Abstract
Recent studies have indicated that the Shaker potassium channel regulates sleep in Drosophila. The Drosophila quiver (qvr) gene encodes a novel potassium channel subunit that modulates the Shaker potassium channel. The Qvr peptide contains a signal sequence for extracellular localization. Qvr may regulate a unique feature of the Shaker IA current that confers special neuronal excitability patterns. Studies of the Shaker channel properties in the qvr mutation background should provide an opportunity to uncover physiologic modulation of potassium channels. We have begun to investigate the impact of qvr protein on the Shaker channel properties and its implications in synaptic function in vivo. We studied synaptic transmission at the larval neuromuscular junction and characterized the transient potassium current IA in larval muscles. We identified two different functional states of IA in qvr larval muscles, as reflected by two distinct components, IAF and IAS, differing in their kinetics of recovery from inactivation and sensitivity to a K+ channel blocker. Correspondingly, qvr mutant larvae exhibit multiple synaptic discharges following individual nerve stimuli during repetitive activity.
INTRODUCTION
Potassium channels are ubiquitous in organisms from bacteria to humans (Hille, 2001). The demand for appropriate membrane excitability is met by a large repertoire of potassium channels individually distinct in current amplitude, as well as temporal dynamics of activation, inactivation and recovery from inactivation. There are several well-established mechanisms to generate this functional diversity. First, there are multiple genes encoding potassium channel subunits with different properties, some of which are gated by membrane potential, while others gated by ligand binding, e.g. to calcium or cyclic nucleotides. Second, the coding region of some potassium channel genes exhibits alternative splicing, yielding isoforms of a subunit. Third, different pore-forming subunits can form heteromultimeric assembly. Furthermore, auxiliary subunits can interact with the channel assembly to modulate potassium channel properties under different cellular conditions. Much of the insight into the functional diversity of potassium channels has been gained from in vivo studies of potassium channel mutations in conjunction with in vitro heterologous expression of single or multiple potassium channel subunits.
Studies of several Drosophila mutants that exhibit the peculiar leg-shaking phenotype have identified: Shaker (Sh) and ether a go-go (eag) as genes encoding the pore-forming α subunit of potassium channels, as well as Hyperkinetic (Hk) as a gene encoding the auxiliary β subunit capable of interacting with Sh and eag subunits (Chouinard, Wilson, Schlimgen, & Ganetzky, 1995; Kamb, Tseng-Crank, & Tanouye, 1988; Kaplan & Trout, 1969; Pongs et al., 1988; Schwarz, Tempel, Papazian, Jan, & Jan, 1988; Wang & Wu, 1996; Warmke, Drysdale, & Ganetzky, 1991). Genetic dissections have revealed the contribution of each subunit to the biophysical properties of a potassium channel and its role in controlling membrane excitability (Wu & Ganetzky, 1992). Computer assisted behavioral analysis reveals quantifiable distinctions in defects of larval locomotion behaviors caused by such K+ channel mutations (Wang, Soll, & Wu, 2002; Wang et al., 1997). Physiological experiments have shown that mutations of these three genes impair the transient K+ current (IA) in Drosophila larval muscles (Salkoff & Wyman, 1981; Wang & Wu, 1996; Wu, Ganetzky, Haugland, & Liu, 1983; Wu & Haugland, 1985; Zhong & Wu, 1991a) and neurons (Baker & Salkoff, 1990; Tanouye & Ferrus, 1985; Yao & Wu, 1999; Zhao, Sable, Iverson, & Wu, 1995). Furthermore, these channel mutations enhance synaptic transmission at the larval neuromuscular junctions (Ganetzky & Wu, 1983; Jan, Jan, & Dennis, 1977; Stern & Ganetzky, 1989; Ueda & Wu, 2009a; Wu et al., 1983), suggesting that these K+ channel subunits play a role in terminating neurotransmitter release at presynaptic terminals. Studies of additional Drosophila mutants with phenotypes similar to Sh, eag and Hk should generate more insight into the specific biophysical properties of the potassium channel that are crucial for individual behavioral phenotypes.
In a previous study, we demonstrated that another Drosophila leg-shaking mutant displays enhanced neurotransmission at the larval neuromuscular junction, reduced and slower IA current in larval muscles, which is caused by mutation in the gene named quiver (qvr) (Wang, Humphreys, Phillips, Hilliker, & Wu, 2000). Two recent studies have indicated that normal IA current is necessary for regulating sleep in Drosophila. First, Sh mutant flies exhibit reduction in sleep amount (Cirelli et al., 2005). Second, a large scale genetic screen for abnormal sleep behavior in Drosophila has identified an extreme mutant called sleepless which turns out to be an allele of qvr (Koh et al., 2008). The qvr gene encodes a putative glycosylphosphatidylinositol (GPI)-anchored membrane peptide enriched in fly brain. The Qvr peptide contains a signal sequence for extracellular localization, which is confirmed by immunostaining of cultured cells expressing qvr (Koh et al., 2008). These results immediately suggest that physiologic states such as wakefulness may have a functional link to the biophysical properties of the Sh channel via Qvr. Furthermore, through this unprecedented interaction with the Sh channel from an extracellular domain, Qvr may regulate a unique feature of IA current that confer special neuronal excitability patterns.
Studies of the Sh channel properties in the qvr mutation background should provide an opportunity to uncover physiologic modulation of potassium channels. We have begun to investigate the impact of qvr protein on the Sh channel properties and its implications in synaptic function in vivo. We studied synaptic transmission at the larval neuromuscular junction and characterized the properties of transient potassium current IA in larval muscles. We identified two different functional states of IA in qvr larval muscles, as reflected by two distinct components, IAF and IAS, differing in their kinetics of recovery from inactivation and sensitivity to a K+ channel blocker. Correspondingly, qvr mutant larvae exhibit multiple synaptic discharges following individual nerve stimuli during repetitive activity. This work has been described in part in a Ph.D. thesis (Wang, 1997).
METHODS
Drosophila Mutants
All flies were raised at room temperature (20–23°C) and fed with standard medium. The parental stock, qvr+; ry+5, for generating the qvr1 mutant, was originally derived from Oregon-R strain and used in this study as a control. qvrΔ43-1 is a homozygous lethal allele of qvr, generated by mobilization and imprecise excision of a nearby P-element P[17en43] (Humphreys, 1996).
Sh5, ShM, g sd ShrKO120 (abbreviated as Sh120 in the text), Hk1 and eag1 were originally from the collection of Dr. Seymour Benzer at the California Institute of Technology, California. ShM is a null allele (Zhao et al., 1995) and eliminates IA in larval muscles (Wu & Haugland, 1985). Sh5 is a point mutation in the S4–S5 linker (Gautam & Tanouye, 1990), and alters the voltage dependence of IA (Gautam & Tanouye, 1990; McCormack et al., 1991; Wu & Haugland, 1985). eag4pm has been identified as a spontaneous mutation in the original stock ShrKO120 (Ganetzky & Wu, 1983). ShrKO120 has a reduced IA current in larval muscles (Haugland & Wu, 1990), and produces a detectable level of Sh polypeptide (Zhao et al., 1995); its mutation site may lie in the 5' portion of the constant region (Gautam & Tanouye, 1990). Compound mutants were all confirmed by scoring leg-shaking phenotype and electrophysiological experiments.
Synaptic Transmission
As previously described, excitatory junctional currents (EJCs) were recorded intracellularly from muscles of abdominal segment 3–5 in third-instar larvae at 16°C in standard saline containing 4 mM MgCl2 (Zhong & Wu, 1991a). Larval dissection was performed in Ca2+-free saline to minimize muscle contraction. For wild-type control, the saline contained 0.2 mM Ca2+. Because of a drastically increased transmission caused by qvr mutations (Wang et al., 2000), [Ca2+]o was lowered from 0.2 to 0.1 mM to allow a quantitative analysis of the altered synaptic mechanism in the qvr1 mutant. Importantly, synaptic transmission at wild-type neuromuscular junctions did not show frequency-dependent enhancement either at 0.2 mM Ca2+ (Fig. 1) or at 0.1 mM Ca2+ (data not shown).
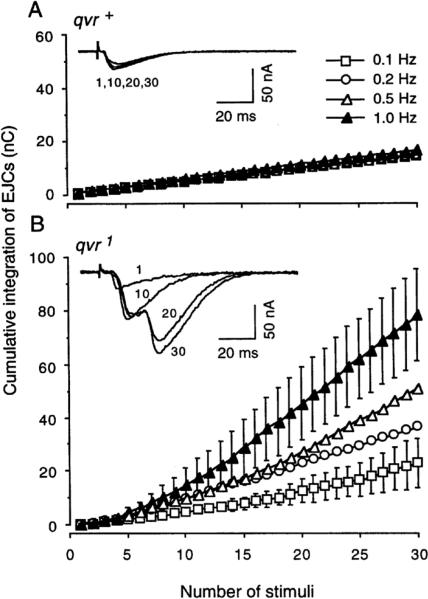
Figure 1.
Frequency-dependent enhancement in synaptic transmission at qvr1 mutant neuromuscular junctions. The integration of EJCs over time rather than a simple measurement from the peak EJC is more indicative of the amount of transmitter release in this case. Segmental nerve fibers were stimulated at indicated repetition rates for a duration of 0.1 ms with a voltage above the higher of the two thresholds that could evoke synaptic transmission in the two motor axon terminals. Representative traces were obtained at 0.8 Hz. A. Larval preparation was immersed in standard saline containing 0.2 mM CaCl2 and 4 mM MgCl2. SEM (n = 4) are smaller than the symbols, therefore not shown. B. Repetitive stimulations of mutant neuromuscular junctions gradually led to multiple EJCs. Standard saline contained 0.1 mM CaCl2 and 4 mM MgCl2. Under this condition, the initial EJCs were about the same as those from qvr+ larvae. Mean ± SEM (n = 4) is shown at 0.1 and 1.0 Hz, and error bars are omitted from plots at 0.2 and 0.5 Hz for simplicity. 16°C.
Muscle fibers were maintained at −80 mV with two-electrode voltage clamp. A suction pipette with a tip opening of about 1 μm was employed to stimulate the segmental nerve to evoke synaptic transmission. Stimulations with a duration of 0.1 ms were delivered at a low repetition rate from 0.1 to 0.5 Hz with a Grass stimulator (Model S88). Normally, two discrete EJCs were evoked at two different thresholds, representing signals generated by Is and Ib boutons (Ueda & Wu, 2009b). In all the experiments presented in this study, a stimulus voltage slightly higher than the upper threshold was used. Signals were low-pass filtered at 2 kHz (Model 3202R, Krohn-Hite, Avon, MA). Temperature deviation from room temperature was controlled by a Peltier stage (Cambion, Cambridge, MA).
Voltage-gated K+ Currents in Larval Muscles
The two-electrode voltage clamp technique for measuring IA has been described previously (Haugland & Wu, 1990; S. Singh & Wu, 1989; Wang & Wu, 1996). In brief, third instar larvae were dissected to make body wall muscles accessible, and the voltage-gated IA and IK were recorded in Ca2+-free standard saline containing 128 mM NaCl, 2 mM KCl, 14 mM MgCl2, 35 mM sucrose, 5 mM EGTA, and 5 mM HEPES (pH 7.1) at 11°C. A two-second pre-conditioning pulse to −20 mV from a holding potential of −80 mV inactivates IA but does not affect IK. The subtraction of the current with pre-conditioning pulse from the one without produces IA. Data acquisition was performed with an IBM-compatible computer equipped with PClamp software (Version 5) in conjunction with a Master-8 programmable stimulator (AMPI) for generating depolarizing voltage. Data were analyzed off-line on Macintosh computers with AxoGraph 2.0 software (Axon Instrument). For measuring the sensitivity of IA to 4-aminopyridine (4-AP, from Sigma), muscle fibers were incubated in saline containing the indicated concentrations of drug for at least 15 min.
RESULTS
Abnormal Synaptic Transmission in qvr1 Mutant Larvae
The larval neuromuscular junction is easily accessible to electrophysiological measurements, which has been well established to reveal the importance of a given gene in controlling neurotransmission (DiAntonio & Schwarz, 1994; Umbach et al., 1994; Wu & Ganetzky, 1992; Yoshihara, Adolfsen, Galle, & Littleton, 2005; Zhong & Wu, 1991b). We found that the qvr1 mutant displayed an abnormal form of frequency-dependent enhancement in synaptic transmission (Figure 1). In the wild-type control, excitatory junctional currents (EJCs) were very regular with little fluctuation when the segmental nerve fiber was stimulated at a rate of 0.8 Hz. As can be seen in the inset of Figure 1A, the EJCs in response to the first, 10th, 20th, and 30th stimuli were approximately the same in their size and kinetics. In contrast, the amplitude of EJCs in qvr1 mutant larvae increased progressively as the nerve was stimulated repetitively, which led to multiple releases as indicated by multi-peak EJCs. Figure 1B inset presents an example of EJCs in qvr1 mutant larvae when the nerve was stimulated at a rate of 0.8 Hz. The first stimulus generated a small EJC, followed with a larger EJC for the 10th stimulus, and multiple-peaked responses for the 20th and the 30th stimuli. The multiple peaks seen in qvr1 EJCs require an integration of EJCs over time to appropriately measure the amount of neurotransmitter release. As shown in Figure 1B, the rate of synaptic enhancement was higher when the nerve was stimulated at higher frequency.
Fast- and Slow-Recovery Components in Sh IA Currents Revealed by Mutation in the qvr Gene
Most invertebrate muscles, including that of Drosophila, do not express Na+ channel, and rely on Ca2+-mediated action potentials for muscle contraction (Schwartz & Stuhmer, 1984). A step depolarizing potential generates five major currents in Drosophila larval muscles. Four outward K+ currents including the voltage-gated transient IA and the delayed rectifier IK, and the Ca2+-dependent fast ICF and the slow ICF, plus an inward Ca2+ current (S. Singh & Wu, 1989). Genetic and pharmacological studies have shown that some of these currents consist of distinct components (Gielow, Gu, & Singh, 1995; A. Singh & Singh, 1999). In a previous study, we showed that the qvr mutations affect only the transient IA, but not IK, ICF, ICS or the calcium current (Wang et al., 2000).
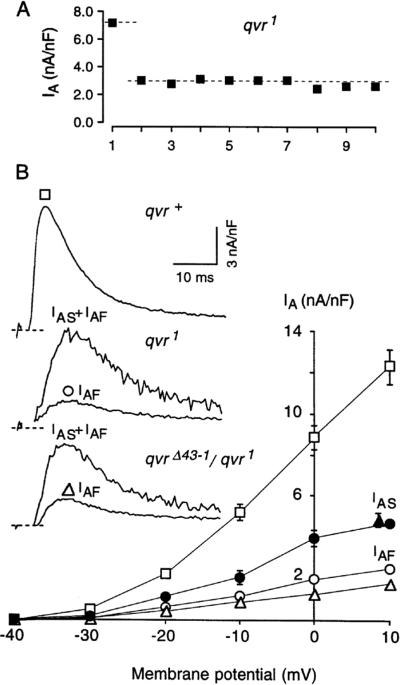
When ten episodes of a depolarizing step to +10 mV were applied to a qvr mutant muscle, the first outward transient current (IA) was more than twice that of the subsequent nine currents, but not as large as the wild-type current. The subsequent nine currents were almost the same size in amplitude (Figure 2A) with the same kinetics, suggesting that there were two components of IA in qvr mutant muscles. This contrasts with the use-dependent inactivation of a homogeneous component, which should display a gradual decay in the current amplitude upon repetitive depolarization. The first and the average of the subsequent nine IA were shown in Figure 2B for qvr1 and qvrΔ43-1/qvr1, in comparison with the IA amplitude of the wild-type control qvr+. As shown in Figure 2, the first and the subsequent IA currents displayed the same time to peak and the same inactivation kinetics, which could be visualized when the two traces were normalized (not shown). The fast- and slow-recovery components are therefore named IAF and IAS, respectively. Operationally, the first IA in Figure 2 is assumed to represent the sum of IAF and IAS, and the average of subsequent nine IA currents includes only IAF. The voltage dependence of IAF and IAS for qvr1 and qvrΔ43-1/qvr1 is presented in Figure 2B. The similarity in I-V curve between qvr1 and qvrΔ43-1/qvr1 suggests the observed phenotype is attributable to the qvr locus and not likely an effect of an unidentified second-site mutation in the background.
Figure 2.
Two discrete components IAF and IAS in qvr mutations. A. Ten episodes of +10 mV depolarizing steps were applied to a qvr1 muscle at a repetition rate of 0.05 Hz from a holding potential of −80 mV. The first depolarization generated a transient current of 7.2 nA/nF, however the subsequent depolarizations generated only a transient current of 3.0 nA/nF. The first IA includes IAF and IAS, and the average of the subsequent nine IA is defined as IAF. B. I-V curves for IAF and IAS. I-V curve for the IA of qvr+ is also plotted for comparison. Inset depicts typical traces from qvr+, qvr1 and qvrΔ43-1/qvr1 when muscles were depolarized to +10 mV. qvrΔ43-1 is homozygous lethal and generated from mobilization and imprecise excision of a nearby P element. n = 8, for qvr+. For mutants (qvr1 and qvrΔ43-1/qvr1), each muscle fiber was used for measurement at only one voltage; each data point was from 4–5 muscle fibers. Values are shown as mean ± SEM. Recordings at approximately the first 3 ms include a capacitance surge and were omitted from the figure. For this and the following figures, experiments were done in standard saline containing 5 mM EGTA and 14 mM MgCl2 (pH 7.1) at 11°C.
Differential Sensitivity of IAF and IAS to 4-AP
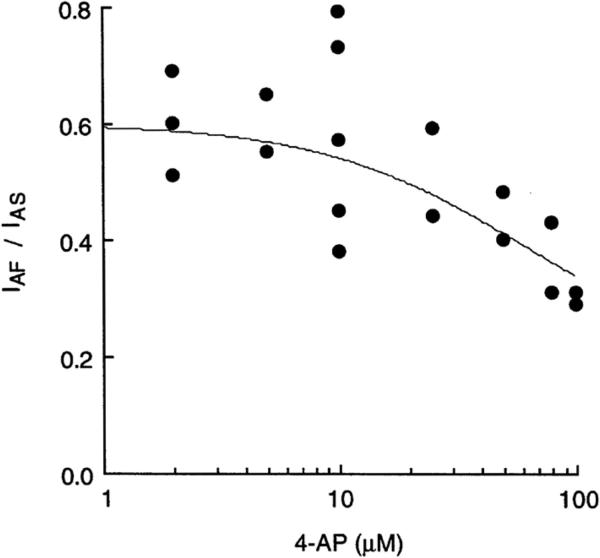
IA but not IK is blocked by 4-AP at micromolar concentrations in Drosophila larval muscles (Haugland & Wu, 1990). It is known that the binding affinity and the mode of action of a channel blocker often depend on the conformational state of the channel (Hille, 2001). Previous experiments have shown that mutations in the S4–S5 linker of the Sh α subunit (Haugland & Wu, 1990; Kirsch, Shieh, Drewe, Vener, & Brown, 1993; McCormack et al., 1991) or in the Hk β subunit (Wang & Wu, 1996; Yao & Wu, 1999) confer abnormal sensitivity to 4-AP, which has been shown to bind to the cytoplasmic pore region of the Sh channel. As shown in Figure 3, IAF was more sensitive to 4-AP than IAS, resulting in a smaller ratio of IAF/IAS when the concentration of 4-AP increased. The ratio IAF/IAS was about 0.3 when 100 μM 4-AP was applied, in contrast to a ratio of 0.58 ± 0.05 (n = 7) before drug treatment. The decreasing ratio of IAF to IAS at higher concentrations of 4-AP indicates that IAF and IAS have differential sensitivity to 4-AP and that they might have different channel conformations.
Figure 3.
Differential sensitivity to 4-AP for IAF and IAS. Each data point represents the ratio of IAF/IAS for one muscle fiber. The ratio was 0.58 ± 0.05 (n = 7) before drug treatment; it became smaller at higher concentrations, because IAF was more sensitive than IAS to 4-AP. The curve line indicates IC50 = 60 μM for IAF, and IC50 = 200 μM for IAS. Muscles were incubated for at least 15 min in saline containing indicated concentrations of 4-AP. Muscles were depolarized to +10 mV from a holding potential of −80 mV.
Genetic Dissection of IAF and IAS
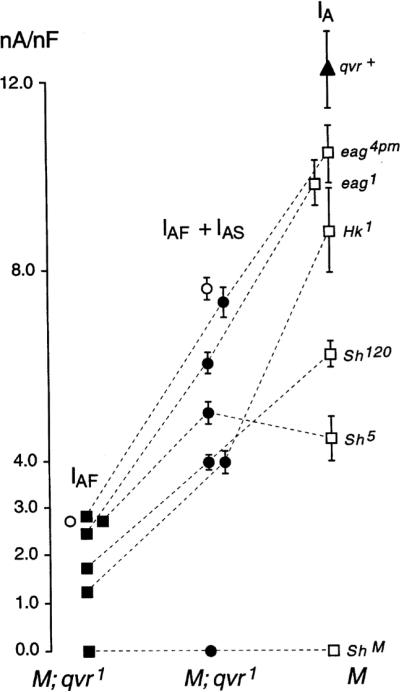
We next used a repertoire of IA channel mutants in Drosophila to further investigate channel assembly in double mutants with qvr. The sum of IAF and IAS of the double mutants, when compared with the IA of the corresponding single mutants, might yield information about the nature or constraints of interactions with the qvr product within the channel (see Figure 4). IAF + IAS in Sh5 qvr1 double mutant was about 5.0 ± 0.2 nA/nF, similar to the amplitude of IA in Sh5 mutant muscles (4.5 ± 0.5 nA/nF). In contrast, IAF + IAS in Sh120 qvr1 double mutant was only around 3.9 ± 0.2 nA/nF, significantly smaller than the observed 6.2 ± 0.6 nA/nF in Sh120 mutant muscles. Notably, as shown in Figure 4, (IAF + IAS)/IA reduction fell consistently in the range of 44–70% in Hk1qvr1, eag1qvr1 and eag4pmqvr1, as seen in Sh120qvr1 double mutants, compared with nearly unchanged for Sh5 qvr1 double mutants. Apparently, Sh5 mutation prevented the amplitude reduction of IA current in qvr1 mutant larvae. This discrepancy in the reduction of conductance conferred by the qvr1 mutation in different molecularly identified mutants may stem from the structural differences between Sh5 and Sh120, which have been shown to be a point mutation in the S4–S5 linker and a mutation with defect in the 5' portion of the constant region, respectively (Gautam & Tanouye, 1990).
Figure 4.
The peak amplitudes of IAF and IAS were differentially modulated by different K+ channel subunits. The sum of IAF and IAS, i.e., the IA current in response to the first depolarization, and IAF of double mutants are plotted in comparison to the IA currents seen in single mutants of Sh, Hk and eag. Sh5qvr1 mutation exhibited no statistically significant reduction in IAF and IAS from the IA current in Sh5. Open circles indicate qvr1; the filled triangle indicates qvr+ control. Muscles were depolarized to +10 mV from a holding potential of −80 mV. Sample size: qvr+, n = 8; eag4pm, 4; eag1, 4; Hk1, 4; Sh120, 6; Sh5, 5; qvr1, 13; eag4pmqvr1, 9; eag1qvr1, 12; Sh5qvr1, 11; Sh120qvr1, 8; Hk1qvr1, 10.
DISCUSSION
The involvement of the qvr K+ channel subunit in sleep regulation and its potential interaction with the Sh K+ channel from extracellular domain (Koh et al., 2008) call for more biophysical studies of the Qvr function. The present study shows that Qvr is important for the maintenance of neuronal excitability. Frequent stimulations of the motor axons in qvr mutant larvae generate discretely increased neuromuscular transmission. In a detailed biophysical characterization of the Sh potassium channel, we found that the transient IA current in qvr mutant muscles exhibits two discrete components, IAF and IAS, which are not observed in normal muscles. IAF displays faster kinetics of recovery from inactivation and more sensitivity to 4-AP than IAS. Furthermore, analysis of IAF and IAS in double mutants, Sh qvr, eag qvr and Hk qvr, identified a potential conformational change in the Sh K+ channel conferred by Qvr.
Our previous study shows that qvr mutations affect only the IA channel in both conductance and kinetics, without altering IK, ICF, ICS, and ICa, suggesting that the Qvr assumes a role in modulating the α-subunit of the IA channel (Wang et al., 2000). Its phenotypic similarities to the three known K+ channel mutants Sh, Hk and eag provide hints that the qvr gene might code for a distinct K+ channel subunit. The Qvr protein is predicted to contain a GPI-attachment site and the GPI anchor can be cleaved by PLC (Koh et al., 2008). We have investigated the potential conformational change conferred by the interaction between Qvr and the Sh K+ channel. First, we used a pharmacological approach. 4-AP has been well established as a Sh channel blocker that binds to the cytoplasmic pore region of the channel (Haugland & Wu, 1990; Kirsch et al., 1993; McCormack et al., 1991). The sensitivities to 4-AP of IAF and IAS showed an IC50 of 60 and 200 μM, respectively, which were much higher than the IC50 of only 7 μM in wild-type muscles (Wang & Wu, 1996). The differential 4-AP sensitivities in IAF and IAS imply different conformation in the cytoplasmic pore region. We then used several K+ channel mutants to identify potential site of conformational change. The Sh5 mutation, which has an amino acid replacement in the S4–S5 linker of the Sh polypeptide (Gautam and Tanouye, 1990), caused an increase in 4-AP sensitivity (Haugland & Wu, 1990). Among several different double mutants, Sh5qvr1 exhibited a special property: a sum amplitude of IAF and IAS is on par with the IA amplitude in Sh5 mutant muscles. These results suggest that the interaction between Qvr and the Sh channel confers conformational change at the Sh5 mutation site.
The excitability control of the motor neurons can be attributed to the function of the Sh channel. It is interesting to note that the 4-AP sensitive IA current plays an important role in the gating of action potentials in hippocampal CA3 pyramidal neurons (Debanne, Guerineau, Gahwiler, & Thompson, 1997). Action potentials in the pyramidal neurons can be blocked by the activation of the IA current with a brief hyperpolarization a few milliseconds before the induction of an action potential. The discrete increase in EJC at the neuromuscular junction in Drosophila larvae suggests that Qvr is important in the controlling the propagation of action potentials. This result is in accord with previous findings that efficient membrane repolarization is required to suppress supernumerary action potentials in the motor axon (Ueda & Wu, 2006).
Sleep is thought as a physiological state that increases the efficiency of behavior by regulating its timing and energy use (Siegel, 2009). Studies of rat cerebral energy consumption suggest that about half of the brain energy is used to drive signals along axons and across synapse (Laughlin & Sejnowski, 2003). Our study suggests that modulation of the Sh K+ channel by Qvr is a potential gating mechanism for the generation of action potentials in the nervous system. One can speculate that physiologic modulation of Qvr function plays an important role in controlling action potentials in relevant neural circuit for sleep. However, qvr expression does not fluctuate with the circadian cycle (Koh et al., 2008). Therefore, future experiments to demonstrate whether and how wakefulness/sleep states modulate the biophysical properties of the Sh K+ channel, reminiscent of the comprehensive studies in the mammalian thalamocortical systems (McCormick & Bal, 1997), will be important to provide a mechanistic link between sleep and K+ channel function. Alternatively, it is possible that wakefulness/sleep states do not modulate Sh K+ channel and the abnormal sleep phenotype in K+ channel mutant flies is due to their hypersensitivity to sensory stimulations and a hypersensitive motor system. Future studies of Qvr function in Drosophila central brain with optical imaging may shed light on the role of Qvr in sleep regulation (Root et al., 2008; Wang et al., 2003).
REFERENCES
- Baker K, Salkoff L. The Drosophila Shaker gene codes for a distinctive K+ current in a subset of neurons. Neuron. 1990;4(1):129–140. doi: 10.1016/0896-6273(90)90449-p. [DOI] [PubMed] [Google Scholar]
- Chouinard SW, Wilson GF, Schlimgen AK, Ganetzky B. A potassium channel beta subunit related to the aldo-keto reductase superfamily is encoded by the Drosophila hyperkinetic locus. Proc Natl Acad Sci U S A. 1995;92(15):6763–6767. doi: 10.1073/pnas.92.15.6763. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cirelli C, Bushey D, Hill S, Huber R, Kreber R, Ganetzky B, et al. Reduced sleep in Drosophila Shaker mutants. Nature. 2005;434(7037):1087–1092. doi: 10.1038/nature03486. [DOI] [PubMed] [Google Scholar]
- Debanne D, Guerineau NC, Gahwiler BH, Thompson SM. Action-potential propagation gated by an axonal I(A)-like K+ conductance in hippocampus. Nature. 1997;389(6648):286–289. doi: 10.1038/38502. [DOI] [PubMed] [Google Scholar]
- DiAntonio A, Schwarz TL. The effect on synaptic physiology of synaptotagmin mutations in Drosophila. Neuron. 1994;12(4):909–920. doi: 10.1016/0896-6273(94)90342-5. [DOI] [PubMed] [Google Scholar]
- Ganetzky B, Wu CF. Neurogenetic analysis of potassium currents in Drosophila: synergistic effects on neuromuscular transmission in double mutants. J Neurogenet. 1983;1(1):17–28. doi: 10.3109/01677068309107069. [DOI] [PubMed] [Google Scholar]
- Gautam M, Tanouye MA. Alteration of potassium channel gating: molecular analysis of the Drosophila Sh5 mutation. Neuron. 1990;5(1):67–73. doi: 10.1016/0896-6273(90)90034-d. [DOI] [PubMed] [Google Scholar]
- Gielow ML, Gu GG, Singh S. Resolution and pharmacological analysis of the voltage-dependent calcium channels of Drosophila larval muscles. J Neurosci. 1995;15(9):6085–6093. doi: 10.1523/JNEUROSCI.15-09-06085.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haugland FN, Wu CF. A voltage-clamp analysis of gene-dosage effects of the Shaker locus on larval muscle potassium currents in Drosophila. J Neurosci. 1990;10(4):1357–1371. doi: 10.1523/JNEUROSCI.10-04-01357.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hille B. Ionic channels of excitable membranes. Third ed. Sinauer; Sunderland: 2001. [Google Scholar]
- Humphreys JM. University of Guelph. 1996 [Google Scholar]
- Jan YN, Jan LY, Dennis MJ. Two mutations of synaptic transmission in Drosophila. Proc R Soc Lond B Biol Sci. 1977;198(1130):87–108. doi: 10.1098/rspb.1977.0087. [DOI] [PubMed] [Google Scholar]
- Kamb A, Tseng-Crank J, Tanouye MA. Multiple products of the Drosophila Shaker gene may contribute to potassium channel diversity. Neuron. 1988;1(5):421–430. doi: 10.1016/0896-6273(88)90192-4. [DOI] [PubMed] [Google Scholar]
- Kaplan WD, Trout WE., 3rd. The behavior of four neurological mutants of Drosophila. Genetics. 1969;61(2):399–409. doi: 10.1093/genetics/61.2.399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kirsch GE, Shieh CC, Drewe JA, Vener DF, Brown AM. Segmental exchanges define 4-aminopyridine binding and the inner mouth of K+ pores. Neuron. 1993;11(3):503–512. doi: 10.1016/0896-6273(93)90154-j. [DOI] [PubMed] [Google Scholar]
- Koh K, Joiner WJ, Wu MN, Yue Z, Smith CJ, Sehgal A. Identification of SLEEPLESS, a sleep-promoting factor. Science. 2008;321(5887):372–376. doi: 10.1126/science.1155942. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Laughlin SB, Sejnowski TJ. Communication in neuronal networks. Science. 2003;301(5641):1870–1874. doi: 10.1126/science.1089662. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McCormack K, Tanouye MA, Iverson LE, Lin JW, Ramaswami M, McCormack T, et al. A role for hydrophobic residues in the voltage-dependent gating of Shaker K+ channels. Proc Natl Acad Sci U S A. 1991;88(7):2931–2935. doi: 10.1073/pnas.88.7.2931. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McCormick DA, Bal T. Sleep and arousal: thalamocortical mechanisms. Annu Rev Neurosci. 1997;20:185–215. doi: 10.1146/annurev.neuro.20.1.185. [DOI] [PubMed] [Google Scholar]
- Pongs O, Kecskemethy N, Muller R, Krah-Jentgens I, Baumann A, Kiltz HH, et al. Shaker encodes a family of putative potassium channel proteins in the nervous system of Drosophila. Embo J. 1988;7(4):1087–1096. doi: 10.1002/j.1460-2075.1988.tb02917.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Root CM, Masuyama K, Green DS, Enell LE, Nassel DR, Lee CH, et al. A presynaptic gain control mechanism fine-tunes olfactory behavior. Neuron. 2008;59(2):311–321. doi: 10.1016/j.neuron.2008.07.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Salkoff L, Wyman R. Genetic modification of potassium channels in Drosophila Shaker mutants. Nature. 1981;293(5829):228–230. doi: 10.1038/293228a0. [DOI] [PubMed] [Google Scholar]
- Schwartz LM, Stuhmer W. Voltage-dependent sodium channels in an invertebrate striated muscle. Science. 1984;225(4661):523–525. doi: 10.1126/science.6330898. [DOI] [PubMed] [Google Scholar]
- Schwarz TL, Tempel BL, Papazian DM, Jan YN, Jan LY. Multiple potassium-channel components are produced by alternative splicing at the Shaker locus in Drosophila. Nature. 1988;331(6152):137–142. doi: 10.1038/331137a0. [DOI] [PubMed] [Google Scholar]
- Siegel JM. Sleep viewed as a state of adaptive inactivity. Nat Rev Neurosci. 2009;10(10):747–753. doi: 10.1038/nrn2697. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Singh A, Singh S. Unmasking of a novel potassium current in Drosophila by a mutation and drugs. J Neurosci. 1999;19(16):6838–6843. doi: 10.1523/JNEUROSCI.19-16-06838.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Singh S, Wu CF. Complete separation of four potassium currents in Drosophila. Neuron. 1989;2(4):1325–1329. doi: 10.1016/0896-6273(89)90070-6. [DOI] [PubMed] [Google Scholar]
- Stern M, Ganetzky B. Altered synaptic transmission in Drosophila hyperkinetic mutants. J Neurogenet. 1989;5(4):215–228. doi: 10.3109/01677068909066209. [DOI] [PubMed] [Google Scholar]
- Tanouye MA, Ferrus A. Action potentials in normal and Shaker mutant Drosophila. J Neurogenet. 1985;2(4):253–271. doi: 10.3109/01677068509102322. [DOI] [PubMed] [Google Scholar]
- Ueda A, Wu CF. Distinct frequency-dependent regulation of nerve terminal excitability and synaptic transmission by IA and IK potassium channels revealed by Drosophila Shaker and Shab mutations. J Neurosci. 2006;26(23):6238–6248. doi: 10.1523/JNEUROSCI.0862-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ueda A, Wu CF. Effects of social isolation on neuromuscular excitability and aggressive behaviors in Drosophila: altered responses by Hk and gsts1, two mutations implicated in redox regulation. J Neurogenet. 2009a;23(4):378–394. doi: 10.3109/01677060903063026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ueda A, Wu CF. Role of rut adenylyl cyclase in the ensemble regulation of presynaptic terminal excitability: reduced synaptic strength and precision in a Drosophila memory mutant. J Neurogenet. 2009b;23(1–2):185–199. doi: 10.1080/01677060802471726. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Umbach JA, Zinsmaier KE, Eberle KK, Buchner E, Benzer S, Gundersen CB. Presynaptic dysfunction in Drosophila csp mutants. Neuron. 1994;13(4):899–907. doi: 10.1016/0896-6273(94)90255-0. [DOI] [PubMed] [Google Scholar]
- Wang JW. Electrophysiological and genetic analyses of Drosophila behavioral mutants: the functional roles of voltage-gated potassium channel subunits. University of Iowa; Iowa City: 1997. [Google Scholar]
- Wang JW, Humphreys JM, Phillips JP, Hilliker AJ, Wu CF. A novel leg-shaking Drosophila mutant defective in a voltage-gated K(+)current and hypersensitive to reactive oxygen species. J Neurosci. 2000;20(16):5958–5964. doi: 10.1523/JNEUROSCI.20-16-05958.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang JW, Soll DR, Wu CF. Morphometric description of the wandering behavior in Drosophila larvae: a phenotypic analysis of K+ channel mutants. J Neurogenet. 2002;16(1):45–63. doi: 10.1080/01677060213106. [DOI] [PubMed] [Google Scholar]
- Wang JW, Sylwester AW, Reed D, Wu DA, Soll DR, Wu CF. Morphometric description of the wandering behavior in Drosophila larvae: aberrant locomotion in Na+ and K+ channel mutants revealed by computer-assisted motion analysis. J Neurogenet. 1997;11(3–4):231–254. doi: 10.3109/01677069709115098. [DOI] [PubMed] [Google Scholar]
- Wang JW, Wu CF. In vivo functional role of the Drosophila hyperkinetic beta subunit in gating and inactivation of Shaker K+ channels. Biophys J. 1996;71(6):3167–3176. doi: 10.1016/S0006-3495(96)79510-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang JW, Wong AM, Flores J, Vosshall LB, Axel R. Two-photon calcium imaging reveals an odor-evoked map of activity in the fly brain. Cell. 2003;112(2):271–282. doi: 10.1016/s0092-8674(03)00004-7. [DOI] [PubMed] [Google Scholar]
- Warmke J, Drysdale R, Ganetzky B. A distinct potassium channel polypeptide encoded by the Drosophila eag locus. Science. 1991;252(5012):1560–1562. doi: 10.1126/science.1840699. [DOI] [PubMed] [Google Scholar]
- Wu CF, Ganetzky B. Neurogenetic studies of ion channels in Drosophila. Ion Channels. 1992;3:261–314. doi: 10.1007/978-1-4615-3328-3_9. [DOI] [PubMed] [Google Scholar]
- Wu CF, Ganetzky B, Haugland FN, Liu AX. Potassium currents in Drosophila: different components affected by mutations of two genes. Science. 1983;220(4601):1076–1078. doi: 10.1126/science.6302847. [DOI] [PubMed] [Google Scholar]
- Wu CF, Haugland FN. Voltage clamp analysis of membrane currents in larval muscle fibers of Drosophila: alteration of potassium currents in Shaker mutants. J Neurosci. 1985;5(10):2626–2640. doi: 10.1523/JNEUROSCI.05-10-02626.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yao WD, Wu CF. Auxiliary Hyperkinetic beta subunit of K+ channels: regulation of firing properties and K+ currents in Drosophila neurons. J Neurophysiol. 1999;81(5):2472–2484. doi: 10.1152/jn.1999.81.5.2472. [DOI] [PubMed] [Google Scholar]
- Yoshihara M, Adolfsen B, Galle KT, Littleton JT. Retrograde signaling by Syt 4 induces presynaptic release and synapse-specific growth. Science. 2005;310(5749):858–863. doi: 10.1126/science.1117541. [DOI] [PubMed] [Google Scholar]
- Zhao ML, Sable EO, Iverson LE, Wu CF. Functional expression of Shaker K+ channels in cultured Drosophila “giant” neurons derived from Sh cDNA transformants: distinct properties, distribution, and turnover. J Neurosci. 1995;15(2):1406–1418. doi: 10.1523/JNEUROSCI.15-02-01406.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhong Y, Wu CF. Alteration of four identified K+ currents in Drosophila muscle by mutations in eag. Science. 1991a;252(5012):1562–1564. doi: 10.1126/science.2047864. [DOI] [PubMed] [Google Scholar]
- Zhong Y, Wu CF. Altered synaptic plasticity in Drosophila memory mutants with a defective cyclic AMP cascade. Science. 1991b;251(4990):198–201. doi: 10.1126/science.1670967. [DOI] [PubMed] [Google Scholar]