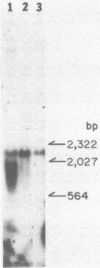
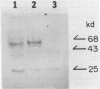
Abstract
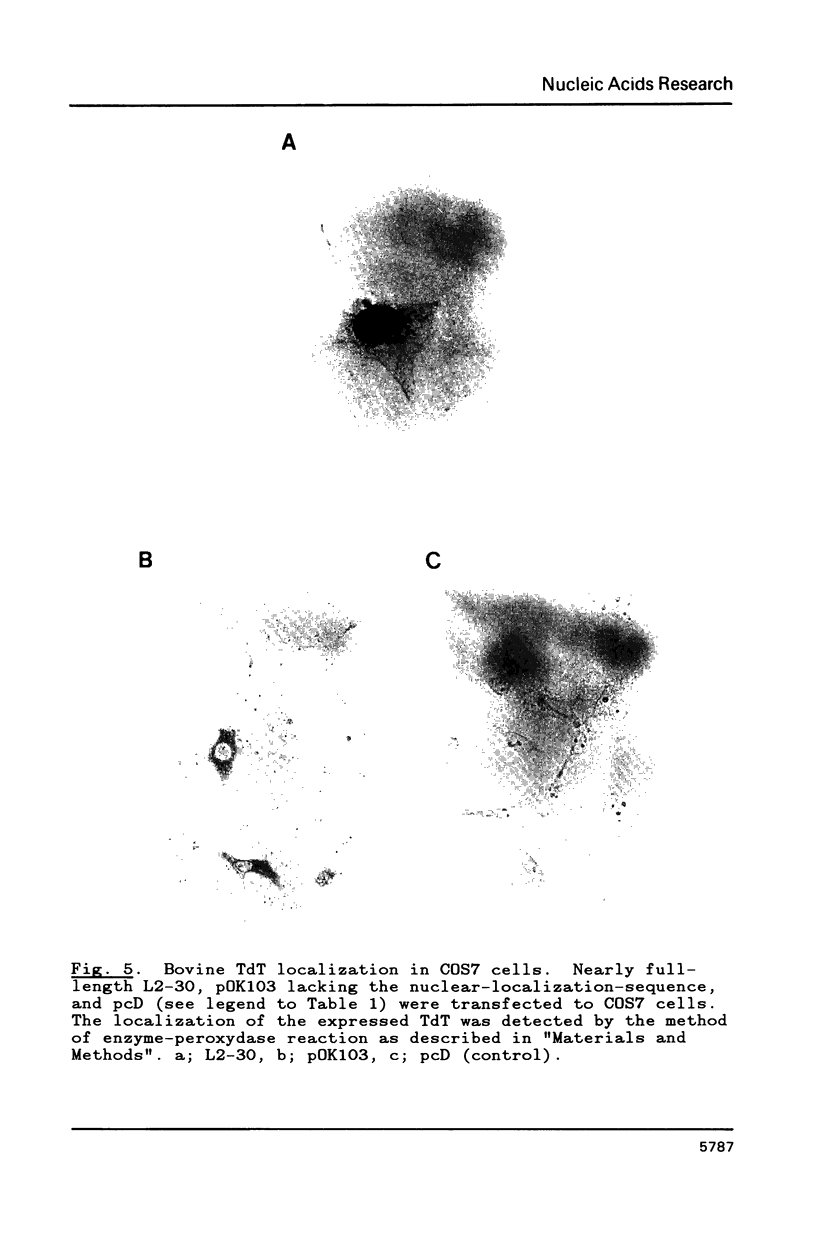
We have isolated nearly full-length cDNA clones of terminal deoxynucleotidyltransferase (TdT) from calf thymus and mouse lymphoma cDNA libraries. The libraries were constructed using the pcD vector system which permits the expression of cDNA inserts in mammalian cells. The bovine TdT cDNA clone contains an open reading frame coding for 520 amino acids, Mr 59,678. The mouse TdT cDNA clone contains an open reading frame of 1,587 bp, whose translated cDNA encodes a 60,004 dalton protein. The mouse TdT cDNA clone contains 60 bp in the 3' end region of the coding sequence not found in the bovine TdT cDNA sequence, otherwise, the clones share about 80% homology. A possible nuclear-localization-sequence (Pro-Arg-Lys-Lys-Arg-Pro-Arg) was conserved in the N-terminal region in the mouse and bovine cDNA clones. Bovine and mouse cDNAs transfected into COS7 monkey fibroblasts directed the synthesis of enzymatically active protein of Mr 60,000 which was detected immunologically using polyclonal rabbit antibody against bovine TdT. Bovine TdT expressed in COS7 cells by nearly full-length cDNA clone was localized in the nucleus and the translational product of pOK103 lacking the nuclear-localization-sequence was localized in the cytoplasm.
Full text
PDF














Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Alt F. W., Baltimore D. Joining of immunoglobulin heavy chain gene segments: implications from a chromosome with evidence of three D-JH fusions. Proc Natl Acad Sci U S A. 1982 Jul;79(13):4118–4122. doi: 10.1073/pnas.79.13.4118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beach C. M., Chan S. K., Vanaman T. C., Coleman M. S. Terminal deoxynucleotidyltransferase. Alignment of alpha- and beta-subunits of the core enzyme along the primary translation product. Biochem J. 1985 May 1;227(3):1003–1007. doi: 10.1042/bj2271003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chang L. M., Bollum F. J. Cyclic AMP-dependent phosphorylation of terminal deoxynucleotidyl transferase. J Biol Chem. 1982 Aug 25;257(16):9588–9592. [PubMed] [Google Scholar]
- Chang L. M., Bollum F. J. Deoxynucleotide-polymerizing enzymes of calf thymus gland. V. Homogeneous terminal deoxynucleotidyl transferase. J Biol Chem. 1971 Feb 25;246(4):909–916. [PubMed] [Google Scholar]
- Gluzman Y. SV40-transformed simian cells support the replication of early SV40 mutants. Cell. 1981 Jan;23(1):175–182. doi: 10.1016/0092-8674(81)90282-8. [DOI] [PubMed] [Google Scholar]
- Gregoire K. E., Goldschneider I., Barton R. W., Bollum F. J. Intracellular distribution of terminal deoxynucleotidyl transferase in rat bone marrow and thymus. Proc Natl Acad Sci U S A. 1977 Sep;74(9):3993–3996. doi: 10.1073/pnas.74.9.3993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ihle J. N., Rebar L., Keller J., Lee J. C., Hapel A. J. Interleukin 3: possible roles in the regulation of lymphocyte differentiation and growth. Immunol Rev. 1982;63:5–32. doi: 10.1111/j.1600-065x.1982.tb00409.x. [DOI] [PubMed] [Google Scholar]
- Kalderon D., Roberts B. L., Richardson W. D., Smith A. E. A short amino acid sequence able to specify nuclear location. Cell. 1984 Dec;39(3 Pt 2):499–509. doi: 10.1016/0092-8674(84)90457-4. [DOI] [PubMed] [Google Scholar]
- Kaneda T., Kuroda S., Hirota Y., Kato K. Highly sensitive solid-phase enzyme immunoassay for terminal deoxynucleotidyl transferase. Anal Biochem. 1982 Nov 1;126(2):327–334. doi: 10.1016/0003-2697(82)90523-1. [DOI] [PubMed] [Google Scholar]
- Laemmli U. K., Favre M. Maturation of the head of bacteriophage T4. I. DNA packaging events. J Mol Biol. 1973 Nov 15;80(4):575–599. doi: 10.1016/0022-2836(73)90198-8. [DOI] [PubMed] [Google Scholar]
- Landau N. R., St John T. P., Weissman I. L., Wolf S. C., Silverstone A. E., Baltimore D. Cloning of terminal transferase cDNA by antibody screening. Proc Natl Acad Sci U S A. 1984 Sep;81(18):5836–5840. doi: 10.1073/pnas.81.18.5836. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nakamura H., Tanabe K., Yoshida S., Morita T. Terminal deoxynucleotidyltransferase of 60,000 daltons from mouse, rat, and calf thymus. Purification by immunoadsorbent chromatography and comparison of peptide structures. J Biol Chem. 1981 Aug 25;256(16):8745–8751. [PubMed] [Google Scholar]
- Nakamura S., Nakayama N., Takahashi K., Kaziro Y. Primary structure of the polypeptide chain elongation factor Tu from E. coli. I. Amino acid sequence of fragment B. J Biochem. 1982 Mar;91(3):1047–1063. doi: 10.1093/oxfordjournals.jbchem.a133754. [DOI] [PubMed] [Google Scholar]
- Nakayama N., Arai N., Kaziro Y., Arai K. Structural and functional studies of the dnaB protein using limited proteolysis. Characterization of domains for DNA-dependent ATP hydrolysis and for protein association in the primosome. J Biol Chem. 1984 Jan 10;259(1):88–96. [PubMed] [Google Scholar]
- Okayama H., Berg P. A cDNA cloning vector that permits expression of cDNA inserts in mammalian cells. Mol Cell Biol. 1983 Feb;3(2):280–289. doi: 10.1128/mcb.3.2.280. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peterson R. C., Cheung L. C., Mattaliano R. J., Chang L. M., Bollum F. J. Molecular cloning of human terminal deoxynucleotidyltransferase. Proc Natl Acad Sci U S A. 1984 Jul;81(14):4363–4367. doi: 10.1073/pnas.81.14.4363. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peterson R. C., Cheung L. C., Mattaliano R. J., White S. T., Chang L. M., Bollum F. J. Expression of human terminal deoxynucleotidyl transferase in Escherichia coli. J Biol Chem. 1985 Sep 5;260(19):10495–10502. [PubMed] [Google Scholar]
- Sanger F., Nicklen S., Coulson A. R. DNA sequencing with chain-terminating inhibitors. Proc Natl Acad Sci U S A. 1977 Dec;74(12):5463–5467. doi: 10.1073/pnas.74.12.5463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sasaki R., Bollum F. J., Goldschneider I. Transient populations of terminal transferase positive (TdT+) cells in juvenile rats and mice. J Immunol. 1980 Dec;125(6):2501–2503. [PubMed] [Google Scholar]
- Towbin H., Staehelin T., Gordon J. Electrophoretic transfer of proteins from polyacrylamide gels to nitrocellulose sheets: procedure and some applications. Proc Natl Acad Sci U S A. 1979 Sep;76(9):4350–4354. doi: 10.1073/pnas.76.9.4350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wettenhall R. E., Cohen P. Isolation and characterisation of cyclic AMP-dependent phosphorylation sites from rat liver ribosomal protein S6. FEBS Lett. 1982 Apr 19;140(2):263–269. doi: 10.1016/0014-5793(82)80910-1. [DOI] [PubMed] [Google Scholar]
- Yokota T., Lee F., Rennick D., Hall C., Arai N., Mosmann T., Nabel G., Cantor H., Arai K. Isolation and characterization of a mouse cDNA clone that expresses mast-cell growth-factor activity in monkey cells. Proc Natl Acad Sci U S A. 1984 Feb;81(4):1070–1074. doi: 10.1073/pnas.81.4.1070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van Eerd J. P., Takahshi K. Determination of the complete amino acid sequence of bovine cardiac troponin C. Biochemistry. 1976 Mar 9;15(5):1171–1180. doi: 10.1021/bi00650a033. [DOI] [PubMed] [Google Scholar]