15043, 2006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Shokoji T, Nishiyama A, Fujisawa Y, Hitomi H, Kiyomoto H, Takahashi N, Kimura S, Kohno M, Abe Y. Renal sympathetic nerve responses to tempol in spontaneously hypertensive rats. Hypertension 41: 266–273, 2003 [DOI] [PubMed] [Google Scholar]
- 27. Sumners C, Raizada MK, Kang J, Lu D, Posner P. Receptor-mediated effects of angiotensin II on neurons. Front Neuroendocrinol 15: 203–230, 1994 [DOI] [PubMed] [Google Scholar]
- 28. Sun C, Du J, Raizada MK, Sumners C. Modulation of delayed rectifier potassium current by angiotensin II in CATH.a cells. Biochem Biophys Res Commun 310: 710–714, 2003 [DOI] [PubMed] [Google Scholar]
- 29. Sun C, Sellers KW, Sumners C, Raizada MK. NAD(P)H oxidase inhibition attenuates neuronal chronotropic actions of angiotensin II. Circ Res 96: 659–666, 2005 [DOI] [PubMed] [Google Scholar]
- 30. Sun C, Sumners C, Raizada MK. Chronotropic action of angiotensin II in neurons via protein kinase C and CaMKII. Hypertension 39: 562–566, 2002 [DOI] [PubMed] [Google Scholar]
- 31. Turrens JF. Mitochondrial formation of reactive oxygen species. J Physiol 552: 335–344, 2003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Veerasingham SJ, Raizada MK. Brain renin-angiotensin system dysfunction in hypertension: recent advances and perspectives. Br J Pharmacol 139: 191–202, 2003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Wang G, Anrather J, Glass MJ, Tarsitano MJ, Zhou P, Frys KA, Pickel VM, Iadecola C. Nox2, Ca2+, and protein kinase C play a role in angiotensin II-induced free radical production in nucleus tractus solitarius. Hypertension 48: 482–489, 2006 [DOI] [PubMed] [Google Scholar]
- 34. Wei SG, Zhang ZH, Yu Y, Felder RB. Systemically administered tempol reduces neuronal activity in paraventricular nucleus of hypothalamus and rostral ventrolateral medulla in rats. J Hypertens 27: 543–550, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Zhu M, Gelband CH, Posner P, Sumners C. Angiotensin II decreases neuronal delayed rectifier potassium current: role of calcium/calmodulin-dependent protein kinase II. J Neurophysiol 82: 1560–1568, 1999 [DOI] [PubMed] [Google Scholar]
- 36. Zimmerman MC, Davisson RL. Redox signaling in central neural regulation of cardiovascular function. Prog Biophys Mol Biol 84: 125–149, 2004 [DOI] [PubMed] [Google Scholar]
- 37. Zimmerman MC, Dunlay RP, Lazartigues E, Zhang Y, Sharma RV, Engelhardt JF, Davisson RL. Requirement for Rac1-dependent NADPH oxidase in the cardiovascular and dipsogenic actions of angiotensin II in the brain. Circ Res 95: 532–539, 2004 [DOI] [PubMed] [Google Scholar]
- 38. Zimmerman MC, Lazartigues E, Lang JA, Sinnayah P, Ahmad IM, Spitz DR, Davisson RL. Superoxide mediates the actions of angiotensin II in the central nervous system. Circ Res 91: 1038–1045, 2002 [DOI] [PubMed] [Google Scholar]
- 39. Zimmerman MC, Oberley LW, Flanagan SW. Mutant SOD1-induced neuronal toxicity is mediated by increased mitochondrial superoxide levels. J Neurochem 102: 609–618, 2007 [DOI] [PubMed] [Google Scholar]
- 40. Zimmerman MC, Sharma RV, Davisson RL. Superoxide mediates angiotensin II-induced influx of extracellular calcium in neural cells. Hypertension 45: 717–723, 2005 [DOI] [PubMed] [Google Scholar]
- 41. Zucker IH. Novel mechanisms of sympathetic regulation in chronic heart failure. Hypertension 48: 1005–1011, 2006 [DOI] [PubMed] [Google Scholar]