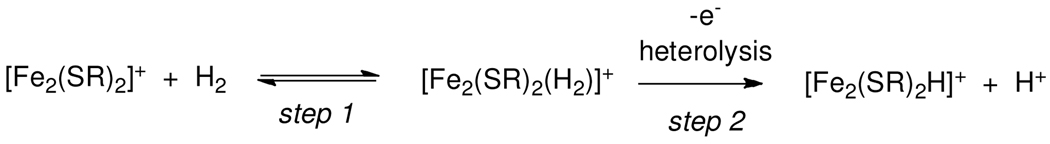Abstract
Mild oxidants such as [Fe(C5Me5)2]+ accelerate the activation of H2 by [Fe2[(SCH2)2NBn](CO)3(dppv)(PMe3)]+ ([1]+). The reaction is first order in [1]+ and [H2] but is independent of the E1/2 and concentration of the oxidant. The analogous reaction occurs with D2 and proceeds with an inverse isotope effect of 0.75(8). The activation of H2 is further enhanced with the tetracarbonyl [Fe2[(SCH2)2NBn](CO)4(dppn)]+ ([2]+), the first crystallographically characterized Hox model containing an amine cofactor. These studies point to rate-determining binding of H2 followed by proton-coupled electron-transfer (PCET). In comparison with [1]+, the rate of H2 activation by [2]+/Fc+ is enhanced by 104 (25 °C).
The hydrogenases (H2ases) are attractive targets for synthetic modeling because they catalyze the redox of H2/H+, an important and topical reaction.1 The [FeFe]- (and [NiFe]-) hydrogenases operate by the combined action of acid-base and electron-transfer. As has been previously shown by both biophysical studies2 and synthetic modeling,3 the catalytic properties of the active site of the [FeFe] enzyme are enabled by the juxtaposition of functional groups dedicated to substrate binding, specifically the azadithiolate cofactor and the distal Fe center. This active site also features two redox-active components, the Fe2(SR)2 and the Fe4S4 subsites, each of which provides 1e− required for the two-electron H2/2H+ couple. In recent years, the advantageous cooperative reactivity of the amine cofactor and one Fe center has been demonstrated in models,4 which enables highly active proton-reduction catalysts. Unsolved in previous models is the ability of the same enzyme to activate H2, an excellent substrate for the enzyme.2b,2c
The activation of H2 by diiron models requires that the Fe2 center be (i) sufficiently electrophilic to attract H2 but (ii) not so electrophilic to induce binding of the amine to Fe.5 For a variety of ligands, the mixed-valence complexes of the type [Fe2[(SCH2)2NR](CO)3-x(PR3)x]+ almost satisfy these criteria, but such models are very slow to activate H2, requiring high pressures and many hours. In this report we show that rapid H2 activation by these Hox models can be achieved by the addition of a mild and fast oxidant.
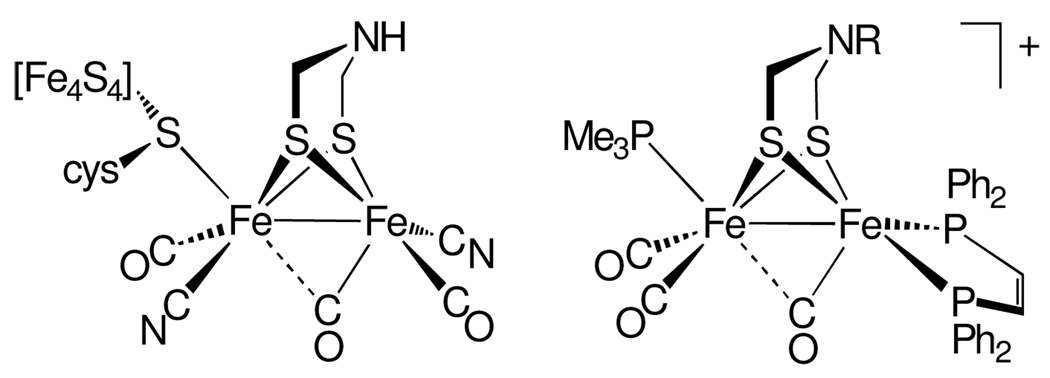
Previously, we showed that the Hox model [Fe2[(SCH2)2NBn](CO)3(dppv)(PMe3)]+ ([1]+, dppv = cis-C2H2(PPh2)2, Figure 1) reacts with H2 only slowly (>26 h, 25 °C, 1800 psi H2).6 We have now discovered that the same complex in the presence of 1 equiv of the mild oxidant [Fe(C5Me5)2]BArF4 (ArF = 3,5-C6H3(CF3)2) reacts with 2 atm of H2 quantitatively at 25 °C in hours to give the diferrous hydride product. The nearly isosteric complex that lacks the amine cofactor, [Fe2(S2C3H6)(CO)3(dppv)(PMe3)]+,5 is unreactive toward H2 under the same conditions.
Figure 1.
Active site of the Hox state of [FeFe]-hydrogenase (left) and the model [1]+ (right, R = CH2Ph).
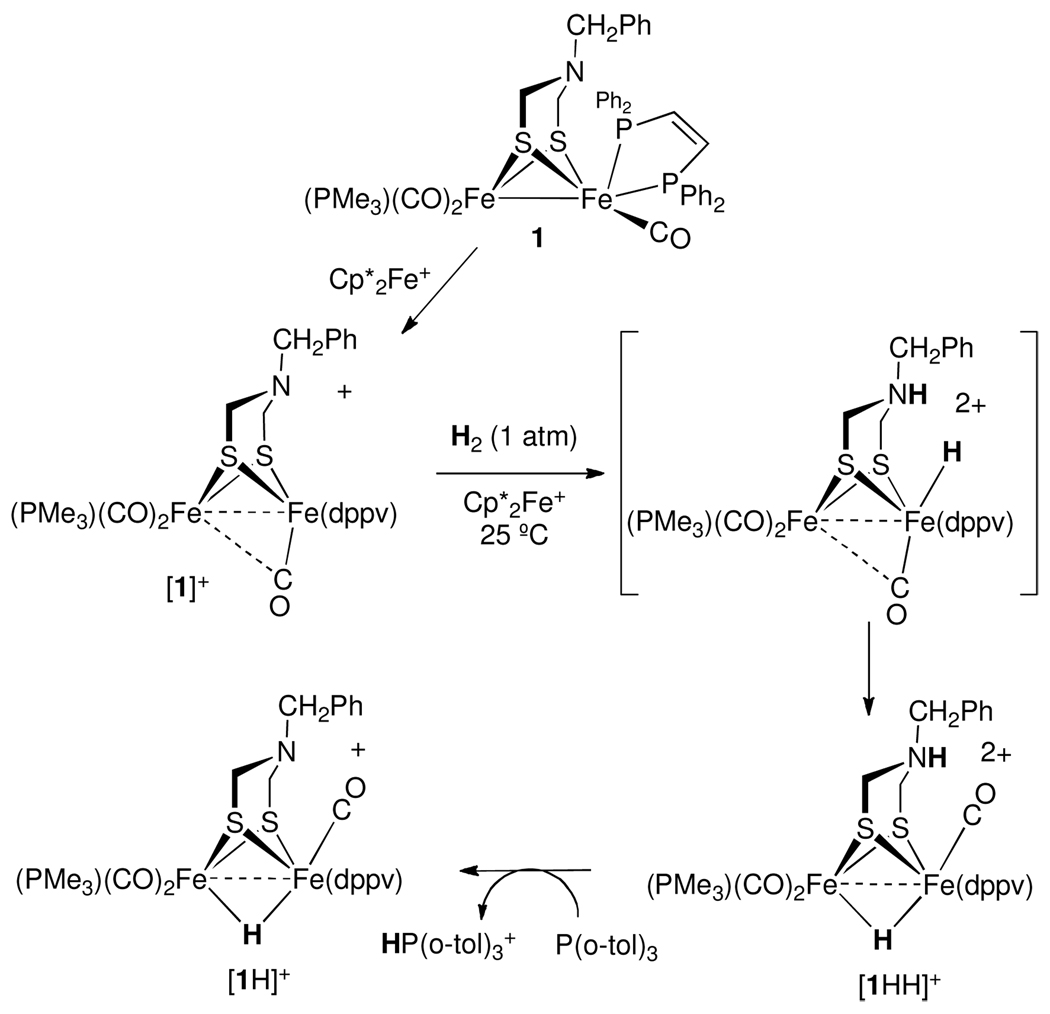
To simplify the analysis of the reaction by proton was trapped as [HP(o-tol)3]+. Heterolytic cleavage in the presence of P(o-tol)3 produced known hydride [1H]+ and [HP(o-tol)3]+, which undergoes slow proton transfer on the NMR timescale and displays distinctive 1H and 31P NMR signals. 2H NMR analysis of the same reaction in CH2Cl2 solution using D2 showed equal deuterium incorporation into [1D]+ and coproduct [DP(o-tol)3]+ (Supplementary Information). H2 activation is proposed to initially produce the diferrous ammonium hydride complex [1HH]2+. Subsequent deprotonation and rearrangement of the incipient terminal hydride complex leads to the final product that contains a bridging hydride, [1H]+ (Scheme 1).
Scheme 1.
Activation of H2 by [1]+ and Cp*2Fe+ to form [1H]+.
To investigate the role of the oxidant, we carried out reactions with various ferrocinium-derivatives,7 [MenFc]BArF4 ([MenFc]+ = [Fe(C5Me5)2]+, [Fe(C5Me4H)2]+, [Fe(C5Me5)(C5H5)]+, Table 1. Monitoring product formation by 1H NMR spectroscopy, we found that the rate of reaction was independent of oxidant strength (for the BArF4− salts: −593 to −313 mV vs Fc/FcBArF4)8. Furthermore the rate is unaffected by the concentration of the oxidant. These findings imply that electron transfer does not occur in or before the rate-determining step.
Table 1.
Observed Pseudo First-Order Rate Constants for the Conversion of 1 to [1]+ with Various Ferrocenium Oxidants. Conditions: 2 atm H2, 0 °C, CD2Cl2 solution, [1]o = [P(o-tol)3] = 4.67 mM.
| Oxidant | equiv MenFc+ |
E(MenFc+/0) vs E(Fc+/0) |
kobs (s−1)× 105 |
Conversion |
|---|---|---|---|---|
| [Me10Fc]+ | 2 | −593 mV | 2.2(3) | 100% |
| [Me10Fc]+ | 2* | −593 mV | 2.2(4) | 100% |
| [Me10Fc]+ | 4 | −593 mV | 2.7(3) | 100% |
| [Me8Fc]+ | 2 | −512 mV | 4.2(6) | >75% |
| [Me8Fc]+ | 4 | −512 mV | 4.2(5) | >75% |
| [Me5Fc]+ | 2 | −313 mV | 3.3(8) | >50% |
P(o-tol)3 omitted from reaction.
Having observed that oxidant did not affect the rate of H2 oxidation, we probed the effect of hydrogen pressure. When one equiv of oxidant was used, the appearance of product [1H]+ was strictly first order. Under these conditions, H2 dissolves quickly and was present in large excess. As a result, [H2] remains constant over the course of each experiment. A plot of kobs vs [H2] was linear, verifying a first order dependence on [H2]. These observations imply a rate law that only includes terms in [H2] and [Fe2].
| (1) |
The experimental rate law in eq 1 is consistent with two kinetic situations (Scheme 2). The first involves rate-determining binding of H2 followed by rapid oxidation and/or heterolysis. In the second mechanism, fast H2 binding is followed by rate-determining heterolysis to form the mixed-valence hydride, which is rapidly oxidized in a subsequent step. The acidity of transition metal dihydrogen complexes is well established.9 The favorability of H2 cleavage is expected to depend on the hydride-acceptor ability of the Fe2+ fragment. Also, numerous precedents show that the electrophilicity of metal centers correlates with their affinity for H2.9d Thus, more electrophilic diiron models should result in a faster reaction. An obvious choice of an electrophilic diiron center would be [Fe2[(SCH2)2NBn](CO)4(dppv)]+, a tetracarbonyl relative of [1]+.10 This mixed-valence compound was found to be unstable, probably owing to disproportionation caused by amine binding.5 We discovered however that a more rigid bulky diphosphine allowed isolation of the sought-for electrophilic, amine-containing Hox model. Specifically [Fe2[(SCH2)2NBn](CO)4(dppn)]+ ([2]+) (dppn = 1,8-bis(diphenylphosphino)naphthalene) was accessed in two steps from Fe2[(SCH2)2NBn](CO)6. According to cyclic voltammetric measurements on CH2Cl2 solutions (Figure S4), the [2]+/0 couple occurs at −254 mV, 390 mV more positive than the [1]+/0 couple.
Scheme 2.
The rate law for oxidation of H2 by [1]+ and ferrocenium is consistent with the rate determining step being H2 addition (step 1) or redox/heterolysis of the H2 complex (step 2).
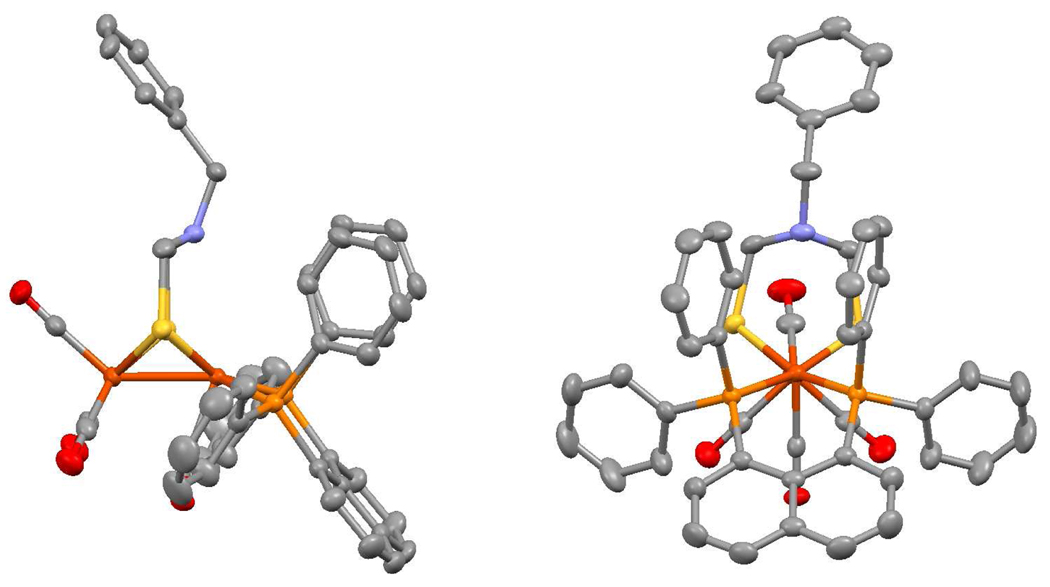
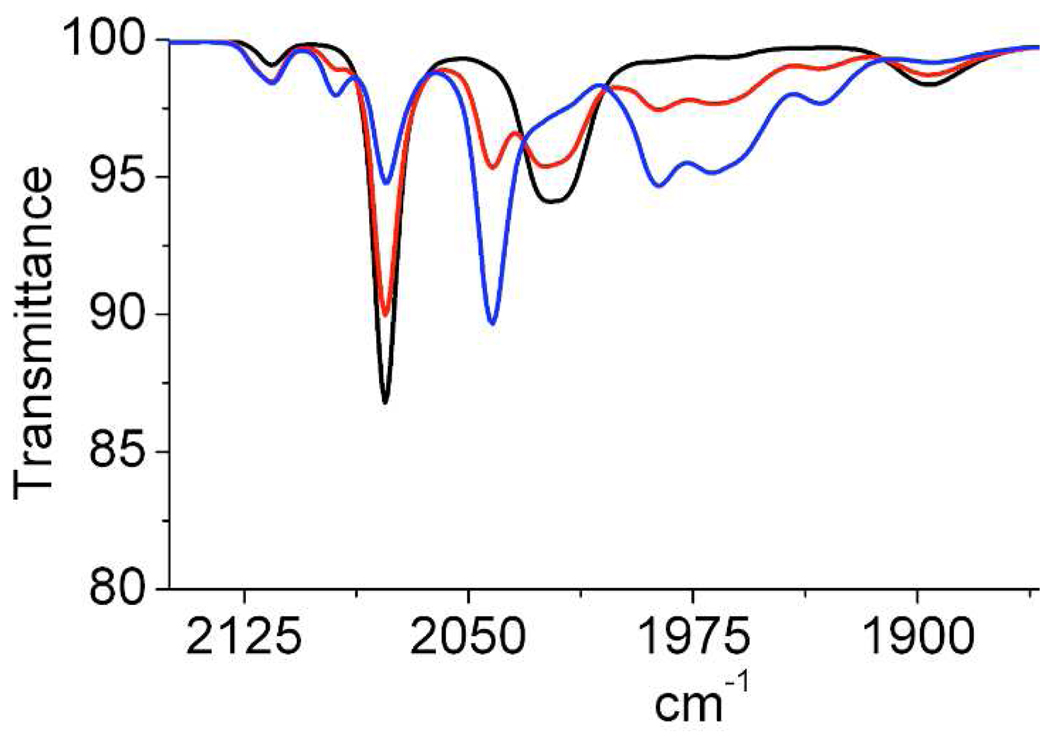
Treatment of a CH2Cl2 solution of 2 with 1 equiv of [Fc]BArF4 gave the mixed-valence salt [2]BArF4, solutions of which remained unchanged for up to 24 h at room temperature. The stability of this mixed-valence amine allowed us to obtain single crystals. Crystallographic analysis shows that the amine (proton acceptor) is poised over the electrophilic Fe center (hydride acceptor) only 3.2 Å away. The high stability of this organometallic frustrated Lewis pair11 is attributed to the steric shielding provided by a pair of phenyl rings that project axially from the dppn ligand (Figure 2). IR spectra in the υCO region show that [2]+ is far more electrophilic than is [1]+:υCO = 1896, 2022, 2078 vs 1870, 1965, 2017 cm−1 respectively (Figure 3).
Figure 2.
Structure of the cation in [Fe2[(SCH2)2NBn](CO)4(dppn)]BArF4. Selected bond lengths: (Fe-N) = 3.234(3), (Fe-Fe) = 2.568(1), (Fe-P) = 2.231(1) Å.
Figure 3.
IR spectra of a CH2Cl2 solution of [2]BArF4 and [Fc]BArF4 before (black) and 5 (red) and 14 min. (blue) after introducing H (1atm).
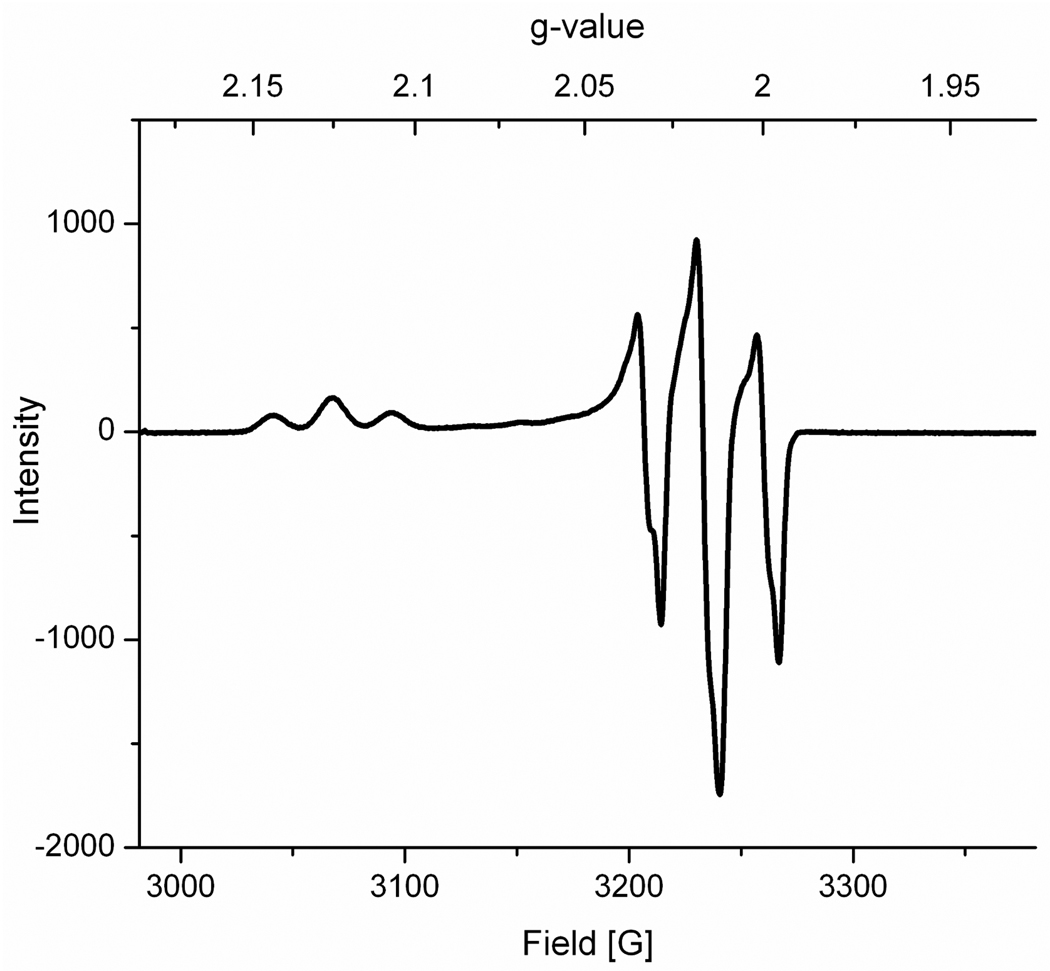
The EPR spectrum of [2]BArF4 features an axial pattern and exhibits triplets indicative of large hyperfine coupling to 31P. This suggests the assignment of [2]+ as formally [(dppn)(CO)FeI(μ-SR)2FeII(CO)3]+. The spectrum is similar to that reported for related cations such as [Fe2(S2C3H6)(CO)4(dppv)]+. It is likely that, as previously reported for [1]+, the electrophilic site is the iron center formally assigned as Fe(I).12
Gratifyingly, in the presence of one equiv of [Fc]BArF4, [2]+ was found to rapidly react with 1 atm H2 (t1/2 < 13 min at 20 °C). Monitoring the reaction by IR and 1H NMR spectroscopies confirmed the formation of the same ammonium hydride produced by treatment of 2 with 2 equiv of [H(OEt2)2]BArF4. Rate measurements showed H2 activation by [2]+/[Fc]+ to be 10× faster than by [1]+/[Fc]+ and 104× faster than [1]+ in the absence of a supplemental oxidant.
We measured the rates of reaction of a 1:1 mixture of [2]BArF4 and [Fc]BArF4 with H2 and D2 using UV-vis spectroscopy at 20 °C. The kinetic isotope effect was found to be kH/kD = 0.71(5). Reactions in which H2 cleavage is known to be rate determining typically exhibit normal kinetic isotope effect (eg kH/kD ~ 2.0).9d Although few reports describe kinetic isotope effects for H2 binding, 13 an inverse kinetic isotope effect has been observed for H2 binding to Ir(H)2Cl(PBut2Me)2.14 The inverse isotope effect measured for our reaction is inconsistent with rate-determining heterolytic cleavage of H2.
An enigmatic aspect of the present results is the observation that [1]+ and [2]+ do not serve as oxidants for the oxidation of H2 by a second equiv of the same cations. This finding may be explicable if the activation of H2 occurs via a concerted proton-coupled electron transfer (PCET), whereby intramolecular heterolysis of the H2 ligand depends on the rate of the electron-transfer. 15 Proton-transfer associated with the intramolecular heterolysis of the H2 adducts is expected to be extremely rapid,16 requiring a rapid oxidant. The rate of self-exchange for Fc+/0 is indeed fast (5 × 106 M−1s−1),17 but we propose that self-exchange for [1]+/0 and [2]+/0 are probably far slower owing to the substantial structural changes that accompany this redox process.18 Further work is required on these self-exchange rates.
We have shown H2 activation by the organometallic radical [Fe2[(SCH2)2NR](CO)xL6-x]+ requires the addition of an oxidant. It is intriguing that for heterolysis the oxidant must be both mild and fast. Kinetic measurements show that H2 binding is rate-determining. The present results point to the important role of PCET for the heterolytic activation of dihydrogen in this class of enzyme mimics.
Supplementary Material
Figure 4.
X-Band EPR spectrum of [2]BArF4 (110 K, 1:1 CH2Cl2:toluene frozen solution). Parameters: gz = 2.1260 with Az(31P) = 78 MHz, gx ≈ gy = 2.0165 with Ax(31P) ≈ Av(31P) = 79 MHz.
ACKNOWLEDGMENT
This research was supported by NIH. We thank Danielle Gray for solving the structure of [2]BArF4, Mark Nilges for assistance with EPR measurements, and Matthew Olsen for advice.
Footnotes
SUPPORTING INFORMATION. Experimental details. This material is available free of charge via the Internet at http://pubs.acs.org.
REFERENCES
- 1.(a) Rakowski Dubois M, Dubois DL. Acct. Chem. Res. 2009;42:1974–1982. doi: 10.1021/ar900110c. [DOI] [PubMed] [Google Scholar]; (b) Karunadasa HI, Chang CJ, Long JR. Nature. 2010;464:1329–1333. doi: 10.1038/nature08969. [DOI] [PubMed] [Google Scholar]; (c) Yang JY, Chen S, Dougherty WG, Kassel WS, Bullock RM, DuBois DL, Raugei S, Rousseau R, Dupuis M, Rakowski DuBois M. Chem. Commun. 2010;46:8618–8620. doi: 10.1039/c0cc03246h. [DOI] [PubMed] [Google Scholar]; (d) Dempsey JL, Brunschwig BS, Winkler JR, Gray HB. Acc. Chem. Res. 2009;42:1995–2004. doi: 10.1021/ar900253e. [DOI] [PubMed] [Google Scholar]
- 2.(a) Vincent KA, Parkin A, Armstrong FA. Chem. Rev. 2007;107:4366–4413. doi: 10.1021/cr050191u. [DOI] [PubMed] [Google Scholar]; (b) Fontecilla-Camps JC, Volbeda A, Cavazza C, Nicolet Y. Chem. Rev. 2007;107:4273–4303. doi: 10.1021/cr050195z. [DOI] [PubMed] [Google Scholar]; (c) Fontecilla-Camps JC, Amara P, Cavazza C, Nicolet Y, Volbeda A. Nature. 2009;460:814–822. doi: 10.1038/nature08299. [DOI] [PubMed] [Google Scholar]
- 3.(a) Gloaguen F, Rauchfuss TB. Chem. Soc. Rev. 2009;38:100–108. doi: 10.1039/b801796b. [DOI] [PMC free article] [PubMed] [Google Scholar]; (b) Tard C, Pickett CJ. Chem. Rev. 2009;109:2245–2274. doi: 10.1021/cr800542q. [DOI] [PubMed] [Google Scholar]
- 4.Barton BE, Olsen MT, Rauchfuss TB. J. Am. Chem. Soc. 2008;130:16834–16835. doi: 10.1021/ja8057666. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Olsen MT, Rauchfuss TB, Wilson SR. J. Am. Chem. Soc. 2010;132:17733–17740. doi: 10.1021/ja103998v. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Olsen MT, Barton BE, Rauchfuss TB. Inorg. Chem. 2009;48:7507–7509. doi: 10.1021/ic900850u. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Connelly NG, Geiger WE. Chem. Rev. 1996;96:877–922. doi: 10.1021/cr940053x. [DOI] [PubMed] [Google Scholar]
- 8.Geiger WE, Barrière F. Acc. Chem. Res. 2010;43:1030–1039. doi: 10.1021/ar1000023. [DOI] [PubMed] [Google Scholar]
- 9.(a) Jia G, Lough AJ, Morris RH. Organometallics. 1992;11:161–171. [Google Scholar]; (b) Chinn MS, Heinekey DM. J. Am. Chem. Soc. 1987;109:5865–5867. [Google Scholar]; (c) Heinekey DM, Oldham WJJ. Chem. Rev. 1993;93:913–926. [Google Scholar]; (d) Kubas GJ. Metal Dihydrogen and σ-Bond Complexes. New York: Kluwer Academic/Plenum; 2001. [Google Scholar]
- 10.Justice AK, Zampella G, De Gioia L, Rauchfuss TB, van der Vlugt JI, Wilson SR. Inorg. Chem. 2007;46:1655–1664. doi: 10.1021/ic0618706. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Stephan DW. Dalton Trans. 2009:3129–3136. doi: 10.1039/b819621d. and references therein.
- 12.Justice AK, De Gioia L, Nilges MJ, Rauchfuss TB, Wilson SR, Zampella G. Inorg. Chem. 2008;47:7405–7414. doi: 10.1021/ic8007552. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Heinekey DM. J. Labelled Compd. Radiopharm. 2007;50:1063–1071. [Google Scholar]
- 14. Hauger BE, Gusev D, Caulton KG. J. Am. Chem. Soc. 1994;116:208–214. Although the inverse KIE for H2 binding is not explicitly reported in this paper, it can be calculated from the EIE for H2 complex formation and the KIE for H2 dissociation.
- 15.(a) Huynh MHV, Meyer TJ. Chem. Rev. 2007;107:5004–5064. doi: 10.1021/cr0500030. [DOI] [PMC free article] [PubMed] [Google Scholar]; (b) Mayer JM. Ann. Rev. Phys. Chem. 2004;55:363–390. doi: 10.1146/annurev.physchem.55.091602.094446. [DOI] [PubMed] [Google Scholar]
- 16.Anslyn EV, Dougherty DA. Modern Physical Organic Chemistry. Sausalito: University Science Books; 2004. [Google Scholar]
- 17.(a) Nielson RM, Hupp JT. Inorg. Chem. 1996;35:1402–1404. doi: 10.1021/ic951191n. [DOI] [PubMed] [Google Scholar]; (b) Nielson RM, McManis GE, Safford LK, Weaver MJ. J. Phys. Chem. 1989;93:2152–2157. [Google Scholar]
- 18.(a) Liu T, Darensbourg MY. J. Am. Chem. Soc. 2007;129:7008–7009. doi: 10.1021/ja071851a. [DOI] [PubMed] [Google Scholar]; (b) Justice AK, Rauchfuss TB, Wilson SR. Angew. Chem., Int. Ed. 2007;46:6152–6154. doi: 10.1002/anie.200702224. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.