Abstract
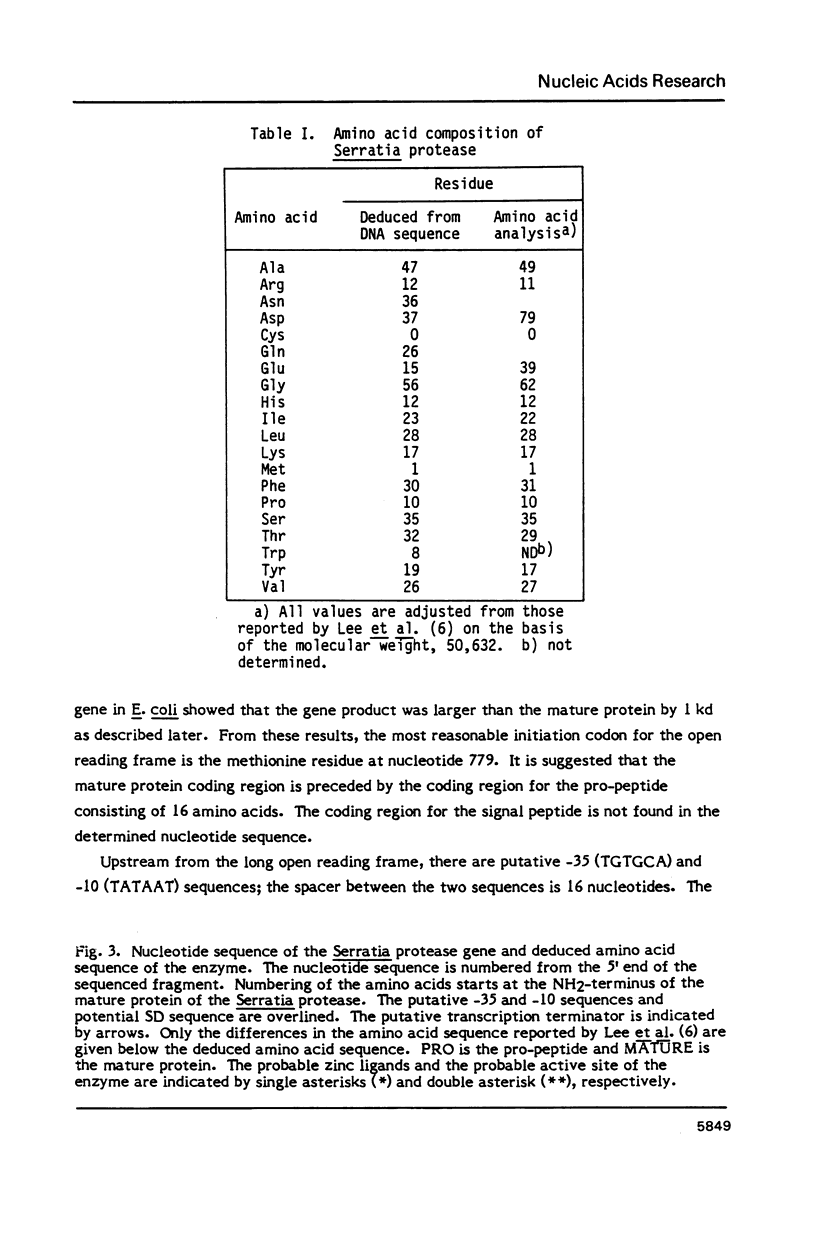
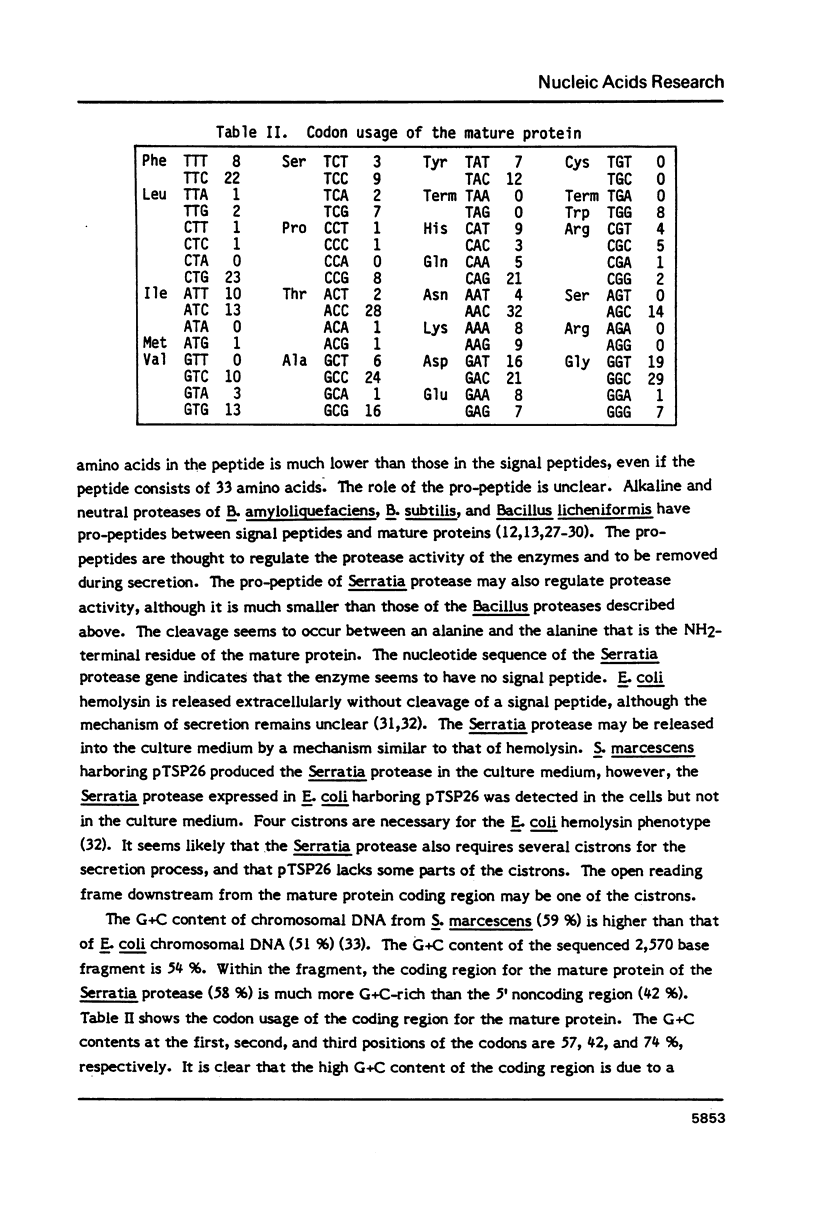
The gene encoding an extracellular metalloproteinase from Serratia sp. E-15 has been cloned, and its complete nucleotide sequence determined. The amino acid sequence deduced from the nucleotide sequence reveals that the mature protein of the Serratia protease consists of 470 amino acids with a molecular weight of 50,632. The G+C content of the coding region for the mature protein is 58%; this high G+C content is due to a marked preference for G+C bases at the third position of the codons. The gene codes for a short pro-peptide preceding the mature protein. The Serratia protease gene was expressed in Escherichia coli and Serratia marcescens; the former produced the Serratia protease in the cells and the latter in the culture medium. Three zinc ligands and an active site of the Serratia protease were predicted by comparing the structure of the enzyme with those of thermolysin and Bacillus subtilis neutral protease.
Full text
PDF







Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Birnboim H. C., Doly J. A rapid alkaline extraction procedure for screening recombinant plasmid DNA. Nucleic Acids Res. 1979 Nov 24;7(6):1513–1523. doi: 10.1093/nar/7.6.1513. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burnette W. N. "Western blotting": electrophoretic transfer of proteins from sodium dodecyl sulfate--polyacrylamide gels to unmodified nitrocellulose and radiographic detection with antibody and radioiodinated protein A. Anal Biochem. 1981 Apr;112(2):195–203. doi: 10.1016/0003-2697(81)90281-5. [DOI] [PubMed] [Google Scholar]
- Chou P. Y., Fasman G. D. Prediction of protein conformation. Biochemistry. 1974 Jan 15;13(2):222–245. doi: 10.1021/bi00699a002. [DOI] [PubMed] [Google Scholar]
- Felmlee T., Pellett S., Lee E. Y., Welch R. A. Escherichia coli hemolysin is released extracellularly without cleavage of a signal peptide. J Bacteriol. 1985 Jul;163(1):88–93. doi: 10.1128/jb.163.1.88-93.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Felmlee T., Pellett S., Welch R. A. Nucleotide sequence of an Escherichia coli chromosomal hemolysin. J Bacteriol. 1985 Jul;163(1):94–105. doi: 10.1128/jb.163.1.94-105.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jacobs M., Eliasson M., Uhlén M., Flock J. I. Cloning, sequencing and expression of subtilisin Carlsberg from Bacillus licheniformis. Nucleic Acids Res. 1985 Dec 20;13(24):8913–8926. doi: 10.1093/nar/13.24.8913. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jones J. D., Grady K. L., Suslow T. V., Bedbrook J. R. Isolation and characterization of genes encoding two chitinase enzymes from Serratia marcescens. EMBO J. 1986 Mar;5(3):467–473. doi: 10.1002/j.1460-2075.1986.tb04235.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaplan J. B., Merkel W. K., Nichols B. P. Evolution of glutamine amidotransferase genes. Nucleotide sequences of the pabA genes from Salmonella typhimurium, Klebsiella aerogenes and Serratia marcescens. J Mol Biol. 1985 Jun 5;183(3):327–340. doi: 10.1016/0022-2836(85)90004-x. [DOI] [PubMed] [Google Scholar]
- Katsuya Y., Hamada K., Hata Y., Tanaka N., Sato M., Katsube Y., Kakiuchi K., Miyata K. Preliminary X-ray studies on Serratia protease. J Biochem. 1985 Oct;98(4):1139–1142. doi: 10.1093/oxfordjournals.jbchem.a135364. [DOI] [PubMed] [Google Scholar]
- LENNOX E. S. Transduction of linked genetic characters of the host by bacteriophage P1. Virology. 1955 Jul;1(2):190–206. doi: 10.1016/0042-6822(55)90016-7. [DOI] [PubMed] [Google Scholar]
- Laemmli U. K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970 Aug 15;227(5259):680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- Lee I. S., Wakabayashi S., Miyata K., Tomoda K., Yoneda M., Kangawa K., Minamino N., Matsuo H., Matsubara H. Serratia protease. Amino acid sequences of both termini, the 53 residues in the middle region containing the sole methionine residue, and a probable zinc-binding region. J Biochem. 1984 Nov;96(5):1409–1418. doi: 10.1093/oxfordjournals.jbchem.a134969. [DOI] [PubMed] [Google Scholar]
- Levy P. L., Pangburn M. K., Burstein Y., Ericsson L. H., Neurath H., Walsh K. A. Evidence of homologous relationship between thermolysin and neutral protease A of Bacillus subtilis. Proc Natl Acad Sci U S A. 1975 Nov;72(11):4341–4345. doi: 10.1073/pnas.72.11.4341. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lovett P. S., Keggins K. M. Bacillus subtilis as a host for molecular cloning. Methods Enzymol. 1979;68:342–357. doi: 10.1016/0076-6879(79)68025-4. [DOI] [PubMed] [Google Scholar]
- Messing J., Crea R., Seeburg P. H. A system for shotgun DNA sequencing. Nucleic Acids Res. 1981 Jan 24;9(2):309–321. doi: 10.1093/nar/9.2.309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- POLLOCK M. R., RICHMOND M. H. Low cyst(e)ine content of bacterial extracellular proteins: its possible physiological significance. Nature. 1962 May 5;194:446–449. doi: 10.1038/194446a0. [DOI] [PubMed] [Google Scholar]
- Sanger F., Nicklen S., Coulson A. R. DNA sequencing with chain-terminating inhibitors. Proc Natl Acad Sci U S A. 1977 Dec;74(12):5463–5467. doi: 10.1073/pnas.74.12.5463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schmitz G., Braun V. Cell-bound and secreted proteases of Serratia marcescens. J Bacteriol. 1985 Mar;161(3):1002–1009. doi: 10.1128/jb.161.3.1002-1009.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vasantha N., Thompson L. D., Rhodes C., Banner C., Nagle J., Filpula D. Genes for alkaline protease and neutral protease from Bacillus amyloliquefaciens contain a large open reading frame between the regions coding for signal sequence and mature protein. J Bacteriol. 1984 Sep;159(3):811–819. doi: 10.1128/jb.159.3.811-819.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weaver L. H., Kester W. R., Matthews B. W. A crystallographic study of the complex of phosphoramidon with thermolysin. A model for the presumed catalytic transition state and for the binding of extended substances. J Mol Biol. 1977 Jul;114(1):119–132. doi: 10.1016/0022-2836(77)90286-8. [DOI] [PubMed] [Google Scholar]
- Wells J. A., Ferrari E., Henner D. J., Estell D. A., Chen E. Y. Cloning, sequencing, and secretion of Bacillus amyloliquefaciens subtilisin in Bacillus subtilis. Nucleic Acids Res. 1983 Nov 25;11(22):7911–7925. doi: 10.1093/nar/11.22.7911. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang M. Y., Ferrari E., Henner D. J. Cloning of the neutral protease gene of Bacillus subtilis and the use of the cloned gene to create an in vitro-derived deletion mutation. J Bacteriol. 1984 Oct;160(1):15–21. doi: 10.1128/jb.160.1.15-21.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]